Highlights
-
•
DOACs are associated with a reduced risk of stroke or systemic embolism and major bleeding events compared to VKAs in AF patients with cancer.
-
•
DOACs are associated with lower rates of intracranial and gastrointestinal bleeding in cancer patients with AF.
-
•
Real-world evidence favors DOACs as a favorable treatment option for AF patients with cancer.
Keywords: Direct oral anticoagulants, Atrial fibrillation, Cancer, Outcomes, Meta
Abstract
Background
Several observational cohort studies have been conducted to investigate the effectiveness and safety of direct oral anticoagulants (DOACs) compared with vitamin K antagonists (VKAs) in patients who have both atrial fibrillation (AF) and cancer. Herein, we conducted a meta-analysis to present a comprehensive overview of the real-world evidence on DOACs in patients with AF and cancer.
Methods
A comprehensive search strategy was performed in PubMed and Embase until February 2024 for studies that enrolled AF patients with cancer who received DOACs or VKAs. The adjusted risk ratios (RRs) and 95% confidence intervals (CIs) of each outcome were extracted and pooled by a random-effects model.
Results
Seven observational cohort studies were eligible for data extraction. The random-effects model analysis indicated that compared with VKA use, the use of DOACs was significantly associated with reduced risks of stroke or systemic embolism (RR=0.79, 95 % CI 0.64–––0.97), major bleeding (RR=0.84, 95 % CI 0.71–––0.99), intracranial bleeding (RR=0.61, 95 % CI 0.54–––0.69), and gastrointestinal bleeding (RR=0.87, 95 % CI 0.80–––0.95) in AF patients with concurrent cancer.
Conclusions
Compared with VKAs, the use of DOACs was associated with decreased risks of thrombotic and bleeding events in AF patients with cancer. Data from real-world scenarios support the use of DOACs as a favorable treatment option for this specific patient population.
1. Introduction
AF is one of the leading causes of cardiovascular diseases and related morbidity [1]. AF patients are at a nearly fivefold higher risk of stroke-related death than non-AF patients [2]. Besides, cancer and cancer-related treatments are associated with an elevated risk of thrombotic and hemorrhagic complications. Several risk factors are associated with AF, such as aging, genetic susceptibility, smoking, alcohol, obesity or overweight, diabetes, and coronary artery disease[3], [4], [5]. Also, coronary artery disease has emerged as a relevant risk factor for cancers given that inflammation is the common theme[6]. Accordingly, inflammation is the common theme among AF, coronary artery disease, and cancer. Among patients with AF and cancer, the risk of stroke or cerebrovascular death is increased, emphasizing the importance of early anticoagulant therapy to reduce stroke risk and mortality.
Vitamin K antagonists (VKAs), such as warfarin, have historically been extensively used in clinical practice for anticoagulation in patients with AF. However, the use of warfarin is limited by the narrow therapeutic window. Due to the advantages of not requiring routine monitoring and being less affected by other medications, direct oral anticoagulants (DOACs) have been demonstrated as a favorable treatment option compared to warfarin [7]. DOACs are recommended as the first-line anticoagulant drug in non-valvular AF patients [8].
However, it is still essential to conduct further research to examine the effectiveness and safety of DOACs in patients with both AF and cancer currently. Previous meta-analyses have been conducted on this matter, but these analyses included both randomized controlled trials (RCTs) and observational cohort studies. The decision to include RCTs in these studies could potentially limit the representativeness of the population under investigation and make it difficult to generalize the results to real-world scenarios. To address these limitations, we conducted a meta-analysis that encompassed recently published observational cohort studies to assess the real-world evidence on DOACs versus VKAs in patients with AF and cancer.
2. Methods
We aimed to conduct this meta-analysis according to the guidance from the Cochrane Handbook for Systematic Reviews and presented the findings according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklists.
