Abstract
Long non-coding RNA (LncRNA), with transcripts over 200 nucleotides in length, play critical roles in numerous biological functions and have emerged as significant players in the pathogenesis of osteoarthritis (OA), an inflammatory condition traditionally viewed as a degenerative joint disease. This review comprehensively examines the influence of LncRNA on the inflammatory processes driving OA progression, focusing on their role in regulating gene expression, cellular activities, and inflammatory pathways. Notably, LncRNAs such as MALAT1, H19, and HOTAIR are upregulated in OA and exacerbate the inflammatory milieu by modulating key signaling pathways like NF-κB, TGF-β/SMAD, and Wnt/β-catenin. Conversely, LncRNA like MEG3 and GAS5, which are downregulated in OA, show potential in dampening inflammatory responses and protecting against cartilage degradation by influencing miRNA interactions and cytokine production. By enhancing our understanding of LncRNA’ roles in OA inflammation, we can better leverage them as potential biomarkers for the disease and develop innovative therapeutic strategies for OA management. This paper aims to delineate the mechanisms by which LncRNA influence inflammatory responses in OA and propose them as novel targets for therapeutic intervention.
Keywords: osteoarthritis, LncRNA, inflammation, chondrocytes, pro-inflammatory factors
Introduction
Osteoarthritis (OA) represents the most common type of joint disorder encountered in orthopedics. It presents clinically with a range of symptoms, including joint swelling and deformation, pain and stiffness, impaired joint mobility, and severe disability.1 The number of OA patients is rising with the aging global population. According to epidemiological studies, 46.3% of middle-aged and older persons in China have primary OA,2 and OA places a heavy burden on global public health.3 OA is characterized by progressive degeneration and loss of articular cartilage, accompanied by secondary bone metaplasia. Clinical manifestations of OA include changes in the internal architecture of joints, alterations in periarticular ligaments, sclerosis of subchondral bone, and inflammation of the synovial membrane.4 Even though the exact cause of OA is still unknown, an increasing amount of research points to the inflammatory response as a key factor in the disease’s progression. Inflammation causes joint pain and stiffness and accelerates the degradation of cartilage and surrounding tissues by various pathways, leading to intra-articular neovascularization and fibrotic changes that accelerate the degeneration of joint structures.
In this complex inflammatory environment, long non-coding RNAs (LncRNAs) have emerged as key regulators. A family of transcripts made from RNA longer than 200 nucleotides, LncRNAs are a subset of non-coding RNAs (ncRNAs).5 LncRNAs have recently garnered increased attention due to their pivotal role in regulating gene expression. LncRNAs are crucial in numerous biological processes, particularly in regulating apoptosis and inflammatory responses.6,7 LncRNAs play an essential function in OA, participating in the inflammatory response to OA by regulating cytokine expression, regulating signaling pathways, and interacting with other non-coding RNAs.8–10 Thus, a thorough examination of the precise roles played by LncRNAs in the inflammatory response of OA will not only shed light on the disease’s pathophysiology but also offer fresh approaches to both diagnosis and treatment.
Inflammatory Responses in OA
Inflammation is a complex pathophysiological process involving a variety of genes and signaling pathways. It is an adaptive response of the organism to various stimuli or harmful factors.11 Depending on the duration and nature of the inflammation, it is referred to as acute or chronic. Acute inflammation is a rapid defense reaction designed to eliminate the noxious stimulus and initiate the healing process quickly; when acute inflammation fails to eradicate the noxious stimulus completely and persists, it evolves into chronic inflammation, leading to long-term tissue damage and repair imbalance, which can lead to a series of chronic diseases.12,13
While traditionally viewed as a condition resulting from mechanical wear and tear OA involves chronic loading and flawed joint biomechanics that culminate in the breakdown of articular cartilage. This degradation initiates a pathological cycle of stiffness, swelling, and reduced joint mobility.14,15 Recently, an expanding volume of research has highlighted the crucial role that chronic inflammation plays in the development of OA. It is common to observe heightened levels of inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the synovial fluid and joint tissues of those affected by OA.16–19 In osteoarthritis models, the state of the cartilage, synovial membrane, and subchondral bone has been improved as a result of suppressing the expression of these inflammatory cytokines.20–24
These inflammatory cytokines induce cartilage catabolism and inhibit the anabolic processes essential to cartilage homeostasis.25 For example, IL-1β, TNF-α, and IL-6 can stimulate the secretion of catabolic enzymes-matrix metal-degrading proteases MMP-1, MMP-3, MMP-9, MMP-13,26–28 and encourage the production of ADAMTS, which is responsible for the aggregator molecules’ proteolysis.29 Significant amounts of cartilage matrix are degraded because of elevated MMP and ADAMTS activity and expression.30,31 Simultaneously, inflammatory mediators hinder the synthesis of type II collagen, aggregated proteoglycans, and other essential components, leading to reduced production of the cartilage matrix.32–34 They also induce chondrocyte apoptosis and senescence, leading to impaired migration of chondrogenic progenitor cells, thus depriving cartilage of its regenerative potential.35 Inflammatory cells, secreted by macrophages, dendritic cells, and fibroblasts in the synovial layer, infiltrate the synovium. This infiltration leads to hyperplasia and increased vascularization of the synovium, therefore encouraging the development of OA.
IL-1β induces autocrine secretion in the form of autocrine secretion as a means of stimulating the synthesis of other chemokines (TNF-α, IL-8, CCL5, CCR1, CCR2, etc)., which induce the recruitment and transport of inflammatory cells and mesenchymal progenitor cells,36–38 which induce the recruitment and transport of inflammatory and mesenchymal progenitor cells, releasing MMPs that destroy peripheral cartilage and promote osteoclast-mediated periarticular bone remodeling. Inhibition of IL-1β expression reversed MMP, ADAMTS, IL-6, and TNF-α expression, and attenuated chondrocyte apoptosis.39 IL-1RI, the TLR receptor family, is the component of the IL-1β receptor and mediates the pro-inflammatory effects of IL-1β.40 The IL-1 type I receptor (IL-1RI) and TNF-R1 receptor expression is significantly upregulated in OA chondrocytes relative to normal chondrocytes.41,42
TNF-α, meanwhile, is secreted by the same cells that secrete IL-1β in the joints, and its biological effects are exerted when TNF-α binds to TNF-R1 and TNF-R2 receptors present on the surface of individual cell membranes. The presence of the structural death domain (DD) in the TNF-R1 receptor, capable of recruiting two different TNF-R1 complexes, is functionally primarily involved in activating inflammatory responses and cell-cell transduction, leading to cell lysis.43 IL-1β and TNF-α each promote the phosphorylation and subsequent degradation of the IκB kinase complex (IKK) through unique pathways, resulting in the liberation of previously restrained NF-κB. Once released, NF-κB moves to the nucleus, initiating the transcription of a broad array of pro-inflammatory genes,44,45 including IL-6, IL-8, MMPs, cyclooxygenase-2 (COX-2). These gene products not only intensify the inflammatory response within the synovium but also elevate the levels of Inflammatory factors through a positive feedback loop, thereby perpetuating a self-sustaining cycle of inflammation.46 In addition, p38MAPK and terminal kinase (JNK) are also activated, leading to the activation of transcription factors such as AP-1, further amplifying the intensity of inflammatory signals. Through direct and indirect methods, the combined action of these signaling pathways induces the expression of genes associated with inflammation and leads to degenerative changes in chondrocytes and synoviocytes.47–51
IL-6 is low in resting cells but is highly expressed in the OA synovium, synovial fluid, cartilage, and subchondral bone after being stimulated by TNF-α and IL-1β.52,53 After binding to its IL-6R receptor, highly expressed IL-6 activates inflammatory signaling pathways such as JAK kinase phosphorylation, STAT3 transcription factor phosphorylation, and nuclear translocation via the gp130 signal transducer receptor and ultimately induces a series of pro-inflammatory genes to form a highly inflammatory microenvironment.54,55 The result is a highly inflammatory microenvironment, which leads to proliferation and pathological changes in synovial cells, resulting in proliferation and hypertrophy of synovial tissue. The release of large quantities of MMPs disrupts chondrocyte homeostasis.56,57 At the same time, activation of JAK and STAT3 by IL-6 induces an osteoblast-dependent inflammatory response,58 promoting osteoclast formation and affecting subchondral bone homeostasis.59,60
In OA, excess reactive oxygen species (ROS) result in oxidative stress, which provokes inflammatory responses in intra-articular tissues and affects the homeostatic regulation of cartilage and synovium.61 Multiple pro-inflammatory factors act synergistically to exacerbate oxidative stress in various ways, inducing overproduction of reactive ROS and exacerbating OA.62–64
Furthermore, the interaction of inflammatory cytokines, induces the expression of enzymes such as phospholipase A2 (PLA2), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). This leads to the production of inflammatory mediators, including nitric oxide (NO) and prostaglandin E2 (PGE2), which exacerbate chondrocyte apoptosis and matrix degradation by promoting chondrocyte matrix degradation enzyme expression65,66 and also activate synoviocytes and other immune cells.67,68 The inflammatory response is enhanced, leading to structural damage and joint dysfunction.69–71
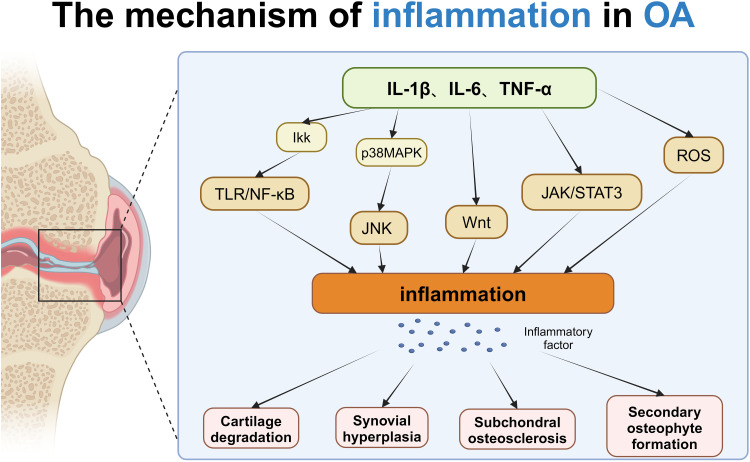
In conclusion, the inflammatory response in osteoarthritis (OA) is a multifaceted and layered process that involves numerous cytokines and signaling pathways. These inflammatory elements play crucial roles in maintaining joint homeostasis and health within OA contexts. By stimulating the expression of pro-inflammatory genes, these mediators intensify the damage to joint tissues. Targeting these inflammatory pathways, and inhibiting the mediators and their signaling processes, has shown to alleviate symptoms of OA, suggesting that this approach could be an effective strategy for treating the condition (Figure 1).
Figure 1.
The mechanism of inflammation in OA: In osteoarthritis (OA), inflammatory factors such as IL-1β, IL-6, and TNF-α trigger inflammation by activating multiple signaling pathways, including the TLR/NF-κB, p38 MAPK, JAK/STAT3, and Wnt pathways. The TLR/NF-κB pathway promotes inflammation through the activation of IKK, while the p38 MAPK pathway exacerbates inflammation via JNK and Wnt signaling, and the JAK/STAT3 pathway is influenced by TNF-α and ROS. These inflammatory signals work together to cause pathological changes such as cartilage degradation, synovial hyperplasia, subchondral osteosclerosis, and osteophyte formation, thus accelerating the progression of OA. Created with BioRender.com.
LncRNA Structure and Function
The Encyclopedia of DNA Elements (ENCODE) project has revealed that less than 3% of the human genome is translatable into proteins., and more than 80% is transcribed into various non-coding RNAs (ncRNAs).72 Among these ncRNAs, some are processed into small RNAs, while the majority are transformed into RNA molecules exceeding 200 nucleotides in length. These longer sequences, which do not overlap with known coding genes and are autonomously transcribed, are referred to as long non-coding RNAs (LncRNAs).73
The principal LncRNAs can be categorized based on their genomic origins, structural characteristics, and functional roles into categories such as Intergenic LncRNAs (LincRNAs), which do not cross any protein-coding sites and are located on genomic sites between protein-coding genes or intergenic regions;74 Intronic LncRNAs (Intronic LncRNAs) located in intronic protein-coding regions in either orientation;75 Justice LncRNA (Sense LncRNA), is transcribed from the exonic region of the coding strand of a protein-coding gene;76 Conversely, Antisense LncRNA is characterized by its partial overlap with the antisense strand of a coding gene;77 Bidirectional LncRNA that overlaps the opposite reverse promoter region of neighboring coding genes and is transcribed in the opposite direction,78 etc.
