Abstract
Purpose
The purpose of this study was to determine the association between lens thickness and cataract in participants aged 0 to 5 years.
Design
This was a prospective, multicenter, case–control study.
Participants
We enrolled 118 participants (171 eyes) aged 0 to 5 years, mean age 14.6 ± 17.0 months, range 0 to 60 months.
Methods
Lens thickness was measured on 342 ultrasound biomicroscopy (UBM) images.
Main Outcome Measures
Lens thickness; feasibility of lens thickness measurement from UBM images.
Results
The mean lens thickness among noncataracts was 3.60 ± 0.17 mm, compared with 3.16 ± 0.61 mm among cataracts (P < 0.0001). Lens thickness <3.5 mm was significantly associated with increased odds of cataract; adjusted odds ratio = 5.99 (95% confidence interval, 2.41–14.88; P < 0.0003) among participants age 0 to 7 months. Lens thickness was significantly associated with cataract laterality among participants age 0 to 7 months (P < 0.0001).
Conclusions
Quantitative UBM can be used to evaluate lens thickness in infants and children with congenital cataracts. The lens in congenital cataract eyes was thinner than that of controls among infants. Abnormal lens thickness was significantly associated with cataract. Future longitudinal studies will examine the association between lens thickness and postcataract surgery outcomes.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Ultrasound biomicroscopy, Congenital cataract, Lens development, High frequency ultrasound, Lens thickness
Cataract in children is among the most common causes of blindness, accounting for up to a fifth of the 1.4 million blind children worldwide.1 Pediatric cataracts can result in poor visual outcomes and decreased vision-related quality of life, even after surgical treatment, because of amblyopia and other complications inherent to intraocular surgery in the early years of life.2, 3, 4
Congenital cataracts are a final common pathway for a diverse gamut of interruptions to normal lens development, most commonly genetic, infectious, or environmental. At the genetic level, more than 115 genes are linked to congenital cataracts.5 The complex and multifactorial process of lens development and cataract formation results in extreme phenotypic and genotypic heterogeneity as a hallmark of congenital and childhood cataracts.
Previous studies suggest lens structural changes are associated with pediatric anterior segment diseases. Features of the lens have been linked to glaucoma without cataract (primary congenital glaucoma)6,7 and specific subtypes of cataract,8, 9, 10 but no previous study has evaluated lens thickness in the context of cataract in a prospective case–control study using high-resolution ultrasound biomicroscopy (UBM) imaging. Structural changes in the lens associated with congenital cataract hold promise in their potential as a predictive biomarker for future adverse events after congenital cataract surgery.
This study was designed to answer the question: is thinner lens associated with cataracts in infants and children? We hypothesized that the lens is thinner in cataract eyes compared with noncataract eyes. We further hypothesized that unilateral and bilateral cataracts differ in lens thicknesses. We proposed to (1) describe the distribution and association of lens thickness among participants aged 0 to 5 years with and without cataracts; (2) compare the structural profiles of bilateral compared with unilateral cataracts in this cohort; and (3) report the rate of successful imaging and analysis using quantitative UBM in participants age 0 to 5 years for lens analysis.
Our study used a case-control study design for comparison of noncataract eyes to congenital cataract eyes before surgery. This study provides the baseline features of a cohort of childhood cataracts and age-matched controls, including normative data for future longitudinal studies aimed at determining risk of complications associated with lens structure. The findings of this study offer improved understanding of the relationship between lens structure and congenital cataracts.
Methods
Study Design
This was an observational case–control study. Participants with cataracts were stratified by age and compared with participants without cataracts.
Setting and Participants
Participants were recruited in this multicenter study in the pediatric ophthalmology outpatient or inpatient setting when they presented with an ocular complaint requiring surgery. Participants with amblyogenic cataracts in 1 or both eyes were offered to enroll as cases. Participants without cataracts with normal ocular anatomy and function were offered to enroll as controls. Inclusion and exclusion criteria are detailed in Table S1 (available at www.ophthamologyscience.org). Verbal and written informed consent were obtained from the parent of each participant. Institutional Review Board Ethics Committee approval was obtained before initiating this study. This research adhered to the tenets of the Declaration of Helsinki.
