Abstract
Spinal motor neurons have the longest axons that innervate the skeletal muscles of the central nervous system. Motor neuron diseases caused by spinal motor neuron cell death are incurable due to the unique and irreplaceable nature of their neural circuits. Understanding the mechanisms of neurogenesis, neuritogenesis, and synaptogenesis in motor neurons will allow investigators to develop new in vitro models and regenerative therapies for motor neuron diseases. In particular, small molecules can directly reprogram and convert into neural stem cells and neurons, and promote neuron-like cell differentiation. Prostaglandins are known to have a role in the differentiation and tissue regeneration of several cell types and organs. However, the involvement of prostaglandins in the differentiation of motor neurons from neural stem cells is poorly understood. The general cell line used in research on motor neuron diseases is the mouse neuroblastoma and spinal motor neuron fusion cell line NSC-34. Recently, our laboratory reported that prostaglandin E2 and prostaglandin D2 enhanced the conversion of NSC-34 cells into motor neuron-like cells with neurite outgrowth. Moreover, we found that prostaglandin E2-differentiated NSC-34 cells had physiological and electrophysiological properties of mature motor neurons. In this review article, we provide contemporary evidence on the effects of prostaglandins, particularly prostaglandin E2 and prostaglandin D2, on differentiation and neural conversion. We also discuss the potential of prostaglandins as candidates for the development of new therapeutic drugs for motor neuron diseases.
Keywords: Differentiation, Motor neuron, NSC-34 cells, Prostaglandin D2, Prostaglandin E2
Introduction
Motor neurons are neural cells that control muscle movements, and they are divided into two main types based on the location of their cell bodies in the central nervous system: corticospinal upper motor neurons and spinal lower motor neurons (Stifani, 2014). As lower motor neurons receive glutamate from upper motor neurons, the spinal motor neurons fire action potentials and then release acetylcholine (ACh). In the developing spinal cord, motor neurons are generated from the progenitor cell pool in the ventral neural tube. The dividing progenitor cells are stimulated by several triggering factors responsible for the subsequent differentiation process. These signals lead to the division of progenitor domains (Davis-Dusenbery et al., 2014), particularly retinoic acid (RA) and sonic hedgehog (SHH), which tightly regulate the exit from the cell cycle and entry into the differentiation of motor neuron progenitors (Novitch et al., 2003; Lee et al. 2009). As motor neuron progenitor cells deviate from the cell cycle, the expression of transcription factors in the motor neuron, pancreas homeobox 1 (HB9), and Islet-1, begins to increase (Lee et al., 2005). These transcription factors induce a switch toward motor neuron specification and the expression of genes involved in cholinergic neurotransmissions, such as vesicular acetylcholine transporter and choline acetyltransferase (ChAT) (Arber et al. 1999; Lee et al. 2012).
Recently, motor neurons derived from embryonic stem (ES) cells and induced pluripotent stem (iPS) cells have been proposed as potential ways to facilitate understanding of the pathogenesis of motor neuron diseases and progress drug discovery for therapies to treat these conditions (Jaiswal, 2017). To date, numerous protocols have been devised for the differentiation of stem cells into populations derived from specialized subtypes of motor neurons, most of which use growth factors and small molecules for differentiation due to their reasonable price and cost-effectiveness (Hu & Zhang, 2009). Morizane et al. reported that purmorphamine, a small molecule activator of the SHH signal pathway, can ventralize neural precursors (Morizane et al., 2011), and that LY294002, a phosphatidylinositol 3-kinase inhibitor, enhances the viability and differentiation of stem cells into motor neuron-like cells (Ebrahimi-Barough et al., 2017). The combination of AMP-kinase inhibitor dorsomorphin, β-catenin-mediated transcription inhibitor XAV939, and TGFβ signaling pathway inhibitor A8301 has enhanced the ability to direct human ES cells into the motor neuron lineage, with 39% of total cells differentiating into HB9/Islet-1 positive motor neurons (Valizadeh-Arshad et al., 2018). However, given that small molecule-induced motor neurons have not been shown to exhibit sufficient electrophysiological maturation, there is still a need to improve and accelerate the differentiation and functional maturation of such motor neurons. We have summarized several publications on the generally used protocols for the differentiation from human stem cells into motor neurons (Table 1).
Table 1.
Protocols for the differentiation from human stem cells into motor neurons
| Starting cells | Neural induction | Motor neuron differentiation | Motor neuron maturation | Cell features | Total induction period | Differentiation rate | References | |
|---|---|---|---|---|---|---|---|---|
| Motor neuron-specific marker protein | Action potential | |||||||
| Endometrial stem cells | LY294002 and RA | BDNF | None | ChAT, ISL1, NF-H, TUJ1 | Non-tested | 15 days |
Approximately 80% (ISL1 positive) |
Ebrahimi-Barough et al. 2017 |
| ESCs and iPSCs | Dorsomorphin, SB431542, BIO, RA and purmorphamine | RA, purmorphamine, cAMP,BDNF, GDNF, IGF-1 and ascorbic acid | RA, purmorphamine, cAMP, BDNF, GDNF, IGF-1 and ascorbic acid | HB9, ISL1, ChAT | Non-tested | 42 days |
