Abstract
Q fever and chlamydiosis often affect ovine and caprine flocks simultaneously or successively. Combination vaccines effective against these 2 diseases would be of great value in veterinary medicine. Unfortunately, the current effective vaccines are a live vaccine for chlamydiosis and killed vaccine for Q fever. Vaccination of mice with live chlamydiosis vaccine 1B and killed phase I vaccine against Q fever at 2 points on the back at the same time produced good protection against chlamydial abortion. This suggests that it may be possible to vaccinate ewes and goats against chlamydiosis and Q fever simultaneously.
Résumé
La fièvre Q et la chlamydiophilose peuvent souvent affecter des troupeaux ovins et caprins simultanément ou de manière successive. Des combinaisons de vaccins efficaces contre ces deux maladies seraient grandement bénéfiques en médecine vétérinaire. Malheureusement, les vaccins actuels qui sont efficaces sont un vaccin vivant pour la chlamydiophilose et un vaccin tué pour la fièvre Q. La vaccination simultanée à 2 endroits sur le dos de souris avec le vaccin vivant 1B de la chlamydiophilose et le vaccin tué de la phase I de l’agent de la fièvre Q a permis d’induire une bonne protection contre l’avortement par Chlamydophila. Ces résultats suggèrent qu’il serait possible de vacciner simultanément les brebis et les chèvres contre la chlamydophilose et la fièvre Q.
(Traduit par Docteur Serge Messier)
The main causes of infectious abortion in small ruminants include Brucella melitensis, Chlamydophila abortus, Coxiella burnetii, Salmonella abortusovis, and Toxoplasma gondii. They also present a zoonotic potential. Collective and repeated antibiotic treatments during an outbreak are expensive, very often ineffective, and sometimes dangerous, as they can result in antibiotic resistance. Therefore, prevention with antibiotics should be discouraged. Vaccination of animals with an effective vaccine that stops abortion and prevents vaginal excretion at lambing is the recommended control measure. However, 2 or more contagious organisms can simultaneously or successively affect a flock during an outbreak of abortion (1,2). The simultaneous use of 2, 3, or more valences in polyvalent vaccines could, therefore, greatly reduce the cost of veterinary prophylaxis and the incidence of such infections (3). Killed adjuvant vaccines are easy to produce and killed multivalent vaccines are widely used in pediatric vaccination (4). Similarly the combined vaccination of ewes with killed Chlamydophila and Campylobacter strains (1) or Chlamydophila and Coxiella strains (5) has been proposed. Nevertheless, although such vaccination sometimes decreases the rate of abortion, shedding of at least Chlamydophila persists, which is very dangerous from both epidemiological and public health points of view. Only live vaccines are effective against brucellosis (Rev 1 strain), chlamydiosis (1B strain), salmonellosis (Rv6 strain) and toxoplasmosis (S48 strain). Combination of live attenuated vaccines has also been successfully developed. Simultaneous administration of Rev 1, 1B, and Rv 6 vaccines preserves preserves the immunogenicity of the all-3 vaccinal valences in mice (6). Similarly, the association of 3 live Chlamydophila, Brucella, and Salmonella vaccines did not decrease antichlamydophila immunity in ewes (7). The live chlamydiosis vaccine is also compatible with the live toxoplasmosis vaccine if they are injected at 2 separate injection sites, whereas injection at the same site decreases the efficacy of the 1B vaccine (8).
Live C. burnetii vaccine is not yet available, whereas infection of ruminants with C. burnetii represents the major reservoir for human Q fever. A killed C. burnetii vaccine combined with a killed vaccine against chlamydiosis is commercially available in France (Chlamyvax FQ; Mérial, Lyon, France). However, the Coxiella in this vaccine are in phase II and it has previously been shown that only killed vaccines with Coxiella in phase I are effective against the infectious form of C. burnetii, which is in phase I (9–12). A killed phase I-derived vaccine, compounded of highly purified corpuscular antigen C. burnetii strain Nine Mile inactivated by formaldehyde without adjuvant, has been developed in Slovakia (13) and could be marketed by CEVA Santé Animale (Libourne, France). To our knowledge, the effects of combining a live and a killed vaccine have never previously been studied. The aim of this study was to evaluate, in a mouse model, the effects of simultaneous vaccination with the live C. abortus 1B vaccine and the killed phase I-Coxiella vaccine administrated at 2 separate injection sites on the effectiveness against subsequent chlamydia challenge.
