Abstract
Through their hemagglutinin-neuraminidase glycoprotein, parainfluenza viruses bind to sialic acid-containing glycoconjugates to initiate infection. Although the virus-receptor interaction is a key factor of infection, the exact nature of the receptors that human parainfluenza viruses recognize has not been determined. We evaluated the abilities of human parainfluenza virus types 1 (hPIV-1) and 3 (hPIV-3) to bind to different types of gangliosides. Both hPIV-1 and hPIV-3 preferentially bound to neolacto-series gangliosides containing a terminal N-acetylneuraminic acid (NeuAc) linked to N-acetyllactosamine (Galβ1-4GlcNAc) by the α2-3 linkage (NeuAcα2-3Galβ1-4GlcNAc). Unlike hPIV-1, hPIV-3 bound to gangliosides with a terminal NeuAc linked to Galβ1-4GlcNAc through an α2-6 linkage (NeuAcα2-6Galβ1-4GlcNAc) or to gangliosides with a different sialic acid, N-glycolylneuraminic acid (NeuGc), linked to Galβ1-4GlcNAc (NeuGcα2-3Galβ1-4GlcNAc). These results indicate that the molecular species of glycoconjugate that hPIV-1 recognizes are more limited than those recognized by hPIV-3. Further analysis using purified gangliosides revealed that the oligosaccharide core structure is also an important element for binding. Gangliosides that contain branched N-acetyllactosaminoglycans in their core structure showed higher avidity than those without them. Agglutination of human, cow, and guinea pig erythrocytes but not equine erythrocytes by hPIV-1 and hPIV-3 correlated well with the presence or the absence of sialic acid-linked branched N-acetyllactosaminoglycans on the cell surface. Finally, NeuAcα2-3I, which bound to both viruses, inhibited virus infection of Lewis lung carcinoma-monkey kidney cells in a dose-dependent manner. We conclude that hPIV-1 and hPIV-3 preferentially recognize oligosaccharides containing branched N-acetyllactosaminoglycans with terminal NeuAcα2-3Gal as receptors and that hPIV-3 also recognizes NeuAcα2-6Gal- or NeuGcα2-3Gal-containing receptors. These findings provide important information that can be used to develop inhibitors that prevent human parainfluenza virus infection.
Human parainfluenza viruses are important respiratory tract pathogens. Human parainfluenza virus type 1 (hPIV-1) causes most cases of laryngotracheobronchitis (croup) in children, and human parainfluenza virus type 3 (hPIV-3) is second only to respiratory syncytial virus as a cause of pneumonia and bronchiolitis in infants younger than 6 months old (3, 27). These viruses, which belong to the genus Respirovirus and the family Paramyxoviridae, have two spike glycoproteins, the hemagglutinin-neuraminidase (HN) glycoprotein and the fusion (F) glycoprotein, embedded in the envelope. Parainfluenza virus infection is initiated by the attachment of the HN glycoprotein to sialic acid-containing receptors of target cells (23, 32, 44). It is thought that both sialoglycoproteins (33, 48) and gangliosides (15, 20–23, 39, 45) can act as viral receptors.
The binding specificity of influenza viruses for sialic acid-containing receptors has been well characterized. Influenza A viruses isolated from various animal species recognize different terminal sialic acid sequences (4). Avian influenza A viruses bind to N-acetylneuraminic acid (NeuAc) linked to galactose (Gal) by an α2-3 linkage (NeuAcα2-3Gal) but not by an α2-6 linkage. In contrast, human influenza A viruses display the opposite receptor-binding specificity: they prefer NeuAcα2-6Gal- and not NeuAcα2-3Gal-containing receptors (25). These receptor specificities have been suggested to be one of the factors associated with viral host range and tissue tropism (29).
Among the respiroviruses, only Sendai virus (SV) (murine parainfluenza virus type 1) has been characterized in detail for its receptor determinants in several model systems. SV binds to both ganglio-series (Galβ1-3GalNAc containing) and neolacto-series (Galβ1-4GlcNAc containing) gangliosides with terminal NeuAcα2-3Gal as isoreceptors (15, 20–22, 39, 45). Although the deduced amino acid sequences of the HNs of hPIV-1 and hPIV-3 are similar to that of the HN of SV (e.g., 72 and 62% identical with hPIV-1 and hPIV-3 HNs, respectively) (10, 26), little is known about the receptor specificities of these human parainfluenza viruses. In this study, we evaluated the abilities of hPIV-1 and hPIV-3 to bind to different types of gangliosides. We found that the receptor specificity of respiroviruses varies among subtypes and that the core structure of the sugar chain constitutes an important part of the receptor recognized by hPIV-1 and hPIV-3.
MATERIALS AND METHODS
Viruses and cells.
We obtained hPIV-1 strain C35 (ATCC VR-94) and hPIV-3 strain C243 (ATCC VR-93) from the American Type Culture Collection (Manassas, Va.). The hPIV-1 clinical isolates Cl-5, Cl-11, and Cl-14 were kindly provided by Kelly Henrickson (Medical College of Wisconsin, Milwaukee). Cl-5, Cl-11, and Cl-14 were isolated in 1973, 1979, and 1983, respectively, from infected children (13). These isolates had been passaged three to five times in Lewis lung carcinoma-monkey kidney (LLC-MK2) cells in serum-free HB101 medium with 5 μg of acetylated trypsin/ml before we received them.
Confluent monolayers of LLC-MK2 cells were infected with hPIV-1 strain C35 or clinical isolates (approximately 10 PFU per cell) in serum-free Eagle minimal essential medium containing acetylated trypsin (1 μg/ml). Three days after infection, virions in the culture medium were collected. The same culture conditions were used to grow and harvest hPIV-3; however, acetylated trypsin was not used. SV (Enders strain) was grown in 11-day-old embryonated chicken eggs. Each virus was purified by sedimentation through 30 to 50% sucrose gradients (11, 30).
Gangliosides.
Total gangliosides of bovine brain, human placenta, and human meconium were prepared by the methods of Ledeen et al. (19) and Taki et al. (41, 42). GM1a, GD1a, and GQ1b were isolated from bovine brain (14, 39). GM3, IV3NeuAcαnLc4Cer (NeuAcα2-3 lactoneotetraosylceramide [NeuAcα2-3PG]), VI3NeuAcαnLc6Cer (NeuAcα2-3 blood group i-type ganglioside NeuAcα2-3lactoneohexaosylceramide [NeuAcα2-3i]), and VIII3NeuAcα, VI3NeuAcα-IV6kladoLc8Cer (NeuAcα2-3 blood group I-type ganglioside [NeuAcα2-3I]) were isolated from human placenta (41). IV6NeuAcαnLc4Cer (NeuAcα2-6lactoneotetraosylceramide ceramide [NeuAcα2-6PG]) and IV6NeuAcα-IV6kladoLc8Cer (NeuAcα2-6 blood group I-type ganglioside [NeuAcα2-6I]) were isolated from human meconium (42). VIII3Galα, VI3NeuGcα-kladoLc8Cer (NeuGcα2-3 blood group I-type ganglioside [NeuGcα2-3I]; NeuGc is N-glycolylneuraminic acid) was isolated from bovine erythrocytes (47). GD1b and GT1b were purchased from Sigma (St. Louis, Mo.).
Preparation of lipid-free BSA.
