Abstract
Background
The increasing use and anticipated future adoption of antibody–drug conjugates (ADCs) present a significant challenge in identifying and monitoring patients for the development of potentially fatal drug-induced interstitial lung disease (ILD). We sought to apply a tissue-specific methylation analysis of circulating cell-free DNA (cfDNA) to measure lung damage in patients with trastuzumab deruxtecan (T-DXd)-related ILD.
Patients and methods
We describe a patient with metastatic human epidermal growth factor receptor 2 (HER2)-positive endometrial cancer who developed ILD during T-DXd treatment. Blood samples collected at the time of ILD diagnosis, after recovery, and following rechallenge were studied for lung damage using lung-specific methylation markers in cfDNA. To validate the findings, we also tested plasma samples from an additional cohort of patients with HER2-positive metastatic breast cancer treated with T-DXd.
Results
In patients with HER2-positive metastatic cancer treated with T-DXd, the presence of an active ILD, as assessed clinically and using chest computed tomography, was associated with increased levels of lung-derived cfDNA.
Conclusions
This proof-of-concept study demonstrates that liquid biopsy can be developed as a valuable tool for detecting and monitoring ADC-related ILD. Its low cost and simplicity make it a potential alternative to current imaging methods, warranting further clinical development.
Key words: HER2, antibody–drug conjugate, interstitial lung disease, liquid biopsy, early detection, breast cancer
Highlights
-
•
Novel ADCs pose a risk of potentially fatal ILD.
-
•
In patients treated with T-DXd, detection of lung-derived circulating cfDNA was associated with ADC-induced ILD.
-
•
Liquid biopsy using tissue-specific methylation analysis may serve as a cost-effective detection and monitoring tool for ADC-induced ILD.
Introduction
Antibody–drug conjugates (ADCs) are reshaping treatment algorithms for multiple cancer types, with dramatic prognostic improvements and 11 ADCs approved to date for solid or hematologic malignancies. Together with improving outcomes in oncology, ADCs raised novel challenges in terms of toxicity management, including fatal toxicities observed in multiple trials. One key side-effect of concern, observed with several novel ADCs, is represented by interstitial lung disease (ILD).1 All-grade ILD has been reported in ∼10.6% of patients receiving treatment with approved ADCs,2 with the highest rate (15%) observed in patients receiving trastuzumab deruxtecan (T-DXd). Among these, ∼1% experienced fatal ILD across clinical trials of T-DXd.3 The mechanism of ILD is currently unknown, although preclinical data suggest a potential role for the activation of alveolar macrophages.4 Elegantly, studying single-cell RNA sequencing failed to find a correlation between ADC target expression in lung tissue and ILD incidence.5 A pooled analysis found that ILD with T-DXd is more common in patients with underlying lung disease, low baseline oxygen saturation, worse kidney function, older age, or Japanese ancestry.3 Recently, the five ‘S’ rule was suggested to tackle ILD in patients receiving T-DXd.6 According to these recommendations, no screening strategy is available to detect ILD before its radiological appearance, and there is a general recommendation to carry out computed tomography (CT) scan of the chest every 6-12 weeks to identify early signs of ILD. Furthermore, according to current guidelines, the ADC should be permanently interrupted in patients who develop symptomatic ILD (grade ≥2).
Liquid biopsy using circulating cell-free DNA (cfDNA) is an emerging modality to assist in tumor genotyping, disease monitoring, and guide therapy selection. Recent technology advances suggested a promising role for the use of cfDNA analysis in the challenging task of early cancer detection. Previously, we and others have shown that cfDNA from nontumor tissues can be investigated in the plasma to provide valuable information on tissue damage, such as cardiomyocyte death in myocardial infarction, intestinal damage secondary to chemotherapy and radiation, and brain damage due to stroke.7,8 More relevantly, we have shown that using a comparative methylome analysis approach, lung-specific methylation markers can be identified and successfully detect the death of normal lung cells, resulting in an increase in circulating lung-derived cfDNA.9 Moreover, this approach enabled us to differentiate between various benign and malignant lung pathologies.9
Herein, we present the first report utilizing a quantitative lung-specific methylation-based cfDNA analysis to study ADC-related ILD.
