Abstract
Once considered unique to the lung, surfactant proteins have been clearly identified in the intestine and peritoneum and are suggested to exist in several other organs. In the lung, surfactant proteins assist in the formation of a monolayer of surface-active phospholipid at the liquid–air interface of the alveolar lining, reducing the surface tension at this surface. In contrast, surface-active phospholipid adsorbed to articular surfaces has been identified as the load-bearing boundary lubricant of the joint. This raises the question of whether surfactant proteins in synovial fluid (SF) are required for the formation of the adsorbed layer in normal joints. Proteins from small volumes of equine SF were resolved by 1- and 2-dimensional polyacrylamide gel electrophoresis and detected by Western blotting to investigate the presence of surfactant proteins. The study showed that surfactant proteins A and D (SP-A and SP-D) are present in the SF of normal horses. We suggest that, like surface-active phospholipid, SP-A and SP-D play a significant role in the functioning of joints. Next will be clarification of the roles of surfactant proteins as disease markers in a variety of joint diseases, such as degenerative joint disease and inflammatory problems.
Résumé
Déjà considéré comme unique au poumon, les protéines du surfactant ont clairement été identifiées dans l’intestin et le péritoine et il est suggéré qu’elles existent dans plusieurs autres organes. Dans le poumon, les protéines du surfactant aident à la formation d’une mono-couche de phospholipides tensio-actif à l’interface air-liquide à l’intérieur des alvéoles, réduisant ainsi la tension de surface à cette surface. À l’opposé, les phospholipides tensio-actifs adsorbés aux surfaces articulaires ont été identifiés comme étant un lubrifiant du support de charge de l’articulation. Ceci soulève la question à savoir si les protéines du surfactant dans le liquide synovial (SF) sont requises pour la formation de la couche adsorbée dans une articulation normale. Les protéines provenant de petites quantités de liquide synovial équin ont été analysées par électrophorèse à 1 et 2 dimensions sur gel de polyacrylamide et détectées par immunobuvardage afin d’étudier la présence de proteins du surfactant. L’étude a montré que les protéines A et D du surfactant (SP-A et SP-D) sont présentes dans le SF de chevaux normaux. Il est suggéré que, tout comme les phospholipides tensio-actifs, SP-A et SP-D jouent un rôle significatif dans le fonctionnement des articulations. Il faudra éclaircir les rôles des protéines du surfactant comme marqueurs de maladie dans un variété de maladies articulaires, telles que les maladies dégénératives des articulations et les problèmes inflammatoires.
(Traduit par Docteur Serge Messier)
Pulmonary surfactant is a surface-active material, consisting of phospholipids (about 90%) and proteins (5% to 10%), covering the alveolar epithelium. Lipids, particularly phospholipids, make up the main fraction of the alveolar surfactant that is obtained by bronchoalveolar lavage (BAL) (1). Dipalmitoylphosphatidylcholine (DPPC) is the main surface-active agent in lung surfactant, but phosphatidylglycerol and proteins are also required for the formation and the function of the surfactant surface film within the airways. Four surfactant proteins in the lung have been identified and characterized: surfactant protein A (SP-A), surfactant protein B (SP-B), surfactant protein C (SP-C), and surfactant protein D (SP-D). Two, SP-B and SP-C, are hydrophobic and promote very rapid adsorption of lipids to the air–liquid interface. The other 2 are members of a family of collagenous carbohydrate-binding proteins (collagenous C-type lectins) now generally called collectins; SP-A and SP-D have similar structures, with a short intersubunit disulfide-forming N-terminal region, a collagen-like domain, a coiled-coil motif neck domain, and a carbohydrate-recognition domain (2). The lung is the major site of synthesis of SP-A and SP-D, both molecules being produced by alveolar type II cells and unciliated bronchial epithelial cells (Clara cells). There is growing evidence that SP-A and SP-D are also produced outside the lung.
Synovial fluid (SF) has 2 major functions: lubrication of joints and nutrition of the joint’s superficial tissues, especially cartilage. Two types of cell organization are seen within the synovial tissue. Type A cells are responsible for the synthesis and transport of hyaluronic acid. Type B cells, which are less numerous, are responsible for the synthesis and transport of proteins into the SF.
The main surfactant protein is SP-A. This hydrophilic glycoprotein has a molecular mass of 28 to 36 kDa and isoelectric points of 4.8 to 5.2 (3). Hobo and colleagues (4,5) have shown the presence of SP-A and SP-D in the lungs of horses and have purified and characterized the structure and function of these proteins.
