Abstract
It is unclear how Toll-like receptor (TLR) 4 signaling affects protein succinylation in the brain after intracerebral hemorrhage (ICH). Here, we constructed a mouse ICH model to investigate the changes in ICH-associated brain protein succinylation, following a treatment with a TLR4 antagonist, TAK242, using a high-resolution mass spectrometry-based, quantitative succinyllysine proteomics approach. We characterized the prevalence of approximately 6700 succinylation events and quantified approximately 3500 sites, highlighting 139 succinyllysine site changes in 40 pathways. Further analysis showed that TAK242 treatment induced an increase of 29 succinyllysine sites on 28 succinylated proteins and a reduction of 24 succinyllysine sites on 23 succinylated proteins in the ICH brains. TAK242 treatment induced both protein hypersuccinylations and hyposuccinylations, which were mainly located in the mitochondria and cytoplasm. GO analysis showed that TAK242 treatment-induced changes in the ICH-associated succinylated proteins were mostly located in synapses, membranes and vesicles, and enriched in many cellular functions/compartments, such as metabolism, synapse, and myelin. KEGG analysis showed that TAK242-induced hyposuccinylation was mainly linked to fatty acid metabolism, including elongation and degradation. Moreover, a combined analysis of the succinylproteomic data with previously published transcriptome data revealed that most of the differentially succinylated proteins induced by TAK242 treatment were mainly distributed throughout neurons, astrocytes, and endothelial cells, and the mRNAs of seven and three succinylated proteins were highly expressed in neurons and astrocytes, respectively. In conclusion, we revealed that several TLR4 signaling pathways affect the succinylation processes and pathways in mouse ICH brains, providing new insights on the ICH pathophysiological processes. Data are available via ProteomeXchange with identifier PXD025622.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10571-021-01144-w.
Keywords: Intracerebral hemorrhage, Brain injury, Succinylation, Toll-like receptor 4
Introduction
Protein posttranslational modifications (PTMs) are considered exceedingly efficient biological mechanisms due to their ability to expand the functions of proteins (Walsh et al. 2005; Witze et al. 2007; Karve and Cheema 2011). They have been shown to be involved in regulating biological processes in human diseases, such as Alzheimer’s disease (Marcelli et al. 2018), brain injury (Klimova et al. 2018), and cancer (Hsu et al. 2018). Protein succinylation is a novel type of PTM that was identified in 2011; the tricarboxylic acid (TCA) cycle intermediate succinate-derived succinyl-CoA has been shown to be a cofactor for lysine succinylation (Zhang et al. 2011). In addition, succinate is a metabolite that plays an important role in contributing to innate immune signaling by increasing protein succinylation (Tannahill et al. 2013). For example, studies have shown that TLR4 activation in macrophages by LPS induces succinate accumulation (Everts et al. 2014; Mills and O'Neill 2014; Tannahill et al. 2013; Wang et al. 2017) and protein succinylation (Tannahill et al. 2013). Meanwhile, inhibiting protein succinylation could also suppress the pro-inflammatory response in macrophages (Wang et al. 2017). Furthermore, protein succinylation has been shown to contribute to cellular energy regulation by increasing the activity of the enzymes involved in glucose and lipid metabolism (Park et al. 2013; Mills and O'Neill 2014). Overall, through the addition of succinyl groups to lysine residues, protein succinylation is critical for regulating metabolic processes, immunity, and inflammation (Mills and O'Neill 2014; Liu et al. 2016).
Intracerebral hemorrhage (ICH) is a life-threatening disease with a poor prognosis and for which few effective treatments exist (Cordonnier et al. 2018). Increasing evidence shows that ICH-induced secondary brain injury can be aggravated by inflammation (Fang et al. 2013; Fei et al. 2019; Zhou et al. 2014). For example, we previously showed that glial TLR4 activation markedly exacerbated brain injury in ICH mice, whereas inhibition by its antagonist, TAK242, significantly alleviated brain injury by reducing neuroinflammation (Wang et al. 2013). These results were consistent with a later conclusion that TAK242 protects against acute cerebral ischemia/reperfusion injury by targeting TLR4 signaling (Hua et al. 2015). Glial cells, such as microglia, have similar inflammatory profiles to those of activated macrophages. These results suggest that ICH-induced brain neuroinflammation also involves protein succinylation, which may be an important underlying mechanism of neuroinflammation-mediated secondary brain injury after ICH.
Therefore, in this study, we aimed to characterize how neuroinflammation influenced protein succinylation in TAK242-treated ICH and control mouse brains using a high-resolution mass spectrometry-based quantitative succinylproteomics approach. Bioinformatic analysis showed that TAK242 treatment induced the hypersuccinylation and hyposuccinylation of proteins, which are mainly located in the mitochondria and cytoplasm and are enriched in many processes, in the ICH brains. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that the TAK242-induced downregulation of succinylation was significantly linked to fatty acid metabolism. Moreover, a combined analysis of our succinylproteomic data with previously published transcriptome data revealed that the mRNAs of seven and three succinylated proteins were specific to neurons and astrocytes, respectively, and exhibited high expression, when compared with those of other neural cells, suggesting that they may play important roles in regulating the biofunctions of neurons and astrocytes after ICH. In conclusion, our analyses reveal that several TLR4 signaling pathways affect the succinylation processes and pathways in mouse ICH brains, providing new insights for understanding the ICH pathophysiological processes.
Materials and Methods
Collagenase-Induced Mouse Model of ICH and TAK242 Treatment
An ICH mouse model was constructed according to our previously developed method (Pan et al., 2018). Briefly, mice were immobilized on a stereotaxic apparatus (RWD Life Science Co., Shenzhen, China) after being anesthetized with 3% isoflurane for anesthesia induction, and with 1.5% isoflurane, for maintenance. A volume of 0.5 μL of 0.075 IU type VII collagenase (Sigma Aldrich) was injected into the left striatum (0.8 mm anterior and 2 mm lateral to bregma, at a depth of 3.5 mm) using a syringe pump (Hamilton, Bonaduz, AG) at a rate of 0.0625 μL/min. The control (sham) group was injected with 0.5 μL of saline. During this surgical operation, the body temperature of the animals was maintained at 37 °C. Unsuccessful ICH models, such as those that were asymptomatic or had died, as evaluated using a behavioral test (Corner task), were excluded from this study.
According to our previous report (Wang et al. 2013; Xiong et al. 2016), TAK242 (CLI-095, MedChemExpress) was formulated using 1% dimethyl sulfoxide (σ) and double-distilled water to a final concentration of 0.4 mg/mL, and then injected intraperitoneally at a dose of 3 mg/kg once daily for three successive days, beginning 6 h after ICH. Mice were randomly assigned to three groups: a sham operation group (sham group), an ICH + vehicle group (administration of the same volume of solvent used to formulate the TAK242 solution), and an ICH + TAK242 group. Perihematomal and contralateral brain tissues were collected three days after ICH for succinylproteomic analysis.
