Abstract
Purpose
This study aimed to explore the inaugural experience of using a continuous glucose monitoring (CGM) system in patients with type 2 diabetes.
Patients and Methods
This study employed a qualitative design. Thirty-one patients with type 2 diabetes were recruited from a national university hospital and underwent CGM for two weeks. Individual interviews with 28 participants were conducted between August 1 and October 17, 2022, after the CGM. Thematic analysis was used to examine the data.
Results
The results revealed transformative shifts in aspects of participants’ lives due to CGM use, including alterations in dietary management and interpersonal relationships. During the two-week journey with CGM, participants were able to visually observe exercise effects and other benefits, leading to the discovery of a new utility for this innovative medical device. However, unavoidable drawbacks such as high cost, inaccurate results, and skin irritation have been identified, prompting suggestions for improvement.
Conclusion
This study determined that CGM is both feasible and valuable for facilitating lifestyle adjustments to manage diabetes. Nevertheless, the challenge of discomfort associated with CGM use should be addressed in the future. To ensure effective utilization and overcome potential obstacles, it is recommended that a comprehensive and user-friendly CGM education manual be created, with the scope of CGM insurance coverage extended to include this research in the future.
Keywords: diabetes mellitus, type 2, blood glucose self-monitoring, continuous glucose monitoring, qualitative research
Introduction
Globally, approximately 537 million people suffer from diabetes, with projections indicating an increase to 643 million by 2030 and 783 million by 2045.1 The prevalence of diabetes in South Korea has markedly increased from 11.8% in 2012 to 16.7% in 2020. Patients with type 2 diabetes who experience prolonged poor glycemic control and inadequate self-management may exhibit hypoglycemia risks and glycemic variability patterns similar to those observed in type 1 diabetes.2 Complications of diabetes include both macrovascular and microvascular complications such as diabetic foot lesions, necessitating regular blood glucose monitoring for prevention and management.3 A five-year follow-up study of 1124 patients with type 2 diabetes from nine general hospitals in South Korea revealed that among lipid, glucose, blood pressure, and weight management, blood glucose monitoring and management were the most deficient.4 The American Association of Diabetes Educators delineates seven components of diabetes self-management—“healthy eating”, “physical activity”, “blood glucose monitoring”, “medication”, “problem-solving”, “healthy coping”, and “reducing risks of diabetes complications”—and particularly emphasizes the importance of systematic education for patients with diabetes on the use of continuous glucose monitoring systems.5
CGM systems eliminate the inconvenience and pain associated with frequent finger pricking required for self-monitoring of blood glucose (SMBG), accurately reflecting the overall state, variability, and hypoglycemic responses of blood glucose levels.2 A CGM system, a device that attaches a sensor to the skin to measure interstitial glucose levels in real time, allows for continuous tracking of blood glucose trends. This enables individuals to understand their personal glucose patterns in response to daily activities such as diet and exercise, thus aiding glucose control. A meta-analysis of 15 randomized clinical trials, including 2461 patients with type 1 and type 2 diabetes, found that CGM usage led to a reduction in HbA1c levels and a significant increase in the time in range (TIR, 70–180 mg/dl).6 CGM also reduces the risk of hypoglycemia, increases treatment satisfaction, and proves beneficial during periods requiring short-term glycemic improvement, such as the initiation of insulin therapy, medication changes, dietary and lifestyle education, exercise, and hospitalization.2,7,8 The American Diabetes Association (ADA) recommends CGM for patients with type 1 diabetes over 65 years of age or those with hypoglycemia unawareness, frequent hypoglycemia, or at risk of hypoglycemia, as well as exceeding their HbA1c targets. Furthermore, they advise its use for all patients with diabetes willing to wear the device, regardless of diabetes type, age, or duration.9 The global trend shows an increasing use of CGM, which is recommended for all adults, children, and adolescents with type 1 diabetes, and for patients with type 2 diabetes receiving multiple daily insulin injections.2 In South Korea, insurance coverage for CGM electrodes in patients with type 1 diabetes commenced in January 2019. However, according to domestic statistics from 2020, only 7.3% of patients with type 1 diabetes have ever been prescribed CGM, and while recent expansions in insurance coverage have potentially increased this to at least 15%, the number of patients continuously using CGM is likely to be much lower.2
The ADA emphasizes the necessity of providing continuous education and training to patients with diabetes when they first start using CGM systems and throughout their usage.10 The experiences of CGM users vary. A study that interviewed 23 patients with type 1 diabetes in Norway about their CGM experiences revealed that while participants recognized CGM as an effective and important tool in diabetes management, enhancing their daily life and satisfaction with treatment, they also found its use cumbersome and challenging.11 In a study of 25 German working professionals, including patients with type 1 diabetes and those with type 2 diabetes undergoing insulin therapy, it was reported that CGM allowed for less disruptive and more focused work environments as it enabled them to monitor their blood glucose levels without disclosing their diabetes diagnosis to colleagues.12 Additionally, a study in the United States involving 11 elderly individuals aged over 65 years using real-time CGM reported that users experienced a sense of safety regarding hypoglycemia.13
Understanding the experiences and perspectives of CGM users is crucial to alleviate the stress and barriers faced by patients using CGM and enhance their self-efficacy in its use. However, research conducted on CGM usage experiences has primarily been limited to a few countries such as Norway, Germany, and the United States, and the participants have predominantly been parents of children and adolescents with diabetes, working professionals, and elderly patients with diabetes.12–14
Research on CGM in South Korea, especially concerning user experiences, is in its early stages. A review of 68 papers from the past 20 years indicates that the focus has predominantly been on device development and validating health outcomes of CGM use.15 The role of healthcare professionals is vital for the efficient use of CGM.16 Especially with the increasing use of CGM among patients with diabetes, discussions on education and counseling about the operation and precautions of CGM, resolving barriers, efficient use, and issues arising in various clinical situations are important areas in nursing, aiming for self-management and capacity development of patients with diabetes. Therefore, this study aims to understand the experiences of adult patients with type 2 diabetes who are first-time users of Continuous Glucose Monitors (CGM). By elucidating these experiences, we intend to provide foundational data crucial for the future development of targeted CGM educational materials and programs that enhance glucose management. This refinement underscores the necessity of the research and directly addresses how it contributes to improving diabetes educational initiatives.
