Abstract
Exploring the microRNAs and aptamers for their therapeutic role as biological drugs has expanded the horizon of its applicability against various human diseases, explicitly targeting the genetic materials. RNA-based therapeutics are widely being explored for the treatment and diagnosis of multiple diseases, including neurodegenerative disorders (NDD). Latter includes microRNA, aptamers, ribozymes, and small interfering RNAs (siRNAs), which control the gene expression mainly at the transcriptional strata. One RNA transcript translates into different protein types; hence, therapies targeted at the transcriptional sphere may have prominent and more extensive effects than alternative therapeutics. Unlike conventional gene therapy, RNAs, upon delivery, can either altogether abolish or alter the synthesis of the protein of interest, therefore, regulating their activities in a controlled and diverse manner. NDDs like Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, Prion disease, and others are characterized by deposition of misfolded protein such as amyloid-ß, tau, α-synuclein, huntingtin and prion proteins. Neuroinflammation, one of the perquisites for neurodegeneration, is induced during neurodegenerative pathogenesis. In this review, we discuss microRNAs and aptamers' role as two different RNA-based approaches for their unique ability to regulate protein production at the transcription level, hence offering many advantages over other biologicals. The microRNA acts either by alleviating the malfunctioning RNA expression or by working as a replacement to lost microRNA. On the contrary, aptamer act as a chemical antibody and forms an aptamer-target complex.
Keywords: MiRNAs, Neuroinflammation, Aptamer, Neurodegenerative disorders, Antagomir, Sponges
Introduction
The discovery of catalytic RNA and RNA interference dates back in 1980 and 1990, respectively. The horizon of RNA’s biological role has widened from DNA & protein to a flexible and complex molecule that controls the events at the genes and cellular level in all living organisms (Kruger et al. 1982; Guerrier-Takada et al. 1983; Fire et al. 1998). There is potential to reach the exact targets with the biologicals, one of the main constraints of pharmacological drugs (Melnikova 2007). The first in this category was fomivirsen; an antisense oligonucleotide approved in 1998 by the US FDA for the treatment of eye inflammation caused by a virus and an aptamer pegaptanib, antagonist of the vascular endothelial growth factor for treating macular degeneration associated with neovascular aging (Bonetta 2009; Jadeja et al. 2020). Since then, an array of the potential molecule and approaches comprising a new generation of antisense oligos, aptamers, ribozymes, small interfering RNAs (siRNAs), and microRNAs (miRNAs) have seen the development targeting disease-causing element at the RNA level. Unlike traditional gene therapy, that function only by increasing or decreasing the target protein output, the RNA-based treatments regulate gene expression and hence can enhance, reduce or remove the synthesis of a specific protein that regulates its related activities more diversely (Chu et al. 2016). Their specificity in approach is based on their distinct and different mechanism of action such as mRNA (messenger RNA) translation inhibitors (antisense), RNA interference agents (RNAi), catalytically active RNA molecules (ribozymes) and RNAs acts by binding proteins and other ligands (aptamers) at cellular and tissue level. Many RNA or RNA-based therapeutics have achieved the stage of clinical investigation. The major roadblock in RNA-based approaches is their targeted delivery, specificity, stability, and host immune responses. Hence, to combat the same, various advances in synthetic and natural nucleic acid carriers and chemically modified oligonucleotide production is under investigation (Sanghvi 2011; Peer and Lieberman 2011; Salim et al. 2020). Neurodegeneration involves structural and functional changes of the neurons, such as decreased neuronal survival and increased neuronal death. The progressive pathogenic events are under inflammatory and neurotoxic mediators (Ransohoff 2016). Neuroinflammation is initially a defensive response in the brain for the regeneration of damaged CNS glial and neuronal cells. Microglia-resident brain macrophages, astrocytes, neurons, and inflammatory mediators are released from these cells. Unlike other cells, they cannot rebuild or regenerate in the CNS once the neurons are damaged or degenerated (Ransohoff 2016; Russo and McGavern 2016).
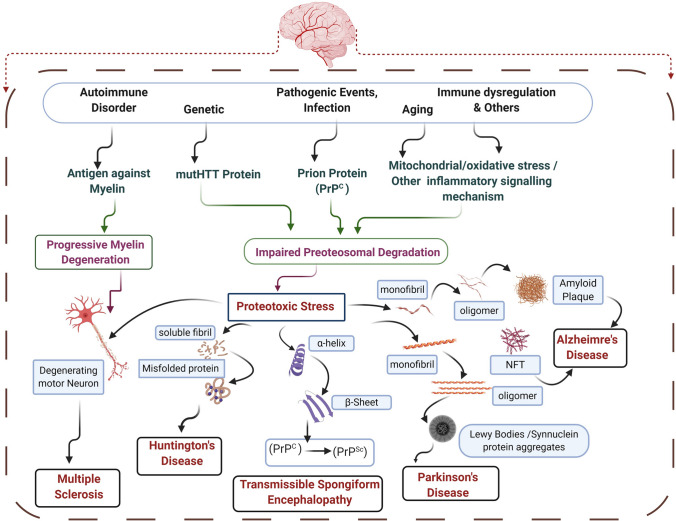
Further, as the neuron degeneration progresses, it fuels excess inflammatory responses, also preventing neuronal regeneration (Kempuraj et al. 2016). Providing various theories of disease pathogenesis, the absolute and exact mechanism behind NDDs is still obscure. However, these diseases' pathology is mainly characterized by the deposition of misfolded proteins like amyloid-ß, tau, α-synuclein, Huntington’s, and prion proteins (Ross and Poirier 2004) (Fig. 1). Therefore, preventing these misfolded proteins' deposition may be a potential approach for the treatment of these diseases. In preclinical models, multiple RNA therapy classes were investigated, and the results were encouraging enough for paving the root for their potential development (Zhou et al. 2019b). In this review, we summarize the popular therapeutic RNA-based approaches and highlights their application in the treatment of neurodegenerative disease (NDD) diseases like Alzheimer’s disease (AD), Parkinson’s disease (PD) and others NDD like Huntington disease (HD), Multiple Sclerosis, Transmissible spongiform encephalopathy (TSE), Amyotrophic Lateral Sclerosis (ALS). It will also discuss miRNAs' interaction, aptamers at various pathological events of NDDs affecting the disease outcome. It will help to enhance our understanding in exploring the biological roles of essential pathogenic genes in these diseases are regulated by these, which potentially control multiple pathways involved in neurodegeneration development. Besides, considering the increasing interest in miRNAs' therapeutic potential and aptamers, we will address current clinical challenges for developing NDD therapies based on RNA.
Fig. 1.
Overview of proteotoxic stress-mediated pathophysiology behind common neurodegenerative disorder (NDD). The various causative agents respective to different NDDs lead to impaired proteasomal degradation. The proteotoxic stress led to the deposition of misfolded protein, oligomer, conformation changes as in Huntington’s disease (HD), α-synuclein deposition in Parkinson’s disease (PD), Amyloid plaque deposition in Alzheimer’s Disease (AD), PrPSc (an abnormal isoform of prion protein) in TSE. These abnormal proteins and their receptors present important targets for RNA-based therapeutics such as aptamer and miRNA
miRNAs: Implication and Therapeutic Strategies
Biogenesis and Functions
The miRNAs were initially discovered while studying the development of nematode C. elegans and led to the discovery of lin-4 and let-7 family (Ambros and Horvitz 1984; Ambros 1989; Lee et al. 1993; Reinhart et al. 2000).The miRNAs are a novel class of small evolutionary preserved (18–25 nucleotides), single-stranded non-coding RNAs that are involved in post-transcriptional gene expression regulation, found in all living organisms, including humans (Bushati and Cohen 2007; Femminella et al. 2015; Maoz et al. 2017; Wang et al. 2019). The biosynthesis and activity of miRNA is a complicated process. In the cellular nucleus, RNA polymerase II/III produces a primary transcript (pri-miRNA); subsequently, RNase Drosha/DGCR8 (DiGeorge syndrome critical region 8) digests the pri-miRNA to release pre-miRNA hairpin structures that move to the cytoplasm with the help of Exportin 5 (Gwizdek et al. 2003; Yi et al. 2003; Lund et al. 2004; Bohnsack et al. 2004). Further, RNA Dicer cleaves the pre-miRNA to create double-stranded miRNA, which then attaches to the proteins of Argonaute (Ago) and finally enters the RNA-induced silencing complex (RISC). Now the mature RISC regulates the mRNA translation by binding to their complementary sequences, located within the untranslated regions (3′-UTRs) (Ambros et al. 2003; Bartel 2004).
Consequently, the targeted mRNAs are either degraded or cannot be translated further into their respective proteins. Tissue-specific and disease-specific expression of each miRNA (Prasad 2017) and their functions are mediated by depletion or translational suppression of the mRNA, further altering their corresponding translated protein levels, ultimately regulating their respective cellular processes (Goodall et al. 2013). As per (Lewis et al. 2005) the human cell represents more than 1000 miRNAs, each having the potential for binding to hundreds of messenger RNAs (mRNAs) (Lewis et al. 2005). The efficiency of one miRNA acting on more than 100 targets has their biochemical basis explained elaborately by the researcher from Bartel's lab. Earlier, there were many reports of supporting the canonical binding between the miRNA with their mRNA target, such as the Watson–Crick pairing of 6–8 nucleotide long fragments seed region at the 5′ end of miRNA to the mRNA, binding of nucleotides beyond the seed region not influencing the miRNA biding efficacy (Bartel 2009; Garcia et al. 2010). However, later the possibility of noncanonical binding was also explained in human miRNA to mRNA interaction (Helwak et al. 2013). The AGO2-miRNA complex and their accessibility to the site define the canonical binding of miRNA to mRNA, including the aftereffects of non-canonical site binding. Recently, the biochemical-based model revealed the miRNA occupancy to their target is predicted by Kd (dissociation constant) for 12 nucleotide sequences (McGeary et al. 2019; Briskin et al. 2020). Alternatively, for specific miRNAs, individual mRNA contains multiple binding sites. Presently, more than 3,400 miRNAs have been recognized in the human genome to date (Grimes et al. 2016). The miRNA is mainly involved in all biological processes such as apoptosis, inflammation, and expression are regulated either by post-transcriptional modification such as histone modification or DNA methylation or by enzyme that stabilizes mature miRNAs (Catalanotto et al. 2016).
In general, such small RNAs have shown to be stable in cerebrospinal fluid (CSF) and blood, possibly because they can be forwarded by liposomes or lipoproteins that stop their degradation (Dorval et al. 2013, 2014). Besides liposome, extracellular circulating miRNA, which acts as an essential biomarker, can also be found as AGO1 and AGO2 protein complex. AGO protein stabilizes the miRNA in a slicing-independent manner, as it decreases the degradation of miRNA and hence increases their half-life. There is no correlation between AGO1 and AGO2 miRNA as various tissue contributes to plasma miRNAs (Winter and Diederichs 2011; Turchinovich and Burwinkel 2012). The miRNAs also act as diagnostic markers. Different methods like microarray and quantitative PCR (Polymerase Chain Reaction) are used to evaluate their biofluids level (Blondal et al. 2013; Roberts et al. 2014; Thorsen et al. 2017). The NGS (next-generation sequencing) is another way to assess the miRNA, which can process millions of sequences read in very few days. The NGS technologies do not get interference of background noise and cross-hybridization. It offers the advantage of generating comprehensive and definitive analysis of all miRNAs from various samples like plasma and sera. However, it also has its limitations, such as being on the expensive side for routine work and the need for computational infrastructure assistance for data analysis and interpretation (Dave et al. 2019). A miRNA can target several genes, and multiple miRNAs can control one gene (Wu et al. 2010; Peter 2010), making miRNAs a possible resource for investigating multifactorial diseases, such as AD (Iqbal and Grundke-Iqbal 2010), PD (Kaur et al. 2019) and other NDDs.
miRNA-Mediated Neuroinflammation in Neurodegenerative Disorder
The miRNAs regulate the neuroinflammatory process and are very well explored at many instances in context of neuroinflammation. The miRNAs can regulate innate and adaptive immunity. They do so by regulating microglia activation, astrocyte reactivity, besides controlling the egressing peripheral immune cells like neutrophils, macrophages, leucocytes, along with T& B cell activities (Gaudet et al. 2018). Their role in initiating and maintaining neuroinflammation is discussed at many places (Su et al. 2016b). Many of the miRNAs also possess the anti-inflammatory properties, such as miRNA-155 promoting the inflammatory process, while miRNA-146a, miRNA-124, and miRNA-21 suppress the inflammatory process. Some miRNAs, such as the let-7 family, act either by promoting or inhibiting the inflammatory process (Thounaojam et al. 2013). The miRNA-124 shows neuroprotection by preventing the microglia polarization. The miRNA-181 is involved in AD (Cogswell et al. 2008; Zhang et al. 2017) and stroke (Ma et al. 2016) by promoting neural death and proinflammatory signals. Some of the crucial miRNAs and their involvement in different neuroinflammatory processes are illustrated in Fig. 2.
Fig. 2.