Procedures
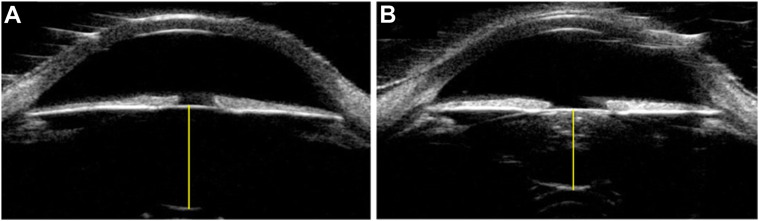
Imaging was performed while the participant was under general anesthesia immediately before necessary surgical procedure (removal of cataract or, for controls, at the time of eyelid, nasolacrimal, or contralateral trauma procedure). Ultrasound biomicroscopy imaging was performed by a single sonographer per center using the Aviso Ultrasound Platform A/B UBM with 50 MHz linear transducer (Quantel Medical) or the Accutome UBM Plus 48 MHz transducer (Keeler) platform. Imaging tools and conditions included use of Alfonso eyelid speculum, hypromellose ophthalmic solution (2.5%) coupling gel, ClearScan probe cover, and standardized lighting conditions without pharmacologic dilation. The imaging protocol has been previously published.11 Two axial scans per eye were collected with the ultrasound probe at the corneal center, marker directed horizontal and vertical, at optimal depth of focus for lens resolution. The average of the 2 axial scans were used for lens thickness measurement (Fig 1).
Figure 1.
Axial UBM image with lens thickness measurement (yellow line) in (A) Control and (B) Cataract. UBM = ultrasound biomicroscopy.
Lens Measurements
Quantitative data for anterior-posterior lens thickness were extracted from each UBM image by a masked observer using ImageJ. Caliper endpoints were placed at the black-white interface at the anterior capsule and the posterior capsule in the center of the pupil (Fig 1). The lens thickness value for each eye was determined by taking the mean measurement from the 2 axial (horizontal and vertical cross section) UBM images.
Covariates
Participant age, gestational age, birthweight, race, ethnicity, and syndrome status were identified as potential effect modifiers or confounders. Age has an established influence on ocular anatomy. Gestational age and birthweight have not been independently linked to cataract development, but extreme prematurity associated with retinopathy of prematurity has been associated with cataract.12
Statistical Methods
Study population characteristics were compared between cataracts and age-matched noncataract eyes. The primary outcome was cataract. A generalized estimating equation (GEE) was used to adjust the correlation between eyes, allowing both eyes of the same participant to be included in the analysis. Associations between covariates (age, gestational age, birthweight, presence of syndrome) and structure (lens thickness) and outcome (cataract) were determined based on P values from the Student’s t test and the Fisher exact test.
Crude Analysis: Cataract–Lens Thickness Association
We calculated crude odds ratios (ORs), with 95% confidence intervals (CIs) and P values, based on logistic regression with GEEs to adjust the correlation between eyes to evaluate association between lens thickness and cataract.
Confounding and Effect Modification
Confounding was determined according to criteria of P value <0.20 for both exposure (lens thickness)-covariate and outcome (cataract)-covariate association or if >10% difference between adjusted and crude log ORs values for associations between covariates for both outcomes and exposures.13 Crude ORs were stratified by age, gestational age, birthweight, and presence of syndrome and were compared using the Breslow–Day test for homogeneity to assess for effect measure modification. Evaluation of these covariates was based on a priori rationale for age, prematurity, and presence of syndrome as potentially related to cataract and lens structure. We used stratified results and P values for interaction to assess potential effect modification.
Final Model
Logistic regression was used for multivariable analysis and crude and adjusted ORs (95% CIs) were calculated and reported by age strata due to effect modification. The final model was an age-adjusted logistic regression for the outcome of cataract in this case–control study. Cutoffs were selected from natural transitions in the data for ease of clinical interpretation of reference points for the thickness of the lens at 3.5 mm.
We performed analysis using R (4.0.2 R Foundation for Statistical Computing) and SAS (9.4 SAS Institute Inc).
Results
Lens measurements were performed on 342 UBM images from 118 participants (171 eyes) age 0 to 5 years (mean age, 14.6 ± 17.0 months; range, 0–60 months). The study flow diagram is detailed in Figure S2 (available at www.ophthalmologyscience.org).
Participant demographics, birth history, and cataract laterality are provided in Table 2. The difference in age distribution was accounted for by controlling for age in analysis. The control cohort had similar birthweight, gestational age, and demographic characteristics to the cataract cohort.
Table 2.
