Abstract
The prenatal stress (PNS) model in rodents can induce different abnormal responses that replicate the pathophysiology of depression. We applied this model to evaluate the efficacy of piromelatine (Pir), a novel melatonin analog developed for the treatment of insomnia, in male and female offspring. Adult PNS rats from both sexes showed comparable disturbance associated with high levels of anxiety and depressive responses. Both males and females with PNS demonstrated impaired feedback inhibition of the hypothalamic–pituitary–adrenal (HPA) axis compared to the intact offspring and increased glucocorticoid receptors in the hippocampus. However, opposite to female offspring, the male PNS rats showed an increased expression of mineralocorticoid receptors in the hippocampus. Piromelatine (20 mg/kg, i.p., for 21 days injected from postnatal day 60) attenuated the high anxiety level tested in the open field, elevated plus-maze and light–dark test, and depressive-like behavior in the sucrose preference and the forced swimming tests in a sex-specific manner. The drug reversed to control level stress-induced increase of plasma corticosterone 120 min later in both sexes. Piromelatine also corrected to control level the PNS-induced alterations of corticosteroid receptors only in male offspring. Our findings suggest that the piromelatine treatment exerts beneficial effects on impaired behavioral responses and dysregulated HPA axis in both sexes, while it corrects the PNS-induced changes in the hippocampal corticosteroid receptors only in male offspring.
Keywords: Prenatal stress, Piromelatine, Sex differences, Behavior, HPA axis, Corticosteroid receptors
Introduction
The prenatal period represents a critical time for brain development. Exposure to stressful events during pregnancy can provoke a net of devastating processes with a high impact on epigenetic factors. The latter can lead to re-programming in brain maturation resulting in long-term consequences in the offspring. Major depressive disorder represents a delayed outcome resulting from the suppressive effects of stress on prenatal development (Darnaudéry and Maccari 2008; Heim et al. 2009; Lupien et al. 2009; Weinstock 2008). The model of prenatal stress (PNS) is associated with many neurobiological disturbances resembling the pathophysiology of patients with depression. Offsprings of PNS rats have been characterized by abnormal circadian rhythmicity of important parameters linked with dysfunction of the hypothalamic–pituitary–adrenal (HPA) axis (Maccari et al. 1995), a condition considered a relevant model of depression (Mairesse et al. 2015). In this line, experimental studies have revealed that in rodents PNS causes long-term harmful effects on brain functions associated with behavioral changes (Głombik et al. 2015; Schmidt et al. 2018; Vallée et al. 1997; Weinstock 2001), desynchronized circadian rhythms of the sleep–wake cycle, motor activity and corticosterone (CORT) secretion (Koehl et al. 1999; Morley-Fletcher et al. 2019). Being age-dependent, neurobiological disturbances have been found during ontogenesis as a consequence of PNS exposure (Henry et al. 1994). Furthermore, abnormal responses have been reported to be sex-specific with female rats exhibiting stronger sensitivity and more vulnerability to PNS (Morley-Fletcher et al. 2019; Mueller and Bale 2008; Schmidt et al. 2018; Sickmann et al. 2015). It is well-known that a dysfunctional negative feedback control mechanism of plasma corticosteroid receptors (CSs) release is closely related to an overactive HPA axis, including a disturbed balance in the activity of mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs) in the hippocampus (Anacker et al. 2012; Kloet et al. 2008; Gulyaeva et al. 2019). Both MRs and GRs have an important role in the inhibitory and regulatory control of the HPA axis. The MRs expression depends on the circadian fluctuations of CSs in the physiological state, while the stress-associated surge in plasma CORT is associated with increased occupancy of GRs (Anacker et al. 2012). The impact of PNS on the changes in the regulation of the HPA axis in offspring has been explored in both animal models and humans (Darnaudéry and Maccari 2008; Maccari et al. 2003).
The PNS model is useful for the finding of new therapeutic strategies aimed to treat the clinical symptoms of depression and anxiety and the underlying disturbed mechanism of regulation of important signaling systems related to stress, including the regulatory feedback pathway of the HPA axis, abolished hippocampal neurogenesis (Maccari et al. 1995; Weinstock 2008) and abnormal changes in important transcriptional factors and receptors associated with depression. Recent reports suggest that pharmacological and alternative postnatal treatment procedures can attenuate the impairments of the PNS manipulation (Basta-Kaim et al. 2014; Mairesse et al. 2013; Morley-Fletcher et al. 2011; Trojan et al. 2017; Wakshlak and Marta 1990).
Piromelatine (N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-4-oxo-4H-pyran-2-carboxamide) (Pir) has multiple actions as MT1/MT2 receptor agonist, serotonin (5-HT)1A/1D receptor agonist, and 5-HT2B receptor antagonist, respectively (Liu et al. 2014). Compared to melatonin, this novel melatonin analog has a longer duration of activity and although it is unknown whether it could directly interact with γ-aminobutyric acid (GABA) receptors, it can potentiate the classical inhibitory neurotransmitter system (Carocci et al. 2014; Liu et al. 2014; Yalkinoglu et al. 2010). Experimental studies suggest that Pir possess multiple beneficial effects, including antinociceptive (Chen et al. 2014), anxiolytic and antidepressant (Spadoni et al. 2011; Tian et al. 2010), antihypertensive (Huang et al. 2013), anti-diabetic (She et al. 2009) and sleep-improving effect (Spadoni et al. 2011). Currently, this novel melatonin analog is at Phase II Clinical Trial for primary insomnia and the treatment of mild Alzheimer’s disease (http://www.neurim.com/products/piromelatine/).
In the present study, we aimed to explore the efficacy of chronic treatment for three weeks with Pir in a rat model of PNS, which properties can be extrapolated to the etiology of major depression. To ascertain the antidepressant effect of this novel melatonin analog on behavior, offspring have been subjected to a battery of tests at the end of the treatment procedure. Piromelatine effectiveness on the HPA axis activity has been evaluated by measuring the stress-induced CORT release and changes in the hippocampal MRs and GRs expression in the PNS rat model.
Materials and Methods
Animals
Male and female Sprague–Dawley animals (220–250 g) (Charles River, Italy) were housed at standard conditions (temperature 21 ± 1 °C; 50–60% humidity; 12-h light/dark cycle; 3–4 per cage) with sawdust bedding and with food and water ad libitum. All experiments were performed in accordance with the European Communities Council Directives of 24 November 1986 (86/609/EEC).
Prenatal Stress Procedure
Before breeding, rats were adapted for a week. Subsequently, female rats were mated with male breeders. The gestation day 0 (E0) was detected by the appearance of a positive vaginal smear (a copulation plug). Then, pregnant females were randomly assigned to the control and PNS group. Six dams from each group were used. Pregnant PNS rats were set in a different room to avoid stress procedures affecting the control dams. The dams were exposed to two different stressors per 24 h according to the protocol of Sickmann et al. (2015) with little modifications: one short-term stressor during the day (e.g. 15-min forced swimming, 4-h crowding and social stressor, 4-h wet bedding, 60-min restraint stress) and one long-term stressor overnight (e.g. light exposure, fasting, home cages tilted at 45°) during E7–20. The stressors were applied in random way every day to escape habituation. Matched control dams were left in their home cages without exposure to stressors. The delivery day was designated as postnatal day (P) 0. The dam and its pups were left undisturbed until weaning on P21. Afterward, a total of 9 litters with more than 8 pups with a similar sex ratio were used. Rat pups of each PNS litter were randomly assigned to vehicle (veh) and Pir groups, respectively. Each experimental and control (C) group (male and female) consisted of young adult offspring rats (P60) with n = 10–12 for the weight measurement and the behavioral tests, and n = 6–8 for CORT, MRs and GRs, respectively.
Experimental Design and Piromelatine Treatment
The experimental design is shown in Fig. 1. The injection of veh/Pir (kindly gifted by Neurim Pharmaceuticals Ltd., Israel) started at P60. It continued for 21 days at a dose of 20 mg/kg, i.p. in a volume of 0.1 ml per 100 g dissolved in hydroxyethyl cellulose (1%), administered at 4:00 p.m. (two hours before the onset of the dark phase). Time and dose regime was determined based on previous studies considering the effects of piromelatine on behavioral responses (He et al. 2013; She et al. 2014; Tian et al. 2010). The controls (C) and matched PNS groups received vehicle treatment. The following six groups were assigned as follows: C-veh male rats; PNS-veh male rats; PNS-Pir male rats; C-veh female rats; PNS-veh female rats; PNS-Pir female rats.
Fig. 1.
Experimental design of all procedure, including the first day of pregnancy (gestational day 0, E0), handling or stress procedure (E7-E20) and delivery (E21) of female rat, postnatal (P) day of weight measurement, behavioral tests, tail vein blood collection for measurement of corticosterone, decapitation and isolation of hippocampi for measurement of mineralocorticoid and glucocorticoid receptors of male and female offspring
Body Weight and Behavioral Tests
Offspring male and female rats with veh/Pir treatment were weighed for 21 days starting from the first day of treatment (P60-P81). Behavioral tests were carried out between P74 and P81. All behavioral tests were performed between 10:00 a.m. and 1:00 p.m. under artificially diffused light in a soundproof room by a researcher in a blinded manner. Rats were moved there at least 30 min before the test procedure. Behavioral tests for anxiety (open field test, OF, elevated plus-maze test, EPM, light–dark test, LDT) and depressive responses (sucrose preference test, SPT and forced swimming test, FST) were conducted starting from the next day after the 13th injection of veh/Pir (P74). The behavior in the OF and EPM were analyzed by a video tracking system (SMART PanLab software, Harvard Apparatus, USA).