Materials and Methods
Research Design
This qualitative study explored the main question of how adults with type 2 diabetes experienced using continuous glucose monitoring (CGM) for the first time.
Participants
The participants in this study were individuals diagnosed with type 2 diabetes who had never used a CGM system. The specific selection criteria included: 1) age between 19 and 69 years, 2) administration of insulin injections at least once a day or oral administration of glucose-lowering agents, and 3) consent to use CGM and participate in the study. The exclusion criteria were as follows: 1) individuals who had not surpassed two weeks after the attachment of the CGM device and 2) individuals unable to use smartphone applications.
Introduction to CGM
In this study, the CGM system used is the FreeStyleⓇLibreTM flash glucose monitoring system by Abbott (Chicago, IL, USA). Unlike other sensors, the Libre is calibrated such that it does not require self-glucose correction by the user and can be used for a duration of two weeks. The CGM system consists of a sensor that measures glucose levels in the interstitial fluid between cells under the skin, a transmitter that sends these measurements, and a monitor that receives and displays these values. The accuracy of recently developed CGM systems has improved to a level comparable with that of self-monitoring blood glucose devices. However, because they measure glucose concentration in the interstitial fluid rather than in the capillary blood, there is a known delay of 5–15 minutes in reflecting actual blood glucose levels, with variations among patients. This delay tends to be more significant in adults than in children and varies among different devices (Figure 1).17
Figure 1.
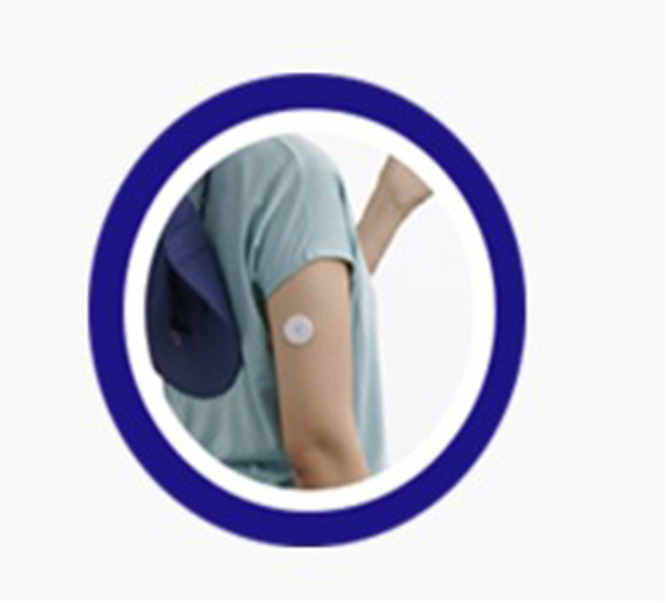
This is an image of a CGM device attached to an arm.
Notes: Adapted with permission from Adela Healthcare Co., Ltd; https://www.diabetesmall.co.kr/main/index.php.18
Recruitment of Study Participants
For the recruitment of study participants, a call for participants was posted from August 1, 2022, to October 17, 2022, at the endocrinology outpatient department and on the notice boards of Jeonbuk University Hospital. Patients who showed interest upon seeing the notice were provided with detailed explanations of the research objectives and methods, and their questions were answered before obtaining their consents. Those who agreed to participate were assessed for their ability to integrate a smartphone into the CGM system. Information including gender, age, highest level of education, occupation, number of cohabitants, duration of diabetes diagnosis, HbA1c levels, and presence of any complications was collected. Participants meeting the study selection criteria were included in the study.
CGM Application and Precautions
Before applying the CGM system, participants who consented to participate in the study were educated on the principles, precautions, and usage methods of CGM using educational materials and library training videos in the endocrinology consultation room by the research staff. After attaching the system, the Libre application was downloaded on the participant’s smartphone, an account was created under the participant’s name, and it was set up to be linked to a professional account. After CGM education, participants were instructed on how to maintain a daily CGM usage diary (freely recording meal types, blood glucose results, exercise amount, and user feedback) and were given the option to choose between paper and digital formats. One hour after installing the application, we checked whether the app was properly linked and whether the CGM device functioned correctly. Participants were then given a pack of glucose candy (sports glucose candy for hypoglycemia prevention), and they signed off and returned home.
During the CGM usage period, to consult on any arising issues, phone calls and texts were sent to participants on days #2, #6, and #11 of usage, with a specific call on day #6 to check on the diary maintenance. Additionally, participants were informed that they could contact the researcher at any time if they experienced discomfort, bleeding, or infection while wearing the CGM device. Monitoring for any issues during use, an interview date was set two weeks later at a convenient time for the participant. During the visit two weeks later, participants submitted the usage diaries they had maintained. Within five days of removing the device, a personal interview was conducted, and a small gift voucher was provided as a token of appreciation for participation.
Data Collection Methods
In the study, two trained interviewers conducted the interviews. Initially, a pilot interview was carried out to refine and standardize the interview guide. All interviews were conducted in a quiet and comfortable place of the participants’ choice to facilitate relaxed sharing of their experiences. Open-ended questions were posed to encourage participants to comfortably share their experiences, and attention was paid to their nonverbal responses, expressions, and attitudes, with notes taken. Consent for recording was obtained to capture the participants’ words verbatim. At the beginning of the interview, participants were prompted to talk about “how they have been doing recently” and “their feelings about their diabetes”. The main questions focused on their experiences using the CGM system, changes in their daily lives, and perceived benefits and challenges. The specific list of questions and topic of interviews is provided in the Table 1. In addition to the interview recordings, 27 participants’ usage diaries were utilized as qualitative data along with the interview materials. Individual interviews lasted from a minimum of 30 min to a maximum of 67 min, with a total of 1280 min of interviews conducted across 28 participants.
Table 1.