Approximately 50% (HB9 or ISL1 positive) |
Shimojo et al. 2015 |
| iPSCs | SB431542, LDN-193189, CHIR99021, Y-27632, RA, SAG,BDNF and GDNF | SB431542, LDN-193189, CHIR99021, Y-27632, RA, SAG, BDNF, GDNF and DAPT | BDNF, GDNF, and CNTF | HB9, SMI32, TUJ1, ChAT |
Spontaneous repetitive action potentials Spontaneous postsynaptic currents |
38 days |
Approximately 70–95% (ISL1, SMI32 or ChAT-positive) Approximately 30–60% (cells with spontaneous repetitive action potentials) |
Guo et al. 2017 |
| ESCs | Dorsomorphin, XAV939, A8301, RA, and bFGF | Dorsomorphin, XAV939, A8301, ascorbic acid, purmorphamine and RA | Dorsomorphin, XAV939, A8301, bFGF, purmorphamine, RA and BDNF | TUJ1, MAP2, HB9, ISL1 | Single action potential | 32 days | 39% (HB9/ISL1 double-positive) | Valizadeh-Arshad et al. 2018 |
BDNF brain-derived neurotrophic factor, bFGF basic fibroblast growth factor, cAMP cyclic adenosine monophosphate, ChAT choline acetyltransferase, CNTF ciliary neurotrophic factor, ESCs embryonic stem cells, GDNF glial cell-derived neurotrophic factor, IGF-1 insulin-like growth factor-1, iPSCs induced pluripotent stem cells, ISL1 islet-1, MAP2 microtubule-associated protein 2, RA retinoic acid
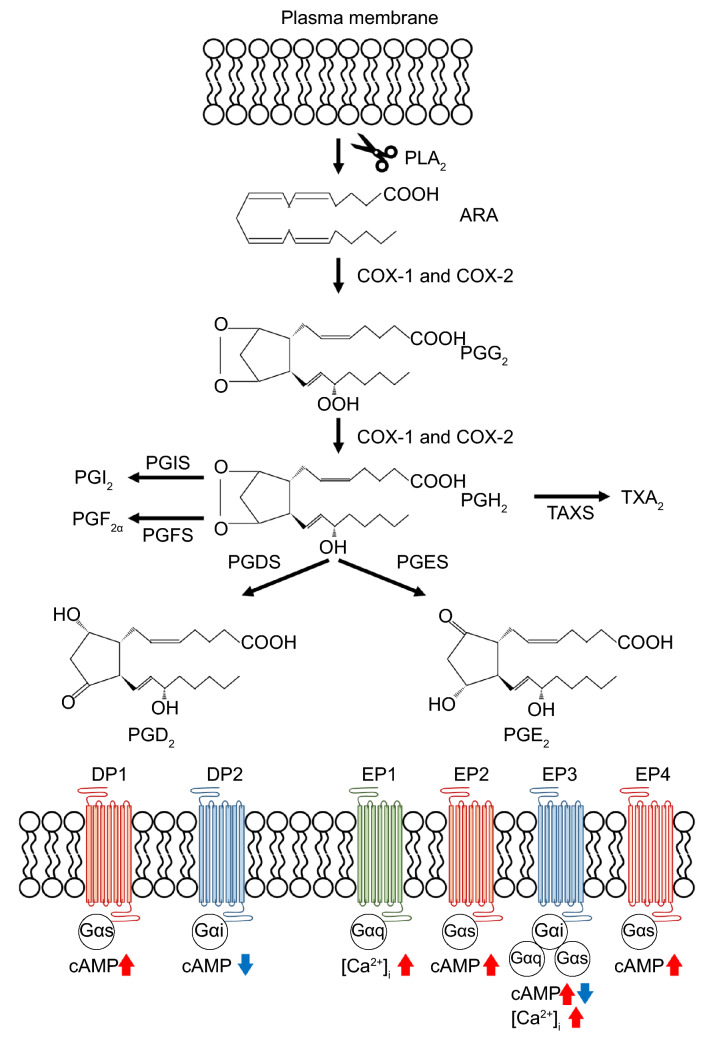
Prostaglandins are potent eicosanoid lipid mediators derived from the arachidonic acid cascade (Phillis et al., 2006) (Fig. 1). Arachidonic acid is specifically released by cytosolic phospholipase A2 and is metabolized by cyclooxygenase (COX) into prostaglandin H2 (PGH2). Subsequently, specific synthetases convert PGH2 into a variety of eicosanoids such as prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), prostaglandin F2, prostaglandin I2, and thromboxane A2 (Ricciotti & Fitzgerald, 2011; Peebles, 2019). Their biological functions have been attributed to interactions with specific G-protein-coupled receptors (GPCRs) at the plasma membrane, termed E-prostanoid (EP) receptor-1 to 4, D-prostanoid (DP) receptor-1 and 2, F-prostanoid (FP) receptor, I-prostanoid (IP) receptor, and thromboxane-prostanoid (TP) receptor (Ricciotti & Fitzgerald, 2011; Peebles, 2019). Of these, EP2, EP4, IP, and DP1 increase intracellular levels of cyclic adenosine monophosphate (cAMP) via Gαs; EP1 and FP increase intracellular levels of calcium via Gαq; DP2 decreases intracellular levels of cAMP via Gαi; and EP3 and TP increase or decrease intracellular cAMP and Ca2+ via Gαs, Gαi, or Gαq (Mohan et al., 2012).
Fig. 1.
The biosynthesis and signaling pathway of prostaglandins. PLA2 phospholipase A2, ARA arachidonic acid, COX cyclooxygenase, PGG2 prostaglandin G2, PGH2 prostaglandin H2, PGI2 prostaglandin I2, PGF2α prostaglandin F2α, PGD2 prostaglandin D2, PGE2 prostaglandin E2, TXA2 thromboxane A2, PGIS prostaglandin I synthase, PGFS prostaglandin F synthase, PGDS prostaglandin D synthase, PGES prostaglandin E synthase, DP1 D-prostanoid receptor-1, DP2 D-prostanoid receptor-2, EP1 E-prostanoid receptor-1, EP2 E-prostanoid receptor-2, EP3 E-prostanoid receptor-3, EP4 E-prostanoid receptor-4
It has been demonstrated that activated Gαs translocated from the plasma membrane promotes neurite outgrowth in PC12 cells (derived from a rat pheochromocytoma) and rat primary hippocampal neurons (Sarma et al., 2015), and that Gq-dependent Ca2+ mobilization promotes neurite outgrowth in primary mouse cortical neurons (Peterson et al., 2013). Prior in vivo studies have reported that the COX-2 inhibitors meloxicam and nimesulide inhibit progenitor cell proliferation in the subventricular zone and hippocampus of young adult mice (Goncalves et al., 2010) and that the brain isolated from embryonic day 18.5 embryos in phospholipase A2-activating protein homozygous null mice had decreased tissue PGE2 levels and less mature and differentiated neurons (Falik Zaccai et al., 2017). This suggests that prostaglandins play an important role in neuritogenesis and neurogenesis in the central nervous system through GPCRs. Thus, it is possible that a prostaglandin-based agent targeting neuritogenesis and neurogenesis in the spinal cord could provide a potential therapeutic approach for motor neuron degeneration and motor neuron diseases.
This article summarizes our investigations that have focused on the effect of prostaglandins on motor neuron differentiation and reviews previous original research and review articles that have investigated the effect of prostaglandins and signal transduction pathways possibly regulated by prostaglandins on neurite outgrowth and neural differentiation. Keeping in mind these results from previous studies and reviews, we discuss the prospects of the prostaglandin signaling cascade as a target for therapeutic regeneration in motor neuron diseases.
PGE2 Accelerated Conversion and Maturation Into Motor Neurons
In vitro studies have reported that PGE2 promotes neurite growth in cultured mouse dorsal root ganglion neurons via EP2 (Hiruma et al., 2000) and ND7/23 mouse sensory neuron-like cells via EP4 (Mitani et al., 2016). PGE2 has also been reported to retract neurites of mouse neuroblastoma Neuro-2 cells by an unknown mechanism (Tamiji & Crawford, 2010). These reports suggest that PGE2 has opposing effects on neurite outgrowth and different cell types may be involved in different EP subtypes. Therefore, it is necessary to investigate the effect of PGE2 on neurite outgrowth and neuronal differentiation in each target cell.