Neurological Deficit Score (NDS) Assessment
According to our previously reported methods (Wang et al. 2013; Xiong et al. 2016), the NDS values were assessed one and three days after TAK242 administration using a 28-point neurological deficit scale, including circling behavior, climbing, front limb symmetry, and body symmetry. Scoring was performed by two trained investigators who were blinded to the animal grouping, and the mean score of the subscales was the final score of each mouse.
Brain Sample Preparation for LC–MS/MS
The brain samples were ground using liquid nitrogen, transferred to centrifuge tubes with four volumes of lysis buffer (8 M urea, 1% protease inhibitor cocktail, 3 μM TSA, and 50 mM NAM), and sonicated three times on ice. The remaining debris was removed via centrifugation at 12,000 × g and 4 °C for 10 min to collect the supernatant. The protein concentration was determined using a bicinchoninic acid assay (BCA) kit, according to the manufacturer’s instructions. The sample was slowly added to a final concentration of 20% v/v TCA to precipitate the protein, then vortexed, and incubated for 2 h at 4 °C. The precipitate was collected via centrifugation at 4500×g for 5 min at 4 °C. The precipitated protein was washed three times with pre-cooled acetone and dried for 2 h. Then, the protein sample was redissolved in 100 mM TEAB and ultrasonically dispersed. Trypsin (Promega, Madison, WS, USA) was added at a 1:50 trypsin: protein mass ratio for the first overnight digestion. The sample was reduced using 5 mM dithiothreitol for 60 min at 37 °C and alkylated with 11 mM iodoacetamide for 45 min at room temperature in the dark. Finally, the peptides were desalted using a C18 SPE column.
Succinyllysine Peptide Enrichment
The tryptic peptides were dissolved in NETN buffer (100 mM NaCl, 1 mM EDTA, 50 mM Tris–HCl, 0.5% NP-40, pH 8.0) and affinity enrichment of succinylpeptides was carried out according to a previous report (Colak et al. 2013). Briefly, peptides were incubated with pre-washed succinyllysine antibody beads (PTM-402, PTM Bio, China) at 4 °C overnight with gentle shaking. The bound peptides were eluted from the beads using 0.1% trifluoroacetic acid, and the eluted fractions were combined and vacuum-dried. For LC–MS/MS analysis, the resulting peptides were desalted using C18 ZipTips (Millipore), according to the manufacturer’s instructions.
LC–MS/MS Analysis
LC–MS/MS analysis was performed according to a previously described protocol (Jin and Wu, 2016). The tryptic peptides were dissolved in solution A (0.1% formic acid, 2% acetonitrile) and separated using a gradient from 9 to 25% of solution B (0.1% formic acid in 90% acetonitrile) within 36 min at a constant flow rate of 500 nL/min using an EASY-nLC 1200 UPLC system (Thermo Fisher Scientific). The separated peptides were analyzed using Q ExactiveTM HF-X (Thermo Fisher Scientific) and a nano-electrospray ion source. The electrospray voltage used was 2.2 kV. The full MS scan resolution was set to 120,000 for a scan range of 350–1600 m/z. HCD fragmentation was performed at a normalized collision energy of 28%. The fragments were detected using an Orbitrap at a resolution of 15,000. The fixed first mass was set to 100 m/z. The automatic gain control target was set to 1E5, with an intensity threshold of 5E4 and a maximum injection time of 50 ms.
Database Search
MaxQuant (v1.6.15.0) was used to retrieve the secondary mass spectral data. Trypsin/P was specified as the cleavage enzyme, and up to four missing cleavages were allowed. The mass tolerance values for precursor ions in the first and main searches were set to 20 ppm and 4.5 ppm, respectively, and the mass tolerance for fragment ions was set to 0.02 Da. Carbamidomethyl on Cys was set as a fixed modification, and succinylation and oxidation on Met were specified as variable modifications. The false discovery rate (FDR) was adjusted to < 1%.
The mass spectrometry proteomics data were deposited on the ProteomeXchange consortium via the PRIDE (Perez-Riverol et al. 2019) partner repository with the dataset identifier PXD025622.
Bioinformatic Analysis
A pipeline written using Perl and R was used for bioinformatic analysis. The MoMo analysis tool (Cheng et al. 2019), based on the Motif-X algorithm (Schwartz and Gygi 2005), was used to analyze the motif characteristics of the succinyllysine sites. Statistical analysis of the succinylproteome was performed using logarithmic intensities of the values that were found to be quantified in one experimental condition. All the sequences of the protein database were used as context sequences. The motif width was then set to 21; the other parameters were set to default values. To identify the modified succinylpeptides with significant changes, three-sample replicate analyses were performed using a P-value cutoff of 0.05. The differentially modified succinyllysine sites (fold change > 1.5) were obtained by comparing the relative quantitative value of each modification site between the TAK242- and vehicle-treated ICH brains. Categorical annotation was supplied in the form of Gene Ontology (GO) cellular components, biological processes, and molecular functions, and KEGG was used for pathway annotation. For each category, a two-tailed Fisher’s exact test was employed to test the enrichment of the differentially modified proteins against all identified proteins. The categories with a corrected P-value < 0.05 were considered significant.
Hierarchical Clustering
According to previously reported methods (Bai et al. 2020), hierarchical clustering of the differentially expressed (DE) succinylopeptides was performed to determine the differences among the ICH groups. Hierarchical clustering was carried out using the heatmap.2 function in the R statistical analysis package (version 3.4.0) (Ihaka and Gentleman, 1996). The clustering for heatmap.2 was performed using Ward’s algorithm and the Euclidean distance. Groups were clustered and visualized using heat maps based on the relative expression profiles.
Mfuzz Cluster Analysis
Through quantitative research and analysis, 139 differentially modified succinyllysine sites were identified. To screen out the succinyllysine sites with significant protein abundance changes in the sham, ICH + TAK242, and ICH + vehicle samples, the relative protein expression values were first converted to log2 values, and then the proteins with an SD > 0.2 were screened out. After screening, the remaining 137 succinyllysine sites were used for the expression pattern cluster analysis using the Mfuzz method (Kumar and Futschik 2007). The Mfuzz analysis parameters used were the following: the number of clusters, k, was set to four and the cluster fuzzing degree, m, was set to two. To study the biological characteristics of the proteins in the different clusters, we performed an enrichment analysis of the proteins in the different clusters of the KEGG pathway, using Fisher’s exact test. A P-value lower than 0.05 indicated a significant enrichment. When the number of proteins in the enriched pathways was greater than three, those proteins were further used to analyze the protein–protein interaction (PPI) network.
PPI Network Analysis
The succinylproteins in each cluster were superimposed onto a composite PPI database by combining STRING (v10) (Szklarczyk et al. 2015), BioPlex (Huttlin et al. 2017), and In Web_IM (Li et al. 2017). The details of the PPI network analysis were based on a previously reported method (Bai et al., 2020).