Glycosphingolipids contained in bovine serum albumin (BSA) were eliminated by chloroform-methanol extraction. Briefly, BSA (5 g; Nacalai Tesque, Kyoto, Japan) was suspended in 100 ml of chloroform-methanol (1:1, vol/vol) at 4°C for 2 h and then filtered through a Buchner funnel. After the BSA had been washed three times with 100 ml of this solvent, the BSA was dried under vacuum (0.1 mm Hg) at room temperature for 3 h and dissolved in 100 ml of distilled water containing 0.005% (wt/vol) MEGA-10 (Dojindo Laboratories, Mashiki, Japan) to mask the lipid-binding sites. The solution was incubated at 4°C for 12 h, dialyzed against distilled water at 4°C for 3 days, and lyophilized.
Antibodies.
Anti-SV HN monoclonal antibodies (MAbs) (S2, S16, and M20), anti-hPIV-1 HN MAbs (P37, P43, and P44), and antinucleoprotein (anti-NP) MAb (P2E) were prepared as described previously (10, 30, 43). Anti-hPIV-3 HN MAb (240/12D) was purchased from Chemicon International (Temecula, Calif.). Antiserum to human blood group I-type ganglioside (human anti-I serum) was obtained from the Central Blood Center, Japanese Red Cross Society.
Blood.
Equine blood was purchased from Cosmo Bio Co., Ltd. (Tokyo, Japan). Guinea pig blood was obtained from the Animal Center, University of Shizuoka. Bovine blood was collected at the Shizuoka Municipal Meat Works. Human blood was collected from a healthy adult.
TLC.
Total gangliosides (5 nmol of each sialic acid) and individual types of gangliosides (1 nmol) were subjected to thin-layer chromatography (TLC) on silica gel plastic plates (Polygram Sil G; Nagel, Düren, Germany) by using a solvent system of either chloroform–methanol–0.2% aqueous calcium chloride (65:35:8) (solvent system 1) or chloroform–methanol–0.2% aqueous calcium chloride (5:4:1) (solvent system 2). The chromatograms were sprayed with a resorcinol-hydrochloric acid reagent for detection of the gangliosides (40).
Virus overlay assay.
Gangliosides were subjected to chromatography as described above. The virus overlay and the immunochemical detection of the viruses on the plates were performed by using a modification of a method described previously (36, 37). Briefly, the chromatograms were blocked with phosphate-buffered saline (PBS) containing 1% egg albumin (crystallized; Taiyo Kagaku Company, Yokkaichi, Japan) and 1% polyvinylpyrrolidone (blocking solution 2) at 4°C for 16 h. The plates were washed three times with PBS and incubated on ice for 3 h with purified virus (20 μg/ml) resuspended in blocking solution 2. The virus suspension was removed by suction, and each plate was washed five times with ice-cold PBS to remove unbound virus. Anti-SV HN MAb, anti-hPIV-1 HN MAb, or anti-hPIV-3 HN MAb diluted 1:1,000 in blocking solution 2 was added to individual plates. After the plates were incubated on ice for 2 h, each MAb solution was removed by suction. The plates were washed five times with ice-cold PBS and incubated on ice for 2 h with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antiserum diluted 1:2,000 in blocking solution 2. The plates were again washed five times with ice-cold PBS, and the viruses bound to the plates were revealed by incubation with an immunostaining reagent containing N,N-diethylphenylenediamine monohydrochloride and 4-chloro-1-naphthol (5).
Solid-phase binding assay.
Each type of ganglioside was dissolved in an ethanol solution containing 200 pmol of l-α-dipalmitoylphosphatidylcholine (Sigma); each ganglioside solution (250 to 1,000 pmol/50 μl) was then serially diluted twofold with the ethanol solution. Fifty microliters of each ganglioside dilution was added to wells of microtiter plates (F96 Polysorp; Nalge Nunc International, Rochester, N.Y.), and ethanol was evaporated at room temperature for 3 h. The remaining binding site on the wells was blocked with 100 μl of PBS containing 0.1% lipid-free BSA (blocking solution 1) at 4°C for 24 h. After the plates were washed five times with ice-cold PBS, 50 μl of each virus suspension (20 μg/ml) in blocking solution 1 was added to the wells and incubated on ice for 3 h. As a control, several wells were incubated without viruses. Unbound viruses were removed by washing with ice-cold PBS. Anti-SV HN MAbs, anti-hPIV-1 HN MAbs, or anti-hPIV-3 HN MAb (50 μl) diluted 1:1,000 with blocking solution 1 was added to the wells. After a 2-h incubation on ice, the plates were washed five times with ice-cold PBS and again incubated on ice for 2 h with 50 μl of horseradish peroxidase-conjugated goat anti-mouse IgG antiserum (Bio-Rad, Hercules, Calif.) diluted 1:2,000 with blocking solution 1. The amount of bound virions was determined by measuring the absorbance at 490 nm with O-phenylenediamine as a substrate (35).
Hemagglutination tests.
Each virus (50 μl, 1 μg of viral protein) was diluted serially with 50 μl of PBS on a microtiter plate. Fifty microliters of a 0.5% (vol/vol) erythrocyte suspension was added to each well. The hemagglutination titer was defined as the maximum dilution of virus that caused hemagglutination after 2 h. The plates were kept on ice during the assays.
Preparation of sialidase-treated erythrocytes.
Erythrocytes from different species were prepared as a 1% (vol/vol) suspension (2 ml) in PBS. Arthrobacter ureafaciens sialidase (10 mU/ml; Nacalai Tesque) was added to the erythrocyte suspension and incubated for 1 h at 37°C. The sialidase-treated erythrocytes were washed three times with PBS.
Fluorescence-activated cell sorting (FACS) analysis of oligosaccharides on the surface of erythrocytes.
Native and sialidase-treated erythrocytes (1% suspension in PBS) were fixed with 1% glutaraldehyde (in PBS) at room temperature for 15 min, washed three times with PBS, and suspended in 1 ml of PBS. Human anti-I serum and biotin-labeled Ricinus communis agglutinin (Seikagaku Corporation, Tokyo, Japan) diluted 1:50 with 100 μl of PBS were added to each suspension of fixed erythrocytes (100 μl). As a negative control, fixed erythrocytes were incubated without human anti-I serum and R. communis agglutinin. After incubation at room temperature for 30 min, the erythrocytes were washed three more times with PBS and again incubated at room temperature for 30 min with 100 μl of a 1:20 dilution (in PBS) of a fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragment of rabbit anti-human IgM for flow cytometry (Dako Japan Co., Ltd., Kyoto, Japan) or a 1:100 dilution (in PBS) of FITC-conjugated streptavidin (Dako Japan Co.). After the cells were washed three more times, their fluorescence intensity was analyzed with an EPICS XL SYSTEM II (Beckman Coulter, Inc., Fullerton, Calif.).
Neutralization of human respirovirus infection by gangliosides.
Various amounts of gangliosides (100 to 20,000 pmol) were evaporated under a stream of nitrogen and dissolved in 100 μl of PBS containing 0.001% lipid-free BSA. The solutions of gangliosides were incubated on ice for 1 h with 100 μl of culture medium containing each respirovirus (200 to 300 infectious units/100 μl). Confluent monolayers of LLC-MK2 cells (1.9 cm2) in 24-well plates (Corning Costar Corporation, Cambridge, Mass.) were inoculated with 200 μl of each mixture of viruses and gangliosides at room temperature. After 1 h, the inoculum was removed from each plate, and the monolayers were washed three times with PBS and incubated for 2 days at 34°C in 1 ml of Eagle minimal essential medium containing 5% fetal bovine serum. The monolayers in each well were washed three times with PBS, fixed with 1 ml of methanol at room temperature for 5 min, and washed three more times with PBS. Anti-SV HN MAbs, anti-hPIV-1 HN MAbs, or anti-hPIV-3 HN and anti-NP MAb diluted 1:500 with 200 μl of PBS containing 0.5% BSA and 0.05% Tween 20 (blocking solution 3) was added to wells. After incubation at room temperature for 30 min, each MAb solution was removed by suction. The wells were washed three times with PBS and incubated at room temperature for 30 min with horseradish peroxidase-conjugated goat anti-mouse IgG antiserum diluted 1:500 with blocking solution 3. After the plates were washed three times with PBS, the viral antigen-positive cells in each well were detected by incubation with 0.5 ml of 3,3′-diaminobenzidine tetrahydrochloride reagent (DAB tablets; Sigma). The wells were washed three times with deionized water. Mock-infected LLC-MK2 cells were fixed and stained as negative controls. Infectious units were defined as the mean of three counts of cells stained brown within an area of 3.8 mm2. For counting purposes, the cells were magnified 200 times with an inverted microscope (ECLIPSE TE300; Nikon Inc., New York, N.Y.).
