Abstract
The effect of a novel bovine mastitis trivalent vaccine, containing Staphylococcus aureus capsular polysaccharide type 5 (T5), 8 (T8), and 336 (T336), on lymphocyte subpopulations, antibody production, and neutrophil phagocytosis was evaluated. Twenty pregnant heifers were immunized with either the trivalent alone, trivalent emulsified in Freund’s incomplete adjuvant (FICA), trivalent in aluminum hydroxide, or adjuvant only (FICA). Immunization was done 30 d before the expected calving date followed by 2 boosts in a 2-week interval. Compared to FICA, serum antigen-specific immunoglobulin (Ig)G1 and IgG2 were significantly increased in all the vaccinated groups before parturition and sustained until 3 wk postpartum. In comparison with the trivalent alone, formulation with either adjuvant enhanced production of IgG2, but not IgG1. Immune sera, which contained the highest amount of antibodies, slightly increased neutrophil phagocytosis to the 3 serotypes of killed S. aureus, but most of the differences were not significant due to large variation between the cows. The percentage of CD4+ lymphocyte was significantly higher in vaccinated groups than that of FICA 4 wk after the primary immunization. In comparison with FICA, cows inoculated with trivalent vaccine and adjuvants had an increased percentage of CD8+ lymphocytes at 2 time points, 2 wk before and after calving. Our results indicated that the whole cell trivalent vaccine, with or without adjuvants, is able to elicit antibody responses specific to the 3 capsular polysaccharide antigens. The increase of T8-specific IgG2 was more noticeable when the vaccine was emulsified with adjuvants.
Résumé
Un vaccin trivalent contre la mammite, contenant du matériel capsulaire polysaccharidique de Staphylococcus aureus type 5 (T5), 8 (T8) et 336 (T336), a été évalué pour ses effets sur les sous-populations lymphocytaires, la production d’anticorps et la phagocytose par les neutrophiles. Au total, 20 taures en gestation ont été immunisées avec soit le vaccin trivalent seul, le vaccin trivalent émulsifié dans de l’adjuvant incomplet de Freund (FICA), le vaccin trivalent dans de l’hydroxyde d’aluminium, ou l’adjuvant seulement (FICA). L’immunisation a été faite 30 j avant la date prévue de mise-bas, suivie de 2 injections de rappel dans un intervalle de 2 semaines. Comparativement au groupe témoin (FICA), les anticorps sériques spécifiques de type IgG1 et IgG2 étaient augmentés de manière significative chez tous les groupes d’animaux vaccinés avant la parturition et se sont maintenus jusqu’à 3 sem post-partum. Chez les animaux ayant reçu le vaccin combiné à l’un ou l’autre des adjuvants, la production d’IgG2, mais pas IgG1, était augmentée par rapport aux animaux n’ayant reçu que le vaccin sans adjuvant. Du sérum immun contenant la plus grande quantité d’anticorps augmenta légèrement la phagocytose par les neutrophiles des 3 sérotypes de S. aureus tués, mais la plupart des différences n’étaient pas significatives étant donné les variations marquées entre les vaches. Quatre semaines après la première immunisation, le pourcentage de lymphocytes CD4+ était significativement plus élevé dans les groupes d’animaux vaccinés comparativement au témoin FICA. En comparaison au groupe FICA, les vaches inoculées avec le vaccin trivalent et les adjuvants avaient un pourcentage plus élevé de lymphocytes CD8+ 2 sem avant et après le vêlage. Les résultats indiquent que le vaccin trivalent à base de cellules entières, avec ou sans adjuvants, est en mesure d’induire une réponse humorale spécifique aux 3 antigènes capsulaires polysaccharidiques. L’augmentation des IgG2 T8 spécifique était plus marquée lorsque le vaccin était émulsifié avec de l’adjuvant.
(Traduit par Docteur Serge Messier)
Introduction
Staphylococcus aureus, a contagious, gram-positive coccus, is the most frequently isolated pathogen from cases of mastitis (1,2). It accounts for 19% to 40% of intramammary infections (IMI) caused by major pathogens, usually subclinical, and is responsible for approximately 35% of economic loss due to mastitis (3). In Canada, it has been estimated that S. aureus infection may be present in as many as 90% of Ontario dairy farms (4). The cure rate of antibiotic treatment is low and the infection often becomes chronic. This could be attributed to the ability of S. aureus to locate intracellularly, including epithelial cells and macrophages, and survive antibiotic treatments (5–7). To date, effective prevention of S. aureus mastitis has not been achieved. Many efforts have been made to develop an effective vaccine to prevent S. aureus mastitis in the past decades. Numerous strategies, including immunization with killed bacteria cells or their virulent factors, have been practiced. However, these vaccines have shown poor responses or decreased prevalence and severity of mastitis only, but never efficiently prevented new IMIs caused by S. aureus (8,9).
Phagocytes, mostly neutrophils, are recruited to sites of infections and play a crucial role in bacterial clearance. Staphylococcus aureus is able to produce a capsular polysaccharide (CP) that masks recognition by phagocytes of antigenic cell wall components thus interfering with opsonization and phagocytosis of phagocytes. This makes S. aureus more resistant to the host immune system (7,10). It has been estimated that 94% to 100% of S. aureus isolated from mastitic cows are encapsulated (11). A total of 11 serotypes of S. aureus capsular polysaccharide (CP) have been identified (12). The result of serotyping S. aureus isolated from 178 dairy farms in the United States indicated that only 3 serotypes, T5 (18%), T8 (23%), and T336 (59%), were responsible for bovine S. aureus mastitis (13,14). In addition, the distribution of serotypes varies geographically. In comparison with the distribution in the United States, the percentage of serotypes from European samples were, T5 = 34%, T8 = 34%, T336 = 30%, and nontypable = 2% (14).
