Abstract
We evaluated renal melamine–cyanurate crystal spherulite formation after single and repeated ingestion of both melamine (MEL) and cyanuric acid (CYA) in catfish and trout. MEL and CYA were co-administered orally over a range of doses, 0.1–20 mg/kg body weight (bw) of each compound, either once or repeatedly for 4, 14 or 28 days (d). In catfish, the No Observable Adverse Effects Levels (NOAELs) for crystal formation for single, 4 d or 14 d dosing were 10, 2.5 and 0.5 mg/kg bw, respectively. In trout, the respective NOAELs were 2.5, 2.5 and 0.5 mg/kg bw. No renal crystals formed in catfish fed 0.1 mg/kg bw of each compound for 28 d. Sequential administration of 20 mg/kg bw of MEL followed by 20 mg/kg bw of CYA or vise-versa, with waiting periods of 1, 3, 7, 14 or 21 d between compound dosing also induced renal crystal formation in fish. These studies show that both catfish and trout are sensitive, non-mammalian models, for renal crystal formation following MEL and CYA ingestion. Since fish generally excrete chemicals more slowly than mammals, they may provide a “worst case scenario” model for higher risk populations, such as infants or persons with compromised renal function.
Keywords: Melamine, Cyanuric acid, Kidney, Crystal, No observable adverse effect level, Fish
1. Introduction
During 2007, a large number of pets in the US, Canada, and South Africa died of renal failure caused by a crystal nephropathy which was induced by co-ingestion of melamine (MEL) and cyanuric acid (CYA) in contaminated pet foods (Brown et al., 2007; Cianciolo et al., 2008; Reyers, 2007; Thompson et al., 2008; USFDA, 2007a). These nitrogen rich triazine compounds were added to feed ingredients to falsely boost the apparent protein levels and were used not only in pet food, but also in livestock and fish feeds (Reimschuessel et al., 2008a; Reyers, 2008; USFDA, 2007b; Luengyosluechakul, 2007). This caused major concern for public health because there was little information available regarding uptake and depletion of MEL or its analogues, such as CYA, in edible tissues (USFDA, 2007c). Recently, the depletions of MEL and CYA in edible tissues were reported for trout and catfish (Reimschuessel et al., 2009).
A second, important area of concern is the general lack of information regarding the dosage of MEL and CYA which causes renal crystal formation, specifically the lowest dose that causes this effect. Experiments in fish, pigs, rats and cats demonstrated that ingestion of MEL and CYA at high concentrations, similar to levels found in the adulterated feeds, caused renal melamine–cyanurate crystal spherulites to form concurrently with clinical signs of renal failure (Reimschuessel et al., 2008a; Dobson et al., 2008; Puschner et al., 2007). These high dose studies could not derive a No Observable Adverse Effect Level (NOAEL) for crystal formation. The United States Food and Drug Administration’s (USFDA) Center for Veterinary Medicine (CVM) therefore initiated a study designed to determine if a threshold dose could be established for the formation of renal crystals following oral co-administration of MEL and CYA. In a previous study we had shown that fish were very susceptible to triazine-induced renal crystal formation (Reimschuessel et al., 2008a). Since we were concurrently conducting a MEL and CYA residue depletion study using trout and catfish, we used those same species as non-mammalian models for our initial NOAEL studies. A sequential dosing regimen was also conducted to evaluate whether both triazines need to be administered at the same time for renal crystals to form.
The studies described here were begun during the summer of 2008. In September 2008, the first news reports of MEL in Chinese infant formula came to our attention (Bradsher, 2008; Xin and Stone, 2008). Milk products had been adulterated with relatively pure MEL, causing urinary tract stones in over 300,000 Chinese children (Sun et al., 2008; Guan et al., 2009; Kuehn, 2009; Shen et al., 2009). World-wide recalls as well as bans of Chinese imports of contaminated dairy products ensued (Barboza, 2008; Parry, 2008; Qing, 2008; Bhalla et al., 2009). As a result, many nations conducted risk assessments and established limits for the amount of MEL that can be present in infant formula and other foods (Anon, 2008; AQSIQ, 2008; Centre for Food Safety, Hong Kong, 2008; Commission Decision 2008/757/EC, 2008; New Zealand Food Safety Authority, 2008; USFDA, 2008).
During the autumn of 2008, preliminary data from these ongoing fish studies was provided to USFDA risk assessors evaluating MEL toxicity because no mammalian NOAEL studies of combined triazine toxicity had been conducted (Reimschuessel et al., 2008b). This information was also provided to The World Health Organization (WHO) which assembled an expert panel in December 2008 to review the toxicological aspects of MEL and CYA and to derive a tolerable daily intake (TDI) for these compounds. Recommendations by WHO for further research included “determine the threshold dose and time course for crystal development in the kidney and urine for MEL alone and in combination with CYA and other triazines” (WHO, 2008).
We describe here the results of oral toxicity studies following simultaneous MEL and CYA (1:1 wt) administration in catfish and trout as single or repeated doses. Presented also are the results of sequential dosing studies, in which MEL was given first, followed by a waiting period of days to weeks before administering CYA. Administering the triazines in the reverse order was also evaluated, with CYA being administered first, followed by MEL. These studies were conducted in accordance with Good Laboratory Practices standards (USFDA, 2009).
2. Materials and methods
2.1. Animals and husbandry
Adult channel catfish, Ictalurus punctatus, and rainbow trout, Oncorhynchus my-kiss, were obtained from commercial fish farms and acclimated to tanks in the aquaculture research laboratory at the USFDA’s Center for Veterinary Medicine, Office of Research. Trout were maintained in recirculating aquaculture systems which consisted of 1800 L round fiberglass tanks receiving partial water changes via automated filter-backwashes, while catfish were housed in either 200 L flow-through glass aquaria or identical recirculating systems as the trout. Fish were monitored for a minimum of one month to ensure they were disease free prior to use in experiments. The holding and experimental systems were supplied with the same source of filtered freshwater which is routinely tested for heavy metals, pesticides, and volatile organic compounds (VOCs). In addition, tank water parameters including temperature, dissolved oxygen (DO), pH, ammonia, and nitrite were monitored to ensure optimal conditions for fish. Commercially available aquaculture feeds were used throughout the study with catfish receiving an extruded diet of 35% protein, 8% fat, and 5% fiber (Burris Aquaculture, Franklinton, LA USA) at 1–2% bw/day, and trout receiving a diet of 40% protein, 14% fat, and 4% fiber (Rangen Inc., Buhl, ID, USA) at 1–4% bw/day. Prior to use, all feeds were tested for MEL and CYA by liquid chromatography tandem mass spectrometry (LC–MS/MS) (Heller and Nochetto, 2008). No MEL or CYA contaminants were detected above the LOQ of the method (0.5 ppm). The use of fish in this study was approved by the CVM Institutional Animal Care and Use Committee.
2.2. Test Articles and treatments
Melamine (MEL; CAS 108-78-1; 99% purity) and cyanuric acid (CYA; CAS 108-80-5; 98% purity) were obtained from Sigma–Aldrich (St Louis, MO, USA). MEL and/or CYA were administered to fish by loading the compound(s) into gelatin capsules (Torpac, Fairfield, NJ, USA or Capsuline, Pompano Beach, FL, USA). Capsules were tested for MEL and CYA using the same LC–MS/MS method as for feeds. No MEL or CYA contamination was detected above the limit of quantification (LOQ) of the method (0.5 ppm). At higher doses (20, 10, 5 and 2.5 mg/kg bw), MEL and CYA were individually weighed into separate gelatin capsules then poured into one capsule just prior to dosing. Lower doses (2.5, 1.0, and 0.5 mg/kg bw) were prepared by the following procedure: MEL and CYA were dissolved using 99.9% methanol (CAS 67-56-1; 99.9% purity, Fisher Chemical, Fairlawn, NJ, USA) into stock solutions at concentrations of 0.6 mg/ml and 0.3 mg/ml, respectively. Aliquots of stock solution were then pipetted into one half of a gelatin capsule. The capsule halves were then loaded into a TurboVap LV drying unit (Zymark, Hopkinton, MA, USA) where the solvent was evaporated using nitrogen gas and a warm water bath. Combination doses (MEL and CYA, 1:1 wt) were thus prepared by loading and drying the individual compounds into separate halves of a single capsule. The two halves were joined to form one complete capsule just prior to dosing.
A single capsule containing the test article(s) was administered to each fish either by embedding it in a gelatin feed nugget that the fish was observed to swallow whole or via an intra-gastric feeding tube. Gelatin feed nuggets were prepared using the same pelleted feeds that fish had previously received (Shaikh et al., 2003).
Control fish received an empty gelatin capsule. Fish were observed for approximately 1 h after dosing. If capsules were regurgitated they were retrieved and if intact, used to dose again. Conversely, if the capsule was not intact, a new dose was prepared and administered.
2.3. Experimental systems and design
Individual fish (sex not determined,) were randomly removed from acclimation tanks, weighed, and transferred to 60L flow-through glass aquaria (1 fish/tank). The fish were allowed to acclimate in the experimental tanks for at least 1 day prior to administering test articles. Mean experimental water parameters for trout were 12.2 ± 1.6 °C, 9.7 ± 1.5 ppm DO and 7.3 ± 0.4 pH. Parameters for catfish were 23.7 ± 0.9 °C, 8.2 ± 1.3 ppm DO and 7.2 ± 0.3 pH. Ammonia and nitrite were monitored weekly during treatments. Mean values for total ammonia and nitrite were 0.046 ± 0.03 ppm and 0.0030 ± 0.002 ppm, respectively.