There may be a correlation between lamellar bodies and surfactant secretion. The presence of lamellar bodies in joints and SF has been reported (6). Dobbie and associates (7) and Schwarz and Hills (8) have reported the presence of lamellar bodies in type B synoviocytes, and synovial B cells seem to be the source and secretion site of synovial surfactant within the joint. Dobbie and associates (7) also demonstrated the existence of an SP-A-like protein in human synovium, which provided new physiological and pathological insight into these tissues. This raises the question of whether surfactant proteins in SF are required for the formation of the adsorbed layer in normal joints.
We have demonstrated, for the first time, SP-A and SP-D in equine SF by 1- and 2-dimensional electrophoresis and Western blotting within known molecular and isoelectric-point ranges.
We studied 5 thoroughbred horses (3 male and 2 female), 22 to 36 mo old. They did not have a history of clinical signs of degenerative joint disease or any other joint diseases and were housed on pasture. The study was carried out with approval obtained from the Ethics Review Committee of the University of Queensland, Brisbane, Australia.
Samples of SF were aspirated from the horses’ carpal joints with 12-gauge needles into Vacutainer tubes containing ethylenediamine tetra-acetate (Becton Dickinson, Rutherford, New Jersey, USA) during autopsy at the Department of Pathology for educational purposes and stored at −20°C. The samples were centrifuged at 1200 × g for 10 min to remove cellular debris and then treated with hyaluronidase for 1 h at 37°C. These samples were used for all immunoblotting analyses.
Bronchoalveolar lavage fluid (BALF) was taken during autopsy from the same animals, with the use of 100 mL of BN buffer (0.01 M sodium borate, pH 7.4; 0.15 M NaCl; 3 mM CaCl2) to lavage each lung. The lavage fluid was centrifuged at 250 × g for 10 min at 4°C to remove cellular debris. The supernatant was then centrifuged at 27 000 × g for 2 h at 4°C, and the resulting pellet was resuspended in 100 μL of BN buffer and kept at −20°C (9).
One-dimensional sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) was performed on samples containing 15 to 250 μg of protein (SDS–PAGE 12%–15% w/v, 1.5-mm-thick polyacrylamide gel) by the method of Laemmli (10) with a Mini-PROTEAN II apparatus (Bio-Rad Life Sciences Research Group, Hercules, California, USA) in the presence of 5% β-mercaptoethanol. After electrophoresis, the gels were used in Western blotting of antigens by transferring separated proteins onto the nitrocellulose membrane. The blots were probed with monoclonal antibody PE-10 directed against human SP-A (DAKO, Kyoto, Japan), polyclonal antibody PO-B (8 μg/mL) against human SP-B (Dr. Ian Doyle, Flinders University, Adelaide, Australia), and a recombinant polyclonal SP-D antibody (IgG, 1 mg/mL) (Dr. Dennis R. Voelker, National Jewish Medical and Research Center, Denver, Colorado, USA). After the blots were incubated with peroxidase-conjugated secondary antibodies (DAKO), bands were detected by enhanced chemiluminescence.
Two-dimensional electrophoresis was carried out using the same method as Bjellqvist and coworkers (11). A nonlinear immobilized pH gradient (NL IPG) (pH 3 to 10) was used as the first dimension. Samples of SF (10 μL for SP-A and 25 μL for SP-D analysis) were diluted to 200 μL in rehydration solution [containing 8 M urea, 0.5% 3-[3-Cholamidopropyl] dimethylammonia-1-propane sulfonate (CHAPS), 15 mM dithiothreitol (DTT), and 0.2% ampholyte (pH 3 to 10)] and were applied directly to the surface of the cathodal end of 110-mm NL IPG strips (Amersham-Pharmacia Biotech, Little Chalfont, England); about 15 μg total protein was applied for SP-A and about 38 μg total protein for SP-D analysis. The samples were focused for a total of 99.9 kVh (Bio-Rad 3000 apparatus) at 20°C. For improved sample entry, low voltages (150 to 300 V) were used for the first few hours. Electrophoresis was then continued with a maximum setting of 3500 V to the steady state. After the focusing, the strips were equilibrated for 15 min at 37°C in 0.375 M Tris-HCl (pH 8.8), 6 M urea, 2% (w/v) SDS, 20% (w/v) glycerol, and 2% (w/v) DTT and then for 15 min in the same buffer but with 2% (w/v) iodoacetamide instead of DTT.
For second-dimension electrophoresis, approximately 0.5 cm was cut from the cathodal end of the strip, and the arrow tip was cut from the anodal end. The strips were transferred to the top of 12% polyacrylamide gel, 10 × 8 cm and 1.5 mm thick, and held in position on the SDS–gel surface with molten 0.5% (w/v) agarose in cathode buffer containing a trace of bromophenol blue. The anode buffer was 0.375% (w/v) Tris-acetate, pH 8.8, and the cathode buffer was 192 mM glycine–Tris (pH 8.3)–0.1% (w/v) SDS, as recommended by Herbert and colleagues (12). The gels were run at 100 mA/gel at a constant temperature of 10°C in a Bio-Rad PROTEAN XI apparatus until the bromophenol blue reached the bottom of the gel. After electrophoresis, the samples were subjected to Western blotting as previously described.