RESULTS
hPIV-1 and hPIV-3 bind to neolacto-series gangliosides.
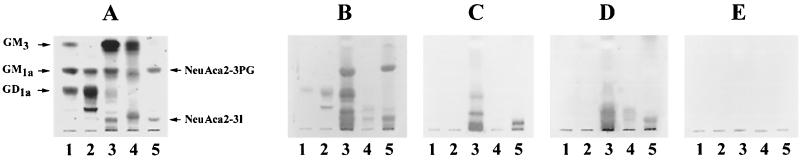
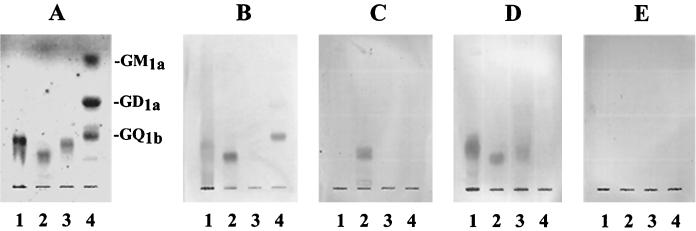
The sialic acid-containing glycoconjugate is the component of the cellular receptors that participates in respirovirus infection (23). SV was reported to bind to both ganglio-series and neolacto-series gangliosides containing terminal NeuAcα2-3Gal. In contrast, the receptor specificity of the ubiquitous human respiroviruses that cause upper- and lower-respiratory-tract illnesses has not been determined. We first determined whether human respiroviruses recognize receptors different from those bound by SV. Ganglioside mixtures isolated from bovine brain, human placenta, and human meconium were subjected to chromatography, and reactivity with SV, hPIV-1, and hPIV-3 was detected by immunostaining with specific anti-HN antibodies (Fig. 1). SV strongly bound to purified NeuAcα2-3PG and NeuAcα2-3I (Fig. 1B, lane 5) containing terminal NeuAcα2-3Gal, as previously reported. SV also bound strongly to the human placenta ganglioside mixture (Fig. 1B, lane 3), which also contained the neolacto-series ganglioside with terminal NeuAcα2-3Gal. Smaller amounts of SV bound to the bovine brain ganglioside mixture, which contained the ganglio-series gangliosides GD1a, GT1b, and GQ1b (Fig. 1B, lane 2), which have been shown to be isoreceptors for SV (20, 22). Additionally, SV weakly bound the slower-migrating gangliosides from the human meconium (Fig. 1B, lane 4). This ganglioside mixture contained the neolacto-series gangliosides with the terminal sialic acid linked to Gal by an α2-6 linkage (NeuAcα2-6Gal). In contrast to SV, which reacted with various types of gangliosides, the human respiroviruses hPIV-1 and hPIV-3 preferentially bound to purified NeuAcα2-3I (Fig. 1C and D, lanes 5) and the slower-migrating gangliosides from the human placenta (Fig. 1C and D, lanes 3). The slower-migrating gangliosides from the human meconium were recognized by hPIV-3 but not by hPIV-1 (Fig. 1C and D, lanes 4). No other gangliosides tested were bound by hPIV-1 and hPIV-3. These results suggest that hPIV-1 and hPIV-3 recognize only limited types of neolacto-series gangliosides as receptors, whereas SV can bind to various types of neolacto- and ganglio-series gangliosides.
FIG. 1.
Binding of respiroviruses to mixtures of gangliosides in virus overlay assays. Total gangliosides (each 5 nmol as sialic acid) and specific kinds of gangliosides (1 nmol) were spotted on silica gel plastic plates and subjected to chromatography with solvent system 1. (A) Gangliosides were detected with resorcinol-hydrochloric acid reagent. (B to D) Ganglioside binding by SV (B), hPIV-1 (C), and hPIV-3 (D) was detected by using virus overlay assays and anti-HN MAbs specific for each virus. (E) A chromatogram was incubated without virus and later incubated with a mixture of anti-HN MAbs. Lanes 1, GM3, GM1a, and GD1a; lanes 2, total gangliosides from bovine brain; lanes 3, total gangliosides from human placenta; lanes 4, total gangliosides from human meconium; lanes 5, NeuAcα2–3PG and NeuAcα2–3I.
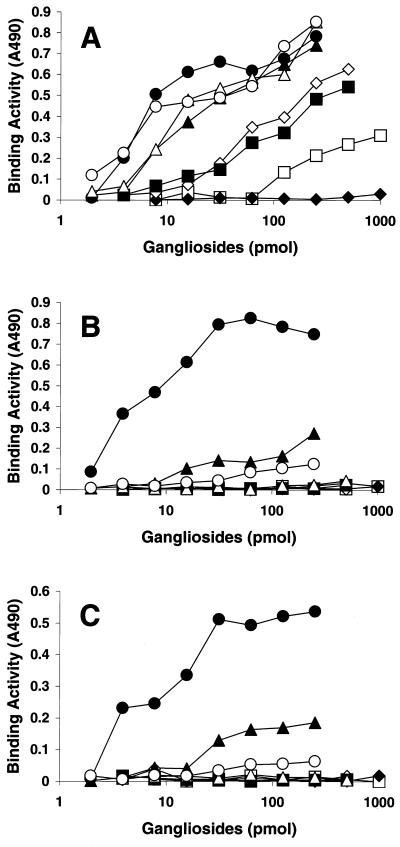
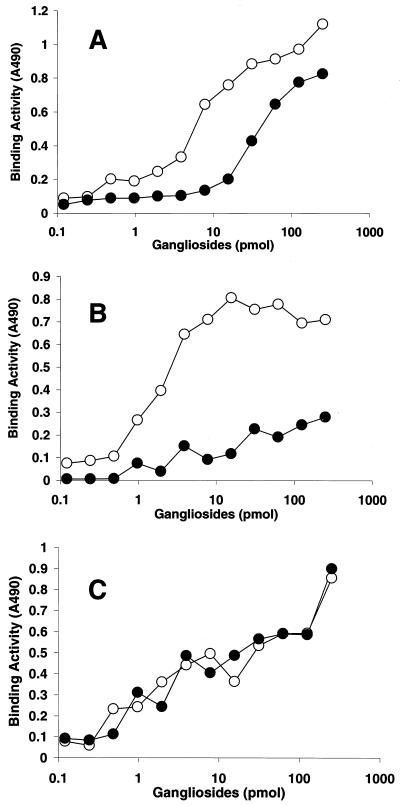
The binding properties of SV, hPIV-1, and hPIV-3 were further evaluated by use of a solid-phase binding assay with microtiter plates coated with various purified gangliosides. SV strongly bound not only to GQ1b, which is a high-affinity receptor for SV (15, 20, 22), but also to the neolacto-series gangliosides containing NeuAcα2-3Gal (NeuAcα2-3PG, NeuAcα2-3i, and NeuAcα2-3I). Moderate quantities of SV bound to GT1b and GD1a. SV also bound to GM3 bearing a short sugar chain with terminal NeuAcα2-3Gal, but the binding was weak. Neither GM1a nor GD1b, each of which lacked terminal NeuAcα2-3Gal, was bound by SV (Fig. 2A). The two human viruses preferentially bound to NeuAcα2-3I and did not bind to any of the ganglio-series gangliosides tested (GQ1b, GT1b, GD1a, or GM3). In addition, these viruses bound to NeuAcα2-3i and NeuAcα2-3PG containing terminal NeuAcα2-3Gal; however, these binding reactions were weaker than those with NeuAcα2-3I (Fig. 2B and C).