Interview Questions
| Phase | Topic | Example Questions |
|---|---|---|
| Introduction | Introduction |
|
| Main Interview | Challenges |
|
| Changes in daily lives |
- Can you describe any specific changes in your work, school, or home life? - How did using the CGM affect your diet, exercise, etc.? - Has there been any change in your medication or insulin usage since using the CGM? |
|
| Benefits |
|
|
| Feeling |
|
|
| Suggestions |
|
|
| Closing | Closing |
|
Data Analysis
Following each interview, the researcher transcribed the recordings to obtain 685 pages of data in A4 format. The CGM usage diary submitted by participants were integrated with interview data. The analysis was conducted using the thematic qualitative analysis method by Hsieh and Shannon,19 following these steps: 1) The transcribed data were repeatedly reviewed to grasp the overall meaning of the content. 2) Significant sentences or phrases were used to generate codes that were marked while reviewing the data. 3) The generated codes were grouped into subcategories based on differences and associations. 4) Subcategories were further classified into higher categories based on their differences and relationships. 5) The relationships between codes, subcategories, and categories were repeatedly compared to name the categories. Through this process, main themes were identified. Two researchers, who conducted the interviews, collaboratively analyzed the data to accurately capture and articulate the emergent themes, ensuring the expressions were closely aligned with the participants’ experiences. To validate our findings, drafts of the analysis were reviewed multiple times by an endocrinologist with extensive experience in diabetes care and a professor with a background in qualitative research. The researcher led the data analysis, and upon completion, many times of meeting were held with a qualitative research expert to review the categories and themes, until finalizing the data analysis. Analysis was conducted using the qualitative data analysis software, Atlas. ti version 24.
Robustness
This study applied the criteria of credibility, consistency, applicability, and neutrality proposed by Guba and Lincoln to evaluate rigor.20 To ensure data credibility, interviews were conducted in a natural and comfortable atmosphere using open-ended questions. The recordings were transcribed verbatim to reflect the participants’ experiences accurately. To ensure consistency, cyclic data collection and analysis were conducted while considering the research themes and key questions. To include the diverse experiences of participants with different diabetes backgrounds and to establish neutrality, the data provided by participants, literature materials, and the researcher’s preconceptions were clearly distinguished. Additionally, to enhance reliability, participant review methods were applied to ensure the analysis results accurately reflected participants’ experiences and thoughts.
Ethical Considerations
This study was approved by the Institutional Review Board (IRB) of Jeonbuk University Hospital (IRB No. 2022-05-017-004). Before the interview commenced, participants who voluntarily expressed their willingness to participate were thoroughly informed about the study’s purpose and methods, the duration of involvement, and the assurances of anonymity. They were also informed about the use of recording devices during the interview, their right to withdraw from the study at any time without consequences, the potential benefits and disadvantages of their participation, and the possibility of their anonymized direct quotes being published. Consent was obtained voluntarily, and a consent form was completed. The interview was then conducted. Participants were informed that data would be stored for three years after the conclusion of the study and would be destroyed thereafter, in accordance with the Declaration of Helsinki. At the end of the interview, a small honorarium was given to express gratitude for their participation in the study.
Results
Characteristics of Study Participants
A total of 31 participants initially participated in the study and were fitted with the CGM device. However, three participants (#10 (difficulty due to mental illness), #26 (removed the sensor), and #29 (withdrew voluntarily)) dropped out of the study, resulting in 28 participants. Of them, 17 (60.7%) were female and 11 (39.3%) were male, with an average age of 51.4 years. Educationally, 14 participants (50.0%) had completed high school and 11 (39.3%) had higher education. Twelve participants (42.9%) were unemployed, 23 (82.1%) were health insurance subscribers, and 4 (14.3%) were medical aid type 1 beneficiaries. The participants had been diagnosed with diabetes for an average of 11 years; 9 (32.1%) did not check their blood sugar regularly, and the average HbA1c level was 8.60%. (Table 2)
Table 2.
Characteristics of All Participants (N=31)
| ID | Sex | Age | Education Level | Employed | Domestic Partners | Duration of Diabetes (years) | SMBG (times/day) | Complication or Chronic Illness | HbA1c (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 21 | High school | No | 3 | 4 | 1 | 8.3 | |
| 2 | Female | 58 | College or more | Yes | 0 | 16 | 0 | 7.5 | |
| 3 | Female | 37 | College or more | No | 3 | 3.5 | 0 | Hypertension | 6.5 |
| 4 | Male | 56 | High school | No | 3 | 3 | 2 | Hypertension | 7.8 |
| 5 | Female | 51 | High school | No | 0 | 10 | 1 | Hypertension | 8.8 |
| 6 | Female | 54 | High school | Yes | 3 | 22 | 1 | Diabetic retinopathy | 9.8 |
| 7 | Male | 35 | High school | Yes | 3 | 3 | 0 | Hypertension | 9.6 |
| 8 | Male | 52 | High school | No | 3 | 7.5 | 2 | Hypertension | 13.8 |
| 9 | Male | 58 | High school | Yes | 4 | 20 | 2 | Hypertension | 6.7 |
| 10 | Male | 36 | College or more | No | 2 | 9 months | 1 | Hypertension | 17.7 |
| 11 | Female | 59 | Middle school | Yes | 1 | 4 | 4 | 10.7 | |
| 12 | Female | 57 | High school | Yes | 1 | 15 | 4 | Hypertension | 11.8 |
| 13 | Female | 24 | College or more | No | 1 | 10 months | 2 | Fatty liver | 9.0 |
| 14 | Male | 55 | College or more | Yes | 4 | 1 | 2 | 9.1 | |
| 15 | Female | 56 | High school | No | 1 | 13 | 3 | 8.8 | |
| 16 | Female | 55 | College or more | No | 2 | 15.6 | 4 | Leg tingling | 5.9 |
| 17 | Male | 49 | College or more | Yes | 2 | 16.8 | 1 | Hypertension | 5.4 |
| 18 | Male | 55 | High school | No | 0 | 2 | 2 | Hypertension | 7.0 |
| 19 | Male | 53 | College or more | Yes | 1 | 25 | 0 | Stroke | 9.9 |
| 20 | Female | 52 | College or more | Yes | 1 | 3 | 0 | Diabetic retinopathy | 7.6 |
| 21 | Female | 60 | Elementary School | No | 1 | 15 | 4 | Hypertension | 7.3 |
| 22 | Female | 59 | High school | Yes | 3 | 13 | 1 | Hypertension | 7.3 |
| 23 | Female | 47 | High school | Yes | 2 | 15 | 0 | 6.8 | |
| 24 | Male | 59 | College or more | Yes | 1 | 5 | 0 | 8.0 | |
| 25 | Male | 55 | High school | Yes | 3 | 10 | 1 | 8.0 | |
| 26 | Male | 29 | College or more | Yes | 0 | 2 months | 0 | 8.8 | |
| 27 | Female | 54 | High school | Yes | 1 | 15 | 0 | Macular edema | 10.1 |
| 28 | Male | 67 | None | No | 1 | 30 | 0 | Cerebrovascular disease | 9.0 |
| 29 | Male | 72 | High school | No | 0 | 20 | 0 | Chronic heart disease | 10.3 |
| 30 | Female | 51 | College or more | Yes | 3 | 3 | 1 | Hypertension | 6.7 |
| 31 | Female | 38 | College or more | Yes | 2 | 4 | 2 | 8.1 |
Notes: Dropped: 10, 26, and 29.