Schematic representation of inflammatory signaling and their role in neuroinflammation involving immune cells in the central nervous system. Aging and other factors may cause an increase in BBB (Blood Brain Barrier) permeability which led to the migration of peripheral immune cells to CNS, possibly initiating the immune-mediated events. The TLR/MyD88/NF-κβ and JAK-STAT pathways work together in the activation of various inflammatory reactions. Microglia and astrocyte can be neuroprotective or neuroinflammatory based on the underlying events of up or down-regulation of different miRNAs. The C/EBP families of transcription factors are important for various inflammatory processes such as M1/M2 polarization. The effects of some miRNAs like miRNA-155, miRNA-124, miRNA-146a, miRNA-21, and let-7 on inflammatory signaling pathways are described in the text. Anti and proinflammatory miRNAs work together to “fine-tune” the neuroinflammatory response. However, the effect of individual miRNAs in individual cell types is context-dependent as miRNAs may only target transcripts actively being expressed. Some miRNAs have roles in the CNS outside of regulating inflammatory signaling, such as regulating neurogenesis. The presence of miRNAs in extracellular vesicles such as exosomes allows them to participate in intracellular communication. miRNA-124, miRNA-21, and let-7 have been found in exosomes released from neurons, regulating nearby cells such as microglia and astrocyte. Hence miRNAs expressed in neurons can also contribute to inflammatory signaling if they act on neighboring immune cells. TLR (Toll-like Receptor),MyD88 (Myeloid differentiation factor 88), IRAK1/4(interleukin-1 receptor-associated kinase),TRIF[TIR(Toll/interleukin-1receptor)domain-containing adaptor protein inducing interferon beta)], TRAF(TNF receptor-associated factor, TAK(Transforming growth factor β-activated protein kinase 1), TAB2/3(TAK1)-binding protein), NEMO (NF-κβ essential modulator), IKKβ(IKK-related kinase), IRF(Interferon regulatory factors), ARE(Antioxidant response element), ISG(Interferon-stimulated gene) NF- κβ (Nuclear factor kappa-light-chain-enhancer of activated B cells), JAK (Janus kinases), STAT (signal transducer and activator of transcription proteins), C/EBP (CCAAT enhancer-binding proteins)
miRNA-155 acts as a proinflammatory mediator of CNS. It’s get induced in macrophages and microglia through (Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κβ)-dependent Toll-Like Receptor (TLR) signaling (Lippai et al. 2013). Their targets include (Suppressor of Cytokine Signaling (SOCS1) (Cardoso et al. 2016; Wang et al. 2018), (SH2 Domain-Containing Inositol 5′-Phosphatase 1) SHIP1 (Cardoso et al. 2016; Ahmadi et al. 2018; Slota and Booth 2019), and Interleukin 13 receptor alpha 1 (IL13Ra1) (Ahmadi et al. 2018). The miRNA-155 functions in inflammatory signaling by suppressing these factors and inducing neuroinflammation. The miRNA-155 in microglia works by another pathway by involving transcriptional factor p53 (Tumor Protein P53), where it targets the c-Maf (musculoaponeurotic fibrosarcoma), which acts as an anti-inflammatory in immune cells (Jablonski et al. 2016). Furthermore, p53 also suppresses the c-Maf by introducing miRNA-34a and miRNA-145, which targets Twist2 (Twist Family BHLH Transcription Factor 2), an activator of c-Maf expression (Su et al. 2014; Wang et al. 2018). Another isoform of 155, i.e., miRNA-155p along with miRNA-142a-3p also inhibit the p38 MAPK (Mitogen-Activated Protein Kinase) and NF-κB pathways within the microglia cells via specific interference with the miRNA142a-3p/miRNA155-5p-Peli1 axis, which plays a critical role in neuroinflammation, making it a potential therapeutic target (Yu et al. 2019; Yang et al. 2020).
miRNA-146a present in neurons, microglia, and astrocytes (Prada et al. 2018; Hosseini et al. 2020). It acts as a negative regulator of inflammation. Glial cell activation provokes the release of proinflammatory mediator NF-κB, which in turn upregulates miRNA-146a (Yu et al. 2019). The miRNA-146a upon induced by NF-κβ, suppresses its activity by activating TLR (Toll-like receptor) signaling. Its other targets also include proinflammatory mediators like STAT-1(Signal transducer and activator of transcription 1) (Zhou et al. 2019a), IRF-5 (Interferon regulatory factor 5) (Tang et al. 2009). Through the Notch1 pathway, miRNA-146a modulates macrophage polarization also (Huang et al. 2016).
miRNA-124 acts as an anti-inflammatory in the brain and regulates neuronal differentiation (Sun et al. 2015; Huang et al. 2018), specifically expressed in microglia (Ponomarev et al. 2011; Ma et al. 2014). Its anti-inflammatory action is due to TLR-6 and MyD88 (Myeloid differentiation factor 88) downregulation (Ma et al. 2014; Kim et al. 2019).The miRNA-124 has shown a decrease in microglia-mediated neuroinflammation by downregulating TNF-α (Tumor necrosis factor- α) and MHC-II (Major histocompatibility complex-II) (Louw et al. 2016). Indeed, miRNA-124 seems to control microglia's inflammatory phenotype, hence acting as a negative regulator of neuroinflammation.
miRNA-21 expression was observed in immune cells like macrophages, mast cells (Sheedy 2015), including CNS resident microglia (Zhang et al. 2012), neurons (Han et al. 2015). The miRNA-21 also displays anti-inflammatory properties through MyD88 and NF-κβ by activating TLR signaling. Its other target is PDCD4 (Programmed cell death protein 4), which led to downregulation of NF- κβ and increases anti-inflammatory cytokines IL-10 (Sheedy 2015). Furthermore, miRNA-21 acts as an anti-inflammatory by decreasing TNF-α levels (Barnett et al. 2016). However, in another study, M2 polarization was inhibited through the STAT-3(Signal transducer and activator of transcription 3) pathway (Chen et al. 2019).
Let-7 comes under the family of evolutionarily conserved miRNA, in which nine is present in humans, acting as suppressors of tumor cells and maintaining developmental processes (Lee et al. 2016).The Let-7 miRNA suppresses neuroinflammation by promoting macrophages polarization into anti-inflammatory M2 phenotype through the (CCAAT/enhancer-binding protein) C/EBP-δ transcription factor (Li et al. 2018b; Shanzhong Yang et al. 2020) whereas it also inhibits apoptosis and activates the M2 phenotype of microglia as well (Li et al. 2018b). The Let-7 is further able to modulate inflammation by targeting the NF-κβ pathway (Kang et al. 2017), cytokines IL-6, IL-10 production (Schulte et al. 2011), and also by regulating TLR4 receptor downstream (Teng et al. 2013).The miRNA-125 and let-7 both helps in the differentiation of astrocytes by targeting negative regulators of this process within glial progenitor cells (Shenoy et al. 2015). The let-7 families are important controller of various inflammatory processes in the CNS where they may show either pro-inflammatory or anti-inflammatory roles. To summarize the important miRNAs targeting neuroinflammation and neurodegenerative disorders, their source, target gene, we used various search criteria like miRNA, target gene, target organ, upregulation/downregulation and tabulated in Tables 1 and 2.
Table 1.
Summarizing the role of important miRNAs in neurodegenerative disorders
| miRNAs | Diseases | Sources | Functional role/molecular target | Up/down regulation | Search term | References |
|---|---|---|---|---|---|---|
| miRNA-155 | AD | CSF | Effects on complement factor H (CFH) | Downregulation | miRNA in NDD, molecular targets | Lukiw (2012) |
| miRNA-146a | AD | CSF | Acts as an inflammatory response repressor in the brain | Upregulation | – | Sethi and Lukiw (2009) |
| miRNA-124 | AD | Brain | BACE1 (beta-site APP cleaving enzyme 1) | Downregulation | – | An et al. (2017) |
| miRNA-21 | PD | White blood cells |
LAMP2A (Lysosome-associated membrane proteins), α-synuclein |
Upregulation | – | Su et al. (2016a) |
| Let-7 | AD | CSF | TLR7(Toll-like receptor 7) | Upregulation | – | Derkow et al. (2018) |
| miRNA-30e | PD | Serum | NLRP3 (Nod-Like Receptor family pyrin domain containing 3), caspase1 | Upregulation | – | Li et al. (2018a) |
| miRNA-107 | AD | Brain | Tyrosine kinase | Upregulation | – | Wang et al. (2008b) |
| miRNA-125b | AD | CSF, Brain | CDKN2A (cyclin-dependent kinase inhibitor 2A) | Upregulation | – | Pogue et al. (2010) |
| miRNA-29a | AD | Blood, Serum, Cortex | BIM ((bcl-2 associated X protein), BMF ((Bcl2 Modifying Factor), PUMA (p53-upregulated modulator of apoptosis), BACE1 | Downregulation | – | Delay et al. (2012) |
| miRNA-106 | AD | Brain | APP (β-amyloid precursor protein) and ABCA1 (ATP-binding cassette transporter) | Downregulation | – | Qiu et al. (2015), Kim et al. (2012) |
| miRNA-9 | AD | Blood | FGFR1 (Fibroblast Growth Factor Receptor 1), NF-κβ and Sirt1 | Downregulation | – | Krichevsky et al. (2003) |
| miRNA-181a | AD | CSF | Sirt1 | Downregulation | – | Schonrock et al. (2012) |
Table 2.
miRNAs in neurodegenerative disorders including their
source and important gene regulations
| miRNAs | Diseases | Gene regulation | Method | Source | Search term | References |
|---|---|---|---|---|---|---|
| miRNA-9, miRNA-196a | HD | REST-coREST, Mut-Htt | Microarray | Hippocampus, cortex | miRNA as neuroregulator, biomarkers and therapeutic agent in NDD | Martí et al. (2010), Cheng et al. (2013); |
| miRNA-206, miRNA-9 | ALS | HDAC4 (Histone Deacetylase), NEFH (neurofilament heavy polypeptide) | Microarray, qRT-PCR | Blood | – | Zhu et al. (2009), Haramati et al. (2010) |
| miRNA-339-5p, miRNA-202 |
Multiple system atrophy |
SLC1A4 (Solute Carrier Family 1 Member 4), COQ2 (Parahydroxybenzoate-polyprenyltransferase) |
TaqMan low-density arrays, TaqMan assay, Microarray |
Serum, Cerebellum | – | Vallelunga et al. (2014), Lee et al. (2015b) |
| miRNA-203, miRNA-191 | Prion diseases | EGR1 (Early Growth Response 1) | Microarray, qRT-PCR | Brain | – | Saba et al. (2008) |
| miRNA-133b, miRNA-34a | AD | Pitx3 (Pituitary homeobox 3), Tau | qRT-PCR, Microarray | Blood, CSF | – | Kim et al. (2007) |
| miRNA-34b, miR-34c, miRNA-433 | PD | SNCA (Synuclein Alpha), FGF20 (Fibroblast growth factor 20) |
TruSeq small RNA Sequencing, Microarray, qRT-PCR |
Brain, CSF | – | Wang et al. (2008a), Kabaria et al. (2015) |
SNCA Synuclein Alpha, FGF20 fibroblast growth factor 20, Pitx3 pituitary homeobox 3, EGR-1 early growth response protein 1, COQ2 para-hydroxybenzoate-polyprenyl transferase, SLC1A4 neutral amino acid transporter A, HDAC4 nuclear histone deacetylase 4, CoREST corepressor for element-1-silencing transcription factor, REST-coREST RE1-silencing transcription factor, IRF5 interferon regulatory factor 5, MYD88 Myeloid differentiation primary response 88, STAT3 Signal transducer and activator of transcription 3, LRRK2 leucine-rich repeat kinase 2, SH2 Src homology 2, domain-containing inositol polyphosphate 5-phosphatase 1 (SHIP1), The microprocessor complex subunit DGCR8 (DiGeorge syndrome critical region 8)
miRNAs-Based Therapeutics in Neurodegenerative Disorders
The miRNA-based therapeutics can be defined into two groups: either miRNA inhibition to decrease the expression of dysfunctional miRNA as their aberrant expression could also lead to disease or replacement therapy to re-establish the lost miRNA levels within the diseased patients (Bernardo et al. 2015). Determining which miRNA needs to be targeted for a specific therapeutic purpose is an investigational process and requires proper knowledge of the miRNA subtypes. The latter also becomes significant as reports have confirmed the opposite mechanism for different subtypes of the same miRNA and the unpredictable mechanism for some miRNAs (Simonson and Das 2015; Paul et al. 2020). The miRNA mimetics are also known as agomiR, are artificial miRNAs that function as an endogenous counterpart by directly binding to the RISC complex (Lin et al. 2018), which further binds to their targeted mRNA in vivo and provides a mimetic action (Bernardo et al. 2015). O9n the contrary, miRNA inhibitors (maybe genetically or chemically prepared) are opposite to the mimetics are known as antagomirs or anti-miRNA (Mattes et al. 2007; Yang and Mattes 2008) and miRNA sponges (Ebert et al. 2007) which are discussed ahead in brief. Additionally, Circular RNA (circRNA) also acts as a potent regulator of miRNA by acting at transcription steps (Memczak et al. 2013).
