Abstract
Prevention of the nuclear translocation of ANXA1 with Tat-NTS was recently reported to alleviate neuronal injury and protect against cerebral stroke. However, the role that Tat-NTS plays in the occurrence and development of gliomas still needs to be elucidated. Therefore, human glioblastoma (GB) cells were treated with various concentrations of Tat-NTS for 24 h, and cell proliferation, migration and invasion were assessed with CCK-8 and Transwell assays. The nuclear translocation of ANXA1 was evaluated by subcellular extraction and immunofluorescence, and protein expression levels were detected by Western blot analysis. In addition, the activity of MMP-2/9 was measured by gelatin zymography. The results revealed that Tat-NTS significantly inhibited the nuclear translocation of ANXA1 in U87 cells and inhibited the proliferation, migration and invasion of GB cells. Tat-NTS also suppressed cell cycle regulatory proteins and MMP-2/-9 activity and expression. Moreover, Tat-NTS reduced the level of p-p65 NF-κB in U87 cells. These results suggest that the Tat-NTS-induced inhibition of GB cell proliferation, migration and invasion is closely associated with the induction of cell cycle arrest, downregulation of MMP-2/-9 expression and activity and suppression of the NF-κB signaling pathway. Thus, Tat-NTS may be a potential chemotherapeutic agent for the treatment of GB.
Keywords: Tat-NTS, Glioblastoma cells, MMP-2/9, NF-κB
Introduction
Gliomas are common malignant brain tumors in the central nervous system, and glioblastoma (GB) is the most common type of glioma (Xu et al. 2020). The median overall survival time for GB is approximately 14.6 months, and with advanced diagnostic modalities and therapeutic strategies, the 5-year survival rate of patients after diagnosis is less than 9.8% (Fatehi et al. 2018), indicating poor prognosis. Although the tumor can be treated by maximal safe surgical resection combined with radiotherapy, chemotherapy and biological therapy, the tumor can still relapse quickly, which threatens the lives of GB patients. GB is still the most difficult problem in neurosurgery. In essence, it is a polygenic disorder, which affects its occurrence and development. Therefore, exploration of the underlying pathogenesis of GB and the discovery of effective biological products are urgently required.
Annexin-A1 (ANXA1)-mediated signaling has received increasing attention in the development of human tumors (Foo et al. 2019). Recent investigations have indicated that ANXA1 plays an essential role in membrane aggregation, proliferation, apoptosis, phagocytosis and carcinogenesis (Lim and Pervaiz 2007). ANXA1 is highly expressed in hepatocellular carcinoma (Masaki et al. 1996), adenocarcinomas of the esophagus (Kan and Meltzer 2009) and pancreas (Bai et al. 2004), lung adenocarcinoma (Liu et al. 2011) and gliomas (Cheng et al. 2013) but downregulated or lost in adenocarcinoma of prostate (Patton et al. 2005), cervical cancer (Wang et al. 2008), thyroid cancer (Petrella et al. 2006) and head and neck cancer (Garcia Pedrero et al. 2004). Specifically, ANXA1 is overexpressed in GB and plays a role in promoting tumors in GB.
Tat-NTS is a newly discovered and synthesized small molecular peptide that inhibits ANXA1 nuclear translocation. Our previous studies have demonstrated that the amino acid residues from R228 to F237 in the repeat III domain of ANXA1 serve as a unique nuclear translocation signal (NTS) and are necessary for nuclear translocation of ANXA1 (Li et al. 2019). More importantly, we synthesized a cell-penetrating peptide derived by combining the transactivator of transcription (Tat) domain with the NTS sequence. This peptide has a good neuroprotective effect on nerve injury induced by oxygen and glucose deprivation in neurons in vitro and could significantly improve prognosis in a rat model of cerebral ischemia. Moreover, Tat-mediated transduction has become a promising strategy for the treatment of numerous diseases, such as HIV-related diseases (Yuan et al. 2019b) and cerebral stroke (Jin et al. 2020). However, whether Tat-NTS inhibits ANXA1 translocation into the nucleus in GB cells and the regulatory role it plays in the occurrence and development of gliomas remain to be further studied.
In this study, we explored the efficacy and potential functional mechanisms of Tat-NTS in GB cells and elucidated the molecular mechanism by which Tat-NTS affects the migration and invasion of GB to provide an experimental basis for the study of Tat-NTS as a potential small-molecule drug for the treatment of GB. Our results indicated that Tat-NTS significantly inhibited the proliferation, migration and invasion of U87 and U251 human glioma cells, which was partly due to the downregulation of MMP-2/-9 expression and activity and suppression of the NF-κB signaling pathway.
Materials and Methods
Cells and Reagents
Human GB cell lines (U87 and U251) were obtained from the Institute of Biochemistry and Cell Biology of Chinese Academy of Sciences (Shanghai, China), and normal human astrocytes (NHAs, Cat. #CC2565) were obtained from Lonza Group Ltd. (Basel, Switzerland). All cells were cultured in DMEM (Gibco, cat. #11965092, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, cat. #16140089, USA) at 37 °C with a 5% CO2 humidified atmosphere. Cells in logarithmic growth were used for experiments, and the maximum number of passages for cell lines was < 10. The Tat-NTS (NH2-YGRKKRRQRRR-RSFPHLRRVFCONH2) and the Tat-Scr (NH2-YGRKKRRQRRRFRLPSVHRFR-CONH2) peptides were synthesized with 99% purity according to our previous study (Li et al. 2019). Antibodies against MMP-2 (#40994), MMP-9 (#3852), ANXA1 (#3299), p-p65NF-κB (#3031), p65NF-κB (#3034), β-actin (#3700), α-tubulin (#2144), Histone 3 (#4499), P21 (#2947), CDC2 (#28439), Cyclin A2 (#4656), Cyclin B1 (#4138), and Ki67 (#9949) and horseradish peroxidase (HRP)-conjugated secondary antibody (#7074 or #7076) were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-ANXA1 (sc-12740) was purchased from Santa Cruz Biotechnology Company. Alexa Fluor 594-conjugated AffiniPure goat anti-mouse IgG (H + L) was purchased from Jackson ImmunoResearch (West Grove, Pennsylvania, USA). Crystal violet dye (#DC079-1G) was obtained from Beijing Dingguo Biological Co., Ltd., and Transwell chambers (#3422; 8-μm pore size) were purchased from Corning Lifesciences (Kennebunk, ME, USA). Matrigel (#356234) was obtained from BD Biosciences (Franklin Lakes, NJ, USA).