It has been suggested to use CP as the antigen for development of a successful vaccine against S. aureus (15). Theoretically, the interference of CP against phagocytosis can be circumvented by the production of CP-specific antibodies. However, CP is categorized as a T-cell independent (TI) antigen, which is poorly immunogenic (16). Indeed, injection of pure T5 CP failed to provoke an immune response in cows (17). Two subsets of T lymphocytes, T helper cells (CD4+ ), and T cytotoxic cells (CD8+ ), modulate immune responses in different ways. The CD4+ cells produce a variety of cytokines which lead to 2 different types of immune responses (Th1 and Th2) and antibody production from B cells (18). Interferon (IFN)-γ and interleukin (IL)-4 are the key cytokines promoting Th1 and Th2 type immunity, respectively. Generally speaking, Th2 type activates mainly antibody-mediated immune responses, including B cells proliferation and antibody secretion. On the other hand, Th1 type elicits both antibody-mediated (specific to IgG2) and cell-mediated immune responses. In cell-mediated immune responses, the cytotoxic activity of CD8+ cells is capable of eliminating altered self-cells, including intracellular pathogen-infected cells. Nevertheless, the T-cell independent (TI) nature of an antigen does not imply that T cells, or their cytokines, can not influence the immunogenicity of TI antigens. Using a CP-protein carrier conjugate has been shown to increase production of CP-specific antibody (17,19). Presumably, the conjugated protein antigens activate T cells through a classical T-cell dependent pathway, and the cytokines produced by activated T cells augment the anti-CP responses (20). Recently, the design of whole cell vaccines has been shown to elicit stronger immune responses than CP-protein conjugate (21). Therefore, a trivalent vaccine, containing killed whole cells from the 3 dominant serotypes of S. aureus in the United States, has been developed by scientists in United States Department of Agriculture (USDA).
In addition to the natural immunogenicity of an antigen, the efficacy of a vaccine is also determined by its adjuvant. Application of different adjuvants on a vaccine has been shown to elicit different types of immune responses (22), different levels of antibody production, and side effects (23). The most common adjuvants used in veterinary vaccines are aluminum hydroxide (ALUM) and Freund’s incomplete adjuvant (FICA). Both adjuvants are inexpensive and have a good safety record (24). However, due to the unique “T-cell independent” nature of CP antigens, the performance of these adjuvants when formulated into the trivalent vaccine needs to be investigated. In the present study, the trivalent vaccine was inoculated to pregnant heifers with or without adjuvants. The effects of different formulations were evaluated based on leukocyte-associated immune functions, including alteration of lymphocyte subpopulations, antigen-specific antibody secretion, and antibody-mediated phagocytosis. Since leukocytes, especially lymphocytes, play a pivotal role in the subsequent response after vaccination, the information might be useful to improve the efficacy of vaccines against S. aureus mastitis.
Materials and methods
Preparation of vaccines
Three serotypes of S. aureus, T5, T8, and T336 (kindly supplied by Dr. A. Fattom, Nabi, Rockville, Maryland, USA), were grown in 10 mL of Columbia broth (T5 and T8) or tryptic soy broth (TSB) (T336) overnight at 37°C. Then 100 μL of T5 or T8 in Columbia broth were streaked on a Columbia agar plate (2%) and incubated overnight at 37°C. Bacteria were harvested by washing the microorganisms off the plate with phosphate buffered saline solution (PBSS). For T336, 100 μL of suspension was inoculated to TSB in a 250 mL flask. Bacteria were killed by 1% formalin overnight at room temperature. Killed bacteria were washed twice with PBSS and resuspended in 10 mL PBSS. The viability and encapsulation of bacteria were verified by plating a small volume of the suspension on a Columbia agar plate and capsular staining. The concentration of bacteria was determined by the optical density (OD) value. Equal amounts of each serotype were mixed and emulsified 1:1 with PBSS or the adjuvant, either FICA or ALUM. The final concentration of each serotype was 109/mL of vaccine.
Animals and immunization protocol
Twenty clinically healthy and pregnant heifers were selected and randomly assigned into 4 groups: (1) adjuvant FICA only (FICA) (n = 5); (2) trivalent only (T) (n = 6); (3) trivalent + FICA (T + F) (n = 4); and (4) trivalent + ALUM (T + A) (n = 5). Heifers received the primary immunization 1 mo (D0) before the expected calving date followed by 2 boosts with the same formulation in a 2-week interval, D14 and D28, respectively. The immunization was administered intramuscularly (2 mL) and in the area of supramammary lymph node (2 mL). Use of animals for this study was approved by the Beltsville Agriculture Research Center’s Animal Care and Use Committee.
Sampling of cows
Blood samples were collected from each heifer before immunization on D0, D14, and D28, as well as 3 times after calving, on C7, C14, and C21, respectively. Briefly, 100 mL of blood was drained from the jugular vein into 2 50-mL syringes containing 6 mL of 40 mM ethylenediaminetetraacetic acid (EDTA) (Sigma, St. Louis, Missouri, USA). At each time point, blood samples were also taken from the tail vein, and serum was collected and stored at −20°C.