Integrative Analysis of the Succinylproteome and Transcriptome Data
Integrative analysis was performed by comparing the succinylproteome data with the previously published transcriptome data (Zhang et al. 2014). The fragments per kilobase of transcript per million mapped reads (FPKM) were used as a proxy for mRNA abundance. Based on these transcriptomic data, the four most studied neural cells (neurons, astrocytes, microglia, and endothelial cells) in stroke were examined. We first defined the relatively high expression of genes in neural cells. For example, when the ratio of the FPKM of gene A in neurons to the FPKM of gene A in the other three neural cells (astrocytes, microglia, and endothelial cells) was > 1.0, we considered gene A to be highly expressed in neurons, and when this ratio > 10, we considered gene A to be highly specific to neurons. Next, to further investigate the close relationships between neural cells (neurons, astrocytes, microglia, and endothelial cells) and the identified succinylated proteins, we performed an association matching analysis by matching these differential succinylated proteins with the genes highly expressed in neural cells.
Results
Succinylproteome Profiling Analysis of the ICH Brain Tissues
TLR4-mediated inflammation is a major contributor to secondary brain injury after ICH (Fang et al. 2013), and we previously showed that the inhibition of TLR4 signaling by its antagonist TAK242 significantly reduced brain injury by downregulating the inflammatory response (Wang et al. 2013). Additionally, some pathological processes, such as inflammation, have been shown to be closely related to protein succinylation. We aimed to investigate how neuroinflammation affects protein succinylation in the perihematomal brain tissue via TAK242 treatment of the ICH samples. After confirming the neuroprotective effects of TAK242 on ICH mice using the NDS (Supplementary Fig. 1A), brain tissues were collected from mice with ICH after receiving three consecutive days’ treatment with TAK242 or the vehicle and from the sham group, which was used as a control.
The succinylproteome profile identification was performed using label-free LC–MS/MS after the succinylated peptide affinity enrichment with anti-succinyllysine antibody-conjugated beads. The resulting MS/MS data were processed using the MaxQuant search engine, and the mouse Swiss-Prot database (17,045 entries), concatenated with a reverse decoy database, was searched using tandem mass spectra. The identification accuracy of the spectrum, peptide, and protein levels was set to an FDR < 1% (Supplementary Fig. 1B). The quality of the succinylproteomics data was confirmed using peptide length distribution (Supplementary Fig. 1C) and the first-order mass error of spectra (Supplementary Fig. 1D). The principal component analysis (PCA) revealed clear separations among the three groups (Supplementary Fig. 1E). Motif analysis of the succinyl-peptide sequences showed that valine, alanine, and isoleucine occurred frequently upstream of succinyllysine (Supplementary Fig. 1F, G).
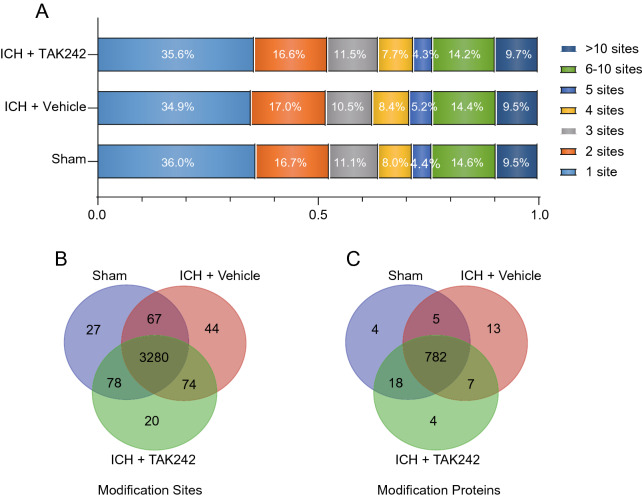
In this study, we identified approximately 6,700 succinyllysine sites, among which 3425 were quantified (Supplementary Fig. 1B). Approximately 36%, 16.7%, 11.1%, 36.2%, and 10% of the succinylproteins with one, two, three, more than three, and more than 10 succinyllysine sites, respectively, were identified in the control (sham) brains, being comparable to the percentage of succinylproteins in the TAK242- or vehicle-treated ICH brains (Fig. 1A). The Venn diagram showed that more than 90% of the succinyllysine sites (3280/3590; Fig. 1B and Supplementary Table 1) and succinylated proteins (782/833; Fig. 1C and Supplementary Table 2) were identical among the sham, TAK242-, and vehicle-treated ICH brains. Conversely, 20 succinyllysine sites on four succinylated proteins were found to be succinylated exclusively in the TAK242-treated ICH brains, but not in the control and vehicle-treated brains (Fig. 1B, C), and 44 succinyllysine sites on 13 succinylated proteins were exclusively found in the vehicle-treated brains, but not in the control and TAK242-treated ICH brains (Fig. 1B, C).
Fig. 1.
