Summary
Background
The proper management of suspicious radiologic findings is crucial to optimize the effectiveness of low-dose computed tomography (LDCT) lung cancer screening trials. In the BioMILD study, we evaluated the utility of combining a plasma 24-microRNA signature classifier (MSC) and LDCT to define the individual risk and personalize screening strategies. Here we aim to assess the utility of repeated MSC testing during annual screening rounds in 1024 participants with suspicious LDCT findings.
Methods
The primary outcome was two-year lung cancer incidence in relation to MSC test results, reported as relative risk (RR) with 95% confidence interval (CI). Lung cancer incidence and mortality were estimated using extended Cox models for time-dependent covariates, yielding the respective hazard ratios (HR). Clinicaltrials.gov ID: NCT02247453.
Findings
With a median follow-up of 8.5 years, the full study set included 1403 indeterminate LDCT (CTind) and 584 positive LDCT (CT+) results. A lung cancer RR increase in MSC+ compared to MSC- participants was observed in both the CTind (RR: 2.5; 95% CI: 1.4–4.32) and CT+ (RR: 2.6; 95% CI: 1.81–3.74) groups and was maintained when considering stage I or resectable tumors only. A 98% negative predictive value in CTind/MSC− and a 30% positive predictive value in CT+/MSC+ lesions were recorded. At seven years’ follow-up, MSC+ participants had a cumulative HR of 4.4 (95% CI: 3.0–6.4) for lung cancer incidence and of 8.1 (95% CI: 2.7–24.5) for lung cancer mortality.
Interpretation
Our study shows that MSC can be reliably performed during LDCT screening rounds to increase the accuracy of lung cancer risk and mortality prediction and supports its clinical utility in the management of LDCT findings of uncertain malignancy.
Funding
Italian Association for Cancer Research; Italian Ministry of Health; Horizon2020; National Cancer Institute (NCI); Gensignia LifeScience.
Keywords: LDCT screening, Biomarkers, Early detection
Research in context.
Evidence before this study
As of May 2024, a PubMed search was conducted using the query “lung cancer screening reduced mortality”, limited to European randomized controlled trials from the last five years. The two main studies, NELSON and MILD, have demonstrated that low-dose computed tomography (LDCT) screening significantly reduces lung cancer mortality, with even better outcomes when the intervention is extended beyond five years. However, LDCT is known to produce suspicious findings in about 9%–15% of cases, but less than 10% of these turn out to be cancer, leading to unnecessary repeat scans and invasive procedures. Effective management of patients with lung nodules and the adoption of proper screening intervals are crucial to maximize the benefits of these programs.
Added value of this study
In 2013, we launched the BioMILD trial, combining a plasma 24-microRNA signature classifier (MSC) with LDCT to assess individual risk and personalize screening. Baseline results showed that participants with a double-negative result had a low 4-year lung cancer incidence of 0.8%, allowing their screening interval to be safely extended to 3 years. By assessing high-risk BioMILD participants with suspicious lung lesions over ten years, the present study reports the performance of repeated MSC testing alongside LDCT for accurate risk assessment. Overall, a positive MSC profile significantly increased the lung cancer risk as the onset of disease, including early-stage and resectable tumors, was approaching, with a notable impact on mortality prediction.
Implications of all the available evidence
Serial blood testing might enhance the management of suspicious LDCT findings, potentially leading to timely interventions or preventive neo-adjuvant or adjuvant therapy. Ultimately, its implementation in screening programs may lead to earlier cancer diagnosis and improve screening compliance while reducing costs and harm.
Introduction
The findings of randomized low-dose computed tomography (LDCT) lung cancer screening trials have provided clear evidence that mortality can be reduced with improved results by extending the intervention beyond five years.1, 2, 3 In all these trials a remarkable increase (>50%) in stage I-II tumors was observed in the screening arm as compared to the 25% detected in the clinical setting in most western countries.4 Nevertheless, although the United States Preventive Services Task Force (USPSTF) recently lowered the risk criteria of eligibility for screening, adherence to screening in the US has only modesty increased to 5.0%.5
Despite the recent introduction of more precise volumetric measurement for solid nodule size in the Lung CT Screening Reporting & Data System (Lung-RADS) guidelines, LDCT screening still yields a high rate of false positives.6 These results lead to unnecessary repeat scans and invasive procedures that often return non-malignant results, causing avoidable morbidity and mortality.6, 7, 8 The proper management of patients with lung nodules is therefore crucial to optimize the effectiveness of LDCT screening.9
Identification of complementary non-imaging-based tests is a priority in this respect, especially given the new adjuvant and neo-adjuvant treatment options for patients with early-stage and resectable lung cancer.10 However, neither diagnostic nor prognostic biomarkers have yet been adopted in LDCT lung cancer screening programs.11,12
Circulating microRNAs (miRNA) reflecting a protumorigenic status of the lung microenvironment or the presence of early lesions could represent ideal candidates as biomarkers for early lung cancer detection.13, 14, 15, 16 In our previous research, we utilized a plasma miRNA assay in LDCT screening series, resulting in the development of a 24-miRNA signature classifier (MSC).17,18 The clinical utility of MSC was initially assessed in a large retrospective trial.19 Based on our findings, an independent research group reported on the cost-effectiveness of the combined LDCT and MSC screening strategy compared to LDCT alone.20
In 2013, we initiated the BioMILD prospective clinical trial, a single-arm LDCT screening study that enrolled 4119 participants (clinicaltrials.gov ID: NCT02247453).21 The baseline results of the BioMILD trial indicated that the combined use of LDCT and MSC predicted individual lung cancer incidence and mortality. Specifically, we demonstrated the feasibility of extending the screening interval to three years for double-negative participants without a significant decrease in stage I and curable tumors.21
According to the BioMILD study design, participants with indeterminate (CTind) or positive (CT+) nodules on LDCT returned for subsequent annual rounds until lung cancer diagnosis or study completion. To gain further insight into the utility of the MSC test in a screening context, we here report the final results on the performance of repeated blood MSC testing in BioMILD participants with suspicious LDCT findings across all screening rounds. Our ultimate goal is to assess whether there is an improvement in the estimation of lung cancer risk and lung cancer-specific mortality after recurrent MSC testing.
