Abstract
Epigenetics play an essential role in the occurrence and improvement of many diseases. Evidence shows that epigenetic modifications are crucial to the regulation of gene expression. DNA methylation is closely linked to embryonic development in mammalian. In recent years, epigenetic drugs have shown unexpected therapeutic effects on neurological diseases, leading to the study of the epigenetic mechanism in neurodegenerative diseases. Unlike genetics, epigenetics modify the genome without changing the DNA sequence. Research shows that epigenetics is involved in all aspects of neurodegenerative diseases. The study of epigenetic will provide valuable insights into the molecular mechanism of neurodegenerative diseases, which may lead to new treatments and diagnoses. This article reviews the role of epigenetic modifications neurodegenerative diseases with dyskinesia, and discusses the therapeutic potential of epigenetic drugs in neurodegenerative diseases.
Keywords: Nervous system, Epigenetic, Neurodegeneration, Motor dysfunction, Parkinson’s disease
Introduction
In general, the patterns and phenotypes of epigenetic modifications still exist, although there is no change in the primary DNA sequence. Epigenetic modifications have, therefore, been described as a mechanism to control gene expression. Recently, researchers have been drawn to the potential mechanism of epigenetic modifications and its crucial role in neurodegenerative diseases. DNA methylation and histone modifications have been reported as drivers of epigenetic mechanisms. Epigenetic modification can participate in many molecular biological processes, including gene expression, protein–protein interactions, cell differentiation, and embryonic development (Berson et al. 2018; Hwang et al. 2017).
In mammals, epigenetic modifications are required for various gene expressions during development (Fraga et al. 2005; Kaminsky et al. 2009; Rideout et al. 2001). Researchers have revealed that many diseases are caused by the wrong pattern of epigenetic modifications at the wrong time or place (Esteller 2002). In adulthood, epigenetic modifications can still regulate brain function. The role of epigenetic modifications is mainly reflected in genetic inheritance and information transmission of neurons. Failure of these processes may disrupt cognitive and motor functions and lead to neurodegenerative diseases (Hwang et al. 2017). Increasing research has shown that epigenetic modifications are proven associated with the plasticity, homeostasis, the stress response of central nervous system, and several motor disorders. This paper introduces the role of epigenetic modifications in a series of neurodegenerative disorders, such as amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD) and so on.
Epigenetic Modifications
The Main Regulatory Mechanism of Epigenetics
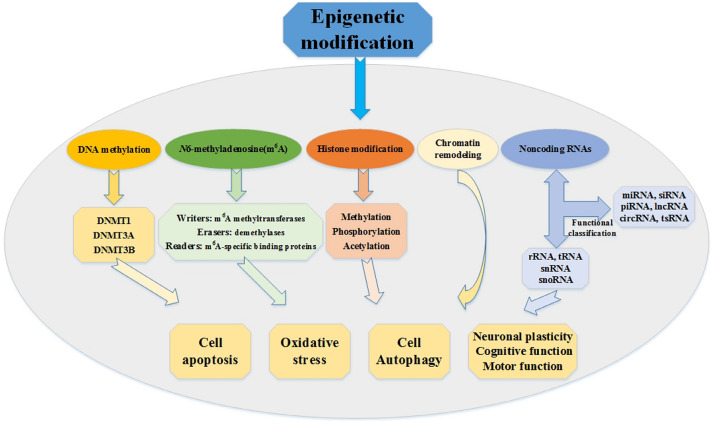
Epigenetic modifications can regulate complicated biological functions without altering the nuclear sequence of a gene. This is a mechanism of gene transcriptional regulation, and studies have shown that this process is usually reversible. Epigenetic modifications mainly include DNA methylation (Iyer et al. 2011), histone modifications (Hassig and Schreiber 1997), chromatin remodeling, N6-methyladenosine (m6A) modification, and regulation of related genes by non-coding RNA (Suh and Blelloch 2011). After birth, the central nervous system (CNS) is affected by the external environment and drugs, resulting in erroneous epigenetic modifications and changes in gene expression, which inhibits neuronal functions (Arney and Fisher 2004; Dias and Ressler 2014) (Fig. 1).
Fig. 1.
The schematic diagram of epigenetics. Several types of epigenetic mechanisms have been discovered, including DNA methylation, histone modifications, chromatin remodeling, m6A modification, and the regulation of non-coding RNA. Epigenetic modifications can regulate various biological functions, such as cell apoptosis, oxidative stress, autophagy, neuronal plasticity, cognitive, and motor function
DNA Methylation
In mammals, 5-methylcytosine (5mC) is the basic form of DNA modification, which is associated with the occurrence and development of diseases (Li and Zhang 2014; Smith and Meissner 2013). DNA methyltransferase (DNMT), which includes DNMT1, DNMT3A, and 3B, can transfer the methyl of S-adenosyl methionine (SAM) to cytosine (Bestor 2000; Cheng 1995; Pradhan et al. 1999; Szyf 2005). DNA methylation is one of the complex mechanism in gene regulation. Cytosine-phosphate-guanine (CpG) dinucleotide, as an epigenetic regulator, is involved in the regulation of gene silence. CpG island contains with a 200 bp region (Bird 2002; Brenner and Fuks 2006), in which the GC content is more than 50%, and the expected CpG ratio is greater than 0.5 (Gardiner-Garden and Frommer 1987). Mammalian genomes contain millions of 5-methylcytosine residues, and 40% of the gene promoters contain CpG islands (Bestor 2000). Most CpG sites are modified by 5mC1, which can mediate the regulation of transcription in the mammalian genome (Bird 2002; Brenner and Fuks 2006). Generally, 5mC is chemically stable, and a barrier for methyl removal is formed through stable carbon–carbon bonds. Based on the stable chemical structure, 5mC can nonetheless be dynamically reversed to an unmodified state in numerous ways. The process of active DNA demethylation mainly includes repeated oxidation of 5mC, excision of 5-carboxylcytosine (5caC) and 5-formylcytosine.
Dynamic DNA demethylation can be regulated at different levels, such as transcription, post-transcription, and post-translation. In addition, genomic-specific demethylation regulators can also regulate this dynamic process (Shen et al. 2014). Researchers found the process of demethylation other than TET protein (Bochtler et al. 2017; Wu and Zhang 2010, 2014). The first mechanism is similar to the process of converting thymine to uracil. Decarboxylase can directly remove the 5-carboxyl group of 5caC (Smiley et al. 2005). The second mechanism is the direct removal of 5-hydroxymethyl from 5-hydroxymethyl cytosine (5hmC) to form unmodified cytosine. In vitro studies have shown that DNA methyltransferases can restore 5hmC to unmodified state without methyl donor (Chen et al., 2012; Liutkeviciute et al., 2009). The third mechanism is that DNMT increases the conversion of 5mC to 5hmC, which promotes the possibility of replication-dependent DNA demethylation (Hashimoto et al. 2012; Valinluck and Sowers 2007).
m6A Modification
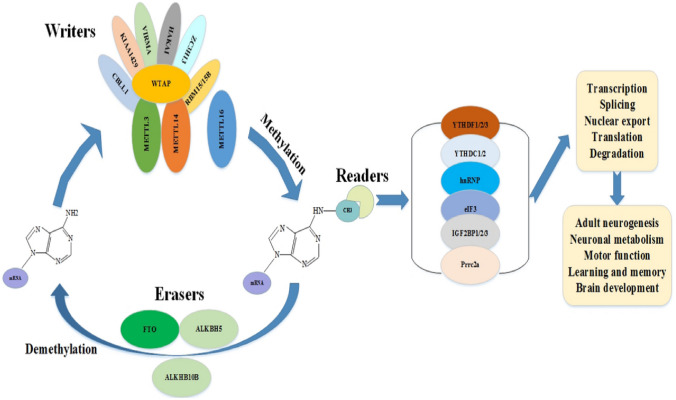
m6A modification is a ubiquitous modification in all eukaryotic mRNA, and it is a dynamic and reversible modification (Wei et al. 1975). It represents over 80% of RNA methylation and exists in various species (Adams and Cory 1975; Bodi et al. 2010; Canaani et al. 1979; Chen-Kiang et al. 1979; Levis and Penman 1978; Narayan et al. 1987; Zhong et al. 2008). m6A regulates many aspects of RNA, including RNA splicing, output, translation, and decay (Roundtree et al. 2017a, b; Roundtree et al. 2017a, b; Wang et al. 2014a, b; Wang et al. 2015; Xiao et al. 2016). m6A methyltransferase includes Wilms tumor-associated protein, KIAA1429, RNA binding motif protein 15/15B, methyltransferase-like 3/14/16 (METTL3/14/16), CBL proto-oncogene-like 1, virus-like m6A methyltransferase related, E3 ubiquitin-protein ligase, and zinc finger CCCH-type containing 13 (Guo et al. 2018a, b; Lence et al. 2016; Liu et al. 2014; Schwartz et al. 2014; Sledz and Jinek 2016; Warda et al. 2017). Recently, researchers have found that cells lacking fat mass and obesity-associated protein (FTO) lead to the up-regulation of m6A density in the 5’UTR region, indicating that FTO is an m6A demethylase (Zhou et al. 2015). In arabidopsis thaliana, AlkB homologue 10B (ALKHB10B) was also found to be m6A demethylase of mRNA (Jia et al. 2011; Zheng et al. 2013). The transferred methyl group can be recognized by different reader proteins, including YTH domain-containing family protein 1/2/3 (YTHDF1/2/3), YTH domain-containing protein 1/2 (YTHDC1/2), heterogeneous ribonucleoprotein (HnRNP), eukaryotic initiation factor 3 (eIF3), insulin-like growth factor 2mRN binding protein 1/2/3 (IGF2BP1/2/3), and proline-rich curl 2A (PRRC2A) (Alarcon et al. 2015; Hsu et al. 2017; Huang et al. 2018; Meyer et al. 2015; Shi et al. 2017; Wang et al. 2015; Xiao et al. 2016; Zhang et al. 2018). Studies have shown that YTHDF1 improves the translation efficiency of m6A-modified mRNAs (Wang et al. 2015). On the other hand, YTHDF2 mediates the instability and degradation of m6A-related mRNAs (Wang et al. 2014a, b). It has been demonstrated that other recognition proteins have different functions in m6A modified mRNA (Xiao et al. 2016; Shi et al. 2017; Kennedy et al. 2017a, b). Some studies focused on the distribution, function, and mechanism of RNA methylation modification, which provided a theoretical basis for disease-related research and aroused widespread concern in academic circles (Kennedy et al. 2017a, b; Willyard 2017) (Fig. 2).
