Abstract
This report describes the diagnosis of porcine circovirus 2 (PCV2)-associated enteritis in 6 weaned pigs without postweaning multisystemic wasting syndrome by histopathology, virus isolation, and in situ hybridization. The most unique lesions were granulomatous inflammation affecting Peyer’s patches, characterized by infiltrates of epithelioid macrophages and giant multinucleated cells. Large, multiple, basophilic or amphophilic, grape-like intracytoplasmic inclusion bodies were often seen in the cytoplasm of histiocytic cells and giant multinucleated cells. No microscopic lesions were observed in the lymphoid tissue, such as lymph node, spleen, and tonsil. A strong hybridization signal for PCV2 was detected in the cytoplasm of histiocytes and giant multinucleated cells in Peyer’s patches. Porcine circovirus 2 was isolated from homogenates of the small and large intestines in 2 weaned pigs. The presence of diarrhea and granulomatous enteritis, and abundant PCV2 DNA associated with the microscopic lesions is suggestive of PCV2-associated enteritis. Thus, PCV2-associated enteritis could be a distinct clinical manifestation of PCV2.
Résumé
Nous décrivons ici le diagnostic d’entérite associée au circovirus porcin 2 (PCV-2) sans le syndrome de dépérissement multi-systémique en post-sevrage chez 6 porcs sevrés au moyen de l’histopathologie, l’isolement viral et l’hybridation in situ. Les lésions les plus caractéristiques étaient une inflammation granulomateuse des plaques de Peyer, caractérisée par une infiltration de macrophages épithéloïdes et de cellules géantes multinucléées. De gros corps d’inclusion intra-cytoplasmiques basophiles ou amphophiles en grappe ont souvent été vus dans le cytoplasme des cellules histiocytaires et des cellules géantes multinucléées. Aucune lésion microscopique n’a été vue dans les tissus lymphoïdes, tel que les nœuds lymphatiques, la rate et les amygdales. Un fort signal d’hybridation pour le PCV2 a été détecté dans le cytoplasme des histiocytes et des cellules géantes multinucléées dans les plaques de Peyer. Le PCV2 a été isolé d’homogénats du petit et du gros intestin de 2 porcs sevrés. La présence de diarrhée et d’entérite granulomateuse, et une quantité abondante d’ADN de PCV2 associées aux lesions microscopiques sont fortement suggestives d’une entérite associée à PCV2 et cette entérite pourrait être une manifestation clinique distincte de l’infection par PCV2.
(Traduit par Docteur Serge Messier)
Porcine circovirus (PCV), a member of the family Circoviridae, is the smallest known nonenveloped, single-stranded, circular DNA virus that replicates autonomously in mammalian cells (1). The family Circoviridae is classified into 2 genera: Circovirus and Gyrovirus (2). Porcine circovirus (PCV), beak and feather disease virus, columbid or pigeon circovirus, canary circovirus, and goose circovirus belong to the genus Circovirus (3–8). The genus Gyrovirus is represented by chicken anemia virus (CAV), the type virus for this group (9). Two types of PCV are now recognized. The first, PCV1, originally identified as a contaminant of the porcine kidney cell line PK-15, is considered to be nonpathogenic (10–12). The second, PCV2 has been associated with postweaning multisystemic wasting syndrome (PMWS) (13,14). Postweaning multisystemic wasting syndrome is characterized by progressive weight loss, respiratory signs, and jaundice (13,14). The characteristic microscopic lesion of PMWS is granulomatous inflammation with giant multinucleated cells and variable numbers of intracytoplasmic inclusion bodies in lymohoid tissues, such as lymph node, spleen, and tonsil (13,14). In addition to PMWS, PCV2 has been associated with various diseases, syndromes in pigs, or both, including porcine dermatitis and nephropathy syndrome (PDNS), reproductive failure, porcine respiratory disease complex, and exudative epidermitis (15–18). This report describes another clinical manifestation, enteritis, associated with PCV2 in weaned pigs.
