Abstract
The morphological features of blood and milk neutrophils from peak lactating goats were compared using light microscopy, scanning electron microscopy and flow cytometry in order to investigate the cytological changes of neutrophils after migration into the mammary gland. The kinetics of reactive oxygen intermediates (ROI) generation and gelatinase release of blood and milk neutrophils, with or without stimulation of phorbol 12-myristate, 13-acetate ester (PMA), were used to characterize their responses to inflammatory stimuli. Neutrophils isolated from goat milk were highly segmented and contained multi-lobed nuclei. Ultrastructurally, milk neutrophils were more ruffled on the surface compared to blood neutrophils. Approximately 30% of milk neutrophils were undergoing cell death, either necrosis or apoptosis, in contrast to 8% of blood neutrophils. The ROI production of activated milk neutrophils peaked earlier than blood neutrophils, but the duration and the intensity were much less. Neutrophils from both sources augmented the release of gelatinase in response to PMA (1 ng/mL). However, the amount of gelatinase released from milk neutrophils was lower (P < 0.05) than that of blood neutrophils. In summary, more neutrophils become apoptotic and necrotic in the mammary gland, presumably due to spontaneous aging, the process of diapedesis, and the interaction with milk components. Milk neutrophils have impaired functionalities in comparison with blood neutrophils. The information is relevant when studying mammary gland immunity and related diseases, such as mastitis.
Résumé
Les caractéristiques morphologiques des neutrophiles sanguins et du lait provenant de chèvres au pic de lactation ont été comparées par microscopie photonique, microscopie électronique à balayage et par cytométrie de flux afin d’étudier les changements cytologiques des neutrophiles suite à leur migration dans la glande mammaire. Les cinétiques de production d’intermédiaires réactifs de l’oxygène (ROI) et du relâchement de gélatinase par les neutrophiles du sang et du lait, avec ou sans stimulation par le 12-myristate, 13-acétate ester de phorbol (PMA), ont été utilisées pour caractériser les réponses aux stimuli inflammatoires. Les neutrophiles isolés du lait de chèvre étaient très segmentés et contenaient des noyaux multi-lobulés. Du point de vue ultrastructure, la surface des neutrophiles du lait avaient une apparence plus ondulée que les neutrophiles sanguins. Environ 30 % des neutrophiles du lait avaient entrepris le processus de mort cellulaire, soit de la nécrose ou de l’apoptose, comparativement à 8 % pour les neutrophiles sanguins. La production de ROI par les neutrophiles du lait activés a atteint un pic plus tôt que les neutrophiles sanguins mais la durée et l’intensité étaient beaucoup moindres. Le relâchement de gélatinase en réponse au PMA (1 ng/mL) était augmenté chez les neutrophiles des deux sources. Toutefois, la quantité de gélatinase relâchée par les neutrophiles du lait était plus faible (P < 0,05) que celle des neutrophiles sanguins. En résumé, plus de neutrophiles deviennent apoptotiques et nécrotiques dans la glande mammaire, probablement due à un vieillissement spontanée, au processus de diapédèse, et à l’interaction avec les composantes du lait. La fonctionnalité des neutrophiles du lait était diminuée par rapport aux neutrophiles sanguins. Cette information est pertinente lors de l’étude de l’immunité de la glande mammaire et des maladies reliées, telle la mammite.
(Traduit par Docteur Serge Messier)
Introduction
Milk neutrophils originate from migration of blood neutrophils into the mammary gland. Circulating neutrophils routinely migrate into the mammary gland of dairy animals, acting against invading bacteria that penetrate the physiological barrier of the teat canal. In healthy dairy goats, the somatic cell count (SCC) in milk is usually higher than that in bovine milk (1), neutrophils make up 50% to 70% of the SCC in normal goat milk, in contrast to only 5% to 20% of the SCC in normal bovine milk (2,3). Therefore, the role played by neutrophils in mammary gland immunity might be affected by these species variations.
It has been shown that the neutrophil in bovine milk is more advanced in its stage of development than its blood neutrophil counterparts. Band cells and immature neutrophils, for example, appear in blood but not in bovine milk (4). The viability of milk neutrophils is also much lower than that of blood neutrophils (4). In addition to spontaneous aging, other factors, such as interaction with milk components and process of migration, might be responsible for the lower viability of milk neutrophils as well. During diapedesis, neutrophils have to extravasate the endothelium, move through the extracellular matrix (ECM), and finally migrate across the mammary epithelium into the milk cistern. Neutrophils are capable of performing a variety of bactericidal functions, including phagocytosis, production of reactive oxygen intermediates (ROI), and degranulation. It has been reported that bovine neutrophils, when transmigrating across an epithelial monolayer in a biological basal-to-apical direction, have compromised phagocytosis and oxidative burst (5,6). In addition, the functional impairment of milk neutrophils increases during an intramammary infection. Milk neutrophils isolated from infected quarters of dairy cows displayed decreased respiratory burst activity compared to those from noninfected quarters (7). Therefore, the poor functionality of milk neutrophils could be associated with the pathogenesis of intramammary infections.
Blood and milk neutrophils have been extensively characterized in bovine species. However, similar studies on neutrophils in dairy goats are rare in the literature. We previously demonstrated that goat milk neutrophils isolated during late lactation undergo prominent apoptosis in comparison with those isolated during peak lactation (8). The present study compares blood neutrophils and milk neutrophils from peak lactating goats in terms of morphology, functionality in response to inflammatory stimuli, and cell death.