Abbreviations: HbA1c, glycated hemoglobin; SMBG, self-monitoring of blood glucose.
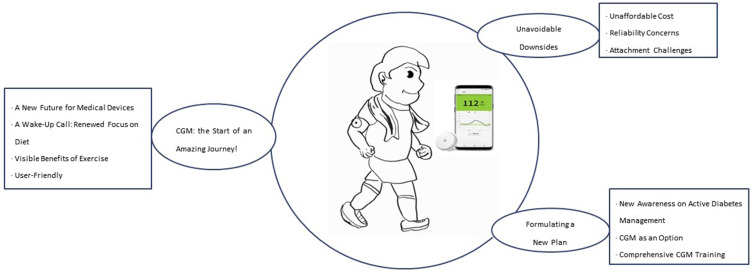
Analysis of the data collected through the individual interviews yielded 70 codes. These 70 codes were categorized into three categories under the theme “A Two-Week Journey with CGM”: “CGM, the Start of an Amazing journey!”; “Unavoidable Downsides”; and “Formulating a New Plan”. The journey of the participants spanned from their first encounter with diabetes to a two-week journey with CGM and to the process of establishing a new diabetes management plan (Figure 2).
Figure 2.
This illustration provides a brief summary of the first experience of a patient with type 2 diabetes using a CGM (Continuous Glucose Monitoring) system. It depicts a person actively exercising with a CGM device attached.
Notes: Smartphone image at the center adapted with permission from Adela Healthcare Co., Ltd; https://www.diabetesmall.co.kr/main/index.php.18
Abbreviation: CGM, Continuous Glucose Monitoring.
CGM, the Start of an Amazing Journey!
A New Future for Medical Devices
The evolution of medical devices has led to significant advancements. The mere existence of a blood sugar measuring sensor is astonishing, and its greatest advantage is the convenience of measurement. Being able to check blood sugar without the pain of pricking one’s finger, even while asleep, felt like a dream come true. This technology, which allows blood sugar levels to be ascertained anytime and anywhere using a phone, is especially helpful during travel. The device, capable of responding quickly in situations of hypo- or hyperglycemia, transcended a mere mechanical function to become a health partner. Most of the participants (25 out of 28) expressed gratitude for the opportunity to encounter such a new machine, and many mentioned that this experience prompted them to think more deeply about the management of diabetes.
Above all, it’s so convenient, and I can check it immediately whenever I need to, so I really want to give it a high score. It’s really great, not having to prick my fingers all the time, being able to check it whenever I feel a bit off, not just when fasting or before and after meals. Anytime I want, and that’s what I really liked about it. (Participant 12, Female, 57 years old, 15 years with diabetes)
I really loved this device. (laughs) Until now, I just thought taking medicine was enough and didn’t manage it at all. Even when told ‘it’s dangerous,’ since I wasn’t immediately feeling sick or dying, I was too complacent. After becoming complacent, using this and seeing my levels go over 200, I would get really shocked and start figuring out how to deal with it. (Participant 22, Female, 59 years old, 13 years with diabetes)
A Wake-Up Call: Renewed Focus on Diet
After attaching the CGM device, the participants restarted their dietary management, noting that their blood sugar levels had stabilized and were better controlled. They resisted the temptation of sugar with black coffee and sought the pure taste of vegetables and cabbage. They chose appropriate amounts of food for their health, even amid busy lives, and started managing their diet. With CGM, they adjusted food quantities more often, opting for balanced blood sugar over sweet fruits or bread. Twelve participants appreciated being able to measure blood sugar immediately after eating in order to understand which foods raised their blood sugar levels.
I almost stopped eating my favorite fruits or bread. So, I ate half a bowl of rice or half a serving of noodles, replacing the rest with vegetables. During these two weeks, I paid a lot of attention to what I ate. (Participant 24, Male, 59 years old, 5 years with diabetes)
While using this, I realized how much my blood sugar could rise and fall. After eating, I noticed that fruits really did increase it a lot. Then there’s instant food. I was surprised this time; I ate a sandwich, and it didn’t raise my levels. The same thing happened again. But rice cakes raised it significantly. As I saw, there’s a record here - fast food, instant food, rice cakes, but surprisingly, things like ramen didn’t raise it, even when I tried it again. (Participant 14, Male, 55 years old, 1 year with diabetes)
Visible Benefits of Exercise
Eleven participants observed the importance of exercise firsthand after wearing the CGM device, as their blood sugar levels decreased following physical activity. They felt that even simple exercises, such as post-meal walking or cycling, were beneficial for blood sugar management.
When I first used this (CGM) and my blood sugar was high, I would walk several rounds in the living room or ride the exercise bike at home for about 20 to 30 minutes, and then it would return to normal. When it’s high after eating, doing these simple exercises without needing to draw blood, and being able to quickly see the results on the device, made me realize the importance of exercising to take care of myself. It was really great. (Participant 22, Female, 59 years old, 13 years with diabetes)
User-Friendly
Half of the participants (14 out of 28) found the functionality and services of the CGM device extremely convenient, particularly appreciating the memo feature that allowed them to record changes in blood sugar levels in real-time, thereby gaining confidence in its accuracy. While there were some inconveniences associated with wearing the device, they were felt to be minor compared to the overall functionality and convenience.
