Abstract
Recombinant bovine/human parainfluenza virus type 3 (rB/HPIV3), a recombinant bovine PIV3 (rBPIV3) in which the F and HN genes were replaced with their HPIV3 counterparts, was used to express the major protective antigens of respiratory syncytial virus (RSV) in order to create a bivalent mucosal vaccine against RSV and HPIV3. The attenuation of rB/HPIV3 is provided by the host range restriction of the BPIV3 backbone in primates. RSV G and F open reading frames (ORFs) were placed under the control of PIV3 transcription signals and inserted individually into the rB/HPIV3 genome in the promoter-proximal position preceding the nucleocapsid protein gene. The recombinant PIV3 expressing the RSV G ORF (rB/HPIV3-G1) was not restricted in its replication in vitro, whereas the virus expressing the RSV F ORF (rB/HPIV3-F1) was eightfold restricted compared to its rB/HPIV3 parent. Both viruses replicated efficiently in the respiratory tract of hamsters, and each induced RSV serum antibody titers similar to those induced by RSV infection and anti-HPIV3 titers similar to those induced by HPIV3 infection. Immunization of hamsters with rB/HPIV3-G1, rB/HPIV3-F1, or a combination of both viruses resulted in a high level of resistance to challenge with RSV or HPIV3 28 days later. These results describe a vaccine strategy that obviates the technical challenges associated with a live attenuated RSV vaccine, providing, against the two leading viral agents of pediatric respiratory tract disease, a bivalent vaccine whose attenuation phenotype is based on the extensive host range sequence differences of BPIV3.
Respiratory syncytial virus (RSV) and human parainfluenza virus type 3 (HPIV3) are nonsegmented negative-strand RNA viruses of the paramyxovirus family. RSV is the leading cause of severe viral respiratory disease in infants and children, followed by HPIV3 and the influenza viruses as the next most important agents. Together, RSV and HPIV3 are responsible for approximately one-third of all cases of pediatric respiratory tract disease leading to hospitalization (12, 22, 42), and in the United States RSV alone is estimated to account for between 73,000 and 126,000 annual hospitalizations of infants younger than 1 year (47). Although the estimated number of RSV-associated hospitalizations did not change significantly over the past 2 decades, the number of RSV-associated deaths in the United States has decreased over the same period from 4,500 to no more than 510 per annum, suggesting that medical care of patients with RSV bronchiolitis has improved (48).
A formalin-inactivated RSV vaccine developed in the 1960s failed to provide protection against RSV infection and indeed led to immune-mediated enhanced disease upon subsequent infection by wild-type (wt) RSV (38). Retrospective analysis and studies with rodent models suggest that this likely involved two factors: denaturation of the protective epitopes in the vaccine, resulting in the induction of antibodies that were poorly neutralizing (43, 45), and the nonreplicating nature of the vaccine, which might have resulted in stimulation of CD4+ but not CD8+ T cells (51). Since disease enhancement has never been associated with natural RSV infection or experimental live RSV vaccine candidates (61), our laboratory has focused on developing a live attenuated RSV vaccine to be administered intranasally to infants beginning in their first or second month of life (13, 15). To date, several live attenuated RSV vaccine candidates have been evaluated in clinical trials (31, 37, 60, 61), but a licensed RSV vaccine is still not available. One of the challenges in developing a live attenuated RSV vaccine is to achieve an appropriate balance between attenuation and immunogenicity (58). All of the RSV vaccine candidates tested to date were either overattenuated and insufficiently immunogenic (32, 60) or underattenuated, retaining some virulence in infants (34, 61). The most promising candidate, a cold-passaged (cp) temperature-sensitive (ts) RSV designated cpts248/404, was infectious, immunogenic, and protective against a second vaccine dose. However, this virus retained the ability to induce brief (∼24-h) upper respiratory tract congestion that interfered with feeding in some subjects in this 1- to 2-month-old target population and thus was considered to be somewhat underattenuated (61).
Protection against reinfection with RSV and HPIV3 is mainly conferred by serum and mucosal antibodies (17). The RSV G and F proteins and the HPIV3 HN and F proteins are the only significant neutralization antigens and are the major protective antigens. Cytotoxic CD8+ T lymphocytes are important in clearing viral infection and can confer resistance to reinfection, but this resistance is very short-lived and does not appear to be an important consideration for immunoprophylaxis (17, 39, 54). However, CD8+ T lymphocytes appear to be important in regulating the CD4+ T-lymphocyte response (29, 51).
Live attenuated vaccine candidates are also being developed against HPIV3, and two different vaccine candidates appear to have satisfactory levels of attenuation and immunogenicity in 1- to 2-month-old infants. One candidate is HPIV3 cp-45, a cold-passaged temperature-sensitive HPIV3 (18, 36), and the other is the Kansas strain of bovine PIV3 (BPIV3) (12, 19, 33, 35, 57). BPIV3 is attenuated for replication in humans due to a natural host range restriction. The use of host range restriction, i.e., the Jennerian or modified Jennerian approach, in the development of live attenuated virus vaccines was successfully employed to eradicate smallpox in nature, and it forms the basis for simian-human and bovine-human rotavirus vaccines (8, 30). It has also been employed as the basis of experimental vaccines against influenza A virus (7, 52, 53), parainfluenza virus type 1 (28), and hepatitis A virus (26). The attenuation phenotype of Jennerian vaccines in general appears to be very stable. Sequence comparison of BPIV3 and HPIV3 suggests that the evolution of the two viruses in their respective hosts has resulted in many host-specific nucleotide and amino acid differences that likely contribute to the host range restriction (2, 3).
We previously constructed a recombinant version of BPIV3 and replaced its F and HN glycoprotein genes with those of HPIV3 in order to improve the vaccine candidate's immunogenicity against HPIV3. This recombinant chimeric bovine/human PIV3 (rB/HPIV3) retained the attenuation phenotype of BPIV3 and was highly immunogenic in rhesus monkeys (46). In the present study, we used this chimeric rB/HPIV3 as a vector to express individually the RSV G or F open reading frame (ORF), inserted as an additional gene unit preceding the rB/HPIV3 N ORF. In consideration of the gradient of transcription in paramyxoviruses, this promoter-proximal position was chosen in order to maximize the level of expression of the RSV G and F ORFs. rB/HPIV3 recombinants expressing RSV G or F (rB/HPIV3-G1 or rB/HPIV3-F1, respectively) were fully viable, replicated efficiently in vitro and in the respiratory tract of hamsters, and induced protective immunity against RSV and HPIV3. This paper describes a new vaccine strategy that obviates the difficulty associated with attenuation, propagation, and handling of a live RSV vaccine and provides a bivalent vaccine against the two most important respiratory viruses of infants and children.
MATERIALS AND METHODS
Viruses and cells.
HEp-2 and simian LLC-MK2 monolayer cell cultures were maintained in minimum essential medium (Life Technologies, Gaithersburg, Md.) supplemented with 5% fetal bovine serum (Summit Biotechnology, Fort Collins, Colo.), 50 μg of gentamicin sulfate/ml, and 4 mM glutamine (Life Technologies). Vero cells were maintained in virus production serum-free medium (Life Technologies) supplemented with 50 μg of gentamicin sulfate/ml.
The wt BPIV3 strain Kansas/15626/84 (clone 5-2-4, lot BPI3-1) (BPIV3 Ka), its recombinant version (rBPIV3), and the rBPIV3 containing HPIV3 glycoprotein genes (rB/HPIV3, previously named rBPIV3-FHHNH) were previously described (6, 35, 46). The biological HPIV3 strain JS was also described previously (23). Parainfluenza viruses were propagated at 32°C in LLC-MK2 cells (ATCC CCL-7) or Vero cells (ATCC CCL-81), as previously described (27). The modified vaccinia strain Ankara (MVA) recombinant virus that expresses bacteriophage T7 RNA polymerase (MVA-T7) was generously provided by L. Wyatt and B. Moss (62).