Fig. 2.
The methylation-associated proteins of mRNA m6A. m6A methyltransferase, also referenced as the “writers”, its main function is to catalyze the RNA methylation process. The demethylase of m6A, also referred to as the “erasers”, can reverse the methylation modification that has been formed. The methylation binding protein is a reader of m6A, its main function is to selective bind m6A, affecting RNA transcription, splicing, nuclear export, translation, and stability. The m6A modificantion can participate in various biological functions, such as adult neurogenesis, neuron metabolism, learning and memory, motor function, and brain development
Histone Modifications and Chromatin Remodeling
Chromatin structure is dynamic, and the histone post-transcriptional modifier (PTM) usually regulates gene expression and cellular characteristics by regulating chromatin structure (Felsenfeld and Groudine 2003). At present, the research on histone modification is mainly focused on acetylation, methylation, and phosphorylation. However, histones can additionally be modified in different ways, including ubiquitination, formylation, and total acylation (Audia and Campbell 2016; Tan et al. 2011). The main target group of histone modification is histone octamer, which consists of four basic core histone H2A, H2B, H3, and H4, (Berger 2002; Dion et al. 2005; Govin et al. 2004; Kouzarides 2007). Histone methylation depends on the residues and the degree of methylation, and interactions with other modifications (Martin and Zhang 2005). Histone acetylation and histone phosphorylation are usually associated with transcriptional activation (Grunstein 1997; Imhof et al. 1997). Histone modification is dynamic and reversible process, which is regulated by acetyltransferase and deacetyltransferase, methyltransferase and demethylase (Kuo and Allis 1998; Trojer and Reinberg 2006). Recent studies have shown that JumonjiC (JmjC) family and lysine-specific demethylase (LSD) family can regulate the dynamic histone methylation. The LSD family can remove lysine labeling of monomethyl and dimethyl histones by FAD-dependent amine oxidase (Zheng et al. 2015). Proteins containing JmjC domains can hydroxylate lysine and arginine residues, which is needed for N-terminal histone methylation (Kooistra and Helin 2012). Researchers have found that JmjC proteins are associated with histone demethylation (Shmakova et al. 2014). Histone acetylation is mostly regulated by histone acetyltransferase (HATs) and histone deacetylase (HDAC) (Guo et al. 2018a, b). HDAC can mediate the elimination of acetyl groups on histone. HATs catalyze the addition of acetyl (de Ruijter et al. 2003) to lysine residues, thereby changing the structure of chromatin and forming the transcriptional active region of euchromatin. The dynamic balance of HAT/HDAC activity determines the histone acetylation level, thereby regulating the level of transcriptional (McKinsey et al. 2001). Ubiquitin is a polypeptide, which performs an essential role in cell DNA damage. Ubiquitination of histone H2A and H2B is a necessary post-translational modification in chromatin modification (Uckelmann and Sixma 2017). Polycomb repressive complex 1 (PRC1) can modify histone H2A at three different sites, including K13/K15, K119, and K127/129 sites. The monoubiquitylation of histone H2A C terminus (H2AK119ub1) is modified by K119 site, thereby regulating gene silence (Wang et al. 2004). Ubiquitin ligase Bre1 and ubiquitin-binding enzyme Rad6 can modify histone H2B in the gene coding region to produce the monoubiquitylation of the H2B C terminus (H2BK123ub1). H2BK123ub1 can regulate the initiation and extension of transcript (Fleming et al. 2008). Studies have shown that ubiquitination is an extremely dynamic modification. Isopeptidase, a deubiquitin enzyme, can remove ubiquitin modification, thereby regulating gene expression (Bannister and Kouzarides 2011). Sumoylation can occur in core histones and bind ubiquitin-like modifier molecules to histone lysine, thereby antagonizing ubiquitination and acetylation (Nathan et al. 2006; Seeler and Dejean 2003). However, further research is needed on the molecular mechanism of sumoylation.
Chromatin undergoes extensive remodeling during nuclear reprogramming. Genes, and more generally, chromatin are regulated by covalent modifications. The N-terminal amino acid residues of histone can be acetylated, methylated, or phosphorylated under the catalysis of various enzymes, to change chromatin structures, thereby activating or inhibiting gene transcription. There are two main structures that mediate chromosome remodeling: ATP-dependent nucleosome remodeling complex and histone modified complex. ATP can alter nucleosome structures through nucleosome remodeling and histone acetylation. Among chromatin modification factors, the heterogeneous nucleosome recombinant deacetylase (NURD) complex with ATP-dependent nucleosome remodeling and histone deacetylase activity has special research value.
Noncoding RNAs
Non-coding RNA includes long non-coding RNA (LncRNAs, transcription length more than 200 bp) and short non-coding RNA (transcription length less than 200 bp). LncRNAs can regulate gene expression via post-transcriptional pathways. Short non-coding RNA can affect chromatin structure, thereby regulating cell differentiation and gene expression (Aumiller and Forstemann 2008; Carissimi et al. 2009; Dharap et al. 2009; Jeyaseelan et al. 2008; Li et al. 2015; Merkerova et al. 2008; Sorensen et al. 2014). In addition, according to the classification of functions, non-coding RNA can be divided into two categories: one includes micronucleus RNA (snRNA), small nucleolus RNA (snoRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA), which are expressed in all types of cells and performs basic functions in cells. The other includes Piwi-related RNA (piRNA), circular RNA (circRNA), microRNA (miRNA), lncRNA, small interference RNA (siRNA), and tRNA derived small RNA (tsRNA). The most studied molecules are miRNA, lncRNA, and CircRNA (Ferlita et al. 2018).
Epigenetic Modifications and Motor Function
Effect of DNA Methylation on Motor Function
PD is characterized by bradykinesia, muscle rigidity, tremor, and paralysis. Recent studies have shown that epigenetic modifications are involved in the pathogenesis of PD. The expression of α-synuclein (SNCA) significantly changed in PD patients. The methylation of SNCA intron 1 decreased in substantia nigra and striatum of PD patients (Jowaed et al. 2010). This study shows that DNA methylation is involved in the regulation of SNCA levels, thereby inducing the pathogenesis of PD. In addition, the occurrence of PD is also related to the lack of DNMT1 (Desplats et al. 2011). Spinal muscular atrophy (SMA) is induced by the deletion or mutation of surviving motor neuron 1 (SMN1) gene, thereby impairing motor function (Lefebvre et al. 1995). Interestingly, the DNA methylation of SMN gene occurs in SMA patients, which is positively correlated with the severity of disease. (Hauke et al. 2009). Amyotrophic lateral sclerosis (ALS) is one of the fatal neurodegenerative diseases with no known genetic cause. Researchers conducted a genome-wide analysis of the midbrain in ALS patients and confirmed that various genes experienced abnormal DNA methylation (Morahan et al. 2007). In addition, Barry et al. reported that the expression of DNMT1 and DNMT3A increased in motor cortex and spinal cord motoneurons of ALS patients (Chestnut et al. 2011). In summary, these observations suggest that aberrant DNA methylation may be associated with neurodegenerative diseases.