I found it incredibly easy to use, almost as if this device was made especially for me (laughs). Because, initially, when going out or doing something, I used to carry a blood glucose meter with me all the time. But now, I’ve become familiar with the symptoms of high or low blood sugar, so I don’t carry it around much these days. But having this app, being able to use it anytime, anywhere, I found it tremendously convenient. (Participant 15, Female, 56 years old, 13 years with diabetes)
Unavoidable Downsides
Unaffordable Cost
Half of the participants (14 out of 28) mentioned the burdensome cost as a disadvantage of using CGM. The expense of approximately 200,000 KRW per month without insurance coverage was significant, especially because it was for checking blood sugar levels rather than for treatment. The cost was felt to be burdensome, even compared with regular test sticks, and many expressed that affording CGM was challenging. Numerous participants said they would use it if insurance coverage was provided, as both medication and CGM costs were heavy burdens.
Even when I know how to use it, paying 80,000–90,000 KRW for two weeks just to check blood sugar levels is too much of a financial burden. (Participant 22, Female, 59 years old, 13 years with diabetes)
Exactly! Even now, people I know think it’s expensive. I only got to experience this because it was part of the trial. If I had to pay for it, I probably wouldn’t have bought it. That’s what other people say too. If it was covered by insurance, something that costs 100,000 KRW could drop to 30,000 or 40,000 KRW. It would be better if there was insurance coverage for it. (Participant 7, Male, 35 years old, 3 years with diabetes)
Reliability Concerns
Approximately 11 participants reported significant discrepancies between readings from the CGM system and those from a self-monitoring blood glucose device, leading to reduced trust in the CGM system. The differences ranged from 20–40 mg/dl, with some participants experiencing variations as high as 100 mg/dl. Conversely, others initially skeptical about the accuracy of CGM later gained confidence as they found the difference in readings to be approximately 20–30 or quite similar.
At first, the difference was about 10 to 20, but as time went on, it became more inconsistent. I wrote down both the readings from the blood glucose meter and CGM daily, and the discrepancy was so significant that it made me doubt its accuracy. (Participant 15, Female, 56 years old, 13 years with diabetes)
Initially, I had a lot of doubts. The reason was that there was always a difference of about 20–30 between the readings I got from pricking my finger and those from the CGM. But after 2–3 days, I got curious and thought that since I had the sensor on one hand, I should try the other hand. When I did, the readings were the same. (Participant 12, Female, 57 years old, 15 years with diabetes)
The accuracy of measurement was emerged as a priority desire to improve trust in CGM for participants. The development of methods to reduce the discrepancy between SMBG and CGM readings was a predominant suggestion. Two participants mentioned that even if there were differences in readings, managing blood sugar levels in accordance with the CGM readings would not pose a significant issue.
The biggest thing, if I had to point out, is the difference in readings, but since I can’t do much about it, it’s inevitable for now. But maybe in the future, as the technology advances, this could be improved with more investment and development.(laughs). (Participant 13, Female, 24 years old, 10 months with diabetes)
Attachment Challenges
Participants reported encountering several challenges associated with the attachment of CGM to the skin. Nine participants experienced sensations such as imperceptible irritation or itching, typically between 3 and 6 days after the CGM device was attached. For some, the sensation at the attachment site felt like a needle piercing, whereas others likened it to a cool stream of water. The attachment of something to the body inevitably leads to these sensory changes. However, these sensations are sometimes perceived as discomfort or, at other times, as a part of the adaptation process.
When the attachment site is pressed, I felt a sensation like an electric current flowing, similar to what you mentioned. But after 2–3 days, when I stopped paying attention to it, I didn’t feel it anymore. (Participant 8, Male, 52 years old, 7 years and 6 months with diabetes)
During the use of CGM, nine participants experienced the device falling off. This was often because of occupations or situations involving heavy sweating, active movement, or carelessness while changing clothes. While some were concerned that the device would fall off while bathing or showering, others found that the adhesive was sufficiently strong to prevent this. Participants with shoulder pain, such as those with a frozen shoulder, experienced discomfort when attaching the device to their arm and suggested alternative attachment sites. There was also anxiety regarding the device falling off during sleep, and participants with sensitive skin at the attachment site experienced discomfort. In particular, during summer, the visibility of the CGM device with short-sleeved clothing led to self-consciousness of others’ gazes.
People who are more sedentary or office workers may not have much trouble, but those who are more active tend to see the device fall off. Despite good adhesion, it starts to peel off after approximately 4–5 days due to sweat. It seems comfortable for less active individuals to wear. (Participant 7, male, 35 years old, 3 years old, with diabetes)
There was a fear that it might fall off even while sleeping. Since I often wake up during the night, I check it around 12 AM, 1 AM, or 2 AM to see if it has become loose. Even while sleeping well, I check whether it is still securely attached. (Participant 2: female, 58 years old, 16 years old, with diabetes)
Participants experiencing skin discomfort hoped for alternative attachment methods beyond the current style, such as re-band styles, ingestible chips, and transformations into patches or bands that could last for a year once attached. Some suggested attaching a patch over the CGM device for more stable fixation, whereas others preferred a thinner and smaller design to prevent the device from catching on clothing during dressing or undressing. There were also suggestions for a less-protruding attachment site, making it easier to wear as a patch.