Co-administration NOAEL experiments – dose determination:
The goal of these experiments was to obtain a single and 14 day NOAEL for renal crystal formation in catfish and trout. Our previous residue depletion study showed renal crystals were present in about 50% of the fish sacrificed 3 days after receiving a single oral dose of each triazine: 20 mg/kg bw MEL and 20 mg/kg bw CYA (20 MEL + 20 CYA mg/kg bw). Thus the starting dose level for our current study was 10 MEL + 10 CYA mg/kg bw. Subsequent dosages would be approximately half of the previous dose level (5 MEL + 5 CYA, 2.5 MEL + 2.5 CYA, 1.0 MEL + 1.0 CYA, 0.5 MEL + 0.5 CYA mg/kg bw). Fish were sacrificed 3 days after the last (or only) dose to fully allow crystal formation.
To reduce the number of animals used for NOAEL determination, we conducted the experiments in a stepwise fashion, starting with 3–6 fish to test the initial dosage. If no crystals were observed in the first group of test animals, more animals were given that dosage, for a total n = 12 to confirm the data. If, however, crystals were found in any of the first 6 fish, no more animals were given that dosage. A new cohort of 6 fish was then treated with the next lower dose level.
Once we determined the concentration at which no crystals formed after a single dose, we used that concentration for the repeated dose study. This was also done in a stepwise fashion. Using the single dose NOAEL level, we treated 6 fish daily for 4 days. If crystals formed, we reduced the dose level and started over. If no crystals formed in 6 fish given 4 daily doses, we used that dosage for the 14 day NOAEL. Again, using the stepwise approach, 6 fish were treated for 14 days. If crystals formed in any of those fish, the dosage was reduced and another 6 fish were treated for 14 days. Once a dose level had been determined that caused no renal crystals in 6 fish, an additional 6 fish were treated to confirm the 14 day NOAEL (see Table 1a for the dose levels tested).
Table 1a.
Intensity of crystal spherulites in catfish kidney wet mount sections.
| DoseMEL + CYA (mg/kg bw each) | Estimated equivalent in feed (ppm) | # Daily doses | Initial phase (intensity) | Confirmatoryphase (intensity) | Comment |
|---|---|---|---|---|---|
| 20 | 500 | 1 | 0,2,2,2,4,4 | (results from depletion study) | |
| 10 | 250 | 1 | 0,0,0 | 0,0,0,0,0,0,0,0,0 | NOAEL |
| 10 | 250 | 4 | 0,0,1,2,2,3 | ||
| 5 | 125 | 4 | 0,0,0,1,1,2 | ||
| 2.5 | 62.5 | 4 | 0,0,0,0,0,0 | Starting test dose for 14 day study | |
| 2.5 | 62.5 | 14 | 0,1,2,2,2,22,2,2,2,2,2 | ||
| 1 | 25 | 14 | 0,0,0,0,1,1 | ||
| 0.5 | 12.5 | 14 | 0,0,0,0,0,0 | 0,0,0,0,0,0 | NOAEL |
| 0.1 | 2.5 | 28 | 0,0,0,0,0,0 | None | NOAEL-depletion study data |
Deviation in the experimental plan:
While conducting these studies, the Chinese infant formula incident was reported, and in order to obtain potential NOAEL values faster, several doses were run concurrently or with the full cohort of 12 fish. This was deemed necessary to provide critically needed preliminary information for a risk assessment being prepared by the USFDA. One additional dosage was tested in six catfish, 0.1 mg/kg bw for 28 days as part of a residue accumulation study. This concentration corresponds to approximately 2.5 ppm in the feed, which has been proposed by many risk assessors as the level of “no concern” for MEL in foods or feeds other than infant formula. The kidneys of these fish were examined for crystals and will be reported here.
Sequential administration experiments:
In these experiments 20 mg/kg bw, the dosage used in our previous depletion study, of either MEL or CYA was administered to 3 fish followed by waiting periods of 1, 3, 7, 14 or 21 days. The second triazine (CYA after MEL or MEL after CYA) was administered after the waiting period. Fish were sacrificed 3 days after the second dose to allow for full crystal formation. If no crystals were present in the kidneys of the first 3 fish, 3 additional fish were tested to confirm the results, for a total n = 6. The purpose was to determine the waiting period at which no crystals would form, called the ‘No response waiting time.’ (see Table 2a for outline).
Table 2a.
Sequential dosing experiments – catfish.
| Triazine order | Waiting period (days) | Initial phase (intensity) | Confirmatory phase (intensity) | Comments |
|---|---|---|---|---|
| MEL | 1 | 0,1,3 | ||
| then | ||||
| CYA | ||||
| 3 | 0,0,4 | |||
| 7 | 0,0,1 | |||
| 14 | 0,0,0 | 0,0,0 | ||
| 21 | 0,0,0 | These were done concurrently w/14 day | ||
| CYA | 1 | 0,0,1 | ||
| then | ||||
| MEL | ||||
| 3 | 0,0,0 | 0,0,0 | ||
| 7 | 0,0,0 | These were done concurrently w/3 day |
2.4. Clinical observations, necropsy and sampling
Fish were examined twice a day for mortalities or any clinical abnormalities, such as abnormal swimming or position in the water column. After the treatment period fish were removed from treatment tanks, stunned with a sharp blow to the head, and euthanized by severing the cervical spine followed by double pithing as per AVMA guidelines on Euthanasia, 2007. The overall mean weight and length of all experimental catfish (n = 102) was 545.7 ± 247.0 g and 35.0 ± 5.4 cm, while for trout (n = 108) the overall means were 553.1 ± 190.4 g and 33.9 ± 3.6 cm. The caudal kidney was removed and transverse sections were dissected for fresh tissue wet mount (Reimschuessel et al., 2009). The first section was taken from the most posterior part of the kidney. In trout, the second set of samples was taken from a region near the dorsal fin. In channel catfish, which have a bifurcated caudal kidney, the second wet mount section was sampled near the base of one fork. The amount of tissue examined by wet mount was approximately 200 mg. The remaining kidney tissue from each fish was archived in a tissue cassette and stored at −80 °C. The wet mount sections were placed on a microscope slide, covered, and flattened with another slide. Slides were viewed with an inverted microscope (IX7-S8F, Olympus America Inc., Center Valley, PA, USA) for the presence of crystal spherulites previously described in fish given a combined oral dose of MEL and CYA (Reimschuessel et al., 2008a, 2009). The crystal results were described with a subjective scale from 0–5 as follows: 0 – none seen; 1 – only 1 crystal in an entire section; 2 – few with scattered distribution; 3 – moderate numbers seen throughout section; 4 – large numbers seen immediately; and 5 – extensive numbers obliterating the regular tissue architecture.
3. Results
3.1. Co-administration study
Crystal presence in both catfish and trout increased when given the same dosage multiple days in a row (Fig 1). The single dose NOAEL for catfish, a warm water species, was 10 MEL + 10 CYA mg/kg bw, while the single dose NOAEL in the trout, a cold water species, was 2.5 MEL + 2.5 CYA mg/kg bw. The 14 day repeated dose NOAEL for both species was 0.5 MEL + 0.5 CYA mg/kg bw. No crystals were observed in kidneys of catfish given 0.1 MEL + 0.1 CYA mg/kg bw for 28 days (comparable to 2.5 ppm of each triazine in the feed). The prevalence of fish with renal crystals declined with decreasing dose (Figs. 2 and 3) as did the intensity of crystals observed in the wet mount sections (Table 1b).
Fig. 1.
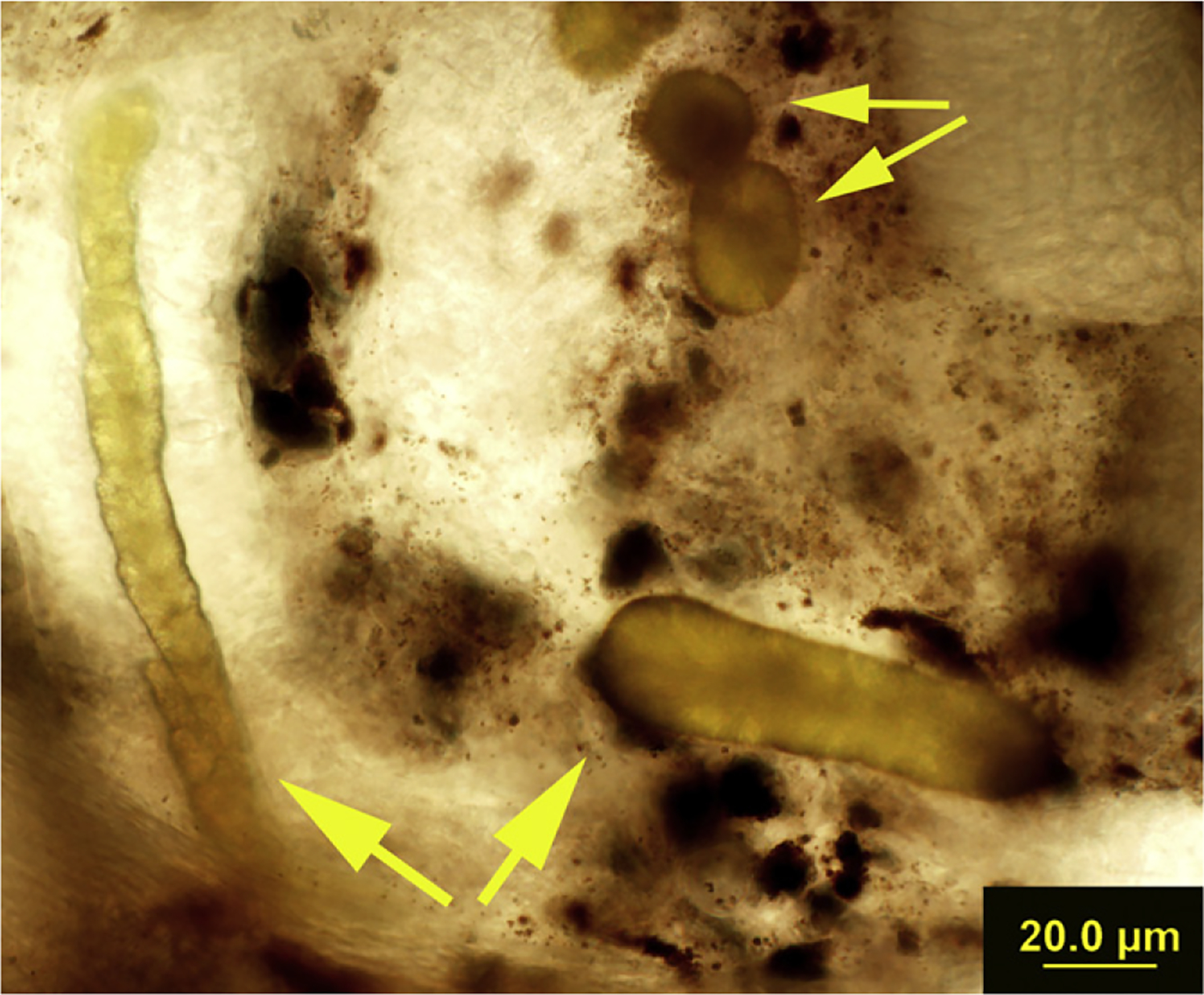
Crystals present in the wet mount section of a trout. Small yellow arrows indicate crystals that have the typical spherulite appearance, large yellow arrows indicate the conglomerate, tubule shape of some of the crystal precipitates. The dark brownish black material is the pigment melanin, found normally in the interstitium of trout kidneys.