Materials and methods
Animals
Eight healthy Toggenberg goats were used in this study. They were Californias Mastitis Test negative in the last 2 milkings. These goats were between 1 and 2 mo in lactation producing 2.5 to 3.0 kg milk per day with an SCC < 3 × 105 mL−1 milk. Feeding and management practice were the same as described previously (8). The experimental protocols were approved by the animal care committee of National Chung-Hsing University, Taiwan.
Milk and blood sampling
Teat ends were disinfected with 70% ethanol solution before milking. The milk (100 mL) from each half was aseptically collected into 50 mL sterile plastic centrifuge tubes. The tubes were placed on ice and transferred immediately to the laboratory. The autologous blood samples (40 mL) were taken after milk sampling by venipuncture from the external jugular vein using heparin as an anticoagulant.
Isolation of neutrophils from milk
Milk neutrophils were isolated from individual milk samples according to the procedure of Hoeben et al (9) with the following modifications. Fresh cells were prepared for each experiment. Milk samples were kept at 4°C during the isolation procedure. Briefly, pooled milk from 1 mammary gland of each goat was diluted (1:1, vol/vol) with Dulbecco’s phosphate-buffered saline solution (DPBS, Ca+2 and Mg+2 free; Sigma Chemical Company, St Louis, Missouri, USA) before centrifugation at 400 × g for 15 min. After decanting of the supernatant, fat and cream layers were wiped off from the wall of the tube. The cell pellet was washed twice with 10 mL DPBS and finally suspended in 1 mL Hank’s balanced salts solution (HBSS, with Ca+2 and Mg+2; Sigma Chemical Company).
Isolation of neutrophils from blood
Blood neutrophils were isolated from peripheral blood by the procedure described by Shoshani et al (10) with minor modifications. Heparinized blood was diluted (1:1, vol/vol) with DPBS. Twenty-five milliliters of the diluted blood was layered on 15 mL Histopaque (1.077 g/mL; Sigma Chemical Company) followed by centrifugation at 400 × g for 30 min at room temperature. Neutrophils, together with erythrocytes, were located at the bottom of the tube after centrifugation. After hypotonic lysis of erythrocytes, the neutrophil pellet was collected (4°C, 250 × g, 10 min), washed twice with DPBS, and finally suspended in 1 mL ice-cold HBSS.
Counting and differentiation of cells
The total number of isolated cells in the final cell suspension prepared from milk and blood were determined on a hemacytometer in triplicate. Differential cell counts of the cell suspension were performed on cytospin slides. Five microliters of the final cell suspension was cytocentrifuged (Kubota 5800; Kubota, Tokyo, Japan) at 60 × g for 3 min. Smears were air-dried, fixed, and stained with Wright Giemsa stain (Sigma Chemical Company). Neutrophils were identified on at least 100 cells at 5 scope fields per slide. The purity of neutrophils was always > 90% for blood cells and 60% to 70% for milk cells.
Morphological assessment
The morphological appearance of blood and milk neutrophils was examined on cytospin slides under a light microscope at 1000 × magnification. In order to investigate the ultrastructural changes of neutrophils, cells were prepared for scanning electron microscopy (SEM) as previously described (11) with modifications. Briefly, freshly isolated neutrophils were prefixed with 2.5% glutaraldehyde for 2 h and were dropped onto poly-L-lysine-coated coverslips for 15 min at room temperature. Neutrophils adhered to coverslips and were washed several times with 0.1 M sucrose in 0.1 M cacodylate buffer (pH 7.4) followed by incubation with 1% OsO4 solution in 0.1 M sucrose, 0.1 M cacodylate buffer, pH 7.4 (1:1) for 1 h. After 3 washings with distilled water, the cells were incubated with 1% thiocarbohydrazine solution for 15 min and then washed 3 times with distilled water. The reaction with 1% OsO4 and 1% thiocarbohydrazine solution was repeated twice and once, respectively. Neutrophils were then dehydrated in ascending grades, 5 min for each concentration of alcohol twice, followed by hexamethyldisilazane for 5 min. Dried cells were coated with carbon in a high vacuum evaporator (JSM-1100; JEOL, Tokyo, Japan) and examined under a scanning electron microscope (JSM-T300; JEOL) equipped with a solid-state backscattered electron detector. Microphotographs were taken with film (Fujifilm 120; Fujifilm, Edison, New Jersey, USA).
Detection of cell death
Cell death of freshly isolated neutrophils was determined by dual labeling of neutrophils with annexin-V and propidium iodide (PI) and analyzed using a flow cytometer (FACS Cablibur flow cytometer; Becton Dickinson, Mountain View, California, USA), as previously described (12). Expression of phosphatidylserine (PS) on the cell surface is considered to be one of the early features of apoptosis. Annexin-V has a high binding affinity to surface PS that is expressed on apoptotic cells (13). On the other hand, PI selectively penetrates into cells that have lost the membrane integrity, such as necrotic cells and cells in late apoptosis. Freshly prepared neutrophils (1 × 106 cells) were suspended in 200 μL of binding buffer containing annexin V-FITC and PI (ApoAlert Annexin V-FITC; Clontech Laboratories, Palo Alto, California, USA), and incubated at room temperature for 5 to 15 min in the dark. Thereafter, neutrophils were gated based on cell size and granularity, and 2-dimensional fluorescence dot-plot profiles were generated to represent a total of 10 000 gated events. Computer software (Cell Quest; Becton Dickinson, Oakville, Ontario) was used to analyze data. Viable cells that do not bind to either annexin-V or PI were those confined to the lower left-hand quadrant. Cells in the early stage of apoptosis maintain their membrane integrity and, therefore, bind to annexin-V but not to PI (lower right-hand quadrant). Cells that bind to PI only (upper left-hand quadrant) are necrotic cells. Cells double-stained with both markers (the upper right-hand quadrant) can be necrotic or late apoptotic cells.