For this purpose, 75 6-week-old OF1 mice (Swiss IFFA; Credo, l’Arbresle, France), average weight 20 g, were divided into 4 groups: a single vaccination group (n = 20), which received 1B chlamydial vaccine alone; a double vaccination group (n = 20), which received the live 1B vaccine and the killed phase I Coxiella vaccine; a virulence group (n = 20), which was unvaccinated and challenged; and a control group (n = 15), which was unvaccinated and unchallenged. The mice were reared in a controlled environment (21°C, 60% relative humidity) on sterilized wood shavings with free access to water and sterilized food (14). They were free from common viral and bacterial pathogens according to routine screening procedures. Maintenance and care of experimental animals were in accordance with National Decree, N° 2001-464, May 2001, concerning animal testing in France.
The virulent C. abortus AB7 strain, isolated from an aborted ewe (14), and the temperature-sensitive vaccine strain 1B (14), used in this study, were propagated in the yolk sacs of chicken embryos and stored at –70°C until use.
Subcutaneous vaccination was performed in the back. Both single vaccination and double vaccination groups were vaccinated with 1 × 105 plaque-forming units (PFU) of the C. abortus 1B vaccine per mouse, which is the dose administered to sheep (15). At the same time, the double vaccination group was vaccinated at another location on the back with 0.2 mL of the formalin inactivated phase I C. burnetii vaccine (1/10 of dose administered to sheep). Three weeks later, according to the manufacturer’s instructions, a boost of 0.2 mL of the killed phase I C. burnetii vaccine was administered.
Two months after the first vaccination, all 3 groups (single vaccination, double vaccination, and virulence) were challenged intraperitoneally at 11 ± 1 d of gestation with a suspension of 3.4 × 104 PFU virulent C. abortus strain AB7/mouse, as previously described (14). Survival of mice pups was monitored daily for 8 to 10 d. Data were analyzed for treatment effect by gross linear means (GLM) software (Stat View, Version 5; SAS Institute, Cary, North Carolina, USA). Mean differences were determined using Fisher’s test of least significance. The level of statistical significance was pre set at P < 0.05.
Inoculation of pregnant mice with virulent C. abortus AB7 strain induced intrauterine infection in fetuses leading to death or the birth of weak pups that did not survive. Numbers of living offspring at birth and during the 1st wk of life can, therefore, be used to estimate the level of infection in mice and the efficacy of the vaccination (14,15). The average number of living offspring in the single vaccination and double vaccination groups were not significantly different from those in the control group, but were significantly different from those in the virulence group (P <0.0001) (Figure 1), showing the effectiveness of the vaccine strain 1B. These results were obtained on mice and not on the target animals (ewes or goats). Moreover, protection was assessed against the parental strain since 1B was obtained by a nitrosogunanidine-induced mutation of strain AB7. The aim of this study was not to check the efficacy of vaccine strain 1B, which has been shown before (6–8,15–19), but to investigate the noninterference of the Q fever vaccine with protection from the live vaccine strain 1B. These results, which were in agreement with those obtained previously for vaccine 1B alone (15,16), indicated that the Q fever vaccine did not affect the protection afforded by the live chlamydia vaccine.
Figure 1.