A continuously farrowing swine unit experienced an epizootic of diarrhea in 150, 40- to 60-day-old weaned Landrace-Large White cross-bred sows. Approximately 30% of the weaned pigs were diarrheic during a 3-week period. A few pigs failed to gain weight and were stunted, but most weaned pigs continued to gain weight. Two weaned pigs (numbers 1 and 2) were submitted from this herd. Another 2 weaned pigs (numbers 3 and 4) were selected from 2 herds and submitted for diagnosis of the intermittent diarrhea. Two weaned pigs (numbers 5 and 6), aged 55 to 67 d old, from different herds were also submitted because of retardation of growth accompanied by diarrhea. Antibiotic theraphy did not show any beneficial results in the 6 cases. All pigs were submitted alive and, immediately upon receipt, they were euthanized for necropsy by electrocution. Samples collected from lung, heart, inguinal lymph node, tonsil, thymus, spleen, small and large intestines, liver, kidney, and pancreas were fixed in 10% (w/v) buffered formaldehyde for 24 h and embedded in paraffin by standard histologic procedures. Small and large intestines from the 6 weaned pigs were examined for viral pathogens such as PCV2, porcine parvovirus (PPV), porcine reproductive and respiratory syndrome virus (PRRSV), and classical swine fever virus (CSFV) and for bacterial pathogens such as Salmonella sp., Brachyspira pilosicoli, and Brachyspira hyodysenteriae. Homogenates of the small and large intestines were inoculated into cultures of PCV-free PK-15 cells, MARC-145 cells, swine testicular cells, primary porcine fallopian tube cells, and Vero cells. In situ hybridization was also performed for PCV2, PRRSV, PPV, and CSFV, as previously described (14,19,20). Positive and negative control tissues for each virus were included by using an in situ hybridization procedure (14,19,20). Polymerase chain reaction (PCR) was carried out as previously described for Lawsonia intracellularis, B. pilosicoli, and B. hyodysenteriae (21–23).
The most consistent and predominant histopathological feature of the diarrheic weaned pigs was granulomatous inflammation and lymphoid depletion in the Peyer’s patches in the small and large intestines. The granulomatous inflammation was characterized by infiltrates of epithelioid cells and giant multinucleated cells (Figure 1). Another typical histopathological feature was the presence of intracytoplasmic inclusion bodies. Large, multiple, basophilic or amphophilic, grape-like intracytoplasmic inclusion bodies were often seen in the cytoplasm of histiocytic cells and giant multinucleated cells. In 2 of the pigs (numbers 4 and 5), the cecum and colon were intensively involved with multifocal necrosis and ulceration with fibrin and neutrophils exuding from the ulcerated areas into the lumen. No microscopic lesions were seen in other organs. No organisms were observed within any of the granulomatous lesions by Ziehl-Neelsen and Grocott’s methenamine silver stains.
Figure 1.
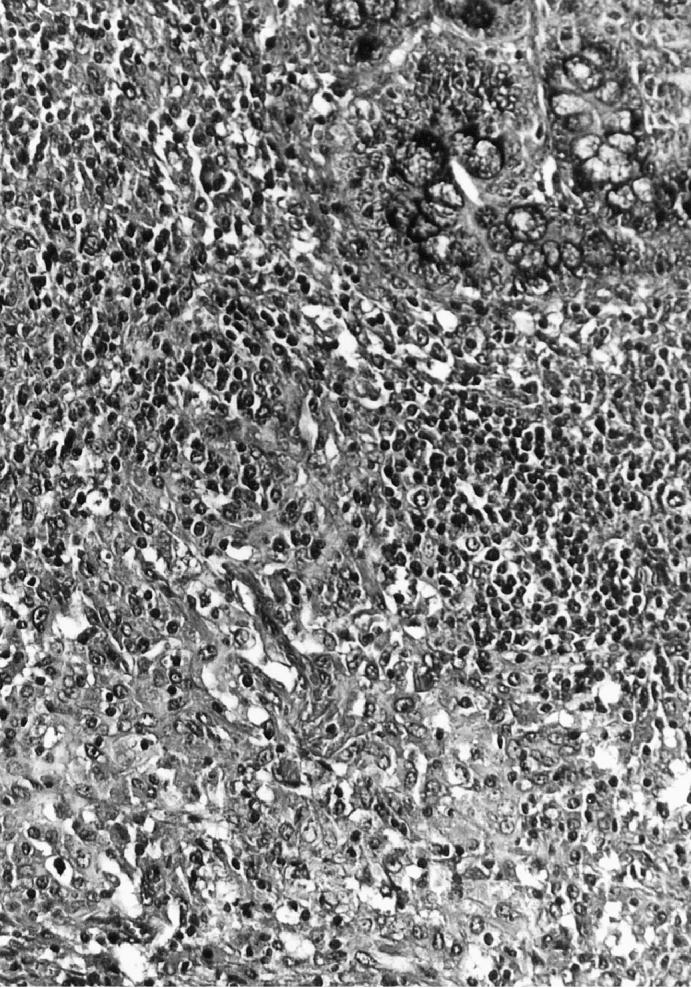
Section of a small intestine from a pig with granulomatous enteritis. A cluster of histiocytes and giant multinucleated cells are seen in Peyer’s patches. Hematoxylin & eosin. 200 ×.