Methods
Study design and selection of participants
The BioMILD trial combined the MSC test and LDCT to improve the lung cancer screening efficacy by personalizing the risk profile and screening intervals. Details on the general enrolment criteria and screening modalities have been described previously.21 Briefly, participants were 50- to 75-year-old current or former heavy smokers who quit smoking less than 10 years before recruitment (≥30 pack-years) or current or former smokers (20 pack-years) with a family history of lung cancer or a prior diagnosis of chronic obstructive pulmonary disease (COPD) or pneumonia. The exclusion criteria were any type of cancer occurring within the last five years and suspicious lung nodules under investigation.
Between January 2013 and March 2016, 4119 participants provided written informed consent and were enrolled at the Fondazione IRCCS Istituto Nazionale dei Tumori. At baseline and at every subsequent screening round, participants underwent LDCT and blood withdrawal for the MSC test. They were then independently classified according to the LDCT and MSC test result in order to better personalize the screening strategies. Participants with a LDCT/MSC double-negative profile were invited to return after a 3 years interval period, while other participants were invited to return after one-year or shorter intervals according to nodule size and independently of the MSC risk level.21
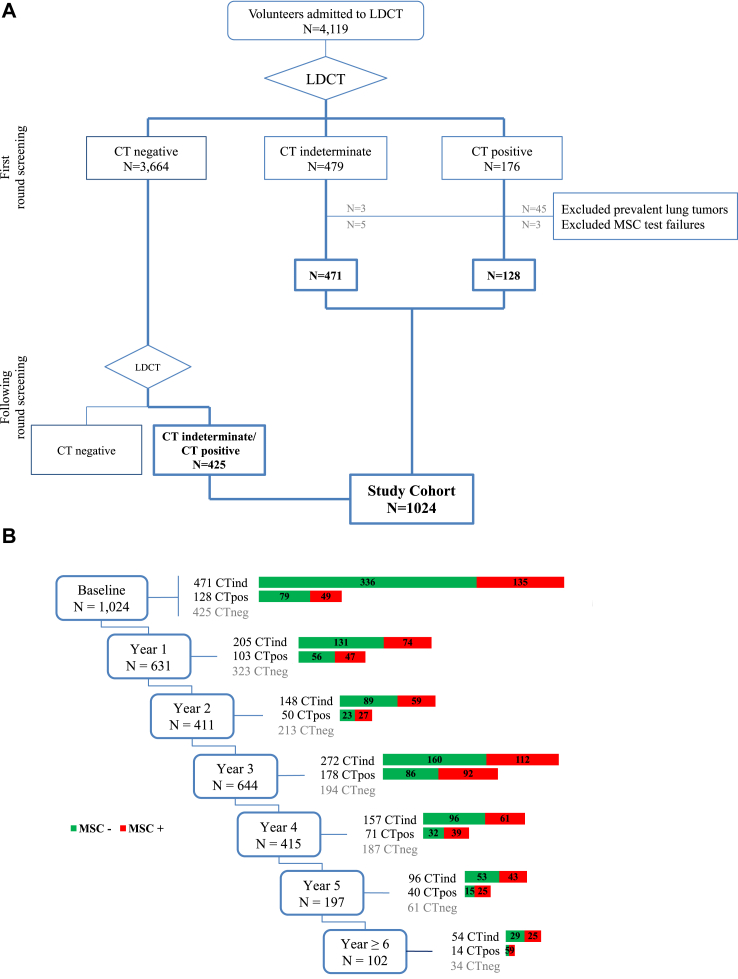
For the present analysis we selected only participants with at least one CTind or CT+ result throughout the screening program, while patients with prevalent lung tumors or MSC test failure were excluded (Fig. 1). The study was approved by the Institutional Review Board and Ethics Committee and complied with the Declaration of Helsinki.
Fig. 1.
Flow chart of the study design. BioMILD screening participants were selected according to the low-dose computed tomography (LDCT) result at the first and subsequent screening rounds (A). At each screening round the selected participants were stratified according to the combined results of LDCT and the microRNA signature classifier (MSC) test (B). LDCT divided patients into CT negative (CT−), CT indeterminate (CTind) and CT positive (CT+), while MSC divided patients into MSC positive (MSC+) and MSC negative (MSC−).
LDCT imaging and nodule classification
LDCT scans were performed on a second-generation dual-source CT scanner (Somatom Definition Flash, Siemens Medical Solutions) as previously described.21 LDCT results were classified according to the MILD screening trial algorithm and similarly to the Lung CT Reporting & Data System guidelines (Lung-RADS version 1.1).3,22 At baseline, LDCT scans with a dominant solid nodule (SN) with a volume of 113–260 mm3; a part-solid nodule (PSN) with a solid component <5 mm in diameter or a nonsolid nodule (NSN) ≥5 mm in diameter were classified as CTind, while LDCT scans with a dominant SN >260 mm3 or a PSN with a solid component ≥5 mm were classified as CT+. At follow-up, LDCT scans with a dominant incident SN ≥60 mm3 or a PSN with a solid component ≥5 mm were classified as CT+, whereas those with a dominant incident SN <60 mm3, a PSN with a solid component <5 mm or a NSN regardless of size were classified as CTind. All remaining scans were classified as LDCT negative (CT−) at both baseline and follow-up.
Nodule management
In accordance with the BioMILD study design, all CTind outcomes were investigated by annual LDCT screening rounds, while CT+ tests were further investigated by LDCT after three months, independently of the MSC test result. When a SN volume-doubling time (VDT) <400 days was observed at the three-month recall, participants underwent 18-fluorodesoxyglucose positron emission tomography (FDG-PET) and/or contrast-enhanced CT. Lesions showing a positive FDG uptake underwent workup by biopsy and/or lung surgery as jointly established by a multidisciplinary team including radiologists and thoracic surgeons.
Plasma microRNA profiling
The MSC test is a custom locked molecular assay with predefined cut-offs based on quantitative real-time polymerase chain reaction (qPCR); it was trained and validated in two retrospectives lung cancer LDCT screening cohorts.17,18 Details about the MSC algorithm can be found in a published methodology paper.19 Starting from 10 mL of whole blood collected in a K2 Ethylenediaminetetraacetic acid (K2EDTA) Vacutainer tube, plasma was separated by two consecutive centrifugation steps at 1258×g and 4 °C for 10 min. RNA was extracted from 200 μL of plasma and eluted in 50 μL of buffer according to the Maxwell RSC miRNA Tissue Kit (Promega) protocol. Starting from 3 μL of eluted RNA, the reverse transcription (RT) phase, 12-cycles pre-amplification process and the qPCR were carried out according to the protocol for running custom RT and preamplification pools on custom TaqMan Array microRNA Cards (ThermoFisher Scientific).