Quantitative succinylproteomics analysis of the ICH brains. A The bar chart shows the proportions of singly and multiply succinylated proteins with the indicated number of succinyllysine sites per protein in the sham, vehicle-treated, and TAK242-treated ICH brains. B and C Venn diagram comparison of the succinyllysine sites (B) and succinylated proteins (C) in the sham, TAK242-treated, and vehicle-treated ICH brains
TAK242 Treatment Changed the ICH-Associated Protein Succinylation
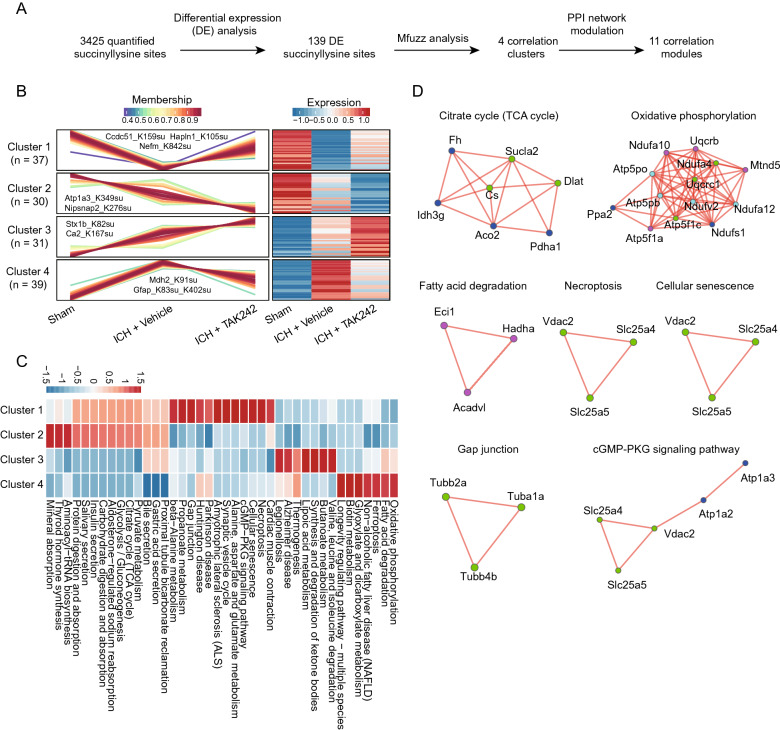
To explore the succinylproteome dynamics after TAK242 treatment, we used a stepwise pipeline (Fig. 2A) to define 139 differentially expressed (DE) succinyllysine sites (121 proteins, Supplementary Table 3) in 40 pathways. We used an Mfuzz cluster analysis (Kumar and Futschik 2007) method to cluster the DE succinyllysine sites, and interpreted the clusters using a PPI network. Most of the sites were identified in four clusters from this analysis (Fig. 2B), except for a few (n = 2) (Supplementary Table 4). Cluster 1 (n = 37) displayed an increase in the hyposuccinylation of proteins, including Ccdc51_K159su, Hapln1_K105su, and Nefm_K842su, caused by ICH after TAK242 treatment (Fig. 2B). Functional enrichment analysis assigned these succinylation events to the following 24 pathways, including β-alanine metabolism, propanoate metabolism, gap junction, synaptic vesicle cycle, necroptosis, and cGMP-PKG signaling pathway, among others (Fig. 2C). Cluster 2 (n = 30) showed a marked decrease in the ICH-associated hyposuccinylation of proteins, including Atp1a3_K349su and Nipsnap2_K276su, after TAK242 treatment (Fig. 2B). Cluster 2 was enriched in 16 pathways; in particular, in the following three pathways: mineral absorption, thyroid hormone synthesis, and aminoacyl-tRNA biosynthesis (Fig. 2C). In contrast, Cluster 3 (n = 31) showed a marked increase in the ICH-associated hypersuccinylation of proteins after TAK242 treatment (Fig. 2B), from which 12 pathways, such as lipoic acid metabolism, synthesis and degradation of ketone bodies, butanoate metabolism, and valine, leucine, and isoleucine degradation were identified (Fig. 2C). Finally, we found that Cluster 4 showed an increase in the ICH-associated hyposuccinylation of proteins, followed by a notable increase after TAK242 treatment (Fig. 2B). Most of these proteins were enriched in different metabolisms, such as oxidative phosphorylation (OXPHOS), fatty acid degradation, glyoxylate and dicarboxylate metabolism, and ferroptosis, among others (Fig. 2C). Furthermore, we integrated the DE succinylproteins in each cluster pattern with the PPI network to define 11 functional modules, providing evidence of the PPI to support these enrichment pathways (Fig. 2D). These results suggest that the metabolic changes mediated by protein succinylation may play an important regulatory role in brain injury reduction when the ICH-induced neuroinflammation was inhibited.
Fig. 2.
Whole-succinylproteome profiling of ICH-related brain tissues reveals the TAK242 treatment-dependent succinyllysine sites and/or pathways. A Bioinformatics pipeline for identifying the DE succinyllysine sites, followed by clustering and module analyses. B Four major clusters (clusters 1–4) of DE succinyllysine sites were defined using Mfuzz cluster analysis. Each line represents one succinyllysine site. The intensity of each protein is log2 Z score transformed (mean-centered and scaled using standard deviation). The line color indicates the degree of pattern correlation between each succinyllysine site and the eigenvalue of the cluster. C Enriched pathways in the cluster succinyllysine sites, as obtained using Fisher’s exact test. When the P-value was lower than 0.05, it was considered significant. D PPI modules detected in the clusters
Analysis of the Succinylproteomic Data of ICH-Associated Brain Tissues Treated with TAK242
Next, we analyzed the succinylproteomic data between the TAK242-treated and the vehicle-treated ICH brains to identify how the TLR4-mediated inflammation affects protein succinylation changes in the perihematoma brain tissue following TAK242 treatment of ICH. First, we found that 29 succinyllysine sites were increased on 28 succinylated proteins, and that 24 succinyllysine sites were downregulated on 23 succinylated proteins, by comparing the TAK242-treated ICH brains with the vehicle-treated groups (Fig. 3A). GO analysis using an A.GO.TOOL (Scholz et al. 2015) showed that the brain succinylproteome was represented by the following GO localization categories: myelin sheath, respiratory chain complex II, succinate dehydrogenase complex, fumarate reductase complex, Golgi membrane, T-tubule, plasma membrane, synapse, neuronal cell body membrane, and postsynaptic density, among others (Fig. 3B). We further analyzed the subcellular localization of these significantly changed succinylproteins and found that approximately 48% of the hyposuccinylated proteins were located in the mitochondria; 30.4% of them were located in the cytoplasm (Fig. 3C). In contrast, the subcellular distribution of hypersuccinylated proteins in the TAK242-treated ICH brains was more extensive than that of hyposuccinylated proteins (Fig. 3D). For example, compared with the hyposuccinylated proteins, the peroxisome, cytoskeleton, cytoplasm, and nucleus were newly identified subcellular compartments where hypersuccinylated proteins were more widely distributed than hyposuccinylated proteins (Fig. 3D). In addition, the percentage of hypersuccinylated proteins located in the mitochondria decreased to approximately 32% in the TAK242-treated ICH brains, with concomitant increases in the percentage of proteins located in the plasma membrane and nucleus (17.9% and 7.1%, respectively), compared with that of hyposuccinylated proteins (Fig. 3D).
Fig. 3.