Cell Counting Kit-8 (CCK-8) Assay
Cell viability was assessed by CCK-8 assay. Briefly, U87 cells were plated at a density of 4 × 103 cells in a 96-well plate. After 24 h of culture, the cells were incubated with various concentrations of Tat-NTS for 24 h or fixed concentrations of Tat-NTS for 12, 24, and 48 h before the CCK-8 assay was performed (#CK-04, Dojindo, Kumamoto, Japan). For the CCK-8 assay, 10 μl of reaction solution was added to each well, and cells were incubated for an additional 4 h. The absorbance was then detected by an EnSpire Manager spectrophotometer plate reader (Turku, Singapore) at 450 nm (excitation) and 600 nm (emission).
Immunofluorescence Staining
For immunofluorescence staining, stimulated U87 cells were gently washed with PBS, fixed in a 4% paraformaldehyde solution for 30 min at room temperature, permeabilized with 0.1% Triton X-100 for 30 min and then blocked for an additional 60 min in PBS containing 10% donkey serum. Cells were then incubated with primary antibodies [anti-ANXA1 (1:200, sc-12740)] or [anti-Ki67 (1:400, #9949, CST)] overnight at 4 °C. Cells were then washed three times with PBS containing 0.25% Tween-20 and subsequently incubated with secondary antibodies for 1 h at room temperature. DAPI was used to counterstain nuclei. The cells were examined and immediately imaged using a fluorescence microscope (IX81, Olympus, Tokyo, Japan).
Protein Extraction and Preparation
For the extraction of whole-cell lysates, U87 cells were lysed using RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing a protease inhibitor cocktail (Roche, Basel, Switzerland). The Nuclear and Cytoplasmic Protein Extraction Kit (Thermo Fisher Scientific) was used to prepare nuclear and cytoplasmic extracts following the protocols provided by the manufacturer. The bicinchoninic acid (BCA) protein assay kit (Beyotime) was used to determine the protein concentrations of the extracts.
Western Blot Analysis
Equal amounts of protein (30 μg) from each sample were loaded for Western blot analysis. The proteins were separated by 10% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes (#ISEQ00010, Millipore, Billerica, Massachusetts, USA). The membranes were washed three times with Tris-buffered saline containing 0.1% Tween-20 (TBST) and then blocked with 5% nonfat milk in TBST at room temperature for 1 h. The membranes were then incubated with the corresponding primary antibodies at 1:1000 in TBST at 4 °C overnight. After washing with TBST three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at 1:10,000 in TBST at room temperature for 1 h. After washing with TBST, the protein on the membranes was visualized by an enhanced chemiluminescence kit (#RPN2232, Amersham, GE Health, UK) and quantitated by using ImageJ 1.4.3 (National Institutes of Health, Bethesda, MD, USA) after normalization to β-actin, α-tubulin or histone H3 expression.
Coimmunoprecipitation (Co-IP) Analysis
Cell lysates (500 μg of protein) were prepared in 1 × IP buffer (Beyotime Institute of Biotechnology) containing protease inhibitor cocktail. The samples were precleared for 1 h with 10 μl of protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology) and then centrifuged to remove proteins that adhered nonspecifically to the protein A/G beads. The supernatant was incubated with 2 μg of anti-ANXA1 antibody or control IgG with gentle rotation overnight at 4 °C. Subsequently, 20 μl of protein A/G PLUS agarose beads (Santa Cruz; sc-2003) was added, and the reaction mixture was incubated at 4 °C for 4 h. The precipitates were then washed three times with wash buffer, denatured in 30 μl of loading buffer, heated at 95 °C for 5 min and subjected to Western blot analysis.
Migration and Invasion Assay
First, the Transwell chamber was placed in DMEM + 0.1% bovine serum albumin (BSA) for hydration in a 5% CO2 incubator at 37 °C for 2 h. The GB cells or NHAs were harvested and counted, and the cells were then inoculated in a chamber at a density of 5 × 104 cells/well. The medium in the upper chamber was DMEM + 0.1% BSA, and the medium in the lower chamber was DMEM + 15% FBS combined with 20 μM Tat-NTS or Tat-Scr. The chamber was removed after 24 h of incubation, and cells on the chamber membrane were fixed with 4% paraformaldehyde and then stained with 0.1% crystal violet. Cells that did not pass through the membrane were wiped with a cotton swab, and the remaining cells were imaged under high magnification (200x). Five random fields of each well were observed, and the results were analyzed. For invasion assays, the chambers were coated with 90 μl of 0.8 mg/ml Matrigel, and the subsequent steps were similar to the migration assays.
Gelatin Zymography
To measure MMP-2 and MMP-9 activity in U87 MG cells, gelatin zymography was performed as described previously (Morioka et al. 2019). Briefly, after incubation with various concentrations (2, 5, 10, 20 and 40 μM) of Tat-NTS or Tat-Src (20 μM) for 24 h, the supernatants of GB cells were collected. Loading buffer (125 mM Tris–HCl, pH 6.8; 1% glycerol; 2% SDS; and 0.01% bromophenol blue) was added to the supernatants without heating or reduction, and the samples were separated by electrophoresis on an 8% SDS–polyacrylamide gel containing 0.1% w/v gelatin. After separation, the gels were gently washed three times with distilled water containing 2.0% Triton X-100 for 1 h to remove SDS. The gels were further incubated with substrate buffer (50 mM Tris–HCl containing 10 mM CaCl2 and 0.02% NaN3, pH 7.6) at 37 °C overnight, followed by staining with Coomassie Brilliant Blue R-250 for 1 h and decolorization in 20% methanol and 10% acetic acid until clear bands appeared. The density was measured with ImageJ software (Fuji Film, Tokyo, Japan).