Neural differentiation of NSC-34 mouse neuroblastoma and the spinal motor neuron fusion cell line has been commonly used as a motor neuron model in previous research. Exposure to PGE2 results in inhibited cell proliferation and increases the percentage of neurite-bearing cells in a concentration-dependent manner without affecting cell viability in NSC-34 cells (Nango et al., 2017). We have previously reported that EP2 and EP3 are expressed in NSC-34 cells and mouse spinal motor neurons (Miyagishi et al., 2013). In this study, an EP2-selective agonist (butaprost) replicated the effect of PGE2 on cell proliferation and neuritogenesis, whereas an EP1 and EP3 agonist (sulprostone) did not. Moreover, co-treatment with an EP2-selective antagonist (PF-04418948) suppressed PGE2-induced neurite outgrowth. It has been reported that EP2 mediates its actions via cAMP signaling. Interestingly, a membrane-permeable cAMP analog (dibutyryl-cAMP) has been shown to mimic PGE2- and butaprost-induced neurite outgrowth. These results suggest that PGE2 suppresses cell proliferation and induces morphological differentiation through EP2 activation; moreover, the Gαs protein-coupled cAMP signaling axis is at least partly involved in the molecular mechanisms of NSC-34 cells.
cAMP signaling is involved in the development of tissues derived from the endoderm, ectoderm, and mesoderm, and thus is highly pleiotropic for differentiation. Although the combination of vascular endothelial growth factor and cAMP synergistically enhances differentiation of human iPS cells into endothelial cells (Ikuno et al., 2017), cAMP along with other small molecules is used to induce motor neurons from human ES cells and human iPS cells (Boulting et al., 2011; Takazawa et al., 2012). cAMP signaling is known to play a role in neural differentiation through the activation of cAMP response element-binding protein in neural stem/progenitor cells (Mantamadiotis et al., 2012). Moreover, an elevation of intracellular cAMP has been shown to promote neurite outgrowth in cultured rat motor neurons (Aglah et al., 2008) as well as survival and neural differentiation of neural stem and progenitor cells in rats with spinal cord injury (Kim et al., 2011). These reports suggest that cAMP stimulates the maturation of lineage-determined progenitor cells rather than regulating the cell lineage. Therefore, PGE2 is presumed to function as an endogenous cAMP activator during motor neuron maturation in vivo. Indeed, we have recently shown that PGE2 induces NSC-34 cells to differentiate into functional motor neuron-like cells, supporting the hypothesis that PGE2 is an endogenous maturation inducer for motor neurons (Nango et al., 2020a).
RA has been shown to inhibit proliferation, promote neuritogenesis, and convert into motor neuron-like cells from proliferating NSC-34 cells (Johann et al., 2011; Maier et al., 2013; Petrozziello et al., 2017) and into motor neurons from iPS cells (Qu et al., 2014; Shimojo et al., 2015). In one study, the percentage of neurite-bearing cells in NSC-34 cells treated with PGE2 for 2 days was significantly higher than that in NSC-34 cells treated with RA for 2 days and was similar to that in NSC-34 cells treated with RA for 7 days; this suggests that PGE2 is more efficient for neural differentiation and neuritogenesis than RA (Nango et al., 2020a). Moreover, we evaluated neuronal electrical properties by whole-cell patch-clamp recordings and showed that the threshold currents for action potential generation were significantly lower in PGE2-treated cells than in RA-treated cells (Nango et al., 2020a). Interestingly, the threshold currents of motor neurons in adult mice lumbar spinal cord slices have been reported to be smaller than those of motor neurons at an early postnatal stage (Mitra & Brownstone 2012). These findings suggest that PGE2-evoked neuron-like cells exhibit higher neuronal maturation than RA-evoked neuron-like cells.
Among the physiological properties of mature neurons, the generation of action potentials is one of the most important features (Davis-Dusenbery et al., 2014). Surprisingly, in NSC-34 cells, the threshold potential, peak potential, and amplitude of the first spike in PGE2-induced neuron-like cells for 2 days were the same as those of RA-induced neuron-like cells for 7 days (Nango et al., 2020a). On the other hand, treatment with dibutyryl-cAMP alone requires at least 21 days to differentiate from NG108-15 cells into action potential-producing cells (Liu et al., 2012). Likewise, the combination of small molecules takes 11 days to generate an action potential in human ES cell-derived neurons (Valizadeh-Arshad et al., 2018). Although differences in cell types and other aspects cannot be straightforwardly compared, PGE2 more rapidly induces differentiation into cells with action potentials. We advocate the possibility that PGE2 is a highly agile and efficient differentiation-inducing factor for mature neurons.
We have also previously assessed the expression of neural differentiation markers using western blot analysis (Nango et al., 2020a). This study showed that the levels of neuronal marker proteins microtubule-associated protein (MAP) 2c and synaptophysin, and motor neuron-specific markers HB9 and Islet-1, were upregulated to the same extent in PGE2-treated cells and RA-treated cells. However, the protein level of ChAT in PGE2-treated cells for 2 days was higher than that in RA-treated cells for 7 days. Consistent with this result, the amount of ACh released from PGE2-treated cells for 2 days was significantly higher than that from RA-treated cells for 7 days. Taken together, these results strongly suggest that PGE2 promoted the conversion of NSC-34 cells into mature motor neuron-like cells (Fig. 2). Hounoum et al. have argued that the differentiated NSC-34 cells induced by RA are unsuitable as experimental models for glutamate excitotoxicity in motor neuron diseases (Hounoum et al., 2016). Although further in vitro studies should be carried out to evaluate glutamate excitotoxicity in PGE2-differentiated NSC-34 cells, PGE2-differentiated cells may be an equal or superior model to unravel the pathological mechanism of motor neuron death compared to RA-differentiated cells.
Fig. 2.
A schematic diagram showing the comparison of PGE2- and RA-induced cell differentiation. PGE2 prostaglandin E2, RA retinoic acid, EP2 E-prostanoid receptor-2, EP3 E-prostanoid receptor-3
PGD2 had Promising Effects on Motor Neuron Differentiation
It is worth noting that PGD2 is the prevailing prostaglandin in the murine spinal cord (Kihara et al., 2009); moreover, DP1 and DP2 are located in adult mouse spinal cord motor neurons (Grill et al., 2008). Although it has been reported that PGD2 reverses serum starvation-induced neurite outgrowth in hypothalamic cell lines (N37 cells) (Tsuchiya et al., 2016), it has not been shown to induce neurite outgrowth in motor neurons. We previously explored whether PGD2 can induce neurite outgrowth in undifferentiated NSC-34 cells (Nango et al., 2020b).