Effect of m6A Modification on Motor Function
m6A is a widespread chemical modification in brain and involves in major biological processes (Meyer et al. 2012). m6A modification disorders are associated with neuronal developmental and degenerative diseases. Recent studies have found that FTO can regulate the occurrence and development of neurodegenerative diseases in motor neurons (Alunni and Bally-Cuif 2016; Engel and Chen 2018; Li et al. 2017). Hess et al. have demonstrated that inhibition of FTO can lead to the reduction of brain volume and the impairment of dopamine receptors (D2 and D3), which may be related to the pathogenesis of PD (Hess et al. 2013). Chen et al. have shown that the m6A modification of N-methyl-d-aspartate receptor 1 (NMDA1) mRNAs was downregulated in PD cells and animal models (Chen et al. 2019), which led to the apoptosis of dopaminergic neurons. These results suggest that m6A modification may be involved in the pathological process of PD. In addition, researchers have reported that the neuron-specific expression of FTO is highly expressed in motor neurons, and the outcomes of this study certified that FTO gene mutation may cause ALS (Mitropoulos et al. 2017). Therefore, the study of mRNA m6A modifications will provide a new target for the treatment of neurodegenerative diseases with dyskinesia.
Effect of Histone Modifications and Chromatin Remodeling on Motor Function
FTD-17 patients with Parkinson’s syndrome exhibits an abnormal accumulation of hyperacetylated microtubule-binding protein TAU in brain (Min et al. 2010). Researchers have shown that HAT-active elongation protein 3 (Elp3) is associated with motor neuron degeneration in ALS (Simpson et al. 2009). Furthermore, HDAC can influence the occurrence and development of ALS (Lazo-Gomez et al. 2013). The decrease of HDAC11 mRNA and the increase of HDAC2 mRNA were found in brain and spinal cord tissues of patients with ALS. (Janssen et al. 2010). Knockout of fusion sarcoma (FUS), a protein involved in motor neuron disease, can lead to the dislocation of HDAC1 (Scekic-Zahirovic et al. 2016). In a mouse model of superoxide dismutase (SOD1), the level of SIRT1 mRNA was increased in muscle, while the level of SIRT2 mRNA was decreased in motor neurons (Valle et al. 2014). In the same mouse model, researchers found that over-expression of HDAC6 delayed the impairment of motor neurons (Du et al. 2015). Studies have shown that the mechanisms of Friedreich ataxia (FRDA) and Huntington’s disease (HD) may involve abnormal histone modifications (Al-Mahdawi et al. 2008; Matsuda et al. 2019; Ryu et al. 2006). FRDA is mainly caused by GAA repetitive amplification mutation in intron 1 of the FXN gene. Researchers have shown that the histone acetylation level of ataxia gene was decreased, while the methylation level of histone H3 lysine 9 (H3K9) was increased in FRDA patients (Al-Mahdawi et al. 2008). HD is mainly caused by pathological prolongation of CAG repeat in exon 1 of Huntington gene (HTT). Some studies have found that HD may be related to the high methylation level of H3K9 and SETDB1HMT (Ryu et al. 2006). Abnormal histone methylation can reduce the levels of H3K27 trimethylation (H3K27me3) in medium spiny neurons, thereby interfering with gene expression (Matsuda et al. 2019). The deletion of polycomb inhibitory complex 2 (PRC2) and H3K27me2/3 will impaire motor performance, and the premature death of neuron (Kaneko et al. 2014; Kennerdell et al. 2018; Li et al. 2013; Piunti et al. 2019; Ragazzini et al. 2019; Sanulli et al. 2015; von Schimmelmann et al. 2016).
Researchers analyzed hundreds of human brain tissues and found that neuronal proteins undergo basic chromatin remodeling in brain (Weng et al. 2012). Hypermethylation of H3K9 and up-regulation of H2A1 (Hu et al. 2011) perform an essential role in the pathogenesis of HD (Stack et al. 2007). Frontotemporal dementia (FTD) is a neurodegenerative disease with sucking and grip reflexes in the early stage, myoclonus, pyramidal tract signs, and parkinson’s syndrome in the late stage (Bourinaris and Houlden 2018; Floris et al. 2015; Greaves and Rohrer 2019). TAR-DNA Binding Protein 43 (TDP-43), a DNA and RNA binding protein, can restrict the expression of chromatin remodeling factor CHD1 by forming insoluble aggregates (Berson et al. 2017; Chou et al. 2018; Fang et al. 2019; Gao et al. 2019; Stoica et al. 2014; Wang et al. 2018). Mass spectrometric analysis showed that the protein level of CHD2 significantly decreased in the temporal lobe cortex of FTD patients after death (Bennett et al. 2019; Che et al. 2018; Cobos et al. 2019). TDP-43 can regulate the protein level of Brama-associated gene 1 (BRG1) (Bronisz et al. 2014; Carey et al. 2016; Tibshirani et al. 2017), which is a component of neuronal BRG1-associated factor complex (nBAF), thereby regulating neuronal differentiation and function (Ronan et al. 2013; Shan et al. 2020; Son and Crabtree 2014; Staahl et al. 2013; Vogel et al. 2017). Therefore, abnormal chromatin remodeling may be one of the resons for the vulnerability of age-dependent neurons.
Effect of Noncoding RNAs on Motor Function
Compared with healthy subjects, 13 miRNAs (8 down-regulated, 5 up-regulated) were found to be associated with the pathogenesis of PD. U1, RP11-46 2G22.1 and RP11-79P5.3 were expressed in leukocytes, amygdala, and substantia nigra. RP11-462G22.1 is involved in muscular dystrophy, suggesting that it may be related to muscle rigidity in PD patients (Li et al. 2018; Soreq et al. 2014). The down-regulation of human accelerated regions 1 (HAR1, a lncRNA) is mediated by abnormal expression of HAR1F, thereby accelerating the pathogenesis of HD (Shimojo 2008; Wu et al. 2013; Zuccato et al. 2003). Taurine up-regulated gene 1 (TUG1, a lncRNA), a target of p53, may be associated with the development of HD (Ehrnhoefer et al. 2014; Intihar et al. 2019; Johnson 2012). In addition, the interaction between mutant Huntingtin and Argonaute 2 (AGO2), a member of Argonaute protein family, is involved in the regulation of gene silencing, which is mediated by miRNA (Savas et al. 2008; Tokiyoshi et al. 2018). Taken together, these findings suggest that noncoding RNAs are closely related to the pathogenesis of neurodegenerative diseases. A better understanding of noncoding RNAs will provide innovative ideas for exploring more effective strategies in the treatment of motor dysfunction (Table 1).
Table 1.
Epigenetic modifications in neurodegenerative diseases induced bradykinesia
| Modifications | Examples of targets affected | Resulting disorders |
|---|---|---|
| DNA methylation | SNCA (SNCA intron 1 methylation); DNMTs (Changes in DNMT1 and DNMT3A levels); | PD, ALS, SMA |
| SMN2 (SMN2 gene silencing) | ||
| N6-methyladenosine | FTO (FTO gene mutation); D2, D3 (The inhibition of FTO could damage to D2 and D3); | ALS, PD |
| NMDA1 (Changes in NMDA1 expression levels) | ||
| Histone modifications | Tau (TAU hyperacetylation); ELP3 (Changes in ELP3 levels, which has HAT activity); | PD, ALS, FRDA, HD, Neurodegeneration |
| HDACs (Changes in HDAC1/2/6/11 levels); SIRTs (Changes in SIRT1/2 levels); H3K9 (H3K9 methylation) | ||
| SETDB1 HMT (Hypermethylation of SETDB1 HMT); PRC2 and H3K27me3 (Deletion of PRC2 and H3K27me2/3) | ||
| Chromatin remodeling | H3K9, H2A1 (Hypermethylation of H3K9 and up-regulation of H2A1); | HD, Age-dependent neuronal vulnerability |
| TDP-43, CHD1/2, BRG1 (TDP-43 restricts the expression of CHDS and BRG1) | ||
| Noncoding RNAs | U1, RP11-46 2G22.1, RP11-79P5.3 (Changes in U1, RP11-46 2G22.1, and RP11-79P5.3 expression levels); | PD, HD |
| HAR1 (Changes in HAR1 levels); TUG1 (Changes in TUG1 levels); | ||
| AGO2 (AGO2 participates in the regulation of gene silence) |
The Role of Epigenetic Drugs in Neurodegenerative Diseases
Over the recent years, due to their highly dynamic nature, epigenetic intervention can be used as a new therapeutic strategy for the treatment of neurodegenerative diseases, such as PD, HD, and ALS. Increasing research has proven that the HDAC inhibitor (HDACi), including trichostatin A, sodium butyrate, sodium phenylbutyrate, and valproic acid, can antagonize progressive neuronal degeneration in HD (Hockly et al. 2003; Tremolizzo et al. 2002). SNCA can inhibit histone acetylation, thereby producing neurotoxicity. Researchers have shown that valproic acid could counteract the neurotoxicity of SNCA and improve the symptoms of PD dyskinesia (Coppede 2014). Furthermore, severa studies have revealed that SNCA-induced neurotoxicity of PD can be antagonized by the administering sodium butyrate or inhibiting of histone deacetylase SIRT2 (Coppede 2014; Outeiro et al. 2007). In HD, anthracycline can improve the acetylation dysfunction of H3 and H4 histones and the hypermethylation of H3K9 (Valor 2015). Recent studies have shown that cysteamine and mithramycin treatment can reduce the hypermethylation of H3 and improve the survival rate of HD patients (Wang et al. 2014a, b). Phenylbutyrate, a type of HDACi, can improve the ALS-associated symptoms of motor dysfunction (Valor 2015; Cudkowicz et al. 2009). Restoring the expression of SAM in ALS can improve neuronal dysfunction (Suchy et al. 2010). At present, several HDACis, DNMTs inhibitors, LSD1 inhibitors, histone methyltransferase dot1-like protein, and enhancer of zeste homolog 2 inhibitors have entered the clinical trial stage (Hsieh and Gage 2005; Audia and Campbell 2016). To treat neurodegenerative diseases, further research is needed on related epigenetic drugs research to overcome neurodegenerative diseases.