The 384-well microfluidic custom cards to analyze eight samples in duplicates were spotted with probes for the 24 miRNAs: hsa-miR-101-2253, hsa-miR-106a-2169, hsa-miR-126-2228, hsa-miR-133a-2246, hsa-miR-140-3p-2234, hsa-miR-140-5p-1187, hsa-miR-142-3p-464, hsa-miR-145-2278, hsa-miR-148a-470, hsa-miR-15b-390, hsa-miR-16-391, hsa-miR-17-2308, hsa-miR-197-497, hsa-miR-19b-396, hsa-miR-21-397, hsa-miR-221-524, hsa-miR-28-3p-2446, hsa-miR-30b-602, hsa-miR-30c-419, hsa-miR-320-2277, hsa-miR-451-1141, hsa-miR-486-5p-1278, hsa-miR-660-1515 and hsa-miR-92a-431. RT-qPCR was performed using the ViiA7 Real-Time PCR System (ThermoFisher Scientific) with proper cycling parameters: 10 min at 94.5 °C and 40 cycles at 97 °C for 30 s and 59.7 °C for 60 s. The background signal was removed setting automatic baseline cycles and a fixed threshold of 0.15 for all 24 assays. The mean cycle threshold (Ct) value of the two duplicates was considered for the analysis. The ratio between couples of miRNAs featuring the MSC signatures (risk of disease—RD; risk of aggressive disease—RAD; presence of disease—PD; presence of aggressive disease—PAD) was calculated as the additive inverse ΔCts value. The number of ratios exceeding the respective cut-offs to be considered positive was nine out of 27 for RD and PD (six out of 27 for samples with high levels of hemolysis), and 14 out of 28 for RAD and PAD. Positivity for at least one of the four signatures determined a positive test outcome. The results of the MSC test were uploaded to the database, if possible within three weeks after blood withdrawal.
Patient management
Clinical data on lung cancers detected as part of the screening study were collected during hospitalization and outpatient follow-up. lung cancers detected after a negative LDCT within the expected interval period or first detected after voluntary withdrawal from screening were defined as interval lung cancers. Information on interval lung cancer and cause of death was collected through phone calls or email contact with the general practitioner or referring hospital and through periodic requests to cancer registries. Only patients with a verified lung cancer diagnosis obtained through one of the mentioned channels were considered here. Vital status was obtained through the National Institute of Statistics (ISTAT platform, SIATEL 2.0), which provides the exact date of death within three months of its occurrence. Participants accumulated person-years from the baseline date until death or the date of last follow-up (last date update: June 2023).
Statistical analysis
In our previous analyses of retrospective series, we observed that the predictive power of MSC was maintained in the two years preceding a lung cancer diagnosis.19,23 Therefore, the primary outcome for the present analysis was the two-year lung cancer incidence after annual CTind and CT+ findings in strata of MSC test results. The percentage of lung cancers detected with specific features (stage, resectability, interval cancer, and adenocarcinoma histology) was calculated relative to the total number of tests and the total number of lung cancers detected. The main comparisons were evaluated calculating the relative risk (RR) between MSC+ and MSC− results and 95% confidence interval (95% CI).
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the MSC test for lung cancer incidence at one year, two years and overall were evaluated generally and stratified by CT results. For the subgroup analyses, we sequentially excluded patients with lung cancer at stages higher than I and former smokers. In lung cancer cases, the sensitivity of the MSC test was calculated at the time of diagnosis and in the previous samples; a swimmer plot of MSC samples from baseline to the time of diagnosis was drawn up. lung cancer incidence overall, one year and two years after the last two annual MSC tests with a maximum interval of two years was reported and selected comparisons were made using the chi-square test.
The seven-year cumulative lung cancer incidence and lung cancer-specific mortality was estimated by extended Kaplan–Meier (KM) curves for time-varying variables and Cox models with hazard ratios (HR) and 95% CIs for time-dependent covariates. Participants were stratified by annual MSC test results, considering the dynamically changing MSC status (positive or negative) for each individual. For lung cancer incidence, the time has been calculated from the baseline to the date of lung cancer diagnosis or last contact. For lung cancer-specific mortality, the time has been calculated from the baseline to the date of death or last vital status follow-up (June 2023). Time-dependent Cox models were also constructed for lung cancer incidence and mortality in strata of MSC test results, COPD and clinical risk models such as the Brock model and the modified Prostate, Lung, Colorectal and Ovarian cancer risk prediction model (PLCOm2012).24,25
All analyses were two-sided and p values < 0.05 were considered significant. Analyses were performed using Statistical Analysis System (SAS) software (release 9.04; SAS Institute, Cary, North Carolina, USA) and RStudio (version 1.4.1106).
Role of the funding source
The funders had no role in the study's design, data collection, analysis, interpretation, writing, or the decision to submit the paper for publication.
Results
Study population
As shown in Fig. 1A, 4119 participants were recruited to the BioMILD screening program. With a median follow-up of 8.5 years, 1088 participants had at least one suspicious LDCT finding at any screening round. In this cohort, 3545 annual MSC tests had been conducted by the end of follow-up and 30 (0.8%) resulted in failures. In 16 participants, the failed test was the only available MSC test (eight at baseline and eight at subsequent screening rounds) and these were therefore excluded from the present analysis. Additionally, to better assess lung cancer incidence and mortality according to the time-varying MSC test results, we also excluded 48 patients with prevalent lung tumors detected at baseline. Of the selected 1024 participants 599 had a suspicious LDCT result already at the first screening round and 425 exclusively over the following years. At every screening round, participants were classified both as CT+, CTind or CT− and as MSC+ or MSC− (Fig. 1B).
The characteristics of the selected participants at the time of enrollment are reported in Supplementary Table S1: 192 (18.8%) were younger than 55 and 321 (31.4%) older than 65 years; 426 (41.6%) were women; 976 (95.3%) had >30 pack-years and 830 (81.1%) were current smokers. Participants were followed until lung cancer diagnosis or study completion for a total of 8594 person-years.
Lung cancer risk prediction by MSC in CTind and CT+ participants
The full study set included 1403 CTind and 584 CT+ results along with the corresponding 1190 MSC- tests and 797 MSC+ results evenly distributed between the two CT groups (Table 1). The median time to lung cancer diagnosis was 1.4 years (interquartile range [IQR]: 1.1–1.6) and 0.4 years (IQR: 0.2–1.0), respectively, in the two CT groups, without any meaningful differences between MSC+ and MSC− participants. lung cancer was diagnosed within two years in 48 (3.4%) CTind and 120 (20.5%) CT+ participants. A significant lung cancer risk increases after CTind (RR: 2.46; 95% CI: 1.40–4.32) or CT+ (RR: 2.60; 95% CI: 1.81–3.74) was observed in the MSC+ group compared to the MSC- group. Similar results were seen when only stage I or resectable tumors were considered. Conversely, in both CT groups, adenocarcinomas were less frequently MSC+ than other histologies, while a non-significant higher risk of interval cancers among MSC+ was observed.