2.1. Inclusion and exclusion criteria
We included studies that met the following inclusion criteria: 1) study design: observational cohort studies reporting adjusted risk ratios (RRs) and 95 % confidence intervals (CIs); 2) population: patients with AF and cancer; 3) comparison: DOACs (dabigatran, rivaroxaban, apixaban, or edoxaban) versus VKAs; 4) outcomes: the effectiveness outcomes included stroke or systemic embolism (SSE), whereas the safety outcomes included major bleeding, intracranial bleeding, and gastrointestinal bleeding. RCTs and post hoc analyses of RCTs were not included. Certain publication types (e.g., reviews, preprint, case series, case reports, editorials) with no sufficient data for analysis were also excluded. We also excluded studies with no adjusted data.
2.2. Literature retrieval
Two authors (XY-L and RK-L) independently and systematically searched the two common databases (PubMed and Embase) until February 2024 for studies reporting the observational data of DOACs compared with VKAs in patients with AF and cancer. The following keywords were applied in the literature searches: (1) “atrial fibrillation”, (2) “cancer” OR “malignancy” OR “tumor”, (3) “non-vitamin K antagonist oral anticoagulants” OR “direct oral anticoagulants” OR “DOACs” OR “DOACs” OR “dabigatran” OR “rivaroxaban” OR “apixaban” OR “edoxaban”, and (4) “vitamin K antagonists” OR “warfarin” OR “coumadin” OR “acenocoumarol” OR “phenprocoumon”. Supplemental Table 1 shows the search strategies of this meta-analysis. In this study, we applied no linguistic restrictions in the literature searches.
2.3. Study selection and data extraction
Two authors (XY-L and RK-L) first screened the titles and abstracts of the searched records using the search strategies mentioned above, and then picked up the relevant studies. Second, the full texts of potential studies found in the first phase were screened to select the final studies of this meta-analysis. If facing disagreements, we could resolve these issues via consultation with the corresponding authors (WG-Z and DX-W). After that, the following characteristics were mainly collected: first author, publication year, data source, study period, study design, age and sex, type or dose of DOACs, VKA type, follow-up period, and outcome data.
2.4. Study quality assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the quality assessment of observational cohort studies. The NOS contained three domains with a total of 9 points, namely the selection of cohorts (4 points), the comparability of cohorts (2 points), and the assessment of the outcome (3 points). According to the previous publications[9], [10], a NOS of < 6 points was regarded as low quality in this meta-analysis.
2.5. Statistical analyses
All the statistical analyses of this study were performed using the Review Manager version 5.4 software (the Cochrane Collaboration 2014, Nordic Cochrane Centre Copenhagen, Denmark). The consistency among the included studies was examined using the Cochrane Q test and I2 statistic. We defined a P-value < 0.1 for the Q statistic, or I2 ≥ 50 % as substantial heterogeneity. In the pooled analysis, the adjusted RRs and 95 %CIs were converted to the natural logarithms (Ln[RR]) and standard errors, which were pooled by a random-effects model with an inverse variance method. In addition, we performed the subgroup analysis based on the DOAC type (dabigatran, rivaroxaban, and apixaban). Edoxaban was not included in the subgroup analysis due to the limited data. We assessed the publication bias for the reported effect estimates using the funnel plots.
3. Results
3.1. Study selection
As shown in Supplemental Figure 1, a total of 880 studies were identified in the electronic databases of PubMed and Embase. We first screened the titles and abstracts of these records and eliminated 852 studies that did not meet our inclusion criteria. Among the remaining 28 studies, we proceeded to review the full texts. By carefully comparing the content of each study to our predetermined criteria for inclusion and exclusion, we finally included 7 studies [11], [12], [13], [14], [15], [16], [17].
The baseline patient characteristics of these included studies are presented in Table 1. This table presents baseline data, such as demographics, DOAC types, and cancer types, allowing for a comprehensive comparison across the included studies. Furthermore, in order to assess the quality of the included studies, we used the NOS tool. All 7 observational cohort studies obtained scores of 6 points or higher, indicating their acceptable quality.