Construction of antigenomic cDNAs encoding recombinant chimeric rB/HPIV3 viruses bearing the RSV G or F ORF as an additional gene insert.
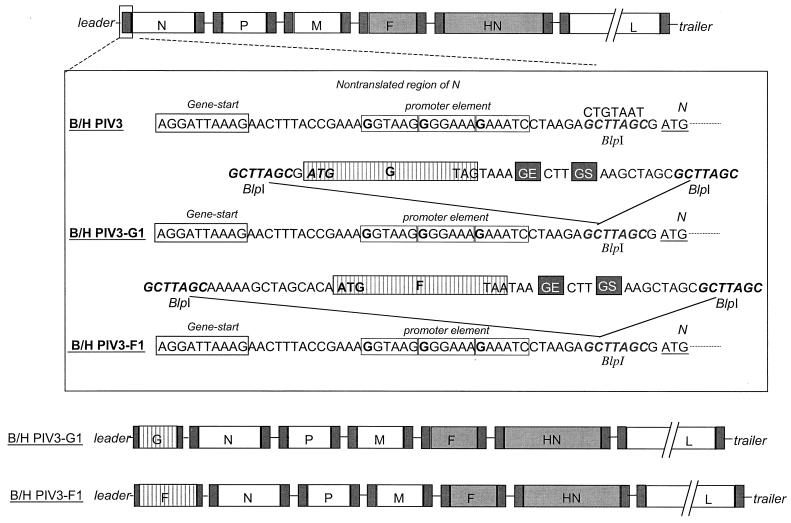
We previously constructed a full-length cDNA of the BPIV3 Kansas strain in which the F and HN glycoprotein genes of the bovine virus had been replaced with the corresponding genes of the HPIV3 JS strain to yield rB/HPIV3. Here, this cDNA was modified to contain three additional unique restriction enzyme recognition sites. Specifically, we used transformer site-directed mutagenesis (Clontech Laboratories, Palo Alto, Calif.) to introduce a BlpI site preceding the N ORF (nucleotides [nt] 103 to 109), an AscI site preceding the N gene end sequence (nt 1676 to 1683), and a NotI site preceding the P gene end sequence (nt 3674 to 3681). These restriction enzyme recognition sites were introduced to facilitate the insertion of foreign genes into the chimeric rB/HPIV3 virus genome: in the present paper, only the BlpI site was used. The sites were designed so that they did not disrupt any of the BPIV3 replication and transcription cis-acting elements.
The previously described RSV subgroup A glycoprotein genes G and F (GenBank accession no. M74568) (14) were modified for insertion into the promoter-proximal BlpI site of rB/HPIV3 (Fig. 1). The BlpI insertion site followed the gene start signal of the N gene and the putative promoter elements (56) at nt 79 to 95 (Fig. 1). For insertion at this site, the RSV ORF was modified by insertion of a BlpI site at its upstream end and addition of a BPIV3 gene end signal, intergenic region, gene start signal, and BlpI site at its downstream end.
FIG. 1.
Insertion of RSV G or F as an additional, promoter-proximal gene. The B/HPIV3 antigenomic cDNA was modified by introduction of a unique BlpI site (bold italics; the original sequence is shown on top) in the nontranslated region of the N gene preceding the start codon of the N ORF. PCR mutagenesis was used to add a PIV3 gene end (GE), intergenic region, and gene start (GS) signal immediately downstream of the G and F ORF stop signals, and BlpI sites were added on either side of the insert. The sequence AAGTAAGAAAAA was used as the gene end signal, and AGGATTAAAG was used as the gene start signal. Elements of the viral promoter that, by analogy to Sendai virus (56), are present in the N gene nontranslated region are indicated by the three boxed hexamers, with G residues in boldface.
For the G ORF of RSV A, the forward PCR primer used was (5′ to 3′) AATTCGCTTAGCGATGTCCAAAAACAAGGACCAACGCACCGC and the reverse primer was (5′ to 3′) AAAAAGCTAAGCGCTAGCCTTTAATCCTA AGTTTTTCTTACTTTTTTTACTACTGGCGTGGTGTGTTGGGTGGAGA TGAAGGTTGTGATGGG (BlpI site underlined, and ORF translational initiation and termination triplets in boldface). For the F ORF of RSV A, the forward PCR primer used was (5′ to 3′) AAAGGCCTGCTTAGCAAAAAGCTAGCACAATGGAGTTGCTAATCCTCAAAGCAAATGCAATTACC and the reverse primer was (5′ to 3′) AAAAGCTAAGCGCTAGCTTCTTTAATC CTAAGTTTTTCTTACTTTTATTAGTTACTAAATGCAATATTATTTATA CCACTCAGTTGATC (BlpI site underlined, and ORF translational initiation and termination triplets in boldface).
The PCR products were digested with BlpI and cloned into the modified full-length cDNA clone using standard molecular cloning techniques. The resulting full-length cDNA containing the G ORF of RSV A was designated pB/HPIV3-GA1, and the plasmid containing the F ORF was designated pB/HPIV3-FA1. The nucleotide sequence of each inserted gene was confirmed by restriction enzyme digestion and automated sequencing (data not shown). All constructs were designed so that the final genome nucleotide length was a multiple of six, which has been shown to be a requirement for efficient RNA replication (5).
Transfection.
HEp-2 cells (approximately 1.5 × 106 cells per well of a six-well plate) were grown to 90% confluency and transfected with BPIV3 support plasmids pTM(N) (0.2 μg), pTM(P) (0.2 μg), and pTM(L) (0.1 μg) and 5 μg of the full-length antigenomic cDNA, using 15 μl of Lipofectamine (Life Technologies) per well. Each transfection mixture also contained 1.5 × 107 PFU of MVA-T7, as previously described (23). The cultures were incubated at 32°C for 12 h before the medium was replaced with minimum essential medium (Life Technologies) containing 5% fetal bovine serum. The supernatants were harvested after incubation at 32°C for an additional 3 days and were then passaged onto Vero cell monolayers in 25-cm2 flasks and incubated for 5 days at 32°C. Virus present in the supernatant was biologically cloned by three sequential terminal dilutions prior to amplification and characterization.
Hamster studies.
Golden Syrian hamsters, which were seronegative for HPIV3 and RSV as determined by hemagglutination-inhibition (HAI) assay and RSV-specific neutralization assay, respectively (9, 57), were anesthetized with methoxyflurane and inoculated intranasally with 106 50% tissue culture infectious doses (TCID50) of rB/HPIV3-G1, rB/HPIV3-F1, rB/HPIV3, HPIV3 JS wt, or BPIV3 Ka wt. One group received both rB/HPIV3-G1 and rB/HPIV3-F1. In the replication study (Table 1), six hamsters per group were sacrificed 4 and 5 days after inoculation by CO2 inhalation, and nasal turbinate and lung tissues were harvested separately for virus quantification by serial dilution on LLC-MK2 cells, as described previously (20, 24). In the serology and challenge study (Tables 2 to 4), hamsters in groups of 12 were inoculated as described above and sera were collected from hamsters 3 days prior to and 26 days after the initial inoculation. They were tested for HPIV3 antibodies by HAI assay using HPIV3 JS as antigen and for RSV antibody by RSV-specific enzyme-linked immunosorbent assay (ELISA) using purified F and G glycoproteins, as previously described (16, 57). Titers of RSV-neutralizing antibodies were determined in a complement-enhanced 60% plaque reduction assay as previously described (10). On day 28 postinoculation, hamsters (six animals per group) were challenged intranasally with 106 TCID50 of HPIV3 JS or 106 PFU of RSV. Titers of challenge virus were determined 5 days after challenge by terminal dilution (HPIV3) or by plaque assay (RSV).
