Abstract
Introduction
Pressurized intraperitoneal aerosol chemotherapy-oxaliplatin (PIPAC-OX) induces direct DNA damage and immunogenic cell death in patients with gastric cancer peritoneal metastases (GCPM). Combining PIPAC-OX with immune checkpoint inhibition remains untested. We conducted a phase I first-in-human trial evaluating the safety and efficacy of PIPAC-OX combined with systemic nivolumab (NCT03172416).
Methods
Patients with GCPM who experienced disease progression on at least first-line systemic therapy were recruited across three centers in Singapore and Belgium. Patients received PIPAC-OX at 90 mg/m2 every 6 weeks and i.v. nivolumab 240 mg every 2 weeks. Translational studies were carried out on GCPM samples acquired during PIPAC-OX procedures.
Results
In total, 18 patients with GCPM were prospectively recruited. The PIPAC-OX and nivolumab combination was well tolerated with manageable treatment-related adverse events, although one patient suffered from grade 4 vomiting. At second and third PIPAC-OX, respectively, the median decrease in peritoneal cancer index (PCI) was −5 (interquartile range: −12 to +1) and −7 (interquartile range: −6 to −20) and peritoneal regression grade 1 or 2 was observed in 66.7% (6/9) and 100% (3/3). Translational analyses of 43 GCPM samples revealed enrichment of immune/stromal infiltration and inflammatory signatures in peritoneal tumors after PIPAC-OX and nivolumab. M2 macrophages were reduced in treated peritoneal tumor samples while memory CD4+, CD8+ central memory and naive CD8+ T-cells were increased.
Conclusions
The first-in-human trial combining PIPAC-OX and nivolumab demonstrated safety and tolerability, coupled with enhanced T-cell infiltration within peritoneal tumors. This trial sets the stage for future combinations of systemic immunotherapy with locoregional intraperitoneal treatments.
Key words: immunotherapy, locoregional therapy, PIPAC, tumor microenvironment, gastric cancer, intraperitoneal, peritoneal metastases, peritoneum, niche
Highlights
-
•
First-in-human study combining PIPAC-OX with immunotherapy for gastric cancer with peritoneal metastases (GCPM).
-
•
This therapeutic regimen demonstrated safety and tolerability with reduction in peritoneal tumor burden.
-
•
Molecular profiling of GCPM samples showed enhancement in T-cell infiltration within peritoneal tumors.
-
•
This trial provides rationale for future systemic immunotherapies to be combined with intraperitoneal treatments for GCPM.
Introduction
Gastric cancer is a leading cause of cancer-related morbidity and mortality worldwide.1 Unresectable gastric cancer is associated with poor survival because of its late presentation, with approximately half of the patients diagnosed at advanced stage2,3 with a median survival of 12 months. Cancers with peritoneal metastases historically portend a poor prognosis even with traditional systemic chemotherapy or surgery.4,5
Systemic chemotherapy has poor penetration of the peritoneal cavity, encountering barriers such as ascites and fibrosis, limiting its effectiveness. Additionally, the complex immune microenvironment of the peritoneal niche, coupled with tumor heterogeneity results in poor responses to systemic immunotherapy.6 Locoregional techniques such as cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) offer a more targeted approach, directly addressing visible tumor nodules and microscopic disease within the peritoneal niche, while reducing systemic toxicity. In patients with histologically proven unresectable or recurrent gastric cancer limited to the peritoneum and/or cancer cells in peritoneal lavage cytology, the combination of intraperitoneal paclitaxel with systemic chemotherapy has shown promise.7,8 Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is an innovative intraperitoneal chemotherapy concept that improves drug delivery by taking advantage of the physical properties of gas and pressure, resulting in superior distribution and depth of penetration of chemotherapy drugs.9,10 The pharmacokinetics of PIPAC have been studied to allow homogenous drug distribution.11
There is limited evidence combining PIPAC with systemic therapy, particularly immunotherapy. Oxaliplatin, as a platinum-based chemotherapy agent, induces cell death by forming DNA adducts and inhibiting DNA repair mechanisms. Additionally, it has been shown to trigger immunogenic cell death, leading to the release of damage-associated molecular patterns (DAMPs), such as calreticulin, high mobility group box 1 (HMGB1), and adenosine triphosphate (ATP).12 The immunogenic cell death process can generate a potential immune response by activating antigen-presenting cells and stimulating the presentation of tumor-specific antigens to T-cells. We hypothesized that the combination of PIPAC-oxaliplatin (PIPAC-OX) and systemic nivolumab is catalyzed by the immunogenic cell death of peritoneal tumor cells initiated by the intraperitoneally delivered oxaliplatin. The release of DAMPs from dying cancer cells acts as a signal to activate immune cells, while nivolumab ensures an optimal immune response by blocking inhibitory immune checkpoints (Figure 1A).13,14 To test this hypothesis, we conducted the PIANO trial, a first-in-human phase I study combining PIPAC-OX with systemic nivolumab in patients with gastric cancer peritoneal metastases (GCPM).
Figure 1.
Study hypothesis and treatment schedule. (A) Study hypothesis: The combination of PIPAC-OX and systemic nivolumab would alter the tumor microenvironment and allow synergistic effects of the two drugs. (B) Treatment schedule: PIPAC-OX was administered at 90 mg/m2 once every 6 weeks for 2 doses. I.V. nivolumab at 240 mg started 1-3 days after the first PIPAC procedure, every 2 weeks. A third dose of PIPAC was permitted for patients demonstrating good response to therapy. PIPAC-OX, pressurized intraperitoneal aerosol chemotherapy-oxaliplatin.
Methods
We evaluated the combination of PIPAC-OX with systemic nivolumab as a prospective single-arm phase I trial conducted in three cancer centers in Singapore and Belgium. This study was registered on ClinicalTrials.Gov (NCT03172416) and EUDRACT (2020-004213-12). All procedures were carried out in compliance with relevant laws and institutional guidelines and have been approved by the National Healthcare Group Domain Specific Review Board, Singapore (2016/01088). This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study subjects before enrolment into the study.