Chemiluminescence assay
The respiratory burst, generation of oxidative radicals, was measured by luminol-dependent chemiluminescence (14). Three representative goats were used in this assay. Briefly, a metal cationic catalyst Cu++ was included to increase light emission and speed of oxidation. A stock solution of 0.1 M luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) in dimethyl sulphoxide was prepared and stored at −20°C until being used. Immediately before use, the solution was thawed and diluted with HBSS to be a 1 mM working solution containing 0.05 M Na2CO3, 0.3 M NaHCO3, 5 mM NH4CO3, and 1.5 mM CuSO4. Freshly prepared cell suspensions (1 × 106 cells) were incubated with 1 mL of luminol working solution in the absence (basal) or presence (stimulated) of 100 μL of 1 μM PMA (diluted with 200 μg/mL dimethyl sulphoxide with HBSS) at a final volume of 2 mL. Reactions were monitored immediately with a luminometer (LB9509; EG&G Berthold, Bundoora, Australia). Relative light units (RLUs), assembled by the measurement of RLU values in time intervals of 5 milliseconds (ms), were given as results of integration over the measurement period.
Release of gelatinase granules
Degranulation of gelatinase granules was demonstrated by gelatinase zymography (15). Three representative goats were used in this assay. Briefly, isolated neutrophils (1 × 106 cells) were incubated with HBSS or PMA (final concentration 1 ng/mL) in a total volume of 0.5 mL at 37°C for 1 h. Thereafter, cells were spun down at 2000 × g for 10 min and 15 μL of the supernatant were loaded for non-reducing sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Four human gelatinase isozymes, with molecular weight 75, 92, 130, and 225 kDa, respectively (16), were loaded as standards in each gel. The photographic negative of each zymogram was scanned and the intensity was analyzed using computer software (BIO-2D; Vilber Lourmat, Marne La Vallee, France). Intensities of the human 92 kDa gelatinase were used to calibrate the relative arbitrary unit for each band.
