Abstract
Chylothorax after esophageal surgery is a rare complication but can lead to death in patients due to malnutrition, fluid imbalance, and immunodeficiency. Multiple treatment options exist for postoperative chylothorax, including conservative treatment, octreotide therapy, and interventions such as thoracic duct embolization and surgical ligation of the thoracic duct. We present a case of lymphatic leakage following laparoscopic esophagectomy for esophageal cancer, confirmed by lymphangiography. The patient underwent an intervention to embolize the thoracic duct under computed tomography after an initial failure under digital subtraction angiography (DSA). One week after the intervention, the patient's pleural fluid output gradually decreased, and the patient was discharged from the hospital. At a 7-month follow-up, the patient remained stable with no recurrence of lymphatic leakage.
Keywords: Lymphatic leakage, Surgery, Esophageal cancer, Embolization
Introduction
Lymphatic leakage following esophagectomy for cancer has reported rates ranging from 1% to 9% [1]. Leakage of chylous fluid from the main lymphatic channels or fistula tract is an uncommon but potentially life-threatening complication of esophagectomy, particularly in cases of esophageal cancer. These complications primarily occur during the thoracic phase of surgery, where damage to the thoracic duct may result from its total resection, injury to small branches, or anatomical variations such as a double thoracic duct. Chest tubes can drain lymph rich in fats, fat-soluble vitamins, proteins, and lymphocytes [2], with flow rates of up to 4 liters/day, leading to serious clinical consequences. Leakage of fats, proteins, and vitamins can cause hypoalbuminemia and lymphopenia, contributing to malnutrition and immunodeficiency, and increasing the risk of infection and sepsis. The clinical impact of chylothorax leakage is significant, with associated morbidity ranging from 0% to 50% and mortality rates as high as 10% [1].
The most common clinical symptom in the postoperative period is lymphatic fluid in pleural drainage. Diagnosis is confirmed when the triglyceride concentration in drainage fluid exceeds 110 mg/dL (6.1 mmol/L). A drainage volume of more than 1 liter per 24 hours may suggest a complete chest tube tear [3].
Various treatment modalities are available for managing postoperative lymphatic leakage, including conservative management, surgical re-intervention for thoracic duct ligation, and endovascular procedures. Conservative management is typically the first-line approach, often supplemented with total parenteral nutrition (TPN), medium-chain triglycerides (MCT), and somatostatin analogues [4]. Re-surgery for thoracic duct ligation is considered for cases that do not respond to conservative measures. However, identifying a torn thoracic duct in the postoperative period can be challenging due to its small size and anatomical changes following surgery. Therefore, endovascular interventions, such as thoracic duct embolization using highly effective coils or embolic agents, are increasingly utilized as less invasive alternatives.
Case presentation
A male patient, 62 years old, with a history of smoking and drinking beer for many years, was hospitalized because of fresh blood in the stool. The patient did not have upper gastrointestinal symptoms such as vomiting, belching, or heartburn. The patient underwent a gastroscopy colonoscopy and endoscopic ultrasound, which detected damage. Biopsy results showed that the patient had squamous cell type esophageal cancer.
After being diagnosed, the patient was consulted and treated with endoscopic esophagectomy.
The patient underwent endoscopic total esophagectomy and esophageal-gastric anastomosis with lymphadenectomy
On the first day after surgery, the amount of fluid through the right pleural drainage tube was 50 mL/14 hours.
On the second day postoperative, the fluid volume stabilized at about 100 mL/24 hours.
On the third day postoperative, the patient's general condition was still stable, lung ventilation was reduced, and pleural drainage was still abundant, about 300 mL/24 hours.
On the fourth postoperative day, the patient was awake, had no fever, could breathe independently, and had a heart rate of 18 times/minute. Clinical examination showed decreased ventilation at the base of the lungs. Pleural drainage produces 1000 mL/24h, opaque yellow fluid. The patient was diagnosed with suspected lymphatic leakage after laparoscopic esophagectomy. At the same time, the patient received parenteral nutrition immediately after the intervention, but the pleural effusion did not decrease.