Isolation of mononuclear cells
Blood samples collected from the jugular vein were poured into 50 mL centrifuge tubes and centrifuged at 1800 × g for 30 min at 4°C. After centrifugation, 5 mL of buffy coat was removed and added to another 50 mL tube containing 20 mL Hank’s balanced salt solution (HBSS) (GIBCO BRL, Gaithersburg, Maryland, USA). Thereafter, the mixture (25 mL) was carefully layered onto the surface of 12.5 mL ficoll-paque solution (1.077 g/mL; Amershan Biosciences, Piscataway, New Jersey, USA) in a 50 mL tube. Mononuclear cells were separated from red blood cells after centrifugation at 1200 × g, 19°C, for 30 min. The mononuclear cell band was removed and mixed with HBSS to the total volume of 45 mL, then centrifuged at 900 × g for 10 min, at 4°C. The pellet was resuspended in 10 mL of HBSS followed by enumeration of cells using a hemacytometer.
Determination of lymphocyte subpopulations
The determination of lymphocyte subpopulations was carried out as previously described (25). Briefly, isolated bovine mononuclear cells (2 × 106) were incubated with one of the following mouse monoclonal antibodies (VMRD, Pullman, Washington, USA), including CD2 (αβ-T cells, 1:100), CD3 (T cell receptor, 1:100), CD4 (T helper cells, 1:160), CD8 (T cytotoxic/suppressor cells, 1:400), MHC II (1:200), B cells (1:100), and CD18 (negative control, 1:100), in 100 μL of FACS (500 mL PBSS, 10 mL FBS, 0.75 mL 20% sodium azide solution) in round bottom 96-well plates. The plates were kept at 4°C for 30 min. Then the plates were centrifuged at 1200 × g at 4°C for 5 min and washed twice with FACS, followed by the addition of 100 μL of fluorescein-isothiocynate (FITC)-conjugated goat secondary antibodies to mouse cells (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). After 30 min of incubation at 4°C, the plates were washed 3 times. The cells were resuspended in 150 μL of the FACS solution and transferred to microtubes containing 350 μL of the FACS (final volume 500 μL). The percentage of each subpopulation was read by flow cytometry (Coulter Electronics, Hialeah, Florida, USA).
Detection of antigen-specific antibodies in serum
The antibody production was measured by enzyme-linked immunosorbent assays (ELISA), as previously described (26). Briefly, 96-well flat bottom plates (Immulon 4; Bynatech, Chantilly, Virginia, USA) were coated with purified T5, T8, or T336 in PBSS (1 μg/mL) and incubated overnight at 22°C. The coating solution was then removed and the plates were blocked with 1% chicken serum albumin (Sigma Chemical Company) in PBSS at 22°C for 1 h. After washing the plates twice with PBSS-T (containing 0.05% Tween 20), samples diluted 500 times with PBSS-T were added into the wells. The plates were incubated at 4°C overnight. The plates were then washed 3 times and incubated with rabbit anti-bovine immunoglobulin (Ig)G1, IgG2, or IgM, diluted in PBSS containing 0.2% Tween 20 for 1 h at 37°C. After washing, alkaline-phosphatase labeled sheep anti-rabbit IgG (1:2000) (Kirkegaard and Perry, Gaithersburg, Maryland, USA) was added to the wells and incubated for another hour at 37°C. Substrate was added and the plates were read at OD405 on a microtiter plate reader (Bio-Tek, Winooski, Vermont, USA).
Neutrophil phagocytosis
Blood neutrophils were isolated as previous described (27). The FITC-labeled bacteria (T5, T8, and T336) were slightly sonicated and 5 × 107 bacteria in 50 μL were added to each vial containing 50 μL of HBSS-diluted sera (collected on either D0 or C7) pooled from each group. The vials were covered by foil and gently shaken at 37°C for 30 min (opsonization). The concentration of isolated neutrophils was adjusted to 10 × 106/mL in HBSS and 250 μL of the suspension (2.5 × 106 neutrophils) were added to each vial. All samples were incubated and rocked at 37°C for 30 min in dark. Thereafter, phagocytosis was ceased by adding 1 mL ice-cold NaCl (0.09%)-EDTA (0.04%). The fluorescent intensity was read by flow cytometry. The percentage of neutrophils with ingested FITC-labeled bacteria was determined after quenching extracellular fluorescence by adding 400 μL of 1% methylene blue. Samples containing neutrophils or bacteria only were used as controls.
Statistical analysis
For lymphocyte subpopulations and antibody responses, comparisons among treatments at each time point were made using mixed models analysis of variance (ANOVA) for repeated measurements (SAS Institute, Cary, North Carolina, USA) (28). For the opsonic effect on neutrophil phagocytosis, comparisons were made between preimmune (D0) and immune (C7) sera for each treatment using the mixed procedure of SAS. Data of antibody response and phagocytosis were analyzed separately for each serotype. Data were presented as least squares means ± standard error of means (sχ̄). Differences were considered statistically significant when P < 0.05.
Results
Lymphocyte subpopulations
The effect of vaccination with different formulations on the expression of selected surface receptors of lymphocytes was presented in Table I. Expression of CD2, a marker of αβ-T lymphocytes, on D14 was significantly (P < 0.05) lowered in the group receiving trivalent only (T) in comparison with groups immunized with vaccines containing either one of the adjuvants (T + F or T + A). Vaccine formulations containing antigen with adjuvant also significantly increased the percentage of CD8+ lymphocytes at certain time points (T + F: D14 and C14; T + A: D14) when compared to trivalent only (P < 0.05). The groups receiving FICA only had a decreased percentage of CD4+ lymphocytes when compared to T and T + A. Expression of MHCII was the lowest in the group immunized with T + F. No significant differences among the treatments were observed on expression of CD3 (T cell receptor) and B cell receptor. Nonspecific binding of the negative control, antibody against CD18, remained low at all time points (data not shown).