Table 1.
Lung cancer incidence in all screening rounds after CTind and CT+ exams in strata of MSC test result.
| TOTAL exams | MSC− | MSC+ | MSC+ versus MSC− RRa (95% CI) | MSC+ versus MSC− RRb (95% CI) | |
|---|---|---|---|---|---|
| CTind | 1403 | 894 | 509 | ||
| LC within 2 years | 48 | 20 | 28 | 2.46 (1.40–4.32) | |
| (% of CTind) | (3.4%) | (2.2%) | (5.5%) | p = 0.0012 | |
| Stage I LC | 35 | 15 | 20 | 2.34 (1.21–4.53) | 0.95 (0.67–1.34) |
| (% of CTind; % of LC) | (2.5%; 72.9%) | (1.7%; 75%) | (3.9%; 71.4%) | p = 0.0093 | p = 0.7837 |
| Resectable LC | 40 | 16 | 24 | 2.63 (1.41–4.91) | 1.07 (0.82–1.40) |
| (% of CTind; % of LC) | (2.9%; 83.3%) | (1.8%; 80%) | (4.7%; 85.7%) | p = 0.0015 | p = 0.7031 |
| Interval cancer | 5 | 2 | 3 | 2.63 (0.44–15.7) | 1.07 (0.20–5.83) |
| (% of CTind; % of LC) | (0.4%; 10.4%) | (0.2%; 10%) | (0.6%; 10.7%) | p = 0.3597 | p = 1.0000 |
| Adenocarcinoma | 32 | 17 | 15 | 1.55 (0.78–3.08) | 0.63 (0.43–0.93) |
| (% of CTind; % of LC) | (2.3%; 66.7%) | (1.9%; 85%) | (2.9%; 53.6%) | p = 0.2073 | p = 0.0312 |
| Median time to diagnosis | 1.4 y | 1.4 y | 1.3 y | ||
| (IQR) | (1.1–1.6) | (1.3–1.6) | (1.0–1.5) | ||
| CT+ | 584 | 296 | 288 | ||
| LC within 2 years | 120 | 34 | 86 | 2.60 (1.81–3.74) | |
| (% of CT+) | (20.5%) | (11.5%) | (29.9%) | p < 0.0001 | |
| Stage I LC | 87 | 29 | 58 | 2.06 (1.36–3.11) | 0.79 (0.65–0.97) |
| (% of CT+; % of LC) | (14.9%; 72.5%) | (9.8%; 85.3%) | (20.1%; 67.4%) | p = 0.0004 | p = 0.0484 |
| Resectable LC | 106 | 29 | 77 | 2.73 (1.84–4.05) | 1.05 (0.90–1.23) |
| (% of CT+; % of LC) | (18.2%; 88.3%) | (9.8%; 85.3%) | (26.7%; 89.5%) | p < 0.0001 | p = 0.5143 |
| Interval cancer | 1 | 0 | 1 | – | – |
| (% of CT+; % of LC) | (0.2%; 0.8%) | (0%; 0%) | (0.3%; 1.2%) | p = 0.4932 | p = 1.0000 |
| Adenocarcinoma | 89 | 30 | 59 | 2.02 (1.34–3.04) | 0.78 (0.64–0.94) |
| (% of CT+; % of LC) | (15.2%; 74.2%) | (10.1%; 88.2%) | (20.5%; 68.6%) | p = 0.0005 | p = 0.0362 |
| Median time to diagnosis | 0.4 y | 0.5 y | 0.4 y | ||
| (IQR) | (0.2–1.0) | (0.2–1.1) | (0.2–0.9) |
CI, confidence interval; CTind, computed tomography indeterminate; CT+, computed tomography positive; IQR, interquartile range; LC, lung cancer; MSC, 24-microRNA signature classifier; RR, relative risk; y, year.
Relative risk on the total number of CTs.
Relative risk on the number of lung cancers detected within two years.
The performance of the MSC test was similar at baseline and further screening rounds, although not always reaching the significance threshold probably due to the low number of events in exploratory subset analyses (Supplementary Tables S2 and S3).
MSC+ and MSC− test results did not significantly differ (p = 0.5325) among participants without lung cancer carrying solid or subsolid nodules (Supplementary Table S4).
Operating characteristics of the MSC test
The MSC test's operating characteristics, sensitivity, specificity, NPV and PPV were analyzed using the one-year, two-year and total lung cancer incidence following a CTind or CT+ result. The findings are reported in Table 2. In CTind the NPV reached 100% for lung cancer diagnosed within one year and 98% for lung cancer diagnosed within two years. In the CT+ group the PPV was 23% for lung cancer detected within one year and 30% for lung cancer detected within two years. Similar results in terms of operating characteristics were reported when considering only stage I tumors (Supplementary Table S5) or current smokers (Supplementary Table S6).
Table 2.
Performance of the MSC test in the CTind and CT+ groups in terms of sensitivity, specificity, positive predictive value and negative predictive value.
| TOTAL exams |
MSC− |
MSC+ |
Sens | Spec | PPV | NPV | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % of total exams | N | % of total exams | N | % of total exams | |||||
| CTind | 1403 | 894 | 509 | |||||||
| LC within 1 year | 9 | 0.6% | 2 | 0.2% | 7 | 1.4% | 78% | 64% | 1% | 100% |
| LC within 2 years | 48 | 3.4% | 20 | 2.2% | 28 | 5.5% | 58% | 65% | 6% | 98% |
| Total LC | 136 | 9.7% | 78 | 8.7% | 58 | 11.4% | 43% | 64% | 11% | 91% |
| CT+ | 584 | 296 | 288 | |||||||
| LC within 1 year | 90 | 15.4% | 24 | 8.1% | 66 | 22.9% | 73% | 55% | 23% | 92% |
| LC within 2 years | 120 | 20.5% | 34 | 11.5% | 86 | 29.9% | 72% | 56% | 30% | 88% |
| Total LC | 156 | 26.7% | 50 | 16.9% | 106 | 36.8% | 68% | 57% | 37% | 83% |
CTind, computed tomography indeterminate; CT+, computed tomography positive; LC, lung cancer; MSC, 24-microRNA signature classifier; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
Time dependency analysis of MSC
To evaluate the diagnostic performance of MSC at different intervals between blood sampling and lung cancer onset, we focused on the 125 patients who had developed lung cancer by the end of follow-up. Indeed, 124 of these (99%) were histologically proven. For the missing case, the adverse clinical conditions prevented the histological diagnosis. Most lung cancers were stage I adenocarcinomas treated with curative surgery and, when considering the MSC test result closest to the lung cancer diagnosis, none of the main clinicopathologic characteristics had a significant impact on the molecular stratification of these patients (Supplementary Table S7).