Antagomirs
The antagomirs are chemically modified single-strand miRNA inhibitor function by blocking microRNA regulation of targeted gene expression, also known as an anti-miRNA antisense oligonucleotide (ASO) (Mattes et al. 2007; Yang and Mattes 2008). The chemical modification of anti-miRNAs as 2′-O-Me (methylation) and 3′-cholesterol-conjugated oligonucleotides is implemented to become the antisense oligonucleotides with full complementarity to the mature miRNA. Their specificity is characterized by high binding affinity, and considering their therapeutic potential, they can be used in clinical studies. Their specific and complementary binding to their mature target miRNA interferes with their function and is proven to be efficient in many tissues. The silencing of endogenous miRNAs by this novel method is specific, efficient, and long-lasting (Davis et al. 2006; Lima et al. 2018a). The intravenous administration of antagomirs against miRNA-16, miRNA-122, miRNA-192, and miRNA-194 has shown the marked reduction of their corresponding miRNA levels in liver, lung, kidney, heart, intestine, fat, skin, bone marrow, muscle, ovaries, and adrenals. To achieve loss of function (LOF) of corresponding miRNA, anti-miRNA oligonucleotides have been developed with complementary sequences to sequester specific endogenous miRNAs in competition with cellular target mRNAs. This induces miRNA repression and prevents mRNA target degradation via RISC, consequently increasing their translation. The biological significance of silencing miRNAs using antagomirs was studied for miRNA-122, found abundantly in the liver. They are being explored in cancer, known as oncomiRs such as antagomir-130a and antagomir-495. Recently antagomirs are also being investigated in asthma, rheumatoid arthritis (RA), epilepsy, and stroke (Krützfeldt et al. 2005; Stenvang et al. 2012; Atri et al. 2019). Intravenous administration of miRNA15a/16-1–specific inhibitor (antagomir) significantly reduced the cerebral water content and infarct volume, improving the neurological stroke outcomes in the miRNA15a/16-1 knockout mice (Yang et al. 2017). In Alzheimer’s case, the intra-hippocampal delivery of miRNA-146a specific inhibitor (antagomir) in the 5Xfad mice (Yang et al. 2017) significantly reduced the tau hyperphosphorylation; and antagomir214-3p promoted autophagy and apoptosis in neurons of SAMP8 mice (Zhang et al. 2016). Both studies showed restored cognitive abilities post-antagomir administration. In another study, miRNA103a-3p inhibition using its antagomir resulted in neuroprotective effects in vitro in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) derived neurotoxin, MPP +-induced SH-SY5Y cell lines, as well as in-vivo (intra-striatal injection of antagomir) via the Parkin/Ambra1 mitophagy pathway (Zhou et al. 2020).
microRNA Sponges
They are competing endogenous RNA as oligonucleotides, containing multiple complementary tandem binding sites to miRNAs of interest. They are comprised of long non-coding RNAs (lncRNAs), pseudogenes, mRNAs, and circRNAs, and acts by competing with endogenous mRNA targets for RISC binding to inhibit miRNA functions and prevent their binding to respective targets. To exhibit optimum position, they require delivery utilizing transgene-encoding vector to induce stable inhibition of the corresponding miRNA. Like antagomirs, miRNA sponges can also be used to target multiple miRNAs. The sponge consists of multiple miRNA-binding sites (MBS) separated by a spacer sequence of 4–6 nucleotides (Ebert et al. 2007; Ebert and Sharp 2010; Kluiver 2012a, b). Hence it owns the advantage of inhibiting all miRNA clusters when multiple MBSs are introduced (Kluiver 2012b). To design a specific miRNA sponge a web-based tool, microRNA sponge generator, and tester (miRNAsong) is available (Barta et al. 2016). The miRNA-277 sponge is used recently to block rCGG repeat-mediated neurodegeneration in Fragile X-associated tremor/ataxia syndrome (FXATS), a late-onset neurodegenerative disorder (Basak et al. 2016).
CircularRNA
The circular RNAs (circRNAs) also belong to non-coding RNAs (ncRNAs) involved in transcriptional and posttranscriptional gene expression regulation. At the initiation step, circRNA acts by interacting with Exon -Intron and interacting with Polymerase II during the elongation step. They partially or fully bind to the complementary oligonucleotides, traps the endogenous mRNA, inhibit its effect in vivo, and sequesters its target transcript (Tang et al. 2017; Lima et al. 2018b). It also acts by sponging miRNAs which controls a large set of biological processes. The circRNAs sponge activity also affects these pathways and thereby participates in human disease initiation and progression. Many circRNA were identified in human and mouse brains, and one of them, ciRS-7 is a circular sponge for miRNA-7 (Hansen et al. 2013). The circMbl is also a circRNA that regulates its own expression by acting as an RBP (RNA binding protein) (Bose and Ain 2018). The expressions pattern and functions of circRNAs have been explored in various disorders, including those of the nervous system, cardiovascular system, cancer, and other common diseases (Lu 2020). As circRNAs are easily detected in many clinical samples, they also present great potential as biomarkers for disease diagnosis and prognostic evaluation (Wu et al. 2018).
Meanwhile, one of the biggest concerns over miRNA-based therapeutics is their targeted delivery and choice of the delivery system that can efficiently surpass the bloodstream or escape the renal excretion and eventually reach to their biological targets (Simonson and Das 2015). Like most gene-based therapy, the delivery system for miRNAs involves using viral and non-viral vectors (Pereira et al. 2013). Adenovirus, retrovirus, and lentivirus are the most commonly used viral vectors (Yang 2015). The retrovirus and lentivirus use reverse-transcription of viral RNA for incorporating the target DNA into the host chromosomes. On the other hand, Adenovirus requires a helper virus to carry out the transfection of miRNA into the host chromosome (Yang 2015; Ojala et al. 2015). The nonviral systems use liposomes and lipid nanoparticles (LNP)-mediated carrier systems (Lee et al. 2019). They are not as efficient as the viral system. Still, they are least toxic without any limitations to the DNA size. These liposomes are made of cationic and polyethyleneglycol (PEG) lipids that increase stability while minimizing toxicity (Wen 2016). A PEG moiety conforms LNPs, an ionizable cationic lipid, i.e., 1,2-dioleyl-3-dimethylammonium propane (DODAP), providing better encapsulation efficiency than liposomes (Campani et al. 2016). Aptamers are also being used as the delivery agent for miRNA besides acting as diagnostic and therapeutic agents in NDD (Chandola and Neerathilingam 2020).
miRNA in Alzheimer’s Disease
The study of the 13-brain-associated miRNAs in human hippocampus specimens from both fetal and older adults has shown that the dysregulation and alterations of tissue-specific miRNAs contribute to the AD pathology can be targeted as a therapeutic strategy for AD. Subsequently, tremendous research has shown changes in several miRNAs between AD patients and age-matched control, further proving that miRNAs can significantly impact AD pathogenesis (Goodall et al. 2013; Henriques et al. 2020). The early stage of AD is characterized by a decline in synaptic functions. Recent reports have focused on the role of miRNA in synaptic alteration using microarray in the AD model. They have reported that the expression of miRNA-30b is significantly altered, which further regulates several targeted gene expressions. The expressions of genes such as Sirt1, ephB2 (Ephrin type-B receptor 2), and GluA2 (Glutamate Ionotropic Receptor AMPA Type Subunit 2) robustly increased as the aftereffect of binding of miRNA-30b at 3′UTRs’ region of their genetic element at DNA. A similar result was confirmed in another study where the expression of these genes increased in the post-mortem hippocampus of AD patients. An increase in the expression of miRNA-30b negatively regulated the expression of these genes, i.e., Sirt1, ephB2, and GluA2 in the hippocampus of AD patients (Song et al. 2019). Therefore, a single miRNA regulates several other mRNAs, and a single molecule can be targeted for modifying its signaling cascade. EphB2 is a protein kinase and plays an imperative role in cognitive function such as synaptogenesis, glutamate receptor expression, dendritic density, axonal guidance, synaptic maturation, and long-term potentiation. Sirt1 is an NADH (nicotinamide adenine dinucleotide (NAD)+ hydrogen (H)-dependent deacetylase and acts as a protective mediator in AD by improving axonal elongation, dendritic branching synaptic plasticity, and memory formation (Reynolds et al. 2018). Additionally, Sirt1 also works as a neuroprotective in AD by declining the cell death induced by inflammation, NF-κB activity, acetylation, and tau phosphorylation. Overall, Sirt1 prevents loss of neurons, reduction in glutamate receptor subunits GluA1, GluN2B (Glutamate [NMDA] receptor subunit epsilon-2), and the PSD-95 (Postsynaptic density protein 95) in the hippocampus in AD. Cell cycle-related neuronal apoptosis (CRNA) is an intracellular event that brings about neuronal death once the mature neuron becomes fully differentiated and re-enter the cell cycle. CRNA may be considered as a possible mechanism for pathological neuronal death (Thubron et al. 2019). The mechanism of cell death includes Aβ accumulation and DNA damage-induced CRNA by activating cyclin D1 (Modi et al. 2012). Cyclin D1 is extremely expressed in the whole brain of AD patients (Yang et al. 2003; Keeney et al. 2012). The miRNA-34a is required to prevent neuronal death by cyclin-dependent apoptosis. The miRNA-34a indorse cell cycle arrest by inhibiting the expression of cyclin D1 that further leads to increased p35-Cdk5 (Cyclin-Dependent Kinase5) activity, and this prevents cell cycle progression (Modi et al. 2012). Activation of cyclin D1 in response to Aβ42 oligomers activates MEK (MAPK/ERK kinase)-ERK (Extracellular Receptor Kinase) pathway, leading to the activation of Aβ42. The miRNA-34a is reversibly controlled by p53 and p73 (TAp73) during cell differentiation (Napoli and Flores 2017). Overall, miRNA-30a and p53 play an important role during neuronal differentiation by decreasing cyclin D1 expression. Neurons treated with Aβ42 activates the MEK-ERK pathway, leading to dysregulation of TAp73/miRNA-34, driving the cell towards apoptosis (Dehghani et al. 2018). ADAM10, an α-secretase, cleaves the amyloid precursors via the non-amyloidogenic pathway in AD. In a clinical study, 700 miRNAs were analyzed, and reportedly miRNA144-5p, miRNA-221, and miRNA-374 were found to be downregulated (Manzine et al. 2018). On the other hand, miRNA-125b participates in AD's pathogenesis via suppression of SphK1 (Sphingosine Kinase 1 receptor). It promoted the APP (Amyloid Precursor Protein) and Aβ peptide production as well as beta-secretase 1 (BACE1) expression (Jin et al. 2018). Another such upregulated miRNA in AD is the miRNA-455-3p (Hsa-miRNA455-3p) which is overexpressed in AD patients reporting mild cognitive impairment (MCI) (Kumar and Reddy 2018). These miRNAs could act as a potential biomarker for Alzheimer’s Disease. Coming to the neuroinflammation led AD changes, there are many miRNAs that can exacerbate the AD by promoting neuroinflammation, such as miRNA-181 promoting neural death and proinflammatory signals in AD. The miRNA-181 family upregulation is seen in AD, including astrocyte reactivity where it regulates the synaptic plasticity through c-FOS (cellular protooncogene) and Sirt (Rodriguez-Ortiz et al. 2014).The miRNA-34 decreases the microglia phagocytosis by targeting TREM2 (triggering receptors expressed on myeloid cells) receptors and hence Aβ clearance (Bhattacharjee et al. 2014). Likewise, miRNA-155 and miRNA-146a mediated regulation of neuroinflammation worsens AD (Alexandrov et al. 2014). The Let 7 acts as a ligand to the TLR-7 receptor, further upregulating the inflammatory pathway (Lehmann et al. 2012).
miRNA in Parkinson’s Disease
Parkinson’s disease is a progressive neurodegenerative disorder with loss of dopaminergic neurons in the nigrostriatal region and the misfolding of α-synuclein protein. Multiple genes reported to be involved in the pathogenesis include SNCA (Lewy body formation), LRRK2 (associated with microtubule binding and vesicular transport), and GBA (encodes glucocerebrosidase enzyme, and is responsible for α-synuclein degradation) (Hershey and Peavy 2015). RNA sequencing, microarray, and QPCR (quantitative polymerase chain reaction) profiling studies have shown that various subtypes of miRNAs; miRNA-30, miRNA-485, miRNA-29, miRNA-26, and let-7 act as a significant contributor to the PD pathology, which may be aversive or neuroprotective (Gui et al. 2015; Goh et al. 2019; Thubron et al. 2019). In the post-mortem of PD patient brains, miRNA-26a, miRNA-30b, and let-7b in the substantia nigra (SN) (Briggs et al. 2015), miRNA-26a, miRNA-30b, and let-7g-3p in the exosomes isolated from the CSF (Gui et al. 2015); let-7e, miRNA-30d, miRNA-29a, miRNA-29b-1 and miRNA-29b-2 in cingulate gyri were upregulated. The miRNA-26b and miRNA-30a have also overexpressed in the LRRK2-KO (Knockout) mice (Tatura et al. 2016), implying the plausible role of LRRK2 in the microRNA regulation. The miRNAs let-7d-5p, let-7f-5p, and let-7g were positively correlated in the prefrontal cortex and the blood of PD patients (Chatterjee and Roy 2017). On the other hand, miRNA29c levels were downregulated in the CSF exosomes (Gui et al. 2015). In addition, miRNA-29 also modulates the T cell-mediated immune response and repression of IFN-γ production (Steiner et al. 2011). Owing to its protective role in inflammation, miRNA-30e also can ameliorate the neuroinflammation by decreasing the proinflammatory mediators i.e. cytokines TNF-α, COX-2 and iNOS, and also inhibit the activation of NLRP3 inflammasome (Li et al. 2018a). Also, miRNA-124 upregulates the microglial activation and targets the p62 (Protein62), p38 MAPK, and autophagy, ameliorating the neuroinflammation in PD patients (Yao et al. 2019). The miRNA485-5p was reportedly downregulated in SN (Cardo et al. 2014), while the complementary strand, miRNA485-3p, was upregulated in the putamen tissues (Nair and Ge 2016) of PD patient’s post-mortem brain. The miRNA-485-associated regulations include apoptosis, synaptic plasticity, and MMP-14 upregulation within the neurons and microglia/macrophages. The miRNA485-3p targets TNF receptor (TNFR)-associated death domain (TRADD) and prevent TNF-α-induced neuronal cell apoptosis (Chen et al. 2016b). The miRNA-26a targets PTEN (phosphatase and tensin homolog), activating PINK1(PTEN-induced kinase 1) and proteolytic cascade for apoptosis (Huse et al. 2009). It also modulates the immune system via dampening the TNF-α/NF-κB signaling axis by silencing HMGA1 (High-mobility group protein A1) and MALT1 (Mucosa-associated lymphoid tissue lymphoma translocation protein 1, the NF-κB signaling factors (Chen et al. 2016a). In a recent study, miRNA-153 and miRNA-223 were also studied in the salivary secretions and were suggested as potential biomarkers for idiopathic parkinsonism (Cressatti et al. 2020). In a recent study, miRNA30-hSNCA was delivered by adeno vector AAV2/8 in a rat model, silencing the SNCA gene which ultimately provided protection against the forelimb defects and decreased the loss of tyrosine hydroxylase neurons within the SN, but unfortunately showed negative effects in the striatum. Besides, it also showed the swelling in the substantia nigra, and thus was withdrawn from the clinical study (Khodr et al. 2014).