Study population
We included patients with gastric cancer with peritoneal metastases and limited extraperitoneal disease. All patients with extraperitoneal metastases were discussed at a multidisciplinary tumor board including surgeons, radiologists, pathologists, and oncologists. Limited extraperitoneal disease included oligometastatic liver/lung disease, retroperitoneal lymph node metastases or extra-abdominal lymph node metastases.15 Patients had to have progressed on at least first-line systemic chemotherapy. Patients had to be of Eastern Cooperative Oncology Group performance status 0-1, have adequate organ function based on biochemical parameters (Supplementary Methods, Protocol, available at https://doi.org/10.1016/j.esmoop.2024.103681), and no standard contraindications for systemic immune checkpoint inhibition or chemotherapy (delivered via PIPAC). Patients were excluded if they had a poor performance status, had an expected survival of <3 months or had predominant extraperitoneal metastases.
Study design
Two doses of PIPAC-OX were administered via laparoscopic surgery 6 weeks apart at a dose of 90 mg/m2, previously shown to be tolerable.16 Nivolumab was dosed intravenously (i.v.) at the standard flat dose of 240 mg every 2 weeks for up to 24 months. The first dose of nivolumab was initiated within 3 days of the first dose of PIPAC-OX. An optional third dose of PIPAC-OX was permitted for those patients who demonstrated prolonged benefit and were keen to undergo the additional procedure. The study and treatment schedule are detailed in Figure 1B. Patients who completed treatment without disease progression were continuously followed-up for tumor response. Upon discontinuation from the study drug nivolumab, patients were contacted 8-weekly by phone to collect overall survival (OS) data until death or up to 2 years, whichever came first.
Outcomes of interest
The primary endpoint was the safety of the combination of PIPAC-OX and i.v. nivolumab. Safety was evaluated in terms of adverse events, serious adverse events, and discontinuation of treatment due to toxicity.
Assessment of safety outcomes
Surgical complications were monitored and graded according to the Clavien–Dindo classification.17 Toxicity was monitored for and graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Adverse events (CTCAE) were assessed as treatment-related by the treating physician if they had developed after PIPAC-OX or nivolumab or, if preexisting, worsened after these procedures.
Secondary outcomes
Secondary efficacy endpoints included the clinical and pathological response to combination treatment, and quality of life of patients. All patients who underwent at least one PIPAC-OX procedure were evaluated for response. During each PIPAC-OX procedure, four-quadrant biopsies were taken where possible. Clinical response was assessed according to the peritoneal cancer index (PCI) documented at each PIPAC-OX procedure. Pathological response was assessed according to the peritoneal regression grade scoring (PRGS) system on tumor samples from each PIPAC-OX procedure.18 Briefly, the PRGS score grade 1 has complete response with absence of tumor cells, grade 2 has major response with major regression features and few residual tumor cells, grade 3 has minor response with some regressive features but predominantly consisting of residual tumor cells, and grade 4 has no response with tumor cells not showing any regressive features. The radiological response to treatment was assessed on cross-sectional imaging according to Response Evaluation Criteria In Solid Tumour (RECIST) v.1.1 criteria. Quality of life was assessed according to the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaires (EORTC) QLQ-C30. Progression-free survival (PFS) was defined as the time from the start of the treatment to the progression of the disease on CT scan or laparoscopy. OS was defined as the time from the start of study treatment to death by any cause.
Pharmacokinetics
Blood samples for pharmacokinetic studies were collected and processed as previously described.16 Pharmacokinetic assays were carried out at the Drug Analysis and Pharmacokinetic Core facility of the Cancer Science Institute of Singapore, National University of Singapore, Singapore. The quantitative analysis of oxaliplatin was carried out using inductively coupled plasma-mass spectrometry (ICP-MS). Methodology development and validation were done based on the assay published by Morrison and colleagues.19 The pharmacokinetic parameters of PIPAC-OX were calculated using noncompartmental analysis with the NonCompart and ncar package in R-4.2.0. Comparisons were undertaken against serum platinum concentrations of three patients treated with 90 kg/m2 of PIPAC-OX in a phase I study previously reported by our group.16
Translational analyses
Before each PIPAC procedure, diagnostic laparoscopy was carried out and peritoneal tumors together with the macroscopically normal adjacent peritoneum were biopsied with laparoscopic biopsy forceps. Where possible, one to two of such paired samples were obtained from each patient. These samples were stored and transported to the laboratory where they were flash frozen and processed for translational analyses.
RNA extraction and whole transcriptome sequencing
For RNA-seq experiments, total RNA was extracted using the RNeasy Mini Kit (Qiagen, Netherlands), and library preparation was conducted using the Tru-Seq Stranded Total RNA with Ribo-Zero Gold kit protocol (Illumina, San Diego, CA). Libraries were sequenced on a HiSeq4000 sequencer using the paired-end 150 bp read option. QC-passed reads were aligned to the human reference CGRh38/hg38 genome using STAR v.2.7.9a. Transcript abundance quantification was carried out using RSEM v1.3.3.20 RNA-seq data were normalized by log2 fragments per kilobase of transcript per million mapped reads.
RNA-seq analyses
Differential analysis of count data was conducted with the DESeq package.21 Dimension reduction of RNA-seq was conducted with Uniform Manifold Approximation and Projection (UMAP). Ellipses were added to UMAP plots for visualization of cluster overlap and/or segregation with the ggbiplot package. For pathway analyses, The Molecular Signatures Database (MSigDB) hallmark gene signature set22 was utilized for the primary analysis; additional gene signature sets utilized are documented in Supplementary Table S1 available at https://doi.org/10.1016/j.esmoop.2024.103681.23, 24, 25, 26 Gene set enrichment analyses (GSEA) of log2FC values were utilized for pathway analyses, where a Bonferroni adjusted P value <0.05 was considered significant. Immune cell subsets were enumerated primarily with the CIBERSORT LM22 immune subset signature.27 Sensitivity analyses were conducted with xCell.28 These were implemented through the immunedeconv package.29 Comparisons were undertaken with the unsupervised t-test. All bioinformatic analyses were undertaken in R-4.2.0.
Statistical analyses
Survival analyses were undertaken with Kaplan–Meier plots and the log-rank test. Median follow-up time was retrieved with the reverse Kaplan–Meier method. All analyses were conducted with R-4.2.0. A two-sided P value of <0.05 was considered significant.