In general, LncRNAs share a large number of features with messenger RNAs (mRNAs), possessing a 5′ cap structure and a 3′ poly(A) tail. RNA polymerase II transcribes these RNA molecules and subsequently undergo splicing and RNA editing to produce isomeric transcripts.79
LncRNAs are crucial for controlling how genes are expressed. They affect the gene transcription process by interacting with transcription factors or modulating their activity.80 This regulation not only activates or inhibits gene transcription but also involves the precise regulation of specific cellular environments and conditions. In addition, LncRNAs have the capability to bind to mRNAs, influencing mRNA stability, splicing, transport, and translation. This interaction plays a crucial role in globally regulating gene expression and protein synthesis. More specifically, ncRNAs protect mRNAs from degradation by forming complexes with mRNAs or by instructing the spliceosome to recognize specific splice sites to produce different mRNA variants.81 LncRNAs can also act as competitive endogenous RNAs (cRNAs), indirectly regulating gene expression by binding to miRNAs and “adsorbing” them, thus reducing the inhibitory effect of miRNAs on target genes. This LncRNA network establishes a complex hierarchy of molecular regulation, enabling cells to precisely regulate gene expression in a dynamic environment.82 LncRNAs are also involved in RNA splicing, editing, and modification, affecting RNA maturation and function. They also interact with specific proteins, affecting their function, localization, and stability, and sometimes even act as molecular scaffolds, organizing and coordinating interactions between multiple molecules.83 Through the above different regulatory pathways, LncRNA is pivotal in regulating various biological processes, including cell state and differentiation. It also plays a crucial role in regulating the onset and progression of numerous inflammatory diseases.
The Role of LncRNA in the Inflammatory Response to OA
Recent research has revealed that lncRNAs are integral to the pathogenesis of osteoarthritis (OA), although their precise functions remain to be fully elucidated. Through microarray and comprehensive transcriptomic analyses, numerous studies have identified variations in lncRNA expression levels in OA patients compared to healthy controls. These findings show that certain lncRNAs are either overexpressed or downregulated, suggesting a potential link to the characteristics of the disease.
LncRNAs Regulated in OA Inflammation
Malat1
The MALAT1 gene, categorized as a LincRNA, is situated in a gene-dense region on human chromosome 11q13.1. This highly conserved, nuclear-resident LncRNA spans approximately 6.7–7 kb and is expressed across a diverse range of cells and tissues. Although MALAT1 lacks introns and Poly A tails, it can be stabilized and prevented from degradation by processing the 3′ end, which contains a triple helix structure.84 As the key member of the LncRNA family, MALAT1 is implicated in numerous diseases and biological processes.85 Research indicates that MALAT1 is significantly overexpressed in the cartilage and synovial tissues of individuals with OA, and this overexpression is closely linked to the upregulation of various inflammation-related genes.
Specifically, MALAT1 regulates key inflammatory signaling pathways, such as the NF-κB and MAPK pathways, significantly promoting the expression and release of inflammatory mediators. For example, overexpression of MALAT1 accelerates LPS-induced pyroptosis and inflammatory responses, while silencing MALAT1 inhibits NF-κB activation, thus weakening these inflammatory responses.86 Furthermore, MALAT1 acts as a competing endogenous RNA (ceRNA), interacting with miRNAs (such as miR-150-5p, miR-146a, and miR-9), indirectly regulating NF-κB activation and consequently leading to the release of large amounts of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α.87,88 Additionally, MALAT1 knockdown can suppress the LPS-induced upregulation of the p38 MAPK pathway, thereby reducing the expression of IL-1, TNF-α.88 Moreover, MALAT1 can regulate the TLR4 signaling pathway to enhance the release of IL-1β and TNFα, further aggravating the inflammatory response.89
During the pathogenesis of OA, MALAT1 expression is significantly upregulated in IL-1β-treated OA chondrocytes. As a ceRNA, MALAT1 competes with miR-150-5p, promoting AKT3 expression. Silencing MALAT1 inhibits cell proliferation and activates the apoptotic pathway, leading to an increase in apoptosis markers such as cleaved-PARP, Bax, and Bcl-2. This knockdown of MALAT1 also increased elevated MMP-13 and ADAMTS-5 expression while reducing collagen II and aggrecan, thus accelerating ECM (extracellular matrix) degradation and contributing to OA progression.90 Additionally, in OA, MALAT1 acts as a ceRNA by binding to miR-146a, activating the PI3K/Akt/mTOR signaling pathway. Studies have shown that silencing MALAT1 promotes type II collagen synthesis and reduces the expression of IL-6, MMP-14, and COX-2, providing significant protection against the production of inflammatory factors and LPS-induced cartilage degradation.91
Apart from its interaction with miR-146a, MALAT1 also binds to miR-145, promoting ADAMTS5 expression while suppressing the expression of key cartilage matrix markers such as COL2A1, ACAN, and COMP, leading to further cartilage degradation.92 Moreover, MALAT1 binds to the transcription factor KLF5, promoting the transcriptional upregulation of CXCL11, which exacerbates inflammatory damage and significantly increases the release of IL-1β and IL-18, accelerating OA progression.93 Further studies have shown that MALAT1 can promote nuclear translocation of NF-κB p65 and β-catenin, thereby upregulating the expression of iNos, Cox2, and MMP13 mRNA in OA chondrocytes, significantly intensifying the inflammatory response.94 As a ceRNA for miR-9, MALAT1 can also indirectly target NF-κB, inducing the release of large amounts of inflammatory mediators from chondrocytes, damaging the ECM, and promoting OA development.95
MALAT1 expression is not only elevated in OA chondrocytes but also significantly increased in osteoblasts of OA patients. In particular, in the serum of OA patients, MALAT1 is positively correlated with DKK1 levels. DKK1 is an endogenous inhibitor of the Wnt/β-catenin signaling pathway, which mediates bone loss by inhibiting osteoblast differentiation and further drives OA pathology.96 Additionally, MALAT1 expression in the synovial tissues of OA patients is significantly higher than in non-OA patients, and the secretion of IL-6, CXCL8, CXCL5, IL1β, and CCL2 by synovial fibroblasts is much higher in OA patients compared to non-OA patients. Silencing MALAT1 not only reduces CXCL8 expression and secretion but also globally affects mRNAs involved in regulating cell growth, proliferation, and inflammatory responses.97 In general, the expression of MALAT1 in OA is increased, which mainly plays a more pro-inflammatory role in OA cartilage, synovial membrane and subchondral bone, inducing inflammatory response.
PVT1
The PVT1 gene is located on human chromosome 8q24.21 and mouse chromosome 15 in the cancer-associated coding region, which comprises multiple exons and introns, does not encode proteins but is functionally significant within the cell through various mechanisms. It plays a vital role in regulating various biological processes such as gene expression, cell proliferation, apoptosis, inflammatory responses, and oncogenesis.98 In addition to its involvement in the development of various diseases, recent studies have demonstrated that PVT1 expression is significantly elevated in OA, particularly in the cartilage and synovial tissues of OA patients. PVT1 plays an important role in OA pathogenesis by regulating inflammatory signaling pathways and ECM metabolism.99
Firstly, PVT1 enhances inflammatory responses by activating classical inflammatory signaling pathways such as MAPK and NF-κB. In OA patients and IL-1β-treated chondrocytes, PVT1 expression is significantly upregulated, accompanied by increased secretion of inflammatory cytokines such as TNF-α and IL-6. Silencing PVT1, however, restores cell viability and significantly reduces the release of inflammatory mediators, thereby protecting chondrocytes from IL-1β-induced apoptosis and inflammatory responses.99,100 This phenomenon may be due to PVT1 acting as a ceRNA by sponging miR-27b-3p, which indirectly regulates the expression of TNF receptor-associated factor 3 (TRAF3). Overexpression of PVT1 leads to increased TRAF3 expression, activating the downstream NF-κB signaling pathway and aggravating the inflammatory response, while silencing PVT1 upregulates miR-27b-3p and inhibits TRAF3 expression, thus reducing inflammation.100
Secondly, PVT1 regulates inflammatory responses and ECM degradation in OA through interactions with multiple miRNAs. As a ceRNA for miR-140 and miR-211-3p, PVT1 regulates the expression of ADAMTS-5 and MMP-13, leading to elevated levels of TNF-α, IL-6, and other inflammatory cytokines, thereby accelerating ECM degradation and promoting OA progression.101,102 Additionally, PVT1 upregulation is closely associated with the increased expression of matrix metalloproteinases (MMP3, MMP9) and inflammatory factors such as NO, PGE2, IL-6, and TNF-α. These elevated levels are accompanied by reduced expression of cartilage matrix components, including aggrecan and type II collagen.103 Studies have shown that silencing PVT1 can inhibit IL-1β-induced chondrocyte metabolic dysfunction and inflammatory responses, indicating that PVT1 plays a crucial role in cartilage degradation and inflammation.
Moreover, PVT1 exacerbates OA inflammatory responses by modulating multiple signaling pathways. PVT1 downregulates miR-146a levels, indirectly amplifying inflammatory responses, while also activating the TGF-β/SMAD4 signaling pathway, further intensifying inflammation. Silencing PVT1 has been shown to increase miR-146a expression and inhibit the TGF-β/SMAD4 signaling pathway, thereby reducing the expression of inflammatory mediators and alleviating OA progression.104 Another study revealed that PVT1 expression is significantly upregulated in the serum of OA patients and in LPS-stimulated chondrocytes. At the same time, miR-93-5p expression is downregulated, while HMGB1 mRNA and protein levels are markedly increased.105 HMGB1, as a damage-associated molecular pattern (DAMP), activates the TLR4/NF-κB signaling pathway, leading to a sustained inflammatory environment and further exacerbating cartilage damage and ECM degradation.106 PVT1 activates the TLR4/NF-κB pathway in C28/I2 cells through the miR-93-5p/HMGB1 axis, significantly increasing the expression of IL-6, IL-1β, TNF-α, and MMP-13, while downregulating aggrecan levels, thereby accelerating OA progression.105
Finally, PVT1 also regulates the proliferation and inflammatory response of fibroblast-like synoviocytes through epigenetic mechanisms. Fibroblast-like synoviocytes play a key role in tissue destruction in OA, and Sirt6 has been identified as a target of PVT1. PVT1 promotes the methylation of the Sirt6 promoter, thereby suppressing Sirt6 expression, which in turn promotes the proliferation and inflammatory response of fibroblast-like synoviocytes. This mechanism has been validated in a mouse collagen-induced arthritis model.107 According to current studies, PVT1 enhances inflammatory response, induces synovial hyperplasia, promotes cartilage degradation, damages chondrocyte function, thus aggravates osteoarthritis and leads to pathological progression of OA.
H19
The H19 gene is in the 11p15.5 region of the human chromosome. After RNA polymerase II is transcribed, spliced, and polyadenylated, the H19 transcript is transported from the nucleus to the cytoplasm.108 H19 is highly expressed in embryonic tissues; however, its expression significantly decreases after birth, leading to inhibited H19 expression in adults.109 In recent years, H19 has been involved in a variety of pathophysiological activities, in particular the inflammatory response. H19 can positively regulate TLR4 expression,110 rising P38 and P65 levels, two important NF-κB factors,111 affect the activity of key kinases in the MAPK pathway (eg, ERK, JNK, and p38)112 and promotes the activation of inflammatory signaling pathways via multiple pathways, releasing large quantities of inflammatory cytokines, thereby promoting inflammatory signaling and playing a key role in the development of OA.99
H19 overexpression treatment raised the level of apoptosis in chondrocytes and hindered cell proliferation. H19 expression was shown to be upregulated in OA samples and chondrocytes, but this was reversed by H19 knockdown, which can be achieved by targeting miR-106a-5p.113 The H19 content of C28/I2 chondrocytes exposed to LPS was significantly elevated compared with the pristine control and increased dose-dependent with LPS. Silencing H19 LncRNA can reduce levels of the IL-1β, IL-6, and TNF-α through miR130a uptake and repair damage to LPS-treated C28/I2 cells.114
H19 was not only highly expressed in OA chondrocytes, but its expression was also significantly higher in OA synovial tissue compared to normal or control groups. In synovial cells transfected with H19, the gene directly targeted JNK2 and NF-κB, leading to increased levels of inflammatory cytokines including TNF-α, IL-6, and IL-1β.115 Upregulation of H19 in the cytoplasm of fibroblast-like synovial cells was found to enhance its miRNA-sponge effect and accelerate miR-103a degradation. Additionally, H19 promoted the production of IL-15 and Dkk-1, IL-15 is a multifunctional pro-inflammatory cytokine that significantly contributes to the induction of pro-inflammatory factors such as TNFα, IL-6, and IL-1β. The expression of Dkk-1 was found to positively correlate with the degree of inflammation, indicating its role as a marker of inflammatory severity.116 In addition, H19 can regulate IL-6 and macrophage infiltration involved in angiogenesis117 and activates the AKT pathway, regulates levels of the vascular endothelial growth factor VEGF and the endothelial-type nitric oxide synthase eNOS, and promotes lumen formation.118 This leads to the formation of synovial and subchondral lumens. The result is increased neovascularization in the synovium and subchondral bone, which paves the way for inflammatory cells and metal matrix-degrading enzymes to exacerbate OA lesions.