miRNA in Other Neurodegenerative Disorders
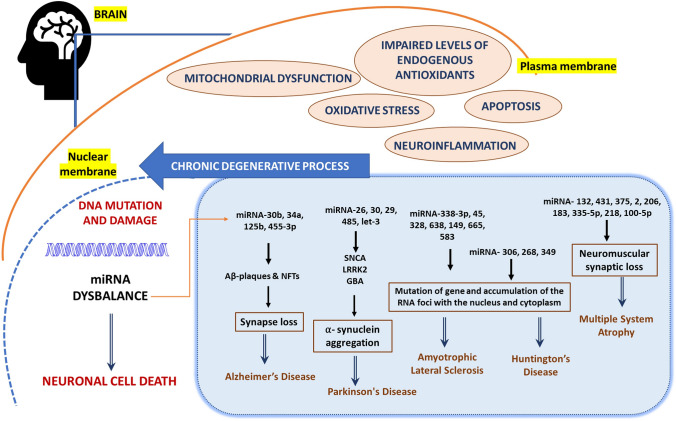
The expression profiles of miRNAs in various tissues and bio fluids are reported to be altered in many other neurodegenerative disorders, including Huntington’s disease, multiple system atrophy (MSA), amyotrophic lateral sclerosis(ALS), multiple sclerosis, epilepsy, and psychiatric disorders (Gilman et al. 2008; Issler and Chen 2015; Vistbakka et al. 2017). In a study, the ALS patients were reported with eight dysregulated miRNAs: miRNA338-3p, -45, -1275, -328, -638, -149, -665, and -583 (De Felice et al. 2012). Mutation in the C9orf72 (c G4C2 repeat in chromosome 9 open reading frame 72) gene of ALS patient, believed to cause RNA foci accumulation within the nucleus and deposition of dipeptide repeat (DPR) proteins within the cytoplasm. Thus, silencing the C9orf72 gene coding the miR-101 and -451 scaffolds using adeno vector (AAV5), helped to ameliorate the toxic accumulation within the nucleus and cytoplasm (Martier et al. 2019). In another study, artificial miRNA molecules: artificial miRNAs: mi268.5 and mi306.12v16G were designed to target the mutant HTT (mHTT) in transgenic mice, where preferential silencing of mutant allele over wild type (WT) was observed (Monteys et al. 2015). Adenovirus-mediated delivery of mHTT (AAV5-miHTT) was also studied recently in a humanized HD mouse model via intrastriatal administration, with successful reduction of HTT in the cortex, and striatum (Caron et al. 2020). In another study, the p53/miRNA34a/Sirt1 axis has shown to positively participate in the R6/2 mice model of Huntington’s disease and could also be another therapeutic target for HD (Reynolds et al. 2018).
Spinal muscular atrophy (SMA), caused by degeneration of α-motor neurons in the spinal cord, is associated with mutation or deletion of the survival motor neuron 1 (SMN1) gene, ultimately leading to progressive skeletal muscle atrophy and symmetric limb paralysis (Groen et al. 2018; Schellino et al. 2019). Several miRNAs have been reported to be involved in SMA, including miRNA-132, miRNA-431, miRNA-375, miRNA-206, miRNA-183, miRNA335-5p, miRNA-218, and miRNA-100-5p (Luchetti et al. 2015; Magri et al. 2018). Out of these, miRNA-9, miRNA-206, miRNA-183, and miRNA-375 have shown the potential to be a therapeutic target for the SMA treatment (Magri et al. 2018). For instance, cell transfection of miR-206-HDAC4- FGFBP1 mimics lowered the HDAC4 (Histone D levels and upregulated the FGFBP1 (fibroblast growth factor-binding protein 1) transcription, which is responsible for regenerating neuromuscular synapses (Valsecchi et al. 2020). The intracerebroventricular (ICV) administration of the miRNA-206 mimics to the pups and mice showed reduced SMA pathology by modulating the sodium–calcium exchanger isoform 2 (NCX2), reduced the disease progression, and also improved the behavioral performance (Valsecchi et al. 2015) (Fig. 3).
Fig. 3.
Overview of pathological evet causing micro-RNA dysbalanced leading to neuronal cell death and various important miRNA and their role in notable neurodegenerative disorder (NDD) like Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease, multiple system atrophy
Aptamer: Implication and Therapeutic Strategies
Origin and Function
Aptamers, a collective term used for the single-stranded DNA or RNA nucleotides of (40–100 nucleotides) in length, small peptides, and peptides comprising 10–30 amino acid residues. It is characterized by one defined structure and can form a different secondary structure such as a loop, kissing hairpin. Owing to the above nature, it transforms into a 3-Dimensional structure. It can interact very precisely and affinity with a varied range of molecular target like small metal ions, organic dyes, amino acids antibodies, proteins, whole cells, organs, viruses, bacteria and possibly can be used in live animal (Mayer 2009; Qu et al. 2017; Zhou and Rossi 2017). Aptamers, like antibody-antigen binding, form aptamer-target complex and hence, may be known as chemical antibodies (Gold 1995). It owns many advantages over classical antibodies in terms of extended half-life, relatively smaller size, lower toxicity, and low immunogenicity. It can distinguish closely related molecules such as conformational isomers. Aptamer’s screening is generally carried out by systematic evolution of ligands by exponential enrichment (SELEX). It employs a combinatorial chemistry technique which helps in the screening of specific ligands by repeated rounds of partition and amplification from a large nucleic acid library of 1014–1016 different candidates (Lakhin et al. 2013). The successful development of therapeutically relevant aptamer and its success in clinical trials demands strong support of medicinal chemistry, physicochemical characterization with production technology, and cost optimization (Zhou and Rossi 2017).
Aptamer-Based Therapeutics in Neurodegenerative Disease
The AD, PD, HD, MS, and TSE are those NDDs whose pathomechanism involves progressive loss of protein homeostasis resulting in accumulation of substantially significant misfolded protein (Fig. 1).
Some of the important RNA chemistry-based aptamer and their application against different pathological hallmarks of NDDs are listed in Table 3.
Table 3.
RNA chemistry-based aptamer in neurodegenerative disorders adapted with modification (Bouvier-Müller and Ducongé 2018b)
| Aptamer | Target | Application | Search term | References |
|---|---|---|---|---|
| Alzheimer’s disease | ||||
| β55 | A-β40 | Detection | Aptamer application, Alzheimer therapeutic, diagnosis, amyloid pathology | Farrar et al. 2014) |
| KM33 | A-β40 oligomers (trimer) | Detection | – | Rahimi et al. (2009) |
| KM41 | A-β40 | Detection | – | Rahimi et al. (2009) |
| E2 | A-β40 | Detection | – | Takahashi et al. (2009) |
| N2 | A-β40 | Inhibition | – | Takahashi et al. (2009) |
| TH14 | BACE1-CT | Inhibition | – | Rentmeister et al. (2006) |
| S10 | BACE1-CT | Inhibition | – | Rentmeister et al. (2006) |
| H15 | BACE1-CT | Inhibition | – | Rentmeister et al. (2006) |
| S15 | BACE1-CT | Inhibition | – | Rentmeister et al. (2006) |
| S24 | BACE1-CT | Inhibition | – | Rentmeister et al. (2006) |
| Tau 1 | Human tau40 oligomers | Inhibition | Aptamer application, Alzheimer therapeutic, diagnosis, Tau pathology | Kim et al. (2016b) |
| USP14–1 | Ubiquitin-Proteasome System | Degradation of Tau | – | Lee et al. (2015a) |
| USP14–2 | Ubiquitin-Proteasome System | Degradation of Tau | – | Lee et al. (2015a) |
| USP14–3 | Ubiquitin-Proteasome System | Degradation of Tau | – | Lee et al. (2015a) |
| Parkinson’s disease | ||||
| Dopa2 | Dopamine | Detection | Aptamer application, Parkinson therapeutic, diagnosis | Mannironi et al. (1997) |
| Dopa2/c.1 | Dopamine | Detection | – | Mannironi et al. (1997) |
| Dopa2 | Dopamine | Detection | – | Mannironi et al. (1997) |
| DL02* | Dopamine | Detection | – | Liew et al. (2011), Annoni et al. (2012) |
| DH05* | Dopamine | Detection | – | Liew et al. (2011), Annoni et al. (2012) |
| DH05* | Dopamine | Detection | – | Liew et al. (2011), Annoni et al. (2012) |
| DHc25* | Dopamine | Detection | – | Liew et al. (2011), Annoni et al. (2012) |
| DHc58* | Dopamine | Detection | – | Liew et al. (2011), Annoni et al. (2012) |
| DHc65* | Dopamine | Detection | – | Liew et al. (2011), Annoni et al. (2012) |
| Multiple sclerosis | ||||
| Apt2-9^ | Myelin basic protein (MBP) autoantibody | Detection | Aptamer application, multiple sclerosis, therapeutic, diagnosis | Vorobjeva et al. (2014) |
| Apt2-9a ^ | MBP Autoantibodies | Detection | – | Vorobjeva et al. (2014) |
| Apt2-9b ^ | MBP Autoantibodies | Detection | – | Vorobjeva et al. (2014) |
| Apt2-9c ^ | MBP Autoantibodies | Detection | – | Vorobjeva et al. (2014) |
| Prion disease (ex: transmissible spongiform encephalopathy) | ||||
| Ap1 | Recombinant Syrian golden Hamster rPrP (rPrPC) | Detection | Aptamer application, Prion Disease, therapeutic, diagnosis | Sekiya et al. (2006) |
| Ap2 | rPrPC | Detection | – | Weiss et al. (1997) |
| Ap1 | rPrPC | Detection | – | Weiss et al. (1997) |
| 60-3* | mPrP | Detection | – | Sekiya et al. (2006) |
| SAF-93* | Bovine PrPSc fibril, | Therapeutic | – | Rhie et al. (2003) |
| apt#1 | bPrp25-241 | Detection | – | Murakami et al. (2008) |
| r(GGA)4-17 | bPrP | Detection | – | Murakami et al. (2008) |
| r(GGA)4 | bPrP-β | Therapeutic | – | Murakami et al. (2008), Mashima et al. (2009) |
| DP7* | Human PrP90–129, and complete human, mouse, hamster PrP | Therapeutic | – | Proske et al. (2002) |
*Ribonucleopeptide (RNP) complex
^2′-Fluoro-Pyrididne-RNA complex
Aptamers in Alzheimer’s Disease
Aptamers can interact with pathologic proteins associated with NDD and to prevent the deposition of this protein. Aptamers are the new molecules that could be useful to understand the mechanism for the pathophysiology of these diseases and further to be used as a therapeutic agent. AD brain is characterized by the deposition of amyloid plaques composed of amyloid-β (Aβ) peptides and neurofibrillary tangles made up of hyperphosphorylated tau protein. Liang et al. group selected one DNA aptamer named A1, which binds to BACE1 with high affinity and reasonable specificity. This A1 had the potential to reduce the formation of Aβ-40 and Aβ-42 (Liang et al. 2015). Furthermore, Rentmeister et al. finalized with RNA aptamers aimed at the short cytoplasmic tail (B1-CT) of BACE1 (Germer et al. 2013). As these RNA aptamers can specifically bind to B1-CT, they have not disturbed other biological functions. Hence, they can potentially be used to stop or inhibit the onset of AD. Bitan et al. in 2009 develop RNA aptamers against Aβ40 oligomers. Since these all aptamers are targeted to oligomers, they can emerge as more efficient and specific means compared to antibodies in the application of identifying the Aβ oligomers, inhibiting Aβ toxicity as well as in the diagnosis of AD (Rahimi and Bitan 2010; Tsukakoshi et al. 2012). As Aβ oligomer exerts its toxic effect by interacting with a specific receptor hence another approach that can inhibit the Aβ-induced toxic effect would be targeting their specific receptor (Sengupta et al. 2016). The normal Prion protein receptor (PrPc) is considered as one of the target receptors which binds with very high affinity to Aβ oligomer (Mashima et al. 2009; Laurén et al. 2009).