Statistical analyses
Statistical analysis was performed using the general linear model procedure (SAS, Cary, North Carolina, USA) (17). Comparisons were conducted between blood and milk neutrophil characteristics. Any P-values of less than 0.05 were considered significantly different.
Results
Morphological assessment
Neutrophils isolated from blood or milk were similar in size, about 10 to 15 μm in diameter, with dark purple-stained nuclear structure (Figure 1). The nuclei of blood neutrophils were 2 to 3 lobed (Figure 1A). On the other hand, milk neutrophils (Figure 1B) were segmented with multi-lobed nuclei. Band cells were occasionally found among blood neutrophils (Figure 1C), but never in milk neutrophils. The SEM demonstrated that blood neutrophils (Figure 2A) were round-shaped. Their surface was smoother with short microvilli and microridges in comparison with milk neutrophils, which had a ruffled surface (Figure 2B).
Figure 1.
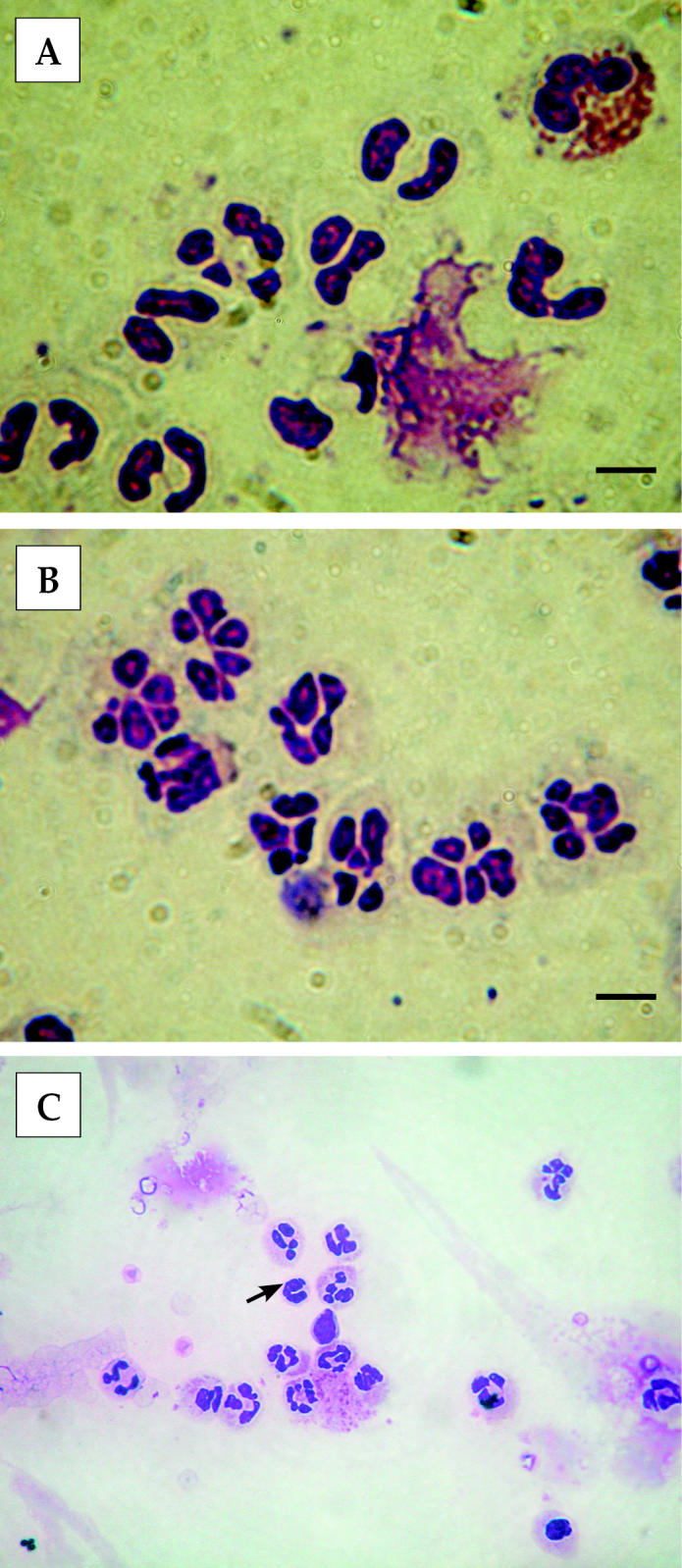
Light micrographs of neutrophils isolated from blood (A) and milk (B) of peak lactation goats. Milk neutrophils possessed multi-lobed nuclei in contrast to 2- to 3-lobed nuclei of blood neutrophils. (C) A band cell in blood neutrophils, as indicated by the arrow. Bar = 10 μm.
Figure 2.
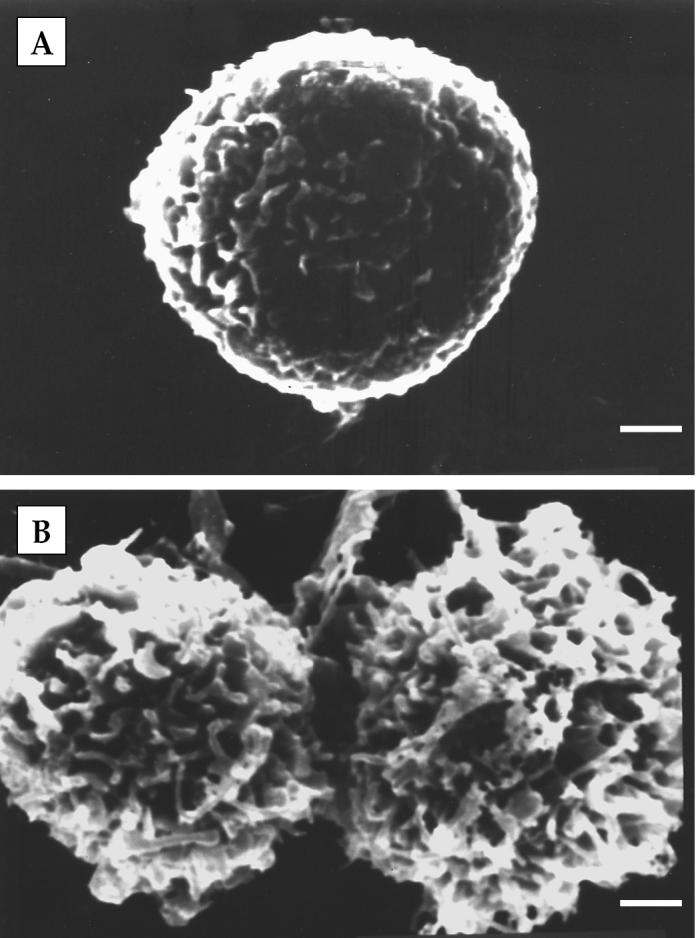
Scanning electron micrographs of neutrophils isolated from blood (A) and milk (B) of peak lactation goats. Milk neutrophils displayed a more ruffled surface compared to blood neutrophils. Bar = 1 μm.
Cell death
Freshly isolated neutrophils were used to assess the incidence of apoptosis and necrosis. The percentage of viable cells was 91.8% for blood neutrophils and 70.34% for milk neutrophils, respectively (Figure 3). The percentages of necrotic or apoptotic neutrophils in terminal stages, the sum of the 2 upper histograms, was apparently much lower in blood neutrophils (7.60%) than that of milk neutrophils (28.47%). The amounts of early apoptotic neutrophils from both sources were very limited.
Figure 3.