Conclusion
Epigenetic modifications have attracted people’s attention in the fields of medicine and pharmacy. Abnormal epigenetic modifications can induce neurodegenerative diseases and motor dysfunction by regulating the expression of different genes (Marques et al. 2011; Migliore and Coppede 2009). Intervention of epigenetic modifications has provide new targets for the treatment of neurodegenerative diseases (Abel and Zukin 2008; Chuang et al. 2009; Dietz and Casaccia 2010; Hahnen et al. 2008; Kazantsev and Thompson 2008; Konsoula and Barile 2012). Collectively, these outcomes suggest that epigenetic modifications perform a crucial role in neurodegenerative diseases, it may be acting as a driver. Abnormal epigenetic modifications can not only lead to motor dysfunction, but also to learning and memory impairment, further research is needed on related epigenetic mechanisms.
The regulatory factors related to epigenetic modifications may be suitable as targets for the direct treatment of neurodegenerative diseases. However, in order to further understand and prove the role of epigenetic modifications in neurodegenerative disorders, many problems still need to be resolved. For example, what is the specific molecular mechanism that causes disease; whether there are other diseases related to it; how the body selects specific mRNA for methylation modification; and whether there are other proteins involved in the formation of disease. It is worth noting that different diseases can interact with different regulatory proteins and different cells have different regulators to affect the expression of specific mRNA. However, there are relatively few animal models and cell models to elucidate the pathogenesis, biochemical disorders, and morphological changes of neurodegenerative diseases. This requires further exploration to clarify how epigenetics regulate motor function through the stimulation of external environment, drugs, and poisons, so as to fully reveal the role of epigenetic modifications on neurodegenerative disorders. This paper will provide new ideas and new therapeutic potential of epigenetic drugs in neurodegenerative diseases with dyskinesia.
Acknowledgements
We would like to acknowledge Hui Yuan, Haiying Wang, Bingchen Liu, Xuda Liu, Yi Wen, Rong Cui, Tingwei Pan, Binbin Liu, and Miaoling Wu for their valuable advices.
Abbreviations
- AGO2
Argonaute 2
- ALS
Amyotrophic lateral sclerosis
- ALKHB5/10B
AlkB homolog 5/10B
- BRG1
Brahma-related gene 1
- circRNA
Circular RNA
- CNS
Central nervous system
- CpG
Cytosine-phosphate-guanine
- D2
Dopamine receptor 2
- D3
Dopamine receptor 3
- DNMT
DNA methyl-transferase
- ELP3
Elongator protein 3
- eIF3
Eukaryotic initiation factor 3
- FRDA
Friedreich ataxia
- FTD
Frontotemporal dementia
- FTO
Fat mass and obesity-associated protein
- FUS
Fused in sarcoma
- HATs
Histone acetyltransferases
- HDACs
Histone deacetylases
- HD
Huntington’s disease
- HDACi
HDAC inhibitor
- H3K9
Histone H3 lysine 9
- HnRNP
Heterogeneous nuclear ribonucleoprotein
- IGF2BP 1/2/3
Insulin-like growth factor 2 mRNA-binding protein 1/2/3
- JmjC
Family and members of the Jumonji C
- lncRNAs
Long noncoding RNA
- LSD
Lysine-specific demethylase
- m6A
N6-Methyladenosine
- METTL3/14/16
Methyltransferase like 3/14/16
- miRNA
MicroRNA
- nBAF
Neuronal BRG1-associated factor complex
- NMDA
N-methyl-d-aspartate
- NURD
Nucleosome remodeling histone deacetylase
- PD
Parkinson’s disease
- piRNA
Piwi related RNA
- PRC2
Polycomb-repressive complex 2
- PTM
Post-transcriptional modifier
- PRRC2A
Proline rich coiled-coil 2A
- rRNA
Ribosomal RNA
- siRNA
Small interfering RNA
- SMA
Spinal muscular atrophy
- SMN1
Survival motor neuron 1
- SNCA
α-Synuclein
- snRNA
Small nuclear RNA
- snoRNA
Small nucleolar RNA
- tRNA
Transfer RNA
- SOD1
Superoxide dismutase
- SIRT
Sirtuins
- TDP-43
TAR-DNA Binding Protein 43
- TET
Ten-eleven translocation
- tsRNA
TRNA derived small RNA
- TUG1
Taurine-upregulated gene 1
- YTHDF1/2/3
YTH domain-containing family protein 1/2/3
- YTHDC1/2
YTH domain-containing protein ½
- 5caC
5-Carboxylcytosine
- 5hmC
5MC to 5-hydroxymethylcytosine
- 5mC
5-Methylcytosine
Author contributions
Each author substantially contributed to the review. ZQ: conception and design, drafting the manuscript; JL, ML, XD, LZ, SW, BX, WL, and ZX: revising the manuscript; YD: conception and design, revising it critically for important intellectual content, and final approval of the version to be published. All authors read and approved the final manuscript.