Fig. 2.
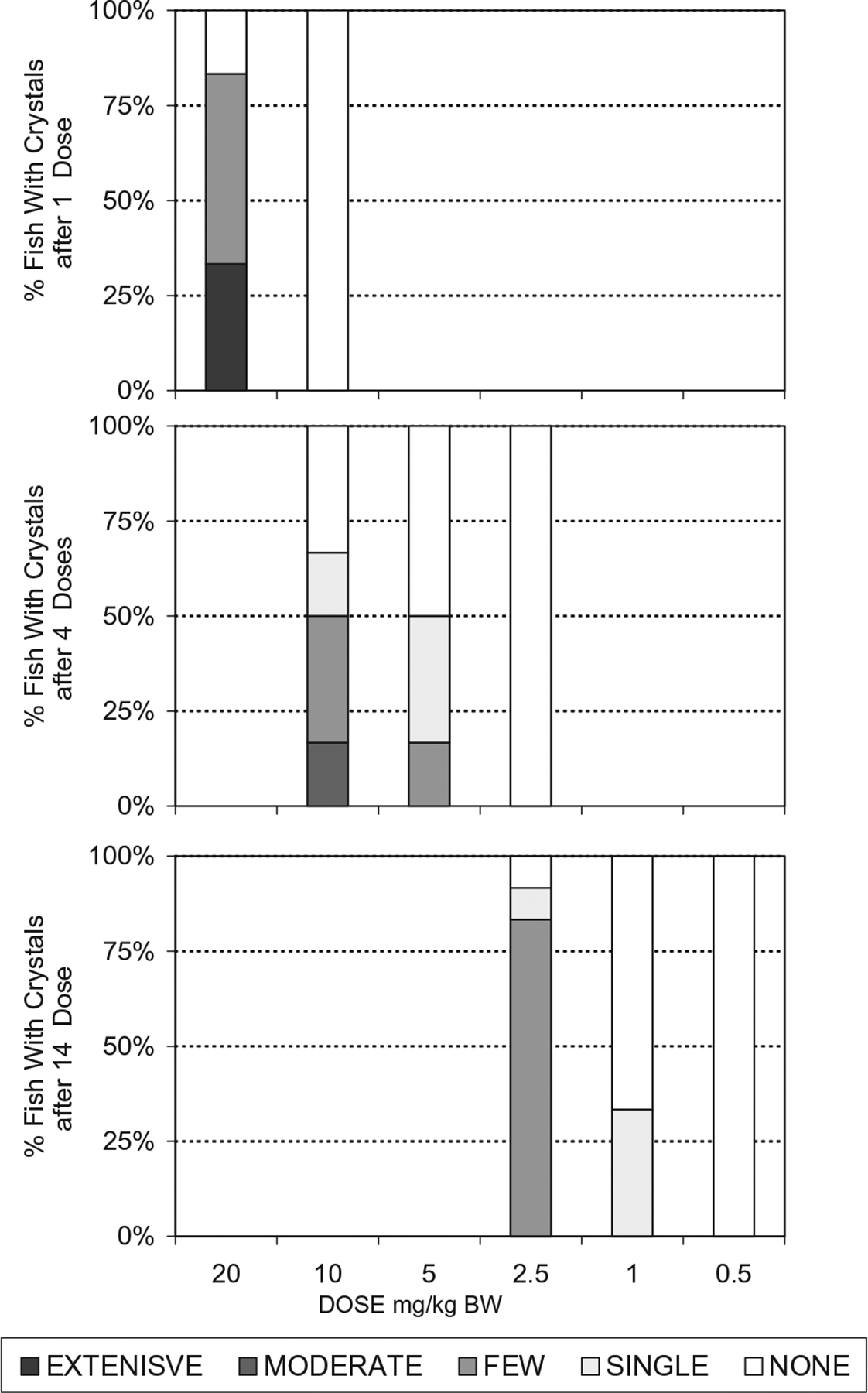
Crystal intensity breakdown observed in kidney wet mount sections from catfish administered melamine and cyanuric acid. A dose response is evident as the number of catfish with crystals decreased as dose decreased. Crystal intensity also decreased as dose decreased. For each individual dosage level:number of doses combination, n = 6–12. The subjective scale for crystal rankings was as follows: None – none seen (numerical rank 0); single – only 1 crystal in an entire section – (numerical rank 1); few – few with scattered distribution -(numerical rank 2); moderate – moderate numbers seen throughout section – (numerical rank 3); extensive – large numbers seen immediately (numerical ranks 4–5).
Fig. 3.
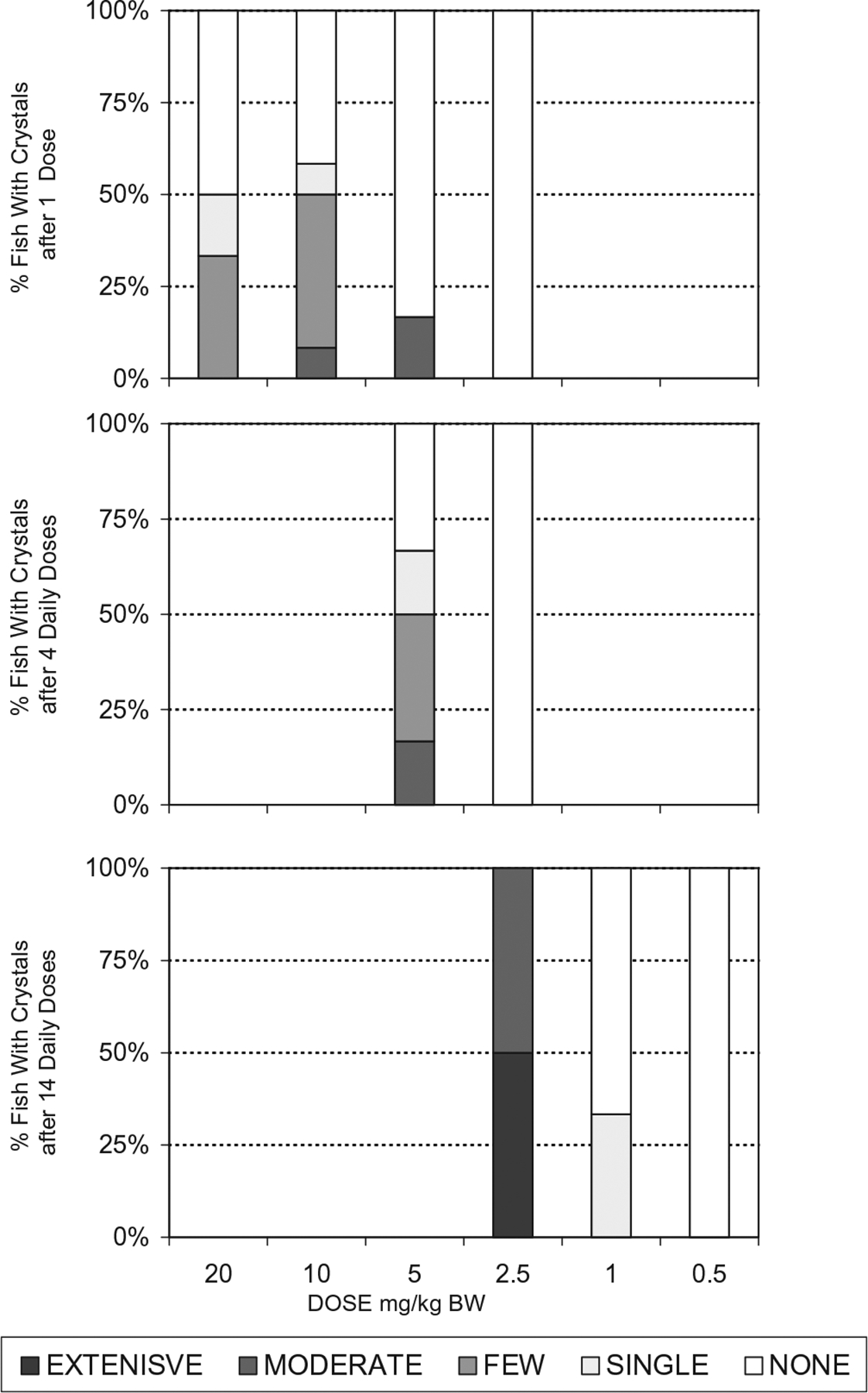
Crystal intensity breakdown observed in kidney wet mount sections from trout administered melamine and cyanuric acid. A dose response is seen in the data for multiple doses as number of trout with crystals as well as crystal intensity decreased as dose decreased. For each individual dosage level:number of doses combination, n = 6–12. The subjective scale for crystal rankings was as follows: None – none seen (numerical rank 0); single- only 1 crystal in an entire section – (numerical rank 1); few – few with scattered distribution – (numerical rank 2); moderate – moderate numbers seen throughout section – (numerical rank 3); extensive – large numbers seen immediately (numerical ranks 4–5).