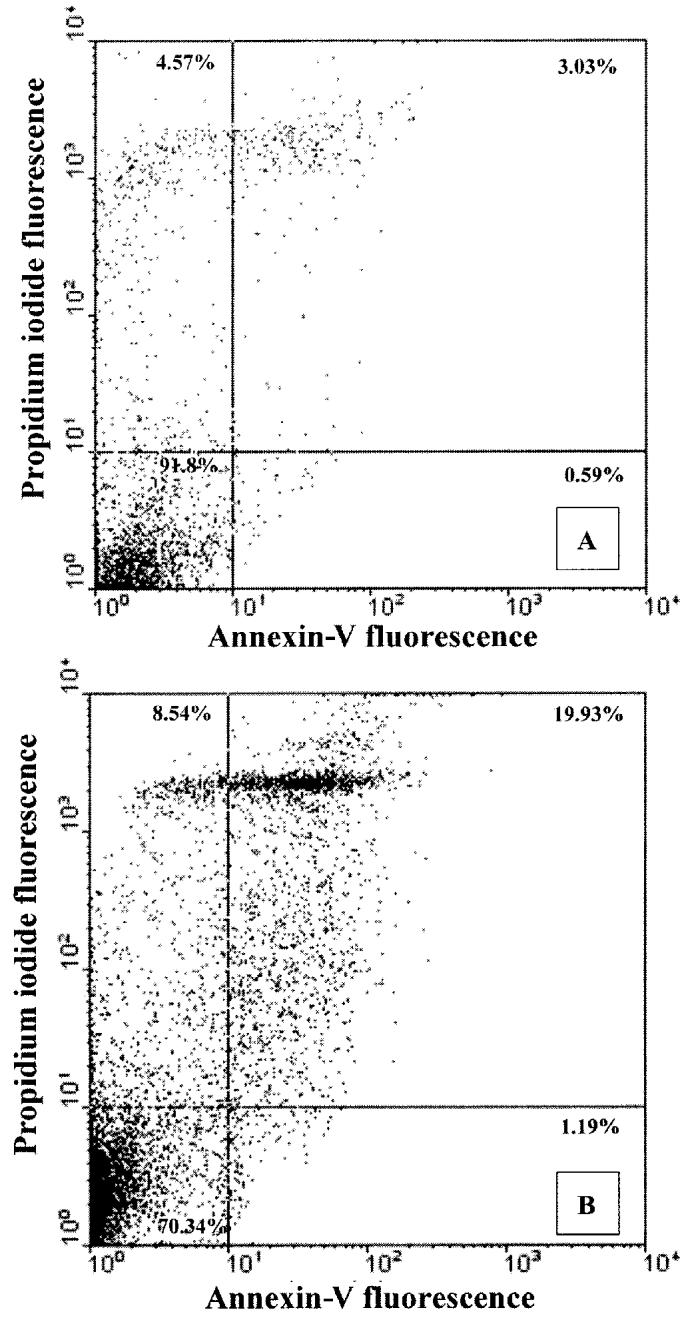
Double staining of neutrophils with annexin-V and propidium iodide (PI). Neutrophils isolated from blood (A) and milk (B) of normal lactating goats were stained with FITC-labeled annexin-V for apoptotic changes and PI for necrotic changes, and the fluorescence intensity was determined by flow cytometry. Both a fluorescence dot plot and the percentage of positive cells are shown. The figure is a representative of 3 separate experiments using neutrophils isolated from 3 different animals.
Production of ROI
Production of ROI, in the presence or absence of PMA stimulation, by blood or milk neutrophils was monitored by luminal-enhanced chemiluminescence (Figure 4). At resting, blood and milk cells showed a very low level of ROI production, although the intensity increased slightly at the end of incubation for milk neutrophils. After activation by PMA, ROI production increased in both blood and milk cells, and peaked at approximately 23 and 13 min, respectively. However, the magnitude of peak respiratory burst in milk neutrophils was only one third of that in blood neutrophils and was less persistent.
Figure 4.
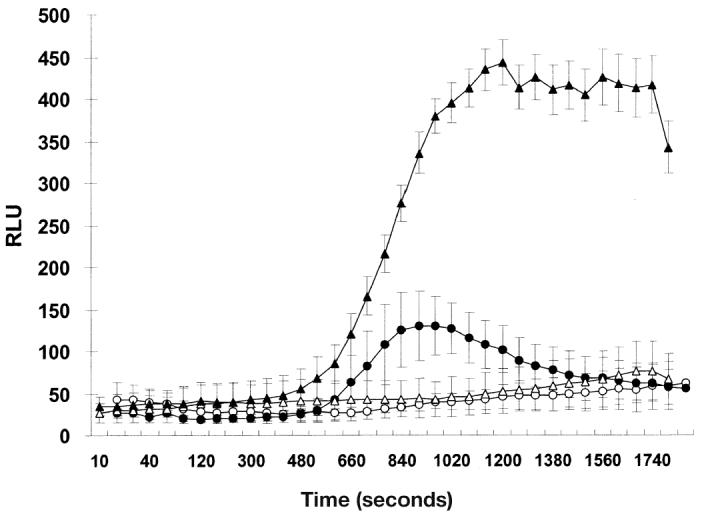
The respiratory burst of neutrophils measured by enhanced luminolchemiluminescence. Neutrophils isolated from blood (triangle) and milk (circle) of normal lactating goats were incubated with 1 mM luminol and 1.5 mM CuSO4 in the absence (resting, open) and presence (stimulated, closed) of 1 μM PMA for 30 min. Data are the means from 3 goats. Vertical bars represent ± sχ̄
Exocytosis of gelatinase granules
The release of gelatinase by blood and milk neutrophils was examined using gelatin zymography (Figure 5A). A 92 kDa gelatinase was released constitutively (PMA 0 ng/mL, incubation time 0). Densitometer scanning results (Figure 5B) indicated that the secretion of gelatinase in neutrophils from both sources increased after 1 h incubation in the absence of PMA (PMA 0 ng/mL, time 1 h), but the differences were not significant (P > 0.05). When incubated for 1 h with PMA (PMA 1 ng/mL, time 1 h), the secretion of gelatinase from blood and milk neutrophils was significantly (P < 0.05) augmented. In addition, the amount of released gelatinase was lower (P < 0.05) in milk neutrophils than in blood neutrophils under all conditions.