Role of the funding source
This study was supported by the National Medical Research Council, Singapore. Funding for this study was used to pay for the costs of some of the treatment of patients. Nivolumab was supplied by Bristol Myers Squibb. This was an investigator-initiated trial and the study team had full access to all data in the study and take final responsibility for the decision to submit this study for publication. The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the manuscript.
Data availability
Genomic data have been uploaded into the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) (accession number: PRJNA1137069). Any other data generated in this study are available upon request from the corresponding author.
Results
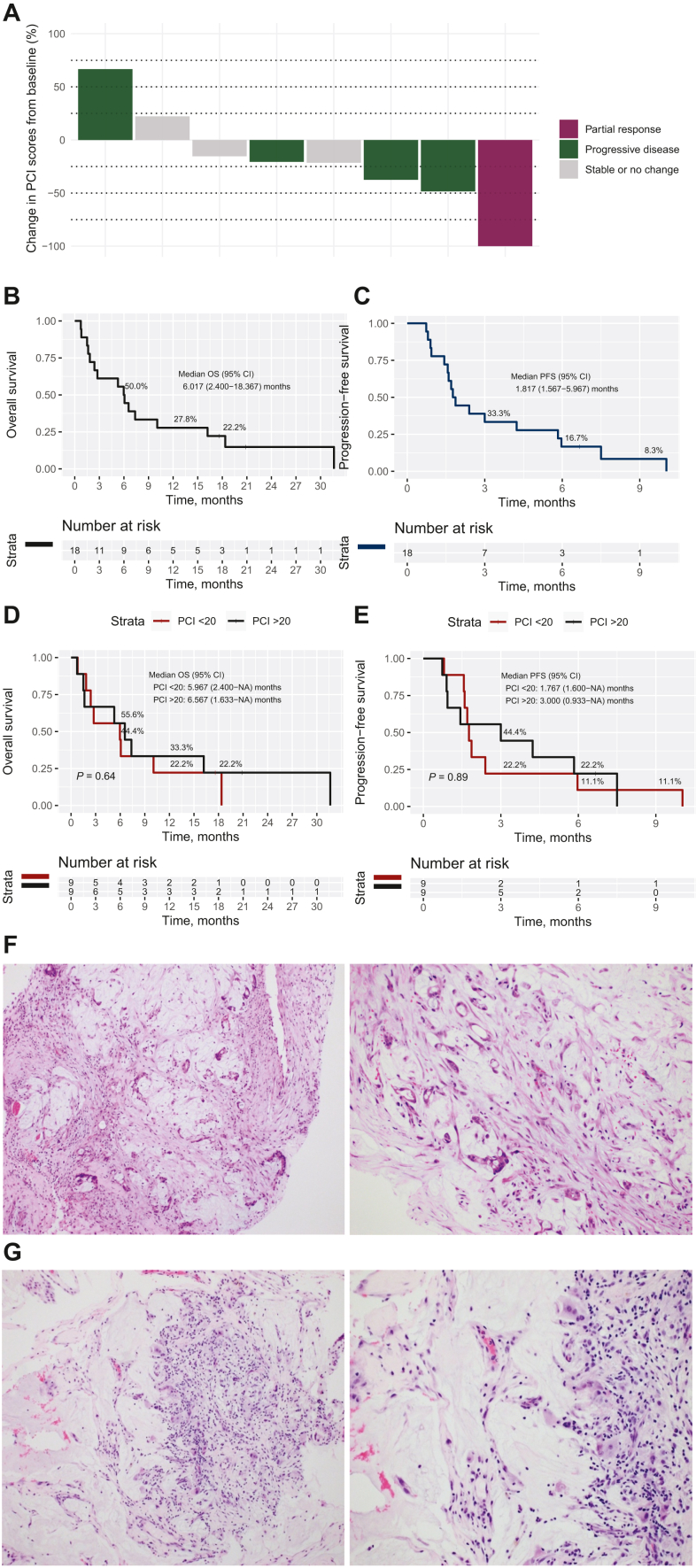
From June 2020 to November 2022, 18 patients with GCPM were prospectively recruited across three centers in Singapore and Belgium, undergoing a median [interquartile range (IQR)] of 1 (IQR: 1-3) cycles of PIPAC-OX and 4 (IQR: 2.25-9) doses of i.v. nivolumab with a median follow-up of 20.8 months. The first dose of PIPAC-OX was successfully administered in all patients, half the patients (50.0%; 9/18) received the second PIPAC-OX dose, and 5 (27.8%) received a third PIPAC-OX dose (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103681). Key characteristics of the 18 patients are presented in Table 1. All participants had progressed on at least one prior line of systemic therapy, with PIPAC-OX and i.v. nivolumab being the second-line treatment in 11 (61.1%), third-line in 5 (27.8%), and fourth-line in 2 (11.1%) patients, respectively.
Table 1.
Key characteristics of included patients
| Variable | |
|---|---|
| Age, years, median [IQR] | 65 [52.5-70.75] |
| Gender, (%) | |
| Male | 7 (38.9) |
| Female | 11 (61.1) |
| Ethnicity, (%) | |
| Chinese | 9 (50) |
| Malay | 1 (5.6) |
| Indian | 3 (16.7) |
| Caucasian | 5 (27.8) |
| ECOG performance status, %a | |
| 0 | 8 (44.4) |
| 1 | 9 (50.0) |
| Primary tumor previously resected, % | 9 (50.0) |
| Positive peritoneal cytology at baseline, % | 14 (77.8) |
| Pre-PIPAC PCI score, median [IQR] | 20 [12.25-30] |
| Cycles of PIPAC, median (range) | 1 (1-3) |
| Doses of i.v. nivolumab, median (range) | 4 (1-16) |
| Previous lines of systemic therapy, % | |
| 1 | 11 (61.1) |
| 2 | 5 (27.8) |
| 3 | 2 (11.1) |
| Limited extraperitoneal metastasis at baseline, % | |
| Liver + distant lymph node | 1 (5.6) |
| Liver | 1 (5.6) |
| Ascites at first PIPAC, % | |
| Present | 13 (72.2) |
ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; PCI, peritoneal cancer index; PIPAC, pressurized intraperitoneal aerosol chemotherapy.