Table 1b.
Intensity of crystal spherulites in trout kidney wet mount sections.
| DoseMEL + CYA (mg/kg bw each) | Estimated equivalent in feed (ppm) | # Daily doses | Initial phase (intensity) | Confirmatory phase (intensity) | Comment |
|---|---|---|---|---|---|
| 20 | 500 | 1 | 0,0,0,1,2,2 | (results from depletion study) | |
| 10 | 250 | 1 | 0,0,0 | 0,0,1,2,2,2,2,2,3 | |
| 5 | 125 | 1 | 0,0,0,0,0,3 | ||
| 2.5 | 62.5 | 1 | 0,0,0,0,0,0 | 0,0,0,0,0,0 | NOAEL |
| 5 | 125 | 4 | 1,2,2,3,0,0 | These were done concurrently w/single dose | |
| 2.5 | 62.5 | 4 | 0,0,0,0,0,0 | Starting test dose for 14 day study | |
| 2.5 | 62.5 | 14 | 4,4,4,3,3,3 | ||
| 1 | 25 | 14 | 3,2,1,1,0,0 | ||
| 0.5 | 12.5 | 14 | 0,0,0,0,0,0 | 0,0,0,0,0,0 | NOAEL |
The intensity of melamine–cyanurate crystal spherulites seen on wet mount sections of kidney. Each intensity value represents the finding in an individual fish, initial phase n = 3–6, confirmatory phase n = 3–9.
Intensity Scale: 1 – only 1 in an entire section; 2 – few with scattered distribution; 3 – moderate numbers seen throughout section; 4 – large numbers seen immediately; and 5 – extensive numbers obliterating the regular tissue architecture.
Estimated equivalent in feed corresponds to the mg/kg bw dose if fish are fed 4% of their body weight per day.
3.2. Sequential dosing study
Crystals formed in fish which ingested MEL and CYA at different times (Table 2b). The longer the waiting times between doses, the fewer fish developed renal crystals. “No response waiting times” in catfish were 3 days for fish given CYA first, and 14 days for fish given MEL first. “No response waiting times” for trout were 7 days for fish given CYA first and 21 days for fish receiving MEL first.
Table 2b.
Sequential dosing experiments – trout.
| Triazine order | Waiting period (days) | Initial phase (intensity) | Confirmatory phase (intensity) | Comments |
|---|---|---|---|---|
| MEL | 1 | 3,3,3 | ||
| then | ||||
| CYA | ||||
| 3 | 0,2,3 | |||
| 7 | 0,0,2 | |||
| 14 | 0,0,0 | 0,0,1 | ||
| 21 | 0,0,0 | 0,0,0 | These were done concurrently w/14 day | |
| CYA | 1 | 0,2,3 | ||
| then | ||||
| MEL | ||||
| 3 | 0,0,0 | 0,0,3 | ||
| 7 | 0,0,0 | 0,0,0 |
The intensity of melamine–cyanurate crystal spherulites seen on a wet mount section of kidney. Each intensity value represents the finding in an individual fish, initial phase n = 3, confirmatory phase n = 3.
Fish were given 20 mg/kg bw of the first triazine followed by a waiting period and then 20 mg/kg bw of the second triazine. Estimated equivalent in feed is approximately 500 ppm of each triazine.
Intensity Scale as in Table 1.
4. Discussion
In 2007, feed ingredients adulterated with MEL and CYA caused renal failure in many pets in the USA, Canada and Africa (Anon, 2008; Barboza and Barrioneuvo, 2007; Reyers, 2007; USFDA, 2007a). Intratubular melamine–cyanurate crystal spherulite formation was the hallmark lesion in these cases (Brown et al., 2007; Cianciolo et al., 2008; Reimschuessel et al., 2008a; Thompson et al., 2008). These spherulites cause intratubular obstruction and subsequent acute renal failure in a mechanism similar to uric acid nephropathy (Conger et al., 1976; Conger, 1990; Dobson et al., 2008; Reimschuessel et al., 2008a; Yarlagadda and Perazella, 2008). Melamine–cyanurate crystal spherulites have also been induced experimentally by co-administration of high doses of MEL and CYA in cats, rats, fish and pigs (Puschner et al., 2007; Dobson et al., 2008; Reimschuessel et al., 2008a). There are, however, no published studies describing a dose response for combined MEL and CYA exposure. We present here a dose response showing that crystal presence increases with repeated dosing.
The toxicity of each of these chemicals when administered alone has been studied (Reimschuessel et al., 2008b; WHO, 2004; OECD, 1998, 1999; IUCLID, 2000a,b). In general, these chemicals have low toxicity except at very high dosages. The primary lesion from long-term repeated high dose exposure is the formation of urinary tract stones, especially for MEL (NTP, 1983; Melnick et al., 1984; Heck and Tyl, 1985; Okumura et al., 1992; Ogasawara et al., 1995) but also for CYA (Hodge et al., 1965; Cohen et al., 1999; Serota et al., 1982). Bladder neoplasms, thought to be primarily due to mechanical irritation by the stones, have also been associated with long-term MEL exposure (NTP, 1983; Ogasawara et al., 1995; Cremonezzi et al., 2001, 2004).
In rats, the stones induced by ingesting MEL alone were composed of melamine–uric acid at a ratio of approximately 1:1 (Ogasawara et al., 1995). In 2008, Chinese infants exposed to high doses of almost pure MEL also developed urinary tract stones composed of melamine–uric acid, with a ratio of approximately 1:2 (Jia et al., 2009; Grases et al., 2009; Guan et al., 2009; WHO, 2008). These stones, visualized by ultrasonography, were located primarily in the renal calyces, ureter, or the renal pelvis (Jia et al., 2009; Lau et al., 2009; Wang et al., 2009; Wen et al., 2009b). Many of the cases were asymptomatic, but some children developed renal failure as a result of post-renal obstruction by the stones (WHO, 2008; Shen et al., 2009; Sun et al., 2009).
It is important to note the difference between stones (melamine–uric acid) formed after ingestion of only MEL, and crystal spherulites (melamine–cyanurate) formed after the ingestion of both MEL + CYA. Melamine–uric acid stones as identified by ultrasonography in the pediatric cases were in the 2–18 mm size range (Grases et al., 2009; Wen et al., 2009a; Hu et al., 2010). The stones caused renal failure due to ureteral obstruction and subsequent hydronephrosis (Wen et al., 2009a; Shen et al., 2009). In contrast, melamine–cyanurate crystal spherulites are microscopic, in the 10–100 μm range. The crystals cause renal failure by forming micro-obstructions within the proximal and distal tubules of the nephrons (Dobson et al., 2008; Reimschuessel et al., 2008a; Chen et al., 2009; Kim et al., 2010). When extensive numbers of crystal spherulites “plug” the renal tubules, intra-renal pressure rises, collapsing capillaries and inducing acute renal failure via crystal nephropathy (Conger et al., 1976). In experimental animals, renal failure can happen within the first few days of exposure to MEL + CYA (Puschner et al., 2007; Dobson et al., 2008; Reimschuessel et al., 2008a; Kim et al., 2010). In contrast, acute renal failure, due to crystal formation and intra-renal obstruction, has not been reported following experimental exposure to either MEL or CYA separately.
One question that has recently arisen is: can ingestion of MEL without CYA cause crystal formation? There were multiple reports of “sandy urine” in children that ingested the adulterated infant formula (Chiu, 2008; Wong and Chiu, 2008; Guan et al., 2009; Jia et al., 2009). This “sand” may have been some type of crystalluria. There is only one report of the typical radial crystal spherulites being found a patient’s urine (Lam et al., 2009). Potential intratubular melamine–uric acid crystal formation was considered a possible contributing factor to the renal failure seen in some infants (Sun et al., 2010), but there is, at present, no data to support this hypothesis.
It is still uncertain what the nidus for stone formation is. Cyanuric acid was actually detected, in small amounts, in the infant formula: melamine (188,000 mg/kg), cyanuric acid (3.2 mg/kg), ammeline (14.9 mg/kg) and ammelide (293 mg/kg) (WHO, 2008). These are the median values reported, some cyanuric values were higher than 3.2. It is unknown if very small amounts of cyanuric acid (either formed by gut bacteria, or in the melamine product) can cause a few crystals to form in the kidney which then can serve as a focus for further precipitation or conglomeration of proteins and uric acid to develop into the stones which formed in the children.