A hybridization signal for PCV2 was detected in Peyer’s patches from all 6 weaned pigs. A strong hybridization signal for PCV2 was detected in the cytoplasm of histiocytes and giant multinucleated cells in Peyer’s patches (Figure 2). Occasionally, PCV2 DNA was also detected in the normal lymph node from 2 of the weaned pigs (numbers 1 and 4). No hybridization signal was seen in tissue sections pretreated with DNase. The probes for PPV, PRRSV, and CSFV were consistently negative in all tissues from the 6 weaned pigs.
Figure 2.
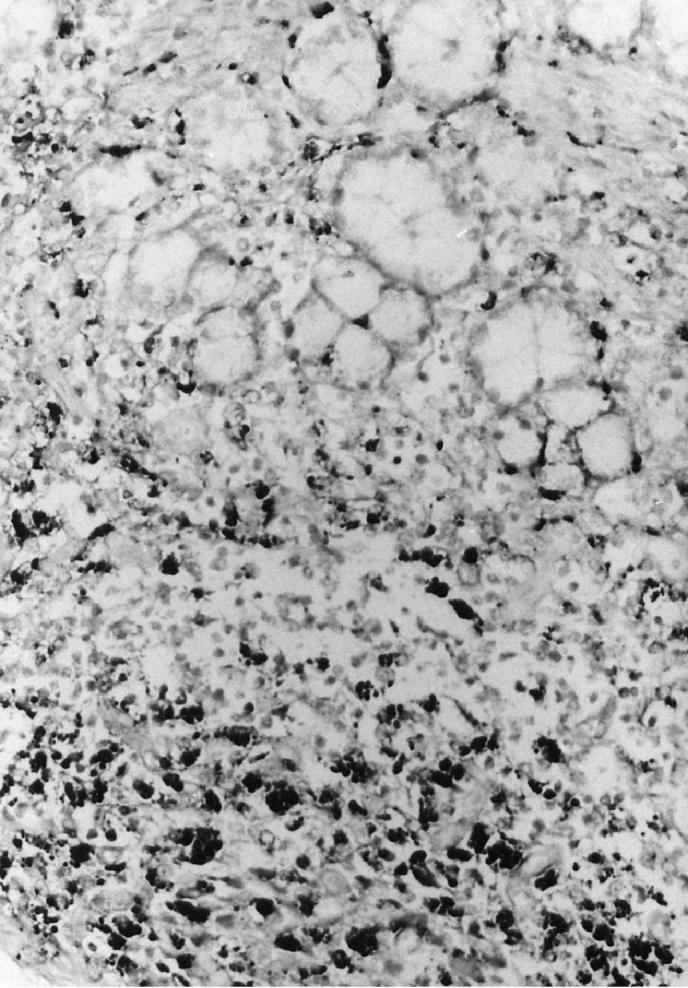
Section of a small intestine from a pig with granulomatous enteritis. Porcine circovirus 2 (PCV2) DNA is detected in macrophages by in situ hybridization. 200 ×.
Lawsonia intracellularis, B. pilosicoli, and B. hyodysenteriae were not detected in the small and large intestines by PCR. Salmonella typhimurium was isolated from the colon of 1 weaned pig (number 5). No other bacterium was isolated from the small and large intestines. Cytopathic viruses were not detected after 5 passages. Porcine circovirus 2 was isolated from the homogenates of small and large intestines in 2 weaned pigs (numbers 2 and 3) and confirmed by in situ hybridization of infected cell cultures, as previously described (24).