HOTAIR
The HOTAIR gene is positioned on human chromosome 12q13.13, situated between the HOXC11 and HOXC12 genes, and is identified as an oncogenic LncRNA. Transcribed by RNA polymerase II, HOTAIR functions through its interaction with chromatin, primarily by associating with the Polycomb Repression Complex 2 (PRC2). Additionally, it can bind to the lysine-specific demethylase complex 1 (LSD1), facilitating the methylation of H3 Lys27 (H3K27me3) and the demethylation of H3 Lys4 (H3K4me2) to repress gene expression. HOTAIR also influences miRNA biosynthesis and function, thereby regulating the expression of downstream target genes.119 HOTAIR is not only pivotal in tumorigenesis and development but also activates the expression of various proteins involved in the inflammatory signaling pathway, such as NF-κB, TNFα, and MAPK. This activation leads to the upregulation of numerous proteins that contain structural protein kinase domains. Additionally, HOTAIR promotes the aggregation of key proteins and complexes within the inflammatory signaling pathway, including the TNFα/NF-κB signaling protein complex, the IκB kinase complex, and the IKKA-IKKB as well as the IKKB kinase complexes.120
The upregulation of HOTAIR results in increased levels of MMP-13 and ADAMTS-5, accompanied by a decrease in type II collagen and aggregated proteoglycans. Additionally, there is an elevation in apoptosis-associated factors such as caspase-3, caspase-9, and Bax. These changes regulate ECM degradation, promote chondrocyte apoptosis, and contribute to the development of OA.121,122 While HOTAIR depletion attenuated LPS-induced chondrocyte apoptosis and CHON-001 inflammatory response, this could be due to the LPS-induced increase in HOTAIR levels, which adsorbs miR-1277-5P and increases SGTB expression,123 and SGTB, as a membrane-associated serine protease, regulates inflammatory cell behavior, inflammatory mediator production, and cell signaling pathways via a mechanism regulating SGTB expression.124 CXCL12 interacts with its receptor CXCR4 to activate inflammatory signaling pathways, leading to the release of inflammatory cytokines such as TNF-α, IL-6, and IL-8. This amplifies the inflammatory response, induces the secretion of MMPs, and leads to the degradation of the periarticular ECM.125 Studies have shown that HOTAIR inhibition decreases CXCL12 expression in chondrocytes by up-regulating miR-107 content and thus up-regulating type II collagen and aggregated proteoglycans and down-regulating MMP-13 and MMP-9 expression.126 In a model of IL-1β-induced arthritic chondrocytes influenced by HOTAIR, there was an observed increase in ADAM10, MMP-13, IL-6, and TNF-α levels, as well as higher reactive ROS levels compared to controls. In this model, miR-222-3p, which is targeted by HOTAIR, plays a crucial role; its overexpression inhibits, while its knockdown exacerbates, the inflammatory damage induced by IL-1β in chondrocytes.127 ETV1, as a target of miR-17-3p, could promote LPS-induced cell damage by activating the MAPK/c-Jun and NF-κB pathways. In the osteoarthritic chondrocyte model, HOTAIR down-regulated miR-17-3p levels, up-regulated ETV1 expression, activated MAPK/c-Jun and NF-κB pathways, and aggravated inflammatory cell injury that promotes, which promotes the OA process.128
Other LncRNAs
NEAT1, transcribed from human genes on chromosome 11, is aberrantly expressed in a wide range of cancers, playing a significant role in tumor formation, treatment response, and prognosis. Additionally, NEAT1 is crucial in immunity and inflammation, influencing these processes through signaling pathways such as Wnt/β-catenin and NF-κB.129 NEAT1 is overexpressed in OA cartilage tissue and IL-1β-induced chondrocytes. This overexpression targets miR-543, leading to the upregulation of inflammation-related genes such as PLA2G4A and the suppression of p-Akt and Bcl-2 expression. Additionally, it enhances the expression of MMPs and interleukin. Conversely, a miR-543 mimic can reverse the effects of NEAT1 overexpression, inhibit PLA2G4A expression, reduce apoptosis and inflammation.120
The downregulation of miR-150-5p by NEAT1 leads to an increase in β-catenin expression in OA chondrocytes. This activation of the Wnt/β-catenin results in the upregulation of MMP and ADAMTS expression, thereby facilitating the development of OA.130 NEAT1 correlates negatively with miR-146a-3p expression and positively with TrkB expression,131 and TrkB has a promoter effect on the production and release of pro-inflammatory chemokines in OA synovium,132 miR-146a/b inhibits the NF-κB pathway, regulates inflammation of cartilage and synovium, and can also be inhibited by adsorption of miR-146a/b,133,134 and also regulates chondrocyte inflammation, apoptosis, and ECM production by adsorbing miR-193a-3p, miR-16-5p, which alleviate OA symptoms.135,136 Overexpression of osteopontin (OPN) activates the NF-κB pathway, essential for producing various inflammatory mediators, including chemokines and cytokines such as IL-1, CXCL1, and CCL2. This activation results in the spontaneous production of NO, PGE2, IL-1β, IL-6, and IL-8, which further amplifies the inflammatory response.137 Additionally, in synoviocytes from OA patients, knocking down NEAT1 reduces OPN protein levels, leading to decreased levels of MMP13, IL-6, and IL-8, inhibiting synoviocyte proliferation and effectively slowing OA progression.138
THRIL, located on human chromosome 12, plays a crucial role in regulating the inflammatory response. It is involved in the modulation of inflammation by interacting with specific signaling pathways and cytokines, highlighting its significance in the broader context of immune regulation. In the LPS-induced OA chondrocyte injury model, THRIL expression was up-regulated by LPS while exacerbating cellular inflammatory injury, as evidenced by loss of cell viability, increased apoptosis and overproduction of pro-inflammatory cytokines, explained by the fact that THRIL overexpression inhibited miR-125b production and exacerbated JAK1/STAT3 and NF-κB pathways activation of JAK1/STAT3 and NF-κB pathways.139 Furthermore, in fibroblastic synoviocytes, THRIL promotes an increase in the levels of pro-apoptotic markers Bax and cleaved caspase-3, while decreasing the levels of the anti-apoptotic protein Bcl-2. It also stimulates synovial proliferation and activates the PI3K/AKT pathway, leading to significant releases of IL-1β and MMP-3. These activities collectively intensify the inflammatory response.140
XIST, located at the X chromosome inactivation center on chromosome Xq13.2, plays a critical regulatory role across a broad spectrum of human diseases. It is implicated in various conditions including cancer, pulmonary fibrosis, inflammation, neuropathic pain, and OA, highlighting its significance in both genetic and epigenetic regulation across different pathological states.141 XIST is expressed at high levels in osteoarthritic cartilage tissue and chondrocytes, interacts negatively with miR-149-5p, targets DNMT3A, and regulates OA progression.142 Furthermore, XIST targets miR-376c-5p by acting as a ceRNA for miR-376c-5p and competing with OPN to target miR-376c-5p, thus counteracting miR-376c-5p-mediated inhibition of OPN. This led to high OPN expression in OA cartilage and synovial membrane, promoted IL-1β, IL-6 and TNF-α production, and reduced anti-inflammatory cytokine in M1 macrophages, exacerbating the inflammatory environment of OA and promoting OA progression.143 In addition, vascular cell adhesion molecule type 1 (VCAM-1) promotes leukocyte aggregation at sites of inflammation and exacerbates the inflammatory response, and its increase also attracts monocytes to adhere to human osteoarthritic synovial fibroblasts (OASF). Research has indicated that elevated levels of XIST in the OA patients’ synovium enhance the expression of VCAM-1. This increase promotes the adhesion of monocytes to OASFs, thereby intensifying the inflammatory environment associated with OA.144
LncRNAs Downregulated in OA Inflammation
MEG3
MEG3 is situated on human chromosome 14q32 and encodes a 1.6 kb RNA sequence, which includes several imprinted genes, covering both maternal and paternal imprints. The DLK1 gene, positioned about 100 kb from the MEG3 locus, interacts closely with MEG3. Together, they form the DLK1-MEG3 imprinted region on chromosome 14q32, highlighting a complex pattern of gene regulation that involves imprinting from both parents.145 MEG3 is expressed in numerous normal tissues and is recognized as the first LncRNA to exhibit tumor suppressor effects. It is found to be underexpressed in a variety of cancers. Additionally, MEG3 is widely implicated in a range of disorders, including metabolic conditions, immune system dysfunctions, cardiovascular diseases, and degenerative diseases, underlining its broad impact on human health.
In OA rat models, LncRNA MEG3 shows a downregulation trend in cartilage tissue, where it regulates OA progression through the miR-16/SMAD7 axis. Studies have shown that miR-16 is overexpressed in systemic inflammatory response syndrome and significantly upregulated in OA rat models.146 miR-16 inhibits the expression of SMAD7, an intracellular antagonist of the TGF-β signaling pathway. The downregulation of SMAD7 promotes the production of inflammatory cytokines, driving the inflammatory response and accelerating OA progression.147,148 Following MEG3 overexpression, there is an upregulation in the expression of chondrocyte-related markers such as MMP-13, collagen II, and ADAMTS5. Additionally, it attenuates IL-1β-induced chondrocyte senescence and apoptosis, offering a potential therapeutic effect on OA.149
Moreover, MEG3 reduces ECM degradation and inhibits OA progression by regulating the miR-93/TGFBR2 axis.150. miR-9-5p is significantly elevated in OA cartilage tissue and promotes cartilage damage.151 KLF4, a transcription factor, shows a downregulation trend in OA and is closely related to the inflammatory response in chondrocytes.152 MEG3, acting as an endogenous sponge, inhibits miR-9-5p expression, positively regulating KLF4 expression. This reverses the damage caused by miR-9-5p to chondrocytes and cartilage tissue, enhancing chondrocyte viability and suppressing apoptosis and inflammation.153
FoxO1, a crucial transcription factor involved in various biological processes, Dephosphorylation and activation of FOXO1 compete with Akt signaling and inhibit AKt-mediated IKK activation, thereby inhibiting NF-κB activity, which lowers the inflammatory response.154 MEG3 negatively regulates miR-361-5p and positively regulates FoxO1 expression, thus mitigating OA progression.155 Meanwhile, vascular endothelial growth factor (VEGF) is highly expressed in OA cartilage tissue, promoting angiogenesis, and exacerbating synovial inflammation in OA. MEG3, by activating p53 and binding to the Sp1 transcription factor site on the VEGFA promoter, negatively regulates VEGF expression, thereby inhibiting OA progression.156 Therefore, MEG3 may be a key target for the treatment of OA.
GAS5
GAS5, located on human chromosome 1q25.1. By selective splicing, these 12 exons are capable of generating two mature RNAs: GAS5a and GAS5b. GAS5 transcripts have upstream 5’ regulatory sequences and, therefore, belong to the class of 5’ TOP genes. These genes include not only genes encoding ribosomal proteins but also a number of ncRNA genes involved in regulating protein synthesis and cell growth arrest. GAS5 is predominantly expressed in the cytoplasm of cells that are in a growth-arrested state, indicating its potential role in regulating cell growth and metabolism. This suggests that GAS5 could be crucial for cellular processes that control the cessation of cell division and metabolic adjustments during growth arrest.157 GAS5 functions as a tumor suppressor gene rather than an oncogene, typically exhibiting reduced expression in various cancers. It plays a significant role in the pathological processes of various diseases, including diabetes and inflammatory conditions. Its underexpression in cancer highlights its potential as a therapeutic target, underscoring its importance in maintaining cellular homeostasis and influencing disease progression.