In our recent melamine depletion study, 1 of 30 fish treated with only MEL had renal crystals typical of those seen in the MEL-CYA treated animals. Likewise, in a study evaluating crystal formation in pigs, one animal treated with only MEL also developed renal crystals (manuscript in preparation). The chemical composition of these crystals is currently being investigated.
Microcrystal formation was described as a gross renal lesion in rats treated with only MEL, but no crystals were found by histopathology (Ogasawara et al., 1995). Those tissues were, however, preserved in formalin, which dissolves uric acid crystals (Vernon, 2006). Melamine–cyanuric acid crystals also dissolve in formalin (Reimschuessel et al., 2008a). If melamine–uric acid crystals do indeed form subsequent to MEL ingestion, then routine formalin processing would probably dissolve those crystals.
Previous histopathologic studies of animals dosed with only MEL may have failed to detect limited crystal formation because the crystals dissolved during fixation. A recent re-evaluation of the histopathology slides from the 1983 NTP melamine oral toxicity study in rats showed evidence of renal pathology that had been missed in the earlier evaluation (NTP, 1983; Hard et al., 2009). Hard et al. (2009) describe a “retrograde nephropathy” which may have occurred when “melamine precipitation in the lower urinary tract created pressure effects through transient obstruction leading to the renal changes, and could be related to some intra-renal obstruction”.
The crystal nephropathy caused by co-administration of MEL and CYA causes renal failure at dosages much lower than the lethal doses of MEL or CYA alone. The LD 50 in rats for MEL alone is approximately 3000 mg/kg and that for CYA is approximately 7000 mg/kg (OCED, 1998, 1999). Puschner et al. (2007) showed that a combined dose of 32 MEL + 32 CYA mg/kg bw induced crystals and renal failure in cats. A 3 day course of 25 MEL + 25 CYA mg/kg bw caused renal failure and crystals in kidneys of 2/10 rats (Kim et al., 2010). In our fish depletion study, we found that a single combined dose of 20 MEL + 20 CYA mg/kg bw induced crystals in approximately half of the animals (Reimschuessel et al., 2009). These findings demonstrate that ingestion of products containing both MEL and CYA poses an even greater risk than ingestion of either chemical alone. Determining a threshold dose for renal crystal formation was thus a priority research area for CVM and USFDA.
Since crystal formation, followed by intra-renal obstruction, as seen with uric acid nephropathy, is the main pathologic feature of combined MEL and CYA ingestion, we chose this feature as the assay for a “No Adverse Effect.” Like uric acid crystals, melamine–cyanurate crystals can dissolve in formalin (Reimschuessel et al., 2008a), although not as rapidly. Therefore we examined tissue using the “wet-mount” technique, to eliminate the possibility of dissolving crystals with the fixative.
Renal melamine–cyanurate crystals were readily observed in the wet mount sections of kidneys of fish ingesting MEL and CYA administered simultaneously. The number of fish which formed crystals, and the intensity of the response was dependant on dose and duration. The 14 day NOAEL for both species was 0.5 MEL + 0.5 CYA mg/kg bw. As can be seen in Table 1b, the dosage levels tested between 0.5 MEL + 0.5 CYA and 10 MEL + 10 CYA mg/kg bw induced crystal formation when given for longer time periods. For example, a dosage of 2.5 MEL + 2.5 CYA mg/kg bw caused crystals to form if given for 14 days, but not if given for only 4 days in both species.
The single dose NOAEL was different for the two fish species. The catfish single dose NOAEL was 10 MEL + 10 CYA mg/kg bw, while that of trout was 2.5 MEL + 2.5 CYA mg/kg bw. This may be due to the fact that trout are held at colder temperatures, and the excretion of a single dose of the triazines from kidney may be slower in trout than in catfish. This has been shown to be the case for muscle tissue, with MEL and CYA residue depletion of a single dose being slower in trout than in catfish (Reimschuessel et al., 2009). In both species, CYA muscle residues deplete faster than MEL. The results of the sequential dosing experiments presented here would suggest that the same is true for CYA in the kidney.
In the case of sequential dosing, fish given CYA first made crystals only if MEL was given within 1 day (catfish) or 3 days (trout). This is presumably due to the rapid excretion of CYA. When MEL was given after those “waiting periods” no crystals were observed in the kidney section. The longer waiting periods essentially created an experiment in which fish were exposed to only one chemical, since the chemical administered earlier was no longer present. Thus, the toxic effects of combined exposure would no longer be expected.
MEL residues, in contrast, take longer to be excreted than CYA, especially in trout which have a slower excretion rate than catfish. In our previous depletion study, after a single 20 mg/kg bw MEL dose, 6 of 6 trout on day 7 and 2 of 6 trout on day 14 had muscle residues which exceeded 0.5 mg/kg (Reimschuessel et al., 2009). Our hypothesis suggested that the residual melamine being excreted in the kidney could bind newly ingested CYA and produce renal crystals. The results of the sequential dosing experiment showed that this is indeed the case in both fish species. Fish treated with MEL first and then CYA developed crystals despite waiting periods of 7 days (catfish) or 14 days (trout).
The results of both sequential dosing experiments corresponded to our residue depletion data (Fig. 4). In the sequential study some catfish and trout developed renal crystals when given CYA 7 days after the single MEL dose, at a time point in the depletion study when muscle MEL residues were still above 0.5 mg/kg in a number of fish. The timeframe for developing crystals was much shorter in the fish given CYA first. Again, in the depletion study, muscle CYA residues in several fish were above 0.5 mg/kg on day 1, and correspondingly, fish in the sequential study developed renal crystals when dosed with MEL on day 1. By day 3, fewer fish had detectable CYA residues in the depletion study, and correspondingly only a few fish developed crystals in the sequential dosing experiments. The residue data are from a different experiment, but the dosage levels were the same, 20 mg/kg bw of each compound, comparable to approximately 500 ppm in the feed.
Fig. 4.
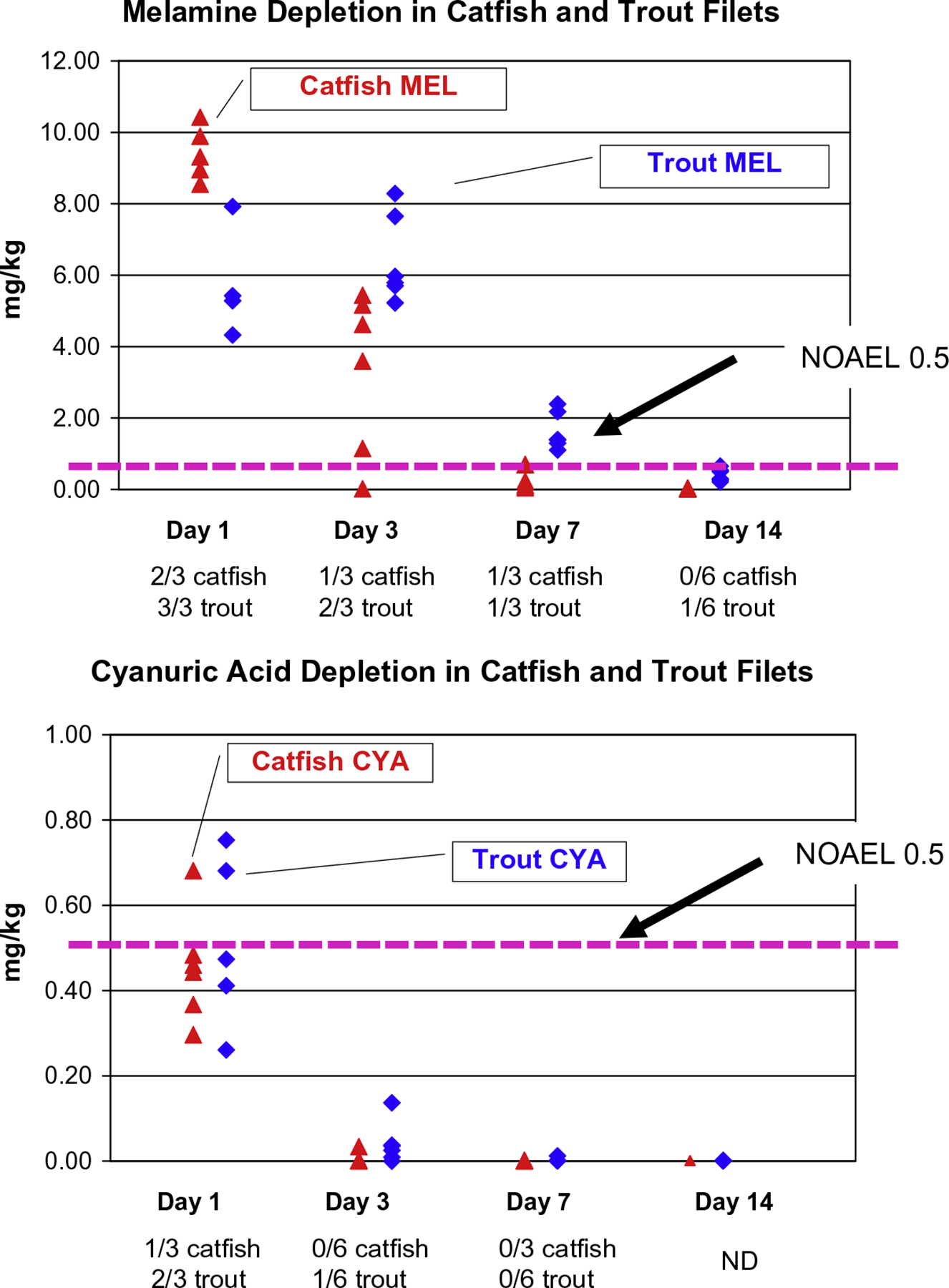
Fourteen day NOAEL and sequential dosing results in relation to residue levels of melamine and cyanuric acid in catfish and trout muscle. This figure presents data from 3 different experiments, (1) the graphs show the residues of melamine and cyanuric acid in muscle following a single 20 mg/kg BW dose (Reimschuessel et al., 2009), (2) the horizontal dashed lines are the NOAELs from the threshold studies, and (3) the fractions below the x-axis of time are the results of the sequential dosing experiments when the compound in the graph was given first and the other compound was given 1, 3, 7, or 14 days after the first. Prevalence of crystals in the sequential dosing experiments roughly parallels the depletion of the initial compound given, as muscle melamine residues fall below the NOAEL at 14 days and little is left to form crystals when cyanuric acid is given. This is also the case with cyanuric acid residues which fall below the level of detection on day 7. A slight lag is expected when apparent residue levels are extrapolated from muscle to kidney as the kidney is the excretory organ for s-triazines and may be the last tissue to deplete these compounds fully. Filets used for residue analysis were prepared as they would be for market.