A small amount of PCV2 DNA was detected in the lymph node without any microscopic lesions in 2 of the weaned pigs (numbers 2 and 3). However, identification of PCV2 may not be significant since PCV2 can be detected in the lymph nodes from infected pigs without clinical PMWS or PCV2-associated disease by PCR and in situ hybridization (25). Therefore, the diagnosis of PCV2-associated enteritis was made on the basis of 3 criteria: (i) presence of diarrhea; (ii) presence of characteristic microscopic lesions in the Peyer’s patches but not in the lymph node; and (iii) presence of PCV2 within these lesions. These 3 criteria individually are nondiagnostic of PCV2-associated enteritis. Porcine circovirus 2 associated enteritis was not diagnosed if lymphoid tissues were not submitted with the case.
Results of the in situ hybridization suggested that PCV2 primarily replicates in macrophages of the Peyer’s patches. Lymphoid depletion by replicating PCV2 is an indication of a direct or indirect pathogenic effect of the virus. Such microscopic changes may have an adverse affect on the antibacterial defence of the intestine and possibly predispose pigs to common secondary bacterial infections.
The presence of diarrhea and granulomatous enteritis and colitis, and abundant PCV2 DNA associated with the microscopic lesions was suggestive of PCV2-associated enteritis. Porcine circovirus 2 associated enteritis should be differentiated from PMWS clinically and histopathologically because of considerable diagnostic overlap between the 2 conditions. Granulomatous inflammation and lymphoid depletion were not seen in the lymph nodes from all 6 of the weaned pigs. Moreover, concurrent outbreak of PMWS or PDNS was not observed in these herds. Thus, PCV2-associated enteritis could be a distinct clinical manifestation of PCV2. Porcine circovirus 2 associated granulomatous enteritis should be considered as a differential diagnosis for grow-finish pigs with antibiotic non-responsive diarrhea. Further studies are needed to define the role of PCV2 in granulomatous enteritis in swine.
Acknowledgments
The research reported here was supported by the Ministry of Agriculture, Forestry and Fisheries-Special Grants Research Program (MAFF-SGRP), and Brain Korea 21 Project, Republic of Korea.
References
- 1.Todd D, Niagro FD, Ritchie BW, et al. Comparison of three animal viruses with circular single-stranded DNA genomes. Arch Virol. 1991;117:129–135. doi: 10.1007/BF01310498. [DOI] [PubMed] [Google Scholar]
- 2.McNulty M, Dale J, Lukert P, et al. Circoviridae. In: van Regenmortel MHV, Fauquet CM, Bishop DHL, et al, eds. Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press, 2000:299–303.
- 3.Mankertz A, Hattermann K, Ehlers B, Soike D. Cloning and sequencing of columbid circovirus (CoCV), a new circovirus from pigeons. Arch Virol. 2000;145:2469–2479. doi: 10.1007/s007050070002. [DOI] [PubMed] [Google Scholar]
- 4.Meehan BM, Creelan JL, McNulty MS, Todd D. Sequence of porcine circovirus DNA: affinities with plant circoviruses. J Gen Virol. 1997;78:221–227. doi: 10.1099/0022-1317-78-1-221. [DOI] [PubMed] [Google Scholar]
- 5.Niagro FD, Forsthoefel AN, Lawther RP, et al. Beak and feather disease virus and porcine circovirus genomes: intermediates between the geminiviruses and plant circoviruses. Arch Virol. 1998;143:1723–1744. doi: 10.1007/s007050050412. [DOI] [PubMed] [Google Scholar]
- 6.Phenix KV, Weston JH, Ypelaar I, et al. Nucleotide sequence analysis of a novel circovirus of canaries and its relationship to other members of the genus Circovirus of the family Circoviridae. J Gen Virol. 2001;82:2805–2809. doi: 10.1099/0022-1317-82-11-2805. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie BW, Niagro FD, Luckert PD, et al. Characterization of a new virus from cockatoos with psittacine beak and feather disease. Virology. 1989;171:83–88. doi: 10.1016/0042-6822(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 8.Todd D, Weston JH, Soike D, Smyth JA. Genome sequence determinations and analyses of novel circoviruses from goose and pigeon. Virology. 2001;286:354–362. doi: 10.1006/viro.2001.0985. [DOI] [PubMed] [Google Scholar]