Funding
We gratefully acknowledge funding from the Natural Science Foundation of Liaoning Province [2020-MS-152], the Basic Research Fund of Young Program of Higher Education of Liaoning Province (Grant No. QNK201735), the National Natural Science Foundation of China (Grant No. 81302406) and the Funds for Distinguished Young Scientists in School of Public Health, China Medical University.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abel T, Zukin RS (2008) Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol 8:57–64. 10.1016/j.coph.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Cory S (1975) Modified nucleosides and bizarre 5’-termini in mouse myeloma mRNA. Nature 255:28–33. 10.1038/255028a0 [DOI] [PubMed] [Google Scholar]
- Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF (2015) N6-methyladenosine marks primary microRNAs for processing. Nature 519:482–485. 10.1038/nature14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mahdawi S, Pinto RM, Ismail O, Varshney D, Lymperi S, Sandi C, Trabzuni D, Pook M (2008) The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum Mol Genet 17:735–746. 10.1093/hmg/ddm346 [DOI] [PubMed] [Google Scholar]
- Alunni A, Bally-Cuif L (2016) A comparative view of regenerative neurogenesis in vertebrates. Development 143:741–753. 10.1242/dev.122796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arney KL, Fisher AG (2004) Epigenetic aspects of differentiation. J Cell Sci 117:4355–4363. 10.1242/jcs.01390 [DOI] [PubMed] [Google Scholar]
- Audia JE, Campbell RM (2016) Histone modifications and cancer. Cold Spring Harb Perspect Biol 8(4):a019521. 10.1101/cshperspect.a019521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller V, Forstemann K (2008) Roles of microRNAs beyond development–metabolism and neural plasticity. Biochim Biophys Acta 1779:692–696. 10.1016/j.bbagrm.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21(3):381–395. 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SA, Tanaz R, Cobos SN, Torrente MP (2019) Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease. Transl Res 204:19–30. 10.1016/j.trsl.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL (2002) Histone modifications in transcriptional regulation. Curr Opin Genet Dev 12:142–148. 10.1016/s0959-437x(02)00279-4 [DOI] [PubMed] [Google Scholar]
- Berson A, Nativio R, Berger SL, Bonini NM (2018) Epigenetic regulation in neurodegenerative diseases. Trends Neurosci 41:587–598. 10.1016/j.tins.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson A, Sartoris A, Nativio R, Van Deerlin V, Toledo JB, Porta S, Liu S, Chung CY, Garcia BA, Lee VM, Trojanowski JQ, Johnson FB, Berger SL, Bonini NM (2017) TDP-43 promotes neurodegeneration by impairing chromatin remodeling. Curr Biol 27:3579–3590. 10.1016/j.cub.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor TH (2000) The DNA methyltransferases of mammals. Hum Mol Genet 9:2395–2402. 10.1093/hmg/9.16.2395 [DOI] [PubMed] [Google Scholar]
- Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21. 10.1101/gad.947102 [DOI] [PubMed] [Google Scholar]
- Bochtler M, Kolano A, Xu GL (2017) DNA demethylation pathways: additional players and regulators. BioEssays 39:1–13. 10.1002/bies.201600178 [DOI] [PubMed] [Google Scholar]
- Bodi Z, Button JD, Grierson D, Fray RG (2010) Yeast targets for mRNA methylation. Nucleic Acids Res 38:5327–5335. 10.1093/nar/gkq266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinaris T, Houlden H (2018) C9orf72 and its relevance in parkinsonism and movement disorders: a comprehensive review of the literature. Mov Disord Clin Pract 5:575–585. 10.1002/mdc3.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Fuks F (2006) DNA methyltransferases: facts, clues, mysteries. Curr Top Microbiol Immunol 301:45–66. 10.1007/3-540-31390-7_3 [DOI] [PubMed] [Google Scholar]
- Bronisz A, Carey HA, Godlewski J, Sif S, Ostrowski MC, Sharma SM (2014) The multifunctional protein fused in sarcoma (FUS) is a coactivator of microphthalmia-associated transcription factor (MITF). J Biol Chem 289:326–334. 10.1074/jbc.M113.493874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani D, Kahana C, Lavi S, Groner Y (1979) Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res 6:2879–2899. 10.1093/nar/6.8.2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HA, Bronisz A, Cabrera J, Hildreth BR, Cuitino M, Fu Q, Ahmad A, Toribio RE, Ostrowski MC, Sharma SM (2016) Failure to target RANKL signaling through p38-MAPK results in defective osteoclastogenesis in the microphthalmia cloudy-eyed mutant. J Cell Physiol 231:630–640. 10.1002/jcp.25108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carissimi C, Fulci V, Macino G (2009) MicroRNAs: novel regulators of immunity. Autoimmun Rev 8:520–524. 10.1016/j.autrev.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Che XQ, Zhao QH, Huang Y, Li X, Ren RJ, Chen SD, Guo QH, Wang G (2018) Mutation screening of the CHCHD2 gene for Alzheimer’s disease and frontotemporal dementia in Chinese Mainland population. J Alzheimers Dis 61:1283–1288. 10.3233/JAD-170692 [DOI] [PubMed] [Google Scholar]
- Chen CC, Wang KY, Shen CK (2012) The mammalian de novo DNA methyltransferases DNMT3A and DNMT3B are also DNA 5-hydroxymethylcytosine dehydroxymethylases. J Biol Chem 287:33116–33121. 10.1074/jbc.C112.406975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yu C, Guo M, Zheng X, Ali S, Huang H, Zhang L, Wang S, Huang Y, Qie S, Wang J (2019) Down-regulation of m6A mRNA methylation is involved in dopaminergic neuronal death. Acs Chem Neurosci 10:2355–2363. 10.1021/acschemneuro.8b00657 [DOI] [PubMed] [Google Scholar]
- Cheng X (1995) Structure and function of DNA methyltransferases. Annu Rev Biophys Biomol Struct 24:293–318. 10.1146/annurev.bb.24.060195.001453 [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S, Nevins JR, Darnell JJ (1979) N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol 135:733–752. 10.1016/0022-2836(79)90174-8 [DOI] [PubMed] [Google Scholar]
- Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ (2011) Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci 31:16619–16636. 10.1523/JNEUROSCI.1639-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I, Liu F, Sayegh M, Donlin-Asp PG, Chen YH, Duong DM, Seyfried NT, Powers MA, Kukar T, Hales CM, Gearing M, Cairns NJ, Boylan KB, Dickson DW, Rademakers R, Zhang YJ, Petrucelli L, Sattler R, Zarnescu DC, Glass JD, Rossoll W (2018) TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci 21:228–239. 10.1038/s41593-017-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT (2009) Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci 32:591–601. 10.1016/j.tins.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos SN, Bennett SA, Torrente MP (2019) The impact of histone post-translational modifications in neurodegenerative diseases. Biochim Biophys Acta Mol Basis Dis 1865:1982–1991. 10.1016/j.bbadis.2018.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F (2014) The potential of epigenetic therapies in neurodegenerative diseases. Front Genet 5:220. 10.3389/fgene.2014.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz ME, Andres PL, Macdonald SA, Bedlack RS, Choudry R, Brown RJ, Zhang H, Schoenfeld DA, Shefner J, Matson S, Matson WR, Ferrante RJ (2009) Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph Lateral Scler 10(2):99–106. 10.1080/17482960802320487 [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370:737–749. 10.1042/BJ20021321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Spencer B, Coffee E, Patel P, Michael S, Patrick C, Adame A, Rockenstein E, Masliah E (2011) Alpha-synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J Biol Chem 286:9031–9037. 10.1074/jbc.C110.212589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Bowen K, Place R, Li LC, Vemuganti R (2009) Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29:675–687. 10.1038/jcbfm.2008.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ (2014) Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 17:89–96. 10.1038/nn.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KC, Casaccia P (2010) HDAC inhibitors and neurodegeneration: at the edge between protection and damage. Pharmacol Res 62:11–17. 10.1016/j.phrs.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion MF, Altschuler SJ, Wu LF, Rando OJ (2005) Genomic characterization reveals a simple histone H4 acetylation code. Proc Natl Acad Sci U S A 102:5501–5506. 10.1073/pnas.0500136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du ZW, Chen H, Liu H, Lu J, Qian K, Huang CL, Zhong X, Fan F, Zhang SC (2015) Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun 6:6626. 10.1038/ncomms7626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Skotte NH, Ladha S, Nguyen YT, Qiu X, Deng Y, Huynh KT, Engemann S, Nielsen SM, Becanovic K, Leavitt BR, Hasholt L, Hayden MR (2014) p53 increases caspase-6 expression and activation in muscle tissue expressing mutant huntingtin. Hum Mol Genet 23:717–729. 10.1093/hmg/ddt458 [DOI] [PubMed] [Google Scholar]
- Engel M, Chen A (2018) The emerging role of mRNA methylation in normal and pathological behavior. Genes Brain Behav 17:e12428. 10.1111/gbb.12428 [DOI] [PubMed] [Google Scholar]
- Esteller M (2002) CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21:5427–5440. 10.1038/sj.onc.1205600 [DOI] [PubMed] [Google Scholar]