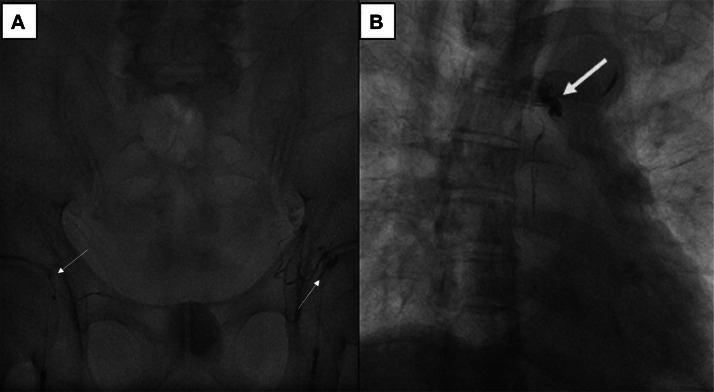
On the 6th day postoperative, the patient underwent intranodal lipiodol lymphangiography under digital subtraction angiography (DSA). DSA results showed that the patient had an anatomical variant of the lymphatic system. Two small lumbar trunks connect to the thoracic duct at the D11 level without cisterna chyli. A completed rupture of the upper 1/3 of the thoracic duct was found, and lipiodol leaked from the lower end into the right pleural space (Fig. 1). The upper part of the thoracic duct was not visible. To embolize the leakage, the retroperitoneal lymph ducts were initially punctured transabdominal using a 22-gauge Chiba needle; however, we could not access the lymphatic duct.
Fig. 1.
DSA imaging of lymphangiography. (A) Bilateral inguinal lymph node puncture with a 22-gauge needle, followed by lipiodol injection into the lymph nodes, showing the lymphatic system in the inguinal region and around the iliac vasculature. (B) The image shows the contrast between lipiodol in the small thoracic duct (arrowhead) and lipiodol leakage due to thoracic duct rupture (empty arrow).
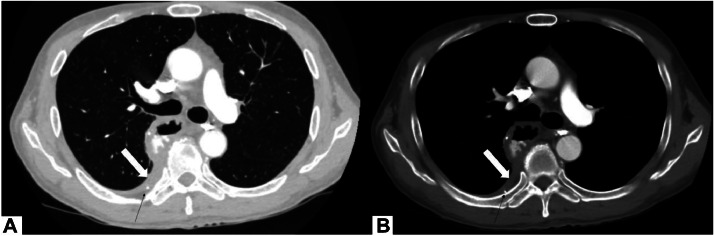
On the eighth day postoperative, a subsequent intervention was performed under computed tomography guidance. The patient was positioned prone and anesthetized with 2% lidocaine. A 22G Chiba needle was used to access the leakage site. Once the needle was correctly positioned, a mixture of N-butyl-2-cyanoacrylate and lipiodol in a 1:3 ratio (4ml in total) was injected through the needle (Fig. 2).
Fig. 2.
TDE by 22G needle under CT. Needle tip (white arrow).
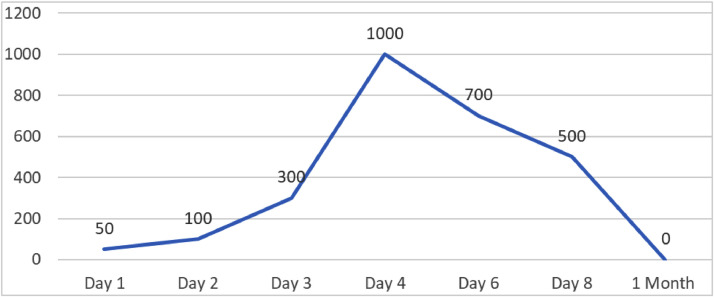
After the intervention, the patient was clinically monitored and the amount of drainage fluid gradually decreased. After intervention under computed tomography, the patient's pleural fluid gradually reduced. The pleural drainage was removed 1 month after the intervention (Fig. 3).