ECOG data of one patient were not available.
Safety and tolerability
The incidence of adverse events and their severity are presented in Table 2. Two patients experienced CTCAE grade 5 toxicities (11.0%), three grade 4 (16.7%), and nine grade 3 (50.0%). The two grade 5 events resulting in mortality were small bowel perforation and sepsis secondary to COVID-19. The event of small bowel perforation occurred 24 days after the first PIPAC-OX dose and two doses of nivolumab. The patient had a PCI score of 13. The CT scan carried out at the time of perforation was reviewed and the bowel wall at the level of perforation appeared thickened, suggestive of disease progression. The case was discussed at great length by an independent data monitoring committee (IDMC) which included an international panel of medical oncologists and surgeons, who were not a part of the study team. The event was deemed to be more likely secondary to disease progression and unlikely secondary to PIPAC-OX. The event of sepsis occurred after the second PIPAC-OX dose and four doses of nivolumab. The patient had an episode of severe COVID after they had come off the trial for disease progression. Bowel perforation occurred in one other patient, who had demonstrated considerable response to PIPAC-OX and nivolumab and was undergoing a resection of an umbilical nodule which was tethered to the transverse colon. Several IDMC reviews of the adverse events confirmed that a majority of grade 3-5 events were unrelated to PIPAC-OX or nivolumab, especially considering the advanced illness of a majority of the patients in the trial.
Table 2.
Treatment-emergent and treatment-related adverse events in the study population. Adverse events that were recorded in at least two patients are reported
| Study population (N = 18) |
||||||
|---|---|---|---|---|---|---|
| Treatment-emergent, n (%) |
Nivolumab-related, n (%) |
PIPAC-OX-related, n (%) |
||||
| Any grade, n (%) | Grade 3-5, n (%) | Any grade, n (%) | Grade 3-5, n (%) | Any grade, n (%) | Grade 3-5, n (%) | |
| Abdominal pain | 10 (55.6) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 5 (27.8) | 0 (0.0) |
| Nausea/vomiting | 9 (50.0) | 1 (5.6) | 1 (5.6) | 0 (0.0) | 2 (11.1) | 1 (5.6) |
| Anorexia/weight loss/cachexia | 6 (33.3) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 5 (27.8) | 0 (0.0) | 2 (11.1) | 0 (0.0) | 2 (11.1) | 0 (0.0) |
| Fatigue | 5 (27.8) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 3 (16.7) | 0 (0.0) |
| Fever | 5 (27.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.1) | 0 (0.0) |
| Constipation | 4 (22.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash/urticaria | 3 (16.7) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 1 (5.6) | 0 (0.0) |
| Dyspnea | 3 (16.7) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypertension | 3 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dysphagia | 3 (16.7) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anemia | 2 (11.1) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Intestinal perforation | 2 (11.1) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Abdominal distension/bloating/ascites | 2 (11.1) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) |
| Cough | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Electrolyte abnormality | 2 (11.1) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hepatitis/deranged liver function tests | 2 (11.1) | 2 (11.1) | 2 (11.1) | 1 (5.6) | 2 (11.1) | 2 (11.1) |
| Hydronephrosis/nephrostomy insertion | 2 (11.1) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Peripheral neuropathy | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) |
| Sepsis | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ureteral stent insertion | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypothyroidism | 1 (5.6) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pleural effusion | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Thrombosis of the neck | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lung infection | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Intestinal obstruction | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bile duct compression | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypotension | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) |
| Hematoma | 1 (5.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neutropenia | 1 (5.6) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dyspepsia | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) |
AE, adverse event; OX, oxaliplatin; PIPAC, pressurized intraperitoneal aerosol chemotherapy.
A total of 32 cycles of PIPAC-OX were administered to the 18 patients within this trial. The most common adverse events after PIPAC-OX were abdominal pain in 10/18 patients (55.6%, all grade 1-2), nausea/vomiting in 10/18 (55.6%, 9 grade 1-2 and 1 grade 3), and anorexia/weight loss in 7/18 (38.9%, 6 grade 1-2 and 1 grade 3). Abdominal pain and nausea typically developed a median of 1 day and anorexia/weight loss a median of 13 days following PIPAC, respectively.
Response to treatment
All 18 patients who underwent at least one PIPAC procedure were evaluated for clinical response using the PCI score, tumor response according to RECIST, and pathological response according to the PRGS. The median PCI was 20 (IQR: 11-31) at baseline. At second and third PIPAC, the median decrease in PCI was −5 (IQR: −12 to +1) and −7 (IQR: −6 to −20), respectively (Figure 2A). PRGS grade 1-2 was seen on pathological assessment in 66.7% (6/9) at second PIPAC and 100% (3/3) at third PIPAC. The median PFS and OS in this cohort was 1.8 months (95% confidence interval 1.6-6.0 months) and 6.0 months (95% confidence interval 2.4-18.4 months), respectively (Figure 2B and C). Fourteen patients had at least two CT scans to report radiological response as per RECIST criteria. Objective response was seen in 7% (1/14) while disease control was achieved in 57% (8/14) of patients. Median follow-up was 20.8 months. No significant difference in survival outcomes were found between patients with high versus low baseline PCI scores (PCI score ≥20 versus <20: OS, log-rank P = 0.640; PFS, log-rank P = 0.890) (Figure 2D and E).
Figure 2.
Survival and response. (A) Overall change in PCI score from baseline after PIPAC for patients who had PCI results after at least two cycles of PIPAC, color-coded by RECIST response. Green bars represent those with progressive disease, burgundy bars represent partial response, and grey bars represent stable or no change. (B) Kaplan–Meier curves of overall survival and (C) progression-free survival, (D) Kaplan–Meier curves of overall survival and (E) progression-free survival stratified by baseline PCI scores, (F) histological images before PIPAC-OX treatment showing viable tumor cells with mucin with some fibrosis (PRGS 3), (G) histological images after PIPAC-OX treatment showing acellular mucin with some inflammatory response (PRGS 1). CI, confidence interval; NA, not available; OS, overall survival; OX, oxaliplatin; PCI, peritoneal cancer index; PFS, progression-free survival; PIPAC, pressurized intraperitoneal aerosolized chemotherapy; PRGS, peritoneal regression grading score.