- Fang MY, Markmiller S, Vu AQ, Javaherian A, Dowdle WE, Jolivet P, Bushway PJ, Castello NA, Baral A, Chan MY, Linsley JW, Linsley D, Mercola M, Finkbeiner S, Lecuyer E, Lewcock JW, Yeo GW (2019) Small-molecule modulation of TDP-43 recruitment to stress granules prevents persistent TDP-43 accumulation in ALS/FTD. Neuron 103:802–819. 10.1016/j.neuron.2019.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M (2003) Controlling the double helix. Nature 421(6921):448–453. 10.1038/nature01411 [DOI] [PubMed] [Google Scholar]
- Ferlita A, Battaglia R, Andronico F, Caruso S, Cianci A, Purrello M, Pietro CD (2018) Non-coding RNAs in endometrial physiopathology. Int J Mol Sci. 10.3390/ijms19072120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AB, Kao CF, Hillyer C, Pikaart M, Osley MA (2008) H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol Cell 31(1):57–66. 10.1016/j.molcel.2008.04.025 [DOI] [PubMed] [Google Scholar]
- Floris G, Borghero G, Cannas A, Di Stefano F, Murru MR, Corongiu D, Cuccu S, Tranquilli S, Cherchi MV, Serra A, Loi G, Marrosu MG, Chio A, Marrosu F (2015) Clinical phenotypes and radiological findings in frontotemporal dementia related to TARDBP mutations. J Neurol 262:375–384. 10.1007/s00415-014-7575-5 [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M (2005) Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A 102:10604–10609. 10.1073/pnas.0500398102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang L, Yan T, Perry G, Wang X (2019) TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration. Mol Cell Neurosci 100:103396. 10.1016/j.mcn.2019.103396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner-Garden M, Frommer M (1987) CpG islands in vertebrate genomes. J Mol Biol 196:261–282. 10.1016/0022-2836(87)90689-9 [DOI] [PubMed] [Google Scholar]
- Govin J, Caron C, Lestrat C, Rousseaux S, Khochbin S (2004) The role of histones in chromatin remodelling during mammalian spermiogenesis. Eur J Biochem 271:3459–3469. 10.1111/j.1432-1033.2004.04266.x [DOI] [PubMed] [Google Scholar]
- Greaves CV, Rohrer JD (2019) An update on genetic frontotemporal dementia. J Neurol 266:2075–2086. 10.1007/s00415-019-09363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M (1997) Histone acetylation in chromatin structure and transcription. Nature 389:349–352. 10.1038/38664 [DOI] [PubMed] [Google Scholar]
- Guo J, Tang HW, Li J, Perrimon N, Yan D (2018a) Xio is a component of the Drosophila sex determination pathway and RNA N(6)-methyladenosine methyltransferase complex. Proc Natl Acad Sci U S A 115:3674–3679. 10.1073/pnas.1720945115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Chen W, Li H, Li M, Li L (2018b) The histone acetylation modifications of breast cancer and their therapeutic implications. Pathol Oncol Res 24:807–813. 10.1007/s12253-018-0433-5 [DOI] [PubMed] [Google Scholar]
- Hahnen E, Hauke J, Trankle C, Eyupoglu IY, Wirth B, Blumcke I (2008) Histone deacetylase inhibitors: possible implications for neurodegenerative disorders. Expert Opin Investig Drugs 17:169–184. 10.1517/13543784.17.2.169 [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Liu Y, Upadhyay AK, Chang Y, Howerton SB, Vertino PM, Zhang X, Cheng X (2012) Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res 40:4841–4849. 10.1093/nar/gks155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassig CA, Schreiber SL (1997) Nuclear histone acetylases and deacetylases and transcriptional regulation: HATs off to HDACs. Curr Opin Chem Biol 1:300–308. 10.1016/s1367-5931(97)80066-x [DOI] [PubMed] [Google Scholar]
- Hauke J, Riessland M, Lunke S, Eyupoglu IY, Blumcke I, El-Osta A, Wirth B, Hahnen E (2009) Survival motor neuron gene 2 silencing by DNA methylation correlates with spinal muscular atrophy disease severity and can be bypassed by histone deacetylase inhibition. Hum Mol Genet 18:304–317. 10.1093/hmg/ddn357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess ME, Hess S, Meyer KD, Verhagen LA, Koch L, Bronneke HS, Dietrich MO, Jordan SD, Saletore Y, Elemento O, Belgardt BF, Franz T, Horvath TL, Ruther U, Jaffrey SR, Kloppenburg P, Bruning JC (2013) The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci 16:1042–1048. 10.1038/nn.3449 [DOI] [PubMed] [Google Scholar]
- Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP (2003) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A 100(4):2041–2046. 10.1073/pnas.0437870100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Gage FH (2005) Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol 17(6):664–671. 10.1016/j.ceb.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J, Cheng Y, Luo G, Dai Q, Liu M, Guo X, Sha J, Shen B, He C (2017) Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27:1115–1127. 10.1038/cr.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chopra V, Chopra R, Locascio JJ, Liao Z, Ding H, Zheng B, Matson WR, Ferrante RJ, Rosas HD, Hersch SM, Scherzer CR (2011) Transcriptional modulator H2A histone family, member Y (H2AFY) marks Huntington disease activity in man and mouse. Proc Natl Acad Sci U S A 108:17141–17146. 10.1073/pnas.1104409108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, Hu YC, Huttelmaier S, Skibbe JR, Su R, Deng X, Dong L, Sun M, Li C, Nachtergaele S, Wang Y, Hu C, Ferchen K, Greis KD, Jiang X, Wei M, Qu L, Guan JL, He C, Yang J, Chen J (2018) Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20:285–295. 10.1038/s41556-018-0045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JY, Aromolaran KA, Zukin RS (2017) The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat Rev Neurosci 18:347–361. 10.1038/nrn.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H (1997) Acetylation of general transcription factors by histone acetyltransferases. Curr Biol 7:689–692. 10.1016/s0960-9822(06)00296-x [DOI] [PubMed] [Google Scholar]
- Intihar TA, Martinez EA, Gomez-Pastor R (2019) Mitochondrial dysfunction in huntington’s disease; interplay between HSF1, p53 and PGC-1alpha transcription factors. Front Cell Neurosci 13:103. 10.3389/fncel.2019.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Abhiman S, Aravind L (2011) Natural history of eukaryotic DNA methylation systems. Prog Mol Biol Transl Sci 101:25–104. 10.1016/B978-0-12-387685-0.00002-0 [DOI] [PubMed] [Google Scholar]
- Janssen C, Schmalbach S, Boeselt S, Sarlette A, Dengler R, Petri S (2010) Differential histone deacetylase mRNA expression patterns in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 69:573–581. 10.1097/NEN.0b013e3181ddd404 [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A (2008) MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39:959–966. 10.1161/STROKEAHA.107.500736 [DOI] [PubMed] [Google Scholar]
- Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C (2011) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7:885–887. 10.1038/nchembio.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R (2012) Long non-coding RNAs in Huntington’s disease neurodegeneration. Neurobiol Dis 46:245–254. 10.1016/j.nbd.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Jowaed A, Schmitt I, Kaut O, Wullner U (2010) Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients’ brains. J Neurosci 30:6355–6359. 10.1523/JNEUROSCI.6119-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, McRae AF, Visscher PM, Montgomery GW, Gottesman II, Martin NG, Petronis A (2009) DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet 41:240–245. 10.1038/ng.286 [DOI] [PubMed] [Google Scholar]
- Kaneko S, Son J, Bonasio R, Shen SS, Reinberg D (2014) Nascent RNA interaction keeps PRC2 activity poised and in check. Genes Dev 28:1983–1988. 10.1101/gad.247940.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM (2008) Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 7:854–868. 10.1038/nrd2681 [DOI] [PubMed] [Google Scholar]
- Kennedy EM, Bogerd HP, Kornepati A, Kang D, Ghoshal D, Marshall JB, Poling BC, Tsai K, Gokhale NS, Horner SM, Cullen BR (2017a) Posttranscriptional m(6)A editing of HIV-1 mRNAs enhances viral gene expression. Cell Host Microbe 22:830. 10.1016/j.chom.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EM, Courtney DG, Tsai K, Cullen BR (2017b) Viral epitranscriptomics. J Virol. 10.1128/JVI.02263-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell JR, Liu N, Bonini NM (2018) MiR-34 inhibits polycomb repressive complex 2 to modulate chaperone expression and promote healthy brain aging. Nat Commun 9:4188. 10.1038/s41467-018-06592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsoula Z, Barile FA (2012) Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J Pharmacol Toxicol Methods 66:215–220. 10.1016/j.vascn.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Kooistra SM, Helin K (2012) Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol 13:297–311. 10.1038/nrm3327 [DOI] [PubMed] [Google Scholar]
- Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Kuo MH, Allis CD (1998) Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays 20:615–626. 10.1002/(SICI)1521-1878(199808)20:8%3c615::AID-BIES4%3e3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- Lazo-Gomez R, Ramirez-Jarquin UN, Tovar-Y-Romo LB, Tapia R (2013) Histone deacetylases and their role in motor neuron degeneration. Front Cell Neurosci 7:243. 10.3389/fncel.2013.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Et A (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80:155–165. 10.1016/0092-8674(95)90460-3 [DOI] [PubMed] [Google Scholar]
- Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, Kreim N, Andrade-Navarro MA, Poeck B, Helm M, Roignant JY (2016) m(6)A modulates neuronal functions and sex determination in drosophila. Nature 540:242–247. 10.1038/nature20568 [DOI] [PubMed] [Google Scholar]