LPS down-regulated GAS5 expression in the LPS-induced chondrocyte model, and the higher the concentration of LPS, the lower the expression of GAS5, strongly suggesting that GAS5 is closely associated with inflammatory responses in OA.158 miR-146a is a miRNA that is upregulated in response to inflammatory stimuli and promotes OA progression by targeting Camk2d and Ppp3r2 as well as pro-inflammatory factors.159 GAS5 can function as an endogenous sponge for miR-146a, absorbing this microRNA to regulate the expression of Smad4. By doing so, GAS5 inhibits chondrocyte apoptosis and potentially slows the progression of OA. This mechanism underscores the importance of GAS5 in modulating cellular responses and maintaining tissue integrity in the context of degenerative joint diseases.160
NKILA
NKILA, an LncRNA spanning 2.6 kb, is located on human chromosome 20q13. It interacts with the NF-κB pathway, playing a crucial role in regulating various biological processes. This interaction is significant in controlling inflammatory responses, cell survival, and apoptosis, highlighting NKILA’s importance in cellular regulation and disease pathology. Under normal conditions, NF-κB binding to IκB remains inactivated. When the cell is stimulated, IκB is degraded, releasing activated NF-κB into the nucleus and triggering transcription of target genes. NKILA inhibits NF-κB activation by binding to the NF-κB/IκB complex, which stabilizes IκB and prevents its degradation. This action of NKILA plays a pivotal role in the down-regulation of the NF-κB signaling pathway, influencing inflammatory and immune responses.161
In the OA chondrocyte model, IL-1β exposure significantly reduces Smad2/3 phosphorylation levels in chondrocytes and decreases NKILA expression. This reduction in NKILA levels leads to an increase in the expression of COX-2, iNOS, MMP-13, NO, PGE-2, and TNF-α. Conversely, NKILA helps to inhibit the phosphorylation of p65 and IκBα, which are part of the NF-κB signaling pathway. By modulating these pathways, NKILA reduces the release of inflammatory mediators and may potentially slow the progression of OA. This dual role highlights NKILA’s complex involvement in regulating inflammatory responses in OA.162 In addition, NKILA, which is also weakly expressed in human cartilage tissue with OA, can act as an LncRNA to uptake miR-145 overexpressed in osteoarthritic tissue, upregulate SP1 expression, and modulate the NF-κB signaling pathway, which in turn affects tissue inflammation.163 Concurrently, overexpression of NKILA significantly reduces the levels of IL-6 and VEGFA by inhibiting the NF-κB signaling pathway. This inhibition directly impacts angiogenesis, curbing excessive vascular growth. NKILA’s role in controlling these key factors highlights its potential therapeutic value in conditions where modulation of angiogenesis and inflammation is crucial.164
Other LncRNAs
ATB is a LncRNA located on human chromosome 14, is activated by transforming growth factor β (TGF-β) and is often overexpressed in various cancers. It plays a significant role in disease development.165 It was found that in the serum of OA patients, the level of LncRNA-ATB was much lower than in healthy subjects and that overexpression of LncRNA-ATB in chondrocytes significantly promoted Akt phosphorylation in chondrocytes, facilitating chondrocyte proliferation and viability.166
In the LPS-induced inflammatory injury model of ATDC5 cells, the expression of lncRNA-ATB was significantly suppressed. Conversely, overexpression of lncRNA-ATB led to the suppression of iNOS, COX-2, IL-6, and TNF-α levels, thereby mitigating the LPS-induced cellular inflammatory injury. The potential therapeutic effect of LncRNA-ATB in OA could stem from its ability to attenuate the inflammatory response and limit OA progression. This is achieved through the regulation of miR-223, a microRNA associated with inflammatory processes. LncRNA-ATB reduces the expression of crucial articulatory proteins such as MyD88 and p38 within the TLR4 signaling pathway, which in turn inhibits the activation of the NF-κB and MAPK pathways. This regulatory effect helps to curb inflammation and may potentially slow the degenerative processes characteristic of OA.167
PACER is located on chromosome 1q32.2, and its transcript is approximately 2.2 kb long. PACER is immediately adjacent to the COX-2 gene (PTGS2). Upstream of COX-2, PACER can regulate transcriptional activation of the COX-2 gene by interacting with the p50 subunit of NF-κB and derepressing p50, which affects the inflammatory response and other related biological processes and participates in various pathophysiological evolutions.168
PACER expression in the serum of OA patients is lower compared to healthy controls, which correlates with increased levels of COX2, PGE2, TNF-α, and IL-1β, and decreased levels of IL-4 and IL-10. Further studies showed that silencing PACER in OA chondrocytes significantly raised COX-2 protein levels, along with PGE2, TNF-α, and IL-1β levels, while the secretion of anti-inflammatory factors IL-4 and IL-10 significantly declined. Conversely, PACER overexpression reversed these effects. Thus, PACER significantly influences the pathogenesis of OA by activating COX2, which enhances PGE2 release and intensifies the inflammatory response, highlighting its critical role in the inflammatory processes that drive the development and progression of OA.169 PACER expression was found to be significantly reduced in osteoarthritic cartilage from hip and knee joints, suggesting that PACER may play a protective role in preventing inflammation-induced cartilage degeneration. This decrease indicates its potential involvement in mitigating the progression of osteoarthritis.170 PACER was found to improve OA symptoms by regulating the expression of HOTAIR LncRNA, a LncRNA known to regulate inflammation and cell proliferation, which exerts a protective effect on OA by inhibiting chondrocyte apoptosis and influencing the course of inflammatory responses through the regulation of HOTAIR LncRNA expression.171
OIP5-AS1, positioned on human chromosome 15q15.1, spans a coding length of approximately 3.5 kb. In recent years, the research focus on OIP5-AS1 has expanded significantly, encompassing a variety of cancers and other diseases. This increased attention highlights its potential role in the molecular mechanisms underlying these conditions and its importance as a target for further investigation. It plays an important role in regulating biological processes such as gene expression, cell proliferation, apoptosis, migration, and invasion. The up-or down-regulation of OIP5-AS1 is closely linked to the onset, development, and prognosis of diseases.172
OIP5-AS1 expression was observed to be lower in OA specimens and IL-1β-induced OA chondrocyte models compared to controls, indicating that its down-regulation may play a significant role in OA progression. Overexpression of OIP5-AS1 in chondrocytes results in decreased secretion of IL-6, IL-8, and TNF-α. This modulation of cytokine levels contributes to enhanced chondrocyte viability and migration and reduced apoptosis, highlighting the protective potential of OIP5-AS1 in OA. These effects collectively suggest a protective role for OIP5-AS1 in maintaining chondrocyte health and potentially mitigating the pathological progression of conditions like osteoarthritis. OIP5-AS1 is also responsible for reduced apoptosis. OIP5-AS1 can up-regulate the inflammatory suppressor PGRN through miR-29b-3p uptake and protect OA chondrocytes from damage by modulating the inflammatory response.173 OIP5-AS1 can also inhibit activation of the PI3K/AKT pathway via miR-338-3p uptake, reduce expression of IL-6, IL-8, MMPs, and attenuate inflammation and ECM degradation.174 It also activates AMPK/Akt/mTOR signaling by promoting PPAR-γ expression, enhances mitochondrial autophagy, attenuates LPS-induced chondrocyte damage and delays the progression of OA.175
Conclusions and Outlook
OA is one of the most common types of degenerative joint disease, endangering human physical and mental health and imposing a considerable economic burden on the patient’s family and public health. Inflammation actively contributes to the pathogenesis of OA, driving processes such as cartilage degeneration, subchondral bone destruction, and synovial proliferation and inflammation. The different tissues of joints affected by OA can interact with a large number of pro-inflammatory mediators via the same or different signaling pathways, which can exacerbate damage to joint tissues and lead to a vicious circle that exacerbates the development of OA.
LncRNAs play a crucial role in the pathogenesis and progression of OA by modulating the inflammatory response through various mechanisms. These binding to miRNAs, interactions with proteins, and effects on chromatin structure. Extensive research has highlighted that the aberrant expression of specific LncRNAs correlates strongly with OA, revealing detailed insights into their mechanisms of action in OA inflammation. For instance, HOTAIR influences chondrocyte inflammatory responses by modulating the Wnt/β-catenin, while MALAT1 affects inflammation through the NF-κB. MEG3 is known to suppress the inflammatory response via the p53 signaling pathway, and H19 exacerbates inflammation by competitively binding to miR-29b-3p, which impacts various inflammatory mediators. Furthermore, many LncRNAs regulate the inflammatory response in OA through epigenetic modifications, including DNA methylation and histone modification, thus affecting inflammation-related gene expression. These mechanisms highlight the complex roles LncRNAs play in both, directly and indirectly, regulating inflammatory processes and modulating the progression of OA. This intricate interplay offers potential targets for therapeutic interventions aimed at mitigating the debilitating effects of OA (Table 1).
Table 1.
Role of LncRNA in OA Inflammation
| LncRNA | Expression in OA | Role in OA Inflammation | Pathways/Targets/Mechanisms | Reference |
|---|---|---|---|---|
| MALAT1 | Upregulated | Enhances the expression and release of inflammatory mediators; modulates cellular responses like pyroptosis and apoptosis | Modulates NF-κB, TLR4, Wnt/β-catenin, PI3K/Akt/mTOR; acts as a ceRNA interacting with miR-146a, miR-145, miR-9; affects cytokine release, ECM degradation, and cell proliferation. | [91–96] |
| PVT1 | Upregulated | Promotes inflammatory responses, ECM degradation, and chondrocyte apoptosis. | Influences TLR4/NF-κB TGF-β/SMAD4 pathways; acts as a ceRNA; targets such as miR −146a, miR-93-5p, miR-27b-3p, miR-140, miR-211-3p influencing MMP and cytokine levels. | [100–102,104,105,107] |
| H19 | Upregulated | Enhance the release of inflammatory cytokines, affect the vitality and proliferation of chondrocytes, and promote angiogenesis | Regulates pathways like NF-κB, TLR4, JNK and AKT; interacts with miR-106a-5p, miR130a, miR-103a affecting cytokine levels and ECM components. | [113–116,118] |
| HOTAIR | Upregulated | Contributes to chondrocyte apoptosis and inflammatory responses. | Regulates pathways like NF-κB/TNFα, MAPK/c-Jun; modulates miRNA function impacting inflammatory signaling and ECM degradation such as miR-1277-5P, miR-107, miR-222-3p, miR-17-3p | [123,125–128] |
| NEAT1 | Upregulated | Targets miRNAs to regulate inflammation and apoptosis in chondrocytes. | Involves Wnt/β-catenin and NF-κB pathways; interacts with miRNAs like miR-543, miR-146a-3p, miR-193a-3p, miR-16-5p affecting inflammation-related gene expression. | [120,130,131,133–136] |
| THRIL | Upregulated | Promotes inflammation and chondrocyte apoptosis, exacerbates inflammatory injury. | Modulates JAK1/STAT3, NF-κB, PI3K/AKT pathways; influences cytokine production and cellular viability. | [139,140] |
| XIST | Upregulated | Regulates gene expression related to inflammation and chondrocyte viability. | Acts as a ceRNA for miR-149-5p, miR-376c-5p; interacts with pathways like PI3K/Akt and affects cytokine levels and ECM components. | [142–144] |
| MEG3 | Downregulated | Plays a role in reducing inflammatory responses, chondrocyte apoptosis and reduces angiogenesis. | Regulation of miR-16, miR-93 and other miRNAs; It affects TGF-β, TLR4/ NF-κB pathway and ECM degradation | [146,150,151,153,155,156] |
| GAS5 | Downregulated | Inhibits chondrocyte apoptosis and modulates inflammatory responses. | Functions as a ceRNA for miR-146a; impacts Smad4 expression and inflammatory signaling pathways. | [158–160] |
| NKILA | Downregulated | Inhibit the activation of NF-κB, reduce the expression of inflammatory mediators and angiogenesis | Stabilizes the NF-κB/IκB complex; modulates inflammation and cellular responses in chondrocytes. | [162–164] |
| ATB | Downregulated | Reduces inflammatory mediators and promotes chondrocyte viability. | Regulates Akt phosphorylation; affects MyD88 and p38 in the TLR4/NF-κB and MAPK pathways. | [166,167] |
| PACER | Downregulated | Modulates affecting inflammatory responses and cartilage degeneration. | Interacts with NF-κB p50 subunit | [169] |
| OIP5-AS1 | Downregulated | Mitigates inflammatory responses and promotes chondrocyte viability. | Regulates PI3K/AKT pathway, AMPK/Akt/mTOR; interacts with miR-338-3p affecting cytokine expression and ECM integrity. | [173–175] |
Growing evidence suggests that LncRNAs play a critical role in the regulation of OA development and progression. Targeted regulation of specific LncRNAs has emerged as a promising strategy to slow OA progression and promote cartilage repair. Targeting LncRNA with drugs is a good treatment method. For instance, docosahexaenoic acid (DHA) exerts anti-inflammatory effects, cartilage protection, and promotes cartilage formation by regulating MALAT1 expression. Studies have shown that MALAT1 plays a crucial regulatory role in OA pathology, providing significant protection against inflammation and degradation in OA chondrocytes.94 Similarly, Achyranthes bidentata polysaccharides (ABPS), an active component of traditional Chinese medicine, inhibit chondrocyte apoptosis by regulating NEAT1 expression, thereby delaying OA progression.176 Further studies indicate that tanshinone IIA (TAN) enhances the IL-1β-suppressed expression of NEAT1_2 and regulates the transcription of cartilage phenotype-related genes, thereby promoting cartilage regeneration in inflammatory environments.177 In addition, xanthohumol (XH) inhibits the expression of LncRNA GAS5, improving mechanically induced MMP-13 expression and reducing ECM degradation, demonstrating its potential protective effect on OA progression.178
In addition to drug-targeted therapies, extracellular vesicles (EVs) have shown great potential as gene delivery vehicles. EVs can selectively deliver downregulated LncRNAs to target tissues, providing a novel gene therapy approach for treating OA and other degenerative diseases. Studies have shown that exosomes derived from bone marrow mesenchymal stem cells (BMSCs), when injected into primary chondrocytes, can deliver MEG3, effectively reducing IL-1β-induced chondrocyte senescence and apoptosis, indicating that MEG3 may be a potential therapeutic target for OA.149 Similarly, EVs derived from human mesenchymal stem cells (HMSCs) can deliver MALAT1, promoting chondrocyte proliferation, reducing inflammation and cartilage degeneration, and enhancing cartilage repair, further highlighting the therapeutic potential of LncRNAs in OA.179 Nanotechnology also provides a novel perspective for targeted LncRNA therapies.180 With their excellent biocompatibility and degradability, nanoparticles have become ideal carriers for delivering LncRNAs to specific tissues. By utilizing the precise targeting capabilities of nanoparticles, not only can the efficiency of LncRNA therapies be improved, but safety can also be enhanced. However, despite the enormous potential of nanotechnology in gene therapy, there is currently no systematic experimental research on nanoparticle-based LncRNA delivery strategies in OA, and further exploration of the feasibility and applications in this field is urgently needed.