Quality of life was assessed according to the EORTC QLQ-C30 for all patients with results presented in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103681. Overall, there was no significant deterioration in quality-of-life scores while patients were receiving treatment (analysis of variance, P = 0.814).
One patient was identified as a responder with regression on PRGS from PRGS 3 to 1. Before PIPAC-OX, histology showed viable tumor cells with mucin, with some fibrosis (PRGS 3) (Figure 2F). Subsequent histology after PIPAC-OX showed acellular mucin with some inflammatory response (PRGS 1) (Figure 2G).
Pharmacokinetics
Out of 18 patients who underwent at least one round of PIPAC, pharmacokinetic analysis was carried out on 7 patients. In all patients except one (patient 7), concentrations of platinum decreased from T = 1 h to T = 30 h. For this patient, an additional sample of ultrafiltrate was tested which found a similar result. Pharmacokinetic modelling was not conducted due to limited sampling. When contrasted against three patients treated with PIPAC-OX alone,16 peak serum platinum concentrations were found to be higher in patients also receiving i.v. nivolumab (Supplementary Figure S3A, available at https://doi.org/10.1016/j.esmoop.2024.103681). Serum platinum concentrations at the 30-h mark was higher compared with the addition of i.v. nivolumab [mean (standard deviation): 46.48 (18.97) versus 7.20 (1.81) ng/ml, P = 0.0014]. No significant differences in Cmax [84.94 (41.60) versus 41.72 (27.34) ng/ml, P = 0.100] were found (Supplementary Figure S3B, available at https://doi.org/10.1016/j.esmoop.2024.103681). Pharmacokinetic modelling and area under the curve (AUC) analysis could not be carried out due to limited sampling.
Microenvironmental characterization of GCPM within PIANO trial
Whole transcriptome sequencing of 43 samples (26 tumor, 17 normal) from seven patients was undertaken. Three patients (PIANO005, PIANO006, and PIANO0016) had paired pre/post-treatment samples.
Treatment with PIPAC-OX and i.v. nivolumab induces microenvironmental alterations
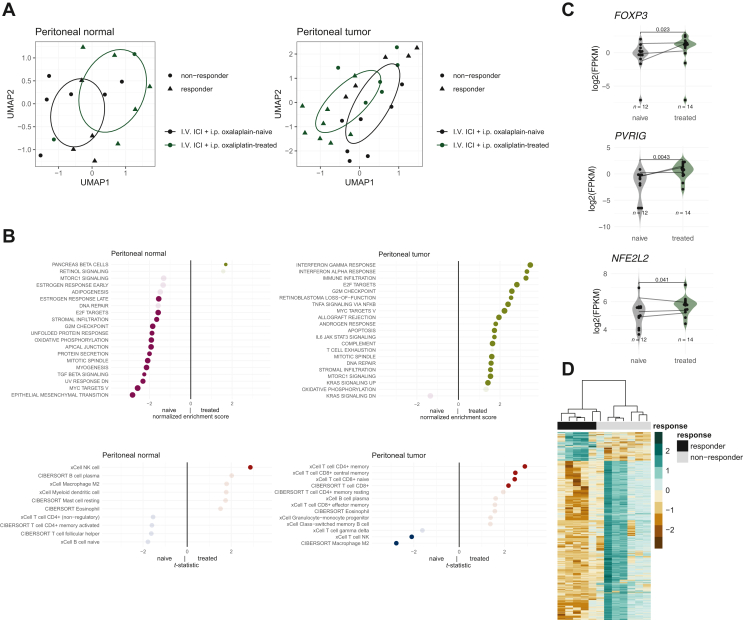
Treatment with PIPAC-OX and i.v. nivolumab demonstrated transcriptomic changes in both peritoneal tumor and normal samples (Figure 3A). Over-enrichment of immune/stromal infiltration and inflammatory signatures were found in treated (after PIPAC-OX) peritoneal tumor samples. Conversely, lower epithelial mesenchymal transition and transforming growth factor-β (TGF-β) signaling signature scores were found in adjacent-normal peritoneal tissue after PIPAC (Figure 3B). M2 macrophages and natural killer (NK) T cells were reduced in treated peritoneal tumor samples while memory CD4+, CD8+ central memory and naive CD8+ T cells were increased. We also inspected several therapeutic targets of interest and their changes before and after treatment. We identified three genes, FOXP3 (P = 0.023), NFE2L2 (P = 0.041), and PVRIG (P = 0.0043) found to have higher levels of expression in post-treatment tumor samples (Figure 3C). Other comparisons are provided in Supplementary Figure S4A, available at https://doi.org/10.1016/j.esmoop.2024.103681.
Figure 3.
Translational analyses. (A) UMAP of peritoneal tumor and normal samples. (B) GSEA pathway comparisons and immune deconvoluted cell type changes before and after PIPAC-OX, i.v. nivolumab treatment. (C) Putative gene changes with treatment in peritoneal tumor samples. P values were retrieved with the Wilcoxon’s test. (D) Gene expression profiles of peritoneal tumor samples. Differential gene expressions were included if log 2-fold change values were >2 or <−2 and adjusted P values were <0.05. FPKM, fragments per kilobase of transcript per million mapped reads; GSEA, gene set enrichment analyses; ICI, immune checkpoint inhibition; PCI, peritoneal cancer index; PIPAC-OX, pressurized intraperitoneal aerosol chemotherapy-oxaliplatin; UMAP, Uniform Manifold Approximation and Projection.
Next, we demonstrate gene expression differences between baseline samples amongst responder and non-responders (Figure 3D). Treatment responders, defined as patients with an OS of >6 months after PIPAC-OX treatment, were uniquely characterized by enriched angiogenesis, TGF-β signaling and hippo pathway (Supplementary Figure S4B, available at https://doi.org/10.1016/j.esmoop.2024.103681). Peritoneal tumors with high M2 macrophages were also noted to be associated with treatment response (Supplementary Figure S4C, available at https://doi.org/10.1016/j.esmoop.2024.103681).