Open Field Test
The OF test was carried out as described earlier (Tchekalarova et al. 2018). The tested rat was placed at the central quadrant of a grey polystyrene box (100 × 100 cm × 60 cm). The following parameters were measured: total distance traveled (cm) in the field, distance traveled and time spent in the aversive central zone (distance center % and time %) for a 5-min period.
Elevated Plus Maze Test
The EPM test was performed as previously (Tchekalarova et al. 2016a, b) in apparatus with two open (50 × 10 cm) and two closed (50 × 10 × 50 cm) arms which were perpendicular to each other and separated by a central zone (10 × 10 cm). The tested rat was placed gently at the center apparatus facing the open arms. The parameters measured were as follows: total distance traveled (cm), the ratio of open to the total number of entries (%) and time (sec) spent in the open arms for a 5-min period.
Light–Dark Test
The apparatus consisted of one open (25 cm × 50 cm × 40 cm) and one covered (dark) (25 cm × 25 cm × 40 cm) compartment, connected with a 7-cm door. The open part of the apparatus was illuminated by a bulb (80 lx) mounted over this area. At the start of the test, the rat was placed into the light compartment. The measured parameters were: total time spent in the light compartment (sec) and the number of transitions for 5 min.
Sucrose Preference Test
Anhedonia, considered a behavioral marker for depression, was estimated in single housed rats as reported previously (Tchekalarova et al. 2018, 2016a). Preference for the sweet solution was calculated as a percentage of total sucrose consumed during a 24-h period. Each tested rat was placed in an individual cage and adapted to drink from two identical, graduated, and plastic bottles with tap water (100 ml) for a week. At the pretest performed for two days, water in one of the bottles was replaced by 1% sucrose. Taste preference was expressed as a percentage of the consumed volume of sucrose solution for a 24-h period.
Forced Swimming Test
The test for estimating the despair-like behavior was carried out as previously (Tchekalarova et al. 2018). The rat was placed in a plastic, cylindrical container with a 45 cm diameter and a height of 60 cm, filled with warm (about 24 °C) water up to 30 cm above the bottom. The time staying immobile (sec) with weak movements only to maintain the head above the water for 5 min was video-recorded and evaluated offline by two independent researchers blind to conditions.
Measurement of Corticosterone (CORT) Levels
CORT levels were determined in three separated blood samples obtained from the tail vein at different time points before and after the stress procedure with forced swimming for 15 min as follows: before stress (0 min), 10 min after stress (10 min) and 120 min (120 min) afterward. The plasma was isolated after centrifugation at 4000 rpm for 10 min in 4 °C. The CORT (ng/mg) was measured by Elisa test kit (Enzo, Switzerland) following the instructions of the manufacturer.
Measurement of Glucocorticoid Receptors and Mineralocorticoid Receptors
MRs and GRs were measured a week after the CORT blood samples were obtained. The brains were rapidly isolated and both hippocampi were dissected and frozen on dry ice. Tissues were preserved at − 80 °C until the biochemical analysis. The tissue samples were homogenized in 10 ml/g tissue in cold buffer containing 10 mM Tris HCl (pH 7,6), 1 mM EGTA, 50 mM NaF, 1 mM EDTA and 1 mM PMSF. MRs and GRs were measured by ELISA kit (DLDevelop, China) in (ng/mg protein) protein according to the procedure recommended by the manufacturer.
Statistical Analysis
Experimental results were presented as mean ± S.E.M. Mixed ANOVA with two between-subjects factors (Sex: male and female, and Treatment: C-veh, PNS-veh and PNS-Pir), and one within-subject factor (Time) were used for body weight (Time: 1–21 day) and CORT (Time: T0, T10 and T120 min) data. Two-way ANOVA with factors Sex (male and female) and Treatment (C-veh, PNS-veh and PNS-Pir) was applied for the behavioral data, MRs and GRs. Post hoc comparisons via the Bonferroni test in case of justification were used. Statistically significant differences were accepted at P ≤ 0.05. The analysis was conducted using the SigmaStat® (version 11.0.) statistical package. The effect size (size of the differences) was estimated with eta squared (η2) for ANOVAs.
Results
Bodyweight
Main Time effect was detected on the repeated measure during the 21-day of vehicle or piromelatine administration [F19,1199 = 65.870, P < 0.001] showing that weight increased over time for all rats. Although male and female rats exhibited different weights [main sex effect: F1,1199 = 21.425, P < 0.001, η2 = 0.70]. Treatment effect was not statistically significant for both male and female PNS offspring [Treatment effect: F2,1199 = 2.022, P = 0.133]. Pir treated PNS offspring showed a tendency in decreasing the body weight gain, but a statistical significance was not found between any group (C-veh, PNS-veh and PNS-Pir rats) (P > 0.05) (Fig. 2a, b).
Fig. 2.
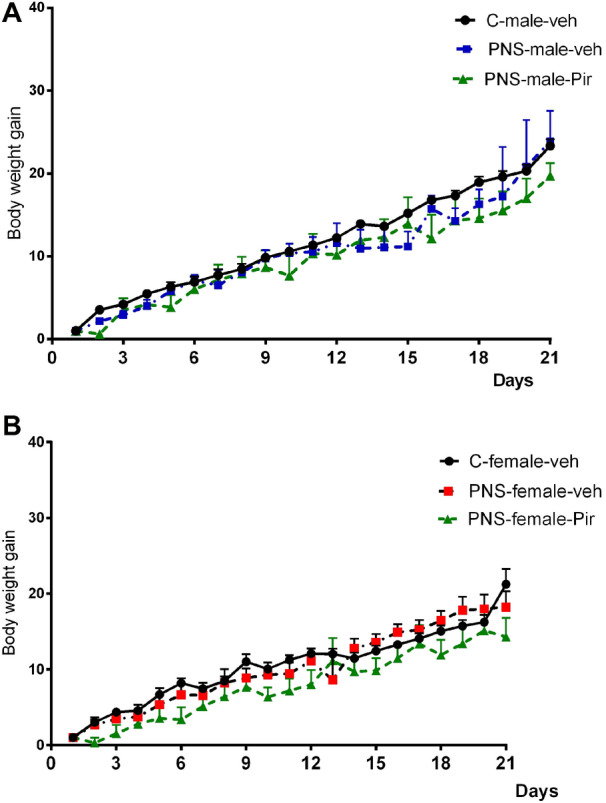
Effects of sex (male and female) and treatment [C-veh, PNS (prenatal stress)-veh and PNS-piromelatine (Pir)] and time (1–21 days) on body weight gain in male (a) and female (b) offspring rats. Changes in bodyweight gain are given as mean ± SEM. Bodyweight was measured (rate of weight increase) from postnatal day 60 to 81. Data from C-veh, PNS-veh and PNS-Pir male and female rats (n = 10–12) were compared using mixed ANOVA followed by Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the C-veh or the respective PNS-veh group; +P < 0.05, ++P < 0.01, +++P < 0.001 in comparison with the matched male group (C-veh, PNS-veh and PNS-Pir)
Behavioral Tests for Motor Activity and Anxiety
Open Field Test
Two-way ANOVA revealed a main sex effect [F2,64 = 14.020, P < 0.001, η2 = 0.68] and Treatment effect [F1,64 = 11.282, P < 0.001 η2 = 0.481] for the total distance traveled. However, Treatment × Sex interaction was not statistically significant [F2,64 = 0.309, P = 0.736]. Post hoc analysis showed that the female control rats were characterized with higher motor activity compared to the matched male controls (P = 0.0032). Both male and female rats exposed to the PNSand treated with vehicle traveled shorter distances in the OF compared to the C-veh matched groups (P = 0.0004 males; P = 0.0009 females) (Fig. 3a). Treatment with Pir tended to revert the motor activity to control level in PNS-treated rats of both sexes, but this effect was not significantly different compared to PNS-veh group (P > 0.05).
Fig. 3.