The 1- and 2-dimensional electrophoresis followed by Western blot analysis provided evidence that the proteins in the equine SF were SP-A and SP-D. Although SP-B had an 8 kDa band in the equine BAL samples, it could not be detected in the expected molecular-weight range (8 to 12 kDa) in the SF samples: the protein in the SF samples reacted with our polyclonal SP-B protein and showed a positive signal around 66 kDa. The detected SP-A molecular weight was around 34 to 36 kDa in the BALF and SF; the detected isoelectric point was around 4.7 in the SF. For SP-D the molecular weight was 43 kDa in BALF and SF, and the isoelectric point was 4.4 in the SF (Figures 1 and 2). These values are consistent with those found in the literature.
Figure 1.
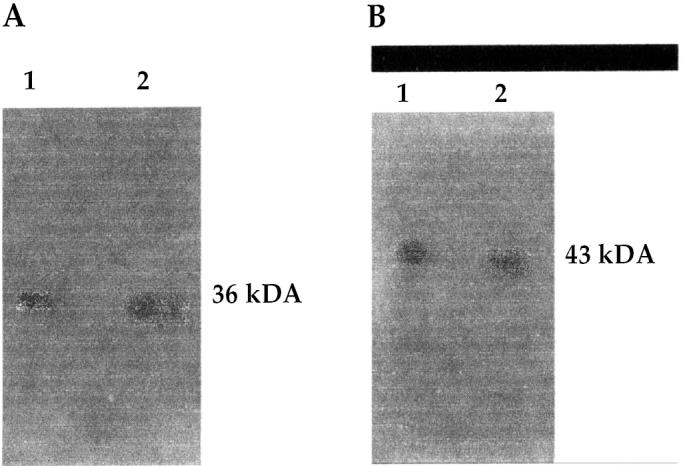
Western blot analysis for surfactant proteins A and D [SP-A (1A) and SP-D (1B)] after 1-dimensional electrophoresis under reducing conditions in which equine synovial fluid (SF) proteins were compared with partially purified surfactant from equine bronchoalveolar lavage fluid as a positive control. Immunodetection was done with anti-SP antibodies, an exposure time of 1 min, and enhanced chemiluminescence.
Figure 2.
Two-dimensional electrophoresis and Western blot analysis of equine SF samples, showing SP-A (2A) and SP-D (2B) molecular weights and isoelectric points (pI). Immunodetection protocol as described for Figure 1.
Ultrastructural and in vivo studies of surfactant biosynthesis have shown surfactant to be produced by extrusion of the lamellar bodies, which occurs in all serosal cells, including those in the synovium and peritoneum. The synthesis of SP-A occurs in mucus-producing cells of the rat stomach and the salivary, sweat, mammary, and lacrimal glands, as indicated by reverse transcriptase polymerase chain reaction (RT-PCR) (13); SP-A has been demonstrated in epithelial cells of the rat small and large intestine by immunohistochemical studies as well as by RT-PCR and cDNA cloning (14).
This study has, for the first time, demonstrated SP-A and SP-D in the SF of horses. We postulate that these proteins play an important role in the lubrication of joints. We suggest that SP-A facilitates the adsorption of surface-active phospholipids to the articular surface and that SP-D regulates phospholipid homeostasis in the joint. The absence of SP-B in SF suggests that it is not required for the adsorption of phospholipid at the articular liquid–solid interface and raises the possibility that its role may be specific for liquid–air interfaces. The SF of normal and diseased equine joints should now be analyzed to determine if there are changes in SP-A and SP-D content with disease. These proteins may be novel markers of disease activity and progression. If so, they could serve as important and reliable markers of joint destruction as well as chronic inflammation in the future.
Two of the antibodies used in Western blotting were monoclonal SP-A antibody (PE-10) and polyclonal SP-D antibody. The SP-A1 and SP-A2 genes both contain the nucleotide sequence corresponding to the peptides (14) that react with monoclonal SP-A antibody (15). Therefore, PE-10 detects SP-A1 and SP-A2 equally well. As described in the specification sheet, PE-10 cross-reacts with the SP-A of a variety of domestic animals. It is therefore safe to conclude that the localization and synthesis of surfactant proteins is not restricted to the respiratory system in horses. In addition, PE-10 is the chosen specific antibody that cross-reacts with the noncollagenous carbohydrate-recognition C-terminal domain of human SP-A (15). The primary structure of the C-terminal domain of equine SP-A is likely homogeneous with the SP-A of rats and horses. Hobo and colleagues (4) showed the similarity in partial amino acid sequences of the noncollagenous carbohydrate-recognition C-terminal domain of rats, humans, and horses. The noncollagenous carbohydraterecognition C-terminal domain of equine joint SP-D may be identical to that in human (16), rat (17), and equine lung SP-D (5).