Although progress has been made on the mechanism of action of LncRNAs in OA inflammation, many questions still need to be answered. Firstly, current research into lncRNA function focuses mainly on a few known LncRNAs, and in the future, we need to broaden the scope of research to systematically screen and identify more LncRNAs with a role in OA inflammation. We need to use technological means such as single-cell sequencing to study whether LncRNAs are specific to different cell types.181 LncRNA as a biomarker is not accurate enough, and more lncRNA models containing lncRNA need to be proposed. The complex interactions between LncRNAs, miRNAs, and circRNAs are crucial areas for future research. Understanding how these non-coding RNAs influence each other could reveal new insights into cellular regulation and disease mechanisms, potentially leading to innovative therapeutic approaches.
In future research, there are several directions worth considering. Firstly, with advances in high-throughput sequencing technology, more LncRNAs will be identified and functionally annotated, and complete resolution of the LncRNA mechanism in OA inflammation will be an important direction for future research. Translational clinical research is also an important direction for future LncRNA research. Although some LncRNAs have been shown to have promising therapeutic potential in laboratory studies, their safety and efficacy in clinical applications have yet to be verified by many studies.
Overall, research into the mechanism of LncRNA’s role in OA inflammation is in full development.
Future studies should focus on expanding basic research and advancing clinical translation to offer new prospects for the diagnosis and treatment of OA.
Funding Statement
The work was supported by Gansu University of Chinese Medicine to introduce a talent research start-up fund (2024YJRC-09) and The 2024 Gansu Basic Research Program-Natural Science Funds-Youth Science and Technology Fund (24JRRA567).
Data Sharing Statement
No Data associated in the manuscript.
Consent for Publication
All authors agreed to publish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
- 1.Du X, Liu Z-Y, Tao -X-X, et al. Research progress on the pathogenesis of knee osteoarthritis. Orthop Surg. 2023;15(9):2213–2224. doi: 10.1111/os.13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Xie W, Xiao W, Dou D. Progress in osteoarthritis research by the National Natural Science Foundation of China. Bone Res. 2022;10(1):41. doi: 10.1038/s41413-022-00207-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. doi: 10.1038/nrrheum.2014.44 [DOI] [PubMed] [Google Scholar]
- 4.Sharma L. Osteoarthritis of the Knee. N Engl J Med. 2021;384(1):51–59. doi: 10.1056/NEJMcp1903768 [DOI] [PubMed] [Google Scholar]
- 5.Yao ZT, Yang YM, Sun MM, et al. New insights into the interplay between long non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun (Lond). 2022;42(2):117–140. doi: 10.1002/cac2.12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WK, Yu XH, Yang W, et al. lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Prolif. 2017;50(1). doi: 10.1111/cpr.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gareev I, Kudriashov V, Sufianov A, et al. The role of long non-coding RNA ANRIL in the development of atherosclerosis. Noncoding RNA Res. 2022;7(4):212–216. doi: 10.1016/j.ncrna.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Liu Q, Zhang J, et al. The emerging role of lncRNAs in osteoarthritis development and potential therapy. Front Genet. 2023;14:1273933. doi: 10.3389/fgene.2023.1273933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Sun Y, Liu J, et al. Roles of long non‑coding RNA in osteoarthritis (Review). Int J Mol Med. 2021;48(1). doi: 10.3892/ijmm.2021.4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Zhang Z, Ma X, Liu X. Advances in Research on the Regulatory Roles of lncRNAs in Osteoarthritic Cartilage. Biomolecules. 2023;13(4):580. doi: 10.3390/biom13040580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Lin H, Zou M, et al. Nicotine in Inflammatory Diseases: anti-Inflammatory and Pro-Inflammatory Effects. Front Immunol. 2022;13:826889. doi: 10.3389/fimmu.2022.826889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques O, Weiss G, Muckenthaler MU. The role of iron in chronic inflammatory diseases: from mechanisms to treatment options in anemia of inflammation. Blood. 2022;140(19):2011–2023. doi: 10.1182/blood.2021013472 [DOI] [PubMed] [Google Scholar]
- 13.Jogpal V, Sanduja M, Dutt R, Garg V, Tinku. Advancement of nanomedicines in chronic inflammatory disorders. Inflammopharmacology. 2022;30(2):355–368. doi: 10.1007/s10787-022-00927-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terkawi MA, Ebata T, Yokota S, et al. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines. 2022;10(5). doi: 10.3390/biomedicines10051109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J, Abu-Amer Y, O’Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58(1):49–63. doi: 10.1080/03008207.2016.1208655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansari MY, Haqqi TM. Interleukin-1β induced stress granules Sequester COX-2 mRNA and regulates its stability and translation in human OA Chondrocytes. Sci Rep. 2016;6:27611. doi: 10.1038/srep27611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansari MY, Ahmad N, Haqqi TM. Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1β stimulated human chondrocytes. Cell Physiol Biochem. 2018;49(3):932–946. doi: 10.1159/000493225 [DOI] [PubMed] [Google Scholar]
- 19.Dai SM, Shan ZZ, Nishioka K, Yudoh K. Implication of interleukin 18 in production of matrix metalloproteinases in articular chondrocytes in arthritis: direct effect on chondrocytes may not be pivotal. Ann Rheum Dis. 2005;64(5):735–742. doi: 10.1136/ard.2004.026088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohzono H, Hu Y, Nagira K, et al. Targeting FoxO transcription factors with HDAC inhibitors for the treatment of osteoarthritis. Ann Rheum Dis. 2023;82(2):262–271. doi: 10.1136/ard-2021-221269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang BW, Jiang Y, Yao ZL, Chen PS, Yu B, Wang SN. Aucubin protects chondrocytes against IL-1β-induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des Devel Ther. 2019;13:3529–3538. doi: 10.2147/dddt.S210220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan X, Zheng D, Lin Q, et al. Confirmation of pain-related neuromodulation mechanism of Bushen Zhuangjin Decoction on knee osteoarthritis. J Ethnopharmacol. 2024;324:117772. doi: 10.1016/j.jep.2024.117772 [DOI] [PubMed] [Google Scholar]
- 23.Ding L, Liao T, Yang N, et al. Chrysin ameliorates synovitis and fibrosis of osteoarthritic fibroblast-like synoviocytes in rats through PERK/TXNIP/NLRP3 signaling. Front Pharmacol. 2023;14:1170243. doi: 10.3389/fphar.2023.1170243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Liu S, Chen Y, et al. Teriparatide ameliorates articular cartilage degradation and aberrant subchondral bone remodeling in DMM mice. J Orthop Translat. 2023;38:241–255. doi: 10.1016/j.jot.2022.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi: 10.1038/nrrheum.2016.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Y, Feng B, Ji X, et al. Complement factor H attenuates TNF-α-induced inflammation by upregulating EIF3C in rheumatoid arthritis. J Transl Med. 2023;21(1):846. doi: 10.1186/s12967-023-04730-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuang H, Ren X, Jiang F, Zhou P. Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol Med. 2023;29(1):17. doi: 10.1186/s10020-023-00614-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayaram P, Mitchell PJT, Shybut TB, Moseley BJ, Lee B. Leukocyte-rich platelet-rich plasma is predominantly anti-inflammatory compared with leukocyte-poor platelet-rich plasma in patients with mild-moderate knee osteoarthritis: a prospective, descriptive laboratory study. Am J Sports Med. 2023;51(8):2133–2140. doi: 10.1177/03635465231170394 [DOI] [PubMed] [Google Scholar]
- 29.Cilek MZ, de Vega S, Shiozawa J, et al. Synergistic upregulation of ADAMTS4 (aggrecanase-1) by cytokines and its suppression in knee osteoarthritic synovial fibroblasts. Lab Invest. 2022;102(1):102–111. doi: 10.1038/s41374-021-00685-4 [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Dong B, Yuan P, Li X. Human umbilical cord mesenchymal stem cells promoting knee joint chondrogenesis for the treatment of knee osteoarthritis: a systematic review. J Orthop Surg Res. 2023;18(1):639. doi: 10.1186/s13018-023-04131-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboudeya HM, Shaker SA, Salama M. Effect of short-term high fat diet on resistin levels and expression of autophagy-related genes in the cartilage of male rats. Sci Rep. 2022;12(1):15313. doi: 10.1038/s41598-022-19481-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bo W, Zhou J, Wang K. Sodium butyrate abolishes the degradation of type II collagen in human chondrocytes. Biomed Pharmacother. 2018;102:1099–1104. doi: 10.1016/j.biopha.2018.03.062 [DOI] [PubMed] [Google Scholar]
- 33.Chen NF, Lin YY, Yao ZK, et al. Oral administration of protease-soluble chicken type II collagen ameliorates anterior cruciate ligament transection-induced osteoarthritis in rats. Nutrients. 2023;15(16). doi: 10.3390/nu15163589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Bai X, Fan Y, Jia Q, Zhang H, Hou H. Structure of type II collagen from sturgeon cartilage and its effect on adjuvant-induced rheumatoid arthritis in rats. Food Funct. 2022;13(11):6152–6165. doi: 10.1039/d1fo03929f [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Yang H, Wang Z, Zhu R, Cheng L, Cheng Q. Low-intensity pulsed ultrasound promotes mesenchymal stem cell transplantation-based articular cartilage regeneration via inhibiting the TNF signaling pathway. Stem Cell Res Ther. 2023;14(1):93. doi: 10.1186/s13287-023-03296-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YH, Wang ML, Tao YC, Wu DB, Chen EQ, Tang H. The high level of IL-1β in the serum of ACLF patients induces increased IL-8 expression in hUC-MSCs and reduces the efficacy of hUC-MSCs in liver failure. Stem Cell Res Ther. 2023;14(1):231. doi: 10.1186/s13287-023-03455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmutovic Persson I, Menzel M, Ramu S, Cerps S, Akbarshahi H, Uller L. IL-1β mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir Res. 2018;19(1):16. doi: 10.1186/s12931-018-0725-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Zhou X, Jiang X, et al. Single-cell profiling identifies IL1B(hi) macrophages associated with inflammation in PD-1 inhibitor-induced inflammatory arthritis. Nat Commun. 2024;15(1):2107. doi: 10.1038/s41467-024-46195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L, Zhang W, Liu T, et al. The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. 2023;62:102663. doi: 10.1016/j.redox.2023.102663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li WW, Fan XX, Xu ZS, et al. BLK positively regulates TLR/IL-1R signaling by catalyzing TOLLIP phosphorylation. J Cell Biol. 2024;223(2). doi: 10.1083/jcb.202302081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabeian H, Betti BF, Dos Santos Cirqueira C, et al. IL-1β Damages Fibrocartilage and Upregulates MMP-13 expression in fibrochondrocytes in the condyle of the temporomandibular joint. Int J Mol Sci. 2019;20(9). doi: 10.3390/ijms20092260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubota A, Hasegawa K, Suguro T, Koshihara Y. Tumor necrosis factor-alpha promotes the expression of osteoprotegerin in rheumatoid synovial fibroblasts. J Rheumatol. 2004;31(3):426–435. [PubMed] [Google Scholar]