FIG. 2.
Binding of respiroviruses to gangliosides in solid-phase binding assays. The binding activities of SV (A), hPIV-1 (B), and hPIV-3 (C) were calculated as the mean values of triplicate measurements of the absorbance at 490 nm (A490) after the subtraction of background values. Symbols: ●, NeuAcα2–3I; ○, NeuAcα2–3PG; ♦, GM1a and GD1b; ⋄, GD1a; ▴, NeuAcα2–3i; ▵, GQ1b; ▪, GT1b; □, GM3.
hPIV-1 and hPIV-3 recognize branched N-acetyllactosaminoglycans (blood group I-type antigens) with a terminal sialic acid.
The results of the solid-phase binding assays suggested that the structure of the oligosaccharide core is also recognized by human respiroviruses; hPIV-1 and hPIV-3 strongly bound to NeuAcα2-3I, but their binding to NeuAcα2-3i or NeuAcα2-3PG was remarkably weak (Fig. 2B and C). The chemical structures of the gangliosides used in this study and their reactivities with the viruses are summarized in Table 1. NeuAcα2-3I contains branched N-acetyllactosaminoglycans (blood group I-type antigens) in its core structure, whereas NeuAcα2-3i and NeuAcα2-3PG do not. Therefore, we next determined the core structure of gangliosides recognized by these viruses. We used erythrocytes from different animal species whose oligosaccharide compositions of glycoproteins and glycolipids vary (16). The results of the hemagglutination of various erythrocytes by SV, hPIV-1, or hPIV-3 are shown in Table 2. SV agglutinated erythrocytes from all species tested (humans, cows, guinea pigs, and horses). In contrast, hPIV-1 and hPIV-3 did not agglutinate equine erythrocytes, which are rich in sialic acid linked to Gal through an α2-3 linkage (16). Therefore, it was suggested that equine erythrocytes do not contain the oligosaccharide core structure that hPIV-1 and hPIV-3 recognize.
TABLE 1.
Ganglioside-binding specificities of SV, hPIV-1, and hPIV-3
| Ganglioside | Structurea | Binding activity withb:
|
||
|---|---|---|---|---|
| SV | hPIV-1 | hPIV-3 | ||
| GM3(NeuAc) | NeuAcα2-3Galβ1-4Glcβ1-1′Cer | + | − | − |
| Ganglio series | ||||
| GM1a | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1′Cer | − | − | − |
| 3 | ||||
| | | ||||
| 2αNeuAc | ||||
| GD1a | NeuAcα2-3Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1′Cer | ++ | − | − |
| 3 | ||||
| | | ||||
| 2αNeuAc | ||||
| GD1b | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1′Cer | − | − | − |
| 3 | ||||
| | | ||||
| 2αNeuAcα2-8NeuAc | ||||
| GT1b | NeuAcα2-3Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1′Cer | ++ | − | − |
| 3 | ||||
| | | ||||
| 2αNeuAcα2-8NeuAc | ||||
| GQ1b | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1-1′Cer | +++ | − | − |
| 33 | ||||
| || | ||||
| NeuAcα2-8NeuAcα2 2αNeuAcα2-8NeuAc | ||||
| Neolacto series | ||||
| NeuAcα2-3PG | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer | +++ | ++ | ++ |
| NeuAcα2-6PG | NeuAcα2-6Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer | ++ | − | ++ |
| NeuAcα2-3i | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer | +++ | ++ | ++ |
| NeuAcα2-3I | NeuAcα2-3Galβ1-4GlcNAcβ1-6 | |||
| Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer | +++ | +++ | +++ | |
| NeuAcα2-3Galβ1-4GlcNAcβ1-3 | ||||
| NeuAcα2-61 | Galβ1-4GlcNAcβ1-6 | |||
| Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer | ++ | − | +++ | |
| NeuAcα2-6Galβ1-4GlcNAcβ1-3 | ||||
| NeuGcα2-3I | Galβ1-3Galβ1-4GlcNAcβ1-6 | |||
| Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer | ++ | ++ | +++ | |
| NeuGcα2-3Galβ1-4GlcNAcβ1-3 | ||||
Sialic acids in the ganglioside structures are shown in bold.
The binding activities of SV, hPIV-1, and hPIV-3 for each ganglioside were estimated as the concentration at which 50% virus binding occurred in a solid-phase binding assay. +++, between 1 and 10 pmol; ++, between 10 and 100 pmol; +, between 100 and 1,000 pmol; −, more than 1,000 pmol.
TABLE 2.
Hemagglutination of erythrocytes from different species by SV and human parainfluenza virusesa
| Virus | Strain | Hemagglutination titer for erythrocytes from:
|
|||
|---|---|---|---|---|---|
| Guinea pig | Human | Cow | Horse | ||
| hPIV-1 | C35 | 512 | 128 | 128 | <2 |
| hPIV-3 | C243 | 512 | 128 | 128 | <2 |
| SV | Enders | 512 | 512 | 512 | 256 |
Each virus was diluted serially with PBS in a microtiter plate, and then a 0.5% (vol/vol) suspension of erythrocytes was added to each well as described in Materials and Methods. The hemagglutination titer was defined as the maximum dilution of virus that caused hemagglutination.
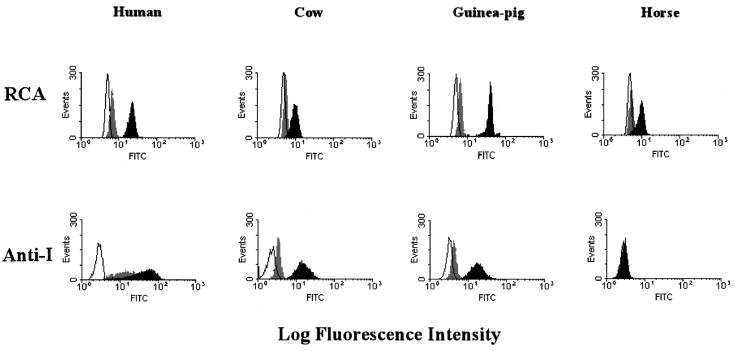
To characterize the oligosaccharide core of various erythrocytes, we determined reactivity with human anti-I serum and biotin-labeled R. communis agglutinin by FACS analysis. The human anti-I serum recognizes branched N-acetyllactosaminoglycans (blood group I-type antigens), and the R. communis agglutinin specifically binds to Galβ1-4GlcNAc- or Galβ1-3GalNAc-containing oligosaccharides (2). FACS analysis of native and sialidase-treated erythrocytes indicated that equine erythrocytes contained ubiquitous sialyl-glycans with Galβ1-4GlcNAc or Galβ1-3GalNAc chains but practically no blood group I-type antigens (Fig. 3). Erythrocytes of humans, cows, and guinea pigs contained blood group I-type antigens with sialic acids. These results agree with the findings of solid-phase binding assays showing that branched N-acetyllactosaminoglycans constitute an important part of the receptors recognized by hPIV-1 and hPIV-3.