Table 1.
Baseline characteristics of the included studies of this meta-analysis.
| Studies | Study type | Data source | Study period | Sample size (N) | Age (years) | Female sex (%) | DOAC type and dose | VKA type | TTR for VKA users | Follow-up time (years) |
Cancer types | NOS tool (points) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ording-2021 | Observational cohort | Danish National Patient Registry |
NA | 1476 | 78 | 41.6 % | DA, API, RIV, EDO; standard dose DOAC (59 %) and reduced dose (41 %) | NA | NA | 1.0 | Gastrointestinal | 7 |
| Shah-2018 | Observational cohort | Market Scan databases,the United States | January 2010-December 2014 | 16,096 | 74 | 40 % | DA, API, RIV | Warfarin | NA | 1.0 | Breast (19.2 %), Gastrointestinal (12.7 %), Lung (12.3 %), Genitourinary (29.2 %), Gyneco-oncological (2.4 %), Hematological (9.8 %), Others (14.4 %) | 8 |
| Pardo Sanz-2019 | Observational cohort | AMBER-AF registry,Oncology and Cardiology Departments,Spain | NA | 637 | 75.4 | 100 % | NA | NA | NA | 2.8 | Breast | 7 |
| Atterman-2021 | Observational cohort | Swedish Patient register | January 2006 and December 2017 | 8228 | 75.1 | 36.5 % | NA | Warfarin | NA | 1.0 | Prostate(27.2 %),Gastrointestinal(19.1 %),Pancreatic(1.0 %),Lung(6.8 %),Breast(9.1 %),Gynecological(4.9 %),Urological(35.6 %),Intracranial(1.3 %),Hematological(10.7 %),Metastasized (9.2 %),Others(14.4 %) | 7 |
| Chan-2021 | Observational cohort | Taiwan (NHIRD and CGMH) | June 2012-December 2017 | 7955[NHIRD]; 2153[CGMH] | 77.0[NHIRD] | 41.9 %[NHIRD] | DA, API, RIV, EDO; 71 %, 91 %, 71 %, and 95 % users taking low-dose API (2.5 mgBID) , DA (110 mg BID), EDO (30 mg QD), and RIV (15/10 mg QD) |
Warfarin | 23.9 %[CGMH] | DOACs:1.45, Warfarin: 1.73 | NA | 8 |
| Deitelzweiget-2021 | Observational cohort | U.S. (Multi-center) | January 2013-September 2015 | 40,271 | NA | NA | DA, API, RIV; API, DA,and RIV users (73 %, 81 %, and 69 %) on standard dose |
Warfarin | NA | Warfarin: 0.63, API: 0.5, DA: 0.61, RIV: 0.59 | Breast (17 %), Genitourinary(14 %), lung (13 %), and Gastrointestinal (13 %) | 8 |
| Mehta-2022 | Observational cohort | SEER-Medicare | 2010–2016 | 7675 | 76.6 | 48.1 % | DA, API, RIV, EDO | Warfarin | 0.64 | Prostate (22.2 %), Breast (19.6 %), Lung (19.3 %), Colorectal (14.5 %), Others | 7 |
AF=atrial fibrillation; DOACs = direct oral anticoagulants; VKAs = vitamin K antagonists; TTR=time in therapeutic range; API=Apixaban; DA=Dabigatran; RIV=Rivaroxaban; EDO=edoxaban; NOS=Newcastle-Ottawa Scale; CGMH=Chang Gung Memorial Hospital; NHIRD=Taiwan National Health Insurance Research Database; SEER=Surveillance Epidemiology and End Results; NA=not available.