TABLE 1.
rB/HPIV3 bearing the RSV G or F ORF as an additional gene in the promoter-proximal position replicates efficiently in the respiratory tract of hamsters
| Immunizing virusa | No. of animals | Mean virus titerb (log10 TCID50/g ± SEc)
|
|||
|---|---|---|---|---|---|
| Day 4
|
Day 5
|
||||
| Nasal turbinates | Lungs | Nasal turbinates | Lungs | ||
| rB/HPIV3-G1 | 6 | 5.9 ± 0.1 (AB) | 5.1 ± 0.6 (A) | 5.5 ± 0.2 (A) | 5.6 ± 0.4 (AC) |
| rB/HPIV3-F1 | 6 | 5.1 ± 0.3 (B) | 4.6 ± 0.2 (A) | 5.7 ± 0.2 (AB) | 3.6 ± 0.2 (BD) |
| rB/HPIV3-G1 and rB/HPIV3-F1 | 6 | 5.7 ± 0.3 (BC) | 4.3 ± 0.8 (A) | 5.6 ± 0.2 (A) | 5.9 ± 0.2 (A) |
| rB/HPIV3 | 6 | 6.2 ± 0.2 (AC) | 5.2 ± 0.6 (A) | 6.5 ± 0.1 (B) | 5.7 ± 0.6 (AC) |
| HPIV3 JS wt | 6 | 6.6 ± 0.1 (A) | 6.5 ± 0.1 (A) | 6.0 ± 0.2 (AB) | 6.0 ± 0.4 (A) |
| BPIV3 Ka wt | 6 | 5.8 ± 0.1 (AB) | 6.1 ± 0.2 (A) | 5.3 ± 0.2 (A) | 4.2 ± 0.5 (CD) |
Hamsters were inoculated intranasally with 106 TCID50 of virus in a 0.1-ml inoculum.
Animals (six per group per day) were sacrificed on day 4 or 5 postinoculation, as indicated, and virus titers in the nasal turbinates and lungs were determined by titration on LLC-MK2 cells at 32°C. The limit of detectability of virus was 102.45 TCID50/g of tissue.
Mean virus titers were assigned to similar groups (A, B, C, and D) by the Tukey-Kramer test. Within each column, mean titers with different letters are statistically different (P < 0.05). Titers indicated with two letters are not significantly different from those indicated with either letter.
TABLE 2.
Immunization of hamsters with rB/HPIV3 expressing the RSV G or F ORF as an additional gene induces a serum antibody response against the RSV G or F protein, respectively
| Immunizing virusa | No. of animals/group | Serum immunoglobulin G ELISA titer (mean reciprocal log, ± SE) against RSV proteinb:
|
|||||
|---|---|---|---|---|---|---|---|
| G protein
|
F protein
|
||||||
| Prec | Day 26 | Log2 fold increase | Prec | Day 26 | Log2 fold increase | ||
| rB-HPIV3-G1 | 12 | 6.0 ± 0.4 | 12.5 ± 0.5 | 6.5 | 6.7 ± 0.5 | 7.5 ± 0.5 | 0.8 |
| rB/HPIV3-F1 | 12 | 6.3 ± 0.3 | 7.2 ± 0.3 | 0.9 | 6.8 ± 0.3 | 16.2 ± 0.5 | 9.4 |
| rB/HPIV3-G1 and rB/HPIV3-F1 | 12 | 6.5 ± 0.6 | 12.0 ± 0.9 | 5.5 | 7.3 ± 0.5 | 14.7 ± 0.4 | 7.4 |
| rB/HPIV3 | 12 | 6.5 ± 0.4 | 8.0 ± 0.4 | 1.5 | 7.3 ± 0.7 | 8.3 ± 0.8 | 1.0 |
| RSV | 12 | 6.8 ± 0.3 | 10.8 ± 0.4 | 4.0 | 7.5 ± 0.5 | 15.7 ± 0.4 | 8.2 |
Hamsters were inoculated intranasally with 106 TCID50 of virus in a 0.1-ml inoculum.
Serum samples were taken 3 days prior to inoculation (Pre) and 26 days postinoculation and analyzed by glycoprotein-specific ELISA for antibodies against RSV G or F protein, as indicated.
Titers in the “Pre” serum specimen represent nonspecific background levels of antibody in this sensitive ELISA.
TABLE 4.
Immunization of hamsters with rB/HPIV3-G1 and/or rB/HPIV3-F1 induces resistance to challenge with HPIV3 and RSV 28 days postinfection
| Immunizing virusa | No. of animals | Mean HPIV3 titerb (log10 TCID50/g ± SEd)
|
Mean RSV titerc (log10 PFU/g ± SEd)
|
||
|---|---|---|---|---|---|
| Nasal turbinates | Lungs | Nasal turbinates | Lungs | ||
| rB/HPIV3-G1 | 6 | 2.3 ± 0.1 (A) | 3.1 ± 0.2 (A) | 1.9 ± 0.2 (AB) | ≤1.7 (A) |
| rB/HPIV3-F1 | 6 | 2.6 ± 0.2 (A) | 3.1 ± 0.1 (A) | 2.9 ± 0.4 (BC) | 2.1 ± 0.2 (A) |
| rB/HPIV3-G1 and rB/HPIV3-F1 | 6 | 2.8 ± 0.2 (A) | 2.8 ± 0.3 (A) | 1.8 ± 0.1 (A) | 1.9 ± 0.4 (A) |
| rB/HPIV3 | 6 | 2.3 ± 0.5 (A) | 3.6 ± 0.4 (A) | 4.1 ± 0.5 (C) | 3.5 ± 0.4 (B) |
| RSV | 6 | 5.6 ± 0.2 (B) | 5.2 ± 0.2 (B) | 1.9 ± 0.3 (AB) | ≤1.7 (A) |
Groups of six hamsters were inoculated intranasally with 106 TCID50 of the indicated PIV3 or 106 PFU of RSV in a 0.1-ml inoculum. Nasal turbinates and lungs were harvested 5 days later for virus titration.
HPIV3 titrations were performed on LLC-MK2 cells. The limit of detectability of virus was 101.7 TCID50/g of tissue.
Quantitation of RSV was determined by plaque enumeration on HEp-2 cells. The limit of detectability of virus was 101.7 PFU/g of tissue.
Mean virus titers were assigned to similar groups (A, B, and C) by the Tukey-Kramer test. Within each column, mean titers with different letters are statistically different (P < 0.05). Titers indicated with two letters are not significantly different from those indicated with either letter.
Expression of RSV G or F glycoprotein in HEp-2 cells.
Serial dilutions of rB/HPIV3-G1 and rB/HPIV3-F1 were used to infect HEp-2 cell monolayers. On day 5 postinfection, the monolayers were fixed with 80% methanol and viral plaques were stained with monoclonal antibodies (MAbs) to RSV G (a mixture of MAbs 1187 and 131-2g), RSV F (a mixture of MAbs 1129, 1269, and 1243 [4]), and HPIV3 HN (MAb 454/11) (11) in an immunoperoxidase system as described previously (44). Plaque reduction neutralization assays were performed using a combination of MAbs (a mixture of MAbs 1129, 1269, and 1243 directed against RSV F protein and a mixture of MAbs 1187, 131-2g, and 130-5f directed against RSV G protein) or using RSV G-specific polyclonal hamster sera. The polyclonal serum was generated by infecting golden Syrian hamsters intraperitoneally with 106 PFU of recombinant vaccinia virus expressing the RSV G protein (25). MAb 1187 was kindly provided by J. Beeler (Food and Drug Administration), and MAbs 131-2g and 130-5f were kindly provided by L. Anderson (Centers for Disease Control and Prevention).