FIG. 3.
Comparison of blood group I antigen on the surface of native and sialidase-treated animal erythrocytes by FACS analysis. Human anti-I serum was used for the detection of blood group I antigen. Native and sialidase-treated erythrocytes from humans, cows, guinea pigs, and horses were fixed and incubated with human anti-I serum. As a control, biotin-labeled R. communis agglutinin (RCA) was used for the detection of ubiquitous glycans on the erythrocytes. As a negative control, fixed erythrocytes were incubated without anti-I serum and R. communis agglutinin. The erythrocytes were washed with PBS and incubated with the FITC-conjugated F(ab′)2 fragment of rabbit anti-human IgM antibody or FITC-conjugated streptavidin. The fluorescence intensities of the cells were analyzed. The white, gray, and solid portions of each histogram indicate results obtained with negative control cells, intact cells, and sialidase-treated cells, respectively.
hPIV-1 and hPIV-3 differ in their specificities for molecular species of terminal sialic acid and its linkage to Gal.
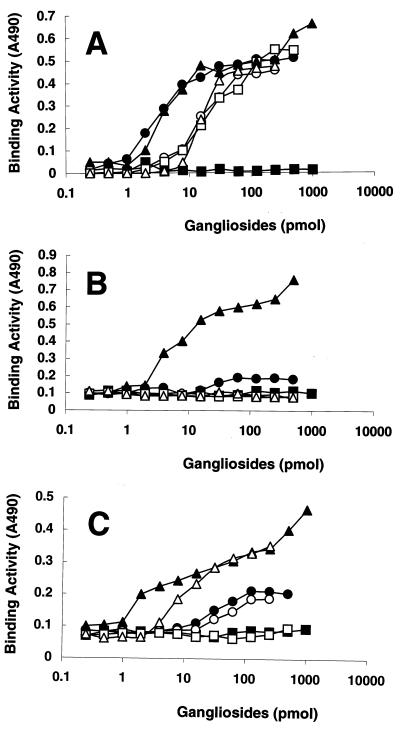
Influenza A viruses bind to sialic acid-containing oligosaccharides with specificities that vary according to the host species of origin (4). Human viruses preferentially bind to α2-6-linked NeuAc, but avian and equine viruses prefer the α2-3 linkage. To determine whether parainfluenza viruses show any preference for the terminal sialic acid sequence (i.e., the molecular species of sialic acid and its linkage to Gal), we evaluated the binding of SV, hPIV-1, and hPIV-3 to various purified gangliosides by using a solid-phase binding assay. The NeuAcα2-6I and NeuAcα2-6PG gangliosides containing α2-6-linked NeuAc were bound by SV; however, the amount of SV bound to those gangliosides was smaller than that bound to α2-3-linked NeuAcα2-3I and NeuAcα2-3PG (Fig. 4A). Although hPIV-3 strongly bound to NeuAcα2-6I (Fig. 4C), hPIV-1 did not bind to NeuAcα2-6I or NeuAcα2-6PG (Fig. 4B). This finding was surprising because most human isolates of influenza A virus preferentially bind to α2-6-linked but not α2-3-linked sialic acid. However, the preferential binding of hPIV-1 to α2-3-linked sialic acid and the preferential binding of hPIV-3 to α2-3-linked sialic acid and to α2-6-linked sialic acid correlate well with their preference for a neuraminidase substrate (1).
FIG. 4.
Binding of respiroviruses to neolacto-series gangliosides containing different terminal sialyl linkages in solid-phase binding assays. The binding activities of SV (A), hPIV-1 (B), and hPIV-3 (C) were calculated as described in the legend to Fig. 2. GM1a and GD1a were used as controls. Symbols: ●, NeuAcα2–3PG; ○, NeuAcα2–6PG; ▴, NeuAcα2–3I; ▵, NeuAcα2–6I; ▪, GM1a; □, GD1a.
We next evaluated the abilities of hPIV-1 and hPIV-3 to bind to gangliosides containing different molecular species of sialic acid (NeuAc and NeuGc) in a TLC virus overlay assay (Fig. 5). The assay showed that hPIV-3 bound not only to NeuAcα2-3I but also to NeuGcα2-3I (Fig. 5D). Unlike hPIV-3, hPIV-1 was unable to bind to NeuGcα2-3I (Fig. 5C). SV bound to both NeuAcα2-3I and NeuGcα2-3I (Fig. 5B, lanes 1 and 2). We also used the solid-phase binding assay to further evaluate the abilities of the viruses to bind to NeuAcα2-3I and NeuGcα2-3I (Fig. 6). SV bound to NeuGcα2-3I; however, its binding to NeuGcα2-3I was weaker than its binding to NeuAcα2-3I (Fig. 6A). In this assay, hPIV-1 bound weakly to NeuGcα2-3I (Fig. 6B), although no binding activity was detected in the virus overlay assay (Fig. 5C); this difference was probably due to the different sensitivities of the assays. The binding activities between hPIV-3 and NeuGcα2-3I and between hPIV-3 and NeuAcα2-3I were nearly identical (Fig. 6C).
FIG. 5.
Binding of respiroviruses to blood group I-type gangliosides containing different terminal molecular species of sialic acid in virus overlay assays. Specific types of gangliosides (1 nmol) were spotted on silica gel plastic plates and subjected to chromatography with solvent system 2. (A) Gangliosides were detected with resorcinol-hydrochloric acid reagent. (B to D) Virus overlay assays with SV (B), hPIV-1 (C), and hPIV-3 (D) were done as described in the legend to Fig. 1 and in Materials and Methods. (E) A chromatogram that was incubated without virus and with a mixture of anti-HN MAbs served as a negative control. Lanes 1, NeuGcα2–3I; lanes 2, NeuAcα2–3I; lanes 3, NeuAcα2–6I; lanes 4, GM1a, GD1a, and GQ1b.
FIG. 6.
Binding of respiroviruses to serial dilutions of blood group I-type gangliosides containing different terminal molecular species of sialic acid in solid-phase binding assays. The binding activities of SV (A), hPIV-1 (B), and hPIV-3 (C) were calculated as described in the legend to Fig. 2. Symbols: ○, NeuAcα2–3I; ●, NeuGcα2–3I.
Binding specificities of hPIV-1 clinical isolates.
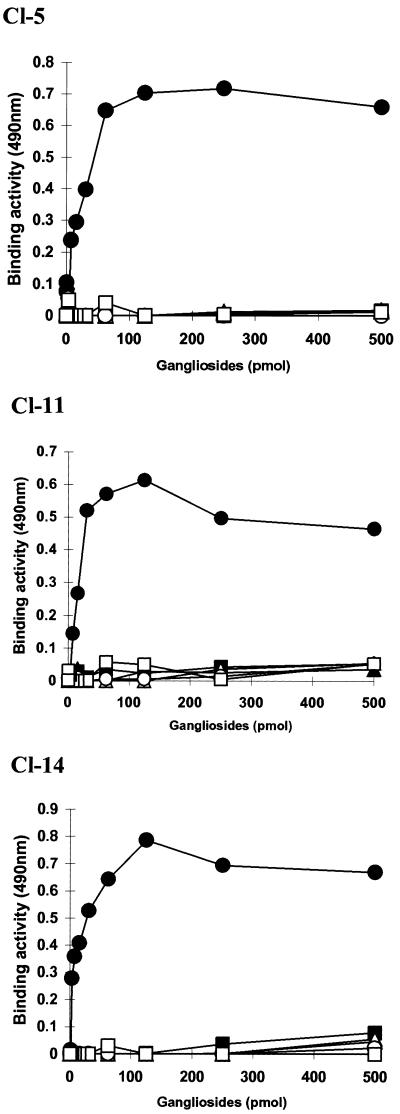
Our finding that hPIV-1 preferentially binds to NeuAcα2-3I but not to NeuAcα2-6I was unexpected because human influenza A viruses preferentially bind to NeuAcα2-6Gal- but not NeuAcα2-3Gal-containing receptors. Therefore, to investigate whether preferential binding to NeuAcα2-3I is the general character of hPIV-1, we obtained hPIV-1 strains isolated from infected patients during different years and determined their binding specificities by using the solid-phase binding assay. The hPIV-1 clinical isolates Cl-5, Cl-11, and Cl-14 were isolated in 1973, 1979, and 1983, respectively. All of the isolates showed the same binding specificities as strain C35: these isolates bound to NeuAcα2-3I but not to other gangliosides containing a NeuAcα2-6Gal linkage (Fig. 7). These results indicate that hPIV-1 preferentially recognizes N-acetyllactosaminoglycans with a terminal NeuAcα2-3Gal linkage. These results also suggest that binding to NeuAcα2-6Gal-containing receptors is not required for infection and maintenance in a human population.