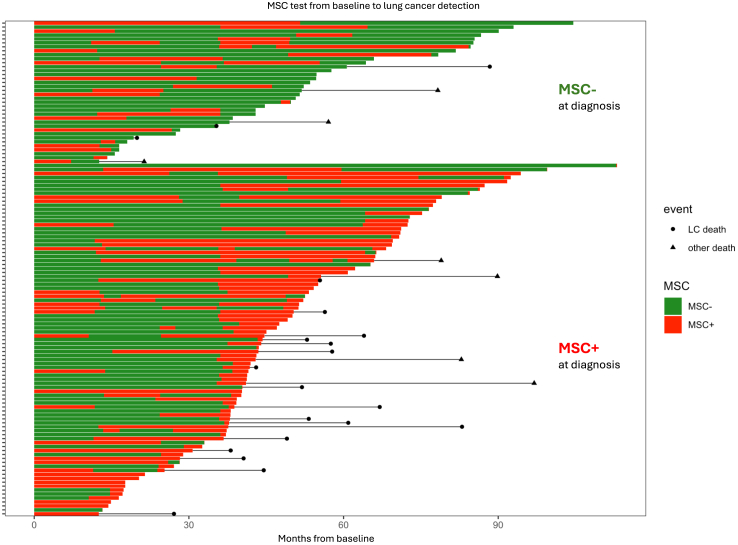
The changes in the MSC test output over time along with the lung cancer diagnosis and mortality are reported in Fig. 2. Time dependency analysis showed an 80% sensitivity of MSC at diagnosis, gradually decreasing to 70% in samples collected within 12 months before diagnosis, 53% between 12 and 24 months, down to 44% between 24 and 36 months (Supplementary Table S8).
Fig. 2.
Swimmer plot reporting the MSC test results over time along with lung cancer diagnosis and mortality. For the 125 patients who developed incident lung tumors during the BioMILD study, the MSC test results at annual recall and at cancer diagnosis were considered. Overall mortality and lung cancer-specific mortality are indicated with black lines starting from the time of lung cancer diagnosis.
Cumulative lung cancer incidence and mortality by annual MSC test repetition
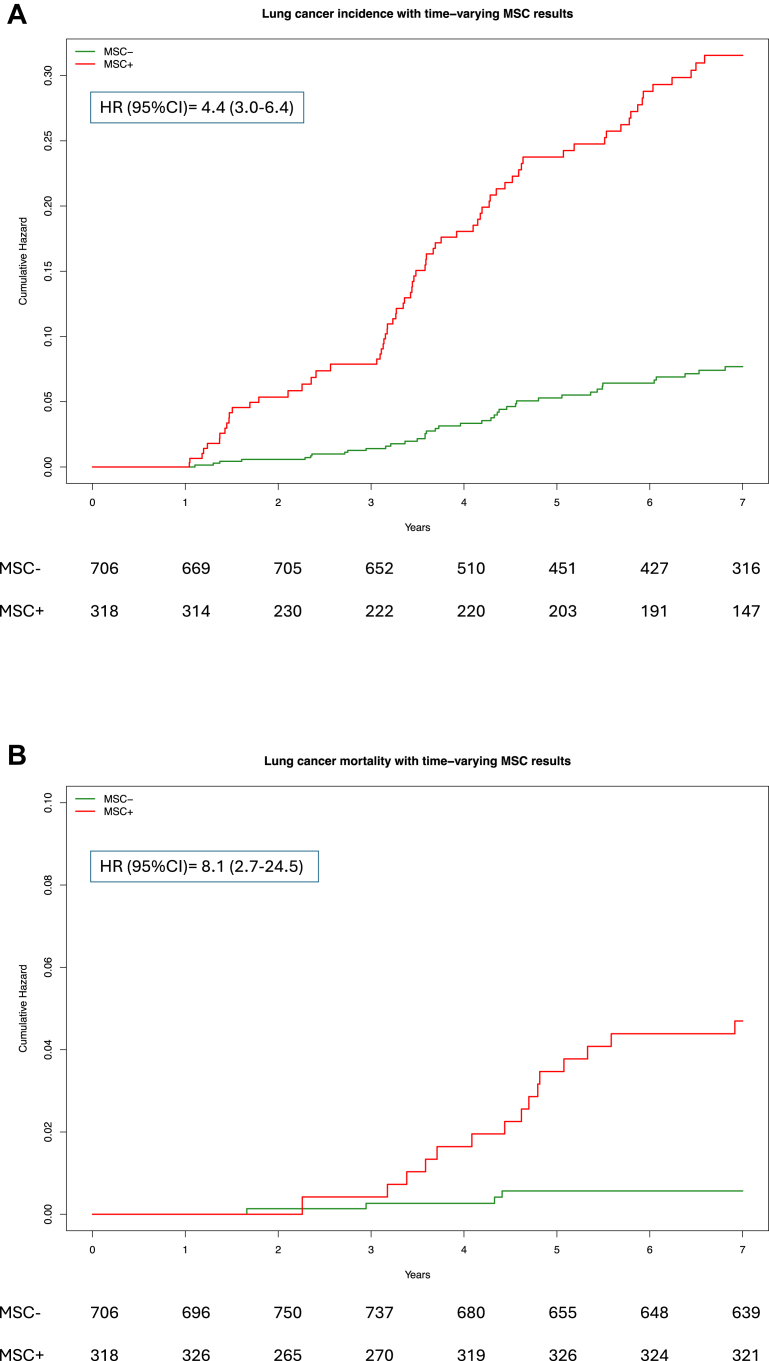
The utility of repeating the MSC test at every screening round was initially assessed by considering the cumulative lung cancer incidence according to the varying MSC test results in annual screening rounds. As shown in Fig. 3A, the lung cancer incidence in MSC+ participants increased significantly and constantly at every screening round compared to MSC− participants, with a cumulative HR of 4.4 (95% CI: 3.0–6.4). Similarly, in Fig. 3B the cumulative lung cancer-specific mortality curves of MSC+ and MSC− participants showed an HR of 8.1 (95% CI: 2.7–24.5).