Statistical Analysis
GraphPad Prism 5 software was used for statistical analysis. All data are expressed as the mean ± standard deviation. One-way analysis of variance was performed to analyze these data, and multiple comparisons between groups were carried out by Bonferroni’s multiple comparison method. P < 0.05 was considered to indicate statistical significance.
Results
Tat-NTS Inhibits the Proliferation of GB Cells
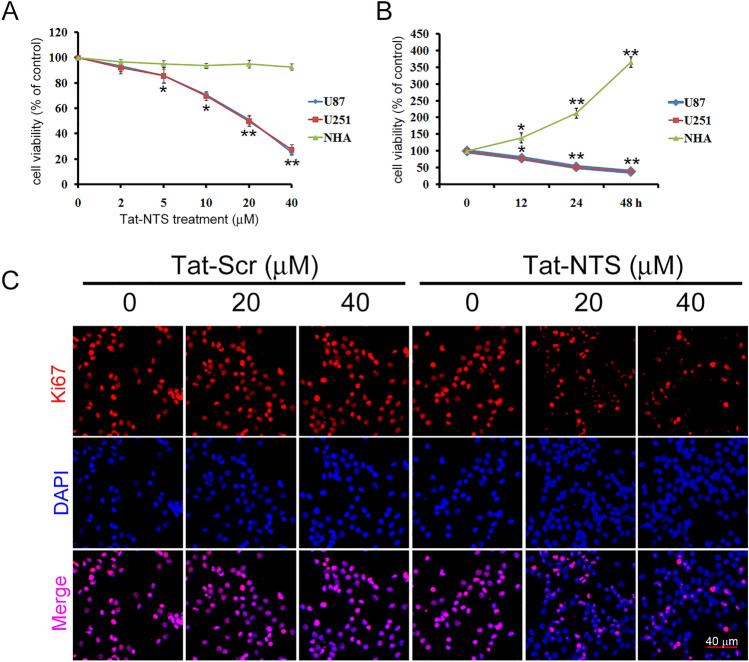
To investigate the influence of Tat-NTS on cell proliferation, cell viability was detected by CCK-8 assay after human GB U87 and U251 cells were treated with vehicle (PBS) or Tat-NTS at different concentrations (2, 5, 10, 20 and 40 μM) for 24 h. The results showed that the proliferation of U87 and U251 cells was inhibited by Tat-NTS in a concentration-dependent manner. Treatment with 10 μM, 20 μM and 40 μM Tat-NTS significantly inhibited cell proliferation (Fig. 1A, P < 0.01), and the IC50 values in U87 and U251 cells treated for after h of treatment were 28.20 μM and 30.06 μM, respectively. Changes in cell viability were then observed after treatment with 20 μM Tat-NTS for different durations. As shown in Fig. 1B, Tat-NTS significantly decreased cell viability after 12 h, 24 h and 48 h. For subsequent experiments, 24 h was selected as the treatment duration. Moreover, we used Tat-NTS treatment to inhibit cellular proliferation in NHAs. The CCK-8 assay revealed that Tat-NTS had little effect on the proliferation of NHAs. Additionally, immunofluorescence staining for Ki67, a proliferation marker, was utilized to examine the effect of Tat-NTS on U87 cell proliferation. Compared to Tat-Scr treatment, Tat-NTS treatment not only inhibited cell viability but also reduced Ki67 expression in the nuclei of U87 cells (Fig. 1C).
Fig. 1.
Effect of Tat-NTS on GB cell viability. Human U87 and U251 GB cells and normal human astrocytes (NHAs) were exposed to various concentrations of Tat-NTS (2, 5, 10, 20 and 40 μM) or PBS (vehicle) for 24 h. Cells were also exposed to 20 μM Tat-NTS for 12, 24 and 48 h. Cell viability was then analyzed by CCK-8 assay. Tat-NTS inhibited GB cell proliferation in a dose-dependent manner (A) and time-dependent manner (B) but had no impact on NHAs. Mean ± SD (n = 5). *P < 0.05 and **P < 0.01 vs 0 μmol/L or 0 h. (C) Expression of the proliferation marker Ki-67 was evaluated using immunofluorescence staining for Ki67 (red) and DAPI (blue). Scale bar, 40 μM
TAT-NTS Specifically Inhibits the Nuclear Translocation of ANXA1
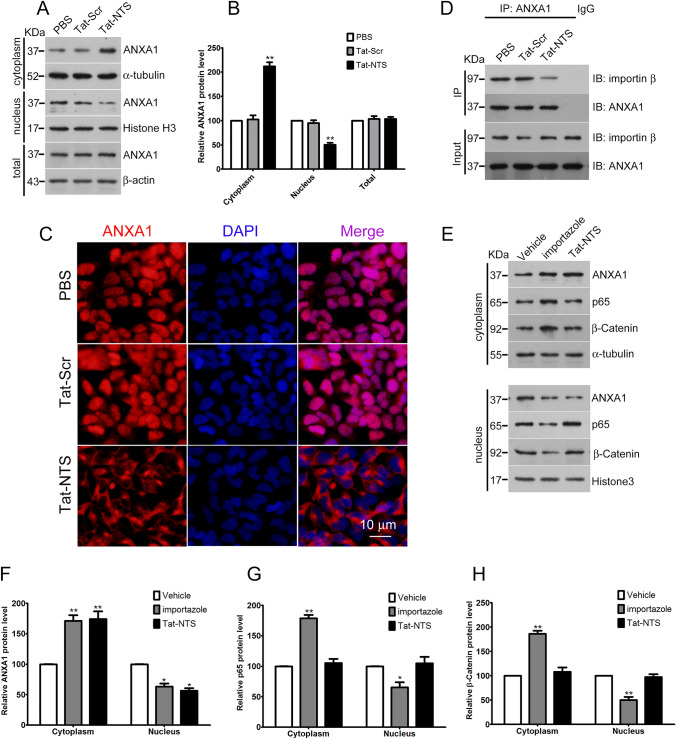
After Tat-NTS treatment for 24 h, the total protein, cytoplasmic components and nuclear components were extracted from the GB cells, and the expression of ANXA1 in each treatment group was detected by Western blot analysis. Compared to that in the PBS group and Tat-Scr group, the amount of nuclear ANXA1 in the Tat-NTS group was significantly reduced, while the amount of cytoplasmic ANXA1 was elevated. Tat-NTS significantly inhibited the incorporation of ANXA1 into the nucleus but had no effect on the total expression of ANXA1 (Fig. 2A, B). Furthermore, immunofluorescence staining analysis also showed that Tat-NTS significantly inhibited ANXA1 nuclear translocation compared to that upon treatment with Tat-Scr or PBS (Fig. 2C). Our previous study demonstrated that Tat-NTS disturbs the interaction between ANXA1 and importin β in neurons. Because it remains unknown whether Tat-NTS exerts a similar function in GB cells, we performed Co-IP experiments to test the efficacy of Tat-NTS in blocking the ANXA1-importin β interaction. As expected, Tat-NTS also markedly decreased the interaction between ANXA1 and importin β (Fig. 2D).
