Abstract
The aim of this study was to evaluate the effect of 3 Brucella ovis subcellular protein fractions: Outer membrane (OMP), inner membrane (IMP), and cytoplasm (CP), on cellular immune response by in vitro production of interleukin (IL)-2, IL-4, and interferon (IFN)-γ. Each fraction was inoculated 3 times into Balb/c mice, primary cultures of mice spleen cells were done, and these were then stimulated with the fractions. Culture supernatants were collected at 24, 48, 72, 96, and 120 h postinoculation. Cytokine concentration was measured by Duoset-enzyme-linked immunosorbent assay (ELISA). The OMP fraction induced highest cellular immune response of 1000 pg/mL of IL-2 at 24 h, which decreased to < 100 pg/mL by 96 h. The IL-2 response for the IMP fraction was low at 24 h, but exceeded that of the OMP fraction at 72, 96, and 120 h. The CP showed a poor IL response. Regarding the IFN-γ production, OMP and IMP induced a high response at 120 h. These results open the possibility for the use of B. ovis outer and inner membrane proteins as a subcellular vaccine.
Résumé
L’objectif de l’étude était d’évaluer l’effet de 3 fractions sub-cellulaires de Brucella ovis, à savoir les protéines de la membrane externe (OMP), les protéines de la membrane interne (IMP), et le cytoplasme (CP), sur la réponse immunitaire cellulaire en déterminant la production in vitro d’interleukine (IL)-2, IL-4 et d’interféron (IFN)- γ. Chaque fraction a été inoculée 3 fois dans des souris Balb/c, des cultures primaires de cellules de rates de souris ont été faites, et celles-ci stimulées avec les fractions. Les surnageants de culture ont été récoltés 24, 48, 72, 96 et 120 h post-inoculation. Les concentrations de cytokines ont été mesurées à l’aide de la méthode ELISA-Duoset. La fraction OMP a induit la plus forte réponse immunitaire cellulaire, 1000 pg/mL d’IL-2 après 24 h avec réduction à > 100 pg/mL après 96 h. La production d’IL-2 induite par la fraction IMP était faible à 24 h, mais dépassait celle de la fraction OMP à 72, 96, et 120 h. La fraction CP n’a induit qu’une faible production d’IL. En ce qui a trait à la production d’IFN-γ, les fractions OMP et IMP ont induit une forte réponse à 120 h. Ces résultats laissent entrevoir la possibilité d’utiliser les OMP et IMP de B. ovis comme vaccin sub-cellulaire.
(Traduit par Docteur Serge Messier)
Introduction
Brucella ovis is the etiological agent of contagious ram epididimitis, a disease of worldwide distribution that is sexually transmitted in the ovine (1). The disease is characterized by a decrease in fertility (2).
The T lymphocytes are differentiated in 2 subclasses, Th1 and Th2. The subclass Th1 mainly secretes interleukin (IL)-2, interferon (IFN)- γ, and tumor necrosis factor (TNF)-β, which participate in cell-mediated immunity (CMI); whereas subclass Th2 secretes IL-4, IL-5, IL-6, and IL-10, promoting a humoral immune response. In addition, there are other cytokines shared by both Th subclasses. It has been reported that B. abortus living cells induce the production of IFN-γ; however, dead cells or cellular fractions induce IL-4 (3). Furthermore, immune response to B. abortus depends on the macrophage activation mediated by antigen-specific T-cells (4).
Cellular immune response is crucial for the host defense against intracellular bacteria. Listeria monocytogenes, Mycobacterium tuberculosis, and B. abortus have been used to study the bacteria-induced cellular immune response of the host (5). There are only a few studies related to the ovine or murine cellular immune response induced by B. ovis (6).
The aim of this study was to evaluate the induction of cytokines IL-2, IL-4, and IFN-γ production, as well as the delayed type hypersensitivity, both stimulated by B. ovis subcellular fractions in the murine model.