FIG. 7.
Binding of hPIV-1 clinical isolates to gangliosides in solid-phase binding assays. The binding activities of hPIV-1 clinical isolates Cl-5, Cl-11, and Cl-14 were calculated as described in the legend to Fig. 2. Symbols: ●, NeuAcα2–3I; ○, NeuAcα2–6I; ▴, NeuAcα2–6SPG; ▵, GD1a; ▪, GM1a; □, GM3.
NeuAcα2-3 blood group I-type ganglioside (NeuAcα2-3I) inhibits human respirovirus infection.
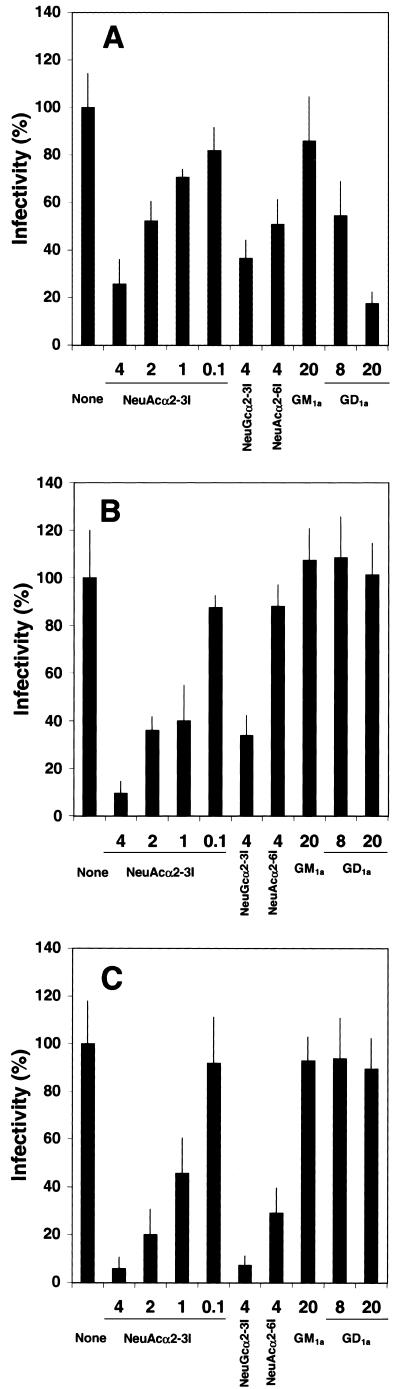
To test the ability of the gangliosides to inhibit viral infection, SV, hPIV-1, and hPIV-3 were preincubated with different types of gangliosides before their adsorption to LLC-MK2 cells. The number of infected cells was scored as a percentage of virus-infected cells that were not pretreated with gangliosides (Fig. 8). Within a range of 0 to 40 μM, NeuAcα2-3I, which showed the strongest binding to respiroviruses, inhibited infection by each virus in a dose-dependent manner. In contrast, GD1a, which bound to SV but not to hPIV-1 or hPIV-3, inhibited only SV infection. NeuAcα2-6I and NeuGcα2-3I inhibited hPIV-3 infection; however, NeuAcα2-6I did not prevent hPIV-1 infection. GM1a, which did not bind to any of these viruses, did not inhibit any infection. These results agree with the above findings for binding specificities as determined by solid-phase binding assays.
FIG. 8.
Ganglioside-mediated inhibition of respirovirus infection of LLC-MK2 cells. The percentage of infectivity is the ratio of the total number of cells infected with viruses (SV [A], hPIV-1 [B], or hPIV-3 [C]) that were pretreated with various gangliosides (y axis; in nanomoles) to the number of cells infected with viruses that were not pretreated with gangliosides. The values are the means and standard deviations for three measurements.
DISCUSSION
Although the deduced amino acid sequences of the HN genes of hPIV-1 and hPIV-3 are similar to that of SV (10, 26), the receptor specificities of hPIV-1 and hPIV-3 do not appear to be identical to those of SV. By using a TLC virus overlay assay and a solid-phase binding assay, we confirmed that SV recognizes neolacto-series gangliosides and ganglio-series gangliosides, both of which contain terminal NeuAcα2-3Gal (15, 20–22, 39, 45). In contrast to SV, hPIV-1 and hPIV-3 do not bind to ganglio-series gangliosides, a finding suggesting that hPIV-1 and hPIV-3 recognize the oligosaccharide core and the terminal sialic acid.
Neolacto-series gangliosides containing blood group I-type gangliosides have been isolated from membranes of human and bovine erythrocytes (7, 17, 28, 46) but not from membranes of horse erythrocytes (12, 50). Neither hPIV-1 nor hPIV-3 hemagglutinated horse erythrocytes (Table 2). Our FACS analysis using sugar sequence-specific antiserum and a biotin-labeled lectin indicated that horse erythrocytes have Galβ1-4GlcNAc- or Galβ1-3GalNAc-containing oligosaccharides to which sialic acid is linked but that the cells have practically no blood group I antigen. Human, bovine, and guinea pig erythrocytes that were hemagglutinated by hPIV-1 and hPIV-3 contained blood group I antigen with sialic acid. These findings show that blood group I antigen with sialic acid on the surface of erythrocytes plays an important role in hemagglutination by hPIV-1 and hPIV-3.
Recently, a cDNA encoding a novel β1,6-N-acetylglucosaminyltransferase that forms I branches was isolated. Northern blot analysis detected transcripts of the enzyme predominantly in human adult tissues where mucin is produced, i.e., the colon, small intestine, trachea, and stomach (51). These findings support our hypothesis that oligosaccharides containing blood group I antigen with terminal NeuAcα2-3Gal may be a significant factor in human parainfluenza virus infection.
The inhibitory effects of gangliosides against parainfluenza virus infection correlated well with the binding specificities of these viruses (Fig. 8). NeuAcα2-3I, which showed the strongest binding to both hPIV-1 and hPIV-3, efficiently inhibited infection by these viruses. These results suggest that gangliosides such as NeuAcα2-3I could be potential inhibitors of type 1 and 3 parainfluenza viruses. How do gangliosides inhibit parainfluenza virus infection? A current model of parainfluenza virus infection shows that HN plays an important role in the process of membrane fusion induced by F protein (18). The first step of virus infection is the binding of HN to its sialic acid-containing receptor. Upon binding its ligand, HN is proposed to undergo a conformational change that, in turn, triggers a conformational change in F protein to release the hydrophobic fusion peptide (18). In fact, recent structural studies of HN revealed that its conformational change is induced when it binds to sialic acid (6). Binding to free gangliosides will therefore induce conformational changes in HN and F protein before the viruses reach target cells, thus reducing the infectivity of the viruses.