- Levis R, Penman S (1978) 5’-terminal structures of poly(A)+ cytoplasmic messenger RNA and of poly(A)+ and poly(A)- heterogeneous nuclear RNA of cells of the dipteran Drosophila melanogaster. J Mol Biol 120:487–515. 10.1016/0022-2836(78)90350-9 [DOI] [PubMed] [Google Scholar]
- Li E, Zhang Y (2014) DNA methylation in mammals. Cold Spring Harb Perspect Biol 6:a19133. 10.1101/cshperspect.a019133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hart RP, Mallimo EM, Swerdel MR, Kusnecov AW, Herrup K (2013) EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia. Nat Neurosci 16:1745–1753. 10.1038/nn.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sun Y, Chen J (2018) Identification of critical genes and miRNAs associated with the development of Parkinson’s disease. J Mol Neurosci 65:527–535. 10.1007/s12031-018-1129-8 [DOI] [PubMed] [Google Scholar]
- Li L, Zang L, Zhang F, Chen J, Shen H, Shu L, Liang F, Feng C, Chen D, Tao H, Xu T, Li Z, Kang Y, Wu H, Tang L, Zhang P, Jin P, Shu Q, Li X (2017) Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Hum Mol Genet 26:2398–2411. 10.1093/hmg/ddx128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Su SY, Liu JL (2015) Differential regulation of microRNAs in patients with ischemic stroke. Curr Neurovasc Res 12:214–221. 10.2174/1567202612666150605121709 [DOI] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10:93–95. 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liutkeviciute Z, Lukinavicius G, Masevicius V, Daujotyte D, Klimasauskas S (2009) Cytosine-5-methyltransferases add aldehydes to DNA. Nat Chem Biol 5:400–402. 10.1038/nchembio.172 [DOI] [PubMed] [Google Scholar]
- Marques SC, Oliveira CR, Pereira CM, Outeiro TF (2011) Epigenetics in neurodegeneration: a new layer of complexity. Prog Neuropsychopharmacol Biol Psychiatry 35:348–355. 10.1016/j.pnpbp.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Martin C, Zhang Y (2005) The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6:838–849. 10.1038/nrm1761 [DOI] [PubMed] [Google Scholar]
- Matsuda T, Irie T, Katsurabayashi S, Hayashi Y, Nagai T, Hamazaki N, Adefuin A, Miura F, Ito T, Kimura H, Shirahige K, Takeda T, Iwasaki K, Imamura T, Nakashima K (2019) Pioneer factor NeuroD1 rearranges transcriptional and epigenetic profiles to execute microglia-neuron conversion. Neuron 101:472–485. 10.1016/j.neuron.2018.12.010 [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN (2001) Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev 11:497–504. 10.1016/s0959-437x(00)00224-0 [DOI] [PubMed] [Google Scholar]
- Merkerova M, Belickova M, Bruchova H (2008) Differential expression of microRNAs in hematopoietic cell lineages. Eur J Haematol 81:304–310. 10.1111/j.1600-0609.2008.01111.x [DOI] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR (2015) 5’ UTR m(6)A promotes cap-independent translation. Cell 163:999–1010. 10.1016/j.cell.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR (2012) Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149:1635–1646. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Coppede F (2009) Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res 667:82–97. 10.1016/j.mrfmmm.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L (2010) Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67:953–966. 10.1016/j.neuron.2010.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K, Merkouri PE, Xiromerisiou G et al (2017) Genomic variants in the FTO gene are associated with sporadic amyotrophic lateral sclerosis in Greek patients. Hum Genomics 11:30. 10.1186/s40246-017-0126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan JM, Yu B, Trent RJ, Pamphlett R (2007) Are metallothionein genes silenced in ALS? Toxicol Lett 168:83–87. 10.1016/j.toxlet.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Narayan P, Ayers DF, Rottman FM, Maroney PA, Nilsen TW (1987) Unequal distribution of N6-methyladenosine in influenza virus mRNAs. Mol Cell Biol 7:1572–1575. 10.1128/mcb.7.4.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D, Ingvarsdottir K, Sterner DE, Bylebyl GR, Dokmanovic M, Dorsey JA, Whelan KA, Krsmanovic M, Lane WS, Meluh PB et al (2006) Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev 20(8):966–976. 10.1101/gad.1404206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317(5837):516–519. 10.1126/science.1143780 [DOI] [PubMed] [Google Scholar]
- Piunti A, Smith ER, Morgan M, Ugarenko M, Khaltyan N, Helmin KA, Ryan CA, Murray DC, Rickels RA, Yilmaz BD, Rendleman EJ, Savas JN, Singer BD, Bulun SE, Shilatifard A (2019) CATACOMB: An endogenous inducible gene that antagonizes H3K27 methylation activity of Polycomb repressive complex 2 via an H3K27M-like mechanism. Sci Adv 5:x2887. 10.1126/sciadv.aax2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S, Bacolla A, Wells RD, Roberts RJ (1999) Recombinant human DNA (cytosine-5) methyltransferase. I. Expression, purification, and comparison of de novo and maintenance methylation. J Biol Chem 274:33002–33010. 10.1074/jbc.274.46.33002 [DOI] [PubMed] [Google Scholar]
- Ragazzini R, Perez-Palacios R, Baymaz IH, Diop S, Ancelin K, Zielinski D, Michaud A, Givelet M, Borsos M, Aflaki S, Legoix P, Jansen P, Servant N, Torres-Padilla ME, Bourc’His D, Fouchet P, Vermeulen M, Margueron R (2019) EZHIP constrains polycomb repressive complex 2 activity in germ cells. Nat Commun 10:3858. 10.1038/s41467-019-11800-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout WR, Eggan K, Jaenisch R (2001) Nuclear cloning and epigenetic reprogramming of the genome. Science 293:1093–1098. 10.1126/science.1063206 [DOI] [PubMed] [Google Scholar]
- Ronan JL, Wu W, Crabtree GR (2013) From neural development to cognition: unexpected roles for chromatin. Nat Rev Genet 14:347–359. 10.1038/nrg3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, He C (2017a) Dynamic RNA modifications in gene expression regulation. Cell 169:1187–1200. 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, He E, Shen B, He C (2017b) YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 10.7554/eLife.31311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J, Hagerty SW, Soh BY, McAlpin SE, Cormier KA, Smith KM, Ferrante RJ (2006) ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington’s disease. Proc Natl Acad Sci U S A 103:19176–19181. 10.1073/pnas.0606373103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanulli S, Justin N, Teissandier A, Ancelin K, Portoso M, Caron M, Michaud A, Lombard B, Da RS, Offer J, Loew D, Servant N, Wassef M, Burlina F, Gamblin SJ, Heard E, Margueron R (2015) Jarid2 methylation via the PRC2 complex regulates H3K27me3 deposition during cell differentiation. Mol Cell 57:769–783. 10.1016/j.molcel.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas JN, Makusky A, Ottosen S, Baillat D, Then F, Krainc D, Shiekhattar R, Markey SP, Tanese N (2008) Huntington’s disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies. Proc Natl Acad Sci U S A 105:10820–10825. 10.1073/pnas.0800658105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scekic-Zahirovic J, Sendscheid O, El OH, Jambeau M, Sun Y, Mersmann S, Wagner M, Dieterle S, Sinniger J, Dirrig-Grosch S, Drenner K, Birling MC, Qiu J, Zhou Y, Li H, Fu XD, Rouaux C, Shelkovnikova T, Witting A, Ludolph AC, Kiefer F, Storkebaum E, Lagier-Tourenne C, Dupuis L (2016) Toxic gain of function from mutant FUS protein is crucial to trigger cell autonomous motor neuron loss. Embo J 35:1077–1097. 10.15252/embj.201592559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, Sanjana NE, Freinkman E, Pacold ME, Satija R, Mikkelsen TS, Hacohen N, Zhang F, Carr SA, Lander ES, Regev A (2014) Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep 8:284–296. 10.1016/j.celrep.2014.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler JS, Dejean A (2003) Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol 4(9):690–699. 10.1038/nrm1200 [DOI] [PubMed] [Google Scholar]
- Shan Y, Zhang Y, Zhao Y, Wang T, Zhang J, Yao J, Ma N, Liang Z, Huang W, Huang K, Zhang T, Su Z, Chen Q, Zhu Y, Wu C, Zhou T, Sun W, Wei Y, Zhang C, Li C, Su S, Liao B, Zhong M, Zhong X, Nie J, Pei D, Pan G (2020) JMJD3 and UTX determine fidelity and lineage specification of human neural progenitor cells. Nat Commun 11:382. 10.1038/s41467-019-14028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Song CX, He C, Zhang Y (2014) Mechanism and function of oxidative reversal of DNA and RNA methylation. Annu Rev Biochem 83:585–614. 10.1146/annurev-biochem-060713-035513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C (2017) YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res 27:315–328. 10.1038/cr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo M (2008) Huntingtin regulates RE1-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF) nuclear trafficking indirectly through a complex with REST/NRSF-interacting LIM domain protein (RILP) and dynactin p150 Glued. J Biol Chem 283:34880–34886. 10.1074/jbc.M804183200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmakova A, Batie M, Druker J, Rocha S (2014) Chromatin and oxygen sensing in the context of JmjC histone demethylases. Biochem J 462:385–395. 10.1042/BJ20140754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Lemmens R, Miskiewicz K, Broom WJ, Hansen VK, van Vught PW, Landers JE, Sapp P, Van Den Bosch L, Knight J, Neale BM, Turner MR, Veldink JH, Ophoff RA, Tripathi VB, Beleza A, Shah MN, Proitsi P, Van Hoecke A, Carmeliet P, Horvitz HR, Leigh PN, Shaw CE, van den Berg LH, Sham PC, Powell JF, Verstreken P, Brown RJ, Robberecht W, Al-Chalabi A (2009) Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum Mol Genet 18:472–481. 10.1093/hmg/ddn375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledz P, Jinek M (2016) Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 10.7554/eLife.18434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JA, Kundracik M, Landfried DA, Barnes VS, Axhemi AA (2005) Genes of the thymidine salvage pathway: thymine-7-hydroxylase from a Rhodotorula glutinis cDNA library and iso-orotate decarboxylase from Neurospora crassa. Biochim Biophys Acta 1723:256–264. 10.1016/j.bbagen.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Smith ZD, Meissner A (2013) DNA methylation: roles in mammalian development. Nat Rev Genet 14:204–220. 10.1038/nrg3354 [DOI] [PubMed] [Google Scholar]