Fig. 2.
Tat-NTS inhibits ANXA1 translocation to the nucleus in U87 cells. U87 cells were treated with PBS, Tat-Scr or Tat-NTS (20 μM) for 24 h. Nuclear and cytosolic protein extracts were then subjected to Western blot analysis with specific antibodies. (A) Representative images of Western blot analysis for ANXA1 levels in the cytoplasmic and nuclear extracts. α-tubulin and histone H3 were used as cytoplasmic and nuclear internal controls, respectively, while β-actin was used as a loading control of total proteins. (B) The relative level of ANXA1 protein was quantified by densitometric analysis. Data are represented as the mean ± SD from three independent experiments. **P < 0.01 vs the Tat-Scr control. (C) Representative immunofluorescence staining results of ANXA1 (red) and nuclei (blue) showing the subcellular distribution of endogenous ANXA1 in U87 cells. Scale bar = 10 μm. (D) Representative Co-IP results showing the interaction of ANXA1 with importin β in GB cells treated with Tat-NTS or Tat-Scr peptide. (E) Representative images following Western blot analysis for ANXA1, p65 NF-κB, and β-catenin in cytoplasmic and nuclear extracts of U87 cells treated with vehicle, importazole or Tat-NTS peptide. α-Tubulin and histone H3 served as cytoplasmic and nuclear loading controls, respectively. (F, G, H) Quantification of the relative protein levels of ANXA1, p65 NF-κB and β-catenin. The intensity of the bands was quantified by densitometric analysis. *P < 0.05 and **P < 0.01 vs the Vehicle group; all data are presented as the mean ± SD from three independent experiments
To examine the specificity of the effect of the Tat-NTS peptide on ANXA1 translocation, we explored the effects of the Tat-NTS peptide and importazole on the nuclear translocation of p65 NF-κB, β-catenin and ANXA1. p65 NF-κB and β-catenin are nucleocytoplasmic shuttling proteins that participate in cell proliferation and apoptosis in an importin β-dependent manner in cancer cells (Lu et al. 2016; Yan et al. 2015). As shown in Fig. 2E–H, importazole (8 μM), a small-molecule inhibitor of the nuclear transport receptor importin-β, significantly decreased the nuclear translocation of p65 NF-κB, β-catenin and ANXA1 as expected. However, Tat-NTS significantly decreased the level of ANXA1 protein in the nuclear fraction but had no observed effect on the subcellular localization of p65 NF-κB and β-catenin. These data suggest that Tat-NTS is a specific inhibitor that blocks ANXA1 nuclear migration but not the nuclear migration of other proteins that are also translocated by this same importin.
TAT-NTS Inhibits the Migration and Invasion of GB Cells
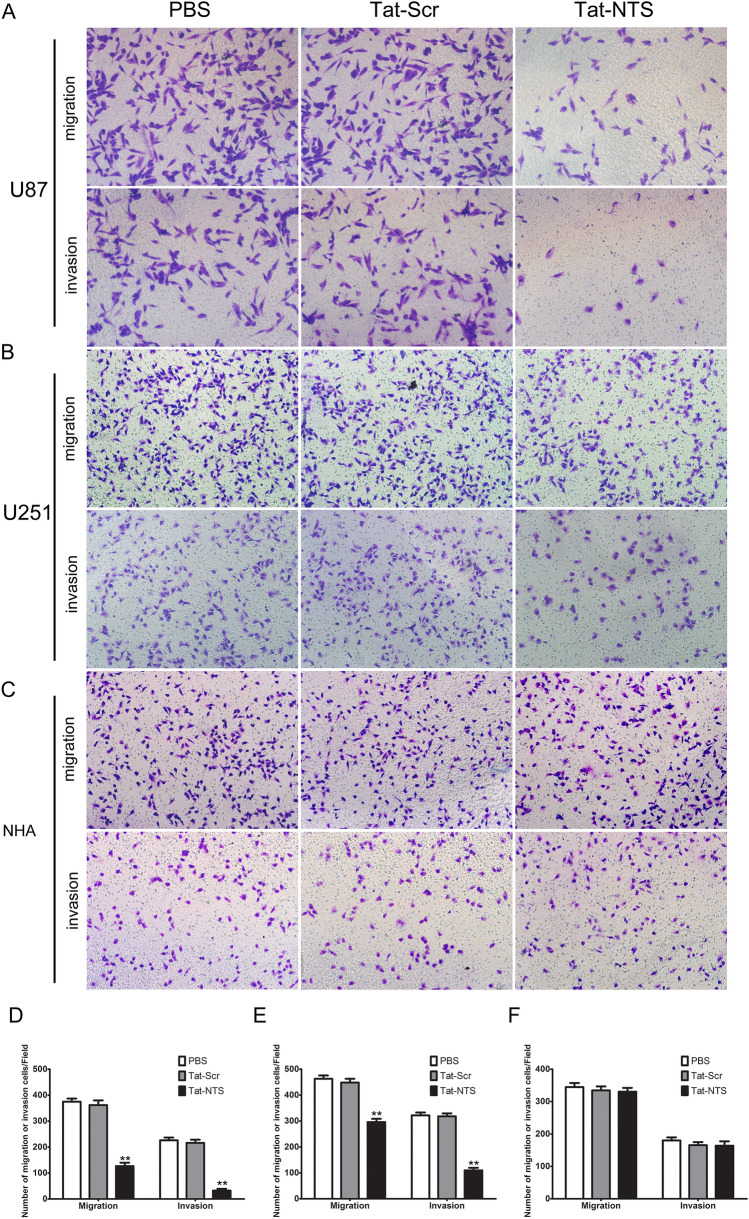
We next explored the effects of Tat-NTS on U87 and U251 cell migration and invasion. Transwell assays were used to detect the migration ability of cells in each group. The results showed that the numbers of penetrating cells in the PBS group and Scr group were similar, and there was no significant difference between these two groups (P > 0.05). However, the number of migrated Tat-NTS-treated U87 and U251 cells were significantly reduced compared to that of the Tat-Scr-treated group (Fig. 3A, B, P < 0.05). In addition, the number of invaded U87 and U251 cells in the Tat-NTS group was also markedly decreased compared to that in the Tat-Scr-treated group, while the numbers of invaded cells in the PBS group and Tat-Scr group were not significantly different (Fig. 3A, C). Thus, these results demonstrated that Tat-NTS inhibits the migratory and invasive abilities of GB cells. Furthermore, to determine whether the effects of Tat-NTS on migration and invasion occur selectively in GB cells, we also treated NHAs for 24 h (Fig. 3C). The results showed that Tat-NTS did not decrease the migration or invasion of NHAs (Fig. 3F), implying that it is safe and a specific inhibitor of GB cells.