3.2. Effectiveness outcomes between DOACs and VKAs
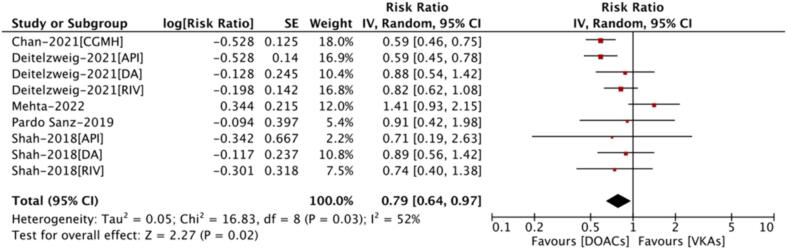
As shown in Fig. 1, a total of 5 included studies reported the effectiveness outcomes between DOACs and VKAs in patients with AF and cancer. Deitelzweig et al and Shah et al reported the data of apixaban, dabigatran, and rivaroxaban, respectively. Chan et al, Mehta et al, and Pardo San et al reported a mixed of DOACs compared with VKAs. Therefore, a total of 9 effect estimates were included in the pooled analysis. The random-effects model analysis indicated that compared with VKA use, the use of DOACs was significantly associated with a reduced risk of SSE (RR=0.79, 95 % CI 0.64–––0.97; I2 = 52 %) (Fig. 1).
Fig. 1.
Stroke or systemic embolism of DOACs versus VKAs in atrial fibrillation patients with cancer DOACs = direct oral anticoagulants; VKAs = vitamin K antagonists; RR=risk ratio; CI=confidence interval; API=Apixaban; DA=Dabigatran; RIV=Rivaroxaban; CGMH=Chang Gung Memorial Hospital.
3.3. Safety outcomes between DOACs and VKAs
A total of 6, 3, and 3 studies were included for major bleeding, intracranial bleeding, and gastrointestinal bleeding, respectively. In the pooled analysis, compared with VKA use, the use of DOACs was significantly associated with decreased risks of major bleeding (RR=0.84, 95 % CI 0.71–––0.99; I2 = 78 %), intracranial bleeding (RR=0.61, 95 % CI 0.54–––0.69; I2 = 0 %), and gastrointestinal bleeding (RR=0.87, 95 % CI 0.80–––0.95; I2 = 0 %) in patients with AF and cancer (Fig. 2).
Fig. 2.
Safety outcomes of DOACs versus VKAs in atrial fibrillation patients with cancer DOACs = direct oral anticoagulants; VKAs = vitamin K antagonists; RR=risk ratio; CI=confidence interval; API=Apixaban; DA=Dabigatran; RIV=Rivaroxaban; CGMH=Chang Gung Memorial Hospital; NHIRD=Taiwan National Health Insurance Research Database.
3.4. Subgroup analysis
We further performed the subgroup analysis based on the DOAC types. As results, dabigatran (RR=0.93, 95 % CI 0.68–––1.26; I2 = 0 %), rivaroxaban (RR=0.86, 95 % CI 0.68–––1.08; I2 = 0 %), or apixaban (RR=0.77, 95 % CI 0.45–––1.31; I2 = 54 %) and VKAs had a similar risk of SSE (Pinteraction = 0.82) (Fig. 3). For the safety outcomes (Fig. 4), dabigatran (RR=0.80, 95 % CI 0.68–––0.94; I2 = 1 %) and apixaban (RR=0.58, 95 % CI 0.50–––0.66; I2 = 0 %) compared with VKAs were associated with a lower risk of major bleeding. However, the use of rivaroxaban did not show a significant reduction in major bleeding risk compared to VKAs.
Fig. 3.
Subgroup analysis of effectiveness outcomes for different DOACs versus VKAs in atrial fibrillation patients with cancer DOACs = direct oral anticoagulants; VKAs = vitamin K antagonists; RR=risk ratio; CI=confidence interval.
Fig. 4.
Subgroup analysis of safety outcomes for different DOACs versus VKAs in atrial fibrillation patients with cancer DOACs = direct oral anticoagulants; VKAs = vitamin K antagonists; RR=risk ratio; CI=confidence interval; NHIRD=Taiwan National Health Insurance Research Database.