We also studied the association of PCI and tumor microenvironment changes. High PCI scores were significantly correlated with increased immune infiltration, T-cell exhaustion, EMT, and PI3K-AKT MTOR signaling within macroscopically normal peritoneal samples (Supplementary Figure S5A, available at https://doi.org/10.1016/j.esmoop.2024.103681). High PCI scores were also associated with increased M2 macrophages in peritoneal tumors, and CD8+ T cells. Plasmacytoid dendritic cells and naive B cells were strongly correlated with high PCI scores in both peritoneal tumor and normal samples (Supplementary Figure S5B, available at https://doi.org/10.1016/j.esmoop.2024.103681).
Discussion
The prognosis for patients with unresectable peritoneal metastases remains grim, even with the current standard of care, which typically involves systemic chemotherapy. One of the major issues faced in treating peritoneal metastases is the limited effectiveness of conventional systemic drug delivery, as it often results in poor penetration into the peritoneal nodules.30 As a result, there is a pressing and unmet need for cancer therapeutics that can strike a balance between efficacy and tolerability in the treatment of cancers with peritoneal metastases. In this context, the combination of PIPAC-OX and systemic nivolumab sheds light on the potential future management landscape of GCPM. PIPAC-OX, when administered directly into the peritoneal cavity, allows for a concentrated and targeted delivery of chemotherapy to the GCPM. This localized treatment approach may overcome the limitations of systemic chemotherapy by ensuring better penetration into the tumor cells in the peritoneal niche with less systemic penetration and toxicity, while inducing immunogenic cell death.
Our study is amongst the first to study how PIPAC-OX may affect the tumor microenvironment of peritoneal metastases. The peritoneum, as a unique anatomical site, is hypothesized to possess a distinct microenvironment that is influenced by malignant cells, allowing them to evade the host immune system and establish an immune niche.6,31,32 Extensive research has identified various genomic drivers, paracrine factors, and immune pathways involved in this immune evasion process.3 Peritoneal metastases have been found to exhibit a different microenvironment compared with the primary tumor site. For instance, previous studies have described a significantly reduced proportion of CD8+ cells in the microenvironment of peritoneal metastases compared with the primary tumor.33 Our study, through whole transcriptome sequencing, unraveled pathways that were enriched or suppressed in both normal and tumor-affected peritoneum. These may offer mechanistic insights into how tumors affect the peritoneal microenvironment and how macroscopically normal peritoneum may have been primed for the establishment of peritoneal metastases. Of note, we also found reduced M2 macrophages and NK T cells and an increase in subsets of CD4+ and CD8+ T cells in tumor samples following treatment with PIPAC-OX, suggesting that this treatment may increase the activity of the adaptive immune system in peritoneal metastases. We also demonstrated changes in several other important genes after PIPAC-OX and nivolumab. FOXP3, also known as scurfin, is a member of the FOX protein family and is involved in the development of regulatory T cells. FOXP3 can act as a co-activator to facilitate the Wnt-b-catenin signaling pathway, inducing EMT and tumor growth and metastasis in non-small-cell lung cancer (NSCLC).34 PVRIG, also known as poliovirus receptor (PVR)-related immunoglobulin domain containing, is involved in NK cell activation. PVRIG blockade significantly enhances NK cell killing of PVRL2+, PVR-low acute myeloid leukemia (AML) cell lines, and significantly increased NK cell activation and degranulation in the context of patient primary AML blasts.35
Our study employed multiple outcome measures of efficacy, evaluating survival outcomes alongside clinical, tumor, and pathological response. While the patients in our study demonstrated good responses to intraperitoneal locoregional therapy with reduction in PCI and PRGS scores, this did not translate into survival advantages. A systematic review and meta-analysis36 highlighted the limitations and variability that criteria such as the RECIST criteria and PCI may have. Despite the PCI being widely used to quantify the extent of peritoneal metastases including in clinical trials investigating peritoneal metastases, it remains an operator-dependent procedure which may introduce subjectivity and variability in interpretation. Our study was not designed to evaluate OS benefit. The lack of survival benefit may be due to the refractory nature of the patients enrolled in the study, after several lines of systemic therapy, with the objective response rate, disease control rate, PFS, and OS remaining modest.37, 38, 39 Other chemotherapy regimens of PIPAC for gastric cancer with peritoneal metastases have been investigated, including cisplatin and doxorubicin. Objective tumor response was observed in 12 patients (50%) receiving PIPAC cisplatin and doxorubicin.40 In another study, the PIPAC-GA2, combination of systemic chemotherapy with XELOX and PIPAC chemotherapy with cisplatin and doxorubicin was also explored. Out of 31 patients with a mean PCI of 13.8, 15 patients were eligible for tumor response assessment, with 4 and 5 patients, respectively, having complete and partial pathological response. The median survival was found to be 13 months. These results suggest that more systemic therapy could be combined with PIPAC-OX, such as chemo-immunotherapy combinations like FOLFOX/nivolumab or CAPOX/pembrolizumab, which are the current standard-of-care first-line regimens for advanced gastric cancer.13,41
One established advantage of PIPAC is the concentrated delivery of chemotherapy drugs into the peritoneal cavity allowing higher doses and deeper penetration into tumor nodules while minimizing systemic dose-limiting toxicity, and preserving quality of life.42,43 To our knowledge, our study is the first to describe the combination of PIPAC and systemic immunotherapy. Whilst the safety findings from our study are favorable, the study’s limited sample size must be taken into account and larger-scale studies evaluating its efficacy are required. Several studies have reported the safety of PIPAC-OX dosed at 92 mg/m2,44, 45, 46 close to our study’s dose of 90 mg/m2, and reported similar safety profiles. The largest study45 included 251 PIPAC-OX treatments in 101 patients with unresectable peritoneal metastases, finding CTCAE grade 3 toxicities in 14 (13.9%) of 101 patients, with grade 4 and 5 toxicities both occurring in 1 patient each. Additionally, our study presents one of the first to report the pharmacokinetic profile of PIPAC-OX on the background of systemic nivolumab. Concentrations of plasma peaked in the first hour before decreasing by the 30th, with data similar to our results reported in our original study of unidirectional PIPAC-OX.16 Pharmacokinetic modelling and AUC analysis were not carried out due to limited sampling. Thus, the regimen of PIPAC-OX employed may result in safe and predictable pharmacokinetics, making its use in combination with systemic nivolumab favorable.