Moving on to the biomarker, SOMAmers (slow off-rate modified aptamer) possess different functional groups on uracil residues and hence enhances the stability and function of aptamer (Gold et al. 2010). Over 200 biomarkers have been identified, which may help the diagnosis and treatment of AD (Blennow and Zetterberg 2018). Besides targeting the amyloid pathology of AD, aptamers are being investigated, aiming at tauopathy. In the line of latter, Kim et al. identified RNA aptamers having the potential to target human tau, which significantly inhibited the tau oligomerization both in vitro and in cultured cell models of tauopathy without impacting the half-life of tau. Cells on aptamer treatment have shown less susceptibility to experimental proteotoxic stress-induced by tau overexpression. The aptamers treated primary hippocampal neurons have also demonstrated decreased synthetic tau oligomer-mediated neurotoxicity and loss of dendritic spine. Hence the above outcome further strengthens the effective potential of RNA aptamers against pathogenic profile emerging from the tau oligomerization (Kim et al. 2016a).
Aptamers in Parkinson’s Disease
PD is one the second most common NDD affecting the population of above 60 years. It is a motor neuron dysfunction initially characterized by postural instability, resting tremor, bradykinesia which in the later stage progresses to autonomic neuropathies, psychiatric disabilities, and cognitive problems. The pathogenesis of PD is characterized by the formation and deposition of Lewy bodies in neurons and loss of specifically dopaminergic neurons in substantia nigra pars com- pacta (Zhao et al. 2017). The Lewy bodies are mainly composed of misfolded and aggregated α-synuclein (α-syn), a presynaptic protein, along with parkin. Besides α-syn, Parkin, PTEN-induced putative kinase 1 (PINK1), protein deglycase (DJ-1), and leucine-rich repeat kinase 2 (LRRK2) also shown its causative role in PD (Beyer et al. 2009). The α-syn can either be in prefibrillar soluble form or can aggregate into fibrils form (Pieri et al. 2016). Most research outcome supports the hypothesis that the prefibrillar soluble form is more toxic than the mono or multi fibrillar aggregates (Volles and Lansbury 2003). However, the hypothesis is still debatable. Various stressors like oxidative stress can promote α-syn oligomer formation. Additionally, the parkin possesses the ability to degrade the protein aggregate; however, the oxidative modification of the parkin further aggravates the protein deposition and LB formation (Zhao et al. 2017).
Aptamers targeted to the α-syn formation would be extremely useful in diagnosing PD progression and treatment (Jang et al. 2020). The specificity and affinity of the aptamer molecule do play an essential role in selecting the relative binding capacity to α- syn oligomer. The M5-15 is the first aptamer designed by the group of Ikebukuro in 2010, against α- syn oligomer, but lacked specificity (Tsukakoshi et al. 2012). Later the same group came up with eight DNA aptamers possessing strong specificity against α-syn oligomer. Besides acting against α-syn, it has also shown specificity against β-sheet structure hence could also act possibility against Aβ too (Tsukakoshi et al. 2012). Surprisingly, the other form of synuclein, the β-Syn derived peptide (GVLYVGSKTR) can bind to both α-syn fibril and soluble oligomer, further preventing its aggregation. When the peptide was administered in the A53T α-syn-induced Drosophila PD model, there was a decrease in α- syn assembly, and the behavioral deficit was observed (Shaltiel-Karyo et al. 2010).
The other approach which could be helpful in preventing PD is showing protection against dopamine (DA) neuron degeneration in the substantia nigra. The DA is involved in neurotransmission processes in basal ganglia, which anchors motor and non-motor symptoms (Rodriguez-Oroz et al. 2009). The normal physiological function of DA happens within the range of 10–8 to 10–6 M (Zheng and Zhou 2007). Hence to execute the normal motor functioning of DA, its concentration needed to be constantly monitored. To do so, the aptamer targeting the DA would be helpful in monitoring the direct relationship of the pathogenic event concerning DA concentration. Tocchini-Valentini and groups develops RNA aptamer dopa2 targeting DA with high affinity and specificity (Bouvier-Müller and Ducongé 2018a). The RNA-based aptamer, an electrochemical biosensor for selective and label-free analysis of DA, was also developed (Farjami et al. 2013). To enhance the sensitivity and ease of detection, DNA aptamers on a multiple-parallel-connected (MPC) silicon nanowire field-effect transistor have also seen the positive light (Li et al. 2013).
Aptamers in Other Neurodegenerative Disorder
Aptamers have also found their applicability in other neurodegenerative disorders like Huntington's disease (HD), Multiple sclerosis (MS), and transmissible spongiform encephalopathy (TSE). HD individuals show progressive motor and cognitive decline characterized by anxiety, loss of awareness, depression, dementia. The pathogenesis event mainly comprises of misfolding and aggregation of mutant Huntington protein (mHTT) (Shacham et al. 2019). In the case of HD also, aptamers are designed to target these aggregates and, at the same time, can degrade as well. In one of the research findings, mHTT overexpression was induced in PC12 cells and further treated with an aptamer, a 20-m G-rich oligonucleotide. The oligonucleotide treatment has successfully increased the survival rate of cells. Hence it could be inferred that more of the aptamer comprising the above property can be synthesized aimed for the HD mechanistic study and to come out with appropriate treatment options (Nastasijevic et al. 2012).
MS is an autoimmune disorder where the immune system targets the myelin sheath and leads to its destruction. Myelin sheath provides the insulating covering to the neuron and plays a vital role in neurotransmission. MS is characterized by visual loss, fatigue, abnormal gait, and cognitive impairment (Ghasemi et al. 2017). The target here is the myelin which could be regulated using aptamer. Nastasijevic and the group have tried 40-nucleotide DNA aptamer against murine myelin. Their findings have reported that aptamer has promoted remyelination in mice. Later a guanosine-rich 40-m DNA aptamer, LJM-3064, has increased myelination. Further, two more aptamers were designed targeting myelin in MS's case (Nastasijevic et al. 2012; Smestad and James Maher 2013; Rozenblum et al. 2014; Wilbanks et al. 2019).
TSE is another form of NDD affecting the human. The main pathogenic event involves converting normal cellular prion protein a (PrPC), an α-helix rich isoform, to abnormal PrPSC, an β-sheet isoform. The latter starts accumulating in the brain, which results in encephalopathy (Scallet et al. 2005). The TSE could be treated or diagnosed by estimating the relative level of PrPC and PrPSC along with inhibiting their conversion step. In 2003, 2′-fluoro-modified RNA oligomers, SAF-93 were designed and tested against both isoforms of PrP. The SAF-3 has shown tenfold more selective affinity towards PrPSc than PrPC and could also inhibit the interconversion. However, the SAF-P3 did not see the light of its clinical application because of its complexity and structural length. Later the drawback of above was rectified by James and the group by synthesizing SAF-93 (1–34, 35bioU, 36–60) (Sayer et al. 2004; Ogasawara et al. 2007; Bouvier-Müller and Ducongé 2018b).
Conclusion
The RNA-based therapy, such as miRNA and aptamers-based biopharmaceuticals, is a new therapy class, presenting significant growth and potential for treating and halting chronic and rare diseases. As either target or as a therapeutic molecule, the miRNAs are in the early stages, and any shred of evidence presented by the RNA-based research data has shown future potential. The miRNAs act either as mimetics or inhibitors, i.e., antagomiRs, sponges, and circular RNA. The miRNAs are also useful as diagnostic and prognostic biomarkers in several NDDs, as they can be detected as intracellular and extracellular circulating RNA in blood plasma, serum, and CSF. These disorders do not have any curative therapies at present, and the main reason for that is their late detection in most cases. The neuroinflammation being strongly associated with neurodegeneration, controlling the neuroinflammatory cascade can help avoid the pathology's progression. One of the most challenging hurdles for its use as a therapeutic strategy is to restore or improve their expression, considering that miRNAs can regulate a single gene and entire cellular pathways and associated processes. Elsewhere, RNA aptamers employ the approach of acting directly via interacting with the cellular receptors upon delivery of therapeutically important oligonucleotides. It has got multiple applications as a diagnostic marker and therapeutic agent. It functions promptly by interacting with the target; therefore, the site-specific targeted delivery is a significant concern and challenge associated with aptamer. Besides, it acts as an efficient, targeted delivering agent for miRNA.
Compared to other RNA-based therapeutics, aptamers own the advantage of having cheaper manufacturing processes, non-immunogenic and thermostable. The early diagnosis and curative treatment of NDDs have always been challenging and still far from complete understanding. Based on this review's presented discussion, it would not be an exaggeration to say that the horizon of RNA-based drugs is limitless. We can expect more RNA-based therapeutics to get their due acceptability with improved drug potency, reduced toxicity, and immunogenicity compared to traditional medicine in the NDD diagnostic and therapeutic in the coming future.
Acknowledgements
Islauddin Khan, including other authors, is thankful to the Department of Pharmacology and Toxicology, NIPER Hyderabad, Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers Govt. of India.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Islauddin Khan, Kumari Preeti, and Valencia Fernandes have contributed equally.
Contributor Information
Dharmendra Kumar Khatri, Email: dkkhatri10@gmail.com.
Shashi Bala Singh, Email: drshashisingh@gmail.com.