Earlier studies showed that SV had a high affinity for sialylglycoprotein (GP-2) isolated from bovine erythrocyte membranes in hemagglutination inhibition assays and in model systems of virus adsorption to sialidase-treated chicken erythrocytes coated with GP-2. The affinity of GP-2 for SV was 2,500 times higher than that of bovine fetuin containing a terminal NeuAcα2-3Galβ1-4GlcNAc sequence on N-linked oligosaccharides (33, 38). GP-2 was found to be an exceptionally rich source of branched sialosyloligosaccharides of N-acetyllactosamine (blood group I-type antigen) on O-glycosidic linkages (8). Because hPIV-1 and hPIV-3 preferentially bind to blood group I-type gangliosides containing lactosamine-repeating units, these viruses may use not only neolacto-series gangliosides but also sialylglycoproteins, such as GP-2, as host cell receptor determinants.
Extensive studies of influenza virus have shown that receptor specificity correlates with the host species of virus origin. Most human influenza A and B viruses preferentially recognize oligosaccharides containing terminal NeuAcα2-6Gal as the receptor determinant, whereas avian and equine influenza A viruses preferentially recognize an α2-3 linkage (NeuAcα2-3Gal) (4, 9, 25, 31, 34, 49). The correlation of receptor specificity with the species of origin suggested receptor-based selective pressure in humans. Influenza virus introduced from an avian species acquired the ability to recognize NeuAcα2-6Gal during circulation among a human population (25). It might be preferable for human influenza virus to bind to NeuAcα2-6Gal-containing sialyloligosaccharides for efficient growth and transmission. A few amino acid changes on the receptor-binding site of the hemagglutinin molecule caused a change in receptor specificity (4). However, human influenza A/HK/156/97 (H5N1), which was isolated from a child in Hong Kong, bound to sialic acid α2-3Gal-containing receptors but not to sialic acid α2-6Gal-containing receptors; H5 viruses from chicken and wild aquatic birds have shown similar receptor specificities (24). That report demonstrated that binding to sialic acid α2-6Gal-containing receptors is not required for initial infection of the human trachea. Similarly, all hPIV-1 clinical isolates characterized in this study preferentially bound to NeuAcα2-3Gal- but not NeuAcα2-6Gal-containing sialyloligosaccharides. This result indicates that binding to a NeuAcα2-6Gal-containing receptor is not required for infection and transmission among humans. However, the fact that hPIV-1 causes only mild infection that is limited to the upper respiratory tract may be explained by the lack of binding to NeuAcα2-6Gal-containing receptors. Further characterization of receptor distribution in the human respiratory tract may reveal the role of receptor specificity in these virus infections. Also, findings that indicate that the HN sequences of viruses in patients are identical to those of viruses cultured in LLC-MK2 cells will be required before a definitive conclusion about the receptor specificity of the viruses can be drawn.
ACKNOWLEDGMENTS
This work was supported by grants AI-38956 and AI-11949 from the National Institute of Allergy and Infectious Diseases, by Cancer Center CORE grant CA-21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
We thank M. Matrosovich for support of this work and helpful discussions.
REFERENCES
- 1.Ah-Tye C, Schwartz S, Huberman K, Carlin E, Moscona A. Virus-receptor interactions of human parainfluenza viruses type 1, 2 and 3. Microb Pathog. 1999;27:329–336. doi: 10.1006/mpat.1999.0313. [DOI] [PubMed] [Google Scholar]
- 2.Baenziger J U, Fiete D. Structure determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979;254:9795–9799. [PubMed] [Google Scholar]
- 3.Collins P L, Chanock R M, McIntosh K. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1205–1241. [Google Scholar]
- 4.Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 5.Conyers S M, Kidwell D A. Chromogenic substrates for horseradish peroxidase. Anal Biochem. 1991;192:207–211. doi: 10.1016/0003-2697(91)90208-b. [DOI] [PubMed] [Google Scholar]
- 6.Crennell S, Takimoto T, Portner A, Taylor G. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat Struct Biol. 2000;7:1068–1074. doi: 10.1038/81002. [DOI] [PubMed] [Google Scholar]
- 7.Feizi T, Childs R A, Hakomori S I, Powell M E. Blood-group-Ii-active gangliosides of human erythrocyte membranes. Biochem J. 1978;173:245–254. doi: 10.1042/bj1730245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feizi T, Gooi H C, Loomes L M, Suzuki Y, Suzuki T, Matsumoto M. Cryptic I antigen activity and Mycoplasma pneumoniae-receptor activity associated with sialoglycoprotein GP-2 of bovine erythrocyte membranes. Biosci Rep. 1984;4:743–749. doi: 10.1007/BF01128815. [DOI] [PubMed] [Google Scholar]
- 9.Gambaryan A S, Tuzikov A B, Piskarev V E, Yamnikova S S, Lvov D K, Robertson J S, Bovin N V, Matrosovich M N. Specification of receptor-binding phenotypes of influenza virus isolates from different hosts using synthetic sialylglycopolymers: non-egg-adapted human H1 and H3 influenza A and influenza B viruses share a common high binding affinity for 6′-sialyl(N-acetyllactosamine) Virology. 1997;232:345–350. doi: 10.1006/viro.1997.8572. [DOI] [PubMed] [Google Scholar]
- 10.Gorman W L, Gill D S, Scroggs R A, Portner A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and Sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology. 1990;175:211–221. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- 11.Gorman W L, Pridgen C, Portner A. Glycosylation of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 1 affects its functional but not its antigenic properties. Virology. 1991;183:83–90. doi: 10.1016/0042-6822(91)90120-z. [DOI] [PubMed] [Google Scholar]
- 12.Hamanaka S, Handa S, Inoue J, Hasegawa A, Yamakawa T. Further studies on gangliosides of erythrocytes from horses and cattle. J Biochem (Tokyo) 1980;87:639–643. [PubMed] [Google Scholar]
- 13.Henrickson K J, Savatski L L. Genetic variation and evolution of human parainfluenza virus type 1 hemagglutinin neuraminidase: analysis of 12 clinical isolates. J Infect Dis. 1992;166:995–1005. doi: 10.1093/infdis/166.5.995. [DOI] [PubMed] [Google Scholar]
- 14.Hirabayashi Y, Nakao T, Matsumoto M, Obata K, Ando S. Improved method for large-scale purification of brain gangliosides by Q-sepharose column chromatography. Immunochemical detection of C-series polysialogangliosides in adult bovine brains. J Chromatogr. 1988;445:377–384. doi: 10.1016/s0021-9673(01)84550-7. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren J, Svennerholm L, Elwing H, Fredman P, Strannegard O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc Natl Acad Sci USA. 1980;77:1947–1950. doi: 10.1073/pnas.77.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- 17.Kundu S K, Samuelsson B E, Pascher I, Marcus D. New gangliosides from human erythrocytes. J Biol Chem. 1983;258:13857–13866. [PubMed] [Google Scholar]
- 18.Lamb R A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 19.Ledeen R W, Yu R K, Eng L F. Gangliosides of human myelin: sialosylgalactosylceramide (G7) as a major component. J Neurochem. 1973;21:829–839. doi: 10.1111/j.1471-4159.1973.tb07527.x. [DOI] [PubMed] [Google Scholar]