Baseline Participant Demographics and Characteristics among Cataract and Controls in a Multicenter Study Population of Children Aged 0–5 Years, Data Collected 2014–2024
| Noncataract (n = 39 participants) | Cataract (n = 79 participants) | P Value∗ | |
|---|---|---|---|
| Age (mos), mean (±SD) | |||
| Age in mos among participants <7 mos | 2.4 (±1.6) | 2.5 (±2.8) | 0.63 |
| Age in mos among participants 7–24 mos | 15.9 (±5.6) | 18.4 (±3.5) | 0.53 |
| Age in mos among participants 24–60 mos | 36.8 (±10.0) | 45.1 (±8.1) | 0.06 |
| Total eyes | 93 | 78 | |
| 0–7 mos, eyes (column %) | 43 (46%) | 49 (63%) | |
| 7–24 mos, eyes (column %) | 24 (26%) | 9 (12%) | |
| 24–60 mos, eyes (column %) | 26 (28%) | 20 (25%) | |
| Sex (female), n (column %) | 22 (56%) | 38 (48%) | 0.03 |
| (male), n (column %) | 17 (44%) | 41 (52%) | |
| Race/ethnicity | |||
| Black, n (column %) | 13 (33%) | 24 (30%) | 0.61 |
| White, n (column %) | 15 (38%) | 40 (51%) | |
| Hispanic, n (column %) | 5 (13%) | 8 (10%) | |
| Asian, n (column %) | 3 (8%) | 2 (3%) | |
| Other, n (column, %) | 3 (8%) | 5 (6%) | |
| Gestational age (wks), mean (±SD) | 37 (±4) | 37 (±3) | 0.85 |
| Birthweight (kg), mean (±SD) | 2.7 (±0.84) | 2.8 (±0.82) | 0.48 |
| Systemic syndrome present,† n (column %) | 2 (6%) | 8 (20%) | 0.06 |
| Bilateral cataract, n (column %) | n/a | 24 (30%) |
SD = standard deviation.
Bolding denotes P < 0.05.
Missing data for 8 participants on syndrome status.
In bivariate analysis, we found thinner lens for cataracts (mean, 3.16 mm; 95% CI, 3.02–3.29) compared with controls (mean, 3.60 mm; 95% CI, 3.56–3.63; P < 0.0001; Table 3, Fig S3, available at www.ophthalmologyscience.org). Mean lens thickness was lower among cataracts compared with controls across all age subgroups but was statistically significant only in participants aged 0 to 7 months and 24 to 60 months (Table 3). Lens measurements demonstrated larger standard deviations in the cataract group.
Table 3.
Mean and Standard Deviation for Lens Thickness among Noncataract Controls and Cataracts, Followed by Age Subgroups and Laterality Subgroups
| Control Eyes, n = 93 |
Cataract Eyes, n = 78 |
P Value∗ | |||
|---|---|---|---|---|---|
| Bilateral Control | Unilateral Control | Unilateral Cataract | Bilateral Cataract | ||
| Age 0–7 mos | 3.65 ± 0.12 | 3.58 ± 0.18 | 3.06 ± 0.54 | 2.99 ± 0.89 | <0.0001 |
| Age 7–24 mos | 3.58 ± 0.12 | 3.57 ± 0.17 | 3.36 ± 0.36 | 3.55 ± 0.22 | 0.27 |
| Age 24–60 mos | 3.62 ± 0.20 | 3.49 ± 0.26 | 3.35 ± 0.32 | 3.31 ± 0.30 | 0.0003 |
| All ages | 3.62 ± 0.16 | 3.57 ± 0.18 | 3.13 ± 0.51 | 3.18 ± 0.69 | <0.0001 |
SD = standard deviation; n = number of eyes.
P values were adjusted for 2 eyes per subject using generalized estimating equations, bolding denotes P < 0.05.
Unilateral cataracts and bilateral cataracts were evaluated as separate subgroups. Unilateral control eyes were compared with bilateral control eyes as well, due to previous literature suggesting subtle anomalies may be present in unilateral cataract’s fellow eye (Fig S4, available at www.ophthalmologyscience.org).14,15 Among participants aged 0 to 7 months, mean lens thickness comparison between unilateral (3.13 mm; 95% CI, 2.96–3.30) and bilateral (3.18 mm; 95% CI, 2.96–3.39) cataracts demonstrated no difference (P = 0.74). Thinner lenses were found in unilateral control eyes (mean, 3.57 mm; 95% CI, 3.52–3.63) compared with bilateral “true” controls with mean thickness of 3.62 mm (95% CI, 3.57–3.66; P = 0.24, Table 3). We found no statistically significant difference between the lens thickness between a unilateral and bilateral cataract, nor between that of a unilateral control eye (contralateral to a unilateral cataract) and a bilateral “true” control eye of a participant with no cataract in either eye for any age group. Although each laterality subgroup did not demonstrate significant differences in pairwise Student t tests, controlling for age and contribution of eyes per participant, regression analysis of the overall association between lens thickness and cataract laterality was indeed significant with P < 0.0001 for age 0 to 7 months and P < 0.0003 for age 24 to 60 months. In addition to a pattern of mean lens thickness association with cataract laterality, the standard deviation of lens thickness demonstrated a significant association with laterality, with the standard deviation increasing from 0.16 among bilateral controls, 0.18 among unilateral controls, 0.51 among unilateral cataracts, and 0.69 among bilateral cataracts.