In our study, PGD2 increased the proportion of cells bearing neurites without affecting cell viability and extended neurite length (Nango et al., 2020b). We also investigated whether BW245C and 15(R)-15-methyl PGD2, which are specific agonists of DP1 and DP2, could regulate neurite outgrowth. Neither BW245C nor 15(R)-15-methyl PGD2-induced neurite outgrowth (Nango et al., 2020b). In agreement with this result, MK0524 and CAY10471, which are specific antagonists of DP1 and DP2, did not inhibit PGD2-induced neurite outgrowth (Nango et al., 2020b). These results imply that neither DP1 nor DP2 contribute to PGD2-evoked neuritogenesis. On the other hand, PGD2 is non-enzymatically metabolized to J2 prostaglandins (Liu et al., 2013a). We have recently demonstrated the conversion of PGD2 into 15d-PGJ2 in a cell-free culture medium (Nango et al., 2020b). 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), a major metabolite of PGD2 (Straus & Glass 2001), is known to enhance neuritogenesis and synaptogenesis in LA-N-5 human neuroblastoma cells (Han et al., 2001) and rat embryonic midbrain cells in a peroxisome proliferator-activated receptor γ (PPARγ)-dependent manner (Park et al., 2004). We have also recently demonstrated that the exogenous application of 15d-PGJ2 leads to significant neurite outgrowth with no influence on cell viability in undifferentiated NSC-34 cells (Nango et al., 2020b). Subsequently, GW9662 (a PPARγ antagonist) suppressed PGD2-induced neuritogenesis in NSC-34 cells (Nango et al., 2020b). Furthermore, we have shown that PGD2 and 15d-PGJ2 significantly upregulate Islet-1 protein expression and that these increases in Islet-1 protein were suppressed by GW9662 in PGD2-treated cells and 15d-PGJ2-treated cells (Nango et al., 2020b). Taken together, these results indicate that PGD2, through conversion to 15d-PGJ2, induces neuritogenesis via a PPARγ-dependent pathway; the results also show that PGD2 and 15d-PGJ2, as well as PGE2, may contribute to the preparation of functionally mature motor neurons from undifferentiated NSC-34 cells.
To the best of our knowledge, no published studies examined the relationship between PPARγ and motor neuron differentiation. The only relevant evidence so far is that of the regulation of motor neuron-specific transcription Islet-1 expression by PPARγ. PPARγ-mediated regulation of Islet-1 gene expression depends on the type of cell. In neural stem cells derived from the neonatal mouse brain, a selective PPARγ agonist (ciglitazone), RA, and 15d-PGJ2 abrogated Islet-1 gene expression (Kanakasabai et al., 2012). In human brain tumor stem cells, ciglitazone has been shown to upregulate the gene expression of Islet-1 (Pestereva et al., 2012). Importantly, Liu et al. predicted transcription factor-target gene interactions between PPARγ and Islet-1 in human extravillous and villous cytotrophoblasts (Liu et al., 2020). In contrast, PPARγ activation promotes differentiation of newborn mouse brain-derived neural stem cells into oligodendrocytes (Kanakasabai et al., 2012). Further studies are needed to determine how PPARγ activation triggers the conversion of mature motor neurons.
Limitations of this Review due to the Lack of In Vivo Studies
Through this review, we explained the effects of PGE2 and PGD2 on motor neuron differentiation and discussed their usefulness. However, one of the main limitations worth noting is that no clear link has yet been reported between prostaglandins and motor neuron differentiation in vivo. To the best of our knowledge, null mutations of prostaglandin-related genes have not been reported to induce motor neuron hypoplasia and subsequent lethal progression. Studies of neonatal lethal phenotypes in mutant mice have shown that deletions of EP4, prostaglandin dehydrogenase, and COX lead to a patent ductus arteriosus (Ushikubi et al., 2000; Turgeon & Meloche 2009). This is consistent with the result of a previous report showing that PGE2 induces mesoderm differentiation in human embryonic cells (Zhang et al., 2018). Moreover, PPARγ-deficiency in neonatal mice is not lethal (O’Donnell et al., 2016). As shown in Fig. 3, PGE2-dependent signaling and the PGD2-15d-PGJ2-mediated cascade are at least partially different from each other in NSC-34 cells. This evidence suggests that a single deficiency of prostaglandin in the central nervous system might be compensated for by other prostaglandin signals and that motor neuron development, even if PGE2 or PGD2 is lost, can still occur. Another explanation for the role of PGE2 and PGD2 in motor neuron development in vivo relates to the induction of differentiation into motor neurons from adult neural progenitor cells but not stem cells.
Fig. 3.
A proposed mechanism underlying PGE2- and PGD2-induced neuritogenesis in motor neuron-like NSC-34 cells. cAMP cyclic adenosine monophosphate, 15d-PGJ215-deoxy-Δ12,14-prostaglandin J2, PGE2 prostaglandin E2, PGD2 prostaglandin D2, RA retinoic acid, DP1 D-prostanoid receptor-1, DP2 D-prostanoid receptor-2, EP2 E-prostanoid receptor-2, EP3 E-prostanoid receptor-3
The second major limitation is the in vivo concentration of prostaglandins. The concentration of PGE2 and PGD2 in the spinal cord is approximately 68 ng/g tissue (193 nM) in a transgenic mouse model of amyotrophic lateral sclerosis (ALS) expressing a mutant form of SOD1 detectable in familial ALS patients (Miyagishi et al., 2017) and 130 pg/mg tissue (369 nM) in spinal cord injury rat model (Redensek et al., 2011), respectively. In addition, although the concentration of 15d-PGJ2 found in the ischemic hippocampus is approximately 18 pmol/g tissue (18 pM) (Liu et al., 2013b), the concentration of 15d-PGJ2 in the spinal cord remains unclear. These in vivo studies suggest that the concentrations of PGE2, PGD2, and 15d-PGJ2 found in the spinal cord and the central nervous system are in the nM and pM range even under pathological conditions. However, the effective concentrations of PGE2, PGD2, and 15d-PGJ2 on neurite outgrowth were in the μM range in NSC-34 cells. It is worth noting that the measured concentrations in in vivo studies represent whole tissue concentrations. Considering that prostaglandins act as autocrine or paracrine factors for their target cells, local tissue concentration is more important than whole tissue concentration. The report that neural progenitor cells migrated to the vicinity of degenerated motor neurons and then differentiated into neurons in the spinal cord of ALS model mouse during disease onset and progression supports the importance of local tissue concentrations of prostaglandin for motor neuron differentiation (Chi et al., 2006).