RESULTS
Construction of antigenomic cDNAs encoding recombinant chimeric rB/HPIV3 viruses bearing the RSV G or F ORF as an additional gene insert.
rB/HPIV3 is a recombinant version of the Kansas strain of BPIV3 in which the BPIV3 F and HN genes were replaced with their counterparts from the JS strain of HPIV3 (46). Thus, rB/HPIV3 encodes the major protective antigens of HPIV3 in the attenuated backbone of BPIV3. The complete antigenomic cDNA encoding rB/HPIV3 was modified by creation of a BlpI site in the upstream noncoding region of the N gene (Fig. 1). This promoter-proximal location was chosen to maximize the expression of an inserted foreign gene. In addition, the site did not disturb cis-acting transcription and replication signals. For insertion at this site, the ORF encoding the RSV G or F glycoprotein was modified by the addition of a BlpI site at its upstream end and the addition of a BPIV3 gene end signal, intergenic region, gene start signal, and BlpI site at its downstream end. This placed the foreign ORF under the control of PIV3 transcription signals so that it would be expressed as a separate mRNA.
Recovery of rB/HPIV3-G1 and rB/HPIV3-F1 viruses from cDNA.
rB/HPIV3-G1 and rB/HPIV3-F1 viruses were recovered by transfecting HEp-2 cells with the respective antigenomic cDNA together with BPIV3 N, P, and L support plasmids. The recovered recombinant viruses were cloned biologically by three sequential terminal dilutions in Vero cells. The presence of the inserted RSV G or F gene in the backbone of each recovered recombinant virus was confirmed by reverse transcription-PCR of viral RNA followed by restriction enzyme digestion and DNA sequencing of the gene junctions. The generation of each PCR product was dependent on the inclusion of reverse transcriptase, indicating that each was derived from viral RNA and not from contaminating cDNA. The sequence of the inserted gene and flanking regions in the recovered recombinant viruses was identical to that of the starting antigenomic cDNA (data not shown).
rB/HPIV3-G1 and rB/HPIV3-F1 viruses replicate efficiently in cell culture.
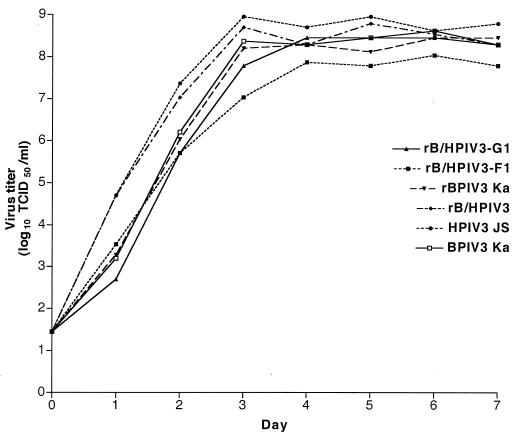
The multicycle growth kinetics of rB/HPIV3-G1 and rB/HPIV3-F1 in vitro were determined by infecting LLC-MK2 cell monolayers in triplicate at a multiplicity of infection (MOI) of 0.01 and harvesting samples at 24-h intervals over a 7-day period, as previously described (2). The replicative efficacies of rB/HPIV3-G1 and rB/HPIV3-F1 were compared to that of rB/HPIV3, rBPIV3, HPIV3 JS, or BPIV3 Ka (Fig. 2). The two parental viruses bearing HPIV3 glycoproteins, namely, HPIV3 and rB/HPIV3, appeared to replicate somewhat more rapidly than the other viruses. However, the final titers achieved for each of the six viruses were similar with one exception: rB/HPIV3-F1 was approximately eightfold reduced compared to rB/HPIV3 (Fig. 2). Nonetheless, rB/HPIV3-F1 and rB/HPIV3-G1 grew to a final titer of at least 107 TCID50/ml in LLC-MK2 cells (Fig. 2) and in Vero cells (data not shown). This indicates that each virus replicates efficiently in cell culture, which is essential for efficient vaccine manufacture.
FIG. 2.
Multicycle replication of rB/HPIV3-G1, rB/HPIV3-F1, and their parental viruses in simian LLC-MK2 cells. Triplicate cultures of LLC-MK2 cells were infected at an MOI of 0.01 with rB/HPIV3-G1, rB/HPIV3-F1, rB/HPIV3, rBPIV3, BPIV3, and HPIV3. Aliquots of cell culture medium were taken at 24-h intervals, and virus titers were determined by serial dilution. Titers are shown as mean log10 TCID50/ml of triplicate samples. The limit of detection of this assay is 101.45 TCID50/ml.
Expression of the RSV G and F glycoproteins.
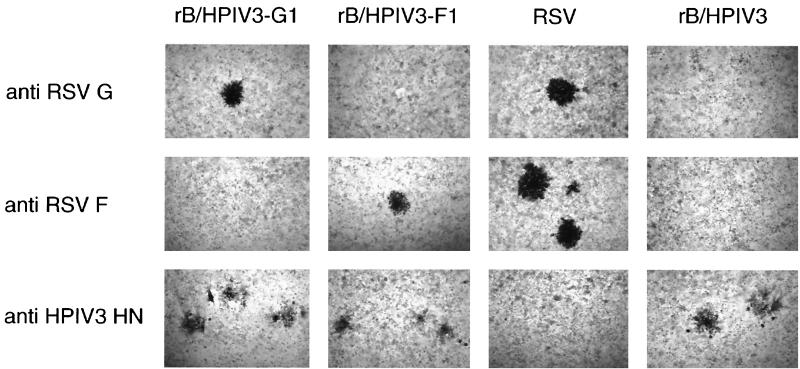
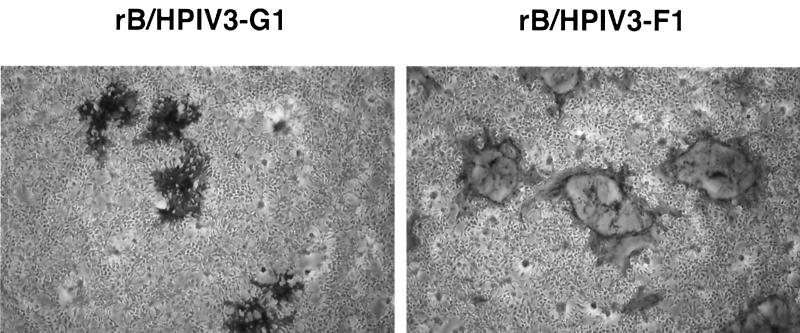
HEp-2 cells were infected with serial dilutions of the rB/HPIV3-G1 or rB/HPIV3-F1 virus, and plaques were visualized by immunostaining with MAbs specific for HPIV3 HN, RSV G, and RSV F proteins. The virus titers determined with HPIV3-specific and RSV-specific antibodies were identical, indicating that most or all of the infectious virions of rB/HPIV3-G1 or rB/HPIV3-F1 expressed RSV G or RSV F glycoproteins, respectively (data not shown). In HEp-2 cell monolayers, the morphology of plaques induced by rB/HPIV3-G1 and rB/HPIV3-F1 was similar to, and slightly smaller than, that of RSV and rB/HPIV3 (Fig. 3). In Vero (Fig. 4) and LLC-MK2 (data not shown) cell monolayers, however, rB/HPIV3-G1 induced a cytopathic effect resembling that of rB/HPIV3 (cell rounding), whereas rB/HPIV3-F1 induced a cytopathic effect resembling that of RSV (large syncytia).
FIG. 3.
rB/HPIV3-G1 and rB/HPIV3-F1 express RSV G and F glycoproteins, respectively. HEp-2 cells were infected with serial dilutions of the indicated virus and incubated for 5 days under methylcellulose. The wells were fixed, and viral plaques were stained in an immunoperoxidase assay using the indicated antibodies: RSV G-specific MAbs 1187 and 131-2g; RSV F-specific MAbs 1129, 1269, and 1243 (4); and HPIV3 HN-specific MAb 454/11 (11).