How do we use this information when evaluating risks of combined triazine exposure to humans? One might first ask, how could one ever extrapolate data obtained from fish to any human health issue, or in general “why study fish?” In a word, diversity. With their myriad anatomical variations and adaptations, fish can provide the necessary tools for a creative scientist to investigate aspects of injury and physiology that cannot be isolated in mammalian models. This is especially true for the kidney. Marshall and Grafflin (1928) used the aglomerular toadfish to demonstrate that basolateral transport is a mechanism by which the kidney removes compounds. These studies could not be performed in mammals, which have glomeruli, the renal filter. Once, however, the proof of concept had been demonstrated in a fish model, researchers were motivated to seek evidence for such a mechanism in mammals.
Currently, fish are used extensively to study human cancer (Bailey et al., 1996; Goessling et al., 2007; Feitsma and Cuppen, 2008), neuromuscular diseases (Dubois-Dalcq et al., 2008; Bassett and Currie, 2003), hematologic and cardiac disorders (Keller and Murtha, 2004; Carradice and Lieschke, 2008; Dahme et al., 2009), aging (Keller and Murtha, 2004), renal injury and development (Reimschuessel, 2001; Drummond, 2005), chemical toxicology and pharmacology (Hill et al., 2005; Kari et al., 2007) and a host of infectious diseases (Dodd et al., 2000; Schmale et al., 2007; Talaat et al., 1998). Fish models, can possess unique adaptations that may make them more suitable for certain types of studies. In addition, using fish models can ultimately reduce the number of higher vertebrates needed for biomedical research.
This is not to say that all the information we need will come from fish studies. However, these studies can provide a substantial foundation for subsequent studies in mammals. Data derived from our fish co-exposure study can provide starting points for traditional rat NOAEL studies. In the present study, catfish receiving 0.1 MEL + 0.1 CYA mg/kg bw orally for 28 days did not develop renal crystals. This dosage is comparable to 2.5 MEL + 2.5 CYA mg/kg (ppm) in the feed. Most risk assessments conducted following the Chinese melamine incident in 2008 concluded that 2.5 ppm is the “level of no concern” for food or feed ingredients. Our data in fish, an extremely sensitive model, would agree with those assessments.
The information derived from the sequential dosing studies may provide insight into potential risks faced by patients with reduced excretory capacity. Fish excrete drugs more slowly than mammals (Reimschuessel et al., 2005; Reimschuessel et al., 2008c). Mammalian models to evaluate triazine-induced crystal formation in kidneys with reduced capacity would require additional experimental variables such as a nephrotoxic agent to diminish tubular function, reduced blood flow or partial nephrectomy. Fish, with their slower excretion rates, can provide a “natural” model for studying effects of drugs or chemicals when there is a prolonged retention time. This is especially important when evaluating the effects of multiple chemicals. With their slower excretion rates, fish may serve as a “worst case scenario” for higher risk groups such as infants or persons with compromised renal function.
In conclusion, we have shown that fish can be a good first-line non-mammalian model to examine the effects of combined MEL and CYA dietary exposure. It is hoped that the data presented here, when viewed in conjunction with other mammalian studies, will broaden our knowledge base and enhance the overall understanding of triazine toxicology in both humans and animals.
Acknowledgements
The authors would like to thank S. Matthews, V. Mills, S. Rill, and M. McDonald for their invaluable assistance in animal care and monitoring.
Abbreviations:
- Bw
body weight
- C
Celsius
- cm
centimeter
- CVM
Center for Veterinary Medicine
- CYA
cyanuric acid
- d
days
- DO
dissolved oxygen
- g
gram
- GLP
good laboratory practices
- K el
elimination rate constant
- L
liter
- LC–MS/MS
liquid chromatography tandem mass spectrometry
- LOQ
limit of quantification
- MEL
melamine
- mg/kg
milligram per kilogram
- min
minutes
- mL
milliliter
- mM
millimolar
- N
normal
- NOAEL
No observable adverse effects level
- ppm
parts per million
- s
seconds
- S.D
standard deviation
- t 1/2
half-life
- TDI
tolerable daily intake
- USFDA
US Food and Drug Administration
- VOCs
volatile organic compounds
- μg/mL
micrograms per milliliter
- μm
micrometer
References
- Anon, 2008. The Government of Canada responds to reports of melamine in food products. Health Canada. Available at: http://www.hc-sc.gc.ca/fn-an/securit/chem-chim/melamine/index-eng.php (accessed 12.03.10). [Google Scholar]
- AQSIQ, 2008. Five Departments Regulated Melamine Limits in Milk Products for the First Time. General Administration of Quality Supervision, Inspection and Quarantine, China. [Google Scholar]
- AVMA Guidelines on Euthanasia, 2007. Available at: <http://www.avma.org/issues/animal_welfare/euthanasia.pdf> (accessed 12.03.10).
- Bailey GS, Williams DE, Hendricks JD, 1996. Fish models for environmental carcinogenesis: the rainbow trout. Environ. Health Perspect 104 (1), 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza D, Barrioneuvo A, 2007. Filler in Animal Feed Is Open Secret in China. New York Times. Available at: <http://www.nytimes.com/2007/04/30/business/worldbusiness/30food.html> (accessed 12.03.10). [Google Scholar]
- Barboza D, 2008. Asia Food Tainting Spreads, Leading to Recall in US. New York Times. Available at: <http://www.nytimes.com/2008/09/27/world/asia/27recall.html> (accessed 12.03.10). [Google Scholar]
- Bassett DI, Currie PD, 2003. The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum. Mol. Genet 5 (12), 265–270. [DOI] [PubMed] [Google Scholar]
- Bhalla V, Grimm PC, Chertow GM, Pao AC, 2009. Melamine nephrotoxicity: an emerging epidemic in an era of globalization. Kidney Int. 75 (8), 774–779. [DOI] [PubMed] [Google Scholar]
- Bradsher K, 2008. China Begins Inquiry into Tainted Baby Formula. New York Times. Available at: <http://www.nytimes.com/2008/09/13/world/asia/13milk.html?hp> (accessed 12.03.10). [Google Scholar]
- Brown CA, Jeong KS, Poppenga RH, Puschner B, Miller DM, Ellis AE, Kang KI, Sum S, Cistola AM, Brown SA, 2007. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J. Vet. Diagn. Invest 19 (5), 525–531. [DOI] [PubMed] [Google Scholar]
- Carradice D, Lieschke GJ, 2008. Zebrafish in hematology: sushi or science? Blood 111 (7), 3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Food Safety, The Government of the Hong Kong Special Administrative Region, 2008. Available at: <http://www.cfs.gov.hk/english/whatsnew/whatsnew_fstr/whatsnew_fstr_Test_dairy_product_FAQ.html> (accessed 12.03.10).
- Chen KC, Liao CW, Cheng FP, Chou CC, Chang SC, Wu JH, Zen JM, Chen YT, Liao JW, 2009. Evaluation of subchronic toxicity of pet food contaminated with melamine and cyanuric acid in rats. Toxicol. Pathol 37 (7), 959–968. [DOI] [PubMed] [Google Scholar]
- Chiu MC, 2008. Melamine-tainted milk product (MTMP) renal stone outbreak in humans. Hong Kong Med. J 14, 424–426. [PubMed] [Google Scholar]
- Cianciolo RE, Bischoff K, Ebel JG, Van Winkle TJ, Goldstein RE, Serfilippi LM, 2008. Clinicopathologic, histologic, and toxicologic findings in 70 cats inadvertently exposed to pet food contaminated with melamine and cyanuric acid. J. Am. Vet. Med. Assoc 233 (5), 729–737. [DOI] [PubMed] [Google Scholar]
- Cohen SM, McKinney L, Wagner BM, Weil C, Goodman JI, Lotti M, Portoghese P, Bernard BK, 1999. An Evaluation of the Long-term Toxicity/Carcinogenicity of Sodium Isocyanurate (No. 44834403) (Submitted as Unpublished Report 25 February 1999 to Occidental Chemical Corporation, provided to US, EPA. Submitted to WHO by the Industry Ad Hoc Committee on Isocyanurates).