Fig. 3.
Extended Kaplan–Meier curves stratified by yearly MSC test repetition. Lung cancer incidence (A) and mortality (B) in MSC+ and MSC− BioMILD screening participants. Cox models with hazard ratio (HR) and 95% confidence interval (95% CI) for time-dependent covariates were adopted.
The MSC test result remained significant for both incidence and mortality after adjusting for risk factors such as COPD or clinical risk models such as the PLCOm2012 and Brock models in multiparametric time-dependent Cox models (Supplementary Table S9).
Multiple MSC tests to improve lung cancer risk prediction
To assess the utility of considering the last two consecutive MSC test results in the presence of a suspicious CT finding, we focused on a group of 810 participants with multiple MSC tests available in the two years prior to the suspicious CT finding. Overall, a delay in the occurrence of lung cancer was observed in the 312 (38.5%) participants with two consecutives negative MSC results (Table 3). Compared to this group, the lung cancer incidence did not differ in the 221 (27.3%) participants who went from MSC+ to MSC− (RR: 1.3; 95% CI: 0.6–3.0), while it increased in the 122 (15.1%) participants who went from MSC− to MSC+ (RR: 4.2; 95% CI: 2.0–8.6) and in the 155 (19.1%) participants with consistently positive MSC test results (RR: 6.6; 95% CI: 3.4–12.6). Despite the increasing trend, the difference between these last two groups was not significant (p = 0.0772). Similar results in terms of RR were observed when considering lung cancer onset within two years after the last MSC test or until the end of follow-up.
Table 3.
Lung cancer diagnosis in 810 screening participants with two consecutive MSC test results within two years.
| MSC test shift | # of participants | Median time of diagnosis from the last MSC test | LC within 1 year (%)a | RR (95% CI) | p-value | LC within 2 years (%)b | RR (95% CI) | p-value | Total LC (%) | RR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSC− ≥ MSC− | 312 | 14.4 m | 11 (3.5) | Reference | 0.5591 | 19 (6.1) | Reference | 0.583 | 24 (7.7) | Reference | 0.5485 |
| MSC+ ≥ MSC− | 221 | 6.8 m | 10 (4.5) | 1.3 (0.6–3.0) | 11 (5.0) | 0.8 (0.4–1.7) | 14 (6.3) | 0.8 (0.4–1.6) | |||
| MSC− ≥ MSC+ | 122 | 3.0 m | 18 (14.8) | 4.2 (2.0–8.6) | 0.0772 | 21 (17.2) | 2.8 (1.6–5.1) | 0.0866 | 24 (19.7) | 2.5 (1.5–4.3) | 0.0943 |
| MSC+ ≥ MSC+ | 155 | 3.0 m | 36 (23.2) | 6.6 (3.4–12.6) | 40 (25.8) | 4.2 (2.5–7.1) | 44 (28.4) | 3.7 (2.3–5.8) | |||
| Total | 810 | 4.0 m | 75 | 91 | 106 |
LC, lung cancer; MSC, 24-microRNA signature classifier; RR, relative risk.
Lung cancer diagnosis within 1 year of last MSC test result.
Lung cancer diagnosis within 2 years of last MSC test result.
Discussion
The ability of molecular testing to refine lung cancer screening by identifying pre-symptomatic, early-stage tumors can only be determined in prospective studies that follow a cohort of high-risk individuals screened with both molecular testing and LDCT screening.
The results of the BioMILD prospective study showed a strong effect on lung cancer risk prediction of baseline LDCT alone, a multiplicative effect when combined with MSC, but also a significant effect of baseline MSC alone with a 2.02-fold higher lung cancer risk at four years.21 However, a single baseline MSC test alone seems not powerful enough to attract individuals as an alternative to LDCT screening. Moreover, in the present-day context, considering the advent of new ultra-low-dose LDCT scanners and AI programs, with an NPV greater than 98% there is little room for proposing a diagnostic blood test for lung cancer as a valid alternative to LDCT.26
The current screening guidelines recommend additional examinations for individuals with suspicious LDCT findings according to nodule volume size, but only 10% are diagnosed with cancer.27,28 In the BioMILD prospective study the MSC result was not used to determine the screening follow-up of CTind and CT+ participants. Nonetheless, the present findings indicate the potential of integrating the biomarker risk level to optimize the clinical management of suspicious LDCT findings.
Here we provide evidence that repeated MSC testing throughout the screening program in participants with suspicious LDCT findings remarkably increased lung cancer risk prediction. The cumulative hazard incidence of around 5% at seven years of follow-up in MSC− participants increased to more than 30% in MSC+ participants. Furthermore, the accuracy of MSC was independent of the most widely used lung cancer risk markers such as COPD or clinical prediction models (Brock and PLCOm2012) and, as required for a biomarker of early cancer diagnosis, it was also independent of tumor stage and resectability. These results, along with the observation that most of the lung cancer-specific mortality was observed in MSC+ individuals, highlight the need for greater clinical care in this group of patients.
Over the years we have gathered evidence that most of the 24 circulating miRNAs composing MSC were expressed and released by hematopoietic and stromal cells rather than tumor cells.15 The modulation of circulating miRNA levels, which define the positivity of the MSC test, is independent of the tumor mutational burden and reflects the shift to a protumorigenic and immunosuppressive phenotype of the lung microenvironment.14, 15, 16,29, 30, 31 These findings could explain why some participants had an MSC+ test result in the absence of a visible tumor and why individuals consistently testing MSC+ over time had a higher risk of developing lung cancer.
In the present study we observed that adenocarcinomas were less frequently MSC positive. This finding aligns with the TRACERx study results, where the absence of preoperative circulating tumor DNA detection identified biologically indolent lung adenocarcinomas with favorable clinical outcomes.32 Our results support the concept that small, asymptomatic adenocarcinomas identified in screening studies have a distinct natural history. These tumors are characterized by a lower mutational burden and potentially fewer neoantigens, likely resulting in a different interaction with the immune and stromal microenvironment.31,32
Others circulating biomarkers for screening and early lung cancer detection are currently under evaluation, although most are being proposed as a “pre-test” for minimizing the risks associated with CT screening. One of these is cell-free plasma DNA evaluation of fragments for early interception (DELFI) which was able to detect lung cancer with an overall ROC AUC of 0.90.33,34 A limitation of this test is the comparatively low sensitivity in stage I lung cancer (50% at 80% specificity) and the inclusion of individuals with symptoms suggestive of lung cancer, rather than a symptom-free screening cohort in the study population where DELFI was tested. Further development of a DELFI classifier is ongoing in screening populations of two registered clinical studies, NCT04825834 and NCT05306288.