Analysis of the succinylproteome data in the TAK242-treated ICH brains. A The heat map shows the differentially succinyllysine sites in the TAK242- and vehicle-treated brains. B GO localization categories enriched in the succinylproteome compared with the proteome, as obtained using a Benjamini–Hochberg false discovery rate (FDR)-corrected P < 0.0001. C and D The subcellular localization of hyposuccinylated (C) and hypersuccinylated (D) proteins in the TAK242-treated ICH brains, when compared with the vehicle-treated brains
Analysis and Annotation of the Differentially Succinylated Proteins in ICH Brains After TAK242 Treatment
We found that ICH-associated hyposuccinylation was comparable with hypersuccinylation in succinylproteins carrying one or two succinyllysine sites per protein after TAK242 treatment, when compared with vehicle treatment (Fig. 4A). GO cellular component enrichment analysis showed that both hyposuccinylation and hypersuccinylation datasets were significantly enriched in the plasma membrane, integral component of membrane, and cell periphery (Fig. 4B and Supplementary Table 5). Hypersuccinylation alone was significantly linked to structures (e.g., synaptic membrane, presynaptic membrane, postsynaptic density, postsynapse), metabolism, and its related enzymes (e.g., succinate dehydrogenase complex, respiratory chain complex II, fumarate reductase complex, among others), somatodendritic compartment, myelin sheath, cytoplasmic ribonucleoprotein granule, contractile actin filament bundle, actomyosin, among others (Fig. 4B). Interestingly, among these, the statistical enrichment of hypersuccinylation was found to be the most significant in the myelin sheath (Fig. 4B), suggesting that it could play an important role in regulating myelin regeneration after treating ICH with TAK242. In contrast, hyposuccinylation alone was enriched in membranes, including the vacuolar membranes, trans-Golgi network membranes, sides of membranes, organelle membranes, membranes, coated vesicles, and clathrin-coated vesicles (Fig. 4B). Consistent with the results of the GO localization enrichment analysis, we found that most of the hyposuccinylated proteins were enriched in pathways associated with metabolism (e.g., sterol metabolism, monocarboxylic acid metabolism, lipid oxidation, cholesterol metabolism, cellular lipid catabolism) and transportation (e.g., regulation of anion transport, positive regulation of ion transport) (Fig. 4C and Supplementary Table 6). For the most part, only proteins with hypersuccinylation were linked with synaptic (trans-synaptic signaling, synaptic vesicle transport, synaptic vesicle localization, synapse organization, among others) and vesicle functions (e.g., vesicle organization, vesicle docking, regulation of secretion by cells) (Fig. 4C). GO molecular function enrichment analysis showed that both hyposuccinylation and hypersuccinylation datasets were significantly enriched in protein kinase binding and kinase binding (Fig. 4D and Supplementary Table 7). However, only hypersuccinylation, but not hyposuccinylation, was significantly linked with succinate dehydrogenase activity, steroid hormone binding, nitric-oxide synthase binding, and G-protein coupled receptor binding, among others (Fig. 4D); hyposuccinylation, but not hypersuccinylation, was enriched in symporter activity, and solute:cation symporter activity, among others (Fig. 4D). Additionally, the KEGG analysis of these significantly altered succinylated proteins showed that hypersuccinylation was enriched in carbon metabolism, synaptic vesicle cycle, citrate cycle (TCA cycle), carbohydrate digestion and absorption, arginine biosynthesis, and SNARE interactions in vesicular transport (Fig. 5A), whereas hyposuccinylation was significantly related to fatty acid elongation, fatty acid degradation, fatty acid metabolism, and lysosomes (Fig. 5B).
Fig. 4.
GO analysis of the differentially succinylated proteins in the TAK242-treated ICH brains. A Distribution of the TAK242-treated ICH-associated hyposuccinylated or hypersuccinylated proteins carrying the indicated number of in vivo succinyllysine sites per protein. B–D GO cellular component (35 upregulated terms, 16 downregulated terms; B), biological process (24 upregulated terms, 20 downregulated terms; C), and molecular function (20 upregulated terms, 7 downregulated terms; D) categories enriched in the ICH-associated hyposuccinylated or hypersuccinylated proteins after TAK242 treatment, when compared to the vehicle treatment. The significant enrichment shown was determined based on a Benjamini–Hochberg FDR-corrected P < 0.05 (outside the dashed line)
Fig. 5.
KEGG pathway analysis of the differentially succinylated proteins in the TAK242-treated ICH brains. A and B KEGG pathway analysis of the ICH-associated hypersuccinylated (A) or hyposuccinylated (B) proteins after TAK242 treatment, when compared with the vehicle treatment
Combined Transcriptome and Succinylproteome Analysis of the ICH Brains Treated with TAK242
We performed a combined analysis of previously reported RNA-seq data of neural cells (Zhang et al. 2014) and our succinylproteome data to reveal cell-type information on the ICH-associated significant alterations in protein succinylation after TAK242 treatment. Given that neurons, astrocytes, microglia, and endothelial cells are the four most studied neural cell types in stroke research, all four types were chosen for this joint analysis.
After matching the highly expressed genes of neural cells (see Materials and Methods section for details, Supplementary Fig. 2) with the obviously changed succinylproteomic data, we found that most of the mRNAs matched by succinylproteins were relatively highly expressed in neurons, astrocytes, and endothelial cells (Fig. 6A): 37% of total hypersuccinylation was identified in neurons, 22% in astrocytes, 16% in endothelial cells, and 7% in microglia (Fig. 6A). Meanwhile, the percentage of hyposuccinylation was decreased to 32% in neurons, with a concomitant increase in the percentage of hyposuccinylation in endothelial cells (Fig. 6A). The ratios of the specific mRNA expression levels in neural cells vary greatly; generally, the higher the ratio, the closer the mRNA and its protein may be linked to the functions of neural cells. Therefore, we further analyzed the specificity ratio of the mRNAs matched by succinylated proteins to further evaluate whether these succinylated proteins could potentially influence the function of neural cells. The pie charts show that seven mRNAs were matched by succinylated proteins (Ina, Atp1a3, Cntn1, Vsnl1, Dnm1, Cend1, and Stxbp1) with a specificity ratio > 10, and five mRNAs (Mapt, Uchl1, Sncb, Slc25a22, and Stx1b) had a specificity ratio > 5 in neurons (Fig. 6B and Supplementary Fig. 3A-D). For astrocytes, there were three mRNAs (Atp1a2, Slc1a3, and Slc6a11) and one mRNA (Acsl6) with specificity ratios > 10 and > 5, respectively (Fig. 6C and Supplementary Fig. 3E, F). In contrast, the ratios of mRNAs matched by succinylated proteins in microglia (Fig. 6D) and endothelial cells (Fig. 6E) were all < 3. Moreover, we found that most of the specificity ratios of matched mRNA and succinylated proteins in the four neural cell types varied from 1 to 3 (Fig. 6B-E), especially for endothelial cells, because 86% of the specificity ratios were < 2 (Fig. 6E).
Fig. 6.
Combined analysis of the succinylproteomic and transcriptomics data. A The bar chart shows the major cellular distributions of all differentially ICH-associated succinyllysine sites, and the significantly hypersuccinylated and hyposuccinylated sites after TAK242 treatment, when compared with the vehicle treatment, respectively, based on the cellular distribution of their matched mRNAs. Matching of the significantly changed proteins (P < 0.05 using Student’s t-test) with the relatively highly expressed genes in neural cells (ratio > 1.0) from the published transcriptome data (Zhang et al. 2014). B–E The pie charts show the specificity ratio distribution of the mRNAs matched with succinylated proteins in neurons (B), astrocytes (C), microglia (D), and endothelial cells (E)
Discussion
TLR4 signaling contributes to brain injury after ICH and promotes protein succinylation, which has been shown to regulate metabolic processes, immunity, and inflammation. In this study, for the first time, we have investigated the contribution of TLR4 signaling to protein succinylation in ICH brains using a high-resolution LC–MS/MS-based quantitative succinylproteomic approach. The results showed that the TAK242-treated ICH brains underwent significant protein succinylation changes. These significantly changed succinylated proteins were mainly distributed in the mitochondria and cytoplasm, and may participate in metabolic processes and neuronal functions. KEGG pathway analysis showed that the TAK242 treatment induced a downregulation of protein succinylation, which was mainly enriched in fatty acid metabolism; thus far, this has been poorly recognized. Further integration of our succinylproteomics data with previously published transcriptome data showed that some highly expressed mRNAs (specificity ratio > 10.0) matched by succinylated proteins were found in neurons and astrocytes after the ICH brains were treated with TAK242, suggesting that the succinylation of these proteins may be involved in the TLR4 signaling-related regulation of the biological functions of neurons and astrocytes. The mouse brain succinylproteome dataset generated in this study constitutes a useful resource for future investigations on the roles of TLR4-mediated inflammation in protein succinylation in the brain after ICH.