Table I.
Flow cytometric analysis of isolated peripheral blood mononuclear cells
| Receptor | Treatment | D0 | D14 | D28 | C7 | C14 | C21 |
|---|---|---|---|---|---|---|---|
| CD2 | FICA | 29.3 ± 5.7 | 27.2 ± 4.0ab | 29.4 ± 4.1 | 29.4 ± 10.4 | 25.9 ± 3.9 | 27.6 ± 6.7 |
| T | 30.5 ± 5.2 | 23.6 ± 4.0b | 30.9 ± 4.9 | 23.2 ± 3.9 | 27.5 ± 4.0 | 23.4 ± 2.6 | |
| T + F | 34.7 ± 5.2 | 37.0 ± 3.5a | 36.0 ± 4.2 | 33.3 ± 5.7 | 34.6 ± 5.7 | 30.5 ± 2.7 | |
| T + A | 36.9 ± 5.2 | 38.6 ± 4.7a | 35.4 ± 4.2 | 32.1 ± 6.0 | 30.5 ± 5.7 | 36.6 ± 4.8 | |
| CD3 | FICA | 38.5 ± 5.5 | 37.2 ± 3.5 | 36.7 ± 6.0 | 39.2 ± 10.5 | 38.6 ± 13.0 | 35.6 ± 6.4 |
| T | 38.9 ± 5.5 | 30.9 ± 3.6 | 36.9 ± 3.2 | 32.4 ± 3.5 | 34.8 ± 6.0 | 31.1 ± 4.6 | |
| T + F | 42.6 ± 7.5 | 36.2 ± 2.4 | 42.2 ± 4.1 | 41.6 ± 3.7 | 36.6 ± 5.1 | 35.7 ± 3.0 | |
| T + A | 43.5 ± 3.7 | 42.0 ± 4.1 | 43.6 ± 6.4 | 36.5 ± 5.3 | 36.0 ± 4.0 | 42.5 ± 7.8 | |
| CD4 | FICA | 10.7 ± 3.9 | 15.1 ± 1.0 | 8.6 ± 3.0b | 8.7 ± 5.6 | 11.2 ± 5.3 | 15.4 ± 6.8 |
| T | 15.3 ± 3.9 | 15.6 ± 2.1 | 20.4 ± 3.3a | 12.4 ± 2.5 | 16.7 ± 1.9 | 15.4 ± 1.7 | |
| T + F | 20.1 ± 4.8 | 16.6 ± 1.2 | 17.7 ± 0.2ab | 18.2 ± 1.2 | 18.0 ± 3.8 | 11.6 ± 3.0 | |
| T + A | 11.9 ± 5.3 | 20.4 ± 6.0 | 20.4 ± 2.7a | 18.8 ± 3.7 | 12.6 ± 4.0 | 15.6 ± 7.3 | |
| CD8 | FICA | 8.3 ± 2.6 | 8.9 ± 2.3ab | 8.1 ± 0.6 | 4.0 ± 2.7 | 7.6 ± 1.3ab | 8.2 ± 2.3 |
| T | 7.5 ± 1.0 | 5.6 ± 0.5b | 7.9 ± 1.0 | 5.6 ± 0.8 | 5.7 ± 0.9b | 8.0 ± 1.7 | |
| T + F | 12.3 ± 3.5 | 11.2 ± 1.6a | 2.5 ± 4.4 | 10.4 ± 3.5 | 12.2 ± 1.6a | 7.0 ± 2.0 | |
| T + A | 8.3 ± 1.8 | 12.1 ± 2.3a | 9.9 ± 1.4 | 10.9 ± 3.2 | 12.0 ± 2.4ab | 15.5 ± 3.1 | |
| MHCII | FICA | 47.0 ± 9.6 | 56.2 ± 6.5a | 47.1 ± 12.9 | 37.2 ± 12.1 | 52.3 ± 9.3 | 58.4 ± 12.4 |
| T | 36.4 ± 9.2 | 48.3 ± 7.2a | 37.3 ± 9.5 | 44.5 ± 8.6 | 50.6 ± 9.9 | 59.1 ± 8.7 | |
| T + F | 27.1 ± 5.5 | 27.1 ± 4.9b | 36.6 ± 4.3 | 36.7 ± 8.3 | 38.7 ± 6.0 | 37.0 ± 3.2 | |
| T + A | 37.6 ± 8.7 | 37.9 ± 6.4ab | 34.0 ± 8.4 | 47.6 ± 6.4 | 42.9 ± 11.1 | 37.4 ± 5.0 | |
| B cell | FICA | 30.7 ± 8.4 | 38.0 ± 13.1 | 40.9 ± 10.3 | 25.8 ± 8.0 | 31.4 ± 7.5 | 45.0 ± 10.3 |