Fig. 3.
Effect of Tat-NTS on the migration and invasion capacity of GB cells in vitro. (A, B, C) U87 GB cells, U251 GB cells and NHAs were treated with PBS, Tat-Scr (20 μM) or Tat-NTS (20 μM) for 24 h, and cell migration was then determined with a Transwell system. Migrated and invaded cells on the bottom surface of the membrane were fixed and stained with crystal violet. Original magnification: 200x. (D, E, F) Quantification of migrating and invading cells corresponding to (A, B, C). The migrating and invading cells were counted under a microscope in five random fields. Data are presented as the mean ± SD from three independent experiments. **P < 0.01 vs the Tat-Src group
Effect of Tat-NTS on Cyclins and the NF-κB Pathway
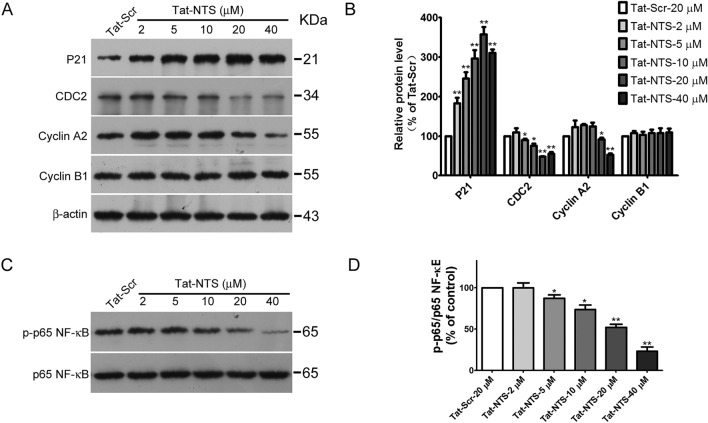
To further explore the mechanisms of Tat-NTS inhibition on GB cell proliferation, we detected the levels of cell cycle regulatory proteins by Western blotting. Previous studies have demonstrated that the NF-κB pathway plays an essential role in GB cell migration and invasion (Wang et al. 2019; Kına et al. 2019). To further investigate whether the inhibitory effect of Tat-NTS on migration and invasion is attributed to NF-κB p65 downregulation, protein extracts were subjected to Western blot analysis. The results showed that Tat-NTS inhibited the expression of Cyclin A2 and CDC2 in GB cells, but the expression of p21 protein was upregulated with increasing Tat-NTS concentration. However, Tat-NTS had no impact on the expression of Cyclin B1 (Fig. 4A, B). Western blot results showed that the phosphorylation level of NF-κB was significantly inhibited by treatment with Tat-NTS for 24 h in a dose-dependent manner, but there was no effect on total NF-κB, suggesting that Tat-NTS suppresses activation of the NF-κB signaling pathway in human GB cells (Fig. 4C, D).
Fig. 4.
Tat-NTS induces G2/M cell cycle arrest and inhibits activation of the NF-κB signaling pathway in GB cells. U87 cells were treated with the indicated concentrations of Tat-NTS (2, 5, 10, 20 and 40 μM) for 24 h, and the expression levels of P21, CDC2, Cyclin A2, CyclinB1, and phosphorylated p65NF-κB in total-cell lysates were determined by Western blot analysis. (A, C) Representative protein blots evaluated by Western blot analysis. (B, D) Densities of the protein bands were normalized to those of β-actin and p65 NF-κB. Data are shown as the mean ± SD from three independent experiments. *P < 0.05 and **P < 0.01 vs the Tat-Scr group
Tat-NTS Inhibits the Expression and Activity of MMP-2 and MMP-9
Matrix metalloproteinases (MMPs) are involved in the degradation of extracellular matrix and components of the basement membrane (King 2016). Furthermore, previous studies have demonstrated that MMP-2 and MMP-9 play a vital role in the migration and invasion of glioma cells (Aroui et al. 2016; Pagliara et al. 2014). Moreover, several reports have indicated that the NF-κB pathway affects the transcription of MMP-2/-9 in cancer cells (Tang et al. 2016; Fong et al. 2010). Therefore, we investigated whether Tat-NTS can regulate the expression and activity of MMP-2 and MMP-9. Western blot analysis showed that Tat-NTS downregulated the expression of MMP-2 and MMP-9 in a concentration-dependent manner (Fig. 5A, B). Gelatinase analysis showed that Tat-NTS inhibited the activity of MMP-2 and MMP-9 released into the cultured medium from GB cells in a similar manner (Fig. 5C, D).
Fig. 5.