- 43.Muendlein HI, Connolly WM, Cameron J, et al. Neutrophils and macrophages drive TNF-induced lethality via TRIF/CD14-mediated responses. Sci Immunol. 2022;7(78):eadd0665. doi: 10.1126/sciimmunol.add0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, Giotti B, Kaluzova M, et al. A paracrine circuit of IL-1β/IL-1R1 between myeloid and tumor cells drives genotype-dependent glioblastoma progression. J Clin Invest. 2023;133(22). doi: 10.1172/jci163802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Ye R, Fan L, et al. A TNF-α blocking peptide that reduces NF-κB and MAPK activity for attenuating inflammation. Bioorg Med Chem. 2023;92:117420. doi: 10.1016/j.bmc.2023.117420 [DOI] [PubMed] [Google Scholar]
- 46.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Zhang B, Zhou Z, et al. Integrated network pharmacology and experimental validation approach to investigate the mechanisms of radix rehmanniae praeparata - angelica sinensis - radix achyranthis bidentatae in treating knee osteoarthritis. Drug Des Devel Ther. 2024;18:1583–1602. doi: 10.2147/dddt.S455006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, He Q, Chen B, et al. Cardamonin protects against iron overload induced arthritis by attenuating ROS production and NLRP3 inflammasome activation via the SIRT1/p38MAPK signaling pathway. Sci Rep. 2023;13(1):13744. doi: 10.1038/s41598-023-40930-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao CW, Song WX, Liu B, et al. Resistin induces chemokine and matrix metalloproteinase production via CAP1 receptor and activation of p38-MAPK and NF-κB signalling pathways in human chondrocytes. Clin Exp Rheumatol. 2022;40(3):501–513. doi: 10.55563/clinexprheumatol/avcj31 [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Shao Y, Yao Z, et al. Mechanical overloading promotes chondrocyte senescence and osteoarthritis development through downregulating FBXW7. Ann Rheum Dis. 2022;81(5):676–686. doi: 10.1136/annrheumdis-2021-221513 [DOI] [PubMed] [Google Scholar]
- 51.Yan J, Feng G, Yang Y, et al. Nintedanib ameliorates osteoarthritis in mice by inhibiting synovial inflammation and fibrosis caused by M1 polarization of synovial macrophages via the MAPK/PI3K-AKT pathway. FASEB J. 2023;37(10):e23177. doi: 10.1096/fj.202300944RR [DOI] [PubMed] [Google Scholar]
- 52.Haseeb A, Ansari MY, Haqqi TM. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J Orthop Res. 2017;35(2):311–320. doi: 10.1002/jor.23262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ansari MY, Khan NM, Ahmad N, Green J, Novak K, Haqqi TM. Genetic Inactivation of ZCCHC6 suppresses interleukin-6 expression and reduces the severity of experimental osteoarthritis in mice. Arthritis Rheumatol. 2019;71(4):583–593. doi: 10.1002/art.40751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi: 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basu A, Das AS, Borah PK, Duary RK, Mukhopadhyay R. Biochanin A impedes STAT3 activation by upregulating p38δ MAPK phosphorylation in IL-6-stimulated macrophages. Inflamm Res. 2020;69(11):1143–1156. doi: 10.1007/s00011-020-01387-1 [DOI] [PubMed] [Google Scholar]
- 56.Symons RA, Colella F, Collins FL, et al. Targeting the IL-6-Yap-Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis. Ann Rheum Dis. 2022;81(2):214–224. doi: 10.1136/annrheumdis-2021-220875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou SM, Chen PC, Lin CM, Fang ML, Chi MC, Liu JF. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res Ther. 2020;22(1):251. doi: 10.1186/s13075-020-02331-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng Z, Zhang R, Li M, et al. STAT3/IL-6 dependent induction of inflammatory response in osteoblast and osteoclast formation in nanoscale wear particle-induced aseptic prosthesis loosening. Biomater Sci. 2021;9(4):1291–1300. doi: 10.1039/d0bm01256d [DOI] [PubMed] [Google Scholar]
- 59.Feng W, Yang P, Liu H, Zhang F, Li M. IL-6 promotes low concentration of RANKL-induced osteoclastic differentiation by mouse BMMs through trans-signaling pathway. J Mol Histol. 2022;53(3):599–610. doi: 10.1007/s10735-022-10077-7 [DOI] [PubMed] [Google Scholar]
- 60.Wu Q, Zhou X, Huang D, Ji Y, Kang F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell Physiol Biochem. 2017;41(4):1360–1369. doi: 10.1159/000465455 [DOI] [PubMed] [Google Scholar]
- 61.Chen B, He Q, Chen C, et al. Combination of curcumin and catalase protects against chondrocyte injury and knee osteoarthritis progression by suppressing oxidative stress. Biomed Pharmacother. 2023;168:115751. doi: 10.1016/j.biopha.2023.115751 [DOI] [PubMed] [Google Scholar]
- 62.Ye H, Long Y, Yang JM, et al. Curcumin regulates autophagy through SIRT3-SOD2-ROS signaling pathway to improve quadriceps femoris muscle atrophy in KOA rat model. Sci Rep. 2024;14(1):8176. doi: 10.1038/s41598-024-58375-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang YF, Wang G, Ding L, et al. Lactate-upregulated NADPH-dependent NOX4 expression via HCAR1/PI3K pathway contributes to ROS-induced osteoarthritis chondrocyte damage. Redox Biol. 2023;67:102867. doi: 10.1016/j.redox.2023.102867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou F, Mei J, Han X, et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9(5):973–985. doi: 10.1016/j.apsb.2019.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586–1592. doi: 10.1016/j.biopha.2018.09.161 [DOI] [PubMed] [Google Scholar]
- 66.Wu J, Li H, Hu F, Luo P. Stevioside attenuates osteoarthritis via regulating Nrf2/HO-1/NF-κB pathway. J Orthop Translat. 2023;38:190–202. doi: 10.1016/j.jot.2022.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caric D, Zekic Tomas S, Filipovic N, et al. Expression Pattern of iNOS, BCL-2 and MMP-9 in the Hip Synovium Tissue of Patients with Osteoarthritis. Int J Mol Sci. 2021;22(3). doi: 10.3390/ijms22031489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen Y, Teng L, Qu Y, et al. Anti-proliferation and anti-inflammation effects of corilagin in rheumatoid arthritis by downregulating NF-κB and MAPK signaling pathways. J Ethnopharmacol. 2022;284:114791. doi: 10.1016/j.jep.2021.114791 [DOI] [PubMed] [Google Scholar]
- 69.Yao N, Chen N, Xu X, et al. Protective effect of Shenmai injection on knee articular cartilage of osteoarthritic rabbits and IL-1β-stimulated human chondrocytes. Exp Ther Med. 2017;13(6):3013–3020. doi: 10.3892/etm.2017.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan T, Ding LG, Lu BY, et al. Combined administration of curcumin and chondroitin sulfate alleviates cartilage injury and inflammation via NF-κB pathway in knee osteoarthritis rats. Front Pharmacol. 2022;13:882304. doi: 10.3389/fphar.2022.882304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li D, Zhang X, Li L, Ma Z, Su F, Wang T. Patellar inward pushing method relieves knee osteoarthritis via regulating cytokines. Transpl Immunol. 2022;72:101534. doi: 10.1016/j.trim.2022.101534 [DOI] [PubMed] [Google Scholar]
- 72.Moore JE, Purcaro MJ, Pratt HE, et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583:699–710. doi: 10.1038/s41586-020-2493-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herman AB, Tsitsipatis D, Gorospe M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell. 2022;82(12):2252–2266. doi: 10.1016/j.molcel.2022.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–157. doi: 10.1038/nrm.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kesner JS, Chen Z, Shi P, et al. Noncoding translation mitigation. Nature. 2023;617:395–402. doi: 10.1038/s41586-023-05946-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez CAG, Adachi S, Nong QD, et al. Sense-overlapping lncRNA as a decoy of translational repressor protein for dimorphic gene expression. PLoS Genet. 2021;17(7):e1009683. doi: 10.1371/journal.pgen.1009683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng F, Chen J, Zhang X, et al. The HIF-1α antisense long non-coding RNA drives a positive feedback loop of HIF-1α mediated transactivation and glycolysis. Nat Commun. 2021;12(1):1341. doi: 10.1038/s41467-021-21535-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartl J, Zanini M, Bernardi F, et al. The HHIP-AS1 lncRNA promotes tumorigenicity through stabilization of dynein complex 1 in human SHH-driven tumors. Nat Commun. 2022;13(1):4061. doi: 10.1038/s41467-022-31574-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. doi: 10.1038/nrg.2015.10 [DOI] [PubMed] [Google Scholar]
- 80.Mattick JS, Amaral PP, Carninci P, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24(6):430–447. doi: 10.1038/s41580-022-00566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mo BY, Guo XH, Yang MR, et al. Long non-coding RNA GAPLINC promotes tumor-like biologic behaviors of fibroblast-like synoviocytes as microRNA sponging in rheumatoid arthritis patients. Front Immunol. 2018;9:702. doi: 10.3389/fimmu.2018.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribeiro DM, Zanzoni A, Cipriano A, et al. Protein complex scaffolding predicted as a prevalent function of long non-coding RNAs. Nucleic Acids Res. 2018;46(2):917–928. doi: 10.1093/nar/gkx1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goyal B, Yadav SRM, Awasthee N, Gupta S, Kunnumakkara AB, Gupta SC. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188502. doi: 10.1016/j.bbcan.2021.188502 [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Li S, Zhang Y, et al. The lncRNA Malat1 regulates microvascular function after myocardial infarction in mice via miR-26b-5p/Mfn1 axis-mediated mitochondrial dynamics. Redox Biol. 2021;41:101910. doi: 10.1016/j.redox.2021.101910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu L, Yan LN, Sui Z. MicroRNA-150 affects endoplasmic reticulum stress via MALAT1-miR-150 axis-mediated NF-κB pathway in LPS-challenged HUVECs and septic mice. Life Sci. 2021;265:118744. doi: 10.1016/j.lfs.2020.118744 [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Mou Q, Zhu Z, Zhao L, Zhu L. MALAT1 promotes liver fibrosis by sponging miR‑181a and activating TLR4‑NF‑κB signaling. Int J Mol Med. 2021;48(6). doi: 10.3892/ijmm.2021.5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin LP, Niu GH, Zhang XQ. Influence of lncRNA MALAT1 on septic lung injury in mice through p38 MAPK/p65 NF-κB pathway. Eur Rev Med Pharmacol Sci. 2019;23(3):1296–1304. doi: 10.26355/eurrev_201902_17025 [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y, Zhang H, Zhang Z, et al. LncRNA MALAT1 cessation antagonizes hypoxia/reoxygenation injury in hepatocytes by inhibiting apoptosis and inflammation via the HMGB1-TLR4 axis. Mol Immunol. 2019;112:22–29. doi: 10.1016/j.molimm.2019.04.015 [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Wang F, Chen G, He R, Yang L. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019;9:54. doi: 10.1186/s13578-019-0302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, Xie S, Li H, Zhang R, Zhang H. LncRNA MALAT1 mediates proliferation of LPS treated-articular chondrocytes by targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sci. 2020;254:116801. doi: 10.1016/j.lfs.2019.116801 [DOI] [PubMed] [Google Scholar]