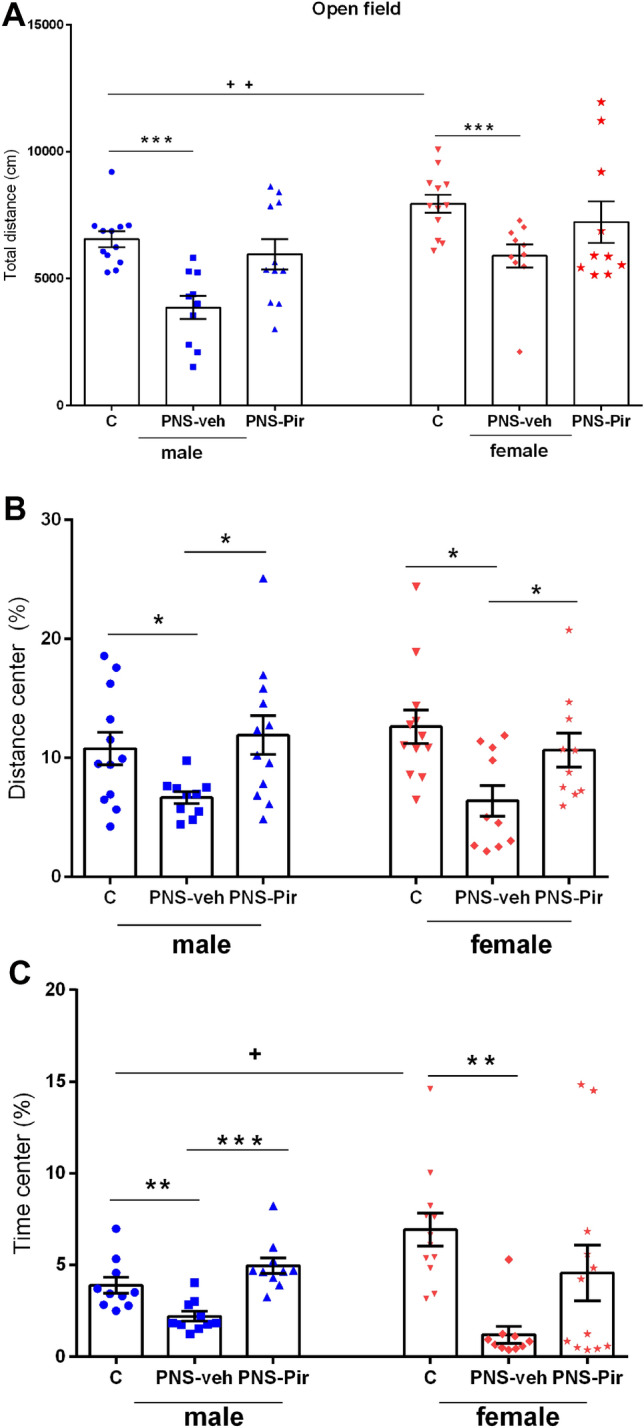
Motor activity and anxiety in male and female Sprague Dawley rats. Effects of sex (male and female) and treatment (C-veh, PNS-veh and PNS-Pir) (chronic 21-day-treatment with Pir 20 mg.kg−1 i.p. or vehicle) on total distance (cm) (a), distance in center (%) (b) and time in center (%) (c) in the open field test. Data are given as mean ± SEM and analyzed by two-way ANOVA followed by Bonferroni post hoc test (n = 10–12). Details as in Fig. 2: *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the C-veh or the respective PNS-veh group; +P < 0.05, ++P < 0.01, +++P < 0.001 in comparison with the matched male group (C-veh, PNS-veh and PNS-Pir)
The anxiety level was assessed by the distance traveled and time spent in the aversive central zone of the OF apparatus. Two-way ANOVA showed a main Treatment effect for distance traveled in the center [F2,63 = 8.440; P < 0.001, η2 = 0.66] and time spent [F2,63 = 10.001, P < 0.001, η2 = 0.58] in the aversive central zone of the open field. No sex effect [F1,63 = 0.00738, P > 0.932] and Treatment × Sex interaction [F2,63 = 0.707, P > 0.497] were detected for distance traveled in the center as well as for time spent there [Sex effect: F1,63 = 0.585, P > 0.447; Treatment × Sex interaction F2,63 = 3.132, P > 0.051]. Exposure to PNS increased the level of anxiety, which was demonstrated by a shorter distance traveled (male: P = 0.036, PNS-veh compared to C-veh group; female: P = 0.045, PNS-veh compared to C-veh group) and time (male: P = 0.01, PNS-veh compared to C-veh group; female: P = 0.002, PNS-veh compared to C-veh group) spent in the central zone (Fig. 3b, c). The post hoc analysis indicated that Pir treatment of PNS rats alleviated the anxiety response to stress (distance, male: P = 0.044, PNS-Pir compared to PNS-veh group; female: P = 0.0398, PNS-Pir compared to PNS-veh; time, male: P = 0.0007, PNS-Pir compared to PNS-veh).
Elevated Plus-Maze Test
Female rats showed higher locomotion compared to male rats [Two-way ANOVA, Sex effect: F1,69 = 14.651, P < 0.001, η2 = 0.42]. Similar to the OF test, the motor activity assessed in the EPM test was significantly decreased by exposure to PNS [Two-way ANOVA, Treatment effect: F2,71 = 7.485, P < 0.001, η2 = 0.49] however, Treatment × Sex interaction was not statistically significant [F2,71 = 0.120, P > 0.872]. The decrease in motor activity due to PNS was verified via the post hoc analysis both in male (P = 0.003 PNS-veh vs. C-veh group) and female (P = 0.01 PNS-veh vs. C-veh group) rats (Fig. 4a). The post hoc test also confirmed that female offspring, either controls, PNS-veh and PNS-Pir exhibited higher locomotion than matched male rats (P = 0.05, P = 0.014; P = 0.024, respectively).
Fig. 4.

Effects of sex (male and female) and treatment (C-veh, PNS-veh and PNS-Pir) on total distance (cm) (a), time spent in the open arms (sec) (b) and ratio of open to total number of entries (%) (c) in the elevated plus maze test. Data are given as mean ± SEM and analyzed by two-way ANOVA followed by Bonferroni post hoc test (n = 10–12). Details as in Fig. 2: *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the C-veh or the respective PNS-veh group; +P < 0.05, ++P < 0.01, +++P < 0.001 in comparison with the matched male group (C-veh, PNS-veh and PNS-Pir)
Anxiety levels were estimated by time-domain and the number of entries in the open arms of the EPM apparatus. Like in the OF, PNS induced increased anxiety levels associated with prolonged time [Two-way ANOVA, Treatment effect: F2,63 = 12.523, P < 0.001, η2 = 0.769] and ratio of open vs total arm entries [Two-way ANOVA, Treatment effect: F2,63 = 13.791, P < 0.001, η2 = 0.78] in the aversive zone. However, Sex effect [time open arms: F1,63 = 0.0360, P = 0.850; ratio of open vs total arm entries F1,63 = 0.630, P = 0.430] as well as Treatment × Sex interaction [time open arms: F2,63 = 0.834, P = 0.440; ratio of open vs total arm entries: F2,63 = 0.462, P = 0.632) were not statistically different. The subsequent post hoc test demonstrated that PNS elevates the anxiety level (P = 0.01, time and P = 0.003, the ratio of open vs total arm entries, respectively, male PNS-veh compared to C-veh rats; P = 0.0011, time and P = 0.006, ratio of open vs total arm entries, female PNS-veh compared to C-veh rats). The chronic treatment with Pir reversed the PNS-induced impaired anxiety response both in males (P = 0.003, time PNS-Pir compared to PNS-veh rats) and in females (P = 0.014, time PNS-Pir compared to PNS-veh rats) (Fig. 4b, c).
Light–Dark Test
The level of anxiety was estimated by two parameters in the LDT, namely time spent in the light compartment and the number of transitions. In agreement with the results, demonstrated in the OF and EPM test, offspring rats with PNS exhibited shorter time in the aversive light compartment of the LD apparatus [Two-way ANOVA, Treatment effect: F2,63 = 20.318, P < 0.001, η2 = 0.68] and a decreased number of transitions [Two-way ANOVA, Treatment effect: F2,63 = 12.481, P < 0.001, η2 = 0.65]. However, Sex effect: [F1,63 = 2.134 P = 0.053] and Treatment × Sex interaction [F2,63 = 0.116, P = 0.891] were not statistically significant for the time in the light compartment. Similarly, no Sex effect: [F1,63 = 2.134, P = 0.053], as well as Treatment × Sex interaction [F2,63 1.004, P = 0.373] was detected for the number of transitions. Post hoc test confirmed that both male PNS-veh male and female groups showed significantly increased anxiety levels (time in the light compartment: P = 0.0031 male PNS-veh compared to C-veh group; P < 0.001 female PNS-veh compared to C-veh group; number of transitions: P = 0.0077 male PNS-veh compared to C-veh group; P = 0.008 female PNS-veh compared to C-veh group). Treatment with Pir alleviated the enhanced anxiety levels both in male (PNS-Pir compared to PNS-veh group: time: P = 0.006 and number of transitions: P = 0.0004, respectively) and female (PNS-Pir compared to PNS-veh group: time: P = 0.0149 and number of transitions: P = 0.0323, respectively) rats (Fig. 5a, b).
Fig. 5.
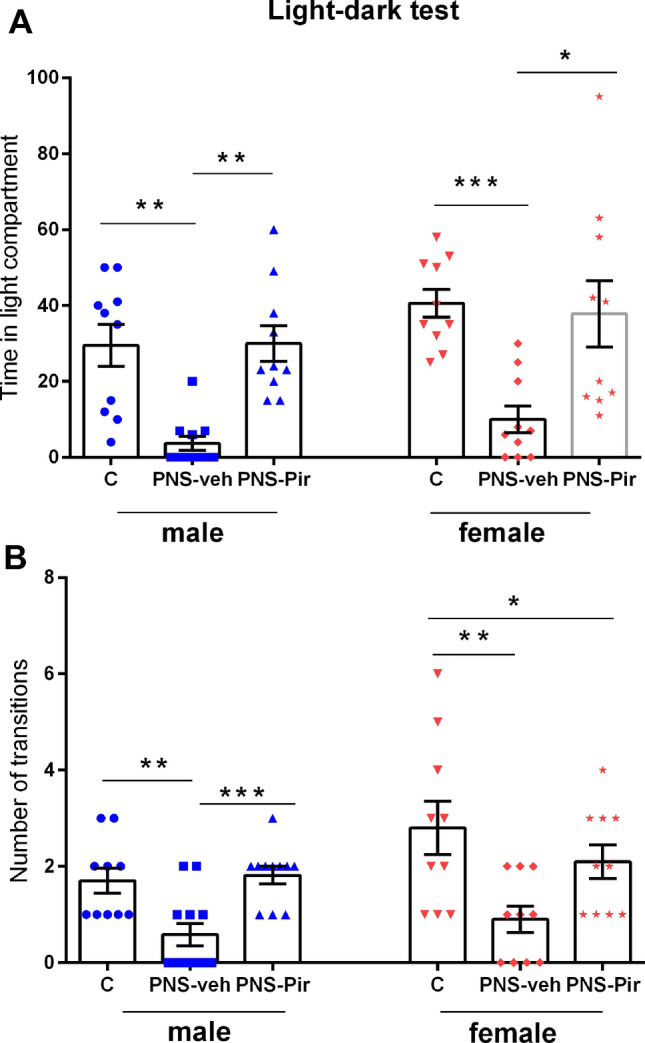
Effects of sex (male and female) and treatment (C-veh, PNS-veh and PNS-Pir) on time in light compartment (sec) (a) and number of transitions (b) in the light–dark test. Data are given as mean ± SEM and analyzed by two-way ANOVA followed by Bonferroni post hoc test (n = 10–12). Details as in Fig. 2: *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the C-veh or the respective PNS-veh group; +P < 0.05, ++P < 0.01, +++P < 0.001 in comparison with the matched male group (C-veh, PNS-veh and PNS-Pir)
Behavioral Tests for Depressive Responses
Sucrose Preference Test
Anhedonia, developed as a sequence of PNS, was determined using the preference to drink sweet solutions in SPT. Offspring rats of both sexes with a history of PNS showed a decreased preference for consumption of sucrose [Two-way ANOVA, Treatment effect: F2,68 = 32.269, P < 0.001, η2 = 0.81] while Sex effect [F1,68 1.530, P = 0.221], as well as Treatment × Sex interaction [F2,68 = 1.680, P = 0.195] did not reach significance. The post hoc test also revealed that rats with a history of PNS demonstrated anhedonia (P < 0.001 male PNS-veh compared to C-veh group; P = 0.01 female PNS-veh compared to C-veh group). The treatment with Pir alleviated depressive responses in both male and female rats with PNS (P < 0.001 and P = 0.0479, respectively) (Fig. 6a).