Fig. 3.
Total fluid output volumes from the chest tube drainage.
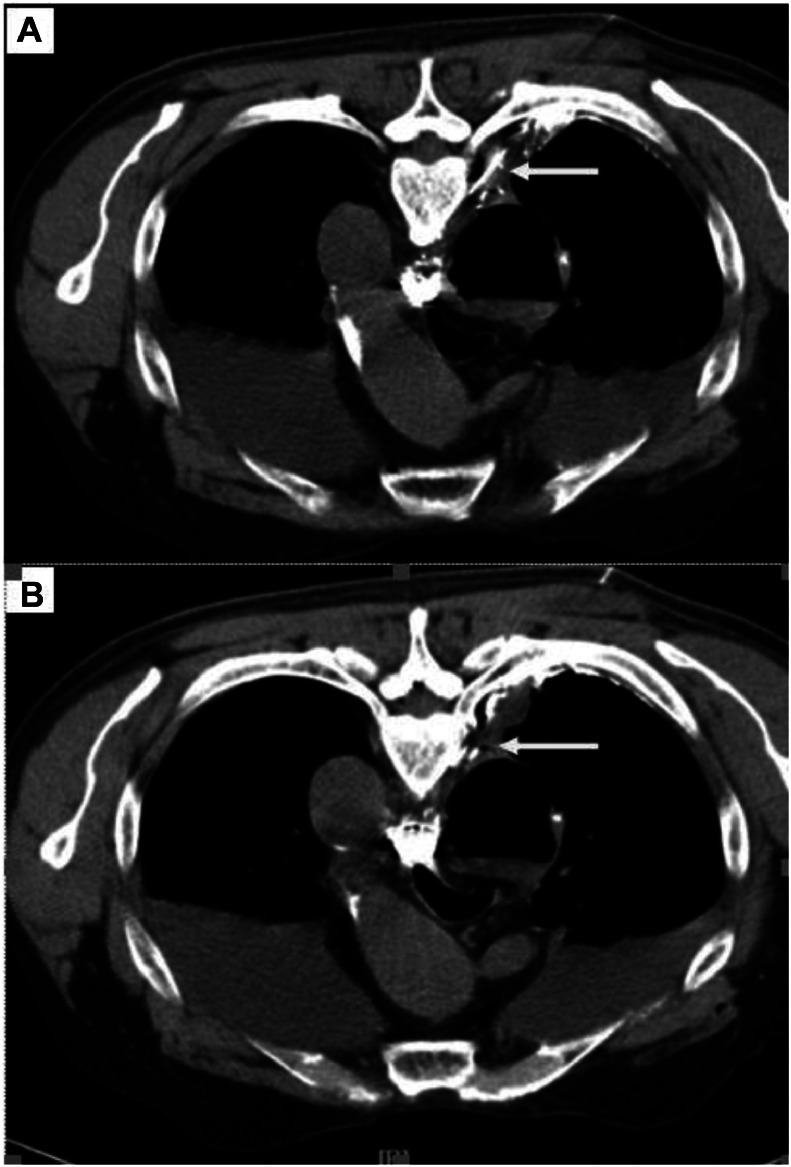
A chest computed tomography scan with contrast injection, performed 7 months postoperative, shows no remaining pleural fluid, and a slight thickening of the pleural wal (Fig. 4).
Fig. 4.
On chest computed tomography contrast-enhanced, parietal pleural thickening (empty arrow) (A, B) and a mixture of histoacryl: lipiodol in a small branch of the thoracic duct (arrow) (A, B).