PTMs are recognized as an extension of metabolic regulation in cell life activities. For example, the TCA metabolite succinate promotes inflammation by increasing protein succinylation (Tannahill et al. 2013). However, to date, little is known about the protein succinylation regulated by inflammation in ICH brains, although the role of inflammation in contributing to brain injury after ICH is now largely clear. Therefore, in this study, we aimed to investigate the effect of TLR4 signaling activation on protein succinylation in ICH-induced brain injury. Using quantitative succinylproteomic analysis, we first revealed approximately 6,700 succinyllysine events and quantified approximately 3,500 sites. Then, 139 DE succinyllysine sites in 40 pathways were defined and grouped into four clusters. Among them, Cluster 1 showed that TAK242 treatment increased the level of hyposuccinylation of proteins caused by ICH, and these succinylated proteins were enriched in metabolism, gap junction, synaptic vesicle cycle, necroptosis, and cGMP-PKG signaling pathways. Protein succinylation in Cluster 4 was upregulated by ICH but decreased after TAK242 treatment. Interestingly, most of these succinylated proteins are involved in metabolic functions, such as OXPHOS, fatty acid degradation, glyoxylate and dicarboxylate metabolism, and ferroptosis. These results suggest that TLR4-mediated inflammation may be involved in the regulation of the metabolic processes after ICH via protein succinylation, which is an important contribution to the study of inflammation and metabolism (Corcoran and O'Neill 2016; Yin et al. 2016; Killen et al. 2019). Although some studies have already shown that LPS stimulation can promote protein succinylation (Mills and O'Neill 2014; Tannahill et al. 2013; Wang et al. 2017), our study provides a useful resource for the details of inflammation-mediated protein succinylation after ICH.
Furthermore, we compared TAK242- and vehicle-treated ICH mouse brains to obtain information about the roles of TLR4 signaling in protein succinylation after ICH. Our data showed that, compared with the vehicle group, 29 succinyllysine sites of 28 succinylated proteins were upregulated and 24 succinyllysine sites of 23 succinylated proteins were downregulated in the TAK242 group, suggesting that TAK242 treatment could significantly change protein succinylation levels. GO and KEGG analyses of these DE succinylated proteins indicated that the TLR4 signaling-associated hypersuccinylation and hyposuccinylation almost independently affect the biological processes after ICH. In particular, KEGG pathway analysis showed that TAK242 reduced the number of succinylated proteins, which were mainly involved in the elongation, degradation, and fatty acid metabolism, indicating that the latter may play an important role in brain injury and may be closely related to inflammation after ICH.
Additionally, as different neural cells respond differently to brain injury after ICH, we performed a joint analysis of a previously published transcriptome with our succinylproteome data to determine which type of neural cell is the major response cell to the protein succinylation regulated by TLR4 signaling. The results showed that the mRNAs matched by succinylated proteins preferentially existed in neurons, endothelial cells, and astrocytes after ICH treatment with TAK242. Furthermore, we found that seven mRNAs matched by succinylated proteins (Ina, Atp1a3, Cntn1, Vsnl1, Dnm1, Cend1, and Stxbp1) were significantly highly expressed in neurons, and that three mRNAs matched by succinylated proteins (Atp1a2, Slc1a3, and Slc6a11) showed markedly higher expression levels in astrocytes than in the other three neural cell types. Among these, we found that contactin-1 (Cntn1) is a neuronal membrane protein that functions as a cell adhesion/recognition molecule (Gennarini et al. 2017; Chatterjee et al. 2019; Falk et al. 2002), and has been shown to regulate myelin formation by orchestrating the organization of the myelin membrane domains around the nodes of Ranvier and conferring signaling between axons and oligodendrocytes (Colakoglu et al. 2014; Boyle et al. 2001; Berglund et al. 1999; Ranscht 1988). In addition, Cntn1 has also been demonstrated to be involved in oligodendrocyte generation by promoting Notch1 activation (Hu et al. 2003). These results suggest that Cntn1 and its succinylation may be involved in the regulation of myelin repair, which is important for recovery from functional disability after ICH, when the inflammation mediated by TLR4 signals is inhibited. Moreover, the other succinylated proteins may also regulate neuroglial function, in response to TAK242 treatment in the ICH brains. For example, α-internexin was found to be localized in reactive axonal structures in physically damaged neurons in experimental trauma models (Dickson et al. 2005) and might play key roles in neurite outgrowth and in the establishment of the neuronal cytoarchitecture (Chien et al. 1996). However, the roles of these succinylated proteins in ICH-induced brain injury remain largely unknown, prompting us to further investigate their roles after ICH in follow-up studies.
In summary, our study is the first to investigate the protein succinylation profiles regulated by TLR4 signaling after ICH, and the changes in TLR4 signaling-associated protein succinylation uncovered in this study indicate that the altered protein succinylation may be a downstream mechanism of inflammation that contributes to brain injury. The identified differential protein succinylation events in the TAK242-treated ICH brains provide new molecular and system-level insights into ICH pathogenesis and into the damage mechanisms of inflammation-related brain injury after ICH.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Fig. 1 Analysis of the succinylproteomic data of ICH brains. (A) NDS in the Sham, ICH + vehicle, and ICH + TAK242 groups at 1 and 3 days after ICH. (B) Overview of protein and succinylation identification. (C and D) Data quality control of the peptide length distribution (C) and peptide mass tolerance distribution (D). (E) PCA analysis shows the dispersion degree of the succinylproteome between the sham, TAK242-treated, and vehicle-treated ICH brains. (F) Amino acid sequence properties of the succinyllysine sites. The heat map shows the significant position-specific under-representation or over-representation of the amino acids flanking the succinyllysine sites. (G) Succinylation motifs and the conservation of the succinyllysine sites. The height of each letter corresponds to the frequency of that amino acid residue at that position. The central K refers to the succinylated Lys. (PDF 3065 kb)
Supplementary file2 Fig. 2 Flow chart of the definition of highly expressed genes in nerve cells. When all the FPKM of gene A in neurons/the FPKM of gene A in the other three neural cell types (astrocyte, microglia, and endothelial cell) ratios were > 1.0, we considered gene A to be highly expressed in neurons; and when the ratio > 10.0, we considered gene A to be highly specific to neurons. (PDF 774 kb)
Supplementary file3 Fig. 3 MS spectrum of the representative succinylated sites. MS/MS spectra of Stxbp1_K120su (A), Cend1_K16su (B), Cntn1_K757su (C), Ina_K438su (D), Atp1a2_K658su (E), and Slc6a11_K610su (F) (PDF 997 kb)
Acknowledgements
This work was supported by the Science Foundation for Distinguished Young Scholars of Science and Technology Department of Sichuan Province (2020JDJQ0046) and the National Natural Science Foundation of China (81701292). We thank the bioinformatic analysis team from Jingjie PTM BioLab Co. Ltd (Hangzhou, China) for the analysis of and advice on the succinylproteome data in this study. We thank Prof. Jia Qian Wu (University of Texas Medical School at Houston) for providing us with the transcriptome data.