Kondo et al. reported that co-localization of COX-2 and 15d-PGJ2 in neuron and glia in the anterior spinal cords of sporadic ALS patients (Kondo et al., 2002). Moreover, we had reported previously that protein expression of microsomal prostaglandin E synthase (mPGES)-1, the enzyme that controls the final step of PGE2 synthesis, is increased in motor neurons and microglia in lumbar spinal cords of G93A mice at the pre-symptomatic and early symptomatic stage (Miyagishi et al., 2012). Although the signaling pathways involved in the upregulation of mPGES-1 are poorly understood and further investigations are needed, interleukin-1β, which is increased in the spinal cord of ALS patients, G93A mice and spinal cord injured rats (Papadimitriou et al., 2010; Wang et al., 1997), has been reported to induce COX-2 expression through protein kinase C and mitogen-activated protein kinases (MAPKs) in mouse astrocytes (Molina-Holgado et al., 2000) and through the extracellular signal-regulated kinase/p38 MAPK pathway in cultured rat dorsal root ganglia neurons (Amaya et al., 2009). These studies suggest that the proinflammatory cytokine-induced local upregulation of COX-2 and mPGES-1 under pathological conditions contributes to increased local concentrations of prostaglandins. However, to the best of our knowledge, the local cellular concentrations of PGE2, PGD2, and 15d-PGJ2 in the spinal cord are unknown, and thus further studies are needed.
Possible Involvement of Other Prostaglandins in Motor Neuron Differentiation
Previous studies reported an increase in COX-2 protein expression in spinal cord motor neurons of spinal cord injury rat (Kim et al., 2017) and ALS patient (Kondo et al., 2002; Maihöfner et al., 2003), and therefore it is assumed that prostaglandins other than PGE2 and PGD2 are also increased in the spinal cord. Hence, this section provides investigations into the effects of prostaglandins other than PGE2, PGD2, and 15d-PGJ2 on neurite outgrowth and discusses their possible involvement in motor neuron differentiation.
Thromboxane A2, which is synthesized by the metabolism of PGH2 by thromboxane synthetase, has been reported to be involved in neurite outgrowth via MAPKs in cultured rat cerebral cortical neurons (Sumimoto et al., 2015). Moreover, PGE1, which is synthesized from dihomo-γ-linolenic acid metabolized by COX, induced neurite outgrowth in mouse neuroblastoma NB2a cell line (Gurwitz & Cunningham. 1988). Although the mechanism of PGE1-induced neurite outgrowth has not been mentioned, the affinity of PGE1 and PGE2 for EP2 is similar (Kiriyama et al., 1997), suggesting that EP2 could be involved in PGE1-induced neurite outgrowth. Conversely, PGA2, PGC2, and PGB2 are non-enzymatic dehydration metabolites of PGE2, similar to the metabolism from PGD2 to 15d-PGJ2 (Straus & Glass, 2001). These metabolites of PGE2 have been reported to be agonists of PPARα or PPARδ, a PPAR family (Forman et al., 1997). The activation of PPARα has been reported to promote neural differentiation in cultured rat forebrain neurons (Bento-Abreu et al., 2007), thus suggesting that these metabolites could also be involved in neural differentiation. These findings suggest that thromboxane A2, PGE1, and metabolites of PGE2 are involved in neural differentiation in brain-derived neurons. However, no study has suggested a relationship between these prostaglandins and the differentiation of motor neurons and their presence in the spinal cord, and further studies are needed.
Conclusion and Future Perspectives
PGE2 levels are increased in various areas in neurodegenerative disorders and not just amyotrophic lateral sclerosis, such as in the cerebrospinal fluid in Alzheimer’s disease (Ho et al., 2000), the substantia nigra in Parkinson’s disease (Mattammal et al., 1995), and the brain in stroke (Yokota et al., 2004). Similar to PGE2, an increase in PGD2 is detected in the post-mortem cerebral cortex of patients with Alzheimer's disease (Iwamoto et al., 1989) and animal models of stroke (Gaudet et al., 1980). Interestingly, activation of EP2 exhibits neuroprotective effects in models of Alzheimer's disease and stroke (Yagami et al., 2016). Combined with reports that COX-2 inhibitors suppress neurogenesis (Goncalves et al., 2010) and PGE2 promotes neural differentiation in neuroectodermal stem cells derived from mouse brains (Wong et al., 2016), prostaglandins may be increased in these neurodegenerative disorders to induce differentiation from endogenous neural stem/progenitor cells into neural cells. Although further in vivo studies using adult neural progenitor cells will be needed to define the role of prostaglandins in the conversion of motor neurons, the prostaglandin signaling cascade can now be identified as a target for the treatment of patients with neurodegenerative disorders. This article may provide new insights into the potency of prostaglandins as differentiation inducers or enhancers, and help develop novel treatment strategies for patients with motor neuron disorders.
Acknowledgements
We are grateful to Dr. Neil Cashman for providing the NSC-34 cell line. We thank all the members of our laboratories for the research work. The author would like to thank Editage (www.editage.com) for English language editing. This work was funded in part by a grant to encourage and promote research projects in the School of Pharmacy, Nihon University (Y.K.), and by a Nihon University Chairman of the Board of Trustees Grant. The funding bodies had no role in the design of the study or the writing of the manuscript.
Author Contributions
HN and YK designed the paper; HN wrote the paper and designed the figures; and YK reviewed the manuscript.
Funding
This work was funded in part by a grant to encourage and promote research projects in the School of Pharmacy, Nihon University (Y.K.), and by a Nihon University Chairman of the Board of Trustees Grant. The funding bodies had no role in the design of the study or the writing of the manuscript.