Patients and methods
Lung-specific cfDNA was detected as previously described.9 In brief, cfDNA was extracted from 4 ml of plasma using the QIAsymphony liquid handling robot (Qiagen) and treated with bisulfite (Zymo Research). DNA concentration was measured using Qubit High Sensitive Double-Strand Molecular Probes (Invitrogen). Bisulfite-treated DNA was subjected to multiplex PCR amplification using primers specific for bisulfite-treated DNA (for 12 lung-specific loci). Sequencing was carried out on PCR products using a NextSeq 500/550 v2 Reagent Kit (Illumina). Sequenced reads were separated by barcode and aligned to the target sequence with Bismark using a computational pipeline available (https://github.com/Joshmoss11/btseq). CpGs were considered methylated if ‘CG’ was read and unmethylated if ‘TG’ was read. To assess the levels of lung epithelial cfDNA, we calculated the fraction of molecules from each marker that contain the lung-specific methylation patterns. We then multiplied the fraction by the total concentration of cfDNA, to obtain the concentration of lung-derived molecules. Given that the mass of a haploid human genome is 3.3 pg, the concentration of cfDNA can be converted from units of ng/ml to haploid genome equivalents per ml plasma (GE/ml) by multiplying by a factor of 303. Control plasma was drawn from a healthy adult. The study was done under institutional review board (Hadassah Medical Center) approval (approval number 0346-12).
Results
First, we describe a 78-year-old woman with a human epidermal growth factor receptor 2 (HER2)-positive (immunohistochemistry +3 score) metastatic endometrial carcinoma (mismatch repair proficient). She was a never smoker and without any additional chronic conditions (normal pulmonary and renal function). For the treatment of her pulmonary and pelvic metastatic disease, the patient has received and progressed on prior treatment with paclitaxel/carboplatin and pembrolizumab/lenvatinib. At the time of disease progression, she had evidence of a malignant pericardial effusion and right pleural effusion. After a multidisciplinary tumor board, the patient started a third-line treatment with T-DXd 5.4 mg/kg and experienced rapid clinical improvement with the disappearance of dyspnea within two cycles. However, after an additional three cycles, she developed a cough that prompted a chest CT scan, revealing signs of bilateral ILD (day 1). T-DXd was interrupted, and oral steroids were initiated (prednisone 60 mg once daily). Following treatment with steroids, the cough waned, and prednisone was tapered off (day 52). Given her excellent response to T-DXd, her strong preference, and the generally limited treatment options for her condition, she decided to resume therapy with T-DXd on day 62 after an additional multidisciplinary discussion. Unfortunately, within 12 days of reintroduction, significant respiratory symptoms emerged and chest CT imaging revealed both disease progression (as recurrent pleural effusions) and signs of bilateral ILD (day 74). T-DXd therapy was immediately interrupted; instead, 60 mg of daily prednisone was prescribed and the patient’s treatment was switched to pegylated doxorubicin, which was administered for an additional 2 months. Because of significant disease progression, the patient chose to focus on palliative care alone.
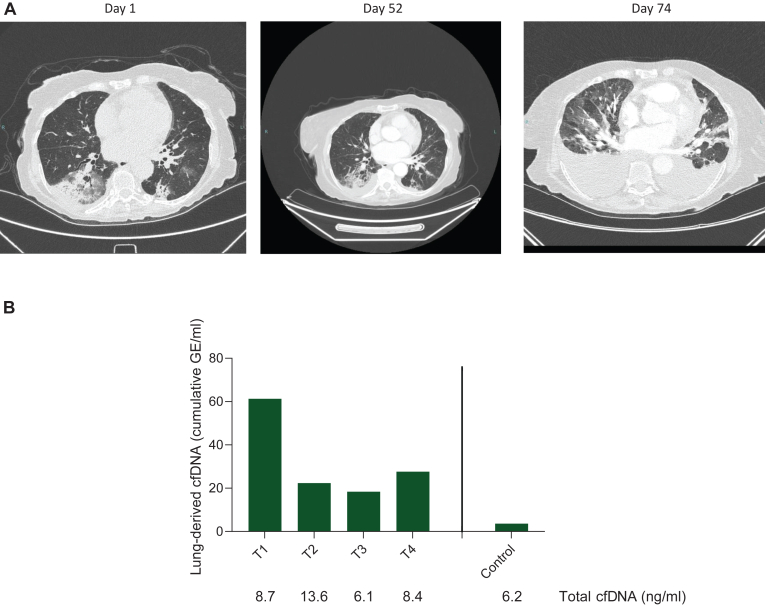
Retrospectively, we analyzed plasma collected at the aforementioned time points, that is, on days 1, 52, 62, and 74 (Figure 1). Upon measuring lung-specific cfDNA, a surrogate for pulmonary damage, we observed markedly elevated levels of lung-derived cfDNA on day 1 (initial ILD event), followed by a decrease after T-DXd was discontinued and steroids were administered. Interestingly, a mild increase in lung-derived cfDNA was observed following the reintroduction of T-DXd, in parallel with the clinical and radiological presentation of recurrent ILD.