Covariates gestational age, birthweight, race, ethnicity, and presence of syndrome were excluded from the model due to nonsignificance (P > 0.2). The final logistic regression model included age as a covariate in the model and results were stratified by age. Crude and adjusted ORs (95% CI) were calculated to determine if thinner lens (<3.5 mm) was associated with increased odds of cataract (adjusted OR with GEE = 5.99, 95% CI, 2.41–14.88; P < 0.0003 in age 0–7 months; adjusted OR = 1.54; 95% CI, 0.31–7.69; P = 0.43 in age 7–24 months; adjusted OR = 4.62; 95% CI, 1.22–17.60; P = 0.008 in age 24–60 months). From this we see that infants (<7 months) with cataracts had 5.99 times the odds of having a lens thickness <3.5 mm (95% CI, 2.41–14.88; P < 0.0003). The wide CIs indicate low precision of the reported estimates (Table 4).
Table 4.
Odds Ratios for Association between Lens Thickness and Cataract, Crude followed by ORs with Age Adjustment and GEEs, Stratified by Age
| Age 0–7 mos | |||||
|---|---|---|---|---|---|
| Noncataract (n = 43 eyes) | Cataract (n = 49 eyes) | Crude OR | Adjusted OR∗, GEE† | ||
| Lens thickness (mm) | Thickness <3.5 mm |
12 | 34 | 5.86 (2.38–14.28), P < 0.0001 | 5.99 (2.41–14.88), P = 0.0003 |
| Thickness ≥3.5 mm |
31 |
15 |
1.00 (REF) |
1.00 (REF) |
|
|
Age 7-24 months | |||||
|
Noncataract (n = 24 eyes) |
Cataract (n = 9 eyes) |
Crude OR |
Adjusted OR∗, GEE† |
||
| Thickness <3.5 mm |
9 | 4 | 1.33 (0.28–6.30), P = 0.72 | 1.54 (0.31–7.69), P = 0.43 | |
| Thickness ≥3.5 mm |
15 |
5 |
1.00 (REF) |
1.00 (REF) |
|
|
Age 24–60 mos | |||||
|
Noncataract (n = 26 eyes) |
Cataract (n = 20 eyes) |
Crude OR |
Adjusted OR∗, GEE† |
||
| Thickness <3.5 mm |
10 | 15 | 4.80 (1.33–17.33), P = 0.01 | 4.62 (1.22–17.60), P = 0.008 | |
| Thickness ≥3.5 mm |
16 | 5 | 1.00 (REF) | 1.00 (REF) | |
Bolding denotes P < 0.05.
GEE = generalized estimating equation; OR = odds ratio; REF = reference.
Adjusted for age.
Age-adjusted and GEE accounted for repeat measures.
Given the above associations, lens thickness as a diagnostic marker for congenital cataract was evaluated using lens thickness cutoffs below 3.3 mm and above 3.9 mm, resulting in a sensitivity of 60.3% for cataract diagnosis, and specificity of 93.6%. Cutoffs of 3.1 mm and 3.9 mm resulted in sensitivity of 47.4% and specificity of 100% (Table S5, available at www.ophthalmologyscience.org).
In terms of UBM imaging success in this pediatric cohort, analyzable UBM images were obtained in 100% of eligible participants and images. No images were excluded for low quality; however, some eyes were not imaged because they were not eligible for testing.
Discussion
Summary of Results
This was an observational case-control study reporting the lens thicknesses associated with cataracts in children aged 0 to 5 years. We found that thinner lens was associated with pediatric cataracts. Overall, mean lens thickness was smaller in the cataract groups, and thicknesses were highest in bilateral controls. We found that pairwise comparison of unilateral and bilateral cataracts and controls had no statistically significant difference but a pattern of stepwise decrease in lens thickness with bilateral being less than that of unilateral, which was less than that of controls. The association between number of cataract eyes per participant (2,1, or 0), and the lens thickness was found to be statistically significant (P < 0.001). Crude and adjusted ORs suggested an association between thinner lens (<3.5 mm) and pediatric cataract in participants <7 months and participants aged 24 to 60 months.
Comparison to Previous Literature
Table S5 provides a summary of the 8 previous studies evaluating pediatric lens thickness.9,10,16, 17, 18, 19, 20, 21 All 8 studies demonstrated lower mean thickness and larger standard deviation in cataracts compared with controls, consistent with the current study. Trivedi and Wilson10 offered the only of the 8 studies with a direct comparison of cataract to controls. They found thinner mean lens thickness in cataract compared with controls, significant only among participants <6 months, with no significant difference in lens thickness between 6 and 200 months. Trivedi and Wilson’s study used control eyes with a contralateral cataract and did not have a bilateral control comparison group.