References
- Ahmadi M, Rahbarghazi R, Shahbazfar AA, Keyhanmanesh R (2018) Monitoring IL-13 expression in relation to miRNA-155 and miRNA-133 changes following intra-tracheal administration of mesenchymal stem cells and conditioned media in ovalbumin-sensitized rats. Thai J Vet Med 48:347–355 [Google Scholar]
- Alexandrov PN, Dua P, Lukiw WJ (2014) Up-regulation of miRNA-146a in progressive, age-related inflammatory neurodegenerative disorders of the human CNS. Front Neurol. 10.3389/fneur.2014.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V (1989) A hierarchy of regulatory genes controls a larva-to-adult developmental switch in C. elegans. Cell 57:49–57. 10.1016/0092-8674(89)90171-2 [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitz HR (1984) Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226:409–416. 10.1126/science.6494891 [DOI] [PubMed] [Google Scholar]
- Ambros V, Bartel B, Bartel DP et al (2003) A uniform system for microRNA annotation. RNA 9:277–279. 10.1261/rna.2183803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F, Gong G, Wang Y et al (2017) MiR-124 acts as a target for Alzheimer’s disease by regulating BACE. Oncotarget 8:114065–114071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni C, Nakata E, Tamura T et al (2012) Construction of ratiometric fluorescent sensors by ribonucleopeptides. Org Biomol Chem 10:8767–8769. 10.1039/c2ob26722e [DOI] [PubMed] [Google Scholar]
- Atri C, Guerfali FZ, Laouini D (2019) MicroRNAs in diagnosis and therapeutics. In: AGO-driven non-coding RNAs. Elsevier, pp 137–177
- Barnett RE, Conklin DJ, Ryan L et al (2016) Anti-inflammatory effects of miR-21 in the macrophage response to peritonitis. J Leukoc Biol 99:361–371. 10.1189/jlb.4a1014-489r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta T, Peskova L, Hampl A (2016) MiRNAsong: a web-based tool for generation and testing of miRNA sponge constructs in silico. Sci Rep 6:1–8. 10.1038/srep36625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297 [DOI] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak I, Patil KS, Alves G et al (2016) MicroRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative diseases. Cell Mol Life Sci 73:811–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo BC, Ooi JYY, Lin RCY, Mcmullen JR (2015) miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart Host transcriptomics and Metatranscriptomics: interactions between pateint, microbes and bacteriophage view project treatment of severe Staphylococcus aureus inf. Futur Sci 7:1771–1792. 10.4155/fmc.15.107 [DOI] [PubMed] [Google Scholar]
- Beyer K, Domingo-Sàbat M, Ariza A (2009) Molecular pathology of lewy body diseases. Int J Mol Sci 10:724–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S, Zhao Y, Lukiw WJ (2014) Deficits in the miRNA-34a-regulated endogenous TREM2 phagocytosis sensor-receptor in Alzheimer’s disease (AD); an update. Front Aging Neurosci. 10.3389/fnagi.2014.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H (2018) Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med 284:643–663 [DOI] [PubMed] [Google Scholar]
- Blondal T, Jensby Nielsen S, Baker A et al (2013) Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 59:S1–S6 [DOI] [PubMed] [Google Scholar]
- Bohnsack MT, Czaplinski K, Görlich D (2004) Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10:185–191. 10.1261/rna.5167604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetta L (2009) RNA-based therapeutics: ready for delivery? Cell 136:581–584. 10.1016/j.cell.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Bose R, Ain R (2018) Regulation of transcription by circular RNAs. Advances in experimental medicine and biology. Springer, New York, pp 81–94 [DOI] [PubMed] [Google Scholar]
- Bouvier-Müller A, Ducongé F (2018a) Application of aptamers for in vivo molecular imaging and theranostics. Adv Drug Deliv Rev 134:94–106. 10.1016/j.addr.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Bouvier-Müller A, Ducongé F (2018b) Nucleic acid aptamers for neurodegenerative diseases. Biochimie 145:73–83. 10.1016/j.biochi.2017.10.026 [DOI] [PubMed] [Google Scholar]
- Briggs CE, Wang Y, Kong B et al (2015) Midbrain dopamine neurons in Parkinson’s disease exhibit a dysregulated miRNA and target-gene network. Brain Res 1618:111–121. 10.1016/j.brainres.2015.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D, Wang PY, Bartel DP (2020) The biochemical basis for the cooperative action of microRNAs. Proc Natl Acad Sci U S A 117:17764–17774. 10.1073/pnas.1920404117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23:175–205. 10.1146/annurev.cellbio.23.090506.123406 [DOI] [PubMed] [Google Scholar]
- Campani V, De Rosa G, Misso G et al (2016) Lipid nanoparticles to deliver miRNA in cancer article in current pharmaceutical biotechnology. Curr Pharm Biotechnol 17:728–736. 10.2174/138920101708160517234941 [DOI] [PubMed] [Google Scholar]
- Cardo LF, Coto E, Ribacoba R et al (2014) MiRNA profile in the substantia Nigra of Parkinson’s disease and healthy subjects. J Mol Neurosci 54:830–836. 10.1007/s12031-014-0428-y [DOI] [PubMed] [Google Scholar]
- Cardoso AL, Guedes JR, De Lima MCP (2016) Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr Opin Pharmacol 26:1–9. 10.1016/j.coph.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Caron NS, Southwell AL, Brouwers CC et al (2020) Potent and sustained huntingtin lowering via AAV5 encoding miRNA preserves striatal volume and cognitive function in a humanized mouse model of Huntington disease. Nucleic Acids Res 48:36–54. 10.1093/nar/gkz976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalanotto C, Cogoni C, Zardo G (2016) MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 10.3390/ijms17101712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandola C, Neerathilingam M (2020) Aptamers for targeted delivery: current challenges and future opportunities. In: Role of novel drug delivery vehicles in nanobiomedicine. IntechOpen
- Chatterjee P, Roy D (2017) Comparative analysis of RNA-Seq data from brain and blood samples of Parkinson’s disease. Biochem Biophys Res Commun 484:557–564. 10.1016/j.bbrc.2017.01.121 [DOI] [PubMed] [Google Scholar]
- Chen CYA, Chang JT, Ho YF, Bin SA (2016a) MiR-26 down-regulates TNF-α/NF-κB signalling and IL-6 expression by silencing HMGA1 and MALT1. Nucleic Acids Res 44:3772–3787. 10.1093/nar/gkw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang Z, Zhang D et al (2016b) Hydrogen sulfide protects against TNF-α induced neuronal cell apoptosis through miR-485-5p/TRADD signaling. Biochem Biophys Res Commun 478:1304–1309. 10.1016/j.bbrc.2016.08.116 [DOI] [PubMed] [Google Scholar]
- Chen Q, Lv J, Yang W et al (2019) Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis. Theranostics 9:6424–6442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PH, Li CL, Chang YF et al (2013) MiR-196a ameliorates phenotypes of huntington disease in cell, transgenic mouse, and induced pluripotent stem cell models. Am J Hum Genet 93:306–312. 10.1016/j.ajhg.2013.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Kohane DS, Langer R (2016) RNA therapeutics: the potential treatment for myocardial infarction. Regen Ther 4:83–91. 10.1016/j.reth.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell JP, Ward J, Taylor IA et al (2008) Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimer’s Dis 14:27–41. 10.3233/JAD-2008-14103 [DOI] [PubMed] [Google Scholar]
- Cressatti M, Juwara L, Galindez JM et al (2020) Salivary microR-153 and microR-223 levels as potential diagnostic biomarkers of idiopathic Parkinson’s disease. Mov Disord 35:468–477. 10.1002/mds.27935 [DOI] [PubMed] [Google Scholar]
- Dave VP, Ngo TA, Pernestig AK et al (2019) MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Lab Investig 99:452–469 [DOI] [PubMed] [Google Scholar]
- Davis S, Lollo B, Freier S, Esau C (2006) Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res 34:2294–2304. 10.1093/nar/gkl183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice B, Guida M, Guida M et al (2012) A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene 508:35–40. 10.1016/j.gene.2012.07.058 [DOI] [PubMed] [Google Scholar]
- Dehghani R, Rahmani F, Rezaei N (2018) MicroRNA in Alzheimer’s disease revisited: implications for major neuropathological mechanisms. Rev Neurosci 29:161–182. 10.1515/revneuro-2017-0042 [DOI] [PubMed] [Google Scholar]
- Delay C, Mandemakers W, Hébert SS (2012) MicroRNAs in Alzheimer’s disease. Neurobiol Dis 46:285–290 [DOI] [PubMed] [Google Scholar]
- Derkow K, Rössling R, Schipke C et al (2018) Distinct expression of the neurotoxic microRNA family let-7 in the cerebrospinal fluid of patients with Alzheimer’s disease. PLoS ONE. 10.1371/journal.pone.0200602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval V, Nelson PT, Hébert SS (2013) Circulating microRNAs in Alzheimer’s disease: the search for novel biomarkers. Front Mol Neurosci. 10.3389/fnmol.2013.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval V, Mandemakers W, Jolivette F et al (2014) Gene and microRNA transcriptome analysis of Parkinson’s related LRRK2 mouse models. PLoS ONE. 10.1371/journal.pone.0085510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA (2010) MicroRNA sponges: progress and possibilities. RNA 16:2043–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR, Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4:721–726. 10.1038/nmeth1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjami E, Campos R, Nielsen JS et al (2013) RNA aptamer-based electrochemical biosensor for selective and label-free analysis of dopamine. Anal Chem 85:121–128. 10.1021/ac302134s [DOI] [PubMed] [Google Scholar]
- Farrar CT, William CM, Hudry E et al (2014) RNA aptamer probes as optical imaging agents for the detection of amyloid plaques. PLoS ONE 9:e89901. 10.1371/journal.pone.0089901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella GD, Ferrara N, Rengo G (2015) The emerging role of microRNAs in Alzheimer’s disease. Front Physiol 6:1–6. 10.3389/fphys.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK et al (1998) Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature 391:806–811. 10.1038/35888 [DOI] [PubMed] [Google Scholar]
- Garcia DM, Baek D, Shin C et al (2010) Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol 18:1139–1146. 10.1038/nsmb.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, Watkins LR et al (2018) MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 24:221–245. 10.1177/1073858417721150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germer K, Leonard M, Zhang X (2013) RNA aptamers and their therapeutic and diagnostic applications. Int J Biochem Mol Biol 4:27–40 [PMC free article] [PubMed] [Google Scholar]
- Ghasemi N, Razavi S, Nikzad E (2017) Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J 19:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh SY, Chao YX, Dheen ST et al (2019) Role of microRNAs in Parkinson’s disease. Int J Mol Sci 20:1–23. 10.3390/ijms20225649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L (1995) Oligonucleotides as research, diagnostic, and therapeutic agents. J Biol Chem 270:13581–13584 [DOI] [PubMed] [Google Scholar]
- Gold L, Ayers D, Bertino J et al (2010) Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 10.1371/journal.pone.0015004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall EF, Heath PR, Bandmann O et al (2013) Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front Cell Neurosci [DOI] [PMC free article] [PubMed]
- Grimes JA, Prasad N, Levy S et al (2016) A comparison of microRNA expression profiles from splenic hemangiosarcoma, splenic nodular hyperplasia, and normal spleens of dogs. BMC Vet Res. 10.1186/s12917-016-0903-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen EJ, Talbot K, Gillingwater TH (2018) Advances in therapy for spinal muscular atrophy: promises and challenges. Nat Rev Neurol 14:214–224 [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C, Gardiner K, Marsh T et al (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:849–857. 10.1016/0092-8674(83)90117-4 [DOI] [PubMed] [Google Scholar]
- Gui YX, Liu H, Zhang LS et al (2015) Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 6:37043–37053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwizdek C, Ossareh-Nazari B, Brownawell AM et al (2003) Exportin-5 mediates nuclear export of minihelix-containing RNAs. J Biol Chem 278:5505–5508. 10.1074/jbc.C200668200 [DOI] [PubMed] [Google Scholar]
- Han Z, Ge X, Tan J et al (2015) Establishment of lipofection protocol for efficient miR-21 transfection into cortical neurons in vitro. DNA Cell Biol 34:703–709. 10.1089/dna.2015.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495:384–388. 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- Haramati S, Chapnik E, Sztainberg Y et al (2010) miRNA malfunction causes spinal motor neuron disease. Proc Natl Acad Sci U S A 107:13111–13116. 10.1073/pnas.1006151107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwak A, Kudla G, Dudnakova T, Tollervey D (2013) Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153:654–665. 10.1016/j.cell.2013.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques AD, Machado-Silva W, Leite REP et al (2020) Genome-wide profiling and predicted significance of post-mortem brain microRNA in Alzheimer’s disease. Mech Ageing Dev. 10.1016/j.mad.2020.111352 [DOI] [PubMed] [Google Scholar]
- Hershey LA, Peavy GM (2015) Cognitive decline in Parkinson disease. Neurology 85:1268–1269. 10.1212/wnl.0000000000002003 [DOI] [PubMed] [Google Scholar]
- Hosseini S, Michaelsen-Preusse K, Grigoryan G et al (2020) Type I interferon receptor signaling in astrocytes regulates hippocampal synaptic plasticity and cognitive function of the healthy CNS. Cell Rep 31:107666. 10.1016/j.celrep.2020.107666 [DOI] [PubMed] [Google Scholar]
- Huang C, Liu XJ, Zhou Q, et al (2016) MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int Immunopharmacol 32:46–54. 10.1016/j.intimp.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Huang S, Ge X, Yu J et al (2018) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32:512–528. 10.1096/fj.201700673R [DOI] [PubMed] [Google Scholar]
- Huse JT, Brennan C, Hambardzumyan D et al (2009) The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev 23:1327–1337. 10.1101/gad.1777409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I (2010) Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimer’s Dement 6:420–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O, Chen A (2015) Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci 16:201–212 [DOI] [PubMed] [Google Scholar]
- Jablonski KA, Gaudet AD, Amici SA et al (2016) Control of the inflammatory macrophage transcriptional signature by miR-155. PLoS ONE. 10.1371/journal.pone.0159724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadeja RN, Jones MA, Abdelrahman AA et al (2020) Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biol 28:101336. 10.1016/j.redox.2019.101336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SJ, Lee CS, Kim TH (2020) α-synuclein oligomer detection with aptamer switch on reduced graphene oxide electrode. Nanomaterials. 10.3390/nano10050832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Tu Q, Liu M (2018) MicroRNA-125b regulates Alzheimer’s disease through SphK1 regulation. Mol Med Rep 18:2373–2380. 10.3892/mmr.2018.9156 [DOI] [PubMed] [Google Scholar]