FIG. 4.
rB/HPIV3-G1 and rB/HPIV3-F1 plaque morphology in Vero cell monolayers. Vero cell monolayers were infected with serial dilutions of rB/HPIV3-G1 or rB/HPIV3-F1, starting at an MOI of 0.01. The wells were fixed, and viral plaques were stained in an immunoperoxidase assay using the indicated antibodies: RSV G-specific MAbs 1187 and 131-2g and RSV F-specific MAbs 1129, 1269, and 1243 (4). rB/HPIV3-G1 induces solid plaques of rounded-up cells similar to rB/HPIV3-induced plaques. rB/HPIV3-F1 induces giant syncytia similar to those seen in RSV-induced plaques.
rB/HPIV3, rB/HPIV3-G1, and rB/HPIV3-F1 were equally sensitive to neutralization by HPIV3-specific MAb 454/11 (data not shown). However, rB/HPIV3-F1 could not be neutralized by an undiluted mixture of RSV F-specific neutralizing MAbs 1129, 1269, and 1243 which has a neutralization titer of 1:256 for RSV. Similarly, rB/HPIV3-G1 could not be neutralized by an undiluted mixture of RSV G-specific neutralizing MAbs 1187, 131-2g, and 130-5f which has a neutralization titer of 1:64 for RSV. RSV G-specific polyclonal serum with a neutralization titer of 1:128 was also unable to neutralize rB/HPIV3-G1. This suggests that RSV G and F glycoproteins are not incorporated into the B/HPIV3-G1 and rB/HPIV3-F1 virions, respectively.
The rB/HPIV3-G1 and rB/HPIV3-F1 viruses replicate efficiently in the respiratory tract of hamsters.
rB/HPIV3-G1 and rB/HPIV3-F1 were evaluated for their ability to replicate in the upper and lower respiratory tract of hamsters following intranasal inoculation. The rB/HPIV3 parental virus and biologically derived BPIV3 and HPIV3 viruses were compared in parallel as controls (Table 1). Each virus was administered intranasally at a dose of 106 TCID50, and one group received both rB/HPIV3-G1 and rB/HPIV3-F1. Animals were sacrificed on days 4 and 5 postinfection, when peak virus titers have been demonstrated for the biological viruses, and the nasal turbinates and lungs were harvested. The level of replication of rB/HPIV3-G1 in the respiratory tract was similar to, and statistically indistinguishable from, that of HPIV3 JS and BPIV3 Ka. Replication of rB/HPIV3-F1 appeared to be somewhat reduced on days 4 and 5 relative to that of rB/HPIV3, rB/HPIV3-G1, and HPIV3, but this difference was not statistically significant in comparison with the biological BPIV3 Ka virus, which in previous primate and clinical studies replicated sufficiently well to induce a protective immune response (33, 57). Also, the titer of virus from the mixed infection of rB/HPIV3-G1 and rB/HPIV3-F1 appeared to be somewhat reduced in the lower respiratory tract on day 4, but this was not statistically significant. Thus, although rB/HPIV3-F1 tended to yield lower titers than did HPIV3 or rB/HPIV3, both rB/HPIV3-G1 and rB/HPIV3-F1 viruses appeared to replicate efficiently in vivo, despite the presence of the 0.9-kb G or 1.8-kb F supernumerary gene next to the promoter.
The rB/HPIV3-G1 and rB/HPIV3-F1 viruses induce serum antibodies to both HPIV3 and RSV.
Hamsters were infected intranasally with rB/HPIV3-G1, rB/HPIV3-F1, or rB/HPIV3 as described above. An additional group received both rB/HPIV3-G1 and rB/HPIV3-F1, and a fifth group received RSV. Serum samples were collected 26 days postinfection and assayed for RSV G- or F-specific antibodies by ELISA (Table 2) and for HPIV3 HN-specific antibodies by HAI assay (Table 3). The titer of G-specific or F-specific RSV antibodies induced by the rB/HPIV3-G1 or rB/HPIV3-F1 virus, respectively, was two- to fourfold higher than that induced by wt RSV. Animals inoculated with both rB/HPIV3-G1 and rB/HPIV3-F1 also had high titers of G-specific and F-specific antibodies.
TABLE 3.
Immunization of hamsters with rB/HPIV3 viruses expressing the RSV G or F ORF induces neutralizing serum antibodies against RSV as well as hemagglutination-inhibiting antibodies against HPIV3
| Immunizing virusa | No. of animals/group | Neutralizing serum antibody response to RSVb (mean reciprocal log2 ± SEd)
|
Serum hemagglutination-inhibiting antibody response to HPIV3c (mean reciprocal log2 ± SEd)
|
||
|---|---|---|---|---|---|
| Pre | Day 26 | Pre | Day 26 | ||
| rB/HPIV3-G1 | 12 | ≤3.3 | 10.0 ± 0.3 (A) | ≤2 | 10.0 ± 0.5 (A) |
| rB/HPIV3-F1 | 12 | ≤3.3 | 9.3 ± 0.5 (A) | ≤2 | 8.8 ± 0.1 (A) |
| rB/HPIV3-G1 and rB/HPIV3-F1 | 12 | ≤3.3 | 10.8 ± 0.4 (A) | ≤2 | 8.8 ± 0.3 (A) |
| rB/HPIV3 | 12 | ≤3.3 | ≤3.3 (B) | ≤2 | 9.5 ± 0.8 (A) |
| RSV | 12 | ≤3.3 | 8.1 ± 1.2 (A) | ≤2 | ≤2 (B) |
Hamsters were inoculated intranasally with 106 TCID50 of the indicated PIV3 or 106 PFU of RSV in a 0.1-ml inoculum.
Serum samples were taken on day 26 postinoculation, and antibody titers were determined by 60% plaque reduction neutralization test.
Serum samples were taken on day 26 postinoculation, and antibody titers were determined by HAI test.
Mean virus titers were assigned to similar groups (A and B) by the Tukey-Kramer test. Within each column, mean titers with different letters are statistically different (P < 0.05).
rB/HPIV3-G1 and rB/HPIV3-F1 induced RSV-neutralizing serum antibody titers that were higher than those induced by wt RSV (Table 3). With regard to HPIV3, each of the viruses induced a titer of PIV3-specific antibody that was indistinguishable from that induced by their parent virus rB/HPIV3. Thus, the rB/HPIV3 vector bearing the G or F gene of RSV induced strong immune responses against both the RSV insert and the PIV3 vector.
The rB/HPIV3-G1 and rB/HPIV3-F1 viruses induce resistance to replication of RSV and HPIV3 challenge virus.
The hamsters infected as described above were challenged 28 days after the initial infection by intranasal inoculation of 106 TCID50 of HPIV3 or 106 PFU of RSV. The animals were sacrificed 5 days later, the nasal turbinates and lungs were harvested, and virus titers were determined (Table 4). Animals that had received the parental rB/HPIV3 virus or the G1 and F1 derivatives exhibited a high level of resistance to the replication of the HPIV3 challenge virus, and there were no significant differences between groups. Animals that received rB/HPIV3-G1, rB/HPIV3-F1, or both viruses exhibited a high level of resistance to replication of the RSV challenge virus. The protective efficacy of the rB/HPIV3-F1 virus against the RSV challenge appeared to be marginally less than that of the rB/HPIV3-G1 virus or of the RSV control. However, this difference was not statistically significant and in any event likely reflected its slightly reduced growth. Therefore, infection with the rB/HPIV3 vector bearing either the G or F gene of RSV induced a level of protection against reinfection with RSV that was essentially equivalent to that conferred by a previous wt RSV infection.