Tat-NTS inhibits the expression and activity of MMP-2 and MMP-9. U87 cells were treated with Scr control (20 μM) or different concentrations of Tat-NTS (2, 5, 10, 20 and 40 μM) for 24 h. Both cell lysates and conditioned media were collected. Cell lysates were analyzed by Western blotting, and conditioned media were analyzed by gelatin zymography assay. (A) Representative images following Western blot analysis for MMP-2 and MMP-9 expression in U87 cells. (B) Quantification of relative protein levels of MMP-2 and MMP-9 in U87 cells. (C) Representative images of gelatin zymography of MMP-2 and MMP-9. (D) Quantification of the relative bands of MMP-2 and MMP-9 in U87 cells (n = 3). *P < 0.05 and **P < 0.01 vs the Tat-Scr group
Discussion
Gliomas are common malignant tumors in the central nervous system (Uhm and Porter 2017) characterized by invasive growth, a high recurrence rate, a high mortality rate and a low cure rate. GB is the most common glioma, and the median overall survival time of GB is approximately 14.6 months. Under standard treatment and diagnosis, the 5-year survival rate is less than 10% (Fatehi et al. 2018). At present, the main treatment methods for GB include surgical resection of the tumor combined with radiotherapy, chemical therapy and biotherapy. GB therapies have not changed in 10 years, resulting in poor prognosis and no significant improvement in median patient survival time. Therefore, the need to identify new agents to assist the surgical treatment of GB is urgent. The NF-κB signaling pathway is widespread in cells and plays an important physiological role by regulating the cell cycle and transcription of genes involved in invasion and metastasis. Previous studies have indicated that the NF-κB signaling pathway is overactivated in gliomas, suggesting that this pathway is closely related to the occurrence and development of gliomas (Ius et al. 2018; Yu et al. 2018). Therefore, the NF-κB pathway may become a promising target candidate for the treatment of gliomas.
ANXA1 is a calcium-dependent and phospholipid-binding protein that is widely involved in anti-inflammatory processes, signal transduction, cell differentiation, cycle regulation, tumor invasion, metastasis and other biological processes (Shao et al. 2019). ANXA1 is composed of a functional N-terminal region and a C-terminal core region. The C-terminal core region consists of four homologous repeats of 70 amino acid residues. In a previous study, we found that the 228–237 amino acid residues in the repeat III domain constitute a nuclear translocation signal (Li et al. 2019). Although ANXA1 plays an important role in promoting or inhibiting cancer in different tumors, its role in breast cancer is still controversial (Tu et al. 2017). A recent study showed that ANXA1 promotes the occurrence and progression of gliomas (Tadei et al. 2018). Moreover, Tadei et al. also showed that ANXA1 protein immunostaining increases as the malignancy grade increases. Moreover, this study observed that ANXA1 overexpression in astrocytomas of all grades associated with increased expression levels of MMPs, especially MMP-9, which promoted the migration and invasion ability of GB cells, all these data indicated that ANXA1 may serve as a diagnostic or prognostic marker for GB. However, another previous study showed that the expression of ANXA1 is not associated with patient survival. Moreover, the number of tumor cells showing nuclear ANXA1 expression increased with the degree of malignancy in astrocytomas, but no association with nuclear ANXA1 and survival was observed. Collectively, these data indicate that the upregulation and nuclear translocation of ANXA1 play a crucial role in the development and/or progression of gliomas but have no association with patient survival.
Transactivator of transcription (Tat) translocates numerous proteins, peptides, plasmid DNA, siRNA, nanoparticles and other substances into cells in a short time with high efficiency (Guidotti et al. 2017; Wadia et al. 2004). Among Tat proteins, human immunodeficiency virus-1 (HIV-1) Tat has been widely studied (Yuan et al. 2019b). HIV-I Tat not only penetrates the cell membrane but also penetrates the blood–brain barrier, which is conducive to in vivo transport. Tat-NTS, as a newly discovered and synthesized inhibitor of ANXA1 nuclear translocation, is mainly composed of transactivator Tat and amino acid residues of the nuclear translocation signal in the repeat III domain of ANXA1 (Li et al. 2019). Our results showed that Tat-NTS inhibits the proliferation, migration and invasion of GB cells by inhibiting the nuclear translocation of ANXA1 and the phosphorylation of NF-κB protein. However, the effect of Tat-NTS treatment on tumor growth in an animal model remains to be determined in future studies.
The aim of this study was to investigate the effects of Tat-NTS on the proliferation, invasion and migration of U87 GB cells. In GB cells, Tat-NTS inhibited the nuclear translocation of ANXA1, inhibited cellular proliferation, caused G2/M phase arrest and inhibited the expression and activity of MMPs by inhibiting the phosphorylation of NF-κB, thus inhibiting the migration and invasion of GB cells. Recent studies have shown that the effect of ANXA1 on the invasion of GB cells is related to the synthesis level of MMPs (Tadei et al. 2018). The present study showed that the protein levels and activities of MMP-2 and MMP-9 in cells treated with Tat-NTS were decreased, suggesting that Tat-NTS can inhibit the synthesis of MMPs by blocking the entry of ANXA1 into the nucleus, thus inhibiting the migration and invasion of GB cells. These findings are similar to previous studies showing that silencing ANXA1 inhibited the invasion of breast cancer cells through the downregulation of MMP-9 (Kang et al. 2012). A change in cell cycle distribution is a basic biological feature of malignant tumors, and disorder of the cell cycle is the essential cause of uncontrolled proliferation (Yuan et al. 2019a). Therefore, the regulation of cell cycle progression has become an important means of tumor treatment and apoptosis or necrosis induction. Cyclins, the main regulatory proteins in the eukaryotic cell cycle, regulate the cell cycle by binding cyclin-dependent kinases (CDKs) to form cyclin-CDK complexes (Malumbres and Barbacid 2009). When tumor cells are treated with chemotherapeutic drugs or radiation, G1/S or G2/M arrest usually occurs to repair damaged DNA. CDC2/cyclin B1 is an important factor that regulates the cell transition from G2 phase to M phase. The dephosphorylation of CDC2 leads to the enhancement of cdc25 protein phosphatase activity and the initiation of mitosis (Butz et al. 2017). In the present study, after treatment with Tat-NTS, the protein level of CDC2 was downregulated, and the activity of the CDC2/cyclin B1 complex was decreased, resulting in G2 phase arrest.