- Kabaria S, Choi DC, Chaudhuri AD et al (2015) Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett 589:319–325. 10.1016/j.febslet.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Lee KH, Lee HS et al (2017) Concurrent treatment with simvastatin and NF-κB inhibitor in human castration-resistant prostate cancer cells exerts synergistic anticancer effects via control of the NF-κB/LIN28/ let-7 miRNA signaling pathway. PLoS ONE. 10.1371/journal.pone.0184644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Mehan S, Singh S (2019) Understanding multifactorial architecture of Parkinson’s disease: pathophysiology to management. Neurol Sci 40:13–23 [DOI] [PubMed] [Google Scholar]
- Keeney JT, Fiorini A, Mitov MI, Perluigi M (2012) Cell cycle proteins in brain in mild cognitive impairment: insights into progression to Alzheimer disease oxidative stress and neurodegenerative disorders view project role of brain insulin resistance in the onset and progression of Alzheimer disease view. Springer 22:220–230. 10.1007/s12640-011-9287-2 [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Thangavel R, Natteru PA et al (2016) Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 1:1–15 [PMC free article] [PubMed] [Google Scholar]
- Khodr CE, Becerra A, Han Y, Bohn MC (2014) Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: positive and negative effects. Brain Res 1550:47–60. 10.1016/j.brainres.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Inoue K, Ishii J et al (2007) A microRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224. 10.1126/science.1140481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yoon H, Ramírez CM et al (2012) MiR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression. Exp Neurol 235:476–483. 10.1016/j.expneurol.2011.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim E, Choi WH et al (2016) Inhibitory RNA aptamers of tau oligomerization and their neuroprotective roles against proteotoxic stress. Mol Pharm 13:2039–2048. 10.1021/acs.molpharmaceut.6b00165 [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee SE, Kim SK et al (2019) Toll-like receptor mediated inflammation requires FASN-dependent MYD88 palmitoylation. Nat Chem Biol 15:907–916. 10.1038/s41589-019-0344-0 [DOI] [PubMed] [Google Scholar]
- Kluiver J (2012a) Generation of miRNA sponge constructs. Methods San Diego Calif 58:113–117 [DOI] [PubMed] [Google Scholar]
- Kluiver J (2012b) Rapid generation of microRNA sponges for microRNA inhibition. PLoS ONE 7:e29275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP et al (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9:1274–1281. 10.1261/rna.5980303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger K, Grabowski PJ, Zaug AJ et al (1982) Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of tetrahymena. Cell 31:147–157. 10.1016/0092-8674(82)90414-7 [DOI] [PubMed] [Google Scholar]
- Krützfeldt J, Rajewsky N, Braich R et al (2005) Silencing of microRNAs in vivo with “antagomirs.” Nature 438:685–689. 10.1038/nature04303 [DOI] [PubMed] [Google Scholar]
- Kumar S, Reddy PH (2018) MicroRNA-455-3p as a potential biomarker for Alzheimer’s disease: an update. Front Aging Neurosci 10:1–11. 10.3389/fnagi.2018.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhin AV, Tarantul VZ, Gening LV (2013) Aptamers: problems, solutions and prospects. Acta Naturae 5:34–43 [PMC free article] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB et al (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-Β oligomers. Nature 457:1128–1132. 10.1038/nature07761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854. 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- Lee JH, Shin SK, Jiang Y et al (2015a) Facilitated tau degradation by USP14 aptamers via enhanced proteasome activity. Sci Rep 5:1–11. 10.1038/srep10757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH et al (2015b) Altered expression of miR-202 in cerebellum of multiple-system atrophy. Mol Neurobiol 51:180–186. 10.1007/s12035-014-8788-4 [DOI] [PubMed] [Google Scholar]
- Lee H, Han S, Kwon CS, Lee D (2016) Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 7:100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SWL, Paoletti C, Campisi M et al (2019) MicroRNA delivery through nanoparticles. J Control Release 313:80–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann SM, Krüger C, Park B et al (2012) An unconventional role for miRNA: let-7 activates toll-like receptor 7 and causes neurodegeneration. Nat Neurosci 15:827–835. 10.1038/nn.3113 [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20 [DOI] [PubMed] [Google Scholar]
- Li BR, Hsieh YJ, Chen YX et al (2013) An ultrasensitive nanowire-transistor biosensor for detecting dopamine release from living pc12 cells under hypoxic stimulation. J Am Chem Soc 135:16034–16037. 10.1021/ja408485m [DOI] [PubMed] [Google Scholar]
- Li D, Yang H, Ma J et al (2018a) MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Hum Cell 31:106–115. 10.1007/s13577-017-0187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jiang T, Li MQ et al (2018b) Transcriptional regulation of macrophages polarization by microRNAs. Front Immunol [DOI] [PMC free article] [PubMed]
- Liang H, Shi Y, Kou Z et al (2015) Inhibition of BACE1 activity by a DNA aptamer in an Alzheimer’s disease cell model. PLoS ONE. 10.1371/journal.pone.0140733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FF, Hasegawa T, Fukuda M et al (2011) Construction of dopamine sensors by using fluorescent ribonucleopeptide complexes. Bioorganic Med Chem 19:4473–4481. 10.1016/j.bmc.2011.06.031 [DOI] [PubMed] [Google Scholar]
- Lima JF, Cerqueira L, Figueiredo C et al (2018a) Anti-miRNA oligonucleotides: a comprehensive guide for design. RNA Biol 15:338–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima JF, Cerqueira L, Figueiredo C et al (2018b) Anti-miRNA oligonucleotides: a comprehensive guide for design. RNA Biol 15:338–352. 10.1080/15476286.2018.1445959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HM, Nikolic I, Yang J et al (2018) MicroRNAs as potential therapeutics to enhance chemosensitivity in advanced prostate cancer. Sci Rep. 10.1038/s41598-018-26050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Csak T et al (2013) Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLoS ONE 8:1–10. 10.1371/journal.pone.0070945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw AM, Kolar MK, Novikova LN et al (2016) Chitosan polyplex mediated delivery of miRNA-124 reduces activation of microglial cells in vitro and in rat models of spinal cord injury. Nanomed Nanotechnol Biol Med 12:643–653. 10.1016/j.nano.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Lu M (2020) Circular RNA: functions, applications and prospects. ExRNA 2:1. 10.1186/s41544-019-0046-5 [Google Scholar]
- Luchetti A, Ciafrè SA, Murdocca M et al (2015) A perturbed MicroRNA expression pattern characterizes embryonic neural stem cells derived from a severe mouse model of spinal muscular atrophy (SMA). Int J Mol Sci 16:18312–18327. 10.3390/ijms160818312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ (2012) Evolution and complexity of micro RNA in the human brain. Front Genet. 10.3389/fgene.2012.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Güttinger S, Calado A et al (2004) Nuclear export of microRNA precursors. Science 303:95–98. 10.1126/science.1090599 [DOI] [PubMed] [Google Scholar]
- Ma C, Li Y, Li M et al (2014) MicroRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol Immunol 62:150–158. 10.1016/j.molimm.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Ma Q, Zhao H, Tao Z et al (2016) MicroRNA-181c exacerbates brain injury in acute Ischemic stroke. Aging Dis 7:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri F, Vanoli F, Corti S (2018) miRNA in spinal muscular atrophy pathogenesis and therapy. J Cell Mol Med 22:755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannironi C, Di Nardo A, Fruscoloni P, Tocchini-Valentini GP (1997) In vitro selection of dopamine RNA ligands. Biochemistry 36:9726–9734. 10.1021/bi9700633 [DOI] [PubMed] [Google Scholar]
- Manzine PR, Pelucchi S, Horst MA et al (2018) MicroRNA 221 targets ADAM10 mRNA and is downregulated in Alzheimer’s disease. J Alzheimer’s Dis 61:113–123. 10.3233/JAD-170592 [DOI] [PubMed] [Google Scholar]
- Maoz R, Garfinkel BP, Soreq H (2017) Alzheimer’s disease and ncRNAs. Advances in experimental medicine and biology. Springer, New York, pp 337–361 [DOI] [PubMed] [Google Scholar]
- Martí E, Pantano L, Bañez-Coronel M et al (2010) A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res 38:7219–7235. 10.1093/nar/gkq575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martier R, Liefhebber JM, Miniarikova J et al (2019) Artificial microRNAs targeting C9orf72 can reduce accumulation of intra-nuclear transcripts in ALS and FTD patients. Mol Ther Nucleic Acids 14:593–608. 10.1016/j.omtn.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T, Matsugami A, Nishikawa F et al (2009) Unique quadruplex structure and interaction of an RNA aptamer against bovine prion protein. Nucleic Acids Res 37:6249–6258. 10.1093/nar/gkp647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, Yang M, Foster PS (2007) Regulation of microRNA by antagomirs: a new class of pharmacological antagonists for the specific regulation of gene function? Am J Respir Cell Mol Biol 36:8–12 [DOI] [PubMed] [Google Scholar]
- Mayer G (2009) The chemical biology of aptamers. Angew Chemie Int Ed 48:2672–2689 [DOI] [PubMed] [Google Scholar]
- McGeary SE, Lin KS, Shi CY et al (2019) The biochemical basis of microRNA targeting efficacy. Science. 10.1126/science.aav1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova I (2007) (2007) RNA-based therapies. Nat Rev Drug Discov 611(6):863–864. 10.1038/nrd2443 [Google Scholar]
- Memczak S, Jens M, Elefsinioti A et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495:333–338. 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- Modi PK, Komaravelli N, Singh N, Sharma P (2012) Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Am Soc Cell Biol 23:3722–3730. 10.1091/mbc.E12-02-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteys AM, Wilson MJ, Boudreau RL et al (2015) Artificial miRNAs targeting mutant huntingtin show preferential silencing in vitro and in vivo. Mol Ther Nucleic Acids 4:e234. 10.1038/mtna.2015.7 [DOI] [PubMed] [Google Scholar]
- Murakami K, Nishikawa F, Noda K et al (2008) Anti-bovine Prion protein RNA aptamer containing tandem GGA repeat interacts both with recombinant bovine prion protein and its β isoform with high affinity. Prion 2:73–80. 10.4161/pri.2.2.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair VD, Ge Y (2016) Alterations of miRNAs reveal a dysregulated molecular regulatory network in Parkinson’s disease striatum. Neurosci Lett 629:99–104. 10.1016/j.neulet.2016.06.061 [DOI] [PubMed] [Google Scholar]
- Napoli M, Flores ER (2017) The p53 family orchestrates the regulation of metabolism: physiological regulation and implications for cancer therapy. Br J Cancer 116:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastasijevic B, Wright BR, Smestad J et al (2012) Remyelination Induced by a DNA aptamer in a mouse model of multiple sclerosis. PLoS ONE 7:e39595. 10.1371/journal.pone.0039595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara D, Hasegawa H, Kaneko K et al (2007) Screening of DNA aptamer against mouse prion protein by competitive selection. Prion 1:248–254. 10.4161/pri.1.4.5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala DS, Amara DP, Schaffer DV (2015) Adeno-associated virus vectors and neurological gene therapy. Neuroscientist 21:84–98 [DOI] [PubMed] [Google Scholar]
- Paul S, Bravo Vázquez LA, Pérez Uribe S, et al (2020) Current status of microRNA-based therapeutic approaches in neurodegenerative disorders. Cells 9 [DOI] [PMC free article] [PubMed]
- Peer D, Lieberman J (2011) Special delivery: targeted therapy with small RNAs. Gene Ther 18:1127–1133 [DOI] [PubMed] [Google Scholar]
- Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CMP (2013) Delivering the promise of miRNA cancer therapeutics. Drug Discov Today 18:282–289 [DOI] [PubMed] [Google Scholar]
- Peter ME (2010) Targeting of mRNAs by multiple miRNAs: the next step. Oncogene 29:2161–2164 [DOI] [PubMed] [Google Scholar]
- Pieri L, Madiona K, Melki R (2016) Structural and functional properties of prefibrillar α-synuclein oligomers. Sci Rep 6:1–15. 10.1038/srep24526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue AI, Cui JG, Li YY et al (2010) Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci Lett 476:18–22. 10.1016/j.neulet.2010.03.054 [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Veremeyko T, Barteneva N et al (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med 17:64–70. 10.1038/nm.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada I, Gabrielli M, Turola E et al (2018) Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol 135:529–550. 10.1007/s00401-017-1803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KN (2017) Oxidative stress and pro-inflammatory cytokines may act as one of the signals for regulating microRNAs expression in Alzheimer’s disease. Mech Ageing Dev 162:63–71. 10.1016/j.mad.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Proske D, Gilch S, Wopfner F et al (2002) Prion-protein-specific aptamer reduces PrPSc formation. ChemBioChem 3:717–725. 10.1002/1439-7633(20020802)3:8%3c717::AID-CBIC717%3e3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- Qiu L, Tan EK, Zeng L (2015) Micrornas and neurodegenerative diseases. Advances in experimental medicine and biology. Springer, New York, pp 51–70 [DOI] [PubMed] [Google Scholar]
- Qu J, Yu S, Zheng Y et al (2017) Aptamer and its applications in neurodegenerative diseases. Cell Mol Life Sci 74:683–695. 10.1007/s00018-016-2345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi F, Bitan G (2010) Selection of aptamers for amyloid β-protein, the causative agent of alzheimer’s disease. J Vis Exp. 10.3791/1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi F, Murakami K, Summers JL et al (2009) RNA aptamers generated against oligomeric Aβ40 recognize common amyloid aptatopes with low specificity but high sensitivity. PLoS ONE 4:e7694. 10.1371/journal.pone.0007694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. 10.1126/science.aag2590 [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M et al (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403:901–906. 10.1038/35002607 [DOI] [PubMed] [Google Scholar]
- Rentmeister A, Bill A, Wahle T et al (2006) RNA aptamers selectively modulate protein recruitment to the cytoplasmic domain of β-secretase BACE1 in vitro. RNA 12:1650–1660. 10.1261/rna.126306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RH, Petersen MH, Willert CW et al (2018) Perturbations in the p53/miR-34a/SIRT1 pathway in the R6/2 Huntington’s disease model. Mol Cell Neurosci 88:118–129. 10.1016/j.mcn.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Rhie A, Kirby L, Sayer N et al (2003) Characterization of 2′-fluoro-RNA aptamers that bind preferentially to disease-associated conformations of prion protein and inhibit conversion. J Biol Chem 278:39697–39705. 10.1074/jbc.M305297200 [DOI] [PubMed] [Google Scholar]