This study differed from previous studies in our prospective case-control design and use of UBM imaging. Previous studies used primarily A-scan biometry.9,10,16, 17, 18, 19, 20, 21 A-scan creates a tracing of ultrasound peaks and valleys associated with tissue boundaries. From the tracing, one can infer the thickness of the structure between the peaks. This method requires anatomic inference, and results can be inaccurate, particularly in the setting of cataract, without subject fixation, and lacking 2-dimensional image reference. Studies have shown that B-mode guidance (with cross sectional view similar to UBM) has more accuracy than A-mode (1-dimensional tracing).22
Diagnostic Utility of Lens Thickness
This study found an association between thinner lens and presence of cataract in 2 age subgroups. Further examination of lens thickness as a diagnostic marker for congenital cataract found sensitivity of 47.4% to 60.3% for cataract diagnosis and specificity of 93.6% to 100%. Use of lens thickness as a diagnostic marker for cataracts is a flawed approach because about half of cataracts have lens thickness in the normal range, resulting in poor sensitivity and high false negatives. Conversely, lens thickness can offer a highly specific test for cataracts. For diagnosis, clinical examination for cataract is more relevant than lens thickness. Lens thickness should not replace current diagnostic approaches but may be relevant in the context of fetal ultrasonography.23 Further study is needed to evaluate the prenatal diagnostic relevance of lens thickness.
Clinical Relevance of Lens Thickness
Lens thickness measurement is not currently standard care for infants and young children with cataracts. Although speculative, lens thickness measurement may have several relevant clinical applications including diagnosis, prognosis, and management. Novel diagnostic approaches include fetal ultrasound diagnosis23 or development of lens thickness as a quantitative marker for pediatrician screening tools, particularly considering extreme values of lens thickness may help identify not only cataracts, but also refractive error.24,25 Lens thickness has demonstrated value in intraocular lens (IOL) power calculations, and is occasionally included as a variable in the IOL power calculation formula (Olsen).26,27 Even among infants not receiving an IOL at the time of surgery, historic lens thickness may be relevant to predict future pseudophakic lens position28 and/or future axial length progression.29,30 Lens thickness may help us predict long-term vision outcome after cataract surgery, or may help us anticipate postoperative complications using risk calculators. Lastly, knowledge of lens thickness can help guide surgical approach. Increased lens thickness has been reported in association with increased risk of capsular tears.31 Thinner lens introduces risks associated with the close proximity of the anterior vitreous during cataract removal.32
Success of Imaging and Analysis
Image acquisition and analysis are essential steps in translational applications of lens thickness data. Image acquisition in a pediatric population is not trivial considering cooperation is unpredictable, and imaging under general anesthesia in this age group carries some risk.33
In previous studies, imaging and analysis were not universally successful. In data obtained from A-scan biometry, lens thickness data were not available for 105/310 (34%) of eyes in 1 study,10 and 18/81 eyes (22%) of eyes in another study were excluded because they were unable to complete the preoperative examinations.9 Li et al9 found that for the 32% of lens measurements not obtained, about half of the participants were unable to complete imaging, and about half had images of poor (nonmeasurable) quality. This suggests that even with general anesthesia lens thickness assessment with A-scan biometry is not universally successful.
The current study had 100% successful imaging and measurement of lens thickness from participants meeting inclusion criteria. Images not acquired were solely due to ineligibility. The success of imaging in the current study is attributed to reliable structure visualization with UBM, prospective knowledge of the structure of interest, and sonographer expertise. General anesthesia helped to eliminate issue of participant cooperation, but most previous studies of lens thickness in congenital cataract also used general anesthesia and still did not have 100% success, suggesting an inherent advantage to 2-dimensional lens visualization (using UBM) at the time of lens thickness measurement.
Directionality
We found that lenses in eyes with congenital cataracts are thinner, with wider variance, than eyes without cataract. The causation question is as follows: do cataractogenic mutations or events lead first to thinner lenses and subsequent opacity, or do mutations lead to opaque lenses that later become thin? Given the multifactorial nature of congenital cataracts, both directions of causation may play a role in the thickness–opacity relationship.
Early fetal events may influence thickness and opacity profoundly. Later onset cataracts (third trimester or perinatal) may influence lens clarity more than thickness. Postbirth lens changes also occur, further complicating directionality. Spontaneous cataractous lens absorption (membranous cataract), and phacomorphic expansion of the cataractous lens are well described, contributing to the wide variance of lens thickness distribution. Clinical correlations such as Lowe syndrome (discoid lens) and microspherophakia (spherical lens) are examples of lens disease associated with abnormal lens thickness, related to both cataract and serious complications like glaucoma. Development may have multifactorial impact on lens thickness and clarity and future complications, suggesting that quantified lens structure in the pediatric population has unique relevance compared with the adult population.
Age-based Trends
This study examined 3 age groups selected a priori because we have established clinical correlations (glaucoma and amblyopia) that have been previously examined at age cutoffs of 7 months, 24 months, and 5 years. These age ranges were used in the major clinical trials in this field (the Infant Aphakia Treatment Study and the IOLunder2 Study).