DISCUSSION
The goal of this study was to examine the suitability of recombinant chimeric bovine/human parainfluenza viruses (rB/HPIV3) expressing RSV glycoproteins for use as bivalent, live attenuated mucosal vaccine against RSV and PIV3. rB/HPIV3 was shown previously to be attenuated and immunogenic in rhesus monkeys (46), and its biological parent virus, BPIV3, was safe and immunogenic in infants 2 to 6 months of age (33, 35). The attenuation phenotype reflects a host range restriction of replication in primates that is specified by the BPIV3 backbone. These properties suggested that rB/HPIV3 would be an appropriate vector for constructing multivalent pediatric vaccines. Here, rB/HPIV3 was evaluated as a vector for the major protective antigens of RSV, the most important cause of pediatric viral respiratory tract disease. This approach offers the possibility of obviating the problems that have been encountered in developing a live attenuated RSV vaccine that is adequately attenuated yet retains sufficient immunogenicity. The live attenuated RSV vaccine candidates that have been produced during 3 decades of research have been either overattenuated and lacking immunogenicity (32, 60) or underattenuated and pathogenic to some degree (34, 61). More recently, reverse genetics has provided a new set of vaccine candidates whose suitability will be evaluated clinically (reference 59 and unpublished data).
The use of rB/HPIV3 that expresses RSV protective antigens as a vaccine against RSV has a number of specific advantages over the previously tested RSV vaccines. First, the expression of RSV glycoproteins as additional, heterologous antigens from rB/HPIV3 makes it possible to vaccinate simultaneously against HPIV3 and RSV. This offers the advantage of simplified vaccine preparation, testing, and administration. In this regard, it is important to note that the F and HN genes of the rB/HPIV3 vector are derived from HPIV3 and thus provide homologous immunity. Multivalent vaccines would reduce the number of necessary separate vaccinations and help simplify the crowded pediatric vaccination schedule.
Second, rB/HPIV3 grows efficiently in vitro and reaches final titers that exceed those of RSV by a factor of 10 to 100. In this respect, vaccine production and evaluation would be more efficient using PIV3-based chimeric viruses. In addition, RSV has the property of unstable infectivity, and a PIV3-based RSV vaccine would facilitate vaccine production, transport, storage, and administration due to increased stability. Thus, use of PIV3 as a vector eliminates the difficulty inherent in attenuating, propagating, handling, and administering RSV.
A third advantage is that the host range restriction phenotype of rB/HPIV3 would be expected to be phenotypically stable, as has been the case with all Jennerian vaccines studied to date (7, 8, 26, 28, 30, 52, 53). Although this will need to be confirmed for BPIV3, clinical studies to date suggest that the attenuation phenotype is indeed stable. This contrasts, for example, with studies of several RSV vaccines in which partial reversion was observed (21). Furthermore, swaps between HPIV3 and BPIV3 involving the N gene, or the HN and F genes together, indicate that the host range restriction of BPIV3 is specified by multiple genes and likely is specified by many of the nucleotide and amino acid differences between BPIV3 and HPIV3 (2, 3, 46). This should contribute to phenotypic stability.
Similar to attenuated RSV vaccine candidates, rB/HPIV3 can infect the respiratory tract efficiently and stimulate local and systemic immunity (33). This route of administration reduces the virus-neutralizing and immunosuppressive effects of maternally derived serum antibodies present in young infants and children (40). These two separate phenomena, neutralization of vaccine virus and antibody-mediated suppression of the immune response, can greatly reduce vaccine efficacy in young infants and children (41). The ability to directly immunize the respiratory tract is critical for the use of rB/HPIV3 as a vector to express antigens for respiratory tract pathogens such as RSV (61).
In the present study, we expressed RSV G and F individually from rB/HPIV3. This was done to assess the effect of each glycoprotein alone on replication of the chimeric virus in vitro and in vivo. Since both RSV glycoproteins are independent neutralization antigens, it would be preferable to express both from a single rB/HPIV3 virus, and this work is in progress. In the present paper, insertion of the 1.8-kb F gene into rB/HPIV3 resulted in an eightfold reduction in growth in vitro. However, the observation that this virus induced extensive syncytium formation suggests that the reduced growth might be due to increased cytopathology resulting from the expression of a second fusion protein, rather than to a limitation of the vector's ability to accommodate large genes. In other work, HPIV3 was shown to accommodate a gene insert of 3.9 kb with little effect on replication in vitro and only a modest attenuation effect in vivo (49). In a separate study, the 15,462-nt HPIV3 genome was increased by nearly 50% with little effect on replication in vitro (M. Skiadopoulos, unpublished data). Thus, the addition of the RSV G gene to rB/HPIV3-F, which would increase the total insert size from 1.8 to 2.7 kb, will likely be tolerated.
rB/HPIV3-G1 and rB/HPIV3-F1 expressed RSV G and F glycoproteins in most or all of the infected cells, as indicated by the similarity in titers determined by RSV or HPIV3 staining of viral plaques. Although we did not formally determine the genome stability of B/HPIV3-G1 and B/HPIV3-F1, we did not detect any loss of activity in biological clones of HPIV3 bearing foreign inserts after eight passages in cell culture (unpublished data). This observation agrees with previously published reports on the genetic stability of modified recombinant parainfluenza viruses (24, 55).
rB/HPIV3-G1 and rB/HPIV-F1 were comparable to RSV in their ability to confer protection against challenge of hamsters with wt RSV. The hamster model was selected over the more common mouse model for RSV infection because PIV3 is severely restricted in mice whereas hamsters are semipermissive for both PIV3 and RSV. The hamster model does not allow evaluation of the attenuation phenotype of BPIV3, since that is specific to primates, but this phenotype was confirmed in earlier work for rB/HPIV3 (46). The level of replication of rB/HPIV3-G1 in the respiratory tract was similar to, and statistically indistinguishable from, that of the control viruses, BPIV3 and HPIV3. rB/HPIV-F1 replicated to a slightly lower titer than did the other viruses on days 4 and 5. This may be an effect of having this large gene in a promoter-proximal position, an effect of the expression of a second fusogenic protein, or both. In any case, this difference was not statistically significant in comparison with the biological BPIV3 virus, which in previous primate and clinical studies replicated sufficiently well to induce a protective immune response (33, 57).
The titer of G-specific or F-specific antibodies induced by the rB/HPIV3-G1 or rB/HPIV3-F1 virus, respectively, was two- to fourfold higher than that induced by wt RSV, and RSV-neutralizing antibody titers induced by B/HPIV3-G1 and -F1 also tended to be higher than those induced by wt RSV. However, since RSV replicated less efficiently in hamsters than did the rB/HPIV3 chimeric viruses, the difference in immunogenicity should be interpreted with caution. It is important to note that the antibody response to rB/HPIV3-G1 or rB/HPIV3-F1 is similar to that induced by RSV in that high RSV glycoprotein-specific ELISA titers are matched by high RSV neutralizing titers, indicating that conformationally correct glycoproteins are expressed. This is fundamentally different from the picture seen following immunization with formalin-inactivated or subunit RSV vaccines, where antibodies with high ELISA titers but low RSV neutralizing activity are induced (16, 43, 44). Each of the rB/HPIV3 viruses induced a titer of PIV3-specific antibody that was indistinguishable from that of their parent virus, rB/HPIV3, indicating that the presence and expression of the insert antigen did not compromise the immunogenicity of the vector glycoproteins. Consistent with the high RSV- and HPIV3-specific antibody titers, rB/HPIV3-G1 and rB/HPIV3-F1, alone or in combination, induced a level of resistance to challenge with RSV that was indistinguishable from that induced by previous infection with wt RSV. Both viruses also induced a level of resistance to challenge with HPIV3 that was similar to that induced by previous infection with rB/HPIV3.
We anticipate that the expression of RSV G and F from rB/HPIV3 will not be associated with immune-mediated enhanced pathogenicity upon RSV challenge in primates, since this was our experience with vaccinia virus recombinants expressing G or F (21). In certain inbred strains of mice, dermal immunization with RSV G alone, expressed from a vaccinia virus vector, primed for enhanced disease upon RSV challenge (1, 50). It is not clear whether this phenomenon is specific to these particular conditions in the mouse model or whether it has wider significance. Since BPIV3 grows poorly in mice, we have not evaluated possible immunopotentiation in that model. However, the possibility of vaccine-specific effects will be carefully evaluated in future studies in primates.