Figure 5.
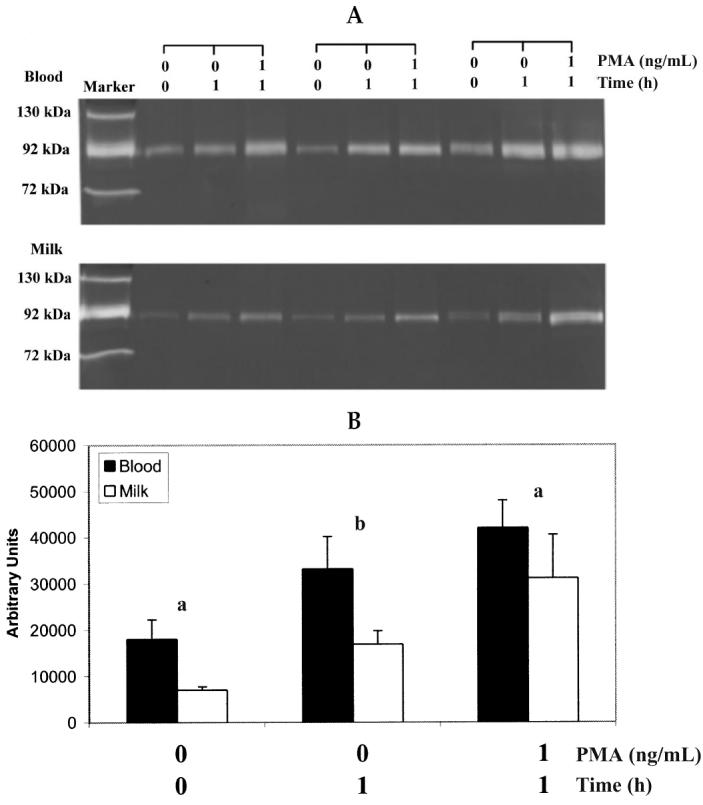
Gelatinase release from blood and milk neutrophils of normal lactating goats. Neutrophil samples were incubated with and without 1 ng/mL PMA at 37°C for 1 h. The supernatants were collected by centrifugation and subjected to gelatin-containing agarose gel electrophoresis. (A) Gelatinase activity detected on the gelatin zymogram for 3 representative goats. (B) Relative gelatinase activity quantified by densitometrically scanning the photographic negative of zymogram for blood (▪) and milk (□) neutrophils. Values represent means ± sχ̄. Comparisons were made between blood and milk neutrophils under the same conditions. a(P < 0.05) b(P < 0.01).
Discussion
In this study, the morphological features, types of cell death, and functionalities of milk neutrophils were compared to those of blood neutrophils in peak-lactating goats. Comparing blood neutrophils and milk neutrophils in this in vivo model is able to fully reflect the interaction among neutrophils, soluble components, endothelium, ECM, and epithelium during a routine diapedesis, conditions that could not be replicated in an in vitro system (6,18–20). The protocol we used to isolate milk cells was not able to increase the purity of milk neutrophils. Thus, only 60% to 70%, according to the differential cell counting, of the isolated milk cells were neutrophils. This is close to the percentage of neutrophils reported previously for SCC from goats without intramammary infections (1). This number is much higher than that reported in bovine milk, which was 3% to 26% (4). Since neutrophils act as the first line of immunological defense against infections, this could explain, at least in part, why goats are more resistant to mastitis.
Our results indicate that milk neutrophils showed cytological and ultrastructural changes in comparison with blood neutrophils. Band cells and less mature neutrophils with 2 or 3 lobed nuclei were only found in blood preparations. In contrast, most milk neutrophils possessed highly lobed nuclei. The cell surface of milk neutrophils was more ruffled and less smooth under the SEM compared to blood neutrophils. This could be a consequence of extensively protruding surface pseudopods to engulf milk components when neutrophils arrive at the mammary gland. Acceleration of senescence of milk neutrophils was further demonstrated in the present study. Dualcolor flow cytometric procedure was used to assess the incidence and the type of cell death in situ. The viability of blood and milk neutrophils was 91.8% and 70.3%, respectively, which showed a similar tendency to that of dairy cows (21). The percentage of necrotic and late apoptotic neutrophils in milk was increased compared to that of the autologous blood (28.47% versus 7.60%). Since necrotic cells usually lose their membrane integrity, their intracellular PS can be stained by annexin-V as well. On the other hand, late apoptotic cells, in the absence of phagocytosis, display necrotic-like disintegration, and can be stained with PI. Therefore, we were not able to differentiate the necrotic cells from late apoptotic cells, since both expressed high binding for both annexin-V and PI. However, if most of the cells in this fraction are late apoptotic cells, then our results are comparable to a recent study, in which the percentages of apoptotic bovine neutrophils in milk and blood during the midlactation were about 35% and 5%, respectively (21).