Although our study has several limitations, it is important to acknowledge that the recruitment and management of patients in this international, multicenter, investigator-initiated trial occurred during the peak of the COVID-19 pandemic, posing unique challenges. Despite these obstacles, the promising translational and safety results strongly support further investigation into novel combinations of immunotherapies and locoregional intraperitoneal interventions.
Conclusion
The combination of PIPAC-OX and nivolumab was safe and well tolerated. Translational data generated from our study show an immune-infiltrated tumor microenvironment and increased T-cell populations and may provide a rationale for combining immunotherapy and locoregional peritoneal therapy in patients with GCPM.
Acknowledgments
Funding
This work was supported by the ExxonMobil-NUS Research Fellowship (no grant number) (to DKAC), the National University Health System [grant number NUHSRO/2022/057/RO5+6/Seed-Mar/02 to DKAC], and the National Medical Research Council [grant number NMRC/RTF/MH 095:003∖008-332 to DKAC]; the National University Health System Seed Fund [grant number NUHSRO/2024/008/RO5+6/Seed-Sep23/01to JJZ], National University Hospital Junior Research Award 2023 [grant number JRA/Sep23/002 to JJZ], and Dean’s Research Development Award (no grant number) awarded by the Yong Loo Lin School of Medicine, National University of Singapore (to JJZ and ARYBL); the National Medical Research Council Clinician Scientist-Individual Research Grant [grant numbers MOH-CIRG21jun-0005 and MOH-CIRG24jan-0044 to CAJO] and Clinician Scientist Award (INV category) [grant number MOH-CSAINV22jul-0005 to CAJO]; the National Medical Research Council [grant numbers NMRC/CIRG23Jul-0035 and NMRC/MOH-000627 to RS]; the National Medical Research Council Centre Grant for the National University Cancer Institute, Singapore [grant number NMRC/CG/M005/2017_NCIS]; the National Research Foundation, Singapore, and Singapore Ministry of Health’s National Medical Research Council under its Open Fund-Large Collaborative Grant (‘OF-LCG’) [grant number MOH-OFLCG18May-0003 to PT], the Singapore Gastric Cancer Consortium (no grant number) (to PT), and the National Medical Research Council [grant number MOH-000967 to PT]. Nivolumab was supplied by Bristol Myers Squibb. The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the manuscript.
Disclosure
PT reports other support from Tempus Healthcare outside the submitted work. RS reports attending advisory board meetings for Bristol Myers Squibb (BMS), Merck, Eisai, Bayer, Taiho, Novartis, Merck Sharp & Dohme (MSD), GSK, DKSH, Astellas, Pierre-Fabre, Tavotek; receiving honoraria for talks from MSD, Eli Lilly, BMS, Roche, Taiho, AstraZeneca, DKSH, Ipsen, Daiichi Sankyo, BeiGene, Astellas; receiving travel support from Roche, AstraZeneca, Taiho, Eisai, DKSH, Ipsen, Paxman Coolers, Cytomed Therapeutics; receiving research funding from Paxman Coolers, MSD, Natera, CytoMed Therapeutics and has patents pending with licensing to Paxman and Auristone outside the submitted work. EC reports speaker fees from Amgen and BMS, travel support from AstraZeneca, Servier, and MSD, participation in advisory board meetings of BeiGene, and stocks in Alligator BioScience, all outside of the submitted work. MCHN reports consultancy fees from AstraZeneca, BeiGene, BMS, Merck, MSD, Novartis, and Pfizer, honoraria from Amgen, BMS, Eli Lilly, MSD, and Taiho, travel support from AstraZeneca, BMS, and MSD, and stocks in AstraZeneca, all outside of the submitted work. WPY reports participation in the advisory boards of Amgen and Astellas Pharma, and in the speakers’ bureau of DKSH, AstraZeneca, BMS, MSD, Novartis, and Daiichi Sankyo, all outside of the submitted work. WC is a senior clinical investigator with the Fund for Scientific Research - Flanders (FWO), outside of the submitted work. All other authors have declared no conflicts of interest.