3.5. Publication bias
In order to evaluate the possible presence of publication bias, we conducted an assessment using funnel plots (Supplemental Figures 2 and 3). These funnel plots provide a visual representation of the distribution of effect sizes observed in different included studies.
4. Discussion
The findings from our meta-analysis suggested that, when compared with VKAs, the use of DOACs was associated with a lower risk of SEE in patients with both AF and cancer. Additionally, it was also found that DOACs were associated with a reduced risk of bleeding events compared to VKAs. Overall, the current real-world evidence supports the use of DOACs as a favorable treatment option for AF patients with concurrent cancer.
In AF patients, the presence of cancer was associated with increased risks of ischemic and bleeding events. Fauchier et al found that cancer can be a powerful predictor of mortality in AF patients[18]. AF is already a high-risk factor for thromboembolism due to the tendency to form atrial thrombi. When AF is combined with malignant diseases, the risk of thromboembolism becomes even higher [19]. Malignant diseases create a high hypercoagulable state in the body, meaning the blood is more prone to clotting. This, in combination with the already increased risk of clot formation due to AF, further enhances the likelihood of a thromboembolic event. Additionally, certain cancer treatments, particularly novel angiogenesis inhibitors, can exacerbate the risk of both thromboembolism and bleeding during anticoagulant therapy for AF.
The use of DOACs in AF patients with concomitant cancer is still not well established. Several studies have examined the effectiveness and safety of DOACs compared with VKAs in patients who have both AF and cancer [20], [21]. A prior meta-analysis conducted by Barbarawi et al, including 3 post hoc analyses of RCTs and 8 retrospective cohorts, found that DOACs reduced the incidence of SSE and major bleeding events compared with warfarin in patients with both AF and cancer [20]. In another meta-analysis including 3 post hoc analyses of RCTs and 5 retrospective cohort studies, there was no significant difference in SSE and major bleeding between the DOAC and VKA groups [21].
The stringent enrollment and exclusion criteria in RCT studies effectively reduce the impact of confounding factors on the estimation of treatment effectiveness and safety, enhancing the reliability of the findings in a restricted and well-defined population cohort [22]. However, due to the stringent criteria, the study population may not always fully represent the comprehensive characteristics of the target population. Particularly, when considering the population with concomitant cancer and AF, this issue becomes even more pronounced. Due to the variations in tumor types and stages among different patients, as well as the presence of multiple comorbidities and diverse medication regimens in this patient population, standardizing anticoagulation treatment interventions becomes challenging, resulting in a high degree of heterogeneity within cohorts. The strict inclusion criteria of RCTs often result in the enrollment of only a small proportion of patients, limiting the generalizability of the study population to real-world settings.
In observational cohort studies, researchers have the freedom to include a broader range of patients with varying characteristics and conditions, replicating the diversity seen in real-world clinical practice. Observational cohort studies can capture a more realistic representation of how medications work in different patient populations[23]. Considering the substantial heterogeneity in both the study population and anticoagulation strategies, observational cohort studies may provide a more realistic representation of drug safety and effectiveness in the real-world settings.
Although our meta-analysis intentionally excluded post hoc analyses of RCTs to focus on real-world observational data, it needs further discussion regarding the differences between findings from RCTs and observational studies. Our current study reported a reduced risk of gastrointestinal bleeding associated with DOACs compared to VKAs, which contrasts with the findings of post hoc analyses of RCTs. This discrepancy may be attributed to confounding factors inherent in observational studies, particularly confounding by indication and patient selection biases. In real-world settings, physicians may preferentially prescribe DOACs to patients perceived to have a lower risk of bleeding, thus leading to a more favorable safety profile for DOACs in these studies. These factors likely contributed to our findings of a lower risk of gastrointestinal bleeding with DOACs. Future research could conduct well-designed prospective studies with larger sample sizes to further clarify the effectiveness and safety of DOACs versus VKAs in patients with both AF and cancer.