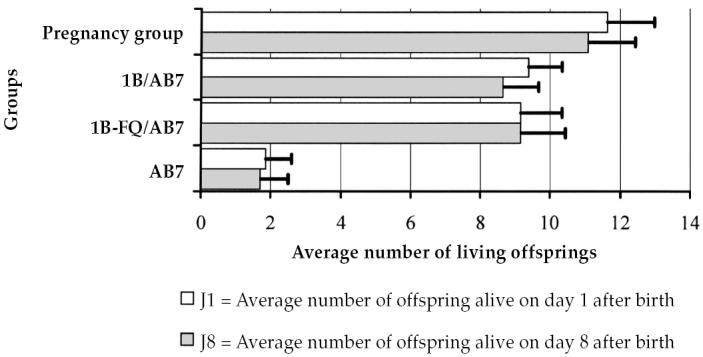
Effectiveness against virulent C. abortus strain AB7 challenge,for live chlamydiosis vaccine strain 1B alone (group 1B/AB7) combined with phase I Q fever vaccine Q VAX (FQ) (CEVA) (group 1B-FQ/AB7) in a mouse model. Two groups of 20 vaccinated and 1 group of 20 unvaccinated pregnant mice (group AB7) were challenged with 1 × 105 plaque-forming units (PFU) of C. abortus strain (AB7), and the average number of living offspring per mouse was recorded daily for 8 d. The number was not significantly different between the unchallenged control group (pregnancy group) and the vaccinated groups, but was significantly different between the 3 groups and the unvaccinated challenged group (P < 0.0001).
The effectiveness of the protection demonstrated in this study was similar to that obtained with the trivalent vaccination (Rev1, 1B, Rv6) using both a mouse model (6) and a ewe model (7). Therefore, it can be hypothesized that the 2 vaccines are compatible in ewes. However, it is necessary to test the absence of negative effects of the double vaccination on the immunity against Coxiella immunity, although it has previously been shown that the combination of this Q fever vaccine with a killed vaccine against chlamydiosis did not affect the production of antibodies against chlamydia or Coxiella antibodies (5). As Q fever and chlamydiosis can easily be confused using traditional diagnosis methods, such as Stamp staining, it is likely that simultaneous vaccination against these 2 abortive diseases would be very effective, as was previously proposed for administration of both chlamydia and toxoplasmosis vaccines (8).
The 2 vaccines were injected at 2 different points on the back to avoid the risk of interference between the formaldehyde used to inactivate the Coxiella in Q fever vaccine and the live 1B strain. Indeed, although combining killed vaccines and live ovine vaccines is frequent and recommended to decrease the number of needle sticks (20), killed and live vaccines cannot be injected with the same syringe. However, the possibility of vaccinating ewes and goats at the same time would be extremely valuable in reducing the number of interventions per animal.
Acknowledgments
The authors thank P. Lechopier and his staff for animal husbandry.
References
- 1.Hansen DE, Hedstrom OR, Sonn RJ, Snyder SP. Efficacy of a vaccine to prevent Chlamydia or Campylobacter-induced abortions in ewes. J Am Vet Med Assoc. 1990;196:731–734. [PubMed] [Google Scholar]
- 2.Schopf KD, Khaschabi, Dackau T. Enzootic abortion in goat herd caused by mixed infection with Coxiella burnetii and Chlamydia psittaci. Case report. Tierarztl Prax. 1991;19:630–634. [PubMed] [Google Scholar]
- 3.Plommet M. Le vaccin Chlamydia ovin : péripéties d’une recherche. Bull Soc Vet Prat de France. 1997;81:171–178. [Google Scholar]
- 4.Kurstak E. Towards the new global vaccinology era in prevention and control of diseases. Vaccine. 2003;2:580–581. doi: 10.1016/s0264-410x(02)00562-5. [DOI] [PubMed] [Google Scholar]
- 5.Dravecky T, Kazar J, Zarsky I, et al. Study of the reactivity and immunogenicity of a combined vaccine against abortion due to coxiellosis and chlamydiosis in sheep. Vet Med (Praha) 1987;32:309–313. [PubMed] [Google Scholar]