| T | 28.7 ± 9.8 | 35.4 ± 8.9 | 27.4 ± 7.7 | 30.8 ± 7.4 | 39.5 ± 10.7 | 51.1 ± 12.7 | |
| T + F | 22.0 ± 5.7 | 24.7 ± 5.3 | 23.8 ± 3.4 | 24.0 ± 2.6 | 31.8 ± 3.0 | 26.6 ± 4.7 | |
| T + A | 24.2 ± 5.4 | 26.6 ± 4.5 | 26.0 ± 5.1 | 31.8 ± 6.4 | 30.2 ± 6.0 | 25.8 ± 3.2 |
Values are mean ± sχ̄ of the percentage of positive-stained cells and are compared with other treatments at each time point
Values with different superscripts are significantly different at P < 0.05
CD — ; FICA — Freud’s incomplete adjuvant; T — trivalent only; T + F — trivalent and Freud’s incomplete adjuvant; T + A — trivalent in aluminum hydroxide; MHC — major histocompatibility complex
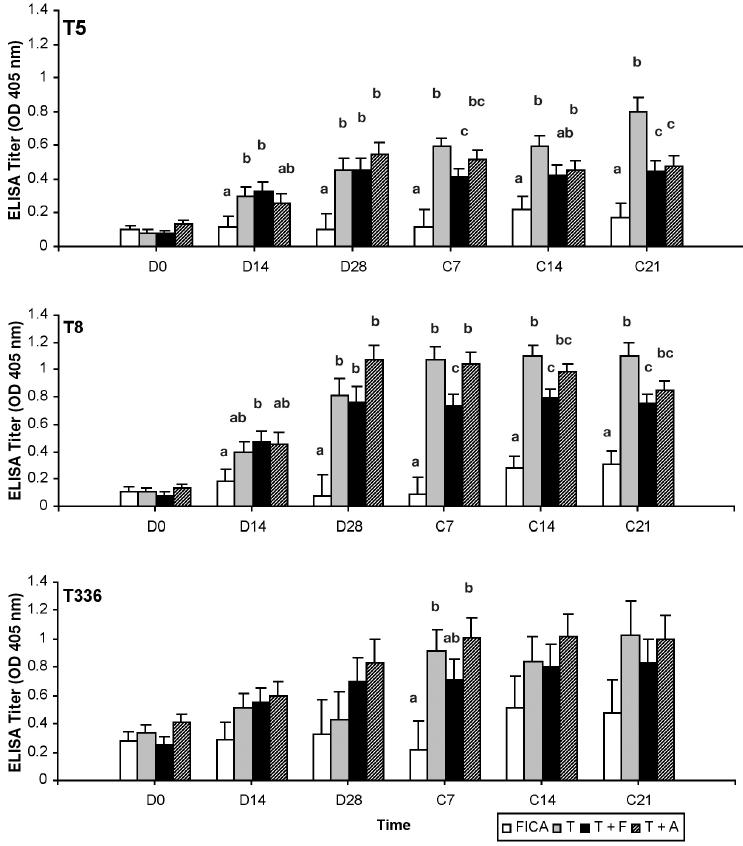
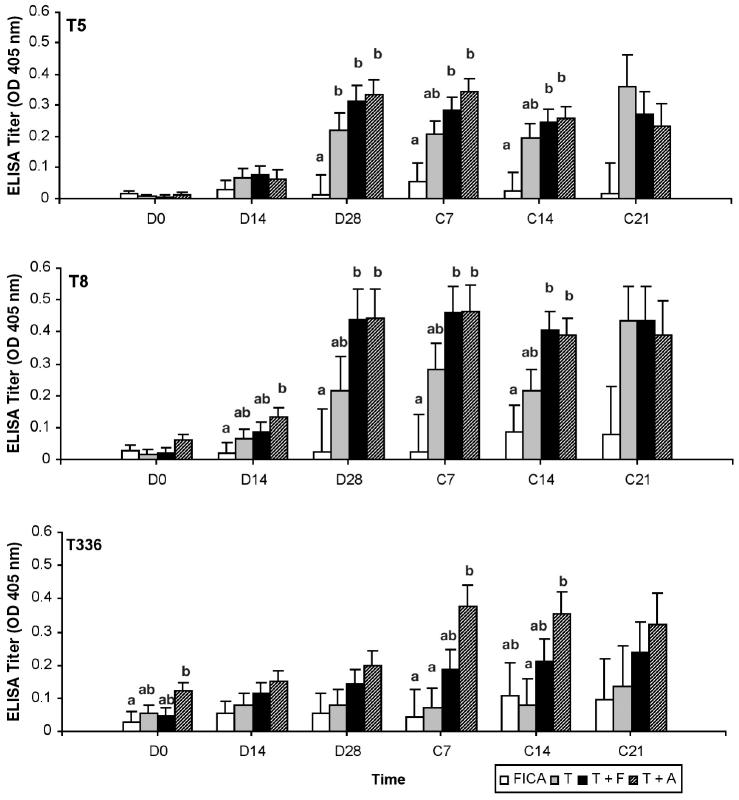
Production of antigen-specific antibodies
In general, the vaccination significantly increased the amount of antigen-specific IgG1 and IgG2 in serum. The response of IgG1 against T5 and T8 was rapid (Figure 1). By D14, vaccinated groups showed an increase in IgG1 to at least 1 of the 3 antigens. However, responses of IgG1 to the 3 antigens were lower in cows immunized with T + F after calving in comparison with T and T + A. Antigen administered with ALUM or FICA did not further augment antigen-specific IgG1 production in comparison with that of the trivalent only. On the other hand, both adjuvants had a positive effect on the production of IgG2 (Figure 2). The T + F and T + A, but not T, had a significantly increased (P < 0.05) IgG2 response to at least 1 of the 3 antigens around parturition when compared to FICA. In addition, the response of T8-specific IgG1 and IgG2 was stronger than those of T5- and T336-specific IgG1 and IgG2. None of the vaccine formulations enhanced the production of antigen-specific IgM (data not shown).
Figure 1.
Antigen specific immunoglobulin (Ig)G1 response in sera as determined by enzyme-linked immunosorbent assay (ELISA). Serum samples were diluted 1/500. Data are expressed as least square mean ± sχ̄ of the optical density (OD) values read at 405 nm. Treatments with different letters at same time point are significantly (P < 0.05) different.