Like previous studies, we found the difference in lens thickness between cataracts and controls in a younger subset of participants. Thinner lens was noted in the 0 to 7 months and 24 to 60 months subgroup and mean lens thickness was similar in the 7 to 24 months group. This is also consistent with previous study of Trivedi and Wilson.10 This was reflected in OR stratified by age for association between lens thickness and cataract (Table 4). This might suggest that cataractous thinning is more likely in the first 7 months of life. Alternatively, the earlier the cataract formation process begins, over the timeline of fetal development and infancy, the greater the effect on lens development. These changes likely must occur either during pregnancy (first, second, or third trimester) or early after birth to affect the lens development, and later onset of cataract has less tendency to result in thinner lens. Later onset cataracts in this cohort demonstrated some lenses were thicker than age-matched controls, whereas others were thinner, suggesting that phacomorphic changes may be more likely after 7 months of age.
We found that the oldest age subgroup had more similar findings to the youngest age subgroup, rather than to the middle age group. Potential reasons for this were smaller sample size in the middle age group, and referral bias. Lag time to cataract diagnosis may occur as the frequency of well child visits and developmental milestones vary considerably at different ages.
The youngest age subgroup had the greatest heterogeneity of lens thickness, likely because of variable etiologies and developmental timing of cataract. The youngest age group had lens thickness standard deviations of 0.5 and 0.9 mm (unilateral and bilateral cataracts, respectively), whereas the combined older subgroups’ lens thickness standard deviation was considerably smaller, between 0.2 and 0.4 mm. This may reflect that the anatomic impact of cataract in the youngest eyes is more pronounced, which is not surprising, given that the lens takes up greater relative proportion of the axial size of the eye at younger ages. Although the observed difference between cataract and control lens thickness was similar between the youngest and oldest groups, the absolute lens thickness differed most in the youngest group.
Limitations
This study was subject to limitations inherent to case-control studies: Causality, sampling bias, and control selection. The study was limited by a small number of cataracts aged 7 to 24 months. Many covariates were evaluated in univariate analysis, all of which could not be controlled for in multivariable analysis because of modest sample size. However, we did not find significant associations between covariates and outcomes. Certain ethnic and racial groups were underrepresented in our local population and therefore in our study. Cataract subtypes (for example, persistent fetal vasculature, lamellar, or nuclear) were not tracked prospectively, and, therefore, we did not have adequate sample size for these subgroups to determine lens thickness associations by cataract subtype. Controls were sampled from the same population as cases and provided a consistent cohort that likely would have been cases if they had cataract. However, our control cohort is sampled from a pediatric ophthalmology referral base and thus does not represent completely healthy normal controls. This would bias our findings in the direction of no effect. Lastly, we cannot determine directionality of our findings to better understand if cataract leads to abnormal structure or if structural abnormality contributes to the development of cataracts.
Strengths
This study provided a modest but sufficient sample size for cataract-control comparison. We utilized objective, quantitative measures of lens thickness gathered by masked analysts. We included variable participant ages but accounted for age in our model, and reported stratified results. Results are likely generalizable, given multicenter recruitment and consistency with previous studies. Successful imaging was achieved in 100% of participants.
Future Directions
More extreme thinning in the bilateral cataracts compared with unilateral cataracts suggests there may be a difference in structural severity in bilateral cataracts compared with unilateral. The finding that the lens is thinner in bilateral compared with unilateral cataracts has been previously reported.10 To our knowledge, this is the first study to report an analogous finding: The unilateral control eye in a patient with contralateral unilateral cataract is thinner than that of an age-matched control. Although the difference in means was not statistically significant, the difference in standard deviation was also much higher in the unilateral control eye of a unilateral cataract compared with an individual with no cataract bilaterally. The concept that subclinical structural anomalies may be present in unilateral control eyes has been previously suggested in the literature based on visual outcomes, but this study provides the first structural evidence of this finding.14 This finding will require further investigation.
Quantitative UBM is an effective imaging technique for the evaluation of the lens thickness in infants and children with congenital cataracts. Thinner lens was associated with cataract in participants with congenital cataracts compared with controls among children aged 0 to 5 years. Future studies will determine the association between lens thickness and complications after congenital cataract surgery.
Manuscript no. XOPS-D-24-00073R1.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s):
I.D.: Payment or honoraria – Novasight.
J.A.: Grants – NIH (K23EY032525, KL2TR003099).
J.L.A.: Research support – K23EY032525, KL2TR003099.
The other authors have no proprietary or commercial interest in any materials discussed in this article.
Dr Janet Alexander acknowledges funding by National Institutes of Health, National Eye InstituteK23EY032525, National Institutes of Health UMB ICTR/Clinical Science and Translational ScienceKL2TR003099, and Knights Templar Eye Foundation Career Starter Grant. Funding for Dr Libby Wei was provided by the Proposed Research Initiated by Students and Mentors (PRISM) Program (University of Maryland, School of Medicine). We acknowledge the support of the University of Maryland, Baltimore, Institute for Clinical & Translational Research (ICTR) and the National Center for Advancing Translational Sciences (NCATS) Clinical Translational Science Award (CTSA) grant number UL1TR003098.