- Conger JD, Falk SA, Guggenheim SJ, Burke TJ, 1976. A micropuncture study of the early phase of acute urate nephropathy. J. Clin. Invest 58 (3), 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JD, 1990. Acute uric acid nephropathy. Med. Clin. North Am 74 (4), 859–871. [DOI] [PubMed] [Google Scholar]
- Commission of the European Communities. COMMISSION DECISION of 26 September 2008 Imposing Special Conditions Governing the Import of Products Containing Milk or Milk Products Originating in or Consigned from China. Official Journal of the European Union September 2008. Available at: <http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:259:0010:0011:EN:PDF> (accessed 12.03.10). [Google Scholar]
- Cremonezzi DC, Silva RA, del Pilar Diaz RA, Valentich MA, Eynard AR, 2001. Dietary polyunsaturated fatty acids (PUFA) differentially modulate melamine-induced preneoplastic urothelial proliferation and apoptosis in mice. PLEFA 64 (3), 151–159. [DOI] [PubMed] [Google Scholar]
- Cremonezzi DC, Díaz MP, Valentich MA, Eynard AR, 2004. Neoplastic and preneoplastic lesions induced by melamine in rat urothelium are modulated by dietary polyunsaturated fatty acids. Food Chem. Toxicol 42 (12), 1999–2007. [DOI] [PubMed] [Google Scholar]
- Dahme T, Katus HA, Rottbauer W, 2009. Fishing for the genetic basis of cardiovascular disease. Dis. Model Mech 2 (1–2), 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson RLM, Motlagh S, Quijano M, Cambron RT, Baker TR, Pullen AM, Regg BT, Bigalow-Kern AS, Vennard T, Fix A, Reimschuessel R, Overmann G, Shan Y, Daston GP, 2008. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. J. Toxicol. Sci 106 (1), 251–262. [DOI] [PubMed] [Google Scholar]
- Dodd A, Curtis PM, Williams LC, Love DR, 2000. Zebrafish: bridging the gap between development and disease. Hum. Mol. Genet 9 (16), 2443–2449. [DOI] [PubMed] [Google Scholar]
- Drummond IA, 2005. Kidney development and disease in the zebrafish: review. J. Am. Soc. Nephrol 16 (2), 299–304. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Williams A, Stadelmann C, Stankoff B, Zalc B, Lubetzki C, 2008. From fish to man: understanding endogenous remyelination in central nervous system demyelinating diseases: review. Brain 131 (7), 1686–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitsma H, Cuppen E, 2008. Zebrafish as a cancer model: review. Mol. Cancer Res 6 (5), 685–694. [DOI] [PubMed] [Google Scholar]
- Goessling W, North TE, Zon LI, 2007. New waves of discovery: modeling cancer in zebrafish: review. J. Clin. Oncol 25 (17), 2473–2479. [DOI] [PubMed] [Google Scholar]
- Grases F, Costa-Bauzá A, Gomila I, Serra-Trespalle S, Alonso-Sainz F, del Valle JM, 2009. Melamine urinary bladder stone. Urology 73 (6), 1262–1263. [DOI] [PubMed] [Google Scholar]
- Guan N, Fan Q, Ding J, Zhao Y, Lu J, Ai Y, Xu G, Zhu S, Yao C, Jiang L, Miao J, Zhang H, Zhao D, Liu X, Yao Y, 2009. Melamine-contaminated powdered formula and urolithiasis in young children. N. Eng. J. Med 360 (11), 1067–1073. [DOI] [PubMed] [Google Scholar]
- Hard GC, Flake GP, Sills RC, 2009. Re-evaluation of kidney histopathology from 13-week toxicity and two-year carcinogenicity studies of melamine in the F344 rat: morphologic evidence of retrograde nephropathy. Vet. Pathol 46 (6), 1248–1257. [DOI] [PubMed] [Google Scholar]
- Heck HD, Tyl RW, 1985. The induction of bladder stones by terephthalic acid, dimethyl terephthalate, and melamine (2,4,6-triamino-s-triazine) and its relevance to risk assessment. Regul. Toxicol. Pharm 5 (3), 294–313. [DOI] [PubMed] [Google Scholar]
- Heller DN, Nochetto CB, 2008. Simultaneous determination and confirmation of melamine and cyanuric acid in animal feed by zwitterionic hydrophilic interaction chromatography and tandem mass spectrometry. Rapid Commun. Mass Spectrom 22, 3624–3632. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE, 2005. Zebrafish as a model vertebrate for investigating chemical toxicity: review. Toxicol. Sci 86 (1), 6–19. [DOI] [PubMed] [Google Scholar]
- Hodge HC, Panner BJ, Downs WL, Maynard EA, 1965. Toxicity of sodium cyanurate. Toxicol. Appl. Pharmacol 7, 667–674. [DOI] [PubMed] [Google Scholar]
- Hu P, Lu L, Hu B, Zhang CR, 2010. The size of melamine-induced stones is dependent on the melamine content of the formula fed, but not on duration of exposure. Pediatr. Nephrol 25 (3), 565–566. [DOI] [PubMed] [Google Scholar]
- IUCLID, 2000a. Chemical Dataset for Cyanuric Acid (International Uniform Chemical Information Database) Dataset Created by European Commission, European Chemicals Bureau.
- IUCLID, 2000b. Chemical Dataset for Melamine (International Uniform Chemical Information Database) Dataset Created by European Commission, European Chemicals Bureau.
- Jia L, Shen Y, Wang X, He L, Xin Y, Hu Y, 2009. Ultrasonographic diagnosis of urinary calculus caused by melamine in children. Chin. Med. J 122 (3), 252–256. [PubMed] [Google Scholar]
- Kari G, Rodeck U, Dicker AP, 2007. Zebrafish: an emerging model system for human disease and drug discovery: review. Clin. Pharmacol. Ther 82 (1), 70–80. [DOI] [PubMed] [Google Scholar]
- Keller ET, Murtha JM, 2004. The use of mature zebrafish (Danio rerio) as a model for human aging and disease: review. Comp. Biochem. Physiol. C. Toxicol. Pharmacol 138 (3), 335–341. [DOI] [PubMed] [Google Scholar]
- Kim CW, Yun JW, Bae IH, Lee JS, Kang HJ, Joo KM, Jeong HJ, Chung JH, Park YH, Lim KM, 2010. Determination of spatial distribution of melamine–cyanuric acid crystals in rat kidney tissue by histology and imaging matrix-assisted laser desorption/ionization quadrupole time-of-flight mass spectrometry. Chem. Res. Toxicol 23 (1), 220–227. [DOI] [PubMed] [Google Scholar]
- Kuehn BM, 2009. Melamine scandals highlight hazards of increasingly globalized food chain. J. Am. Med. Assoc 301 (5), 473–475. [DOI] [PubMed] [Google Scholar]
- Lam CW, Lan L, Che X, Tam S, Wong SS, Chen Y, Jin J, Tao SH, Tang XM, Yuen KY, Tam PK, 2009. Diagnosis and spectrum of melamine-related renal disease: plausible mechanism of stone formation in humans. Clin. Chim. Acta 402(1–2), 150–155. [DOI] [PubMed] [Google Scholar]
- Lau HY, Wong CS, Ma JK, Kan E, Siu KL, 2009. US findings of melamine-related renaldisorders in Hong Kong children. Pediatr. Radiol 39 (11), 1188–1193. [DOI] [PubMed] [Google Scholar]
- Luengyosluechakul S, 2007. Evidence of melamine and related substances contamination to animal feed in Thailand. Thai J. Vet. Med 37 (4), 7–8. [Google Scholar]
- Marshall EK Jr, Grafflin AL, 1928. The structure and function of the kidney of Lophius pisatorius. Bull. Johns Hopkins Hosp 43, 205. [Google Scholar]
- Melnick RL, Boorman GA, Haseman JK, Montali RJ, Huff J, 1984. Urolithiasis and bladder carcinogenicity of melamine in rodents. Toxicol. Appl. Pharmacol 72 (2), 292–303. [DOI] [PubMed] [Google Scholar]
- New Zealand Food Safety Authority, 2008. NZFSA Refines Melamine Response Approach. Available at: <http://www.nzfsa.govt.nz/publications/media-releases/2008/29-sep-melamine-statement.htm> (accessed 12.03.10).