Figure 2.
Antigen specific immunoglobulin (Ig)G2 response in sera as determined by enzyme-linked immunosorbent assay (ELISA). Serum samples were diluted 1/500. Data are expressed as least square mean ± sχ̄ of the optical density (OD) values read at 405 nm.
ab Treatments at same time point are significantly different (P < 0.05).
Neutrophil phagocytosis
To investigate if the sera from vaccinated cows could increase the phagocytosis of S. aureus by blood neutrophils, neutrophils were isolated from healthy cows and incubated with FITC-labeled S. aureus opsonized by pooled sera collected on D0 and C7. In general, all immune sera, regardless of adjuvants, had an increased opsonic effect on neutrophils phagocytosis to killed S. aureus. However, most of the differences were not statistically significant. The C7 sera from cows immunized with T + F significantly increased the phagocytosis of T8 when compared to the sera from D0 (45.88 ± 12.33 versus 39.05% ± 12.33%, P < 0.05) (Figure 3).
Figure 3.
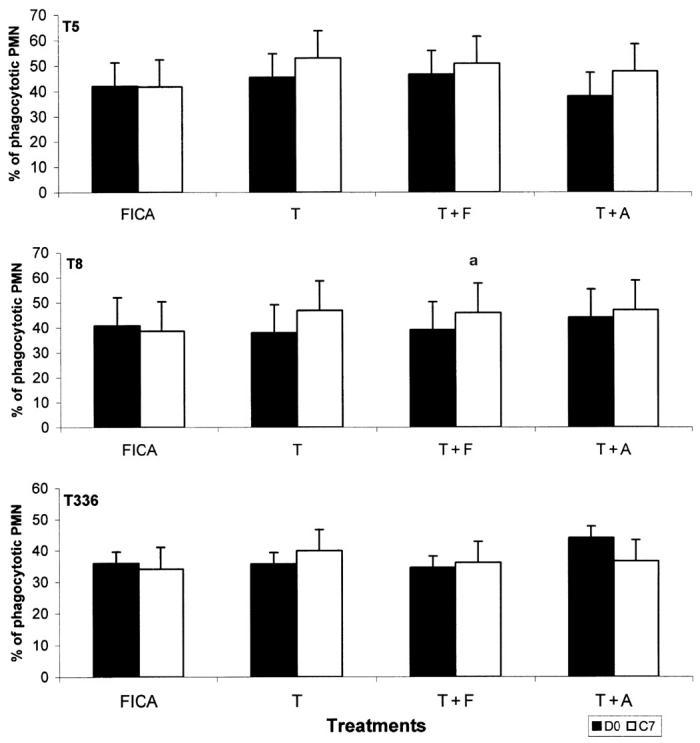
Phagocytosis of Staphylococcus aureus by bovine neutrophils. Bovine neutrophils were incubated with fluoroscein-isothiocynate (FITC)-labeled T5, T8, or T336 S. aureus opsonized by preimmunization (D0) and immune sera (C7). Values are least square mean ± sχ̄ of the percentage of phagocytotic neutrophils. Comparisons were made between the value of D0 and the value of C7 for each treatment.
a Significantly different (P < 0.05).
T — trivalent only; T + F — trivalent and Freud’s incomplete adjuvant; T + A — trivalent in aluminum hydroxide.
Discussion
Staphylococcus aureus is the most frequently isolated pathogen from cases of bovine mastitis (1,2). Since the 3 dominant serotypes of S. aureus responsible for bovine mastitis in the United States were T5 (18%), T8 (23%), and T336 (59%) (13,14), a trivalent vaccine containing whole cells of these serotypes as antigens was developed to prevent intramammary infections induced by S. aureus. However, due to the complicated pathogenesis of S. aureus and the poor immunogenicity of CP, the immunogenicity of this trivalent vaccine needs to be enhanced. Therefore, the objective of this study was to investigate if the most common adjuvants, ALUM and FICA, could promote leukocyte-associated immune responses elicited by the vaccine.
It has been indicated that cows are more susceptible to mastitis during the postpartum period (2 wk after calving), which might be attributed to the suppressed immune responses of lymphocytes (29). Thus, the primary vaccination and 2 boosts were given within a month prior to the expected calving date of each heifer. Theoretically, the maximal effect of vaccination could be reached during the postpartum period by following this immunization protocol. This was supported by the production of IgG1 and IgG2 in serum. Nearly all the antigen-specific IgG1 and IgG2 reached the peak between D28 and C14. The IgM had a high preimmunization level for the antigens. However, vaccination did not have a positive effect on the production of IgM against S. aureus CP antigens, which was in agreement with an earlier study (26). Our data also showed that the response of T8-specific antibody was either stronger (higher OD value) or occurred earlier than T5 and T336. It has been suggested that the flaccid nature of T8 CP increases the exposure of the cell wall (26). Therefore, more protein antigens located on the cell wall of T8 S. aureus were available to augment the “conjugate” effect and subsequent humoral responses.