While the exact mechanisms are not known, a higher apoptotic rate of milk neutrophils could be the outcome of several events. First possibility is spontaneous aging. After a 24 h incubation, more than 50% of neutrophils isolated from peripheral blood undergo apoptosis (22). Neutrophils recovered from milk might be several hours older than those isolated from blood on average. Considering the short lifespan of neutrophils, several hours could make a significant difference in the percentage of apoptotic cells. Alternatively, diapedesis causes apoptosis. It has been demonstrated that chemoattractant (C5a)-induced bovine neutrophil migration was able to either initiate apoptosis directly or accelerate the process of apoptosis induced by spontaneous aging (23). The third possibility is the interaction with milk components. Once in the lumen of alveoli, milk neutrophils ingest fat and casein, which causes a loss in phagocytic and bactericidal functions and leads to death of the neutrophils (24). Moreover, large amounts of immunoglobulins have been shown to bind to milk components, including fat and casein (25). Newly migrated neutrophils, being phagocytes, engulf these “opsonized” particles and become senescent (26).
In addition to morphological differences and a higher rate of cell death, neutrophils derived from goat milk had compromised functions, in terms of production of oxidative radicals (respiratory burst) and extracellular release of gelatinase. In comparison with milk neutrophils, blood neutrophils reacted faster and had a much stronger respiratory burst in response to PMA. Interaction with milk or its components could result in the unresponsiveness of neutrophils. Milk or purified casein has been proven to inhibit the respiratory burst of bovine neutrophils (21). In addition, aging, apoptotic, and migrated neutrophils have been shown to have a reduced respiratory burst (6,23,27).
Bovine blood neutrophils have been demonstrated to constitutively release a 92 kDa gelatinase (MMP-9), and the release is augmented by the presence of PMA (15). According to our results, this is also true for goat blood and milk neutrophils. However, the amount of released gelatinase was always significantly lower in milk neutrophils in various conditions applied in our study, including no incubation and PMA and 1 h incubation with or without PMA. Without stimulation, the gelatinase activity of milk neutrophils was only approximately 39% (7040 versus 18030 arbitrary units) of that of blood neutrophils. Therefore, the lower responsiveness was not simply due to the purity of isolated neutrophils (> 90% in blood and 60% to 70% in milk). However, the higher percentage of cells undergoing apoptosis, necrosis, or both could also be responsible, at least in part, for the impaired function of milk neutrophils. The process of migration might also be involved in the decreased MMP-9 activity of milk neutrophils. MMP-9 is rapidly secreted in activated neutrophils to breakdown extracellular components, which assists neutrophils to migrate across the basement membrane (28). Thus, a significant amount of MMP-9 is released during the diapedesis, which leads to reduced secretion of milk neutrophils.
Taken together, this investigation demonstrated that goat milk neutrophils from normal mammary glands are hypo-functional and have a higher rate of cell death in comparison with autologous blood neutrophils, as seen in bovines and humans.
Acknowledgments
This project is supported in part by a grant (No. 90-2313-B-005-111) from National Science Council, Taiwan.
References
- 1.Paape MJ, Capuco AV. Cellular defense mechanisms in the udder and lactation of goats. J Anim Sci. 1997;75:556–565. doi: 10.2527/1997.752556x. [DOI] [PubMed] [Google Scholar]
- 2.Dulin AM, Paape MJ, Schultze WD, Weinland BT. Effect of parity, stage of lactation, and intramammary infection on concentration of somatic cells and cytoplasmic particles in goat milk. J Dairy Sci. 1983;66:2426–2433. doi: 10.3168/jds.S0022-0302(83)82101-8. [DOI] [PubMed] [Google Scholar]
- 3.Poutrel B, Lerondelle C. Cell content of goat milk: California mastitis test, Coulter counter, Fossomatic for predicting half infection. J Dairy Sci. 1983;66:2575–2579. doi: 10.3168/jds.S0022-0302(83)82129-8. [DOI] [PubMed] [Google Scholar]
- 4.Paape MJ, Schultze WD, Desjardins C, Miller RH. Plasma corticosteroids, circulating leukocytes and milk somatic cell responses to Escherichia coli endotoxin-induced mastitis. Proc Soc Exp Biol Med. 1974;145:553–559. doi: 10.3181/00379727-145-37850. [DOI] [PubMed] [Google Scholar]
- 5.Dulin AM, Paape MJ, Nickerson SC. Comparison of phagocytosis and chemiluminescence by blood and mammary gland neutrophils from multiparous and nulliparous cows. Am J Vet Res. 1988;49:172–177. [PubMed] [Google Scholar]