- NTP, 1983. Carcinogenesis Bioassay of Melamine (CAS No. 108-78-1) in F344/N Rats and B6C3F1 Mice (Feed Study). United States Department of Health and Human Services, Public Health Service, National Institutes of Health, National Toxicology Program, Research Triangle Park, NC, and Bethesda, MD (NTP TR 245; NTP-81–86; NIH Publication No. 83–2501). Available at: <http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr245.pdf> (accessed 12.03.10). [Google Scholar]
- OECD, 1998. Screening Information Dataset (SIDS) for Melamine. (CAS No. 108-78-1) Paris: Organisation for Economic Co-operation and Development. Available at: <http://www.inchem.org/documents/sids/sids/108781.pdf> (accessed 12.03.10). [Google Scholar]
- OECD, 1999. Screening Information Dataset (SIDS) for Isocyanuric Acid. (CAS No. 108-80-5) Paris: Organisation for Economic Co-operation and Development. Available at: <http://www.chem.unep.ch/irptc/sids/OECDSIDS/108805.pdf> (accessed 12.03.10). [Google Scholar]
- Ogasawara H, Imaida K, Ishiwata H, Toyoda K, Kawanishi T, Uneyama C, Hayashi S, Takahashi M, Hayashi Y, 1995. Urinary bladder carcinogenesis induced by melamine in F344 male rats: correlation between carcinogenicity and urolith formation. Carcinogenesis 16 (11), 2773–2777. [DOI] [PubMed] [Google Scholar]
- Okumura M, Hasegawa R, Shirai T, Ito M, Yamada S, Fukushima S, 1992. Relationship between calculus formation and carcinogenesis in the urinary bladder of rats administered the non-genotoxic agents thymine or melamine. Carcinogenesis 13 (6), 1043–1045. [DOI] [PubMed] [Google Scholar]
- Parry J, 2008. China’s Tainted milk scandal spreads around world. Br. Med. J 337:a, 1890. Available at: <http://www.bmj.com/cgi/content/full/337/oct01_1/a1890> (accessed 12.03.10). [DOI] [PubMed] [Google Scholar]
- Puschner B, Poppenga RH, Lowenstine LJ, Filigenzi MS, Pesavento PA, 2007. Assessment of melamine and cyanuric acid toxicity in cats. J. Vet. Diag. Invest 19 (6), 616–624. [DOI] [PubMed] [Google Scholar]
- Qing T, 2008. FDA Announces Recalls of More Melamine Tainted Chinese Products. The Epoch Times. Available at: <http://www.theepochtimes.com/n2/content/view/4876/> (accessed 18.02.09). [Google Scholar]
- Reimschuessel R, 2001. A fish model of renal regeneration and development: review. ILAR J. 42 (4), 285–291. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R, Evans E, Andersen WC, Turnipseed SB, Karbiwnyk CM, Mayer TD, Nochetto C, Rummel NG, Gieseker CM, 2009. Residue depletion of melamine and cyanuric acid in catfish and rainbow trout following oral administration. J. Vet. Pharmacol. Ther 33(2), 172–182. Available at: <http://www3.interscience.wiley.com/journal/122498253/abstract> (accessed 12.03.10). [DOI] [PubMed] [Google Scholar]
- Reimschuessel R, Gieseker CM, Miller RA, Ward J, Boehmer J, Rummel N, Heller DN, Nochetto C, De Alwis GK, Bataller N, Andersen WC, Turnipseed SB, Karbiwnyk CM, Satzger RD, Crowe JB, Reinhard MK, Roberts JF, Witkowski MR, 2008a. Evaluation of the renal effects of experimental feeding of melamine and cyanuric acid to fish and pigs. Am. J. Vet. Res 69 (9), 1217–1228. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R, Hattan DG, Gu Y, 2008b. Background Paper on Toxicology of Melamine and Its Analogues, prepared for Expert Meeting to Review Toxicological Aspects of Melamine and Cyanuric Acid. Health Canada, Ottowa, Canada, 1–4 December 2008. Available at: <http://www.who.int/foodsafety/fs_management/Melamine_5.pdf> (accessed 12.03.10). [Google Scholar]
- Reimschuessel R, Stewart L, Squibb E, Hirokawa K, Brady T, Brooks B, Shaikh B, Hodsdon C, 2008c. Phish-Pharm-2008. Available at: <http://www.fda.gov/AnimalVeterinary/ScienceResearch/ToolsResources/Phish-Pharm/default.htm> (accessed 12.03.10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimschuessel R, Stewart L, Squibb E, Hirokawa K, Brady T, Brooks B, Shaikh B, Hodsdon C, 2005. Fish drug analysis-phish-pharm: a searchable database of pharmacokinetics data in fish. AAPS J. 7(2), E288–E327. Available at: <http://www.aapsj.org/view.asp?art=aapsj070230> (accessed 12.03.10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyers F, 2007. Melamine-contaminated pet food. The South African experience. Vet. News (May issue), 8–12. [Google Scholar]
- Reyers F, 2008. Alert (Heads-up) message for South African Veterinary Livestock Health and Production Group. Melamine contamination of fresh milk in South Africa – Dairy meal adulteration. Livestock Health Prod. Rev 8, 4–5. [Google Scholar]
- Schmale MC, Nairn RS, Winn RN, 2007. Aquatic animal models of human disease. Comp. Biochem. Physiol. C. Toxicol. Pharmacol 145 (1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serota D, Fezio W, Hepner K, et al. , 1982. Thirteen-week Toxicity Study in Mice: Monosodium Isocyanurate: Project No. 2169–100. Final Report. (Submitted as unpublished report from Hazleton Laboratories America to WHO by the Industry Ad Hoc Committee on Isocyanurates). [Google Scholar]
- Shaikh B, Rummel N, Gieseker C, Serfling S, Reimschuessel R, 2003. Metabolism and residue depletion of albendazole and its metabolites in rainbow trout, tilapia and Atlantic salmon after oral administration. J. Vet. Pharmacol. Ther 26 (6), 421–427. [DOI] [PubMed] [Google Scholar]
- Shen Y, Liu XR, Zhang GJ, Zhou N, 2009. Blood purification therapy in treatment of acute renal failure in infants with melamine-induced stones. Chin. Med. J 122 (3), 257–261. [PubMed] [Google Scholar]
- Sun DQ, Zhang XF, Zhang L, Feng H, Yang YH, 2009. The clinical analysis of young children’s urolithiasis due to melamine-tainted infant formula. World J. Urol Available at: <http://www.ncbi.nlm.nih.gov/sites/entrez>. [DOI] [PubMed] [Google Scholar]
- Sun Q, Shen Y, Sun N, Zhang GJ, Chen Z, Fan JF, Jia LQ, Xiao HZ, Li XR, Puschner B, 2010. Diagnosis, treatment and follow-up of 25 patients with melamine-induced kidney stones complicated by acute obstructive renal failure in Beijing Children’s Hospital. Eur. J. Pediatr 169 (4), 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Shen Y, Sun Q, Li XR, Jia LQ, Zhang GJ, Zhang WP, Chen Z, Fan JF, Jiang YP, Feng DC, Zhang RF, Zhu XY, Xiao HZ, 2008. Melamine related urinary calculus and acute renal failure in infants. Chin. J. Pediatr 46 (11), 810–815. [PubMed] [Google Scholar]
- Talaat AM, Reimschuessel R, Waserman SS, Trucksis M, 1998. Mycobacterium marinum and the goldfish, Carassius auratus: a model system for mycobacterial pathogenesis. Infect. Immun 66 (6), 2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ME, Lewin-Smith MR, Kalasinsky VF, Pizzolato KM, Fleetwood ML, McElhaney MR, Johnson TO, 2008. Characterization of melamine-containing and calcium oxalate crystals in three dogs with suspected pet food-induced nephrotoxicosis. Vet. Pathol 45 (3), 417–426. [DOI] [PubMed] [Google Scholar]
- USFDA, 2007a. Available at: <http://www.fda.gov/animalveterinary/safetyhealth/recallswithdrawals/ucm129575.htm> (accessed 12.03.10).
- USFDA, 2007b. Available at: <http://www.fda.gov/AnimalVeterinary/SafetyHealth/RecallsWithdrawals/ucm129932.htm#AnimalFeed> (accessed 12.03.10).
- USFDA, 2007c. Interim Melamine and its Analogues Safety/Risk Assessment. Federal Register, 72, 30014–30015. Available at: <http://www.fda.gov/ScienceResearch/SpecialTopics/PeerReviewofScientificInformationandAssessments/ucm155012.htm> (accessed 12.03.10). [Google Scholar]
- USFDA, 2008. Interim Safety and Risk Assessment of Melamine and its Analogues in Food for Humans. Available at: <http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Melamine/ucm164522.htm> and <http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Melamine/ucm164520.htm> (accessed 12.03.10).
- USFDA, 2009. Code of Federal Regulations Title 21, Part 58 Good Laboratory Practice for Nonclinical Laboratory Studies. Available at: <http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=58&showFR=1> (accessed 12.03.10).
- Vernon SE, 2006. Preservation of tissue urate crystals with the use of a rapid tissue-processing system. J. Histotechnol 29 (1), 17–19. [Google Scholar]
- Wang X, Guo J, Ruan S, Lu Y, Bi Y, Xiao X, Wang G, 2009. Ureteroscopic lithotripsy for the treatment of urinary calculi in infants and young children. Urology 74(4) (Abstract # MP-12.15). [Google Scholar]
- Wen JG, Yang H, Wang Y, Wang G, 2009a. The clinical analysis of urolithiasis in 165 infants and children with history of feeding melamine contaminated milk powder. J. Urolo 181 (4), 380. [Google Scholar]
- Wen JG, Li ZZ, Zang H, Wang Y, Wang JX, Fan YZ, 2009b. The clinical analysis of double renal calculus in 50 infants and children fed melamine contaminated milk powder. J Urolo. 181(4), 380. [Google Scholar]
- WHO, 2004. Food Additives Series No. 52, Safety Evaluation of Certain Food Additives and Contaminants: Sodium Dichloroisocyanurate. The sixty-first Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Rome, Italy, 10–19 June 2003, World Health Organization, Geneva. Available at: <http://whqlibdoc.who.int/publications/2004/924166052X.pdf> (accessed 12.03.10). [Google Scholar]
- WHO, 2008. Expert Meeting to Review Toxicological Aspects of Melamine and Cyanuric Acid. Available at: <http://www.who.int/foodsafety/fs_management/Exec_Summary_melamine.pdf> and <http://www.who.int/foodsafety/fs_management/conclusions_recommendations.pdf> and <http://www.who.int/foodsafety/fs_management/infosan_events/en/index.html> (accessed 07.03.10).
- Wong SN, Chiu MC, 2008. The scare of melamine tainted milk products. Hong Kong J. Paediatr 13 (4), 230–234. [Google Scholar]
- Xin H, Stone R, 2008. Chinese probe unmasks high-tech adulteration with melamine. Science 322 (5906), 1310–1311. [DOI] [PubMed] [Google Scholar]
- Yarlagadda SG, Perazella MA, 2008. Drug-induced crystal nephropathy: an update. Expert Opinion Drug Saf. 7 (2), 147–158. [DOI] [PubMed] [Google Scholar]