Fig. 6.

Depressive-like behavior in male and female Sprague Dawley rats. Effects of sex (male and female) and treatment (C-veh, PNS-veh and PNS-Pir) on preference to sucrose solution (%) (a) in the sucrose preference test and immobility time (sec) (b) in the forced swimming test (FST). Data are given as mean ± SEM and analyzed by two-way ANOVA followed by Bonferroni post hoc test (n = 10–12). Details as in Fig. 2: *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the C-veh or the respective PNS-veh group; +P < 0.05, ++P < 0.01, +++P < 0.001 in comparison with the matched male group (C-veh, PNS-veh and PNS-Pir)
Forced Swimming Test
The despair-like response was evaluated by measuring the time of immobility in the FST. Like in the SPT, the exposure to PNS induced increased immobility in the two sexes [Two-way ANOVA, Treatment effect: F2,63 = 90.677, P < 0.001, η2 = 0.811] with the Treatment × Sex interaction [F2,63 = 4.781, P = 0.012, η2 = 0.37]. Sex effect was not statistically different [F1,63 = 1.357, P = 0.134]. The hopeless behavior was also indicated by the post hoc in PNS-veh male and female rats (P < 0.001 and P < 0.001 PNS-veh compared to C-veh group, respectively) (Fig. 6b). While treatment with Pir succeeded in reversing this depressive response in male rats with PNS (P < 0.001 PNS-Pir compared to PNS-veh group), chronic treatment with this melatonin analog was ineffective in PNS female rats in the FST.
Plasma Corticosterone (CORT) Levels in Basal and Stress-Induced Conditions
Mixed ANOVA revealed a main effect of Treatment [F2,106 = 11.188, P < 0.001, η2 = 0.651], Sex [F1,106 = 11.422, P = 0.001, η2 = 0.778] and Time [F2,106 = 21.861, P < 0.001, η2 = 0.799]. Furthermore, Treatment × Sex [F2,106 = 7.825, P < 0.001, η2 = 0.55], as well as Treatment × Time interaction [F2,106 = 11.004, P < 0.001, η2 = 0.61] were detected. Application of stress procedure increased the CORT concentration. Ten minutes after the stress procedure, elevation of CORT level was detected in all groups with the exception of the female PNS-Pir group (control groups: P < 0.05 C-veh 10 min vs. 0 min), (PNS groups: male: P < 0.01 PNS-veh 10 min vs. 0 min, P = 0.05 PNS-veh vs C-veh at 10 min; female: P < 0.01 PNS-veh 10 min vs. 0 min), (male PNS-Pir group: P < 0.05 PNS-Pir vs. C-veh at 10 min). Surprisingly, the female PNS-Pir group was characterized with enormously elevated CORT levels on the 10 min (P < 0.01 female PNS-Pir 10 min vs. T0 min; P < 0.001 female PNS-Pir vs. male C-veh and male PNS-veh, respectively, at 10 min) (Fig. 7).
Fig. 7.
Plasma corticosterone secretion (ng/mg protein) in basal condition (time in 0 min), and in response to the FST (time at 10 min and 120 min). Effects of sex (male and female) and treatment (C-veh, PNS-veh and PNS-Pir) on corticosterone level. Data are given as mean ± SEM and analyzed by three-way ANOVA followed by Bonferroni post hoc test (n = 6–8). Details as in Fig. 2. *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with time at 0 or 10 min within the respective group or between groups within the same time; +P < 0.05, ++P < 0.01, +++P < 0.001 in comparison with the matched male group (C-veh, PNS-veh and PNS-Pir)
Stress-induced plasma CORT elevation detected in the PNS offspring of both sexes remained stable 120 min later (male: P < 0.001 PNS-veh 120 vs. 0; female: P < 0.001 PNS-veh 120 vs. C-veh 0). The treatment with Pir decreased the stressed-induced elevated CORT on 120 min in male and female offspring with a history of PNS (male: P < 0.05 PNS-Pir 120 min vs. 10 and female: vs. P < 0.001 PNS-Pir 120 min vs. 10 min).
Expression of Glucocorticoid Receptors and Mineralocorticoid Receptors in the Hippocampus
Two-way ANOVA showed a main Treatment effect for GRs [F2.41 = 3.271, P = 0.049, η2 = 0.357] while Sex effect: [F1.41 = 0.834, P = 0.367] as well as the Treatment × Sex interaction [F2.41 = 0.327, P = 0.571] were not statistically significant. Exposure to PNS and vehicle treatment resulted in significantly increased expression of GRs both in male (P = 0.007 PNS-veh compared to C-veh) and in female rats (P = 0.006 PNS-veh compared to C-veh group) (Fig. 8a). Piromelatine suppressed the PNS-induced elevation of GRs to the control level in male rats (P = 0.014 PNS-Pir compared to PNS-veh) but was ineffective in female rats (P = 0.05 PNS-Pir compared to C-veh).
Fig. 8.
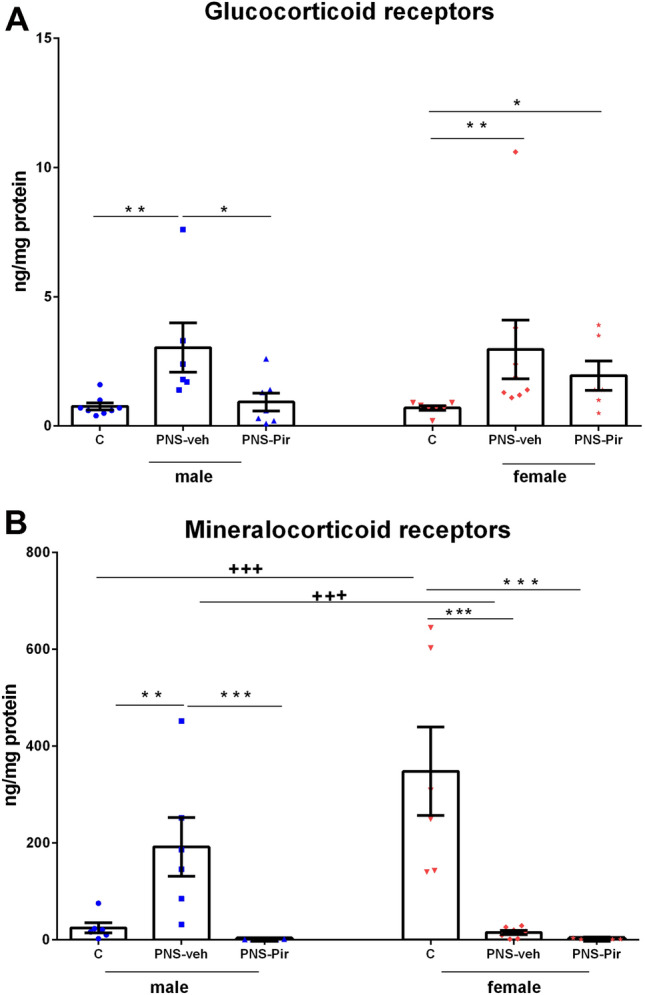
Effects of sex (male and female) and treatment (C-veh, PNS-veh and PNS-Pir) on the expression of mineralocorticoid receptors (MR) (ng/mg protein) (a) and glucocorticoid receptors (GR) (ng/mg protein) (b) in Sprague Dawley rats. Data are given as mean ± SEM and analyzed by two-way ANOVA followed by Bonferroni post hoc test (n = 6–8). Details as in Fig. 2: *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the C-veh or the respective PNS-veh group; +P < 0.05, ++P < 0.01, +++P < 0.001 in comparison with the matched male group (C-veh, PNS-veh and PNS-Pir)
Two-way ANOVA analysis revealed a main Treatment effect [F2,37 = 10.567, P = 0.003, η2 = 0.63], Sex effect [F1,37 = 8.123, P = 0.007, η2 = 0.57] and Treatment × Sex interaction [F2,37 = 24.862, P < 0.001, η2 = 0.73] for MRs. Female C-veh rats demonstrated higher MRs expression than male controls (P = 0.001) (Fig. 8b). The male rats with PNS showed significantly increased the expression of MRs (P = 0.004 PNS-veh compared to C-veh group). Conversely, female rats with PNS were characterized with significantly diminished MR expression in the hippocampus (P = 0.001 PNS-veh compared to C-veh group). While Pir reversed the PNS-induced increase of MRs in male rats (P < 0.001 PNS-Pir compared to PNS-veh), the treatment with this melatonin analog was ineffective in female rats with PNS (P < 0.001 PNS-Pir compared to C-veh).