HUMAN SUBJECTS: Human subjects were included in this study. Verbal and written informed consent were obtained from the parent of each participant. Institutional Review Board Ethics Committee approval was obtained before initiating this study. This research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Wei, Kolosky, Byun, Levin, Dortonne, Martinez, Bazemore, Jaafar, Madigan, Magder, Alexander
Data collection: Wei, Kolosky, Byun, Dolgetta, Levin, Bregman, Manrique, Dortonne, Martinez, Jaafar, Madigan, Magder, Alexander
Analysis and interpretation: Wei, Kolosky, Dolgetta, Levin, Bregman, Manrique, Dortonne, Bazemore, Jaafar, Madigan, Magder, Alexander
Obtained funding: Alexander
Overall responsibility: Wei, Kolosky, Byun, Dolgetta, Levin, Bregman, Manrique, Dortonne, Bazemore, Jaafar, Madigan, Magder, Alexander
Supplementary Data
References
- 1.Kong L., Fry M., Al-Samarraie M., et al. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J AAPOS. 2012;16:501–507. doi: 10.1016/j.jaapos.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Lambert S.R., Cotsonis G., DuBois L., et al. Long-term effect of intraocular lens vs contact lens correction on visual acuity after cataract surgery during infancy: a randomized clinical trial. JAMA Ophthalmol. 2020;138:365–372. doi: 10.1001/jamaophthalmol.2020.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirwan C., Lanigan B., O’Keefe M. Glaucoma in aphakic and pseudophakic eyes following surgery for congenital cataract in the first year of life. Acta Ophthalmol. 2010;88:53–59. doi: 10.1111/j.1755-3768.2009.01633.x. [DOI] [PubMed] [Google Scholar]
- 4.Fox A., O’Keefe M., Lanigan B. A follow-on study on vision-related quality of life assessment using the NEI-VFQ-25 in those with a history of unilateral and bilateral congenital cataracts. Acta Ophthalmol. 2018;96:e596–e599. doi: 10.1111/aos.13692. [DOI] [PubMed] [Google Scholar]
- 5.Berry V., Georgiou M., Fujinami K., et al. Inherited cataracts: molecular genetics, clinical features, disease mechanisms and novel therapeutic approaches. Br J Ophthalmol. 2020;104:1331–1337. doi: 10.1136/bjophthalmol-2019-315282. [DOI] [PubMed] [Google Scholar]
- 6.Engels B.F., Dietlein T.S., Jacobi P.C., Krieglstein G.K. [Ultrasound biomicroscopy diagnosis of congenital glaucoma] Klin Monbl Augenheilkd. 1999;215:338–341. doi: 10.1055/s-2008-1034728. [DOI] [PubMed] [Google Scholar]
- 7.Hussein T.R., Shalaby S.M., Elbakary M.A., et al. Ultrasound biomicroscopy as a diagnostic tool in infants with primary congenital glaucoma. Clin Ophthalmol. 2014;8:1725–1730. doi: 10.2147/OPTH.S66682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin H., Lin D., Liu Z., et al. A novel congenital cataract category system based on lens opacity locations and relevant anterior segment characteristics. Invest Ophthalmol Vis Sci. 2016;57:6389–6395. doi: 10.1167/iovs.16-20280. [DOI] [PubMed] [Google Scholar]
- 9.Li Z., Chang P., Wang D., et al. Morphological and biometric features of preexisting posterior capsule defect in congenital cataract. J Cataract Refract Surg. 2018;44:871–877. doi: 10.1016/j.jcrs.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi R.H., Wilson M.E. Biometry data from Caucasian and African-American cataractous pediatric eyes. Invest Ophthalmol Vis Sci. 2007;48:4671–4678. doi: 10.1167/iovs.07-0267. [DOI] [PubMed] [Google Scholar]
- 11.Maripudi S., Byrd J., Qureshi A., et al. Pediatric corneal structural development during childhood characterized by ultrasound biomicroscopy. J Pediatr Ophthalmol Strabismus. 2020;57:238–245. doi: 10.3928/01913913-20200506-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezisi C.N., Kekunnaya R., Jalali S., et al. Cataract surgery in children with retinopathy of prematurity (ROP): surgical outcomes. Br J Ophthalmol. 2017;101:1128–1131. doi: 10.1136/bjophthalmol-2016-309392. [DOI] [PubMed] [Google Scholar]
- 13.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis T.L., Maurer D., Tytla M.E., et al. Vision in the “good” eye of children treated for unilateral congenital cataract. Ophthalmology. 1992;99:1013–1017. doi: 10.1016/s0161-6420(92)31857-3. [DOI] [PubMed] [Google Scholar]