Discussion
The thoracic duct is the largest lymphatic duct in the body. Thoracic duct fistula after esophageal surgery is a rare but dangerous complication that is potentially life-threatening and requires early treatment. The rate of lymphatic leakage after esophageal surgery is not high. According to a study by Pramod Kumar Mishra and colleagues published in 2013, 9/104 patients had esophageal fistula after esophagectomy due to cancer, accounting for about 8.7% of the studied [5]. Another report by S. Merigliano and colleagues showed that the rate of thoracic duct fistula after thoracic surgery was 2%-3% [6]. The mortality rate of thoracic lymphatic fistula can be up to 50%-82% if conservative treatment is prolonged and without any intervention according to research by SA Wemyss-Holden [7]. If patients undergo early thoracic duct ligation surgery, this rate drops to 10%-16% [7]. Thus, it can be seen that lymphatic leakage after esophageal surgery is not too common, but can have serious consequences, even a relatively high mortality rate.
Lymphatic fistula can cause chylous effusion and should be treated promptly. Thoracic duct leakage can cause pneumonia accompanied by respiratory failure, affecting the patient's life. Besides, losing a large amount of lymph fluid is equivalent to the patient losing a large amount of immunoglobulins, lymphocytes, proteins, and fats, which can lead to malnutrition and immunodeficiency [4]. Pleural effusion is a common symptom of thoracic duct fistula. Among 850 cases of esophageal cancer operated on by Lewis, 2.7% developed pleural effusion even though thoracic duct ligation was performed to prevent chylous effusion [5].
Diagnosis of esophageal fistula should be made after esophagectomy when the amount of fluid passing through the thoracic duct is large and the cause cannot be explained. The fluid is usually milky white and appears within 7 days of surgery (after the start of enteral feeding) [5]. When the drainage fluid changes color to milky white, doctors must diagnose whether the patient has a thoracic duct leak. In our patient's case, the fluid output was stable during the first days after surgery. However, on the fourth day after surgery, the amount of fluid discharged was relatively large, about 1000 mL/24 hours, accompanied by a change in color of the fluid to milky white. These data may suggest postoperative lymphatic leakage in our patient.
Different imaging diagnostic tools are needed to diagnose a thoracic duct fistula, but first, there must be clinical suspicion to direct the leakage of chylous fluid from the lymphatic vessels. Recently, magnetic resonance lymphography has shown its superior advantages compared to CT scans in detecting lymphatic lesions [8]. In addition, a study by De-Xin Yu in 2013 and colleagues was shown to be useful in diagnosing morphological changes in the thoracic lymphatic system and in locating leaks in patients with lesions of the thoracic duct, MRI without contrast is a viable option [9]. However, in our patient's case, the patient did not receive an MRI lymphography but instead had a digital subtraction angiography (DSA) because the patient could have undergone embolization immediately after the procedure.
Treatment of lymphatic leakage after surgery is still a controversial issue. Treatment of thoracic duct fistula after esophagectomy can include both conservative and surgical treatment. Maintenance treatment usually includes intravenous nutrition using somatostatin/octreotide, which is often used when fluid volumes are not large (<500 mL/day) [5]. When fluid volume is <1000 mL/day, drainage is the first line of treatment. Some surgeons advocate early reoperation even though nonsurgical treatment can give good results [5]. Recently, percutaneous thoracic duct embolization using digital subtraction angiography intervention has been proposed and described as a minimally invasive method that avoids complications from surgery. However, the current treatment with DSA intervention or surgery is still controversial because the success rate of DSA intervention in different hospitals is different due to the doctors' qualifications at that hospital. The overall success rate of this method is about 70% while the success rate of thoracic duct ligation surgery is up to about 90% [10]. However, in cases where the thoracic duct is severed after surgery, it is challenging to find the severed thoracic duct segment to tie during surgery. Therefore, percutaneous intervention is the optimal option. In our case, when intervention under DSA failed, intervention under CT guidance was the next choice. The literature documents only a few instances of thoracic duct embolization under computed tomography guidance. However, these are cases of intervention in the abdominal lymphatic vessels [11,12], and there have been no cases of direct intervention in the thoracic duct under computed tomography.