- 92.Liu C, Ren S, Zhao S, Wang Y. LncRNA MALAT1/MiR-145 Adjusts IL-1β-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med J. 2019;60(11):1081–1092. doi: 10.3349/ymj.2019.60.11.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rozi R, Zhou Y, Rong K, Chen P. miR-124-3p sabotages lncRNA MALAT1 stability to repress chondrocyte pyroptosis and relieve cartilage injury in osteoarthritis. J Orthop Surg Res. 2022;17(1):453. doi: 10.1186/s13018-022-03334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng L, Yang Z, Li Y, et al. Malat1 attenuated the rescuing effects of docosahexaenoic acid on osteoarthritis treatment via repressing its chondroprotective and chondrogenesis activities. Biomed Pharmacother. 2022;154:113608. doi: 10.1016/j.biopha.2022.113608 [DOI] [PubMed] [Google Scholar]
- 95.Zhang G, Zhang H, You W, Tang X, Li X, Gong Z. Therapeutic effect of Resveratrol in the treatment of osteoarthritis via the MALAT1/miR-9/NF-κB signaling pathway. Exp Ther Med. 2020;19(3):2343–2352. doi: 10.3892/etm.2020.8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD. Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42(4):669–680. doi: 10.1016/j.bone.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 97.Nanus DE, Wijesinghe SN, Pearson MJ, et al. Regulation of the inflammatory synovial fibroblast phenotype by metastasis-associated lung adenocarcinoma transcript 1 long noncoding RNA in obese patients with osteoarthritis. Arthritis Rheumatol. 2020;72(4):609–619. doi: 10.1002/art.41158 [DOI] [PubMed] [Google Scholar]
- 98.Wu F, Zhu Y, Zhou C, Gui W, Li H, Lin X. Regulation mechanism and pathogenic role of lncRNA plasmacytoma variant translocation 1 (PVT1) in human diseases. Genes Dis. 2023;10(3):901–914. doi: 10.1016/j.gendis.2022.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xing D, Liang JQ, Li Y, et al. Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop Surg. 2014;6(4):288–293. doi: 10.1111/os.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu X, Yu Y, Yin F, et al. Knockdown of PVT1 inhibits IL-1β-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int Immunopharmacol. 2020;79:106052. doi: 10.1016/j.intimp.2019.106052 [DOI] [PubMed] [Google Scholar]
- 101.Yao N, Peng S, Wu H, Liu W, Cai D, Huang D. Long noncoding RNA PVT1 promotes chondrocyte extracellular matrix degradation by acting as a sponge for miR-140 in IL-1β-stimulated chondrocytes. J Orthop Surg Res. 2022;17(1):218. doi: 10.1186/s13018-022-03114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu K, Meng Z, Xian X-M, et al. LncRNA PVT1 induces chondrocyte apoptosis through upregulation of TNF-α in synoviocytes by sponging miR-211-3p. Mol Cell Probes. 2020;52:101560. doi: 10.1016/j.mcp.2020.101560 [DOI] [PubMed] [Google Scholar]
- 103.Zhao Y, Zhao J, Guo X, She J, Liu Y. Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes. Biosci Rep. 2018;38(5). doi: 10.1042/bsr20180576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y-Z, Yao L, Liang S-K, et al. LncPVT1 promotes cartilage degradation in diabetic OA mice by downregulating miR-146a and activating TGF-β/SMAD4 signaling. J Bone Miner Metab. 2021;39(4):534–546. doi: 10.1007/s00774-020-01199-7 [DOI] [PubMed] [Google Scholar]
- 105.Meng Y, Qiu S, Sun L, Zuo J. Knockdown of exosome‑mediated lnc‑PVT1 alleviates lipopolysaccharide‑induced osteoarthritis progression by mediating the HMGB1/TLR4/NF‑κB pathway via miR‑93‑5p. Mol Med Rep. 2020;22(6):5313–5325. doi: 10.3892/mmr.2020.11594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11(7):615–628. doi: 10.1593/neo.09284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang C-W, Wu X, Liu D, et al. Long non-coding RNA PVT1 knockdown suppresses fibroblast-like synoviocyte inflammation and induces apoptosis in rheumatoid arthritis through demethylation of sirt6. J Biol Eng. 2019;13(1):60. doi: 10.1186/s13036-019-0184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hashemi M, Moosavi MS, Abed HM, et al. Long non-coding RNA (lncRNA) H19 in human cancer: from proliferation and metastasis to therapy. Pharmacol Res. 2022;184:106418. doi: 10.1016/j.phrs.2022.106418 [DOI] [PubMed] [Google Scholar]
- 109.Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: roles in tumorigenesis. Biomed Pharmacother. 2020;123:109774. doi: 10.1016/j.biopha.2019.109774 [DOI] [PubMed] [Google Scholar]
- 110.Yang H. Silencing of long non-coding RNA H19 alleviates lipopolysaccharide (LPS)-induced apoptosis and inflammation Injury by Regulating miR-140-5p/TLR4 axis in cell models of pneumonia. Curr Mol Med. 2023;23(3):275–284. doi: 10.2174/1566524022666220407100949 [DOI] [PubMed] [Google Scholar]
- 111.Yang S, Fang F, Yu X, et al. Knockdown of H19 Inhibits the Pathogenesis of Acne Vulgaris by Targeting the miR-196a/TLR2/NF-κB Axis. Inflammation. 2020;43(5):1936–1947. doi: 10.1007/s10753-020-01268-z [DOI] [PubMed] [Google Scholar]
- 112.Yang J, Li Y, Wang L, Zhang Z, Li Z, Jia Q. LncRNA H19 aggravates TNF -α-induced inflammatory injury via TAK1 pathway in MH7A cells. Biofactors. 2020;46(5):813–820. doi: 10.1002/biof.1659 [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Liu X, Ni X, Feng P, Wang YU. Long non-coding RNA H19 modulates proliferation and apoptosis in osteoarthritis via regulating miR-106a-5p. J Biosci. 2019;44(6). doi: 10.1007/s12038-019-9943-x [DOI] [PubMed] [Google Scholar]
- 114.Hu Y, Li S, Zou Y. Knockdown of LncRNA H19 Relieves LPS-Induced Damage by Modulating miR-130a in Osteoarthritis. Yonsei Medical Journal. 2019;60(4):381–388. doi: 10.3349/ymj.2019.60.4.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stuhlmüller B, Kunisch E, Franz J, et al. Detection of oncofetal h19 RNA in rheumatoid arthritis synovial tissue. Am J Pathol. 2003;163(3):901–911. doi: 10.1016/s0002-9440(10)63450-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mu N, Gu J-T, Huang T-L, et al. Blockade of discoidin domain receptor 2 as a strategy for reducing inflammation and joint destruction in rheumatoid arthritis via altered interleukin-15 and Dkk-1 signaling in fibroblast-like synoviocytes. Arthritis Rheumatol. 2020;72(6):943–956. doi: 10.1002/art.41205 [DOI] [PubMed] [Google Scholar]
- 117.Sun Y, Zhong L, He X, et al. LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J Mol Cell Cardiol. 2019;131:66–81. doi: 10.1016/j.yjmcc.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 118.Cheng X-W, Chen Z-F, Wan Y-F, Zhou Q, Wang H, Zhu H-Q. Long Non-coding RNA H19 Suppression protects the endothelium against hyperglycemic-induced inflammation via inhibiting expression of miR-29b target gene vascular endothelial growth factor a through activation of the protein kinase b/endothelial nitric oxide synthase pathway. Front Cell Dev Biol. 2019;7:263. doi: 10.3389/fcell.2019.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raju GSR, Pavitra E, Bandaru SS, et al. HOTAIR: a potential metastatic, drug-resistant and prognostic regulator of breast cancer. Mol Cancer. 2023;22(1):65. doi: 10.1186/s12943-023-01765-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, Yi K, Liu X, et al. HOTAIR Up-regulation activates NF-κB to induce immunoescape in gliomas. Front Immunol. 2021;12:785463. doi: 10.3389/fimmu.2021.785463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu J, Wang Z, Shan Y, Pan Y, Ma J, Jia L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/β-catenin axis. Cell Death Dis. 2018;9(7):711. doi: 10.1038/s41419-018-0746-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dou P, Hu R, Zhu W, et al. Long non-coding RNA HOTAIR promotes expression of ADAMTS-5 in human osteoarthritic articular chondrocytes. Die Pharmazie. 2017;72(2):113–117. doi: 10.1691/ph.2017.6649 [DOI] [PubMed] [Google Scholar]
- 123.Wang B, Sun Y, Liu N, Liu H. LncRNA HOTAIR modulates chondrocyte apoptosis and inflammation in osteoarthritis via regulating miR −1277-5p/ SGTB axis. Wound Repair Regen. 2021;29(3):495–504. doi: 10.1111/wrr.12908 [DOI] [PubMed] [Google Scholar]
- 124.Cao M, Xu W, Yu J, et al. Up-regulation of SGTB is associated with neuronal apoptosis after neuroinflammation induced by lipopolysaccharide. J Mol Histol. 2013;44(5):507–518. doi: 10.1007/s10735-013-9517-4 [DOI] [PubMed] [Google Scholar]
- 125.Lin C, Liu L, Zeng C, et al. Activation of mTORC1 in subchondral bone preosteoblasts promotes osteoarthritis by stimulating bone sclerosis and secretion of CXCL12. Bone Res. 2019;7(1):5. doi: 10.1038/s41413-018-0041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu J, Wu Z, Xiong Y. Knockdown of long noncoding RNA HOTAIR inhibits osteoarthritis chondrocyte injury by miR-107/CXCL12 axis. J Orthop Surg Res. 2021;16(1):410. doi: 10.1186/s13018-021-02547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang J, Luo X, Cai S, Sun J, Wang S, Wei X. Blocking HOTAIR protects human chondrocytes against IL-1β-induced cell apoptosis, ECM degradation, inflammatory response and oxidative stress via regulating miR-222-3p/ADAM10 axis. Int Immunopharmacol. 2021;98:107903. doi: 10.1016/j.intimp.2021.107903 [DOI] [PubMed] [Google Scholar]
- 128.Chen H, Qi J, Bi Q, Zhang S. Expression profile of long noncoding RNA (HOTAIR) and its predicted target miR-17-3p in LPS-induced inflammatory injury in human articular chondrocyte C28/I2 cells. Int J Clin Exp Pathol. 2017;10(9):9146–9157. [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang M, Weng W, Zhang Q, et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J Hematol Oncol. 2018;11(1):113. doi: 10.1186/s13045-018-0656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Papathanasiou I, Balis C, Destounis D, Mourmoura E, Tsezou A. NEAT1-mediated miR-150-5p downregulation regulates b-catenin expression in OA chondrocytes. Funct Integr Genomics. 2023;23(3):246. doi: 10.1007/s10142-023-01139-4 [DOI] [PubMed] [Google Scholar]
- 131.Ning F, Zhu S, Gao H, Deng Y. NEAT1/miR-146a-3p/TrkB/ShcB axis regulates the development and function of chondrocyte. Cell Cycle. 2021;20(20):2174–2194. doi: 10.1080/15384101.2021.1974787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gowler PRW, Li L, Woodhams SG, et al. Peripheral brain-derived neurotrophic factor contributes to chronic osteoarthritis joint pain. Pain. 2020;161(1):61–73. doi: 10.1097/j.pain.0000000000001694 [DOI] [PubMed] [Google Scholar]
- 133.Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li X, Kroin JS, Kc R, et al. Altered spinal microRNA-146a and the microRNA-183 cluster contribute to osteoarthritic pain in knee joints. J Bone Miner Res. 2013;28(12):2512–2522. doi: 10.1002/jbmr.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu F, Liu X, Yang Y, et al. NEAT1/miR-193a-3p/SOX5 axis regulates cartilage matrix degradation in human osteoarthritis. Cell Biol Int. 2020;44(4):947–957. doi: 10.1002/cbin.11291 [DOI] [PubMed] [Google Scholar]