Discussion
The main finding of the present study is that the novel melatonin analog Pir alleviates anxiety and depressive-like responses by normalizing the PNS-induced impairment of HPA axis feedback function in both sexes while it corrects the hippocampal CRs expression in a sex-dependent manner.
In agreement with recent studies (Kurek et al. 2018; Poltyrev et al. 1996) we report that the body weight gain in male and female offspring was not affected by exposure to PNS compared with the normal control groups. Although continuous treatment with Pir showed a tendency towards decreasing body weight, which remained stable throughout the whole period of measurement, it did not significantly affect the body weight gain in prenatally stressed offspring. Increased CORT concentration during pregnancy is associated with offspring born with abnormal body weight in experimental models and in humans (Baum et al. 2003; Darnaudéry and Maccari 2008; Mairesse et al. 2007; Seckl 2004), changes in metabolic parameters and a high risk for obesity in adults (Pilgaard et al. 2011; Saenger et al. 2007). Melatonin and its analogs are known to be involved in cell metabolism, to raise body temperature and to suppress body weight gain or normalize it in pathophysiological conditions (Agil et al. 2011; Puchalski et al. 2003; Tchekalarova et al. 2017, 2016a; Wolden-Hanson et al. 2000). She et al. reported the effects of long-term Pir treatment, as a MT1/MT2 receptors agonist, on weight gain in two quite different models: high-fat diet (2009) and rats with sleep deprivation (2014). Thus, this author found that while Pir was able to normalize body weight gain of rats on a high-fat diet to control level (2009) this melatonin analog did not succeed to attenuate to control level depressed body weight gain in sleep-deprived rats. This drug was also reported to improve glucose intolerance and insulin sensitivity without affecting food intake (Huang et al. 2013; Tian et al. 2010). In another study, Pir has been shown to decrease food intake in diabetic and high-fat diet rats, to enhance both melatonin receptor expression and to positively influence glucose and lipid metabolism (Wang et al. 2013; Zhou et al. 2018).
Mota CMD (2020) reported that 5-HT inhibits brown adipose tissue sympathetic nerve activity via a GABA input to the dorsomedial hypothalamus, without activating the 5-HT1A receptor in raphe pallidus. However, Ootsuka and Blessing (Ootsuka and Blessing 2003) specifically showed that activation of 5HT1a receptors inhibits sympathetic nerve activity to brown fat thermogenesis in raphe pallidus. These controversial results on the mechanism related to the role of serotonin receptors on brown fat thermogenesis also support the view that the mechanism is more complex and deserves further exploration. Our data did not show a substantial influence of Pir on weight gain, suggesting differences in the underlying mechanism of Pir, as a mixed MT1/MT2 receptor and 5-HT1A/1D receptor agonist, on body weight gain, which depends on the model and possibly the underlying mechanism of this drug on this parameter. However, future studies are needed to ascertain the precise mechanism of Pir treatment under PNS.
Consistent with other investigations (Butkevich et al. 2019; Grundwald and Brunton 2015; Hiroi et al. 2016; Koehl et al. 1997; Maccari et al. 2003; Soares-Cunha et al. 2018; Tsunashima et al. 1997), previous reports revealed that offspring of both sexes of prenatally stressed mothers have anxiety and depressive-like changed behavior associated with impaired HPA axis feedback mechanism and changed GRs expression.
Stress during pregnancy produced significant suppression of motor activity in the OF and EPM tests in both sexes, reflecting enhanced fear and anxiety-like responses. In agreement with Lehman et al. (Lehmann et al. 2000), we found that PNS blunted the higher activity of females compared to males. Corresponding to our data, continuous maternal stress decreased the distance moved or the number of crossings in male and female offspring which are also considered signs of less motivation and scare of a novel environment (first exposure to an apparatus) (Poltyrev et al. 1996; Sun et al. 2013; Wakshlak and Marta 1990). In opposite, other authors did not show any influence of the PNS on this behavior (Zuena et al. 2008), or reported an increase only in males (Sickmann et al. 2015; Van den Hove et al. 2013) or a decrease in females (Lehmann et al. 2000). The discrepancies between studies could be due to rather different methods or strains used, differences in the applied stress procedures, the gestational day the stressor was started and the duration of time, or varieties of the time when the test was conducted (Martínez-Téllez et al. 2009; Sickmann et al. 2015). In support of our results is the research of Wiebel et al., who revealed that hypercorticism negatively impacts the physical activity in rats (Weibel et al. 2002). Despite the inconsistencies, the data confirmed that early stress experience impedes reactions to new challenges. Furthermore, the lack of difference among controls and PNS rats of both sexes on motor activity suggests affective response in the OF and EPM test but not mere motor impairment. PNS has also been shown to produce changes in the circadian rhythmicity of motor activity (Maccari and Morley-Fletcher 2007; Maccari and Van Reeth 2007; Mairesse et al. 2013). The deficiency of the circulating melatonin during pregnancy is critical for fetal development and can alter the circadian clock function (Chen et al. 2013). Within distinct models, melatonin and its analogs enhanced physical activity and stabilized the circadian pattern of locomotion (Mairesse et al. 2013; Tchekalarova et al. 2019; Weibel et al. 2000; Wolden-Hanson et al. 2000). In this study, Pir treatment attenuated the affective response associated with decreased motor activity in the novel environment in offspring of both sexes. Even though the restriction of this study is that the effect of a matched Pir-treated control group was not examined to reduce overuse of resources = and animals, other authors reported enough data that this melatonin analog does not affect locomotion per se (Fu et al. 2016; He et al. 2013; Tian et al. 2010).
The anxious behavior of offspring from both sexes was indicated with lower distance traveled and time spent in the center of the OF. Higher anxiety of the PNS-rats was observed in two more tests: decreased time spent and an open to the total number of entries ratio in the EPM, as well as significantly diminished the time spent in light compartment and the number of transitions in the LD tests. Inconsistencies regarding the anxiety-like behavior of PNS offspring are present among authors. Whereas Sickman et al. (Sickmann et al. 2015) did not specify any impact of PNS on anxiety, numerous studies revealed that PNS could influence negatively anxious behavior in males and/or in females (Morley-Fletcher et al. 2011; Poltyrev et al. 1996; Soares-Cunha et al. 2018; Wakshlak and Marta 1990). Additionally, it has been proposed that rats of both sexes could react differently to PNS (Maccari et al. 2003; Sun et al. 2013; Van den Hove et al. 2013; Zuena et al. 2008), which warrants the need for further exploration of interactions between sex and anxiety, on one hand, and between sex, PNS and anxiety on the other. Yet, all these data indicated that prenatal maternal stress exposure, accompanied by prolonged hyperactive HPA axis might have a great impact on the susceptibility of the adult offspring. We report that PNS exacerbates anxiety both in male and female rats, while the chronic treatment with Pir exerted similar beneficial effects on these responses. Though the limitation of this study is that we did not use a reference drug or naive control treated with Pir meant for minimizing the resources and animal use, sufficient literature data have illustrated that melatonin and melatonin agonists, including Pir can produce anxiolytic effects (Chen et al. 2013; Papp et al. 2006; Tchekalarova et al. 2016b; Tian et al. 2010). Further, Tian et al. (2010) demonstrated that the antidepressant and anxiolytic effects of the novel melatonin-derived compound Pir were superior to that of melatonin.
In accordance with reports that PNS represents a risk for the development of depressive disorders (Butkevich et al. 2019; Darnaudéry and Maccari 2008; Soares-Cunha et al. 2018; Wang et al. 2015), we observed that offspring with a history of PNS, irrespective of sex, exhibited depressive-like responses. Following PNS, rats showed a decreased preference for a sweet solution, which reflects anhedonia, as well as hopeless behavior in the FST. The results regarding sex-related differences in depression are also controversial with reports demonstrating this mental state mainly in males (Van den Hove et al. 2013; Zuena et al. 2008), while others, solely in females (Alonso et al. 1991; Sickmann et al. 2015). Despite this, stress experienced during pregnancy appears to be crucial for developing normal behavior in adulthood, and concomitant with neurological, biochemical, and behavioral changes that persist in later life (Darnaudéry and Maccari 2008; Morley-Fletcher et al. 2011; Zuena et al. 2008). Scientific works have proven the important role of melatonin in epigenetic programing (Darnaudéry and Maccari 2008; Korkmaz and Reiter 2008; Maccari and Morley-Fletcher 2007) along with its effectiveness and that of its analogs against depression symptoms in humans (Laudon and Frydman-Marom 2014), and in different animal models (Ilieva et al. 2019; Tchekalarova et al. 2019, 2016a, 2013), including PNS (Chen et al. 2013; Mairesse et al. 2013; Morley-Fletcher et al. 2011). Melatonin was also reported to reduce immobility in rats and mice (Brotto et al. 2000; Estrada-Reyes et al. 2018). Consistent with these findings, acute treatment with the innovative melatoninergic drug Pir decreased the immobile time in male rats in the FST, thus displaying an antidepressant action similar to those of imipramine (Tian et al. 2010). Fu et al. (2016) outlined that the chronic administration of Pir prevented anhedonia and depression-like behavior in chronically stressed male rats. In our study, chronic treatment with Pir mitigated depressive responses in male rats with PNS. This novel compound exerted a sex-specific response by reversing the despair-like behavior in the FST only in PNS male rats, although a clear trend of decrease was also noticed in female rats with PNS. Literature data suggest that the predisposition of females to developing mood disorders, including depression, might be related to the gonadal steroids and HPA axis relationships (Hankin 2009; Morley-Fletcher et al. 2019; Mueller and Bale 2008). To provide a precise explanation, more investigations focused on the effect of Pir on naive females are necessary.