It will definitely get better. The device could be smaller, like the size of a round battery. Even just sticking a tape on it wouldn’t show much, and it wouldn’t be as inconvenient. It’s not too much of an inconvenience now, but a smaller size would definitely be better. (Participant 20, Female, 52 years old, 3 years with diabetes)
One area for improvement could be in the form of a patch or band. Medical device technology needs to evolve, but I think there’s a high possibility. Nowadays, there are devices that come in patch form, so if that could be improved. (Participant 14, Male, 55 years old, 1 year with diabetes)
Formulating a New Plan
New Awareness on Active Diabetes Management
Through their two-week experience with CGM, many participants (16 out of 28) gained a renewed sense of the seriousness of diabetes and the need for active management. Participants who had lived with diabetes for a long time had become complacent about their management; however, various experiences with CGM reignited the realization of its importance. The importance of diet management and exercise was re-emphasized during the two-week journey with CGM, and many planned to continue these practices to maintain a healthy life. Most participants gained confidence in dietary management while using CGM. However, not all patients could easily adjust their diets based on such experiences. Some participants found dietary control challenging, but felt that managing their diet became easier with CGM. There was also a sense of being monitored while wearing the CGM device, which facilitated self-management. Some participants planned consistent medication adherence and weight loss in the hope of enjoying a healthy and happy life without complications.
From now on, especially in this era where people live up to 100 years, I never thought it could happen to me, but seeing my brother suffer, I realized I could be in the same situation. That made me think that I need to manage my blood sugar levels more actively. (Participant 5, Female, 51 years old, 10 years with diabetes)
First of all, (laughs) I think I need to get back to exercising. I’ve been a bit hesitant because I’m moving, but for now, exercise is the plan. As for eating, (laughs) honestly, I find it hard to control. I can reduce the quantity, but not the variety. (laughs) So I plan to focus a lot on exercise. I have a plan to exercise vigorously. (laughs) (Participant 31, Female, 38 years old, 4 years with diabetes)
CGM as an Option
Some participants (12 out of 28) acknowledged CGM as an option for diabetes management after actively deciding to reengage in managing their condition during the study. Most were surprised by the advancements in medical technology that led to the development of CGM, especially the ability to measure blood sugar levels immediately after eating, which they considered a significant advantage. This helped them identify foods that caused their blood sugar levels to spike. Many participants were satisfied with the CGM device and expressed a desire to continue using it, even if it was not covered by insurance. However, a substantial number were concerned about the cost and indicated that their continued use of CGM depended on insurance coverage.
I really want to keep using this Libre continuously for my diabetes control. I feel that only by continuing can I manage my condition well! I can’t manage it just with a blood glucose meter that requires drawing blood. (Participant 22, Female, 59 years old, 13 years with diabetes)
It’s been really convenient; sometimes it shows 80 and so on. So, if the medical expenses are affordable, I really want to continue using it. That’s why I asked to use it again during the Chuseok holiday because I’d be moving around a lot. I really want to keep using it if it’s covered by medical insurance. (Participant 12, Female, 57 years old, 15 years with diabetes)
Comprehensive CGM Training
Nine participants emphasized the need for comprehensive education on the principles of CGM, application usage, and correct attachment methods. They believed that systematic education could reduce mistrust towards the device. Specifically, they felt the need for simple and easy-to-understand video materials for older adults and highlighted the importance of detailed explanations of attachment methods.
I generally like using IT devices, so I didn’t find it particularly difficult or have any aversion to it. Of course, I thought it might be a bit challenging for older people. Attachment definitely requires some training. That much I considered, but otherwise, for those who know how to handle IT devices to some extent, if they’re given a proper initial explanation, they should manage, right? If you buy it from a medical device store, they’ll help you with the attachment. It doesn’t seem too difficult to use, but if it’s sold online, then the attachment might be a bit more challenging for those not familiar with IT devices. (Participant 17, Male, 49 years old, 16 years and 8 months with diabetes)
Discussion
This qualitative study explored the experiences of patients with type 2 diabetes using CGM for the first time. The analysis of interviews and usage diaries revealed three subthemes: “CGM, the start of an amazing journey!”; “unavoidable downsides” and “formulating a new plan”.
The study participants perceived their engagement with CGM as a novel exploration of diabetes management, signifying a leap in the evolution of medical devices. In the context of contemporary digital healthcare, there is a clear trajectory towards personalized disease prevention and management services. This study underscores the transformative potential of such devices in enhancing patient autonomy and revolutionizing traditional approaches to disease management.21 The importance of personalized medicine for effective diabetes treatment was emphasized, and continuous glucose monitors (CGMs) are expected to become central wearable devices in personalized medicine and digital healthcare.22 CGM technology, including software applications, can be used to develop predictive models that identify individuals at high risk of developing diabetes.23
Through their use of CGM, participants began to actively manage their diets again, realizing the importance of meal control, as they could immediately see changes in their blood sugar levels after eating. A qualitative study found that changing eating habits is challenging, and family support plays a significant role in adherence to a diet.24 People with diabetes often view dietary management as a lifelong challenge, and factors related to dietary adherence in patients with diabetes include family support, self-motivation, and self-efficacy.25–27 CGM usage in patients with diabetes has been also suggested to improve physical activity levels and overall health.28 Thus, short-term CGM use can help individuals determine the right amount, timing, and method of exercise, and diet necessary for their blood sugar management and establish it as a habit.
However, there were also drawbacks to CGM use, such as high cost, measurement uncertainty, skin irritation, discomfort at the attachment site, and the device falling off easily. Many participants expressed concerns regarding the cost of CGM. A qualitative study with 13 Canadian participants also reported negative experiences related to high costs and discomfort due to the device’s location.29 As of April 2023, the National Health Insurance Service announced a research project to review detailed insurance criteria for diabetes management devices for patients with gestational and type 2 diabetes.30 There is hope that with future health insurance coverage, patients with type 2 diabetes will be able to use CGM more affordably and effectively.
Study participants also raised concerns about the uncertain accuracy of CGM measurements. Recently, Abbott introduced the FreeStyle Libre 3, acclaimed as the world’s smallest and thinnest sensor, which minimizes the sensation of a foreign body. This model facilitates real-time monitoring without scanning and is recognized for its high accuracy. As technology progresses, it is anticipated that accuracy concerns will diminish with ongoing improvements. Skin irritation has emerged as a major drawback of CGM.