- 15.Birch E.E., Cheng C., Stager D.R., et al. The critical period for surgical treatment of dense congenital bilateral cataracts. J AAPOS. 2009;13:67–71. doi: 10.1016/j.jaapos.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen J.S. The sagittal growth of the eye. II. Ultrasonic measurement of the axial diameter of the lens and the anterior segment from birth to puberty. Acta Ophthalmol. 1971;49:427–440. doi: 10.1111/j.1755-3768.1971.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 17.Garner L.F., Meng C.K., Grosvenor T.P., Mohidin N. Ocular dimensions and refractive power in Malay and Melanesian children. Ophthalmic Physiol Opt. 1990;10:234–238. [PubMed] [Google Scholar]
- 18.Pennie F.C., Wood I.C.J., Olsen C., et al. A longitudinal study of the biometric and refractive changes in full-term infants during the first year of life. Vision Res. 2001;41:2799–2810. doi: 10.1016/s0042-6989(01)00169-9. [DOI] [PubMed] [Google Scholar]
- 19.Zadnik K., Manny R.E., Yu J.A., et al. Ocular Component Data in Schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80:226–236. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Vasavada A.R., Praveen M.R., Nath V., Dave K. Diagnosis and management of congenital cataract with preexisting posterior capsule defect. J Cataract Refract Surg. 2004;30:403–408. doi: 10.1016/S0886-3350(03)00502-9. [DOI] [PubMed] [Google Scholar]
- 21.El Shakankiri N.M., Bayoumi N.H., Abdallah A.H., El Sahn M.M.F. Role of ultrasound and biomicroscopy in evaluation of anterior segment anatomy in congenital and developmental cataract cases. J Cataract Refract Surg. 2009;35:1893–1905. doi: 10.1016/j.jcrs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Bergès O., Puech M., Assouline M., et al. B-mode-guided vector-A-mode versus A-mode biometry to determine axial length and intraocular lens power. J Cataract Refract Surg. 1998;24:529–535. doi: 10.1016/s0886-3350(98)80297-6. [DOI] [PubMed] [Google Scholar]
- 23.Jung E.H., Lee B.J., Yu Y.S., Kim J.H. Postnatal ophthalmological characteristics in patients with congenital cataract diagnosed by fetal ultrasonography. J Matern Fetal Neonatal Med. 2021 Oct;34:3386–3392. doi: 10.1080/14767058.2019.1685963. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez V.M., Cabot F., Ruggeri M., et al. Calculation of crystalline lens power using a modification of the Bennett method. Biomed Opt Express. 2015;6:4501–4515. doi: 10.1364/BOE.6.004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karabulut M., Karabulut S., Simsek H.C., Karalezli A. The iridocorneal angle and related anterior segment structures in pediatric anisohyperopic amblyopic eyes. SAGE Open Med. 2022;10 doi: 10.1177/20503121221107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen T. Prediction of the effective postoperative (intraocular lens) anterior chamber depth. J Cataract Refract Surg. 2006;32:419–424. doi: 10.1016/j.jcrs.2005.12.139. [DOI] [PubMed] [Google Scholar]
- 27.Satou T., Shimizu K., Tsunehiro S., et al. Relationship between crystalline lens thickness and shape and the identification of anterior ocular segment parameters for predicting the intraocular lens position after cataract surgery. Biomed Res Int. 2019 doi: 10.1155/2019/3458548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langenbucher A., Szentmáry N., Cayless A., et al. Prediction of IOL decentration, tilt and axial position using anterior segment OCT data. Graefes Arch Clin Exp Ophthalmol. 2024;262:835–846. doi: 10.1007/s00417-023-06208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Meng Y., Wang Z., et al. Crystalline lens thickness change is associated with axial length elongation and myopia progression in orthokeratology. Cont Lens Anterior Eye. 2022;45 doi: 10.1016/j.clae.2021.101534. [DOI] [PubMed] [Google Scholar]
- 30.Lu T., Song J., Wu Q., et al. Refractive lens power and lens thickness in children (6-16 years old) Sci Rep. 2021;11 doi: 10.1038/s41598-021-98817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Addou-Regnard M., Fajnkuchen F., Bui A., et al. Impact of lens thickness on complications of hypermature cataract surgery: a prospective study. J Fr Ophtalmol. 2016;39:631–635. doi: 10.1016/j.jfo.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Shajari M., Rusev V., Mayer W., et al. Impact of lens density and lens thickness on cumulative dissipated energy in femtosecond laser-assisted cataract surgery. Lasers Med Sci. 2019;34:1229–1234. doi: 10.1007/s10103-019-02715-6. [DOI] [PubMed] [Google Scholar]
- 33.DiMaggio C., Sun L.S., Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.