We hypothesize that the effects of Pir on behavioral responses in the PNS model in the present study might involve simultaneous activation of the 5-HT1A and 5-HT1D receptors. This argument is encouraged by investigations with melatonin and the mixed MT1/MT2 agonist and 5-HT2C antagonist agomelatine, interacting with distinct serotoninergic receptors (Bourin et al. 2004; Monnet 2002). The alteration or deletion of the 5-HT1A receptor has resulted in increased fear, depressive and anxiogenic behavior (Gross et al. 2000; Vinkers et al. 2010). Moreover, stimulation of this receptor has shown anxiolytic activity in animal models and in humans (De Vry et al. 1993; Feighner & Boyer 1989; Gross et al. 2002). The 5-HT1D receptor is involved in locomotion, fear and anxiety and is responsible for the regulation of 5-HT release (Pytliak et al. 2011). Stress, the main trigger for anxiety and depression, leads to increased release of 5-HT and concomitant sensitization and dysfunction of the 5-HT1D receptor, thereby resulting in the pathological state. The PNS-evoked changes on these receptors, as well as their precise role in the effect of Pir on behavioral responses, need further elucidation.
We report sex-dependent differences for CORT levels with higher sensitivity after acute stress in control female rats compared to male rats. However, the basal and stress-induced CORT levels were similar in male and female offspring with a history of PNS. Some authors suggested that sex steroid hormones affect the HPA axis response differently (Green and McCormick 2016; Morley-Fletcher et al. 2019; Zaidan et al. 2013) and because many CORT Elisa assays, including Enzo used in the present study, have cross-reactivity with gonadal hormones (Krasowski et al. 2014) their role on PNS-induced changed HPA axis activity can not be excluded. However, the precise mechanism in conditions of PNS still needs to be elucidated. Further, we report that unlike control rats, the surge of plasma CORT secretion persisted both in male and female PNS rats following 120 min of the stress procedure. These findings indicate that maternal stress can increase the sensitivity to acute stress in adult male and female offspring, suggesting an impaired inhibitory feedback regulation of the HPA axis. Literature data demonstrating the baseline plasma CORT levels in adult offspring of PNS rats are controversial. In agreement with our findings, several other authors have also reported no difference in baseline CORT levels between controls and PNS rats (Butkevich et al. 2019; Sickmann et al. 2015) or a sustained elevation of stress-induced CORT secretion 20 and 60 min after application of the stress procedure in male rats (Dugovic et al. 1999). Koehl et al. (1999) reported an altered circadian pattern of CORT in male and female rats with a history of PNS that is characterized by significantly elevated values of the total and free hormone at the end of the light period. Other authors have demonstrated high plasma CORT levels of PNS offspring under both normal and stress-related conditions (Koehl et al. 1999; Maccari and Morley-Fletcher 2007; Mairesse et al. 2015; Soares-Cunha et al. 2018). The variability in literature data might be due to different factors that can affect the results, such as the age of testing, strain, gestational period of stress procedure, type of stressors or time of testing the hormone. This issue suggests that various factors could lead to different changes in CORT levels in the offspring of stressed mothers, but the typical outcome is an impaired control of the HPA axis with exacerbated vulnerability in stress conditions.
It is accepted that GCs have a crucial impact on the functionality of the hippocampus and, in particular, on neuronal excitability and plasticity (reviewed in: McEwen 1999). Factors that contribute to a sustained elevation of GCs can negatively affect both the morphology and activity of the hippocampus, thereby causing volume shrinkage and/or suppression in neurogenesis and cognitive processes (Lupien et al. 1998; McEwen 1999). Increased GCs may also trigger downregulation of their receptors, MRs and/or GRs, by acting via a negative feedback mechanism. In line with this presumption, Koehl et al. (1999) demonstrated that sex-dependent elevated CORT in PNS rats was associated with an attenuated binding capacity of MRs in both male and female rats. Furthermore, GRs were downregulated specifically at the end of the light phase only in male rats, although elevated CORT levels at the same period were detected in both sexes. These findings suggest that the negative feedback mechanism in the hippocampus is complex, and decreased GR expression in the hippocampus, while expected, is not an inevitable consequence of the sustained elevation of plasma CORT level. In the present study, we report that while the expression of MRs in control and PNS conditions are strictly sex-specific, GRs are upregulated following PNS in both males and females. Intact female rats were characterized by increased MRs in the hippocampus compared to male controls, which is possibly related to their higher sensitivity to stress-induced CORT augmented levels. Surprisingly, except for downregulated MRs detected in female PNS rats, MRs in males, as well as GRs in both sexes, were raised under exposure to PNS. Studies focused on the link between CORT levels under baseline and stress conditions and respective changes in CRs in the hippocampus are few and contradictory (Maccari et al. 1995; Morley-Fletcher et al. 2004). Also, literature data are mostly on male rats. The reported upregulation of GRs in the hippocampus of male and female rats with a history of PNS in the present study is in accordance with other studies conducted with different strains, structures, time and duration of prenatal stress exposure (Kanitz et al. 2003; Wei et al. 2004).
We report that the beneficial effect of chronic treatment with Pir on changes in CRs in the hippocampus of PNS rats is sex-dependent. These results agree with data for clinically used antidepressants that can ameliorate disturbed regulatory mechanisms of HPA axis activity parallel to positive influence on depressive responses (reviewed in Anacker et al. 2012). Zhou et al. (2018) found out that Pir administration alleviated the HPA axis hyperfunctioning in chronically stressed rats on a high-fat diet. Yet, our findings confirm the advantage of PNS as an adequate model for exploring novel pharmacotherapeutic strategies associated with anxiety and depressive-like behavior. We can speculate that the 5-HT1A receptor is implicated in the mechanism of action of Pir in the PNS model as in other atypical antidepressants acting via 5-HTergic system (Srinivas et al. 2001). However, even though this melatonin analog succeeded in normalizing the delayed stress-evoked initiation of the feedback inhibitory regulatory mechanism on plasma CORT level both in male and female rats, it corrected changes in the hippocampal CRs primarily in male offspring. Surprisingly, although Pir alleviated to control level stress-induced CORT after 120 min, this plasma hormone was abnormally elevated at 10th min after stress in female rats with PNS. The sex-dependent difference of Pir treatment on stress-induced CORT level is unknown and deserves a future analysis of the role of estrogen hormones on Pir effects under stress conditions.
In conclusion, we provide evidence for the first time that the novel melatonin analog Pir corrects the anxiety responses and ameliorated the impaired feedback regulation of the HPA axis in offspring with a history of PNS from both sexes. However, this drug modifies the PNS-induced changes of CRs in the hippocampus only in male rats with PNS. Further studies are required to clarify the PNS-induced changes between the MRs and GRs balance in the hippocampus, and also in the amygdala, as well as additional investigations on other putative signaling pathways involved in the HPA axis activity, including the role of gonadal hormones.
Acknowledgements
This work was supported by the National Science Fund of Bulgaria, research Grant № KP-06-H21/10.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Natasha Ivanova, Email: ivanova_nm@yahoo.com.
Jana Tchekalarova, Email: janetchekalarova@gmail.com.