The vaccination did not significantly alter the subpopulations of lymphocytes. However, significant differences among formulations were observed sporadically at certain time points and might have biological relevance with the antibody secretion. For example, the trivalent vaccine, with or without adjuvant, increased the percentage of CD4+ lymphocytes on D28 when compared to the FICA. Cytokines produced by CD4+ cells are important to the maturation of B cells, which secrete antibodies. The increase of CD4+ cells coincided with the stronger response of IgG1 and IgG2 in serum observed on D28, indicating the requirement of CD4+ cells for IgG production. Moreover, increased number of CD4+ may be associated with the initiation of another type of cell-mediated immune responses, delayed-type hypersensitivity (DTH), which is effective on eliminating intracellular pathogens. Addition of adjuvants significantly increased the percentages of αβ-T lymphocytes (on D14) and CD8+ lymphocytes (on D14 and C14) in comparison with cows immunized with the trivalent only. The percentage of αβ-T lymphocytes represents the subpopulations of both CD4+ and CD8+ lymphocytes. The CD8+ cells are associated with the cytotoxic activity of T lymphocytes, which is triggered in Th1 type immune responses. The increased percentage of CD8+ cells suggested that formulation with adjuvants influenced a Th1 response. This is supported by the result that IgG2 production, an indicator of Th1 type responses, was significantly increased in cows receiving vaccines emulsified with adjuvants. The expression of MHCII receptor was significantly lower in groups immunized with trivalent vaccines containing adjuvants. Although MHCII receptors are not required for the presentation of CP antigens, uptake of TI antigens has been shown to inhibit MHCII-restricted antigen presentation in macrophages (30). It is possible that formulated adjuvants increased the ingestion of CP antigens by antigen-presenting mononuclear cells, and this in turn decreased the expression of MHCII receptors. Further investigations will be required to verify this hypothesis.
Comparing opsonic effects of preimmunization sera and sera from C7 revealed that opsonization was slightly enhanced after vaccination. However, only the sera collected from cows immunized with T + F on C7 significantly increased the phagocytosis of neutrophils to T8 S. aureus. Although the overall production of antigen-specific antibodies was generally increased in the serum of cows immunized with vaccines, IgG2, the most effective opsonin promoting neutrophils phagocytosis (14,32), may not be produced sufficiently to enhance phagocytosis. It has been shown that a minimum amount of specific IgG2 antibody is required for promoting phagocytosis (26). Although production of IgG2 was increased in vaccinated cows, the amount was still much lower than that of IgG1 which is not opsonic for bovine neutrophil phagocytosis. In fact, IgG1 was reported to inhibit bovine neutrophil phagocytosis (27). Both ALUM and FICA have been shown to stimulate mainly Th2 type responses (33). Therefore, it was not surprising to see the limited effect of these vaccines on production of IgG2.
Taken together, immunization of the novel trivalent vaccine, with or without adjuvants, increased the amount of antigen-specific antibodies in serum. However, their effects on other parameters, including alteration of lymphocyte subsets and neutrophil phagocytosis were minimal. Neither ALUM nor FICA induced a persistent enhancement on these parameters. The failure could be due to the Th2 type nature of both adjuvants. Nevertheless, adjuvants known to induce Th1 type immune responses, such as Freud’s complete adjuvant (FCA), usually have undesirable side effects, such as tissue damage (34). Further research will be required to design an optimal formulation for the trivalent vaccine to prevent S. aureus mastitis.
References
- 1.Barkema HW, Schukken YH, Lam TJGM, Beiboer ML, Benedictus G, Brand A. Incidence of clinical mastitis in dairy herds grouped in three categories by bulk milk somatic cell counts. J Dairy Sci. 1998;81:411–419. doi: 10.3168/jds.S0022-0302(98)75591-2. [DOI] [PubMed] [Google Scholar]
- 2.Schukken YH, Leslie KE, Lam T. Staphylococcus aureus: incidence, prevalence and risk factors for intramammary infection. In: Proceedings. 32nd Annu Meet Natl Mastitis Counc 1993:19–26.
- 3.Fox LK, Hancock DD. The effect of segregation on prevention of intramammary infections by Staphylococcus aureus. J Dairy Sci. 1989;72:540–544. doi: 10.3168/jds.S0022-0302(89)79138-4. [DOI] [PubMed] [Google Scholar]
- 4.Kelton D, Godkin A, Alves D, et al. Effectiveness of biosecurity practices to limit the spread of Staphylococcus aureus on Ontario Sentinel dairy farms. J Dairy Sci. 1999;82:61. [Google Scholar]
- 5.Almeida RA, Matthews KR, Cifrian E, Guidry AJ, Oliver SP. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 6.Hébert A, Sayasith K, Sénéchal S, Dubreuil P, Lagacé J. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol Letters. 2000;193:57–62. doi: 10.1111/j.1574-6968.2000.tb09402.x. [DOI] [PubMed] [Google Scholar]
- 7.Hensen SM, Pavicic MJAMP, Lohuis JACM, Poutrel B. Use of bovine primary mammary epithelial cells for the comparison of adherence and invasion ability of Staphylococcus aureus strains. J Dairy Sci. 2000;83:418–429. doi: 10.3168/jds.S0022-0302(00)74898-3. [DOI] [PubMed] [Google Scholar]
- 8.Pankey JW, Boddie NT, Watts JL, Nickerson SC. Evaluation of protein A and a commercial bacterin as vaccines against Staphylococcus aureus mastitis by experimental challenge. J Dairy Sci. 1985;68:726–731. doi: 10.3168/jds.S0022-0302(85)80879-1. [DOI] [PubMed] [Google Scholar]
- 9.Watson DL, McColl ML, Davies HI. Field trial of a staphylococcal mastitis vaccine in dairy herds: clinical, subclinical and microbiological assessments. Aust Vet J. 1996;74:447–450. doi: 10.1111/j.1751-0813.1996.tb07567.x. [DOI] [PubMed] [Google Scholar]
- 10.Barrio B, Vangroenweghe F, Dosogne H, Burvenich C. Decreased neutrophil bactericidal activity during phagocytosis of slime-producing Staphylococcus aureus strain. Vet Res. 2000;31:603–609. doi: 10.1051/vetres:2000143. [DOI] [PubMed] [Google Scholar]
- 11.Norcross NL, Opdebeeck JP. Encapsulation of Staphylococcus aureus isolated from bovine milk. Vet Microbiol. 1993;8:397–404. doi: 10.1016/0378-1135(83)90052-4. [DOI] [PubMed] [Google Scholar]
- 12.Sompolinsky D, Samra Z, Karakawa WW, Vann WF, Schneerson R, Malik Z. Encapsulation and capsular types in isolated of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidry AJ, Fattom A, Patel A, O’Brien CN. Prevalence of capsular serotypes among Staphylococcus aureus isolates from cows with mastitis in the United States. Vet Microbiol. 1997;59:53–58. doi: 10.1016/s0378-1135(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 14.Guidry AJ, Fattom A, Patel A, O’Brien CN, Shepherd S, Lohuis J. Serotyping scheme for Staphylococcus aureus isolated from cows with mastitis. Am J Vet Res. 1998;59:1537–1539. [PubMed] [Google Scholar]
- 15.Relyveld EH. Recent developments with vaccines against bacterial protein toxins. In: Alouf JE, Fehrenbach FJ, Freer JH, Jeljaszewicz J, eds. Bacterial Protein Toxins. London: FEMS Symposium 24, Academic Press, 1984:217–226.