Author Contributions
SGY and XYX designed the research studies and wrote the manuscript. YJL, YRY, and CYT conducted the experiments and analyzed the data. SHY, XXZ, JY, YHD, and ZQZ analyzed the data.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The animal protocol was approved by the Animal Care and Ethics Committee of the Chengdu University of Traditional Chinese Medicine, whose standards meet the animal care guidelines of the NIH, USA.
Data Availability
Reviewer account details to access the PXD025622 dataset.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan-Jing Liang, Yuan-Rui Yang, and Chuan-Yuan Tao contributed equally to this work.
Contributor Information
Shu-Guang Yu, Email: ysg28588@126.com, Email: ysg@cdutcm.edu.cn.
Xiao-Yi Xiong, Email: xiongxy_89@163.com, Email: xiongxy1989@cdutcm.edu.cn.
References
- Bai B, Wang X, Li Y, Chen PC, Yu K, Dey KK, Yarbro JM, Han X, Lutz BM, Rao S, Jiao Y, Sifford JM, Han J, Wang M, Tan H, Shaw TI, Cho JH, Zhou S, Wang H, Niu M, Mancieri A, Messler KA, Sun X, Wu Z, Pagala V, High AA, Bi W, Zhang H, Chi H, Haroutunian V, Zhang B, Beach TG, Yu G, Peng J (2020) Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105(6):975e977-991e997. 10.1016/j.neuron.2019.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund EO, Murai KK, Fredette B, Sekerkova G, Marturano B, Weber L, Mugnaini E, Ranscht B (1999) Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron 24(3):739–750. 10.1016/s0896-6273(00)81126-5 [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B (2001) Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30(2):385–397. 10.1016/s0896-6273(01)00296-3 [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Schild D, Teunissen CE (2019) Contactins in the central nervous system: role in health and disease. Neural Regen Res 14(2):206–216. 10.4103/1673-5374.244776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Grant CE, Noble WS, Bailey TL (2019) MoMo: discovery of statistically significant post-translational modification motifs. Bioinformatics 35(16):2774–2782. 10.1093/bioinformatics/bty1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CL, Mason CA, Liem RK (1996) alpha-Internexin is the only neuronal intermediate filament expressed in developing cerebellar granule neurons. J Neurobiol 29(3):304–318. 10.1002/(SICI)1097-4695(199603)29:3%3c304::AID-NEU3%3e3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- Colak G, Xie Z, Zhu AY, Dai L, Lu Z, Zhang Y, Wan X, Chen Y, Cha YH, Lin H, Zhao Y, Tan M (2013) Identification of lysine succinylation substrates and the succinylation regulatory enzyme CobB in Escherichia coli. Mol Cell Proteomics 12(12):3509–3520. 10.1074/mcp.M113.031567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colakoglu G, Bergstrom-Tyrberg U, Berglund EO, Ranscht B (2014) Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc Natl Acad Sci USA 111(3):E394-403. 10.1073/pnas.1313769110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran SE, O’Neill LA (2016) HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest 126(10):3699–3707. 10.1172/JCI84431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C, Demchuk A, Ziai W, Anderson CS (2018) Intracerebral haemorrhage: current approaches to acute management. Lancet 392(10154):1257–1268. 10.1016/S0140-6736(18)31878-6 [DOI] [PubMed] [Google Scholar]
- Dickson TC, Chuckowree JA, Chuah MI, West AK, Vickers JC (2005) alpha-Internexin immunoreactivity reflects variable neuronal vulnerability in Alzheimer’s disease and supports the role of the beta-amyloid plaques in inducing neuronal injury. Neurobiol Dis 18(2):286–295. 10.1016/j.nbd.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, Artyomov MN, Jones RG, Pearce EL, Pearce EJ (2014) TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol 15(4):323–332. 10.1038/ni.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk J, Bonnon C, Girault JA, Faivre-Sarrailh C (2002) F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol Cell 94(6):327–334. 10.1016/s0248-4900(02)00006-0 [DOI] [PubMed] [Google Scholar]
- Fang H, Wang PF, Zhou Y, Wang YC, Yang QW (2013) Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflammation 10:27. 10.1186/1742-2094-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei X, He Y, Chen J, Man W, Chen C, Sun K, Ding B, Wang C, Xu R (2019) The role of Toll-like receptor 4 in apoptosis of brain tissue after induction of intracerebral hemorrhage. J Neuroinflammation 16(1):234. 10.1186/s12974-019-1634-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G, Bizzoca A, Picocci S, Puzzo D, Corsi P, Furley AJW (2017) The role of Gpi-anchored axonal glycoproteins in neural development and neurological disorders. Mol Cell Neurosci 81:49–63. 10.1016/j.mcn.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Hsu JM, Li CW, Lai YJ, Hung MC (2018) Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res 78(22):6349–6353. 10.1158/0008-5472.CAN-18-1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, Takeda Y, Chia W, Sankar N, Ng YK, Ling EA, Maciag T, Small D, Trifonova R, Kopan R, Okano H, Nakafuku M, Chiba S, Hirai H, Aster JC, Schachner M, Pallen CJ, Watanabe K, Xiao ZC (2003) F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell 115(2):163–175. 10.1016/s0092-8674(03)00810-9 [DOI] [PubMed] [Google Scholar]
- Hua F, Tang H, Wang J, Prunty MC, Hua X, Sayeed I, Stein DG (2015) TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab 35(4):536–542. 10.1038/jcbfm.2014.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, Colby G, Gebreab F, Gygi MP, Parzen H, Szpyt J, Tam S, Zarraga G, Pontano-Vaites L, Swarup S, White AE, Schweppe DK, Rad R, Erickson BK, Obar RA, Guruharsha KG, Li K, Artavanis-Tsakonas S, Gygi SP, Harper JW (2017) Architecture of the human interactome defines protein communities and disease networks. Nature 545(7655):505–509. 10.1038/nature22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5(3):299–314 [Google Scholar]
- Jin W, Wu F (2016) Proteome-wide identification of lysine succinylation in the proteins of tomato (Solanum lycopersicum). PLoS ONE 11(2):e0147586. 10.1371/journal.pone.0147586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve TM, Cheema AK (2011) Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease. J Amino Acids 2011:207691. 10.4061/2011/207691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen MJ, Giorgi-Coll S, Helmy A, Hutchinson PJ, Carpenter KL (2019) Metabolism and inflammation: implications for traumatic brain injury therapeutics. Exp Rev Neurother 19(3):227–242. 10.1080/14737175.2019.1582332 [DOI] [PubMed] [Google Scholar]