- 6.Plommet M, Bosseray N, Lantier F, Bernard F, Pardon P, Rodolakis A. Simultaneous vaccination by three living attenuated strains of Brucella, Salmonella and Chlamydia in mice. Vaccine. 1987;5:27–32. doi: 10.1016/0264-410x(87)90005-3. [DOI] [PubMed] [Google Scholar]
- 7.Souriau A, Bosseray N, Rodolakis A, Lantier F, Plommet M. Anti-chlamydial immunity in ewes conferred by vaccination with a combination of three live Chlamydia, Brucella and Salmonella vaccines. Vet Rec. 1988;123:29–32. doi: 10.1136/vr.123.1.12. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers WSK, Simpson J, Lee SJ, Baxendale W. Use of live chlamydial vaccine to prevent ovine enzootic abortion. Vet Rec. 1997;141:63–67. doi: 10.1136/vr.141.3.63. [DOI] [PubMed] [Google Scholar]
- 9.Ormsbee RA, Bell EH, Lackman DB, Tallent G. The influence of phase on protective potency of Q fever vaccine. J Immunol. 1963;92:404–412. [PubMed] [Google Scholar]
- 10.Behymer DE, Biberstein EL, Riemann HP, et al. Q fever (Coxiella burnetii) investigation in dairy cattle: challenge of immunity after vaccination. Am J Vet Res. 1976;37:631–634. [PubMed] [Google Scholar]
- 11.Brooks DL, Ermel RW, Franti CE, et al. Q fever vaccination of sheep: Challenge of immunity in ewes. Am J Vet Res. 1986;47:1235–1238. [PubMed] [Google Scholar]
- 12.Williams JC, Hoover TA, Waag DM, Banerjee-Bhatnagar N, Bolt CR, Scott GH. Antigenic structure of Coxiella burnetiiA comparison of lipopolysaccharide and protein antigens as vaccines against Q fever. Ann New York Acad Sci. 1990;590:370–380. doi: 10.1111/j.1749-6632.1990.tb42243.x. [DOI] [PubMed] [Google Scholar]
- 13.Sadecky E, Brezina R, Kazar J, Schramek S, Urvolgyi J. Immunization against Q fever of naturally infected dairy cows. Acta Virol. 1975;19:486–488. [PubMed] [Google Scholar]
- 14.Rodolakis A. In vitro and in vivo properties of chemically induced temperature-sensitive mutants of Chlamydia psittaci var. ovis: screening in a murine model. Infect Immunol. 1983;42:525–530. doi: 10.1128/iai.42.2.525-530.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodolakis A, Bernard F. Vaccination with temperature sensitive mutant of Chlamydia psittaci against enzootic abortion of ewes. Vet Rec. 1984;114:193–194. doi: 10.1136/vr.114.8.193. [DOI] [PubMed] [Google Scholar]
- 16.Rekiki A, Bouakane A, Bernard F, Hammami S, Rodolakis A. Effectiveness of vaccine strain 1B against Tunisian field strains of Chlamydophila abortus using mouse model. Rev Med Vet. 2003;154:463–468. [Google Scholar]
- 17.Rodolakis A, Souriau A. Response of ewes to temperature-sensitive mutants of Chlamydia psittaci (var ovis) obtained by NTG mutagenesis. Ann Rech Vet. 1983;14:155–161. [PubMed] [Google Scholar]
- 18.Rodolakis A, Souriau A. Response of goats to vaccination with temperature-sensitive mutants of Chlamydia psittaci obtained by nitrosoguanidine mutagenesis. Am J Vet Res. 1986;47:2627–2631. [PubMed] [Google Scholar]
- 19.Rodolakis A, Souriau A. Vaccination contre la chlamydiose bovine avec un mutant thermosensible de Chlamydia psittaci. Ann Rech Vet. 1987;18:439–442. [PubMed] [Google Scholar]
- 20.Ellis RW. Development of combination vaccines. Vaccine. 1999;17:1635–1642. doi: 10.1016/s0264-410x(98)00424-1. [DOI] [PubMed] [Google Scholar]