4.1. Limitations
There were several limitations that should be considered in this meta-analysis. First, all the studies included in this analysis were observational cohort studies. While the studies have been evaluated using the NOS and have achieved scores of at least six points, there may still be the presence of selection bias and uncontrolled confounding factors due to the inherent limitations of this study design. Second, there was clinical heterogeneity among the different studies. This heterogeneity arises from variations in factors such as the type and dosage of DOACs, international normalized ratio levels during VKA use, the specific types of tumors, tumor staging, and the different treatments that patients received for their tumors. Third, it was important to consider the follow-up time of the included studies. In some cases, the follow-up period was relatively short, limiting the ability to comprehensively assess the long-term effects of anticoagulation. Lastly, due to the lack of consistent data on specific cancer types in the included studies, we were unable to perform a subgroup analysis stratified by cancer type. Future studies should address this limitation by exploring the differential effects of anticoagulants in various malignancies.
4.2. Future direction
The available research data comparing edoxaban with VKAs are limited, and we therefore did not perform the corresponding subgroup analysis. Therefore, the conclusions drawn regarding edoxaban's superiority should be interpreted with caution. Although we conducted the subgroup analysis based on the DOAC types regarding SSE and major bleeding events, due to the limited number of studies available for analysis, it was not feasible to conduct a subgroup analysis to examine the specific bleeding types associated with intracranial and gastrointestinal bleeding or other adverse outcomes. In addition, the number of included studies in each DOAC group was also limited. To further elucidate the results and develop more appropriate anticoagulation treatment strategies for AF patients with cancer, well-designed clinical studies with larger sample sizes are needed. Moreover, several factors such as tumor types, tumor staging, and different treatments for tumors should be considered in the further studies.
5. Conclusions
Compared with VKAs, the use of DOACs was associated with decreased risks of thrombotic and bleeding events in AF patients with cancer. Data from real-world scenarios support the use of DOACs as a favorable treatment option for this specific patient population.
Funding.
This work was supported by Guangdong Natural Science Foundation (2024A1515013289).
Authors' contributions: Wengen Zhu and Dexi Wu contributed to the study conception and design. Data search was performed by Xiuying Li and Runkai Li. Study selection and data extraction were performed by and Runkai Li. Data analyses and visualization were performed by Xiuying Li. The first draft of the manuscript was written by Xiuying Li and Runkai Li, and edited by Dexi Wu and Wengen Zhu.
CRediT authorship contribution statement
Xiuying Li: Writing – original draft. Runkai Li: Writing – original draft. Wengen Zhu: Writing – review & editing, Data curation, Conceptualization. Dexi Wu: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101512.
Contributor Information
Xiuying Li, Email: 418787219@qq.com.
Runkai Li, Email: Leerunkai2021@outlook.com.
Wengen Zhu, Email: zhuwg6@mail.sysu.edu.cn.