Figure 1.
Correlation between CT imaging and lung-derived cfDNA. (A) Chest CT at the same level during the clinical course showing the initial diagnosis of interstitial lung disease (day 1), repeated scan after nearly 2 months following clinical and radiologic improvement (day 52), and marked inflammation with malignant effusions soon after rechallenge with T-DXd (day 74). (B) Levels of lung-derived cfDNA measured as cumulative genome equivalents (GE) at various time points. Below are the total levels of cfDNA in plasma, roughly equal at all time points. cfDNA, cell-free DNA; CT, computed tomography; T-DXd, trastuzumab deruxtecan.
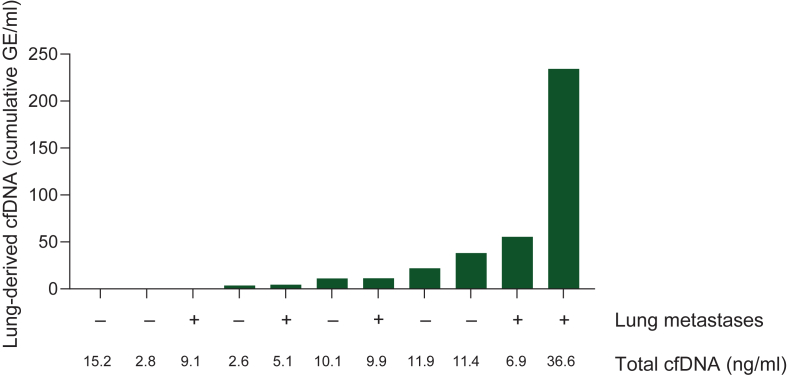
Encouraged by our preliminary findings, we examined our biobank and identified 11 samples from 11 patients with HER2-positive metastatic breast cancer who were treated with T-DXd (details of these patients are presented in Table 1). Analysis of lung-specific cfDNA revealed varying levels that did not correlate with the presence of known pulmonary metastases (Figure 2). In line with previous findings, the patient with the highest level of lung-derived cfDNA had a chronic lung disease and developed ILD, which was attributed to T-DXd. The treatment was discontinued, and steroids were administered. A plasma sample was collected while the patient was improving, 4 weeks after ILD diagnosis, during which the patient was on a daily dosage of 40 mg prednisone. In addition, the patient with the second highest cfDNA level developed radiologic symptoms of ILD on CT 3 weeks after her plasma sampling. A multidisciplinary evaluation of the etiology was inconclusive. To the best of our knowledge, all other patients in this cohort remain ILD-free.
Table 1.
Patients characteristics
| Patient | Duration of T-DXd treatment on the day of plasma collection (months) | ILD details | Current status (as of July 2024) |
|---|---|---|---|
| 1 | 14.80 | None | Ongoing T-DXd therapy |
| 2 | 20.40 | None | Ongoing T-DXd therapy |
| 3 | 19.40 | None | On subsequent treatment line |
| 4 | 5.17 | None | Ongoing T-DXd therapy |
| 5 | 6.47 | None | Ongoing T-DXd therapy |
| 6 | 16.27 | None | Ongoing T-DXd therapy |
| 7 | 20.67 | None | Ongoing T-DXd therapy |
| 8 | 5.73 | None | On subsequent treatment line |
| 9 | 16.27 | None | Ongoing T-DXd therapy |
| 10 | 2.77 | Three weeks before chest CT which demonstrated ILD | On subsequent treatment line |
| 11 | 8.47 | Taken during T-DXd-related ILD resolution using systemic steroids (1 month after ILD diagnosis). | On subsequent treatment line |
CT, computed tomography; ILD, interstitial lung disease; T-DXd, trastuzumab deruxtecan.