Participants in the current study reported itching, pain, and discomfort at the attachment site. In the previous studies, mostly patients with type 1 diabetes, experiences skin problems such as rashes, skin irritation, discomfort at the attachment, and bleeding.31,32 Another drawback is the tendency of the device to easily fall off. Current solutions for skin hypersensitivity include applying “steroid spray” on the skin and using spray-type coating agents and tape-type skin protective films that reduce allergic reactions to the tape on the skin surface.33 It is important to explore various methods to resolve these issues and alleviate discomfort at attachment sites for participants. For improved convenience of attachment, most participants also preferred the sensor to be inserted in less visible areas. In this study, the sensor was attached to the arm, and some participants expressed concerns about it being noticeable, leading to questions about their diabetes. Future research should consider alternative attachment sites, such as the abdomen or thighs.
CGM has emerged as an important management option for patients with diabetes, allowing them to resume effective diabetes management. CGM can be used not only for continuous monitoring but also for understanding one’s glucose patterns, even if used intermittently. This could include applying CGM to patients with a BMI of 30 kg/m² or more or to patients with type 2 diabetes with an HbA1c level above 10.0% for a period of 2–4 weeks to help them understand and maintain their lifestyle habits. Despite the cost, many participants expressed a willingness to continue using CGM, highlighting the need for insurance coverage, education, interpretation, and management guidance for patients with type 2 diabetes. The participants experienced a new and convenient approach to managing diabetes during their two-week trial of CGM. There is a strong hope that all patients with diabetes worldwide will be able to use CGM as a viable option for diabetes management.
For prolonged use of CGM, participants called for comprehensive educational programs. The importance of education on CGM is growing, and diabetes education nurses are often responsible for providing CGM training. Opinions have suggested the use of video materials, as well as more straightforward and effective instructional methods. In South Korea, since June 2022, support for CGM-related counseling and education costs has begun; however, this is limited to patients with type 1 diabetes. Therefore, there is a need to develop systematic educational programs that meet the needs of all patients with diabetes and research to validate their effectiveness.
In our study, we observed significant variations in responses about the use and perception of CGM across different demographic groups. Younger participants adapted quickly to CGM technology, finding it easy to use, whereas older participants required additional support and detailed instruction. Females generally managed their diets more effectively and preferred discreet CGM placement, while males reported difficulties in diet management. Education level further influenced the ability to interpret and utilize CGM data, with higher education correlating with better utilization and comprehension of the technology. Participants diagnosed with diabetes for long years (about more than 15 years) showed a high level of engagement with their health management routines and expressed a strong interest in using CGM. These insights highlight the need for tailored educational and support strategies to optimize CGM use across diverse demographic profiles.
The strength of this study is that it is the first in South Korea to explore the use of CGM among patients with type 2 diabetes. It emphasizes the use of CGM as a tool for diabetes management, fostering a greater interest in CGM among healthcare providers and patients with diabetes, and promoting advancements in self-management of the condition. However, the study had some challenges faced by participants in maintaining a usage diary, potentially leading to the loss of valuable insights into their daily emotions and experiences. The study was conducted during the summer, which might have affected the results because of issues such as sweating or humidity causing the CGM devices to detach more easily.
This study has several limitations. First, the exclusion of individuals who discontinued the use of CGM within the first two weeks may have omitted potentially important insights, particularly regarding reasons for stopping early. This restriction potentially limits the generalizability of our findings. Additionally, the initial two-week period of CGM use provides a limited perspective on user experiences and may not fully capture changes in opinions that could develop with longer usage. Future studies should consider extended monitoring periods and include follow-up interviews with participants who discontinue use to enhance the robustness and generalizability of the results. Another limitation of this study is the absence of data concerning hypoglycemia, which is a critical aspect of diabetes management that could significantly influence the study’s outcomes. Additionally, the study does not address the psychological stress associated with diabetes management, which could affect patient compliance and the effectiveness of CGM use. The study utilized the “Libre 2” for its economic affordability and ease of use over a two-week period. Future studies are suggested to explore experiences with the more advanced “Libre 3” model, potentially offering improved features and user experiences.
Conclusion
This study explored the experience of new diabetes management technology from the users’ perspective through two weeks of CGM usage. Participants were impressed with the novel CGM technology, which enabled them to experience changes in diet and exercise—typically the most challenging aspects of diabetes management. However, they also encountered difficulties related to cost, accuracy of measurements, and skin attachment, and expressed a hope for improvements in these areas. Many stated they would continue to use CGM if the financial aspects were addressed. CGM is a sensation among diabetes patients. Effective policies and programs can be developed when healthcare providers and governments understand experiences and needs from the patients’ perspective. This study provides an important viewpoint in this regard and is expected to be utilized in the treatment, care, education, and counseling of diabetes patients. The limitations identified in this study are expected to be progressively resolved through ongoing research, leading to more robust studies conducted globally.
Acknowledgments
We extend our heartfelt gratitude to all participants of this study, who generously dedicated two weeks to the experience of CGM and shared their invaluable experiences with us. This paper is based on a portion of the first author’s master’s thesis, which is published and accessible via Jeonbuk National University’s digital repository at https://dcoll.jbnu.ac.kr/ezpdfdrm/ezPDFSetupNonax.jsp#.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), Grant/Award Number: 2021R1A2C2092656.
Abbreviations
CGM, Continuous Glucose Monitoring; SMBG, Self-Monitoring of Blood Glucose; ADA, American Diabetes Association.
Data Sharing Statement
The data from which the findings of this study were drawn are available from the corresponding author, Youngran Yang, upon reasonable request. Please contact youngran13@jbnu.ac.kr for access to the data.
Disclosure
The authors declare no conflict of interest.