In summary, the in vitro and in vivo characteristics of rB/HPIV3-G1 and rB/HPIV3-F1 indicate that these viruses are candidates for a bivalent mucosal vaccine against both RSV and HPIV3. Both viruses, as well as a planned virus expressing both G and F, need further characterization with regard to their level of attenuation and their immunogenicity in primates before clinical studies can be initiated. The lack of internal RSV proteins in rB/HPIV3-G1 and rB/HPIV3-F1, and therefore the loss of several cytotoxic T-lymphocyte epitopes, did not seem to impact the immunogenicity and protective efficacy of these viruses (17). In consideration of the complex immune response induced by RSV, a vectored approach to developing an RSV vaccine may help to achieve the fine balance between immunogenicity and attenuation that is needed.
ACKNOWLEDGMENTS
We thank Robert Chanock for his careful review of the manuscript. We also thank Teresa Mulaikal, Fatemeh Davoodi, and Ernest Williams for their excellent technical support and Dan Wenzke for help with the hamster study. Stephen Whitehead and Christine Krempl provided help and advice at various stages. We are grateful to Judy Beeler and Larry Anderson for providing MAbs against RSV G protein.
This research is part of a continuing program of research and development with Wyeth-Lederle Vaccines and Pediatrics through CRADA grants AI-000030 and AI-000087.
REFERENCES
- 1.Alwan W H, Kozlowska W J, Openshaw P J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailly J E, McAuliffe J M, Durbin A P, Elkins W R, Collins P L, Murphy B R. A recombinant human parainfluenza virus type 3 (PIV3) in which the nucleocapsid N protein has been replaced by that of bovine PIV3 is attenuated in primates. J Virol. 2000;74:3188–3195. doi: 10.1128/jvi.74.7.3188-3195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailly J E, McAuliffe J M, Skiadopoulos M H, Collins P L, Murphy B R. Sequence determination and molecular analysis of two strains of bovine parainfluenza virus type 3 that are attenuated for primates. Virus Genes. 2000;20:173–182. doi: 10.1023/a:1008130917204. [DOI] [PubMed] [Google Scholar]
- 4.Beeler J A, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63:2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements M L, Belshe R B, King J, Newman F, Westblom T U, Tierney E L, London W T, Murphy B R. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. J Clin Microbiol. 1991;29:1175–1182. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements M L, Subbarao E K, Fries L F, Karron R A, London W T, Murphy B R. Use of single-gene reassortant viruses to study the role of avian influenza A virus genes in attenuation of wild-type human influenza A virus for squirrel monkeys and adult human volunteers. J Clin Microbiol. 1992;30:655–662. doi: 10.1128/jcm.30.3.655-662.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements-Mann M L, Makhene M K, Mrukowicz J, Wright P F, Hoshino Y, Midthun K, Sperber E, Karron R, Kapikian A Z. Safety and immunogenicity of live attenuated human-bovine (UK) reassortant rotavirus vaccines with VP7-specificity for serotypes 1, 2, 3 or 4 in adults, children and infants. Vaccine. 1999;17:2715–2725. doi: 10.1016/s0264-410x(98)00497-6. [DOI] [PubMed] [Google Scholar]
- 9.Coates H V, Alling D W, Chanock R M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966;83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 10.Coates H V, Forsyth B R, Chanock R M. Biophysical studies of respiratory syncytial virus. I. Density of respiratory syncytial virus and associated complement-fixing antigens in cesium chloride density gradient. J Bacteriol. 1966;91:1263–1269. doi: 10.1128/jb.91.3.1263-1269.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelingh K J, Winter C C, Murphy B R, Rice J M, Kimball P C, Olmsted R A, Collins P L. Conserved epitopes on the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986;60:90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins P L, Chanock R M, McIntosh K. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1205–1243. [Google Scholar]
- 13.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1352. [Google Scholar]
- 14.Collins P L, Olmsted R A, Spriggs M K, Johnson P R, Buckler-White A J. Gene overlap and site-specific attenuation of transcription of the viral polymerase L gene of human respiratory syncytial virus. Proc Natl Acad Sci USA. 1987;84:5134–5138. doi: 10.1073/pnas.84.15.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins P L, Whitehead S S, Bukreyev A, Fearns R, Teng M N, Juhasz K, Chanock R M, Murphy B R. Rational design of live-attenuated recombinant vaccine virus for human respiratory syncytial virus by reverse genetics. Adv Virus Res. 1999;54:423–451. doi: 10.1016/s0065-3527(08)60374-7. [DOI] [PubMed] [Google Scholar]
- 16.Connors M, Collins P L, Firestone C Y, Sotnikov A V, Waitze A, Davis A R, Hung P P, Chanock R M, Murphy B R. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 17.Connors M, Kulkarni A B, Collins P L, Firestone C-Y, Holmes K L, Morse III H C, Murphy B R. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J Virol. 1992;66:1277–1281. doi: 10.1128/jvi.66.2.1277-1281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crookshanks-Newman F K, Belshe R B. Protection of weanling hamsters from experimental infection with wild-type parainfluenza virus type 3 (para 3) by cold-adapted mutants of para 3. J Med Virol. 1986;18:131–137. doi: 10.1002/jmv.1890180205. [DOI] [PubMed] [Google Scholar]
- 19.Crowe J E., Jr Current approaches to the development of vaccines against disease caused by respiratory syncytial virus (RSV) and parainfluenza virus (PIV). A meeting report of the WHO Programme for Vaccine Development. Vaccine. 1995;13:415–421. doi: 10.1016/0264-410x(95)98266-d. [DOI] [PubMed] [Google Scholar]
- 20.Crowe J E, Jr, Bui P T, Firestone C Y, Connors M, Elkins W R, Chanock R M, Murphy B R. Live subgroup B respiratory syncytial virus vaccines that are attenuated, genetically stable, and immunogenic in rodents and nonhuman primates. J Infect Dis. 1996;173:829–839. doi: 10.1093/infdis/173.4.829. [DOI] [PubMed] [Google Scholar]
- 21.Crowe J E, Jr, Collins P L, London W T, Chanock R M, Murphy B R. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine. 1993;11:1395–1404. doi: 10.1016/0264-410x(93)90168-w. [DOI] [PubMed] [Google Scholar]
- 22.Dudas R A, Karron R A. Respiratory syncytial virus vaccines. Clin Microbiol Rev. 1998;11:430–439. doi: 10.1128/cmr.11.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durbin A P, Hall S L, Siew J W, Whitehead S S, Collins P L, Murphy B R. Recovery of infectious human parainfluenza virus type 3 from cDNA. Virology. 1997;235:323–332. doi: 10.1006/viro.1997.8697. [DOI] [PubMed] [Google Scholar]
- 24.Durbin A P, Skiadopoulos M H, McAuliffe J M, Riggs J M, Surman S R, Collins P L, Murphy B R. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J Virol. 2000;74:6821–6831. doi: 10.1128/jvi.74.15.6821-6831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elango N, Prince G A, Murphy B R, Venkatesan S, Chanock R M, Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci USA. 1986;83:1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerson S U, Tsarev S A, Govindarajan S, Shapiro M, Purcell R H. A simian strain of hepatitis A virus, AGM-27, functions as an attenuated vaccine for chimpanzees. J Infect Dis. 1996;173:592–597. doi: 10.1093/infdis/173.3.592. [DOI] [PubMed] [Google Scholar]
- 27.Hall S L, Stokes A, Tierney E L, London W T, Belshe R B, Newman F C, Murphy B R. Cold-passaged human parainfluenza type 3 viruses contain ts and non-ts mutations leading to attenuation in rhesus monkeys. Virus Res. 1992;22:173–184. doi: 10.1016/0168-1702(92)90049-f. [DOI] [PubMed] [Google Scholar]
- 28.Hurwitz J L, Soike K F, Sangster M Y, Portner A, Sealy R E, Dawson D H, Coleclough C. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine. 1997;15:533–540. doi: 10.1016/s0264-410x(97)00217-x. [DOI] [PubMed] [Google Scholar]