Dexi Wu, Email: wudexi@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tzeis S., et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2024;21(9):e31–e149. doi: 10.1016/j.hrthm.2024.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira C., et al. Atrial Fibrillation and Non-cardiovascular Diseases: A Systematic Review. Arq Bras Cardiol. 2015;105(5):519–526. doi: 10.5935/abc.20150142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung M.K., et al. Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement From the American Heart Association. Circulation. 2020;141(16):e750–e772. doi: 10.1161/CIR.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 4.Brandes A., et al. Risk Factor Management in Atrial Fibrillation. Arrhythm Electrophysiol Rev. 2018;7(2):118–127. doi: 10.15420/aer.2018.18.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekhael M., et al. The relationship between atrial fibrillation and coronary artery disease: Understanding common denominators. Trends Cardiovasc Med. 2024;34(2):91–98. doi: 10.1016/j.tcm.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Sun M., et al. Effect of inflammation on association between cancer and coronary artery disease. BMC Cardiovasc Disord. 2024;24(1):72. doi: 10.1186/s12872-023-03613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotalczyk A., et al. Stroke prevention strategies in high-risk patients with atrial fibrillation. Nat Rev Cardiol. 2021;18(4):276–290. doi: 10.1038/s41569-020-00459-3. [DOI] [PubMed] [Google Scholar]
- 8.Van Gelder IC et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024 Aug 30:ehae176. Epub ahead of print. [DOI] [PubMed]
- 9.Zhu W., et al. Comparative Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients. Stroke. 2021;52(4):1225–1233. doi: 10.1161/STROKEAHA.120.031007. [DOI] [PubMed] [Google Scholar]
- 10.Liu F., et al. Reappraisal of Non-vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. Frontiers in Cardiovascular Medicine. 2021;8 doi: 10.3389/fcvm.2021.757188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah S., et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018;2(3):200–209. doi: 10.1182/bloodadvances.2017010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardo Sanz A., et al. Current status of anticoagulation in patients with breast cancer and atrial fibrillation. Breast. 2019;46:163–169. doi: 10.1016/j.breast.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Ording A.G., et al. Bleeding complications in patients with gastrointestinal cancer and atrial fibrillation treated with oral anticoagulants. Cancer Med. 2021;10(13):4405–4414. doi: 10.1002/cam4.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta H.B., et al. Comparative Effectiveness and Safety of Direct Oral Anticoagulants Versus Warfarin Among Adults With Cancer and Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2022;15(12):e008951. doi: 10.1161/CIRCOUTCOMES.122.008951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deitelzweig S., et al. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients With Active Cancer. JACC CardioOncol. 2021;3(3):411–424. doi: 10.1016/j.jaccao.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan Y.H., et al. Clinical Outcomes in Atrial Fibrillation Patients With a History of Cancer Treated With Non-Vitamin K Antagonist Oral Anticoagulants: A Nationwide Cohort Study. Stroke. 2021;52(10):3132–3141. doi: 10.1161/STROKEAHA.120.033470. [DOI] [PubMed] [Google Scholar]
- 17.Atterman A., et al. Atrial Fibrillation, Oral Anticoagulants, and Concomitant Active Cancer: Benefits and Risks. TH Open. 2021;5(2):e176–e182. doi: 10.1055/s-0041-1728670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fauchier L., et al. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart. 2015;2(1):e000290. doi: 10.1136/openhrt-2015-000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ording A.G., et al. Oral anti-coagulant treatment patterns in atrial fibrillation patients diagnosed with cancer: A Danish nationwide cohort study. Br J Haematol. 2022;197(2):223–231. doi: 10.1111/bjh.18060. [DOI] [PubMed] [Google Scholar]
- 20.Barbarawi M., et al. Efficacy and Safety of the Non-Vitamin K Antagonist Oral Anticoagulant Among Patients With Nonvalvular Atrial Fibrillation and Cancer: A Systematic Review and Network Meta-analysis. Curr Probl Cardiol. 2022;47(11) doi: 10.1016/j.cpcardiol.2022.101346. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., et al. Safety and efficacy of new oral anticoagulants compared to those of warfarin in AF patients with cancer: a meta-analysis of randomized clinical trials and observational studies. Eur J Clin Pharmacol. 2021;77(6):849–857. doi: 10.1007/s00228-021-03132-x. [DOI] [PubMed] [Google Scholar]
- 22.Zabor E.C., Kaizer A.M., Hobbs B.P. Randomized Controlled Trials. Chest. 2020;158(1s):S79–S87. doi: 10.1016/j.chest.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Kattan M.W. Cohort Studies: Design, Analysis, and Reporting. Chest. 2020;158(1s):S72–S78. doi: 10.1016/j.chest.2020.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.