A methylation-based multicancer early detection diagnostic test, Galleri (GRAIL), was validated in independent population cohorts.35,36 Overall sensitivity for lung cancer was low in detecting stage I disease, varying from 22% to 8.7%, although with a high specificity (>98%); sensitivity was higher in advanced stages. Given the low sensitivity, a negative test is insufficient to rule out further investigation. Large-scale efforts including prospective trials in the target-use population are underway.37
Recently, in large multicenter cohorts enrolled on the basis of the National Lung Screening Trial eligibility criteria (age 55–75 years; smoking history >30 pack-years), an 18-small-RNA (sRNA) feature consensus signature efficiently discriminate patients with non-lung cancer versus lung cancer nodules, even at a low stage.38 The sRNA analysis was successfully deployed with small dried blood spot collection, opening the possibility for the test to be conducted through home sampling.38 This study adds further evidence of the utility of circulating sRNAs as biomarkers for early detection and screening of lung cancer.39 This class of biomarkers seems indeed more effective in a screening context, since it is based on a host-response concept that integrates tumor- and host-derived information facilitating the detection of early signals.9
An important limiting factor to the implementation of LDCT screening is the large proportion in the eligible population of asymptomatic individuals who lack compliance or cannot undergo LDCT for health reasons.40 Although the availability of a blood-based test may also contribute to overcoming the well-known resistance of heavy smokers to LDCT screening, previous prospective studies with pre-test blood biomarkers have demonstrated the risk of false guarantees generated by a negative blood test with low sensitivity as well as a negative baseline LDCT.41,42 On the other hand, the ability to refine individual lung cancer risk determination through non-invasive validated blood tests could reduce the number of LDCT screening rounds, increase compliance and ultimately reduce costs and potential harm. Clinical studies are now underway to evaluate the complementarity of MSC with radiomics and immunologic markers to better define the timing of intervention.43,44 We envision a broader applicability of MSC for early lung cancer detection in high-risk individuals with incidental nodule findings at LDCT screening or unwilling to undergo upfront radiologic examination.
To our knowledge, BioMILD is the largest prospective lung cancer screening study of heavy smoker asymptomatic individuals that combined radiologic imaging and a blood biomarker with over eight years of follow-up. The low-cost, easy-to-implement blood test could be repeatedly performed during LDCT rounds to increase the accuracy of lung cancer risk definition and contribute to the better management of pulmonary nodules of uncertain malignancy. The baseline data showed that screening participants with a double-negative result could safely be allocated to a three-year LDCT repeat.21 In light of the present results, we posit that with an NPV of 98%, a CTind/MSC− test outcome would allow the screening interval to be extended from one to two years. Conversely, a CT+/MSC+ result might add urgency to the planning of further, closer investigations, including PET, which was successfully used in our previous screening trials,28 or ultimately prompt preventive neo-adjuvant or adjuvant therapy.10
Contributors
MBo, UP and GS designed and conceptualized the study, contributed to data collection, formal analysis, visualization, writing of the original draft, review and editing. FS contributed to formal analysis, visualization, writing of the original draft, review and editing. REL, MBa, PS, MS, AZ, LR and AM contributed to data collection and writing of the original draft. AC, CV and GC: contributed to formal analysis, writing of original draft, review and editing. All authors have read and approved the final version of the manuscript.
Data sharing statement
UP and GS have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All data used in the study are available upon request to the corresponding author.
Declaration of interests
MBo, UP and GS are co-inventors of three patent applications regarding the miRNA signature classifier. These patents were licensed to a private company, Gensignia Life Science, under the regulations of Fondazione IRCCS Istituto Nazionale dei Tumori of Milan. All other authors declare no competing interests.
Acknowledgements
The authors thank A. G. Russo of the Epidemiology Unit, Agency for Health Protection of Milan for data retrieval, Dr S. Sestini for patients' management, C. Banfi for project management, C. Jacomelli for data management, Dr M. Mensah and Dr. C. Borzi for biobanking and molecular analyses, Dr M. Ruggirello and R. Vigorito for radiomics analysis and the BioMILD staff: A. Calanca, C. Ninni and M. El Hayek. The BioMILD trial was supported by grants from the Italian Association for Cancer Research (AIRC 5xmille IG12162, IG18812 and IG23244), the Italian Ministry of Health (RF-2021-12373449 and Ricerca Corrente), the Horizon 2020 4-IN THE LUNG RUN program (n. 848294) the National Cancer Institute (EDRN UO1-CA166905), and Gensignia Life Science.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101070.