In summary, the present study shows that Tat-NTS significantly inhibits the nuclear translocation of ANXA1, thus inducing cell cycle arrest and inhibiting cell invasion abilities. Due to its small molecular weight and ability to readily cross the blood–brain barrier, Tat-NTS provides a new strategy for the clinical treatment of GB.
Author Contributions
Zhenzhao Luo, Li Liu and Xing Li designed the experiments. Zhenzhao Luo performed the in vitro experiments. Zhenzhao Luo, Li Liu and Weiqun Chen analyzed the data. Zhongxin Lu and Zhenzhao Luo conceived of and designed the study, and participated in the data analysis and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
Youth Fund of Health and Family Planning Commission of Wuhan Municipality (Grant No. WX17Q10) and Guidance Project of Wuhan Municipal Health Commission (Grant No. WX21Z22).
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research Involving Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
The original online version of this article was revised: the figure legends of all the figures has been corrected.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenzhao Luo, Li Liu and Xing Li have contributed equally to this work.
Change history
9/3/2021
A Correction to this paper has been published: 10.1007/s10571-021-01143-x
References
- Aroui S, Aouey B, Chtourou Y, Meunier AC, Fetoui H, Kenani A (2016) Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem Biol Interact 244:195–203. 10.1016/j.cbi.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Bai XF, Ni XG, Zhao P, Liu SM, Wang HX, Guo B, Zhou LP, Liu F, Zhang JS, Wang K, Xie YQ, Shao YF, Zhao XH (2004) Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol 10(10):1466–1470. 10.3748/wjg.v10.i10.1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz H, Németh K, Czenke D, Likó I, Czirják S, Zivkovic V, Baghy K, Korbonits M, Kovalszky I, Igaz P, Rácz K, Patócs A (2017) Systematic investigation of expression of G2/M transition genes reveals CDC25 alteration in nonfunctioning pituitary adenomas. Pathol Oncol Res 23(3):633–641. 10.1007/s12253-016-0163-5 [DOI] [PubMed] [Google Scholar]
- Cheng SX, Tu Y, Zhang S (2013) FoxM1 promotes glioma cells progression by up-regulating Anxa1 expression. PLoS ONE 8(8):e72376. 10.1371/journal.pone.0072376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehi M, Hunt C, Ma R, Toyota BD (2018) Persistent disparities in survival for patients with glioblastoma. World Neurosurg 120:e511–e516. 10.1016/j.wneu.2018.08.114 [DOI] [PubMed] [Google Scholar]
- Fong Y, Shen KH, Chiang TA, Shih YW (2010) Acacetin inhibits TPA-induced MMP-2 and u-PA expressions of human lung cancer cells through inactivating JNK signaling pathway and reducing binding activities of NF-kappaB and AP-1. J Food Sci 75(1):H30-38. 10.1111/j.1750-3841.2009.01438.x [DOI] [PubMed] [Google Scholar]
- Foo SL, Yap G, Cui J, Lim LHK (2019) Annexin-A1—a blessing or a curse in cancer? Trends Mol Med 25(4):315–327. 10.1016/j.molmed.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Garcia Pedrero JM, Fernandez MP, Morgan RO, Herrero Zapatero A, Gonzalez MV, Suarez Nieto C, Rodrigo JP (2004) Annexin A1 down-regulation in head and neck cancer is associated with epithelial differentiation status. Am J Pathol 164(1):73–79. 10.1016/s0002-9440(10)63098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G, Brambilla L, Rossi D (2017) Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci 38(4):406–424. 10.1016/j.tips.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Ius T, Ciani Y, Ruaro ME, Isola M, Sorrentino M, Bulfoni M, Candotti V, Correcig C, Bourkoula E, Manini I, Pegolo E, Mangoni D, Marzinotto S, Radovic S, Toffoletto B, Caponnetto F, Zanello A, Mariuzzi L, Di Loreto C, Beltrami AP, Piazza S, Skrap M, Cesselli D (2018) An NF-κB signature predicts low-grade glioma prognosis: a precision medicine approach based on patient-derived stem cells. Neuro Oncol 20(6):776–787. 10.1093/neuonc/nox234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Li D, Lin MH, Li L, Harrich D (2020) Tat-based therapies as an adjuvant for an HIV-1 functional cure. Viruses 12(4):415. 10.3390/v12040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan T, Meltzer SJ (2009) MicroRNAs in Barrett’s esophagus and esophageal adenocarcinoma. Curr Opin Pharmacol 9(6):727–732. 10.1016/j.coph.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Ko J, Jang SW (2012) The role of annexin A1 in expression of matrix metalloproteinase-9 and invasion of breast cancer cells. Biochem Biophys Res Commun 423(1):188–194. 10.1016/j.bbrc.2012.05.114 [DOI] [PubMed] [Google Scholar]
- Kına I, Sultuybek GK, Soydas T, Yenmis G, Biceroglu H, Dirican A, Uzan M, Ulutin T (2019) Variations in toll-like receptor and nuclear factor-kappa B genes and the risk of glioma. Br J Neurosurg 33(2):165–170. 10.1080/02688697.2018.1540764 [DOI] [PubMed] [Google Scholar]
- King SE (2016) Matrix metalloproteinases: new directions toward inhibition in the fight against cancers. Future Med Chem 8(3):297–309. 10.4155/fmc.15.184 [DOI] [PubMed] [Google Scholar]
- Li X, Zheng L, Xia Q, Liu L, Mao M, Zhou H, Zhao Y, Shi J (2019) A novel cell-penetrating peptide protects against neuron apoptosis after cerebral ischemia by inhibiting the nuclear translocation of annexin A1. Cell Death Differ 26(2):260–275. 10.1038/s41418-018-0116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LH, Pervaiz S (2007) Annexin 1: the new face of an old molecule. FASEB J 21(4):968–975. 10.1096/fj.06-7464rev [DOI] [PubMed] [Google Scholar]