Figure 2.
Lung-derived cfDNA analysis in patients with metastatic breast cancer treated with T-DXd. cfDNA, cell-free DNA; GE, genome equivalents; ILD, interstitial lung disease; T-DXd, trastuzumab deruxtecan.
Discussion
In this commonly encountered clinical scenario, we identified an association between the patient’s symptoms, lung imaging, and lung-derived cfDNA. The application of a simple and universal method to measure normal lung-derived cfDNA enabled us to measure the level of ILD. Altogether, these data provide a proof-of-concept for the application of liquid biopsy to successfully detect and potentially monitor treatment-related adverse events in the context of cancer care. The use of such an approach has the potential to revolutionize cancer care by allowing an affordable, minimally invasive, and rapid approach toward the detection and monitoring of drug-related tissue damage. Furthermore, point-of-care technologies (e.g. Nanopore) have the potential to further transform and decentralize liquid biopsy analysis to improve patients’ safety in diverse and resource-limited environments.
Acknowledgements
We deeply acknowledge and honor Yafa Cohen for her invaluable contribution to cancer research, whose legacy will continue to inspire and drive progress.
Funding
None declared.
Disclosure
AG reports honoraria from Novartis and GSK. PT is a Social Media Editor in ESMO Open and reports research funding (to institution) from AstraZeneca and consulting or advisory roles for AstraZeneca, Daiichi Sankyo, Eli Lilly, Roche, Genentech, Gilead, and Novartis. SMT is an Associate Editor of the Breast Cancer section of ESMO Open, and reports consulting or advisory roles for Novartis, Pfizer, Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, CytomX Therapeutics, Daiichi Sankyo, Gilead, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Infinity Therapeutics, Sumitovant Biopharma, Zetagen, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Bio, Bayer, Incyte Corp, Jazz Pharmaceuticals, Natera, Tango Therapeutics, Systimmune, eFFECTOR, and Hengrui USA; research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, Gilead, NanoString Technologies, Seattle Genetics, and OncoPep; and travel support from Eli Lilly, Sanofi, Gilead, and Pfizer. All other authors have declared no conflicts of interest.
Data sharing
Data are available upon reasonable request from the authors.
References
- 1.Tarantino P., Ricciuti B., Pradhan S.M., Tolaney S.M. Optimizing the safety of antibody-drug conjugates for patients with solid tumours. Nat Rev Clin Oncol. 2023;20(8):558–576. doi: 10.1038/s41571-023-00783-w. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Z., Shen G., Li J., et al. Incidence of antibody-drug conjugates-related pneumonitis in patients with solid tumors: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2023;184 doi: 10.1016/j.critrevonc.2023.103960. [DOI] [PubMed] [Google Scholar]
- 3.Powell C.A., Modi S., Iwata H., et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7(4) doi: 10.1016/j.esmoop.2022.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumagai K., Aida T., Tsuchiya Y., Kishino Y., Kai K., Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020;111(12):4636–4645. doi: 10.1111/cas.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters S., Parikh K., Dimou A., Desai A. 3P Correlation between antibody-drug conjugate (ADC) targetable antigen expression and occurrence of interstitial lung disease (ILD) ESMO Open. 2023;8 [Google Scholar]
- 6.Tarantino P., Tolaney S.M. Detecting and managing T-DXd-related interstitial lung disease: the five “S” rules. JCO Oncol Pract. 2023;19(8):526–527. doi: 10.1200/OP.23.00097. [DOI] [PubMed] [Google Scholar]
- 7.Moss J., Magenheim J., Neiman D., et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat Commun. 2018;9(1):5068. doi: 10.1038/s41467-018-07466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grinshpun A., Kustanovich A., Neiman D., et al. A universal cell-free DNA approach for response prediction to preoperative chemoradiation in rectal cancer. Int J Cancer. 2023;152(7):1444–1451. doi: 10.1002/ijc.34392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magenheim J., Rokach A., Peretz A., et al. Universal lung epithelium DNA methylation markers for detection of lung damage in liquid biopsies. Eur Respir J. 2022;60(5) doi: 10.1183/13993003.03056-2021. [DOI] [PubMed] [Google Scholar]