- 6.Smits E, Burvenich C, Guidry AJ, Heyneman R, Massart-Leen A. Diapedesis across mammary epithelium reduces phagocytic and oxidative burst of bovine neutrophils. Vet Immunol Immunolpathol. 1999;68:169–176. doi: 10.1016/s0165-2427(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 7.Piccinini R, Bronzo V, Moroni P, Luzzago C, Zecconi A. Study on the relationship between milk immune factors and Staphylococcus aureus intramammary infections in dairy cows. J Dairy Sci. 1999;66:501–510. doi: 10.1017/s0022029999003751. [DOI] [PubMed] [Google Scholar]
- 8.Su WJ, Chang CJ, Peh HC, Lee SL, Huang MC, Zhao X. Apoptosis and oxidative stress of infiltrated neutrophils obtained from mammary glands of goats during various stages of lactation. Am J Vet Res. 2002;63:241–246. doi: 10.2460/ajvr.2002.63.241. [DOI] [PubMed] [Google Scholar]
- 9.Hoeben D, Burvenich C, Heyneman R. Influence of antimicrobial agents on bactericidal activity of bovine milk polymorphonuclear leukocytes. Vet Immunol Immunopathol. 1997;56:271–282. doi: 10.1016/s0165-2427(96)05759-5. [DOI] [PubMed] [Google Scholar]
- 10.Shoshani E, Leitner G, Hanochi B, Saran A, Shpogel NY, Berman A. Mammary infection with Staphylococcus aureus in cows: progress from inoculation to chronic infection and its detection. J Dairy Res. 2000;67:155–169. doi: 10.1017/s002202990000412x. [DOI] [PubMed] [Google Scholar]
- 11.Labow RS, Erfle DJ, Santerre JP. Neutrophil-mediated degradation of segmented polyurethanes. Biomaterials. 1995;16:51–59. doi: 10.1016/0142-9612(95)91096-h. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Akahoshi T, Jiang S, et al. Induction of neutrophil death resembling neither apoptosis nor necrosis by ONO-AE-248, a selective agonist for PGE2 receptor subtype 3. J Leukocyte Biol. 2000;68:187–193. [PubMed] [Google Scholar]
- 13.Fadok VA, Voelker DR, Campbell PA, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 14.Hoeben D, Monfardini E, Opsomer G, et al. Chemiluminescence of bovine polymorphonuclear leucocytes during the periparturient period and relation with metabolic markers and bovine pregnancy-associated glycoprotein. J Dairy Res. 2000;67:249–259. doi: 10.1017/s0022029900004052. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Zhao X, Ma S. Secretion of 92 kDa gelatinase (MMP-9) by bovine neutrophils. Vet Immunol Immunopathol. 1999;67:247–258. doi: 10.1016/s0165-2427(98)00228-1. [DOI] [PubMed] [Google Scholar]
- 16.Makowski GS, Ramsby ML. Calibrating gelatin zymograms with human gelatinase standards. Anal Biochem. 1996;236:353–356. doi: 10.1006/abio.1996.0179. [DOI] [PubMed] [Google Scholar]
- 17.Statistical analysis system, SAS/STAT user’s guide, Version 6, SAS Institute, Cary, North Carolina, USA, 1990.
- 18.Lin Y, Xia L, Turner JD, Zhao X. Morphologic observation of neutrophil diapedesis across bovine mammary gland epithelium in vitro. Am J Vet Res. 1995;56:203–207. [PubMed] [Google Scholar]
- 19.Lin Y, Cai J, Turner JD, Zhao X. Quantification of bovine neutrophil migration across mammary epithelium in vitro. Can J Vet Res. 1996;60:145–149. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J-W, Zhao X. Recombinant human interleukin-8, but not human interleukin-1β, induces bovine neutrophil migration in an in vitro co-culture system. Cell Biol International. 2000;24:889–895. doi: 10.1006/cbir.2000.0562. [DOI] [PubMed] [Google Scholar]
- 21.Van Oostveldt K, Vangroenweghe F, Dosogne H, Burvenuch C. Apoptosis and necrosis of blood and milk polymorphonuclear leukocytes in early and midlactation healthy cows. Vet Res. 2001;32:617–622. doi: 10.1051/vetres:2001143. [DOI] [PubMed] [Google Scholar]
- 22.Savill JS, Wyllie AH, Henson JE. Macrophage phagocytosis of aging neutrophils in inflammation. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Oostveldt K, Paape MJ, Burvenich C. Apoptosis of bovine neutrophils following diapedesis through a monolayer of endothelial and mammary epithelial cells. J Dairy Sci. 2002;85:139–147. doi: 10.3168/jds.S0022-0302(02)74062-9. [DOI] [PubMed] [Google Scholar]
- 24.Paape MJ, Wergin WP. The leukocyte as a defense mechanism. J Am Vet Med Assoc. 1977;170:1214–1223. [PubMed] [Google Scholar]
- 25.Cooray R. Casein effects on the meyloperoxidase-mediated oxygen-dependent bactericidal activity of bovine neutrophils. Vet Immunol Immunolpathol. 1996;51:55–65. doi: 10.1016/0165-2427(95)05496-0. [DOI] [PubMed] [Google Scholar]
- 26.Frenyo VL, Butler JE, Guidry AJ. The association of extrinsic bovine IgG1, IgG2, SIgA and IgM with the major fractions and cells of milk. Vet Immunol Immunopathol. 1986;13:239–254. doi: 10.1016/0165-2427(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 27.Daniels RH, Elmore MA, Mill ME, Shimizu Y, Lackie JM, Finnen MJ. Priming of the oxidative burst in human neutrophils by physiological agonists or cytochalasin B results from the recruitment of previously non-responsive cells. Immunol. 1994;82:465–472. [PMC free article] [PubMed] [Google Scholar]
- 28.Delclaux C, Delacourt C, D’Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996;14:288–295. doi: 10.1165/ajrcmb.14.3.8845180. [DOI] [PubMed] [Google Scholar]