- 29.Hussell T, Baldwin C J, O'Garra A, Openshaw P J. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 30.Kapikian A Z, Vesikari T, Ruuska T, Madore H P, Christy C, Dolin R, Flores J, Green K Y, Davidson B L, Gorziglia M, et al. An update on the “Jennerian” and modified “Jennerian” approach to vaccination of infants and young children against rotavirus diarrhea. Adv Exp Med Biol. 1992;327:59–69. doi: 10.1007/978-1-4615-3410-5_8. [DOI] [PubMed] [Google Scholar]
- 31.Karron R A, Ambrosino D M. Respiratory syncytial virus vaccines. Pediatr Infect Dis J. 1998;17:919–920. doi: 10.1097/00006454-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Karron R A, Buonagurio D A, Georgiu A F, Whitehead S S, Adamus J E, Clements-Mann M L, Harris D O, Randolph V B, Udem S A, Murphy B R, Sidhu M S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karron R A, Makhene M, Gay K, Wilson M H, Clements M L, Murphy B R. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in two- to six-month-old infants. Pediatr Infect Dis J. 1996;15:650–654. doi: 10.1097/00006454-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Karron R A, Wright P F, Crowe J E, Jr, Clements M L, Thompson J, Makhene M, Casey R, Murphy B R. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus (RSV) vaccines in chimpanzees, adults, infants and children. J Infect Dis. 1997;176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 35.Karron R A, Wright P F, Hall S L, Makhene M, Thompson J, Burns B A, Tollefson S, Steinhoff M C, Wilson M H, Harris D O, et al. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J Infect Dis. 1995;171:1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- 36.Karron R A, Wright P F, Newman F K, Makhene M, Thompson J, Samorodin R, Wilson M H, Anderson E L, Clements M L, Murphy B R, Belshe R B. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J Infect Dis. 1995;172:1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]
- 37.Kim H W, Arrobio J O, Pyles G, Brandt C D, Camargo E, Chanock R M, Parrott R H. Clinical and immunological response of infants and children to administration of low-temperature adapted respiratory syncytial virus. Pediatrics. 1971;48:745–755. [PubMed] [Google Scholar]
- 38.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 39.Kulkarni A B, Collins P L, Bacik I, Yewdell J W, Bennink J R, Crowe J E, Jr, Murphy B R. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J Virol. 1995;69:1261–1264. doi: 10.1128/jvi.69.2.1261-1264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy B R, Alling D W, Snyder M H, Walsh E E, Prince G A, Chanock R M, Hemming V G, Rodriguez W J, Kim H W, Graham B S, Wright P F. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24:894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy B R, Olmsted R A, Collins P L, Chanock R M, Prince G A. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol. 1988;62:3907–3910. doi: 10.1128/jvi.62.10.3907-3910.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy B R, Prince G A, Collins P L, Van Wyke Coelingh K, Olmsted R A, Spriggs M K, Parrott R H, Kim H W, Brandt C D, Chanock R M. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res. 1988;11:1–15. doi: 10.1016/0168-1702(88)90063-9. [DOI] [PubMed] [Google Scholar]
- 43.Murphy B R, Prince G A, Walsh E E, Kim H W, Parrott R H, Hemming V G, Rodriguez W J, Chanock R M. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy B R, Sotnikov A V, Lawrence L A, Banks S M, Prince G A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 45.Murphy B R, Walsh E E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26:1595–1597. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt A C, McAuliffe J M, Huang A, Surman S R, Bailly J E, Elkins W R, Collins P L, Murphy B R, Skiadopoulos M H. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPIV3 in primates. J Virol. 2000;74:8922–8929. doi: 10.1128/jvi.74.19.8922-8929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shay D K, Holman R C, Newman R D, Liu L L, Stout J W, Anderson L J. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 48.Shay D K, Holman R C, Roosevelt G E, Clarke M J, Anderson L J. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children 1979–1997. J Infect Dis. 2001;183:16–22. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- 49.Skiadopoulos H M, Surman S R, Durbin A P, Collins P L, Murphy B R. Long nucleotide insertions between the HN and L protein coding regions of human parainfluenza virus type 3 yield viruses with temperature-sensitive and attenuation phenotypes. Virology. 2000;272:225–234. doi: 10.1006/viro.2000.0372. [DOI] [PubMed] [Google Scholar]
- 50.Sparer T E, Matthews S, Hussell T, Rae A J, Garcia-Barreno B, Melero J A, Openshaw P J. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subbarao E K, Perkins M, Treanor J J, Murphy B R. The attenuation phenotype conferred by the M gene of the influenza A/Ann Arbor/6/60 cold-adapted virus (H2N2) on the A/Korea/82 (H3N2) reassortant virus results from a gene constellation effect. Virus Res. 1992;25:37–50. doi: 10.1016/0168-1702(92)90098-t. [DOI] [PubMed] [Google Scholar]
- 53.Subbarao K, Webster R G, Kawaoka Y, Murphy B R. Are there alternative avian influenza viruses for generation of stable attenuated avian-human influenza A reassortant viruses? Virus Res. 1995;39:105–118. doi: 10.1016/0168-1702(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 54.Tao T, Davoodi F, Cho C J, Skiadopoulos M H, Durbin A P, Collins P L, Murphy B R. A live attenuated recombinant chimeric parainfluenza virus (PIV) candidate vaccine containing the hemagglutinin-neuraminidase and fusion glycoproteins of PIV1 and the remaining proteins from PIV3 induces resistance to PIV1 even in animals immune to PIV3. Vaccine. 2000;18:1359–1366. doi: 10.1016/s0264-410x(99)00406-5. [DOI] [PubMed] [Google Scholar]
- 55.Tao T, Skiadopoulos M H, Davoodi F, Riggs J M, Collins P L, Murphy B R. Replacement of the ectodomains of the hemagglutinin-neuraminidase and fusion glycoproteins of recombinant parainfluenza virus type 3 (PIV3) with their counterparts from PIV2 yields attenuated PIV2 vaccine candidates. J Virol. 2000;74:6448–6458. doi: 10.1128/jvi.74.14.6448-6458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tapparel C, Maurice D, Roux L. The activity of Sendai virus genomic and antigenomic promoters requires a second element past the leader template regions: a motif (GNNNNN)3 is essential for replication. J Virol. 1998;72:3117–3128. doi: 10.1128/jvi.72.4.3117-3128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Wyke Coelingh K L, Winter C C, Tierney E L, London W T, Murphy B R. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988;157:655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]
- 58.Waris E M, Tsou C, Erdman D D, Day D B, Anderson L J. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J Virol. 1997;71:6935–6939. doi: 10.1128/jvi.71.9.6935-6939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitehead S S, Firestone C-Y, Karron R A, Crowe J E, Jr, Elkins W R, Collins P L, Murphy B R. Addition of a missense mutation present in the L gene of respiratory syncytial virus (RSV) cpts530/1030 to RSV vaccine candidate cpts248/404 increases its attenuation and temperature sensitivity. J Virol. 1999;73:871–877. doi: 10.1128/jvi.73.2.871-877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright P F, Belshe R B, Kim H W, Van Voris L P, Chanock R M. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect Immun. 1982;37:397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright P F, Karron R A, Belshe R B, Thompson J, Crowe J E, Jr, Boyce T G, Halburnt L L, Reed G W, Whitehead S S, Anderson E L, Wittek A E, Casey R, Eichelberger M, Thumar B, Randolph V B, Udem S A, Chanock R M, Murphy B R. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 62.Wyatt L S, Moss B, Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology. 1995;210:202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]