- Klimova N, Long A, Kristian T (2018) Significance of mitochondrial protein post-translational modifications in pathophysiology of brain injury. Transl Stroke Res 9(3):223–237. 10.1007/s12975-017-0569-8 [DOI] [PubMed] [Google Scholar]
- Kumar L, Futschik M (2007) Mfuzz: a software package for soft clustering of microarray data. Bioinformation 2(1):5–7. 10.6026/97320630002005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wernersson R, Hansen RB, Horn H, Mercer J, Slodkowicz G, Workman CT, Rigina O, Rapacki K, Staerfeldt HH, Brunak S, Jensen TS, Lage K (2017) A scored human protein-protein interaction network to catalyze genomic interpretation. Nat Methods 14(1):61–64. 10.1038/nmeth.4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Qian C, Cao X (2016) Post-translational modification control of innate immunity. Immunity 45(1):15–30. 10.1016/j.immuni.2016.06.020 [DOI] [PubMed] [Google Scholar]
- Marcelli S, Corbo M, Iannuzzi F, Negri L, Blandini F, Nistico R, Feligioni M (2018) The involvement of post-translational modifications in Alzheimer’s disease. Curr Alzheimer Res 15(4):313–335. 10.2174/1567205014666170505095109 [DOI] [PubMed] [Google Scholar]
- Mills E, O’Neill LA (2014) Succinate: a metabolic signal in inflammation. Trends Cell Biol 24(5):313–320. 10.1016/j.tcb.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Pan C, Liu N, Zhang P, Wu Q, Deng H, Xu F, Lian L, Liang Q, Hu Y, Zhu S, Tang Z (2018) EGb761 ameliorates neuronal apoptosis and promotes angiogenesis in experimental intracerebral hemorrhage via RSK1/GSK3beta pathway. Mol Neurobiol 55(2):1556–1567. 10.1007/s12035-016-0363-8 [DOI] [PubMed] [Google Scholar]
- Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, Lombard DB, Zhao Y (2013) SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 50(6):919–930. 10.1016/j.molcel.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M, Perez E, Uszkoreit J, Pfeuffer J, Sachsenberg T, Yilmaz S, Tiwary S, Cox J, Audain E, Walzer M, Jarnuczak AF, Ternent T, Brazma A, Vizcaino JA (2019) The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47(D1):D442–D450. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B (1988) Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol 107(4):1561–1573. 10.1083/jcb.107.4.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz C, Lyon D, Refsgaard JC, Jensen LJ, Choudhary C, Weinert BT (2015) Avoiding abundance bias in the functional annotation of post-translationally modified proteins. Nat Methods 12(11):1003–1004. 10.1038/nmeth.3621 [DOI] [PubMed] [Google Scholar]
- Schwartz D, Gygi SP (2005) An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat Biotechnol 23(11):1391–1398. 10.1038/nbt1146 [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43(Database issue):D447–D452. 10.1093/nar/gku1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA (2013) Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496(7444):238–242. 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr (2005) Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl 44(45):7342–7372. 10.1002/anie.200501023 [DOI] [PubMed] [Google Scholar]
- Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, Xu Y, Zhou L, Chu Y, Zhang C, Qin X, Yang P, Yu H (2017) SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1beta production and to prevent DSS-induced colitis in mice. Cell Rep 19(11):2331–2344. 10.1016/j.celrep.2017.05.065 [DOI] [PubMed] [Google Scholar]
- Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW (2013) Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 44(9):2545–2552. 10.1161/STROKEAHA.113.001038 [DOI] [PubMed] [Google Scholar]
- Witze ES, Old WM, Resing KA, Ahn NG (2007) Mapping protein post-translational modifications with mass spectrometry. Nat Methods 4(10):798–806. 10.1038/nmeth1100 [DOI] [PubMed] [Google Scholar]
- Xiong XY, Liu L, Wang FX, Yang YR, Hao JW, Wang PF, Zhong Q, Zhou K, Xiong A, Zhu WY, Zhao T, Meng ZY, Wang YC, Gong QW, Liao MF, Wang J, Yang QW (2016) Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation 134(14):1025–1038. 10.1161/CIRCULATIONAHA.116.021881 [DOI] [PubMed] [Google Scholar]
- Yin F, Sancheti H, Patil I, Cadenas E (2016) Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Rad Biol Med 100:108–122. 10.1016/j.freeradbiomed.2016.04.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–11947. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y (2011) Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol 7(1):58–63. 10.1038/nchembio.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 115:25–44. 10.1016/j.pneurobio.2013.11.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Fig. 1 Analysis of the succinylproteomic data of ICH brains. (A) NDS in the Sham, ICH + vehicle, and ICH + TAK242 groups at 1 and 3 days after ICH. (B) Overview of protein and succinylation identification. (C and D) Data quality control of the peptide length distribution (C) and peptide mass tolerance distribution (D). (E) PCA analysis shows the dispersion degree of the succinylproteome between the sham, TAK242-treated, and vehicle-treated ICH brains. (F) Amino acid sequence properties of the succinyllysine sites. The heat map shows the significant position-specific under-representation or over-representation of the amino acids flanking the succinyllysine sites. (G) Succinylation motifs and the conservation of the succinyllysine sites. The height of each letter corresponds to the frequency of that amino acid residue at that position. The central K refers to the succinylated Lys. (PDF 3065 kb)
Supplementary file2 Fig. 2 Flow chart of the definition of highly expressed genes in nerve cells. When all the FPKM of gene A in neurons/the FPKM of gene A in the other three neural cell types (astrocyte, microglia, and endothelial cell) ratios were > 1.0, we considered gene A to be highly expressed in neurons; and when the ratio > 10.0, we considered gene A to be highly specific to neurons. (PDF 774 kb)
Supplementary file3 Fig. 3 MS spectrum of the representative succinylated sites. MS/MS spectra of Stxbp1_K120su (A), Cend1_K16su (B), Cntn1_K757su (C), Ina_K438su (D), Atp1a2_K658su (E), and Slc6a11_K610su (F) (PDF 997 kb)
Data Availability Statement
Reviewer account details to access the PXD025622 dataset.