Further studies are needed to clarify the roles of SP-A and SP-D in equine SF in the diagnosis of variety of diseases.
References
- 1.Pattle RE. Properties, function and origin of the alveolar lining layer. Nature. 1955;175:1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- 2.Kuroki Y, Voelker DR. Pulmonary surfactant proteins. J Biol Chem. 1994;269:25943–25946. [PubMed] [Google Scholar]
- 3.Sueishi K, Benson BJ. Isolation of a major apolipoprotein of canine and murine pulmonary surfactant. Biochemical and immunochemical characteristics. Biochim Biophys Acta. 1981;665:442–453. doi: 10.1016/0005-2760(81)90257-5. [DOI] [PubMed] [Google Scholar]
- 4.Hobo S, Ogasawara Y, Kuroki Y, Akino T, Yoshihara T. Purification and biochemical characterization of pulmonary surfactant protein A of horses. Am J Vet Res. 1999;60:169–173. [PubMed] [Google Scholar]
- 5.Hobo S, Ogasawara Y, Kuroki Y, Akino T, Yoshihara T. Purification and biochemical characterization of equine pulmonary surfactant protein D. Am J Vet Res. 1999;60:368–372. [PubMed] [Google Scholar]
- 6.Hills BA. Oligolamellar lubrication of joints by surface active phospholipid. J Rheumatol. 1989;16:82–91. [PubMed] [Google Scholar]
- 7.Dobbie JW, Tasiaux N, Meýjers P, et al. Lamellar bodies in synoviocytes, mesothelium and specific epithelia as possible site of auto-antigen in rheumatoid disease. Br J Rheumatol. 1994;33:508–519. doi: 10.1093/rheumatology/33.6.508. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz IM, Hills BA. Synovial surfactant: lamellar bodies in type B synoviocytes and proteolipid in synovial fluid and the articular lining. Br J Rheumatol. 1996;35:821–827. doi: 10.1093/rheumatology/35.9.821. [DOI] [PubMed] [Google Scholar]
- 9.Liau DF, Ryan SF. Purification of surfactant protein A from dog lung by reconstitution with surfactant lipids. Chem Phys Lipids. 1991;59:29–38. doi: 10.1016/0009-3084(91)90060-o. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Bjellqvist B, Pasquali C, Ravier F, Sanchez JC, Hochstrasser D. A nonlinear wide-range immobilized pH gradient for 2-dimensional electrophoresis and its definition in a relevant pH scale. Electrophoresis. 1993;14:1357–1365. doi: 10.1002/elps.11501401209. [DOI] [PubMed] [Google Scholar]
- 12.Herbert BR, Molloy MP, Gooley AA, Walsh BJ, Bryson WG, Williams KL. Improved protein solubility in 2-dimensional electrophoresis using tributyl phosphine as reducing agent. Electrophoresis. 1998;19:845–851. doi: 10.1002/elps.1150190540. [DOI] [PubMed] [Google Scholar]
- 13.Rubio S, Lacaze-Masmonteil T, Chailley-Heu B, Kahn A, Bourbon JR, Ducroc R. Pulmonary surfactant protein A (SP-A) is expressed by epithelial cells of small and large intestine. J Biol Chem. 1995;270:12162–12169. doi: 10.1074/jbc.270.20.12162. [DOI] [PubMed] [Google Scholar]
- 14.Katyal SL, Singh G, Locker J. Characterization of a second human pulmonary surfactant-associated protein SP-A gene. Am J Respir Cell Mol Biol. 1992;6:446–452. doi: 10.1165/ajrcmb/6.4.446. [DOI] [PubMed] [Google Scholar]
- 15.Hiraike N, Sohma H, Kuroki Y, Akino T. Epitope mapping for monoclonal antibody against human surfactant protein A (SP-A) that alters receptor binding of SP-A and the SP-A-dependent regulation of phospholipid secretion by alveolar type II cells. Biochim Biophys Acta. 1995;1257:214–222. doi: 10.1016/0005-2760(95)00068-n. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Willis AC, Reid KB. Purification, characterization and cDNA cloning of human lung surfactant protein D. Biochem J. 1992;284:795–802. doi: 10.1042/bj2840795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson A, Chang D, Rust K, Moxley M, Longmore W, Crouch E. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry. 1989;28:6361–6367. doi: 10.1021/bi00441a031. [DOI] [PubMed] [Google Scholar]