Appendix A. Supplementary data
References
- 1.National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 3.Pastorino U., Silva M., Sestini S., et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol. 2019;30:1162–1169. doi: 10.1093/annonc/mdz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 5.Fedewa S.A., Kazerooni E.A., Studts J.L., et al. State variation in low-dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst. 2021;113:1044–1052. doi: 10.1093/jnci/djaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammer M.M., Hunsaker A.R. Strategies for reducing false-positive screening results for intermediate-size nodules evaluated using lung-RADS: a secondary analysis of national lung screening trial data. Am J Roentgenol. 2022;219:397–406. doi: 10.2214/AJR.22.27595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van’t Westeinde S.C., Horeweg N., De Leyn P., et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J Cardiothorac Surg. 2012;42(3):420–429. doi: 10.1093/ejcts/ezs081. [DOI] [PubMed] [Google Scholar]
- 8.El Alam R., Byrne S.C., Hammer M.M. Rate of benign nodule resection in a lung cancer screening program. Clin Imaging. 2023;104 doi: 10.1016/j.clinimag.2023.109984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seijo L.M., Peled N., Ajona D., et al. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol. 2019;14 doi: 10.1016/j.jtho.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garassino M.C., Torri V. Neoadjuvant or perioperative approach in lung cancer. N Engl J Med. 2024;390:1816–1818. doi: 10.1056/NEJMe2403723. [DOI] [PubMed] [Google Scholar]
- 11.Ostrin E.J., Sidransky D., Spira A., Hanash S.M. Biomarkers for lung cancer screening and detection. Cancer Epidemiol Biomarkers Prev. 2020;29:2411–2415. doi: 10.1158/1055-9965.EPI-20-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grannis F.W. Limitations of molecular testing in combination with computerized tomographic for lung cancer screening. Nat Commun. 2022;13:1–2. doi: 10.1038/s41467-022-31419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borzi C., Calzolari L., Centonze G., Milione M., Sozzi G., Fortunato O. mir-660-p53-mir-486 network: a new key regulatory pathway in lung tumorigenesis. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andriani F., Majorini M.T., Mano M., et al. MiR-16 regulates the pro-tumorigenic potential of lung fibroblasts through the inhibition of HGF production in an FGFR-1- and MEK1-dependent manner. J Hematol Oncol. 2018;11 doi: 10.1186/S13045-018-0594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortunato O., Borzi C., Milione M., et al. Circulating mir-320a promotes immunosuppressive macrophages M2 phenotype associated with lung cancer risk. Int J Cancer. 2019;144:2746–2761. doi: 10.1002/ijc.31988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borzi C., Calzolari L., Ferretti A.M., et al. c-Myc shuttled by tumour-derived extracellular vesicles promotes lung bronchial cell proliferation through miR-19b and miR-92a. Cell Death Dis. 2019;10 doi: 10.1038/s41419-019-2003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeri M., Verri C., Conte D., et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108 doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sozzi G., Boeri M., Rossi M., et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol. 2014;32:768–773. doi: 10.1200/JCO.2013.50.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mensah M., Borzi C., Verri C., et al. MicroRNA based liquid biopsy: the experience of the plasma miRNA signature classifier (MSC) for lung cancer screening. J Vis Exp. 2017;2017 doi: 10.3791/56326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z., Wang Y., Wu W., Yang Y., Du L., Dong H. Cost-effectiveness of low-dose computed tomography with a plasma-based biomarker for lung cancer screening in China. JAMA Netw Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastorino U., Boeri M., Sestini S., et al. Baseline computed tomography screening and blood microRNA predict lung cancer risk and define adequate intervals in the BioMILD trial. Ann Oncol. 2022;33:395–405. doi: 10.1016/j.annonc.2022.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Gierada D.S., Rydzak C.E., Zei M., Rhea L. Improved interobserver agreement on lung-RADS classification of solid nodules using semiautomated CT volumetry. Radiology. 2020;297:675–684. doi: 10.1148/radiol.2020200302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeri M., Verri C., Conte D., et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108:3713–3718. doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWilliams A., Tammemagi M.C., Mayo J.R., et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tammemägi M.C., Katki H.A., Hocking W.G., et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vliegenthart R., Fouras A., Jacobs C., Papanikolaou N. Innovations in thoracic imaging: CT, radiomics, AI and x-ray velocimetry. Respirology. 2022;27:818–833. doi: 10.1111/resp.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krist A.H., Davidson K.W., Mangione C.M., et al. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325:962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino U., Bellomi M., Landoni C., et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003;362:593–597. doi: 10.1016/S0140-6736(03)14188-8. [DOI] [PubMed] [Google Scholar]
- 29.Borzi C., Ganzinelli M., Caiola E., et al. LKB1 down-modulation by miR-17 identifies patients with NSCLC having worse prognosis eligible for energy-stress-based treatments. J Thorac Oncol. 2021;16:1298–1311. doi: 10.1016/j.jtho.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Moro M., Fortunato O., Bertolini G., et al. MiR-486-5p targets CD133+ lung cancer stem cells through the p85/AKT pathway. Pharmaceuticals. 2022;15:297. doi: 10.3390/ph15030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verri C., Borzi C., Holscher T., et al. Mutational profile from targeted NGS predicts survival in LDCT screening–detected lung cancers. J Thorac Oncol. 2017;12:922–931. doi: 10.1016/j.jtho.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbosh C., Frankell A.M., Harrison T., et al. Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature. 2023;616:553–562. doi: 10.1038/s41586-023-05776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathios D., Johansen J.S., Cristiano S., et al. Detection and characterization of lung cancer using cell-free DNA fragmentomes. Nat Commun. 2021;12:1–14. doi: 10.1038/s41467-021-24994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal A.I.C., Mathios D., Jakubowski D., et al. Cell-free DNA fragmentomes in the diagnostic evaluation of patients with symptoms suggestive of lung cancer. Chest. 2023;164:1019–1027. doi: 10.1016/j.chest.2023.04.033. [DOI] [PubMed] [Google Scholar]
- 35.Klein E.A., Richards D., Cohn A., et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32:1167–1177. doi: 10.1016/j.annonc.2021.05.806. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson B.D., Oke J., Virdee P.S., et al. Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): a large-scale, observational cohort study. Lancet Oncol. 2023;24:733–743. doi: 10.1016/S1470-2045(23)00277-2. [DOI] [PubMed] [Google Scholar]
- 37.Schrag D., Beer T.M., McDonnell C.H., et al. Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. Lancet. 2023;402:1251–1260. doi: 10.1016/S0140-6736(23)01700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikosek T., Horos R., Trudzinski F., et al. Early detection of lung cancer using small RNAs. J Thorac Oncol. 2023;18(11):1504–1523. doi: 10.1016/J.JTHO.2023.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Sozzi G., Pastorino U. Small RNAs do it better. J Thorac Oncol. 2023;18:1428–1430. doi: 10.1016/j.jtho.2023.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Crosbie P.A.J., Gabe R., Simmonds I., et al. Participation in community-based lung cancer screening: the yorkshire lung screening trial. Eur Respir J. 2022;60 doi: 10.1183/13993003.00483-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan F.M., Mair F.S., Anderson W., et al. Earlier diagnosis of lung cancer in a randomised trial of an autoantibody blood test followed by imaging. Eur Respir J. 2020;57 doi: 10.1183/13993003.00670-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestri G.A., Goldman L., Tanner N.T., et al. Outcomes from more than 1 million people screened for lung cancer with low-dose CT imaging. Chest. 2023;164:241–251. doi: 10.1016/j.chest.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fortunato O., Huber V., Segale M., et al. Development of a molecular blood-based immune signature classifier as biomarker for risks assessment in lung cancer screening. Cancer Epidemiol Biomarkers Prev. 2022;31:2020–2029. doi: 10.1158/1055-9965.EPI-22-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rundo L., Ledda R.E., di Noia C., et al. A low-dose CT-based radiomic model to improve characterization and screening recall intervals of indeterminate prevalent pulmonary nodules. Diagnostics. 2021;11:1610. doi: 10.3390/diagnostics11091610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.