Declarations
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aglah C, Gordon T, Posse de Chaves EI (2008) cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology 55:8–17. 10.1016/j.neuropharm.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Amaya F, Samad TA, Barrett L, Broom DC, Woolf CJ (2009) Periganglionic inflammation elicits a distally radiating pain hypersensitivity by promoting COX-2 induction in the dorsal root ganglion. Pain 142:59–67. 10.1016/j.pain.2008.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S (1999) Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23:659–674. 10.1016/S0896-6273(01)80026-X [DOI] [PubMed] [Google Scholar]
- Bento-Abreu A, Tabernero A, Medina JM (2007) Peroxisome proliferator-activated receptor-alpha is required for the neurotrophic effect of oleic acid in neurons. J Neurochem 103:871–881. 10.1111/j.1471-4159.2007.04807.x [DOI] [PubMed] [Google Scholar]
- Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, Rodolfa CT, Dimos JT, Mikkilineni S, MacDermott AB, Woolf CJ, Henderson CE, Wichterle H, Eggan K (2011) A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol 29:279–287. 10.1038/nbt.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Ke Y, Luo C, Li B, Gozal D, Kalyanaraman B, Liu R (2006) Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells 24:34–43. 10.1634/stemcells.2005-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Dusenbery BN, Williams LA, Klim JR, Eggan K (2014) How to make spinal motor neurons. Development 141:491–501. 10.1242/dev.097410 [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Barough S, Hoveizi E, Yazdankhah M, Ai J, Khakbiz M, Faghihi F, Tajerian R, Bayat N (2017) Inhibitor of PI3K/Akt signaling pathway small molecule promotes motor neuron differentiation of human endometrial stem cells cultured on electrospun biocomposite polycaprolactone/collagen scaffolds. Mol Neurobiol 54:2547–2554. 10.1007/s12035-016-9828-z [DOI] [PubMed] [Google Scholar]
- Falik Zaccai TC, Savitzki D, Zivony-Elboum Y, Vilboux T, Fitts EC, Shoval Y, Kalfon L, Samra N, Keren Z, Gross B, Chasnyk N, Straussberg R, Mullikin JC, Teer JK, Geiger D, Kornitzer D, Bitterman-Deutsch O, Samson AO, Wakamiya M, Peterson JW, Kirtley ML, Pinchuk IV, Baze WB, Gahl WA, Kleta R, Anikster Y, Chopra AK (2017) Phospholipase A2-activating protein is associated with a novel form of leukoencephalopathy. Brain 140:370–386. 10.1093/brain/aww295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 94:4312–4317. 10.1073/pnas.94.9.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet RJ, Alam I, Levine L (1980) Accumulation of cyclooxygenase products of arachidonic acid metabolism in gerbil brain during reperfusion after bilateral common carotid artery occlusion. J Neurochem 35:653–658. 10.1111/j.1471-4159.1980.tb03704.x [DOI] [PubMed] [Google Scholar]
- Goncalves MB, Williams EJ, Yip P, Yáñez-Muñoz RJ, Williams G, Doherty P (2010) The COX-2 inhibitors, meloxicam and nimesulide, suppress neurogenesis in the adult mouse brain. Br J Pharmacol 159:1118–1125. 10.1111/j.1476-5381.2009.00618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill M, Heinemann A, Hoefler G, Peskar BA, Schuligoi R (2008) Effect of endotoxin treatment on the expression and localization of spinal cyclooxygenase, prostaglandin synthases, and PGD2 receptors. J Neurochem 104:1345–1357. 10.1111/j.1471-4159.2007.05078.x [DOI] [PubMed] [Google Scholar]
- Guo W, Naujock M, Fumagalli L, Vandoorne T, Baatsen P, Boon R, Ordovás L, Patel A, Welters M, Vanwelden T, Geens N, Tricot T, Benoy V, Steyaert J, Lefebvre-Omar C, Boesmans W, Jarpe M, Sterneckert J, Wegner F, Petri S, Bohl D, Vanden Berghe P, Robberecht W, Van Damme P, Verfaillie C, Van Den Bosch L (2017) HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat Commun 8:861. 10.1038/s41467-017-00911-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D, Cunningham DD (1988) Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A 85:3440–3444. 10.1073/pnas.85.10.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Greene ME, Pitts J, Wada RK, Sidell N (2001) Novel expression and function of peroxisome proliferator-activated receptor gamma (PPARϒ) in human neuroblastoma cells. Clin Cancer Res 7:98–104 [PubMed] [Google Scholar]
- Hiruma H, Ichikawa T, Kobayashi H, Hoka S, Takenaka T, Kawakami T (2000) Prostaglandin E2 enhances axonal transport and neuritogenesis in cultured mouse dorsal root ganglion neurons. Neuroscience 100:885–891. 10.1016/S0306-4522(00)00347-X [DOI] [PubMed] [Google Scholar]
- Ho L, Luterman JD, Aisen PS, Pasinetti GM, Montine TJ, Morrow JD (2000) Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology 55:323. 10.1212/WNL.55.2.323 [DOI] [PubMed] [Google Scholar]
- Hounoum BM, Vourch P, Felix R, Corcia P, Patin F, Guéguinou M, Potier-Cartereau M, Vandier C, Raoul C, Andres CR, Mavel S, Blasco H (2016) NSC-34 motor neuron-like cells are unsuitable as experimental model for glutamate-mediated excitotoxicity. Front Cell Neurosci 10:1–9. 10.3389/fncel.2016.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Zhang SC (2009) Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc 4:1295–1304. 10.1038/nprot.2009.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuno T, Masumoto H, Yamamizu K, Yoshioka M, Minakata K, Ikeda T, Sakata R, Yamashita JK (2017) Efficient and robust differentiation of endothelial cells from human induced pluripotent stem cells via lineage control with VEGF and cyclic AMP. PLoS ONE 12:e0176238. 10.1371/journal.pone.0176238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto N, Kobayashi K, Kosaka K (1989) The formation of prostaglandins in the postmortem cerebral cortex of Alzheimer-type dementia patients. J Neurol 236:80–84. 10.1007/BF00314401 [DOI] [PubMed] [Google Scholar]
- Jaiswal MK (2017) Therapeutic opportunities and challenges of induced pluripotent stem cells-derived motor neurons for treatment of amyotrophic lateral sclerosis and motor neuron disease. Neural Regen Res 12:723–736. 10.4103/1673-5374.206635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann S, Dahm M, Kipp M, Zahn U, Beyer C (2011) Regulation of choline acetyltransferase expression by 17β-oestradiol in NSC-34 cells and in the spinal cord. J Neuroendocrinol 23:839–848. 10.1111/j.1365-2826.2011.02192.x [DOI] [PubMed] [Google Scholar]