- Liu YF, Zhang PF, Li MY, Li QQ, Chen ZC (2011) Identification of annexin A1 as a proinvasive and prognostic factor for lung adenocarcinoma. Clin Exp Metastasis 28(5):413–425. 10.1007/s10585-011-9380-1 [DOI] [PubMed] [Google Scholar]
- Lu T, Bao Z, Wang Y, Yang L, Lu B, Yan K, Wang S, Wei H, Zhang Z, Cui G (2016) Karyopherinβ1 regulates proliferation of human glioma cells via Wnt/β-catenin pathway. Biochem Biophys Res Commun 478(3):1189–1197. 10.1016/j.bbrc.2016.08.093 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9(3):153–166. 10.1038/nrc2602 [DOI] [PubMed] [Google Scholar]
- Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M, Maeba T, Sogawa K, Konishi R, Taniguchi K, Hatanaka Y, Hatase O, Nishioka M (1996) Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology 24(1):72–81. 10.1053/jhep.1996.v24.pm0008707286 [DOI] [PubMed] [Google Scholar]
- Morioka N, Kodama K, Tomori M, Yoshikawa K, Saeki M, Nakamura Y, Zhang FF, Hisaoka-Nakashima K, Nakata Y (2019) Stimulation of nuclear receptor REV-ERBs suppresses production of pronociceptive molecules in cultured spinal astrocytes and ameliorates mechanical hypersensitivity of inflammatory and neuropathic pain of mice. Brain Behav Immun 78:116–130. 10.1016/j.bbi.2019.01.014 [DOI] [PubMed] [Google Scholar]
- Pagliara V, Adornetto A, Mammì M, Masullo M, Sarnataro D, Pietropaolo C, Arcone R (2014) Protease nexin-1 affects the migration and invasion of C6 glioma cells through the regulation of urokinase plasminogen activator and matrix metalloproteinase-9/2. Biochim Biophys Acta 1843:2631–2644. 10.1016/j.bbamcr.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Patton KT, Chen HM, Joseph L, Yang XJ (2005) Decreased annexin I expression in prostatic adenocarcinoma and in high-grade prostatic intraepithelial neoplasia. Histopathology 47(6):597–601. 10.1111/j.1365-2559.2005.02300.x [DOI] [PubMed] [Google Scholar]
- Petrella A, Festa M, Ercolino SF, Zerilli M, Stassi G, Solito E, Parente L (2006) Annexin-1 downregulation in thyroid cancer correlates to the degree of tumor differentiation. Cancer Biol Ther 5(6):643–647. 10.4161/cbt.5.6.2700 [DOI] [PubMed] [Google Scholar]
- Shao G, Zhou H, Zhang Q, Jin Y, Fu C (2019) Advancements of annexin A1 in inflammation and tumorigenesis. Onco Targets Ther 12:3245–3254. 10.2147/ott.s202271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadei MB, Mayorquim MV, de Souza CB, de Souza CS, Possebon L, Souza HR, Iyomasa-Pilon MM, Geromel MR, Girol AP (2018) Expression of the annexin A1 and its correlation with matrix metalloproteinases and the receptor for formylated peptide-2 in diffuse astrocytic tumors. Ann Diagn Pathol 37:62–66. 10.1016/j.anndiagpath.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Tang Y, Lv P, Sun Z, Han L, Zhou W (2016) 14–3-3β Promotes migration and invasion of human hepatocellular carcinoma cells by modulating expression of MMP2 and MMP9 through PI3K/Akt/NF-κB pathway. PLoS ONE 11(1):e0146070. 10.1371/journal.pone.0146070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Johnstone CN, Stewart AG (2017) Annexin A1 influences in breast cancer: controversies on contributions to tumour, host and immunoediting processes. Pharmacol Res 119:278–288. 10.1016/j.phrs.2017.02.011 [DOI] [PubMed] [Google Scholar]
- Uhm JH, Porter AB (2017) Treatment of glioma in the 21st century: an exciting decade of postsurgical treatment advances in the molecular era. Mayo Clin Proc 92(6):995–1004. 10.1016/j.mayocp.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF (2004) Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med 10(3):310–315. 10.1038/nm996 [DOI] [PubMed] [Google Scholar]
- Wang LD, Yang YH, Liu Y, Song HT, Zhang LY, Li PL (2008) Decreased expression of annexin A1 during the progression of cervical neoplasia. J Int Med Res 36(4):665–672. 10.1177/147323000803600407 [DOI] [PubMed] [Google Scholar]
- Wang B, Zhao CH, Sun G, Zhang ZW, Qian BM, Zhu YF, Cai MY, Pandey S, Zhao D, Wang YW, Qiu W, Shi L (2019) IL-17 induces the proliferation and migration of glioma cells through the activation of PI3K/Akt1/NF-κB-p65. Cancer Lett 447:93–104. 10.1016/j.canlet.2019.01.008 [DOI] [PubMed] [Google Scholar]
- Xu S, Tang L, Li X, Fan F, Liu Z (2020) Immunotherapy for glioma: current management and future application. Cancer Lett 476:1–12. 10.1016/j.canlet.2020.02.002 [DOI] [PubMed] [Google Scholar]
- Yan W, Li R, He J, Du J, Hou J (2015) Importin β1 mediates nuclear factor-κB signal transduction into the nuclei of myeloma cells and affects their proliferation and apoptosis. Cell Signal 27(4):851–859. 10.1016/j.cellsig.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Yu Z, Chen Y, Wang S, Li P, Zhou G, Yuan Y (2018) Inhibition of NF-κB results in anti-glioma activity and reduces temozolomide-induced chemoresistance by down-regulating MGMT gene expression. Cancer Lett 428:77–89. 10.1016/j.canlet.2018.04.033 [DOI] [PubMed] [Google Scholar]
- Yuan R, Liu Q, Segeren HA, Yuniati L, Guardavaccaro D, Lebbink RJ, Westendorp B, de Bruin A (2019a) Cyclin F-dependent degradation of E2F7 is critical for DNA repair and G2-phase progression. EMBO J 38(20):e101430. 10.15252/embj.2018101430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zhou C, Yang Q, Ma S, Wang X, Guo X, Ding Y, Tang J, Zeng Y, Li D (2019b) HIV-1 Tat protein inhibits the hematopoietic support function of human bone marrow mesenchymal stem cells. Virus Res 273:197756. 10.1016/j.virusres.2019.197756 [DOI] [PubMed] [Google Scholar]