- Son EY, Crabtree GR (2014) The role of BAF (mSWI/SNF) complexes in mammalian neural development. Am J Med Genet C Semin Med Genet 166C:333–349. 10.1002/ajmg.c.31416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T (2014) miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res 5:711–718. 10.1007/s12975-014-0364-8 [DOI] [PubMed] [Google Scholar]
- Soreq L, Guffanti A, Salomonis N, Simchovitz A, Israel Z, Bergman H, Soreq H (2014) Long non-coding RNA and alternative splicing modulations in Parkinson’s leukocytes identified by RNA sequencing. Plos Comput Biol 10:e1003517. 10.1371/journal.pcbi.1003517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staahl BT, Tang J, Wu W, Sun A, Gitler AD, Yoo AS, Crabtree GR (2013) Kinetic analysis of npBAF to nBAF switching reveals exchange of SS18 with CREST and integration with neural developmental pathways. J Neurosci 33:10348–10361. 10.1523/JNEUROSCI.1258-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack EC, Del SS, Luthi-Carter R, Soh BY, Goldstein DR, Matson S, Goodrich S, Markey AL, Cormier K, Hagerty SW, Smith K, Ryu H, Ferrante RJ (2007) Modulation of nucleosome dynamics in Huntington’s disease. Hum Mol Genet 16:1164–1175. 10.1093/hmg/ddm064 [DOI] [PubMed] [Google Scholar]
- Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau KF, Vizcay-Barrena G, Lin WL, Xu YF, Lewis J, Dickson DW, Petrucelli L, Mitchell JC, Shaw CE, Miller CC (2014) ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun 5:3996. 10.1038/ncomms4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchy J, Lee S, Ahmed A, Shea TB (2010) Dietary supplementation with S-adenosyl methionine delays the onset of motor neuron pathology in a murine model of amyotrophic lateral sclerosis. Neuromolecular Med 12(1):86–97. 10.1007/s12017-009-8089-7 [DOI] [PubMed] [Google Scholar]
- Suh N, Blelloch R (2011) Small RNAs in early mammalian development: from gametes to gastrulation. Development 138:1653–1661. 10.1242/dev.056234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M (2005) DNA methylation and demethylation as targets for anticancer therapy. Biochemistry (mosc) 70:533–549. 10.1007/s10541-005-0147-7 [DOI] [PubMed] [Google Scholar]
- Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N et al (2011) Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146(6):1016–1028. 10.1016/j.cell.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani M, Zhao B, Gentil BJ, Minotti S, Marques C, Keith J, Rogaeva E, Zinman L, Rouaux C, Robertson J, Durham HD (2017) Dysregulation of chromatin remodelling complexes in amyotrophic lateral sclerosis. Hum Mol Genet 26:4142–4152. 10.1093/hmg/ddx301 [DOI] [PubMed] [Google Scholar]
- Tokiyoshi E, Watanabe M, Inoue N, Hidaka Y, Iwatani Y (2018) Polymorphisms and expression of genes encoding Argonautes 1 and 2 in autoimmune thyroid diseases. Autoimmunity 51:35–42. 10.1080/08916934.2017.1416468 [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A (2002) An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci U S A 99(26):17095–17100. 10.1073/pnas.262658999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojer P, Reinberg D (2006) Histone lysine demethylases and their impact on epigenetics. Cell 125:213–217. 10.1016/j.cell.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Uckelmann M, Sixma TK (2017) Histone ubiquitination in the DNA damage response. DNA Repair (amst) 56:92–101. 10.1016/j.dnarep.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Valinluck V, Sowers LC (2007) Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res 67:946–950. 10.1158/0008-5472.CAN-06-3123 [DOI] [PubMed] [Google Scholar]
- Valle C, Salvatori I, Gerbino V, Rossi S, Palamiuc L, Rene F, Carri MT (2014) Tissue-specific deregulation of selected HDACs characterizes ALS progression in mouse models: pharmacological characterization of SIRT1 and SIRT2 pathways. Cell Death Dis 5:e1296. 10.1038/cddis.2014.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valor LM (2015) Epigenetic-based therapies in the preclinical and clinical treatment of Huntington’s disease. Int J Biochem Cell Biol 67:45–48. 10.1016/j.biocel.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Vogel CA, Kramar EA, Matheos DP, Havekes R, Hemstedt TJ, Magnan CN, Sakata K, Tran A, Azzawi S, Lopez A, Dang R, Wang W, Trieu B, Tong J, Barrett RM, Post RJ, Baldi P, Abel T, Lynch G, Wood MA (2017) Mutation of neuron-specific chromatin remodeling subunit BAF53b: rescue of plasticity and memory by manipulating actin remodeling. Learn Mem 24:199–209. 10.1101/lm.044602.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schimmelmann M, Feinberg PA, Sullivan JM, Ku SM, Badimon A, Duff MK, Wang Z, Lachmann A, Dewell S, Ma’Ayan A, Han MH, Tarakhovsky A, Schaefer A (2016) Polycomb repressive complex 2 (PRC2) silences genes responsible for neurodegeneration. Nat Neurosci 19:1321–1330. 10.1038/nn.4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Fischhaber PL, Guo C, Tang TS (2014a) Epigenetic modifications as novel therapeutic targets for Huntington’s disease. Epigenomics 6(3):287–297. 10.2217/epi.14.19 [DOI] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y (2004) Role of histone H2A ubiquitination in Polycomb silencing. Nature 431(7010):873–878. 10.1038/nature02985 [DOI] [PubMed] [Google Scholar]
- Wang J, Ho WY, Lim K, Feng J, Tucker-Kellogg G, Nave KA, Ling SC (2018) Cell-autonomous requirement of TDP-43, an ALS/FTD signature protein, for oligodendrocyte survival and myelination. Proc Natl Acad Sci U S A 115:E10941–E10950. 10.1073/pnas.1809821115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C (2014b) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505:117–120. 10.1038/nature12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C (2015) N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 161:1388–1399. 10.1016/j.cell.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Hobartner C, Sloan KE, Bohnsack MT (2017) Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. Embo Rep 18:2004–2014. 10.15252/embr.201744940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CM, Gershowitz A, Moss B (1975) Methylated nucleotides block 5’ terminus of HeLa cell messenger RNA. Cell 4:379–386. 10.1016/0092-8674(75)90158-0 [DOI] [PubMed] [Google Scholar]
- Weng MK, Zimmer B, Poltl D, Broeg MP, Ivanova V, Gaspar JA, Sachinidis A, Wullner U, Waldmann T, Leist M (2012) Extensive transcriptional regulation of chromatin modifiers during human neurodevelopment. PLoS One 7:e36708. 10.1371/journal.pone.0036708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willyard C (2017) An epigenetics gold rush: new controls for gene expression. Nature 542:406–408. 10.1038/542406a [DOI] [PubMed] [Google Scholar]
- Wu H, Zhang Y (2014) Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156:45–68. 10.1016/j.cell.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Zuo X, Deng H, Liu X, Liu L, Ji A (2013) Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res Bull 97:69–80. 10.1016/j.brainresbull.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Wu SC, Zhang Y (2010) Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 11:607–620. 10.1038/nrm2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, Wang X, Ma HL, Huang CM, Yang Y, Huang N, Jiang GB, Wang HL, Zhou Q, Wang XJ, Zhao YL, Yang YG (2016) Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell 61:507–519. 10.1016/j.molcel.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Zhang F, Kang Y, Wang M, Li Y, Xu T, Yang W, Song H, Wu H, Shu Q, Jin P (2018) Fragile X mental retardation protein modulates the stability of its m6A-marked messenger RNA targets. Hum Mol Genet 27:3936–3950. 10.1093/hmg/ddy292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RP, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49:18–29. 10.1016/j.molcel.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YC, Ma J, Wang Z, Li J, Jiang B, Zhou W, Shi X, Wang X, Zhao W, Liu HM (2015) A Systematic review of histone lysine-specific demethylase 1 and Its inhibitors. Med Res Rev 35:1032–1071. 10.1002/med.21350 [DOI] [PubMed] [Google Scholar]
- Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, Fray RG (2008) MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20:1278–1288. 10.1105/tpc.108.058883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB (2015) Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 526:591–594. 10.1038/nature15377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, Rigamonti D, Cattaneo E (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet 35:76–83. 10.1038/ng1219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.