- Kanakasabai S, Pestereva E, Chearwae W, Gupta SK, Ansari S, Bright JJ (2012) PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes. PLoS ONE 7:1–14. 10.1371/journal.pone.0050500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Matsushita T, Kita Y, Uematsu S, Akira S, Kira J, Ishii S, Shimizu T (2009) Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc Natl Acad Sci 106:21807–21812. 10.1073/pnas.0906891106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Kweon KJ, Kim P, Kim HJ, Kim SS, Sohn NW, Maeng S, Shin JW (2017) Ginsenoside Rg3 improves recovery from spinal cord injury in rats via suppression of neuronal apoptosis, pro-inflammatory mediators, and microglial activation. Molecules 22:122. 10.3390/molecules22010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Zahir T, Tator CH, Shoichet MS (2011) Effects of dibutyryl cyclic-AMP on survival and neuronal differentiation of neural stem/progenitor cells transplanted into spinal cord injured rats. PLoS ONE 6:1–12. 10.1371/journal.pone.0021744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S (1997) Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol 122:217–224. 10.1038/sj.bjp.0701367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, Sasaki S, Iwata M, Noguchi N, Uchida K (2002) 15-Deoxy-Delta(12,14)-prostaglandin J(2): The endogenous electrophile that induces neuronal apoptosis. Proc Natl Acad Sci U S A 99:7367–7372. 10.1073/pnas.112212599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Cuvillier JM, Lee B, Shen R, Lee JW, Lee SK (2012) Fusion protein Isl1-Lhx3 specifies motor neuron fate by inducing motor neuron genes and concomitantly suppressing the interneuron programs. Proc Natl Acad Sci USA 109:3383–3388. 10.1073/pnas.1114515109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee B, Lee JW, Lee SK (2009) Retinoid signaling and Neurogenin2 function are coupled for the specification of spinal motor neurons through a chromatin modifier CBP. Neuron 62:641–654. 10.1016/j.neuron.2009.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lee B, Ruiz EC, Pfaff SL (2005) Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev 19:282–294. 10.1101/gad.1257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li W, Rose ME, Pascoe JL, Miller TM, Ahmad M, Poloyac SM, Hickey RW, Graham SH (2013a) Prostaglandin D2 toxicity in primary neurons is mediated through its bioactive cyclopentenone metabolites. Neurotoxicology 39:35–44. 10.1016/j.neuro.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Rose ME, Miller TM, Li W, Shinde SN, Pickrell AM, Poloyac SM, Graham SH, Hickey RW (2013b) COX2-derived primary and cyclopentenone prostaglandins are increased after asphyxial cardiac arrest. Brain Res 26(1519):71–77. 10.1016/j.brainres.2013.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Rouault C, Guesnon M, Zhu W, Clément K, Degrelle SA, Fournier T (2020) Comparative study of PPAR γ targets in human extravillous and villous cytotrophoblasts. PPAR Res 2020:1–18. 10.1155/2020/9210748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tu H, Zhang D, Zheng H, Li YL (2012) Voltage-gated sodium channel expression and action potential generation in differentiated NG108-15 cells. BMC Neurosci 13:129. 10.1186/1471-2202-13-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier O, Dahm M, Brück S, Brück S, Beyer C, Johann S (2013) Differentiated NSC-34 motoneuron-like cells as experimental model for cholinergic neurodegeneration. Neurochem Int 62:1029–1038. 10.1016/j.neuint.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Probst-Cousin S, Bergmann M, Neuhuber W, Neundörfer B, Heuss D (2003) Expression and localization of cyclooxygenase-1 and -2 in human sporadic amyotrophic lateral sclerosis. Eur J Neurosci 18:1527–1534. 10.1046/j.1460-9568.2003.02879.x [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Papalexis N, Dworkin S (2012) CREB signalling in neural stem/progenitor cells: Recent developments and the implications for brain tumour biology. BioEssays 34:293–300. 10.1002/bies.201100133 [DOI] [PubMed] [Google Scholar]
- Mattammal MB, Strong R, Lakshmi VM, Chung HD, Stephenson AH (1995) Prostaglandin H synthetase-mediated metabolism of dopamine: Implication for Parkinson’s disease. J Neurochem 64:1645–1654. 10.1046/j.1471-4159.1995.64041645.x [DOI] [PubMed] [Google Scholar]
- Mitani K, Sekiguchi F, Maeda T, Tanaka Y, Yoshida S, Kawabata A (2016) The prostaglandin E2/EP4 receptor/cyclic AMP/T-type Ca2+ channel pathway mediates neuritogenesis in sensory neuron-like ND7/23 cells. J Pharmacol Sci 130:177–180. 10.1016/j.jphs.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Mitra P, Brownstone RM (2012) An in vitro spinal cord slice preparation for recording from lumbar motoneurons of the adult mouse. J Neurophysiol 107:728–741. 10.1152/jn.00558.2011 [DOI] [PubMed] [Google Scholar]
- Miyagishi H, Kosuge Y, Ishige K, Ito Y (2012) Expression of microsomal prostaglandin E synthase-1 in the spinal cord in a transgenic mouse model of amyotrophic lateral sclerosis. J Pharmacol Sci 118:225–236. 10.1254/jphs.11221fp [DOI] [PubMed] [Google Scholar]
- Miyagishi H, Kosuge Y, Takano A, Endo M, Nango H, Yamagata-Murayama S, Hirose D, Kano R, Tanaka Y, Ishige K, Ito Y (2017) Increased expression of 15-hydroxyprostaglandin dehydrogenase in spinal astrocytes during disease progression in a model of amyotrophic lateral sclerosis. Cell Mol Neurobiol 37:445–452. 10.1007/s10571-016-0377-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishi H, Kosuge Y, Yoneoka Y, Ozone M, Endo M, Osada N, Ishige K, Kusama-Eguchi K, Ito Y (2013) Prostaglandin E2-Induced cell death is mediated by activation of EP2 receptors in motor neuron-like NSC-34 cells. J Pharmacol Sci 121:347–350. 10.1254/jphs.12274SC [DOI] [PubMed] [Google Scholar]
- Mohan S, Ahmad AS, Glushakov AV, Chambers C, Doré S (2012) Putative role of prostaglandin receptor in intracerebral hemorrhage. Front Neurol 3:1–17. 10.3389/fneur.2012.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado E, Ortiz S, Molina-Holgado F, Guaza C (2000) Induction of COX-2 and PGE(2) biosynthesis by IL-1beta is mediated by PKC and mitogen-activated protein kinases in murine astrocytes. Br J Pharmacol 131:152–159. 10.1038/sj.bjp.0703557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J (2011) Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res 89:117–126. 10.1002/jnr.22547 [DOI] [PubMed] [Google Scholar]
- Nango H, Kosuge Y, Miyagishi H, Sugawa K, Ito Y, Ishige K (2017) Prostaglandin E2 facilitates neurite outgrowth in a motor neuron-like cell line, NSC-34. J Pharmacol Sci 135:64–71. 10.1016/j.jphs.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Nango H, Kosuge Y, Sato M, Shibukawa Y, Aono Y, Saigusa T, Ito Y, Ishige K (2020a) Highly efficient conversion of motor neuron-like NSC-34 cells into functional motor neurons by prostaglandin E2. Cells 9:1741. 10.3390/cells9071741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nango H, Kosuge Y, Yoshimura N, Miyagishi H, Kanazawa T, Hashizaki K, Suzuki T, Ishige K (2020b) The molecular mechanisms underlying prostaglandin D2-induced neuritogenesis in motor neuron-like NSC-34 cells. Cells 9:1–16. 10.3390/cells9040934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S (2003) A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 40:81–95. 10.1016/j.neuron.2003.08.006 [DOI] [PubMed] [Google Scholar]