References
- Agil A, Navarro-Alarcõn M, Ruiz R, Abuhamadah S, El-Mir MY, Vázquez GF (2011) Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res. 10.1111/j.1600-079X.2010.00830.x [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Arevalo R, Afonso D, Rodríguez M (1991) Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiol Behav. 10.1016/0031-9384(91)90538-Y [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante C (2012) Europe PMC funders group the glucocorticoid receptor : pivot of depression and of antidepressant treatment ? Psychoneuroendocrynology 36:415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta-Kaim A, Szczesny E, Glombik K, Stachowicz K, Slusarczyk J, Nalepa I, Zelek- Molik A, Rafa- Zablocka K, Budziszewska B, Kubera M, Leskiewicz M, Lason W (2014) Prenatal stress affects insulin-like growth factor-1 (IGF-1) level and IGF-1 receptor phosphorylation in the brain of adult rats. Eur Neuropsychopharmacol. 10.1016/j.euroneuro.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Baum M, Ortiz L, Quan A (2003) Fetal origins of cardiovascular disease. Curr Opin Pediatr. 10.1097/00008480-200304000-00005 [DOI] [PubMed] [Google Scholar]
- Bourin M, Mocaër E, Porsolt R (2004) Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci 29(2):126–133 [PMC free article] [PubMed] [Google Scholar]
- Brotto LA, Barr AM, Gorzalka BB (2000) Sex differences in forced-swim and open-field test behaviours after chronic administration of melatonin. Eur J Pharmacol. 10.1016/S0014-2999(00)00491-X [DOI] [PubMed] [Google Scholar]
- Butkevich IP, Mikhailenko VA, Vershinina EA, Barr GA (2019) Differences between the prenatal effects of fluoxetine or buspirone alone or in combination on pain and affective behaviors in prenatally stressed male and female rats. Front Behav Neurosci. 10.3389/fnbeh.2019.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carocci A, Catalano A, Sinicropi MS (2014) Melatonergic drugs in development. Clin Pharmacol. 10.2147/CPAA.S36600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Sheen JM, Tiao MM, Tain YL, Huang LT (2013) Roles of melatonin in fetal programming in compromised pregnancies. Int J Mol Sci. 10.3390/ijms14035380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Fichna J, Laudon M, Storr M (2014) Antinociceptive effects of novel melatonin receptor agonists in mouse models of abdominal pain. World J Gastroenterol. 10.3748/wjg.v20.i5.1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudéry M, Maccari S (2008) Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 10.1016/j.brainresrev.2007.11.004 [DOI] [PubMed] [Google Scholar]
- De Vry J, Benz U, Schreiber R, Traber J (1993) Shock-induced ultrasonic vocalization in young adult rats: a model for testing putative anti-anxiety drugs. Eur J Pharmacol. 10.1016/0014-2999(93)90530-U [DOI] [PubMed] [Google Scholar]
- Estrada-Reyes R, Valdés-Tovar M, Arrieta-Baez D, Dorantes-Barrón AM, Quero-Chávez D, Solís-Chagoyán H, Argueta J, Dubocovich ML, Benítez-King G (2018) The timing of melatonin administration is crucial for its antidepressant-like effect in mice. Int J Mol Sci. 10.3390/ijms19082278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner JP, Boyer WF (1989) Serotonin-1A anxiolytics: An overview. 16th Collegium Internationale Neuro-Psychopharmacologicum Congress Satellite Conference: New Findings with Anxiolytic Drugs (1988, Munich, Federal Republic of Germany). Psychopathology
- Fu W, Xie H, Laudon M, Zhou S, Tian S, You Y (2016) Piromelatine ameliorates memory deficits associated with chronic mild stress-induced anhedonia in rats. Psychopharmacology 233:2229–2239. 10.1007/s00213-016-4272-3 [DOI] [PubMed] [Google Scholar]
- Głombik K, Stachowicz A, Ślusarczyk J, Trojan E, Budziszewska B, Suski M, Kubera M, Lasoń W, Wedzony K, Olszanecki R, Basta-Kaim A (2015) Maternal stress predicts altered biogenesis and the profile of mitochondrial proteins in the frontal cortex and hippocampus of adult offspring rats. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.06.015 [DOI] [PubMed] [Google Scholar]
- Green MR, McCormick CM (2016) Sex and stress steroids in adolescence: Gonadal regulation of the hypothalamic–pituitary–adrenal axis in the rat. Gen Comp Endocrinol. 10.1016/j.ygcen.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R (2000) Altered fear circuits in 5-HT1A receptor KO mice. Biol Psychiat. 10.1016/S0006-3223(00)01041-6 [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R (2002) Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416:396–400. 10.1038/416396a [DOI] [PubMed] [Google Scholar]
- Grundwald NJ, Brunton PJ (2015) Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyaeva NV (2019) Biochemical mechanisms and translational relevance of hippocampal biochemical mechanisms and translational relevance of hippocampal vulnerability to distant focal brain injury : the price of stress response. Biochemistry 84:1306–1328 [DOI] [PubMed] [Google Scholar]
- Hankin BL (2009) Development of sex differences in depressive and co-occurring anxious symptoms during adolescence: descriptive trajectories and potential explanations in a multiwave prospective study. J Clin Child Adolesc Psychol. 10.1080/15374410902976288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Ouyang X, Zhou S, Yin W, Tang C, Laudon M, Tian S (2013) A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’ disease. Horm Behav. 10.1016/j.yhbeh.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB (2009) Effect of childhood trauma on adult depression and neuroendocrine function: sex-specifi c moderation by CRH receptor 1 gene. Front Behav Neurosci. 10.3389/neuro.08.041.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S (1994) Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 10.1111/j.1365-2826.1994.tb00591.x [DOI] [PubMed] [Google Scholar]
- Hiroi R, Carbone DL, Zuloaga DG, Bimonte-Nelson HA, Handa RJ (2016) Sex-dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood. Neuroscience. 10.1016/j.neuroscience.2016.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Zhang C, Hou Y, Laudon M, She M, Yang S, Ding L, Wang H, Wang Z, He P, Yin W (2013) Blood pressure reducing effects of piromelatine and melatonin in spontaneously hypertensive rats. Eur Rev Med Pharmacol Sci 17(18):2449–2456 [PubMed] [Google Scholar]
- Ilieva K, Tchekalarova J, Atanasova D, Kortenska L, Atanasova M (2019) Antidepressant agomelatine attenuates behavioral deficits and concomitant pathology observed in streptozotocin-induced model of Alzheimer’s disease in male rats. Horm Behav. 10.1016/j.yhbeh.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Kanitz E, Otten W, Tuchscherer M, Manteuffel G (2003) Effects of prenatal stress on corticosteroid receptors and monoamine concentrations in limbic areas of suckling piglets (Sus scrofa) at different ages. J Vet Med Series A: Physiol Pathol Clin Med 50(3):132–139 [DOI] [PubMed] [Google Scholar]
- Kloet ER, Han F, Meijer OC (2008) From the stalk to down under about brain glucocorticoid receptors, stress and development. Neurochem Res 33:637–642 [DOI] [PubMed] [Google Scholar]
- Koehl M, Barbazanges A, Le Moal M, Maccari S (1997) Prenatal stress induces a phase advance of circadian corticosterone rhythm in adult rats which is prevented by postnatal stress. Brain Res. 10.1016/S0006-8993(97)00394-6 [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudéry M, Dulluc J, Van Reeth O, Moal ML, Maccari S (1999) Prenatal stress alters circadian activity of hypothalamo-pituitary- adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 10.1002/(SICI)1097-4695(19990905)40:3%3c302::AID-NEU3%3e3.0.CO;2-7 [PubMed] [Google Scholar]
- Korkmaz A, Reiter RJ (2008) Epigenetic regulation: a new research area for melatonin? J Pineal Res. 10.1111/j.1600-079X.2007.00509.x [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Drees D, Morris CS, Maakestad J, Blau J, Ekins S (2014) Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol 14:33. 10.1186/1472-6890-14-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek A, Głombik K, Detka J, Basta-Kaim A, Kubera M, Lasoń W, Budziszewska B (2018) Regulators of glucocorticoid receptor function in an animal model of depression and obesity. J Neuroendocrinol. 10.1111/jne.12591 [DOI] [PubMed] [Google Scholar]
- Laudon M, Frydman-Marom A (2014) Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci. 10.3390/ijms150915924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Stöhr T, Feldon J (2000) Long-term effects of prenatal stress experience and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res. 10.1016/S0166-4328(99)00122-9 [DOI] [PubMed] [Google Scholar]
- Liu YY, Yin D, Chen L, Qu WM, Chen CR, Laudon M, Cheng NN, Urade Y, Huang ZL (2014) Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology. 10.1007/s00213-014-3530-5 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, De Leon M, De Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ (1998) Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 10.1038/271 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S (2007) Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology. 10.1016/j.psyneuen.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Maccari S, Van Reeth O (2007) Circadian rhythms, effects of prenatal stress in rodents, in: encyclopedia of. Stress. 10.1016/B978-012373947-6.00082-9 [Google Scholar]
- Maccari S, Hervk S, Inserm U, De Bordeaux Ii U, De Carreire D (1995) Adoption reverses the long-term impairment feedback induced by prenatal stress in glucocorticoid. J Neurosci 15:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O (2003) Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 10.1016/S0149-7634(03)00014-9 [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Bréant B, Hahn T, Darnaudéry M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O (2007) Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 10.1152/ajpendo.00574.2006 [DOI] [PubMed] [Google Scholar]
- Mairesse J, Silletti V, Laloux C, Zuena AR, Giovine A, Consolazione M, van Camp G, Malagodi M, Gaetani S, Cianci S, Catalani A, Mennuni G, Mazzetta A, van Reeth O, Gabriel C, Mocaër E, Nicoletti F, Morley-Fletcher S, Maccari S (2013) Chronic agomelatine treatment corrects the abnormalities in the circadian rhythm of motor activity and sleep/wake cycle induced by prenatal restraint stress in adult rats. Int J Neuropsychopharmacol 16:323–338. 10.1017/S1461145711001970 [DOI] [PubMed] [Google Scholar]
- Mairesse J, Van Camp G, Gatta E, Marrocco J, Reynaert ML, Consolazione M, Morley-Fletcher S, Nicoletti F, Maccari S (2015) Sleep in prenatally restraint stressed rats, a model of mixed anxiety-depressive disorder. In: Antonelli MC (ed) Advances in neurobiology. Springer, New York [DOI] [PubMed] [Google Scholar]
- Martínez-Téllez RI, Hernández-Torres E, Gamboa C, Flores G (2009) Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 10.1002/syn.20664 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1999) Stress and hippocampal plasticity. Annu Rev Neurosci 22:105–122. 10.1146/annurev.neuro.22.1.105 [DOI] [PubMed] [Google Scholar]