- 20.Markwell M A, Moss J, Hom B E, Fishman P H, Svennerholm L. Expression of gangliosides as receptors at the cell surface controls infection of NCTC 2071 cells by Sendai virus. Virology. 1986;155:356–364. doi: 10.1016/0042-6822(86)90199-6. [DOI] [PubMed] [Google Scholar]
- 21.Markwell M A, Paulson J C. Sendai virus utilizes specific sialyloligosaccharides as host cell receptor determinants. Proc Natl Acad Sci USA. 1980;77:5693–5697. doi: 10.1073/pnas.77.10.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markwell M A, Svennerholm L, Paulson J C. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci USA. 1981;78:5406–5410. doi: 10.1073/pnas.78.9.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markwell M A K. New frontiers opened by the exploration of host cell receptors. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Press; 1991. pp. 407–425. [Google Scholar]
- 24.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matrosovich M N, Gambaryan A S, Teneberg S, Piskarev V E, Yamnikova S S, Lvov D K, Robertson J S, Karlsson K A. Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology. 1997;233:224–234. doi: 10.1006/viro.1997.8580. [DOI] [PubMed] [Google Scholar]
- 26.Morrison T, Portner A. Structure, function and processing of the glycoproteins of Paramyxoviridae. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Press; 1991. pp. 347–382. [Google Scholar]
- 27.Murphy B R. Parainfluenza viruses. In: Gorbach S L, Bartlett J G, Blacklow N R, editors. Infectious diseases. Philadelphia, Pa: The W. B. Saunders Company; 1998. pp. 2125–2131. [Google Scholar]
- 28.Niemann H, Watanabe K, Hakomori S. Blood group i and I activities of “lacto-N-norhexaosylceramide” and its analogues: the structural requirements for i-specificities. Biochem Biophys Res Commun. 1978;81:1286–1293. doi: 10.1016/0006-291x(78)91275-5. [DOI] [PubMed] [Google Scholar]
- 29.Paulson J C. Interactions of animal viruses with cell surface receptors. In: Conn M, editor. The receptors. Vol. 2. Orlando, Fla: Academic Press, Inc.; 1985. pp. 131–219. [Google Scholar]
- 30.Portner A, Scroggs R A, Metzger D W. Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology. 1987;158:61–68. doi: 10.1016/0042-6822(87)90238-8. [DOI] [PubMed] [Google Scholar]
- 31.Rogers G N, Paulson J C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 32.Scheid A, Caliguiri L A, Compans R W, Choppin P W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972;50:640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Harada M, Suzuki Y, Matsumoto M. Incorporation of sialoglycoprotein containing lacto-series oligosaccharides into chicken asialoerythrocyte membranes and restoration of receptor activity toward hemagglutinating virus of Japan (Sendai virus) J Biochem (Tokyo) 1984;95:1193–1200. doi: 10.1093/oxfordjournals.jbchem.a134709. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Horiike G, Yamazaki Y, Kawabe K, Masuda H, Miyamoto D, Matsuda M, Nishimura S I, Yamagata T, Ito T, Kida H, Kawaoka Y, Suzuki Y. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett. 1997;404:192–196. doi: 10.1016/s0014-5793(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Jyono M, Tsukimoto M, Hamaoka A, Suzuki Y. Labeling of influenza virus with alkylamine-modified horseradish peroxidase. Anal Biochem. 1995;228:42–47. doi: 10.1006/abio.1995.1312. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Sometani A, Yamazaki Y, Horiike G, Mizutani Y, Masuda H, Yamada M, Tahara H, Xu G, Miyamoto D, Oku N, Okada S, Kiso M, Hasegawa A, Ito T, Kawaoka Y, Suzuki Y. Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. Biochem J. 1996;318:389–393. doi: 10.1042/bj3180389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki Y, Nakao T, Ito T, Watanabe N, Toda Y, Xu G, Suzuki T, Kobayashi T, Kimura Y, Yamada A, Sugawara K, Nishimura H, Kitame F, Nakamura K, Deya E, Kiso M, Hasegawa A. Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains. Virology. 1992;189:121–131. doi: 10.1016/0042-6822(92)90687-k. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki Y, Suzuki T, Matsumoto M. Isolation and characterization of receptor sialoglycoprotein for hemagglutinating virus of Japan (Sendai virus) from bovine erythrocyte membrane. J Biochem (Tokyo) 1983;93:1621–1633. doi: 10.1093/oxfordjournals.jbchem.a134301. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y, Suzuki T, Matsunaga M, Matsumoto M. Gangliosides as paramyxovirus receptor. Structural requirement of sialo-oligosaccharides in receptors for hemagglutinating virus of Japan (Sendai virus) and Newcastle disease virus. J Biochem (Tokyo) 1985;97:1189–1199. doi: 10.1093/oxfordjournals.jbchem.a135164. [DOI] [PubMed] [Google Scholar]
- 40.Svennerholm L. Estimation of sialic acids. II. Colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- 41.Taki T, Matsuo K, Yamamoto K, Matsubara T, Hayashi A, Abe T, Matsumoto M. Human placenta gangliosides. Lipids. 1988;23:192–198. doi: 10.1007/BF02535457. [DOI] [PubMed] [Google Scholar]
- 42.Taki T, Rokukawa C, Kasama T, Kon K, Ando S, Abe T, Handa S. Human meconium gangliosides. Characterization of a novel I-type ganglioside with the NeuAc alpha 2–6Gal structure. J Biol Chem. 1992;267:11811–11817. [PubMed] [Google Scholar]
- 43.Takimoto T, Bousse T, Coronel E C, Scroggs R A, Portner A. Cytoplasmic domain of Sendai virus HN protein contains a specific sequence required for its incorporation into virions. J Virol. 1998;72:9747–9754. doi: 10.1128/jvi.72.12.9747-9754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tozawa H, Watanabe M, Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973;55:242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- 45.Umeda M, Nojima S, Inoue K. Activity of human erythrocyte gangliosides as a receptor to HVJ. Virology. 1984;133:172–182. doi: 10.1016/0042-6822(84)90436-7. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe K, Hakomori S. Gangliosides of human erythrocytes. A novel ganglioside with a unique N-acetylneuraminosyl-(2 leads to 3)-N-acetylgalactosamine structure. Biochemistry. 1979;18:5502–5504. doi: 10.1021/bi00591a037. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe K, Hakomori S I, Childs R A, Feizi T. Characterization of a blood group I-active ganglioside. Structural requirements for I and i specificities. J Biol Chem. 1979;254:3221–3228. [PubMed] [Google Scholar]
- 48.Wybenga L E, Epand R F, Nir S, Chu J W, Sharom F J, Flanagan T D, Epand R M. Glycophorin as a receptor for Sendai virus. Biochemistry. 1996;35:9513–9518. doi: 10.1021/bi9606152. [DOI] [PubMed] [Google Scholar]
- 49.Xu G, Suzuki T, Hanagata G, Deya E, Kiso M, Hasegawa A, Suzuki Y. Drift of the sialyl-linkage specific recognition of the sialidase of influenza B virus isolates. J Biochem (Tokyo) 1993;113:304–307. doi: 10.1093/oxfordjournals.jbchem.a124043. [DOI] [PubMed] [Google Scholar]
- 50.Yachida Y, Tsuchihashi K, Gasa S. Characterization of novel mono-O-acetylated GM3s containing 9-O-acetyl sialic acid and 6-O-acetyl galactose in equine erythrocytes. Glycoconj J. 1996;13:225–233. doi: 10.1007/BF00731497. [DOI] [PubMed] [Google Scholar]
- 51.Yeh J C, Ong D, Fukuda M. Molecular cloning and expression of a novel β-1,6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J Biol Chem. 1999;274:3215–3221. doi: 10.1074/jbc.274.5.3215. [DOI] [PubMed] [Google Scholar]