References
- 1.Korean Diabetes Association (KDA). Diabetes fact sheet in Korea 2022. Seoul; 2023. Available from: https://www.diabetes.or.kr/bbs/?code=fact_sheet&category. Accessed July 18, 2024. [Google Scholar]
- 2.Kim WJ, Kim JH, Yoo HJ, et al. A position statement of the utilization and support status of continuous glucose monitoring in Korea. J Korean Diabetes. 2021;22(4):225–237. doi: 10.4093/jkd.2021.22.4.225 [DOI] [Google Scholar]
- 3.Diabetes complications. Korea Disease Control and Prevention Agency; 2022. Available from: https://health.kdca.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do?cntnts_sn=2351. Accessed July 18, 2024. [Google Scholar]
- 4.Jung JH, Lee JH, Jang HM, et al. Management status of patients with type 2 diabetes mellitus at general hospitals in Korea: a 5-year follow-up study. J Korean Diabetes. 2022;23(1):64–75. doi: 10.4093/jkd.2022.23.1.64 [DOI] [Google Scholar]
- 5.Kolb L. Association of Diabetes Care and Education Specialists. An effective model of diabetes care and education: the ADCES7 self-care behaviors™. Sci Diabetes Self Manag Care. 2021;47(1):30–53. doi: 10.1177/0145721720978154 [DOI] [PubMed] [Google Scholar]
- 6.Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(5):1146–1156. doi: 10.2337/dc19-1459 [DOI] [PubMed] [Google Scholar]
- 7.Bergenstal RM, Mullen DM, Strock E, Johnson ML, Xi MX. Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabetes Compl. 2022;36(3):108106. doi: 10.1016/j.jdiacomp.2021.108106 [DOI] [PubMed] [Google Scholar]
- 8.Ajjan RA, Jackson N, Thomson SA. Reduction in HbA1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: a pilot, multicentre, randomised controlled trial. Diab Vasc Dis Res. 2019;16(4):385–395. doi: 10.1177/1479164119827456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes—2015 abridged for primary care providers. Clin Diabetes. 2015;33(2):97–111. doi: 10.2337/diaclin.33.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diabetes technology: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement_1):S77–S88. doi: 10.2337/dc20-S007 [DOI] [PubMed] [Google Scholar]
- 11.Sørgård B, Iversen MM, Mårtensson J. Continuous glucose monitoring in adults with type 1 diabetes: a balance between benefits and barriers: a critical incident study. J Clin Nurs. 2019;28(17–18):3318–3329. doi: 10.1111/jocn.14911 [DOI] [PubMed] [Google Scholar]
- 12.Scharf J, Nguyen XQ, Vu-Eickmann P, Krichbaum M, Loerbroks A. Perceived usefulness of continuous glucose monitoring devices at the workplace: secondary analysis of data from a qualitative study. J Diabetes Sci Technol. 2019;13(2):242–247. doi: 10.1177/1932296818789143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litchman ML, Allen NA. Real-time continuous glucose monitoring facilitates feelings of safety in older adults with type 1 diabetes: a qualitative study. J Diabetes Sci Technol. 2017;11(5):988–995. doi: 10.1177/1932296817702657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493–498. doi: 10.1089/dia.2019.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn JH, Kang JH, Pahn JH, Yang YR. Present and future of research on continuous glucose monitoring: a narrative review. Korean J Adult Nurs. 2023;35(4):311–326. doi: 10.7475/kjan.2023.35.4.311 [DOI] [Google Scholar]
- 16.Kim JH. Current status of continuous glucose monitoring among Korean children and adolescents with type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab. 2020;25(3):145–151. doi: 10.6065/apem.2040038.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adela freestyle libre; 2022. Available from: https://www.diabetesmall.co.kr/main/index.php. Accessed July 18, 2024.
- 18.Adela Healthcare Co. Ltd. Freestyle Libre. Korean. Available from: https://www.diabetesmall.co.kr/main/index.php. Accessed July 12, 2024.
- 19.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 20.Guba EG, Lincoln YS. Effective evaluation: improving the usefulness of evaluation results through responsive and naturalistic Approaches. 1st ed. Washington, DC: Jossey-Bass; 1981:1–444. [Google Scholar]
- 21.Jo JG. The state of healthcare sensing technology in wearable devices. Korean Inst Electr Eng. 2016;65(11):23–27. [Google Scholar]
- 22.Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a consensus report from the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. 2020;43(7):1617–1635. doi: 10.2337/dci20-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cichosz SL, Johansen MD, Hejlesen O. Toward big data analytics: review of predictive models in management of diabetes and its complications. J Diabetes Sci Technol. 2016;10(1):27–34. doi: 10.1177/1932296815611680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes J, Tripp-Reimer T, Parker E, Muller B, Laroche H. Factors influencing diabetes self-management among medically underserved patients with type II diabetes. Glob Qual Nurs Res. 2017;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sami W, Ansari T, Butt NS, Ab Hamid MR. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci. 2017;11(2):65–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Kamau MW Factors Influencing Diet Adherence Among Diabetes Mellitus Type 2 Patients Attending Clinic at Moi Teaching and Referral Hospital [thesis]. Kenya: Maseno University; 2022:1–103. [Google Scholar]
- 27.Mishali M, Omer H, Heymann AD. The importance of measuring self-efficacy in patients with diabetes. Fam Pract. 2011;28(1):82–87. doi: 10.1093/fampra/cmq086 [DOI] [PubMed] [Google Scholar]
- 28.Houlder SK, Yardley JE. Continuous glucose monitoring and exercise in type 1 diabetes: past, present and future. Biosensors. 2018;8(3):73. doi: 10.3390/bios8030073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallis M, Ryan H, Berard L, et al. How continuous glucose monitoring can motivate self-management: can motivation follow behaviour? Can J Diabetes. 2023;47(5):435–444. doi: 10.1016/j.jcjd.2023.04.001 [DOI] [PubMed] [Google Scholar]
- 30.Kim EY. Gestational and type 2 diabetes ‘continuous glucose monitoring’ Coverage Review; 2023. Available from: https://www.docdocdoc.co.kr/news/articleView.html?idxno=3004742. Accessed July 18, 2024.
- 31.Berg AK, Olsen BS, Thyssen JP, et al. High frequencies of dermatological complications in children using insulin pumps or sensors. Pediatr Diabetes. 2018;19(4):733–740. doi: 10.1111/pedi.12652 [DOI] [PubMed] [Google Scholar]
- 32.Englert K, Ruedy K, Coffey J, et al. Skin and adhesive issues with continuous glucose monitors: a sticky situation. J Diabetes Sci Technol. 2014;8(4):745–751. doi: 10.1177/1932296814529893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther. 2018;20(S2):S2–54. doi: 10.1089/dia.2018.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from which the findings of this study were drawn are available from the corresponding author, Youngran Yang, upon reasonable request. Please contact youngran13@jbnu.ac.kr for access to the data.