- 136.Li D, Sun Y, Wan Y, Wu X, Yang W. LncRNA NEAT1 promotes proliferation of chondrocytes via down-regulation of miR-16-5p in osteoarthritis. J Gene Med. 2020;22(9):e3203. doi: 10.1002/jgm.3203 [DOI] [PubMed] [Google Scholar]
- 137.Gao S-G, Zeng C, Song Y, et al. Effect of osteopontin on the mRNA expression of ADAMTS4 and ADAMTS5 in chondrocytes from patients with knee osteoarthritis. Exp Ther Med. 2015;9(5):1979–1983. doi: 10.3892/etm.2015.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Q, Wang W, Zhang F, Deng Y, Long Z. NEAT1/miR-181c Regulates Osteopontin (OPN)-mediated synoviocyte proliferation in osteoarthritis. J Cell Biochem. 2017;118(11):3775–3784. doi: 10.1002/jcb.26025 [DOI] [PubMed] [Google Scholar]
- 139.Liu G, Wang Y, Zhang M, Zhang Q. Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol. 2019;66:354–361. doi: 10.1016/j.intimp.2018.11.038 [DOI] [PubMed] [Google Scholar]
- 140.Liang Y, Li H, Gong X, Ding C. Long Non-coding RNA THRIL mediates cell growth and inflammatory response of fibroblast-like synoviocytes by activating PI3K/AKT signals in rheumatoid arthritis. Inflammation. 2020;43(3):1044–1053. doi: 10.1007/s10753-020-01189-x [DOI] [PubMed] [Google Scholar]
- 141.Wang W, Min L, Qiu X, et al. Biological Function of Long Non-coding RNA (LncRNA) Xist. Front Cell Dev Biol. 2021;9:645647. doi: 10.3389/fcell.2021.645647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Y, Liu K, Tang C, Shi Z, Jing K, Zheng J. Long non-coding RNA XIST contributes to osteoarthritis progression via miR-149-5p/DNMT3A axis. Biomed Pharmacother. 2020;128:110349. doi: 10.1016/j.biopha.2020.110349 [DOI] [PubMed] [Google Scholar]
- 143.Li L, Lv G, Wang B, Kuang L. XIST/miR-376c-5p/OPN axis modulates the influence of proinflammatory M1 macrophages on osteoarthritis chondrocyte apoptosis. J Cell Physiol. 2020;235(1):281–293. doi: 10.1002/jcp.28968 [DOI] [PubMed] [Google Scholar]
- 144.Wang YH, Tsai CH, Liu SC, et al. miR-150-5p and XIST interaction controls monocyte adherence: implications for osteoarthritis therapy. Front Immunol. 2022;13:1004334. doi: 10.3389/fimmu.2022.1004334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ghafouri-Fard S, Taheri M. Maternally expressed gene 3 (MEG3): a tumor suppressor long non coding RNA. Biomed Pharmacother. 2019;118:109129. doi: 10.1016/j.biopha.2019.109129 [DOI] [PubMed] [Google Scholar]
- 146.Xu J, Xu Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 2017;7:69. doi: 10.1186/s13578-017-0195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie LX. Evidence for serum miR-15a and miR-16 levels as biomarkers that distinguish sepsis from systemic inflammatory response syndrome in human subjects. Clin Chem Lab Med. 2012;50(8):1423–1428. doi: 10.1515/cclm-2011-0826 [DOI] [PubMed] [Google Scholar]
- 148.Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108(4):601–609. doi: 10.1172/jci12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jin Y, Xu M, Zhu H, et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med. 2021;25(19):9281–9294. doi: 10.1111/jcmm.16860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen K, Zhu H, Zheng MQ, Dong QR. LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage. 2021;13(2_suppl):1274s–1284s. doi: 10.1177/1947603519855759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kopańska M, Szala D, Czech J, et al. MiRNA expression in the cartilage of patients with osteoarthritis. J Orthop Surg Res. 2017;12(1):51. doi: 10.1186/s13018-017-0542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fisch KM, Gamini R, Alvarez-Garcia O, et al. Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthritis Cartilage. 2018;26(11):1531–1538. doi: 10.1016/j.joca.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang Y, Chen D, Yan Z, et al. LncRNA MEG3 Protects Chondrocytes From IL-1β-Induced Inflammation via Regulating miR-9-5p/KLF4 Axis. Front Physiol. 2021;12:617654. doi: 10.3389/fphys.2021.617654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiang M, Liu T, Tian C, et al. Kinsenoside attenuates liver fibro-inflammation by suppressing dendritic cells via the PI3K-AKT-FoxO1 pathway. Pharmacol Res. 2022;177:106092. doi: 10.1016/j.phrs.2022.106092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang A, Hu N, Zhang Y, et al. MEG3 promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-361-5p/FOXO1 axis. BMC Med Genomics. 2019;12(1):201. doi: 10.1186/s12920-019-0649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Su W, Xie W, Shang Q, Su B. The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. Biomed Res Int. 2015;2015:356893. doi: 10.1155/2015/356893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin G, Wu T, Gao X, He Z, Nong W. Research progress of long non-coding RNA GAS5 in malignant tumors. Front Oncol. 2022;12:846497. doi: 10.3389/fonc.2022.846497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cai L, Huang N, Zhang X, Wu S, Wang L, Ke Q. Long non-coding RNA plasmacytoma variant translocation 1 and growth arrest specific 5 regulate each other in osteoarthritis to regulate the apoptosis of chondrocytes. Bioengineered. 2022;13(5):13680–13688. doi: 10.1080/21655979.2022.2063653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang X, Wang C, Zhao J, et al. miR-146a facilitates osteoarthritis by regulating cartilage homeostasis via targeting Camk2d and Ppp3r2. Cell Death Dis. 2017;8(4):e2734. doi: 10.1038/cddis.2017.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang D, Qiu S. LncRNA GAS5 upregulates Smad4 to suppress the apoptosis of chondrocytes induced by lipopolysaccharide. Arch Gerontol Geriatr. 2021;97:104478. doi: 10.1016/j.archger.2021.104478 [DOI] [PubMed] [Google Scholar]
- 161.Wang H, Liu Y, Huan C, et al. NF-κB-Interacting Long Noncoding RNA Regulates HIV-1 Replication and Latency by Repressing NF-κB Signaling. J Virol. 2020;94(17). doi: 10.1128/jvi.01057-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ma X, Cai D, Zhu Y, et al. L-Glutamine alleviates osteoarthritis by regulating lncRNA-NKILA expression through the TGF-β1/SMAD2/3 signalling pathway. Clin Sci (Lond). 2022;136(13):1053–1069. doi: 10.1042/cs20220082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xue H, Yu P, Wang WZ, Niu YY, Li X. The reduced lncRNA NKILA inhibited proliferation and promoted apoptosis of chondrocytes via miR-145/SP1/NF-κB signaling in human osteoarthritis. Eur Rev Med Pharmacol Sci. 2020;24(2):535–548. doi: 10.26355/eurrev_202001_20030 [DOI] [PubMed] [Google Scholar]
- 164.Luo LH, Rao L, Luo LF, Chen K, Ran RZ, Liu XL. Long non-coding RNA NKILA inhibited angiogenesis of breast cancer through NF-κB/IL-6 signaling pathway. Microvasc Res. 2020;129:103968. doi: 10.1016/j.mvr.2019.103968 [DOI] [PubMed] [Google Scholar]
- 165.Li J, Li Z, Zheng W, et al. LncRNA-ATB: an indispensable cancer-related long noncoding RNA. Cell Prolif. 2017;50(6). doi: 10.1111/cpr.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dang X, Lian L, Wu D. The diagnostic value and pathogenetic role of lncRNA-ATB in patients with osteoarthritis. Cell Mol Biol Lett. 2018;23:55. doi: 10.1186/s11658-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ying H, Wang Y, Gao Z, Zhang Q. Long non-coding RNA activated by transforming growth factor beta alleviates lipopolysaccharide-induced inflammatory injury via regulating microRNA-223 in ATDC5 cells. Int Immunopharmacol. 2019;69:313–320. doi: 10.1016/j.intimp.2019.01.056 [DOI] [PubMed] [Google Scholar]
- 168.Gupta SC, Awasthee N, Rai V, Chava S, Gunda V, Challagundla KB. Long non-coding RNAs and nuclear factor-κB crosstalk in cancer and other human diseases. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188316. doi: 10.1016/j.bbcan.2019.188316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.He M, Liu J, Sun Y, Chen X, Wang J, Gao W. FSGT capsule inhibits IL-1β-induced inflammation in chondrocytes and ameliorates osteoarthritis by upregulating LncRNA PACER and downregulating COX2/PGE2. Immun Inflamm Dis. 2024;12(6):e1334. doi: 10.1002/iid3.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pearson MJ, Philp AM, Heward JA, et al. Long Intergenic Noncoding RNAs mediate the human chondrocyte inflammatory response and are differentially expressed in osteoarthritis cartilage. Arthritis Rheumatol. 2016;68(4):845–856. doi: 10.1002/art.39520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jiang M, Liu J, Luo T, Chen Q, Lu M, Meng D. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci Rep. 2019;39(6). doi: 10.1042/bsr20190404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zheng C, Chu M, Chen Q, Chen C, Wang ZW, Chen X. The role of lncRNA OIP5-AS1 in cancer development and progression. Apoptosis. 2022;27(5–6):311–321. doi: 10.1007/s10495-022-01722-3 [DOI] [PubMed] [Google Scholar]
- 173.Zhi L, Zhao J, Zhao H, Qing Z, Liu H, Ma J. Downregulation of LncRNA OIP5-AS1 Induced by IL-1β Aggravates Osteoarthritis via Regulating miR-29b-3p/PGRN. Cartilage. 2021;13(2_suppl):1345s–1355s. doi: 10.1177/1947603519900801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang X, Wang Z, Wang B, Li J, Yuan H. lncRNA OIP5-AS1 attenuates the osteoarthritis progression in IL-1β-stimulated chondrocytes. Open Med (Wars). 2023;18(1):20230721. doi: 10.1515/med-2023-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun Z, Tang J, You T, Zhang B, Liu Y, Liu J. LncRNA OIP5-AS1 promotes mitophagy to alleviate osteoarthritis by up-regulating PPAR-γ to activate AMPK/Akt/mTOR pathway. Mod Rheumatol. 2024. doi: 10.1093/mr/roae015 [DOI] [PubMed] [Google Scholar]
- 176.Fu C, Qiu Z, Huang Y, et al. Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR-377-3p pathway. Biomed Pharmacother. 2022;154:113551. doi: 10.1016/j.biopha.2022.113551 [DOI] [PubMed] [Google Scholar]
- 177.Sun J, Chen W, Zhou Z, et al. Tanshinone IIA facilitates efficient cartilage regeneration under inflammatory factors caused stress via upregulating LncRNA NEAT1_2. Biomedicines. 2023;11(12). doi: 10.3390/biomedicines11123291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zheng T, Zhou Q, Huang J, Lai J, Ji G, Kong D. Xanthohumol inhibited mechanical stimulation-induced articular ECM degradation by mediating lncRNA GAS5/miR-27a Axis. Front Pharmacol. 2021;12:737552. doi: 10.3389/fphar.2021.737552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pan C, Huang W, Chen Q, et al. LncRNA Malat-1 From MSCs-derived extracellular vesicles suppresses inflammation and cartilage degradation in osteoarthritis. Front Bioeng Biotechnol. 2021;9:772002. doi: 10.3389/fbioe.2021.772002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu S, Zhang H, Wang S, et al. Ultrasound-triggered in situ gelation with ROS-controlled drug release for cartilage repair. Mater Horiz. 2023;10(9):3507–3522. doi: 10.1039/d3mh00042g [DOI] [PubMed] [Google Scholar]
- 181.Hu Y, Cui J, Liu H, et al. Single-cell RNA-sequencing analysis reveals the molecular mechanism of subchondral bone cell heterogeneity in the development of osteoarthritis. Rmd Open. 2022;8(2):e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No Data associated in the manuscript.