- Monnet FP (2002) Melatonin modulates [3H] serotonin release in the rat hippocampus: effects of circadian rhythm. J Neuroendocrinol. 10.1046/j.0007-1331.2001.00761.x [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaude´ry M, Mocaer E, Froger N, Lanfumey L, Laviola G, Casolini P, Zuena AR, Marzano L, Hamon M, Maccari S (2004) Chronic Treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT 1A receptor MRNA in prenatally stressed rats. Neuropharmacology 47(6):841–47 [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Mairesse J, Soumier A, Banasr M, Fagioli F, Gabriel C, Mocaer E, Daszuta A, McEwen B, Nicoletti F, Maccari S (2011) Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology 217:301–313. 10.1007/s00213-011-2280-x [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Mairesse J, Van Camp G, Reynaert ML, Gatta E, Marrocco J, Bouwalerh H, Nicoletti F, Maccari S (2019) Perinatal stress programs sex differences in the behavioral and molecular chronobiological profile of rats maintained under a 12-h light-dark cycle. Front Mol Neurosci. 10.3389/fnmol.2019.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota CMD (2020) A new role for serotonin: the 5-HT3 receptor in bladder afferent hypersensitivity. J Physiol. 10.1113/JP279094 [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL (2008) Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 10.1523/JNEUROSCI.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW (2003) 5-Hydroxytryptamine 1A receptors inhibit cold-induced sympathetically mediated cutaneous vasoconstriction in rabbits. J Physiol. 10.1113/jphysiol.2003.048041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Litwa E, Gruca P, Mocaër E (2006) Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav Pharmacol. 10.1097/01.fbp.0000181601.72535.9d [DOI] [PubMed] [Google Scholar]
- Pilgaard KA, Mosbech TH, Grunnet L, Eiberg H, Van Hall G, Fallentin E, Larsen T, Larsen R, Poulsen P, Vaag A (2011) Differential nongenetic impact of birth weight versus third-trimester growth velocity on glucose metabolism and magnetic resonance imaging abdominal obesity in young healthy twins. J Clin Endocrinol Metab. 10.1210/jc.2011-0577 [DOI] [PubMed] [Google Scholar]
- Poltyrev T, Keshet GI, Kay G, Weinstock M (1996) Role of experimental conditions in determining differences in exploratory behavior of prenatally stressed rats. Dev Psychobiol. 10.1002/(SICI)1098-2302(199607)29:5%3c453::AID-DEV4%3e3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Puchalski SS, Green JN, Rasmussen DD (2003) Melatonin effects on metabolism independent of gonad function. Endocrine. 10.1385/ENDO:21:2:169 [DOI] [PubMed] [Google Scholar]
- Pytliak M, Vargová V, Mechírová V, Felšöci M (2011) Serotonin receptors—from molecular biology to clinical applications. Physiol Res. 10.33549/physiolres.931903 [DOI] [PubMed] [Google Scholar]
- Saenger P, Czernichow P, Hughes I, Reiter EO (2007) Small for gestational age: short stature and beyond. Endocr Rev. 10.1210/er.2006-0039 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Braun K, Brandwein C, Rossetti AC, Guara Ciurana S, Riva MA, Deuschle M, Bock J, Gass P, Gröger N (2018) Maternal stress during pregnancy induces depressive-like behavior only in female offspring and correlates to their hippocampal Avp and Oxt receptor expression. Behav Brain Res. 10.1016/j.bbr.2018.06.027 [DOI] [PubMed] [Google Scholar]
- Seckl JR (2004) Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 10.1530/eje.0.151u049 [DOI] [PubMed] [Google Scholar]
- She M, Deng X, Guo Z, Laudon M, Hu Z, Liao D, Hu X, Luo Y, Shen Q, Su Z, Yin W (2009) NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol Res. 10.1016/j.phrs.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Arentzen TS, Dyrby TB, Plath N, Kristensen MP (2015) Prenatal stress produces sex-specific changes in depression-like behavior in rats: Implications for increased vulnerability in females. J Dev Orig Health Dis. 10.1017/S2040174415001282 [DOI] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, Borges S, Domingues AV, Silva D, Sousa N, Rodrigues AJ (2018) Mild prenatal stress causes emotional and brain structural modifications in rats of both sexes. Front Behav Neurosci. 10.3389/fnbeh.2018.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni G, Bedini A, Rivara S, Mor M (2011) Melatonin receptor agonists: New options for insomnia and depression treatment. CNS Neurosci Ther. 10.1111/j.1755-5949.2010.00197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas BN, Subhash MN, Vinod KY (2001) Cortical 5-HT1A receptor downregulation by antidepressants in rat brain. Neurochem Int. 10.1016/S0197-0186(00)00123-6 [DOI] [PubMed] [Google Scholar]
- Sun H, Jia N, Guan L, Su Q, Wang D, Li H, Zhu Z (2013) Involvement of NR1, NR2A different expression in brain regions in anxiety-like behavior of prenatally stressed offspring. Behav Brain Res. 10.1016/j.bbr.2013.08.044 [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, Blazhev A, Dragomirova P, Pechlivanova D, Petkova Z, Atanasova M (2013) Strain-dependent effects of long-term treatment with melatonin on kainic acid-induced status epilepticus, oxidative stress and the expression of heat shock proteins. Pharmacol Biochem Behav 111:44–50. 10.1016/j.pbb.2013.08.006 [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, Nenchovska Z, Atanasova D, Atanasova M, Kortenska L, Stefanova M, Alova L, Lazarov N (2016) Consequences of long-term treatment with agomelatine on depressive-like behavior and neurobiological abnormalities in pinealectomized rats. Behav Brain Res. 10.1016/j.bbr.2015.12.043 [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, Nenchovska Z, Atanasova D, Atanasova M, Kortenska L, Stefanova M, Alova L, Lazarov N (2016) Consequences of long-term treatment with agomelatine on depressive-like behavior and neurobiological abnormalities in pinealectomized rats. Behav Brain Res 302:11–28. 10.1016/j.bbr.2015.12.043 [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, Atanasova D, Nenchovska Z, Atanasova M, Kortenska L, Gesheva R, Lazarov N (2017) Agomelatine protects against neuronal damage without preventing epileptogenesis in the kainate model of temporal lobe epilepsy. Neurobiol Dis. 10.1016/j.nbd.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, Stoynova T, Ilieva K, Mitreva R, Atanasova M (2018) Agomelatine treatment corrects symptoms of depression and anxiety by restoring the disrupted melatonin circadian rhythms of rats exposed to chronic constant light. Pharmacol Biochem Behav. 10.1016/j.pbb.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, Kortenska L, Ivanova N, Atanasova M, Marinov P (2019) Agomelatine treatment corrects impaired sleep-wake cycle and sleep architecture and increases MT1 receptor as well as BDNF expression in the hippocampus during the subjective light phase of rats exposed to chronic constant light. Psychopharmacology. 10.1007/s00213-019-05385-y [DOI] [PubMed] [Google Scholar]
- Tian SW, Laudon M, Han L, Gao J, Huang FL, Yang YF, Deng HF (2010) Antidepressant-and anxiolytic effects of the novel melatonin agonist Neu-P11 in rodent models. Acta Pharmacol Sin. 10.1038/aps.2010.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan E, Ślusarczyk J, Chamera K, Kotarska K, Glombik K, Kubera M, Basta-Kaim A (2017) The modulatory properties of chronic antidepressant drugs treatment on the brain chemokine—chemokine receptor network: a molecular study in an animal model of depression. Front Pharmacol. 10.3389/fphar.2017.00779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G (1997) GABAA receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience 80:1019–1032. 10.1016/S0306-4522(97)00144-9 [DOI] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S (1997) Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci 17:2626–2636. 10.1523/JNEUROSCI.17-07-02626.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hove DLA, Kenis G, Brass A, Opstelten R, Rutten BPF, Bruschettini M, Blanco CE, Lesch KP, Steinbusch HWM, Prickaerts J (2013) Vulnerability versus resilience to prenatal stress in male and female rats; implications from gene expression profiles in the hippocampus and frontal cortex. Eur Neuropsychopharmacol. 10.1016/j.euroneuro.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Oosting RS, van Bogaert MJV, Olivier B, Groenink L (2010) Early-life blockade of 5-HT1A receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biol Psychiat. 10.1016/j.biopsych.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Wakshlak A, Marta W (1990) Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiol Behav. 10.1016/0031-9384(90)90315-U [DOI] [PubMed] [Google Scholar]
- Wang PP, She MH, He PP, Chen WJ, Laudon M, Xu XX, Yin WD (2013) Piromelatine decreases triglyceride accumulation in insulin resistant 3T3-L1 adipocytes: role of ATGL and HSL. Biochimie. 10.1016/j.biochi.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ma Y, Hu J, Cheng W, Jiang H, Zhang X, Li M, Ren J, Li X (2015) Prenatal chronic mild stress induces depression-like behavior and sex-specific changes in regional glutamate receptor expression patterns in adult rats. Neuroscience. 10.1016/j.neuroscience.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H (2004) Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA. 10.1073/pnas.0402208101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel L, Turek FW, Mocaer E, Van Reeth O (2000) A melatonin agonist facilitates circadian resynchronization in old hamsters after abrupt shifts in the light-dark cycle. Brain Res. 10.1016/S0006-8993(00)02806-7 [DOI] [PubMed] [Google Scholar]
- Weinstock M (2001) Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 10.1016/S0301-0082(01)00018-1 [DOI] [PubMed] [Google Scholar]
- Weinstock M (2008) The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 10.1016/j.neubiorev.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen DD (2000) Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 10.1210/endo.141.2.7311 [DOI] [PubMed] [Google Scholar]
- Yalkinoglu O, Zisapel N, Nir T (2010) Phase-I study of the safety, tolerability, pharmacokinetics and sleep promoting activity of Neu-P11, a novel putative insomnia drug in healthy humans. Sleep 33:A220 [Google Scholar]
- Zaidan H, Leshem M, Gaisler-Salomon I (2013) Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biol Psychiat. 10.1016/j.biopsych.2013.04.014 [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang D, Luo XH, Jia X, Li MX, Laudon M, Zhang RX, Jia ZP (2018) Melatonin receptor agonist piromelatine ameliorates impaired glucose metabolism in chronically stressed rats fed a high-fat diet. J Pharmacol Exp Ther. 10.1124/jpet.117.243998 [DOI] [PubMed] [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alemà GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S (2008) Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE. 10.1371/journal.pone.0002170 [DOI] [PMC free article] [PubMed] [Google Scholar]