Our patient had a lymph angiogram under DSA on the fourth day after surgery, after detecting an increase in the amount of pleural drainage fluid and a change in the color of the fluid, and a diagnosis of thoracic duct injury was confirmed. After having the thoracic duct embolized on the sixth day after surgery, the patient gradually stabilized and had pleural drainage removed 4 days after the procedure. Thus, it can be seen that completely occluding the thoracic duct can bring the patient good treatment results, and reduce the patient's hospital stay, especially after the patient has just had a major surgery. Health has not recovered after surgery.
Conclusion
Thoracic duct rupture after esophagectomy is a rare complication but has serious consequences, requiring early diagnosis and appropriate treatment. Direct glue injection to the leakage site under CT guidance could be a feasible treatment for cases of failure with percutaneous transabdominal thoracic duct embolization.
Patient consent
Informed consent for patient information to be published in this article was obtained.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kamarajah SK, Siddaiah-Subramanya M, Parente A, Evans RPT, Adeyeye A, Ainsworth A, et al. Risk factors, diagnosis and management of chyle leak following esophagectomy for cancers. Ann Surg Open. 2022;3(3) doi: 10.1097/AS9.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lv S, Wang Q, Zhao W, Han L, Wang Q, Batchu N, et al. A review of the postoperative lymphatic leakage. Oncotarget. 2017;8(40):69062–69075. doi: 10.18632/oncotarget.17297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power R, Smyth P, Donlon NE, Nugent T, Donohoe CL, Reynolds JV. Management of chyle leaks following esophageal resection: a systematic review. Esophagus. 2021;34(11) doi: 10.1093/dote/doab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg. 2016;49(1):18–24. doi: 10.1093/ejcts/ezv041. [DOI] [PubMed] [Google Scholar]
- 5.Mishra PK, Saluja SS, Ramaswamy D, Bains SS, Haque PD. Thoracic duct injury following esophagectomy in carcinoma of the esophagus: ligation by the abdominal approach. World J Surg. 2013;37(1):141–146. doi: 10.1007/s00268-012-1811-x. [DOI] [PubMed] [Google Scholar]
- 6.Merigliano S, Molena D, Ruol A, Zaninotto G, Cagol M, Scappin S, et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg. 2000;119(3):453–457. doi: 10.1016/s0022-5223(00)70123-1. [DOI] [PubMed] [Google Scholar]
- 7.Wemyss-Holden SA, Launois B, Maddern GJ. Management of thoracic duct injuries after oesophagectomy. Br J Surg. 2001;88(11):1442–1448. doi: 10.1046/j.0007-1323.2001.01896.x. [DOI] [PubMed] [Google Scholar]
- 8.Erden A, Fitoz S, Yagmurlu B, Erden I. Abdominal confluence of lymph trunks: detectability and morphology on heavily T2-weighted images. AJR Am J Roentgenol. 2005;184(1):35–40. doi: 10.2214/ajr.184.1.01840035. [DOI] [PubMed] [Google Scholar]
- 9.Yu DX, Ma XX, Wang Q, Zhang Y, Li CF. Morphological changes of the thoracic duct and accessory lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur Radiol. 2013;23(3):702–711. doi: 10.1007/s00330-012-2642-8. [DOI] [PubMed] [Google Scholar]
- 10.Atie M, Dunn G, Falk GL. Chylous leak after radical oesophagectomy: Thoracic duct lymphangiography and embolization (TDE): a case report. Int J Surg Case Rep. 2016;23:12–16. doi: 10.1016/j.ijscr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinç H, Oğuz Ş, Sarı A. A novel technique in treating retroperitoneal lymphatic leakage is direct percutaneous embolization through the leakage pouch. Diagn Interv Radiol. 2015;21(5):419–422. doi: 10.5152/dir.2015.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itou C, Koizumi J, Myojin K, Yamashita T, Mori N, Imai Y. A case of refractory chylous ascites after nephrectomy successfully treated with percutaneous obliteration using adhesive glue. Jpn J Radiol. 2013;31(1):71–74. doi: 10.1007/s11604-012-0146-8. [DOI] [PubMed] [Google Scholar]