Contributor Information
R. Sundar, Email: mdcragh@nus.edu.sg.
J.B.Y. So, Email: sursbyj@nus.edu.sg.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Wagner A.D., Grothe W., Haerting J., Kleber G., Grothey A., Fleig W.E. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 3.Gwee Y.X., Chia D.K.A., So J., et al. Integration of genomic biology into therapeutic strategies of gastric cancer peritoneal metastasis. J Clin Oncol. 2022;40(24):2830. doi: 10.1200/JCO.21.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chia C.S., You B., Decullier E., et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol. 2016;23(6):1971–1979. doi: 10.1245/s10434-015-5081-3. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker P.H., Ryan D.P. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol. 2012;13(8):e362–e369. doi: 10.1016/S1470-2045(12)70210-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J.J., Ong C.A.J., Srivatsava S., et al. Spatially resolved niche and tumor microenvironmental alterations in gastric cancer peritoneal metastases. Gastroenterology. 2024 doi: 10.1053/j.gastro.2024.08.007. [DOI] [PubMed] [Google Scholar]
- 7.He Z., Zhao T.T., Xu H.M., et al. Efficacy and safety of intraperitoneal chemotherapy in patients with advanced gastric cancer: a cumulative meta-analysis of randomized controlled trials. Oncotarget. 2017;8(46):81125–81136. doi: 10.18632/oncotarget.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan D.Y., Syn N.L., Yap R., et al. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are we ready? J Gastrointest Surg. 2017;21(3):425–433. doi: 10.1007/s11605-016-3336-3. [DOI] [PubMed] [Google Scholar]
- 9.Solaß W., Hetzel A., Nadiradze G., Sagynaliev E., Reymond M.A. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc. 2012;26(7):1849–1855. doi: 10.1007/s00464-012-2148-0. [DOI] [PubMed] [Google Scholar]
- 10.Solass W., Herbette A., Schwarz T., et al. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc. 2012;26(3):847–852. doi: 10.1007/s00464-011-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solaß W., Giger-Pabst U., Zieren J., Reymond M.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol. 2013;20(11):3504–3511. doi: 10.1245/s10434-013-3039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesniere A., Schlemmer F., Boige V., et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 13.Shitara K., Ajani J.A., Moehler M., et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942–948. doi: 10.1038/s41586-022-04508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janjigian Y.Y., Shitara K., Moehler M., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Batran S.E., Goetze T.O., Mueller D.W., et al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction - a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer. 2017;17(1):893. doi: 10.1186/s12885-017-3918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim G., Tan H.L., Sundar R., et al. PIPAC-OX: a phase I study of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy in patients with peritoneal metastases. Clin Cancer Res. 2021;27(7):1875–1881. doi: 10.1158/1078-0432.CCR-20-2152. [DOI] [PubMed] [Google Scholar]
- 17.Clavien P.A., Barkun J., de Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 18.Solass W., Sempoux C., Detlefsen S., Carr N.J., Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS) Pleura Peritoneum. 2016;1(2):99–107. doi: 10.1515/pp-2016-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison J.G., White P., McDougall S., et al. Validation of a highly sensitive ICP-MS method for the determination of platinum in biofluids: application to clinical pharmacokinetic studies with oxaliplatin. J Pharm Biomed Anal. 2000;24(1):1–10. doi: 10.1016/s0731-7085(00)00377-0. [DOI] [PubMed] [Google Scholar]
- 20.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberzon A., Birger C., Thorvaldsdottir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang P., Gu S., Pan D., et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshihara K., Shahmoradgoli M., Martínez E., et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4(1):2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Porath I., Thomson M.W., Carey V.J., et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40(5):499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Xu X., Maglic D., et al. Comprehensive molecular characterization of the hippo signaling pathway in cancer. Cell Rep. 2018;25(5):1304–1317.e5. doi: 10.1016/j.celrep.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen B., Khodadoust M.S., Liu C.L., Newman A.M., Alizadeh A.A. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aran D., Hu Z., Butte A.J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18(1):220. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturm G., Finotello F., List M. Immunedeconv: an R package for unified access to computational methods for estimating immune cell fractions from Bulk RNA-sequencing data. Methods Mol Biol. 2020;2120:223–232. doi: 10.1007/978-1-0716-0327-7_16. [DOI] [PubMed] [Google Scholar]
- 30.So J.B.Y. Bi-directional chemotherapy for peritoneal metastases. Br J Surg. 2023;110(6):627–628. doi: 10.1093/bjs/znad061. [DOI] [PubMed] [Google Scholar]
- 31.Ornella M.S.C., Badrinath N., Kim K.A., et al. Immunotherapy for peritoneal carcinomatosis: challenges and prospective outcomes. Cancers (Basel) 2023;15(8):2383. doi: 10.3390/cancers15082383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao X., Ajani J.A., Song S. Molecular biology and immunology of gastric cancer peritoneal metastasis. Transl Gastroenterol Hepatol. 2020;5:57. doi: 10.21037/tgh.2020.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimori D., Kinoshita J., Yamaguchi T., et al. Established fibrous peritoneal metastasis in an immunocompetent mouse model similar to clinical immune microenvironment of gastric cancer. BMC Cancer. 2020;20(1):1014. doi: 10.1186/s12885-020-07477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S., Liu Y., Li M.-Y., et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16(1):124. doi: 10.1186/s12943-017-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J., Whelan S., Kotturi M.F., et al. PVRIG is a novel natural killer cell immune checkpoint receptor in acute myeloid leukemia. Haematologica. 2021;106(12):3115–3124. doi: 10.3324/haematol.2020.258574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Giorgio A., Macrì A., Ferracci F., et al. 10 Years of pressurized intraperitoneal aerosol chemotherapy (PIPAC): a systematic review and meta-analysis. Cancers (Basel) 2023;15(4):1125. doi: 10.3390/cancers15041125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gamboa A.C., Winer J.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer. Cancers (Basel) 2019;11(11):1662. doi: 10.3390/cancers11111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manzanedo I., Pereira F., Serrano A., Perez-Viejo E. Review of management and treatment of peritoneal metastases from gastric cancer origin. J Gastrointest Oncol. 2021;12(suppl 1):S20–S29. doi: 10.21037/jgo-20-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glehen O., Gilly F.N., Arvieux C., et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 40.Nadiradze G., Giger-Pabst U., Zieren J., Strumberg D., Solass W., Reymond M.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg. 2016;20(2):367–373. doi: 10.1007/s11605-015-2995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rha S.Y., Oh D.Y., Yañez P., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–1195. doi: 10.1016/S1470-2045(23)00515-6. [DOI] [PubMed] [Google Scholar]
- 42.Jacquet P., Sugarbaker P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 43.Mimouni M., Richard C., Adenot P., et al. Pressurized intra-peritoneal aerosol chemotherapy (PIPAC): increased intraperitoneal pressure does not affect distribution patterns but leads to deeper penetration depth of doxorubicin in a sheep model. BMC Cancer. 2021;21(1):461. doi: 10.1186/s12885-021-07955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robella M., De Simone M., Berchialla P., et al. A phase I dose escalation study of oxaliplatin, cisplatin and doxorubicin applied as PIPAC in patients with peritoneal carcinomatosis. Cancers (Basel) 2021;13(5):1060. doi: 10.3390/cancers13051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sgarbura O., Hübner M., Alyami M., et al. Oxaliplatin use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is safe and effective: a multicenter study. Eur J Surg Oncol. 2019;45(12):2386–2391. doi: 10.1016/j.ejso.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Rovers K.P., Wassenaar E.C.E., Lurvink R.J., et al. Pressurized intraperitoneal aerosol chemotherapy (Oxaliplatin) for unresectable colorectal peritoneal metastases: a multicenter, single-arm, phase II trial (CRC-PIPAC) Ann Surg Oncol. 2021;28(9):5311–5326. doi: 10.1245/s10434-020-09558-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genomic data have been uploaded into the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) (accession number: PRJNA1137069). Any other data generated in this study are available upon request from the corresponding author.