- 16.Poolman JT. Polysaccharides and membrane vaccines. In: Miziahi A, ed. Bacterial Vaccines. Advances in the biological process, Volume 13. New York: Wiley-Liss, 1990:57. [PubMed]
- 17.Gilbert FB, Poutrel B, Sutra L. Immunogenicity in cows of Staphylococcus aureus type 5 capsular polysaccharide-ovalbumin conjugate. Vaccine. 1994;12:369–374. doi: 10.1016/0264-410x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 19.Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to pseudomonas aeruginosa recombinant exoprotein A. Infect Immun. 1993;61:1023–1032. doi: 10.1128/iai.61.3.1023-1032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Ann Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 21.Tollersrud T, Zernichow L, Andersen SR, Kenny K, Lund A. Staphylococcus aureus capsular polysaccharide type 5 conjugate and whole cell vaccines stimulate antibody responses in cattle. Vaccine. 2001;19:3896–3903. doi: 10.1016/s0264-410x(01)00124-4. [DOI] [PubMed] [Google Scholar]
- 22.Yip HC, Karulin AY, Tary-Lehmann M, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–3949. [PubMed] [Google Scholar]
- 23.Stieneker F, Kersten G, van Bloois L, et al. Comparison of 24 different adjuvants for inactivated HIV-2 split whole virus as antigen in mice. Induction of titers of binding antibodies and toxicity of the formulations. Vaccine. 1995;13:45–53. doi: 10.1016/0264-410x(95)80010-b. [DOI] [PubMed] [Google Scholar]
- 24.Cox J, Coulter AR. Adjuvants — a classification and review of their modes of action. Vaccine. 1997;15:248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 25.Shafer-Weaver KA, Corl CM, Sordillo LM. Shifts in bovine CD4+ subpopulations increase T-helper-2 compared with T-helper-1 effector cells during the postpartum period. J Dairy Sci. 1999;82:1696–1706. doi: 10.3168/jds.S0022-0302(99)75399-3. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien CN, Guidry AJ, Fattom A, Shepherd S, Douglass LW, Westhoff DC. Production of antibodies to Staphylococcus aureus serotypes 5, 8, and 336 using poly(DL-lactide-co-glycolide) microspheres. J Dairy Sci. 2000;83:1758–1766. doi: 10.3168/jds.S0022-0302(00)75046-6. [DOI] [PubMed] [Google Scholar]
- 27.Guidry AJ, Berning LM, Hambleton CN. Opsonization of Staphylococcus aureus by bovine immunoglobulin isotypes. J Dairy Sci. 1993;76:1285–1289. doi: 10.3168/jds.S0022-0302(93)77458-5. [DOI] [PubMed] [Google Scholar]
- 28.SAS/STAT User’s Guide, Version 8, SAS Institute, Cary, North Carolina, USA, 2000.
- 29.Oliver SP, Sordillo LM. Udder health in the periparturient period. J Dairy Sci. 1988;71:2584–2660. doi: 10.3168/jds.S0022-0302(88)79847-1. [DOI] [PubMed] [Google Scholar]
- 30.González-Fernández M, Carrasco-Marin E, Alvarez-Dominguez C, Outschoorn IM, Leyva-Cobián F. Inhibitory effects of thymus-independent type 2 antigens on MHC class II-restricted antigen presentation: Comparative analysis of carbohydrate structures and the antigen presenting cell. Cellular Immunol. 1997;176:1–13. doi: 10.1006/cimm.1996.1078. [DOI] [PubMed] [Google Scholar]
- 31.Harp JA, Nonnecke BJ. Regulation of mitogenic response by bovine milk leukocytes. Vet Immunol Immunopathol. 1986;11:215–224. doi: 10.1016/0165-2427(86)90002-4. [DOI] [PubMed] [Google Scholar]
- 32.Howard CJ, Taylor G, Brownlie J. Surface receptors for immunoglobulin on bovine polymorphonuclear neutrophils and macrophages. Res Vet Sci. 1980;29:128–130. [PubMed] [Google Scholar]
- 33.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 34.Weeratna RD, McCluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine. 2000;18:1755–1762. doi: 10.1016/s0264-410x(99)00526-5. [DOI] [PubMed] [Google Scholar]