- Roberts TC, Coenen-Stass AML, Betts CA, Wood MJA (2014) Detection and quantification of extracellular microRNAs in murine biofluids. Biol Proced Online 16:5. 10.1186/1480-9222-16-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P et al (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8:1128–1139 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H et al (2014) Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimer’s Dis 42:1229–1238. 10.3233/JAD-140204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10:S10. 10.1038/nm1066 [DOI] [PubMed] [Google Scholar]
- Rozenblum GT, Kaufman T, Vitullo AD (2014) Myelin basic protein and a multiple sclerosis-related MBP-peptide bind to oligonucleotides. Mol Ther Nucleic Acids 3:e192. 10.1038/mtna.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MV, McGavern DB (2016) Inflammatory neuroprotection following traumatic brain injury. Science 353:783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R, Goodman CD, Huzarewich RLCH et al (2008) A miRNA signature of prion induced neurodegeneration. PLoS ONE 3:3652. 10.1371/journal.pone.0003652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim L, Islam G, Desaulniers JP (2020) Targeted delivery and enhanced gene-silencing activity of centrally modified folic acid-siRNA conjugates. Nucleic Acids Res 48:75–85. 10.1093/nar/gkz1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi YS (2011) A status update of modified oligonucleotides for chemotherapeutics applications. Curr Protoc Nucleic Acid Chem Chapter. 10.1002/0471142700.nc0401s46 [DOI] [PubMed] [Google Scholar]
- Sayer NM, Cubin M, Rhie A et al (2004) Structural determinants of conformationally selective, prion-binding aptamers. J Biol Chem 279:13102–13109. 10.1074/jbc.M310928200 [DOI] [PubMed] [Google Scholar]
- Scallet A, Carp R, Ye X (2005) Pathophysiology of transmissible spongiform encephalopathies. Curr Med Chem Endocr Metab Agents 3:171–184. 10.2174/1568013033483483 [Google Scholar]
- Schellino R, Boido M, Vercelli A (2019) JNK signaling pathway involvement in spinal cord neuron development and death. Cells 8 [DOI] [PMC free article] [PubMed]
- Schonrock N, Humphreys DT, Preiss T, Götz J (2012) Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J Mol Neurosci 46:324–335. 10.1007/s12031-011-9587-2 [DOI] [PubMed] [Google Scholar]
- Schulte LN, Eulalio A, Mollenkopf HJ et al (2011) Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. EMBO J 30:1977–1989. 10.1038/emboj.2011.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya S, Noda K, Nishikawa F et al (2006) Characterization and application of a novel RNA aptamer against the mouse prion protein. J Biochem 139:383–390. 10.1093/jb/mvj046 [DOI] [PubMed] [Google Scholar]
- Sengupta U, Nilson AN, Kayed R (2016) The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 6:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi P, Lukiw WJ (2009) Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett 459:100–104. 10.1016/j.neulet.2009.04.052 [DOI] [PubMed] [Google Scholar]
- Shacham T, Sharma N, Lederkremer GZ (2019) Protein misfolding and ER stress in Huntington’s disease. Front Mol Biosci 6:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel-Karyo R, Frenkel-Pinter M, Egoz-Matia N et al (2010) Inhibiting α-synuclein oligomerization by stable cell-penetrating β-synuclein fragments recovers phenotype of parkinson’s disease model flies. PLoS ONE. 10.1371/journal.pone.0013863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanzhong Yang L, Icyuz M, Abraham E et al (2020) Polarization microRNA let-7c regulates macrophage. Am Assoc Immnol. 10.4049/jimmunol.1202496 [Google Scholar]
- Sheedy FJ (2015) Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol [DOI] [PMC free article] [PubMed]
- Shenoy A, Danial M, Blelloch RH (2015) Let-7 and miR-125 cooperate to prime progenitors for astrogliogenesis. EMBO J 34:1180–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson B, Das S (2015) MicroRNA therapeutics: the next magic bullet? Mini-Reviews Med Chem 15:467–474. 10.2174/1389557515666150324123208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slota JA, Booth SA (2019) MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Non-coding RNA 5:1–24. 10.3390/ncrna5020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smestad J, James Maher L (2013) Ion-dependent conformational switching by a DNA aptamer that induces remyelination in a mouse model of multiple sclerosis. Nucleic Acids Res 41:1329–1342. 10.1093/nar/gks1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hu M, Zhang J et al (2019) A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer’s disease. EBioMedicine 39:409–421. 10.1016/j.ebiom.2018.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner DF, Thomas MF, Hu JK et al (2011) MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 35:169–181. 10.1016/j.immuni.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvang J, Petri A, Lindow M et al (2012) Inhibition of microRNA function by antimiR oligonucleotides. Silence 3:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Hopkins S, Nesser NK et al (2014) The p53 transcription factor modulates microglia behavior through microRNA-dependent regulation of c-Maf. J Immunol 192:358–366. 10.4049/jimmunol.1301397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Yang X, Lou J (2016a) Geniposide reduces α-synuclein by blocking microRNA-21/lysosome-associated membrane protein 2A interaction in Parkinson disease models. Brain Res 1644:98–106. 10.1016/j.brainres.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Su W, Aloi MS, Garden GA (2016b) MicroRNAs mediating CNS inflammation: small regulators with powerful potential. Brain Behav Immun 52:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Luo ZM, Guo XM et al (2015) An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci. 10.3389/fncel.2015.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tada K, Mihara H (2009) RNA aptamers selected against amyloid β-peptide (Aβ) inhibit the aggregation of Aβ. Mol Biosyst 5:986–991. 10.1039/b903391b [DOI] [PubMed] [Google Scholar]
- Tang Y, Luo X, Cui H et al (2009) MicroRNA-146a contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum 60:1065–1075. 10.1002/art.24436 [DOI] [PubMed] [Google Scholar]
- Tang L, Chen HY, Hao NB et al (2017) microRNA inhibitors: natural and artificial sequestration of microRNA. Cancer Lett 407:139–147 [DOI] [PubMed] [Google Scholar]
- Tatura R, Kraus T, Giese A et al (2016) Parkinson’s disease: SNCA-, PARK2-, and LRRK2- targeting microRNAs elevated in cingulate gyrus. Park Relat Disord 33:115–121. 10.1016/j.parkreldis.2016.09.028 [DOI] [PubMed] [Google Scholar]
- Teng G, Wang W, Dai Y, et al (2013) Let-7b Is involved in the inflammation and immune responses associated with helicobacter pylori infection by targeting toll-like receptor 4. PLoS ONE. 10.1371/journal.pone.0056709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen M, Blondal T, Mouritzen P (2017) Quantitative RT-PCR for microRNAs in biofluids. In: Methods in molecular biology. Humana Press Inc., pp 379–398 [DOI] [PubMed]
- Thounaojam MC, Kaushik DK, Basu A (2013) MicroRNAs in the brain: it’s regulatory role in neuroinflammation. Mol Neurobiol 47:1034–1044. 10.1007/s12035-013-8400-3 [DOI] [PubMed] [Google Scholar]
- Thubron EB, Rosa HS, Hodges A et al (2019) Regional mitochondrial DNA and cell-type changes in post-mortem brains of non-diabetic Alzheimer’s disease are not present in diabetic Alzheimer’s disease. Sci Rep. 10.1038/s41598-019-47783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukakoshi K, Abe K, Sode K, Ikebukuro K (2012) Selection of DNA aptamers that recognize α-synuclein oligomers using a competitive screening method. Anal Chem 84:5542–5547. 10.1021/ac300330g [DOI] [PubMed] [Google Scholar]
- Turchinovich A, Burwinkel B (2012) Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. RNA Biol 9:1066–1075. 10.4161/rna.21083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallelunga A, Ragusa M, Di Mauro S et al (2014) Identification of circulating microRNAs for the differential diagnosis of Parkinson’s disease and Multiple System Atrophy. Front Cell Neurosci. 10.3389/fncel.2014.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi V, Boido M, De Amicis E et al (2015) Expression of muscle-specific MiRNA 206 in the progression of disease in a murine SMA model. PLoS ONE. 10.1371/journal.pone.0128560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi V, Anzilotti S, Serani A et al (2020) miR-206 reduces the severity of motor neuron degeneration in the facial nuclei of the brainstem in a mouse model of SMA. Mol Ther 28:1154–1166. 10.1016/j.ymthe.2020.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistbakka J, Elovaara I, Lehtimäki T, Hagman S (2017) Circulating microRNAs as biomarkers in progressive multiple sclerosis. Mult Scler J 23:403–412 [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT (2003) Zeroing in on the pathogenic form of α-Synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42:7871–7878. 10.1021/bi030086j [DOI] [PubMed] [Google Scholar]
- Vorobjeva MA, Krasitskaya VV, Fokina AA et al (2014) RNA aptamer against autoantibodies associated with multiple sclerosis and bioluminescent detection probe on its basis. Anal Chem 86:2590–2594. 10.1021/ac4037894 [DOI] [PubMed] [Google Scholar]
- Wang G, van der Walt JM, Mayhew G et al (2008a) Variation in the miRNA-433 BINDING SITE of FGF20 confers risk for Parkinson disease by overexpression of α-synuclein. Am J Hum Genet 82:283–289. 10.1016/j.ajhg.2007.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WX, Rajeev BW, Stromberg AJ et al (2008b) The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J Neurosci 28:1213–1223. 10.1523/JNEUROSCI.5065-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Tang M, Zong P et al (2018) MiRNA-155 regulates the Th17/Treg ratio by targeting SOCS1 in severe acute pancreatitis. Front Physiol. 10.3389/fphys.2018.00686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Qin L, Tang B (2019) MicroRNAs in Alzheimer’s disease. Front Genet 10:1–13. 10.3389/fgene.2019.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Proske D, Neumann M, et al (1997) RNA aptamers specifically interact with the prion protein PrP. J Virol 71 [DOI] [PMC free article] [PubMed]
- Wen MM (2016) Getting miRNA therapeutics into the target cells for neurodegenerative diseases: a mini-review. Front Mol Neurosci 9:1–7. 10.3389/fnmol.2016.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbanks B, Smestad J, Heider RM et al (2019) Optimization of a 40-mer antimyelin DNA aptamer identifies a 20-mer with enhanced properties for potential multiple sclerosis therapy. Nucleic Acid Ther 29:126–135. 10.1089/nat.2018.0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Diederichs S (2011) Argonaute proteins regulate microRNA stability: increased microRNA abundance by Argonaute proteins is due to microRNA stabilization. RNA Biol 8:1149–1157. 10.4161/rna.8.6.17665 [DOI] [PubMed] [Google Scholar]
- Wu S, Huang S, Ding J et al (2010) Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene 29:2302–2308. 10.1038/onc.2010.34 [DOI] [PubMed] [Google Scholar]
- Wu S-L, Wang G-J, Wang J-X (2018) Roles of circular RNAs and their interactions with microRNAs in human disorders Jun-feng Sun. Clin Surg Res Commun 2:1–8 [Google Scholar]
- Yang N (2015) An overview of viral and nonviral delivery systems for microRNA. Int J Pharm Investig 5:179–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Mattes J (2008) Discovery, biology and therapeutic potential of RNA interference, microRNA and antagomirs. Pharmacol Ther 117:94–104 [DOI] [PubMed] [Google Scholar]
- Yang Y, Mufson EJ, Herrup K (2003) Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease [DOI] [PMC free article] [PubMed]
- Yang X, Tang X, Sun P et al (2017) MicroRNA-15a/16-1 antagomir ameliorates ischemic brain injury in experimental stroke. Stroke 48:1941–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Zang S, Wang Y et al (2020) Methamphetamine induced neuroinflammation in mouse brain and microglial cell line BV2: roles of the TLR4/TRIF/Peli1 signaling axis. Toxicol Lett 333:150–158. 10.1016/j.toxlet.2020.07.028 [DOI] [PubMed] [Google Scholar]
- Yao L, Zhu Z, Wu J et al (2019) MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J 33:8648–8665. 10.1096/fj.201900363R [DOI] [PubMed] [Google Scholar]
- Yi R, Qin Y, Macara IG, Cullen BR (2003) Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 17:3011–3016. 10.1101/gad.1158803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Song Y, Xie C et al (2019) MiR-142a-3p and miR-155-5p reduce methamphetamine-induced inflammation: role of the target protein Peli1. Toxicol Appl Pharmacol 370:145–153. 10.1016/j.taap.2019.03.019 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang LI, Dong L-Y et al (2012) MiR-21 represses FasL in microglia and protects against microglia-mediated neuronal cell death following hypoxia/ischemia. Wiley Online Libr 60:1888–1895. 10.1002/glia.22404 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Q, Liu C et al (2016) MiR-214-3p attenuates cognition defects via the inhibition of autophagy in SAMP8 mouse model of sporadic Alzheimer’s disease. Neurotoxicology 56:139–149 [DOI] [PubMed] [Google Scholar]
- Zhang SF, Chen JC, Zhang J, Xu JG (2017) MIR-181a involves in the hippocampus-dependent memory formation via targeting PRKAA1. Sci Rep 7:1–11. 10.1038/s41598-017-09095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yu S, Zheng Y et al (2017) Oxidative modification and its implications for the neurodegeneration of Parkinson’s disease. Mol Neurobiol 54:1404–1418. 10.1007/s12035-016-9743-3 [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhou X (2007) Sodium dodecyl sulfate-modified carbon paste electrodes for selective determination of dopamine in the presence of ascorbic acid. Bioelectrochemistry 70:408–415. 10.1016/j.bioelechem.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Zhou J, Rossi J (2017) Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov 16:181–202. 10.1038/nrd.2016.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zhao L, Wang K et al (2019a) MicroRNA-146a inhibits NF-κB activation and pro-inflammatory cytokine production by regulating IRAK1 expression in THP-1 cells. Exp Ther Med 18:3078–3084. 10.3892/etm.2019.7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L-Y, Qin Z, Zhu Y-H et al (2019b) Current RNA-based therapeutics in clinical trials. Curr Gene Ther 19:172–196. 10.2174/1566523219666190719100526 [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhao Y, Li Z et al (2020) miR-103a-3p regulates mitophagy in Parkinson’s disease through Parkin/Ambra1 signaling. Pharmacol Res 160:105197 [DOI] [PubMed] [Google Scholar]
- Zhu H, Wu H, Liu X et al (2009) Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy 5:816–823. 10.4161/auto.9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.