- O’Donnell PE, Ye XZ, DeChellis MA, Davis VM, Duan SZ, Mortensen RM, Milstone DS (2016) Lipodystrophy, diabetes and normal serum insulin in PPARγ-deficient neonatal mice. PLoS ONE 11:e0160636. 10.1371/journal.pone.0160636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou D, Le Verche V, Jacquier A, Ikiz B, Przedborski S, Re DB (2010) Inflammation in ALS and SMA: sorting out the good from the evil. Neurobiol Dis 37:493–502. 10.1016/j.nbd.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Lee RD, Kang SK, Han SY, Park KL, Yang KH, Song YS, Park HJ, Lee YM, Yun YP, Oh KW, Kim DJ, Yun YW, Hwang SJ, Lee SE, Hong JT (2004) Neuronal differentiation of embryonic midbrain cells by upregulation of peroxisome proliferator-activated receptor-gamma via the JNK-dependent pathway. Exp Cell Res 297:424–433. 10.1016/j.yexcr.2004.03.034 [DOI] [PubMed] [Google Scholar]
- Peebles RS (2019) Prostaglandins in asthma and allergic diseases. Pharmacol Ther 193:1–19. 10.1016/j.pharmthera.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestereva E, Kanakasabai S, Bright JJ (2012) PPARγ agonists regulate the expression of stemness and differentiation genes in brain tumour stem cells. Br J Cancer 106:1702–1712. 10.1038/bjc.2012.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TS, Thebeau CN, Ajit D, Camden JM, Woods LT, Wood WG, Petris MJ, Sun GY, Erb L, Weisman GA (2013) Up-regulation and activation of the P2Y(2) nucleotide receptor mediate neurite extension in IL-1β-treated mouse primary cortical neurons. J Neurochem 125:885–896. 10.1111/jnc.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrozziello T, Secondo A, Tedeschi V, Esposito A, Sisalli M, Scorziello A, Di Renzo G, Annunziato L (2017) ApoSOD1 lacking dismutase activity neuroprotects motor neurons exposed to beta-methylamino-L-alanine through the Ca2+/Akt/ERK1/2 prosurvival pathway. Cell Death Differ 24:511–522. 10.1038/cdd.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, Horrocks LA, Farooqui AA (2006) Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders. Brain Res Rev 52:201–243. 10.1016/j.brainresrev.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Qu Q, Li D, Louis KR, Li X, Yang H, Sun Q, Crandall SR, Tsang S, Zhou J, Cox CL, Cheng J, Wang F (2014) High-efficiency motor neuron differentiation from human pluripotent stem cells and the function of Islet-1. Nat Commun 5:1–13. 10.1038/ncomms4449 [DOI] [PubMed] [Google Scholar]
- Redensek A, Rathore KI, Berard JL, López-Vales R, Swayne LA, Bennett SA, Mohri I, Taniike M, Urade Y, David S (2011) Expression and detrimental role of hematopoietic prostaglandin D synthase in spinal cord contusion injury. Glia 59:603–614. 10.1002/glia.21128 [DOI] [PubMed] [Google Scholar]
- Ricciotti E, Fitzgerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31:986–1000. 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma T, Koutsouris A, Yu JZ, Krbanjevic A, Hope TJ, Rasenick MM (2015) Activation of microtubule dynamics increases neuronal growth via the nerve growth factor (NGF)- and Gαs-mediated signaling pathways. J Biol Chem 290:10045–10056. 10.1074/jbc.M114.630632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo D, Onodera K, Doi-Torii Y, Ishihara Y, Hattori C, Miwa Y, Tanaka S, Okada R, Ohyama M, Shoji M, Nakanishi A, Doyu M, Okano H, Okada Y (2015) Rapid, efficient, and simple motor neuron differentiation from human pluripotent stem cells. Mol Brain 8:1–15. 10.1186/s13041-015-0172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifani N (2014) Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci 8:1–22. 10.3389/fncel.2014.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DS, Glass CK (2001) Cyclopentenone prostaglandins: New insights on biological activities and cellular targets. Med Res Rev 21:185–210. 10.1002/med.1006 [DOI] [PubMed] [Google Scholar]
- Sumimoto S, Muramatsu R, Yamashita T (2015) Thromboxane A2 stimulates neurite outgrowth in cerebral cortical neurons via mitogen activated protein kinase signaling. Brain Res 1594:46–51. 10.1016/j.brainres.2014.07.048 [DOI] [PubMed] [Google Scholar]
- Takazawa T, Croft GF, Amoroso MW, Studer L, Wichterle H, Macdermott AB (2012) Maturation of spinal motor neurons derived from human embryonic stem cells. PLoS ONE 7:1–9. 10.1371/journal.pone.0040154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiji J, Crawford DA (2010) Prostaglandin E2 and misoprostol induce neurite retraction in Neuro-2a cells. Biochem Biophys Res Commun 398:450–456. 10.1016/j.bbrc.2010.06.098 [DOI] [PubMed] [Google Scholar]
- Tsuchiya H, Hohjoh H, Fujiwara Y, Sugimoto Y, Koshimizu TA (2016) Prostaglandin D2 elicits the reversible neurite retraction in hypothalamic cell line. Biochem Biophys Res Commun 470:804-810. 10.1016/j.bbrc.2016.01.091 [DOI] [PubMed] [Google Scholar]
- Turgeon B, Meloche S (2009) Interpreting neonatal lethal phenotypes in mouse mutants: Insights into gene function and human diseases. Physiol Rev 89:1–26. 10.1152/physrev.00040.2007 [DOI] [PubMed] [Google Scholar]
- Ushikubi F, Sugimoto Y, Ichikawa A, Narumiya S (2000) Roles of prostanoids revealed from studies using mice lacking specific prostanoid receptors. Jpn J Pharmacol 83:279–285. 10.1254/jjp.83.279 [DOI] [PubMed] [Google Scholar]
- Valizadeh-Arshad Z, Shahbazi E, Hashemizadeh S, Moradmand A, Jangkhah M, Kiani S (2018) In vitro differentiation of neural-like cells from human embryonic stem cells by a combination of dorsomorphin, XAV, and A. Cell J 19:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Olschowka JA, Wrathall JR (1997) Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res 759:190–196. 10.1016/s0006-8993(97)00254-0 [DOI] [PubMed] [Google Scholar]
- Wong CT, Ussyshkin N, Ahmad E, Rai-Bhogal R, Li H, Crawford DA (2016) Prostaglandin E2 promotes neural proliferation and differentiation and regulates Wnt target gene expression. J Neurosci Res 94:759–775 [DOI] [PubMed] [Google Scholar]
- Yagami T, Koma H, Yamamoto Y (2016) Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol Neurobiol 53:4754–4771. 10.1007/s12035-015-9355-3 [DOI] [PubMed] [Google Scholar]
- Yokota C, Kaji T, Kuge Y, Inoue H, Tamaki N, Minematsu K (2004) Temporal and topographic profiles of cyclooxygenase-2 expression during 24 h of focal brain ischemia in rats. Neurosci Lett 357:219–222. 10.1016/j.neulet.2003.12.109 [DOI] [PubMed] [Google Scholar]
- Zhang B, He L, Liu Y, Zhang J, Zeng Q, Wang S, Fan Z, Fang F, Chen L, Lv Y, Xi J, Yue W, Li Y, Pei X (2018) Prostaglandin E2 is required for BMP4-induced mesoderm differentiation of human embryonic stem cells. Stem Cell Reports 10:905–919. 10.1016/j.stemcr.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]