- 9.Todd D, Creelan JL, Mackie DP, et al. Purification and biochemical characterization of chicken anaemia agent. J Gen Virol. 1990;71:819–823. doi: 10.1099/0022-1317-71-4-819. [DOI] [PubMed] [Google Scholar]
- 10.Allan GM, McNeilly F, Cassidy JP, et al. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet Microbiol. 1995;44:49–64. doi: 10.1016/0378-1135(94)00136-k. [DOI] [PubMed] [Google Scholar]
- 11.Krakowka S, Ellis JA, Meehan B, et al. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet Pathol. 2000;37:254–263. doi: 10.1354/vp.37-3-254. [DOI] [PubMed] [Google Scholar]
- 12.Tischer I, Rasch R, Tochtermann G. Characterization of papovavirus- and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl Bakteriol Mikrobiol Hyg Ser A. 1974;226:153–167. [PubMed] [Google Scholar]
- 13.Allan GM, Ellis, JA Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Choi C, Chae C. Pathogenesis of postweaning multisystemic wasting syndrome reproduced by co-infection with Korean isolates of porcine circovirus 2 and porcine parvovirus. J Comp Pathol. 2003;128:52–59. doi: 10.1053/jcpa.2002.0605. [DOI] [PubMed] [Google Scholar]
- 15.Choi C, Chae C. Colocalization of porcine reproductive and respiratory syndrome virus and porcine circovirus 2 in porcine dermatitis and nephropathy syndrome by double-labeling techniqe. Vet Pathol. 2001;38:436–441. doi: 10.1354/vp.38-4-436. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Chae C. Concurrent presence of porcine circovirus type 2 and porcine parvovirus in retrospective cases of exudative epidermitis in pigs. Vet J. 2004;167:104–106. doi: 10.1016/j.tvjl.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Chung HK, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. 2003;166:251–256. doi: 10.1016/s1090-0233(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor B, Gauvreau H, West K, et al. Multiple porcine circovirus 2-associated abortion and reprodutive failure in a multisite swine production unit. Can Vet J. 2001;42:551–553. [PMC free article] [PubMed] [Google Scholar]
- 19.Choi C, Chae C. Localization of classical swine fever virus from chronically infected pigs by in situ hybridization and immunohistochemistry. Vet Pathol. 2003;40:107–113. doi: 10.1354/vp.40-1-107. [DOI] [PubMed] [Google Scholar]
- 20.Cheon D-S, Chae C. Comparison of virus isolation, reverse transcription-polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine reproductive and respiratory syndrome virus from naturally aborted fetuses and stillborn piglets. J Vet Diagn Invest. 2000;12:582–587. doi: 10.1177/104063870001200619. [DOI] [PubMed] [Google Scholar]
- 21.Kim O, Kim B, Chae C. Prevalence of Lawsonia intracellularis in selected pig herds in Korea as determined by PCR. Vet Rec. 1998;143:587–589. doi: 10.1136/vr.143.21.587. [DOI] [PubMed] [Google Scholar]
- 22.Choi C, Han DU, Kim J, et al. Prevalence of Brachyspira pilosicoli in Korean pigs, determined using a nested PCR. Vet Rec. 2002;150:217–218. doi: 10.1136/vr.150.7.217. [DOI] [PubMed] [Google Scholar]
- 23.Elder RO, Duhamel GE, Mathiesen MR, et al. Multiplex polymerase chain reaction for simultaneous detection of Lawsonia intracellularis, Serpulina hyodysenteriae, and salmonellae in porcine intestinal specimens. J Vet Diagn Invest. 1997;9:281–286. doi: 10.1177/104063879700900309. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Chae C. Differentiation of porcine circovirus 1 and 2 in formalin-fixed, paraffin-wax-embedded tissues from pigs with postweaning multisystemic wasting syndrome by in-situ hybridisation. Res Vet Sci. 2001;70:265–269. doi: 10.1053/rvsc.2001.0471. [DOI] [PubMed] [Google Scholar]
- 25.Calsamiglia M, Segales J, Quintana J, et al. Detection of porcine circovirus types 1 and 2 in serum and tissue samples of pigs with and without postweaning multisystemic wasting syndrome. J Clin Microbiol. 2002;40:1848–1850. doi: 10.1128/JCM.40.5.1848-1850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