Materials and methods
Bacterial strain
Brucella ovis REO198 was maintained on Brucella agar (BA; Beckton-Dickinson, Franklin Lakes, New Jersey, USA), at 37°C.
Subcellular fractions
Subcellular fractions were obtained according to the method described by Morton (7), with some modifications. Bacteria grown on BA were suspended in 10 mM HEPES (Sigma Chemical Company, St. Louis, Missouri, USA), pH 7.2; centrifuged at 1000 × g for 30 min; and the pellet washed twice and resuspended in 10 mM HEPES containing 0.1 M phenyl-methyl-sulphonyl-fluoride (PMSF), 0.1 M p-hydroxy-mercury-benzoate (PHMB), and 3% ethylenediamine-tetraacetic acid (EDTA). Cells were sonicated by 6 cycles of 1 min in a Vibra-cell (Sonics, Newtown, Connecticut, USA), and cellular detritus was removed by centrifugation at 10 000 × g for 20 min. The supernatant was centrifuged at 150 000 × g for 1 h; the new supernatant contained the cytoplasmic proteins (CP), and the pellet contained the total membranes. These membranes were resuspended in 10 mM HEPES with 1% (w/v) sodium N-lauryl-sarcosine and agitated for 30 min at 37°C (8). They were then centrifuged at 150 000 × g for 1 h. The pellet contained the outer membrane proteins (OMP), whereas the inner membrane proteins (IMP) were in the supernatant. The purity of the OMP was verified by the presence of lipopolysaccharides (LPS) by limulus amebocyte lysate (LAL) test (QCL-1000 Chromogenic Limulus Amebocyte Lysate; Bio-Whittaker, East Rutherford, New Jersey, USA) (9). The OMP were recovered according to the method of Wessel and Flügge (10). The IMP and CP were separately precipitated overnight with cold ethanol 1:4 (v/v) at 4°C. They were then centrifuged at 1000 × g for 20 min and the sediment dried by air flow and kept frozen until used. Protein concentration was determined by using the method by Bradford (11).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
Proteins of each subcellular fraction were separated by 15% SDS-PAGE (12). They were transferred (13) to 0.45 μm nitrocellulose membranes (Gibco Laboratories, Carlsbad, California, USA). To identify immunogenic proteins, an antiserum against B. ovis REO198 was used, as mentioned below. As a secondary antibody, a peroxidase-linked goat serum against rabbit immunoglobulin (Ig)G, was used (diluted 1:1000) (Sigma Chemical Company).
Rabbit antiserum against B. ovis
Two New Zealand rabbits were injected, IM, with whole B. ovis cells (1.5 × 108 colony forming units [CFU]) in saline solution, initially with Freund’s complete adjuvant, and after 20 d with Freund’s incomplete adjuvant. After 7 d, they were inoculated 3 times with cells without adjuvant, using the same route, at 1 wk intervals. The titers were determined by double immuno-diffusion using a hot saline (HS) extraction of B. ovis as the antigen (14).
Immunization of mice
Three groups of 6 male 4- to 5-week-old Balb/c mice were immunized with each subcellular fraction. They were initially injected, IM, with complete Freund’s adjuvant and again, 15 d after later by intra-peritoneal inoculation without adjuvant. The protein concentration used was of 11, 54, and 10 μ g/mL for OMP, IMP, and CP, respectively.
Lymphocyte culture and cytokine induction.
Mice spleens were combined from each group, washed 3 times with Hanks’ solution, and placed in a petri dish containing 5 mL of RPMI 1640 medium (Gibco Laboratories), containing 100 U/mL penicillin and 100 μ g/mL streptomycin, over gauze to retain tissue debris. They were then macerated and the cell suspension was transferred to a conical tube with 5 mL of the same medium, and centrifuged at 400 × g for 3 min. The pellet was resuspended in 0.17 M NH4Cl for 5 min at 4°C to lyse the erythrocytes; it was washed 3 times with RPMI; and then resuspended in RPMI enriched with 20% bovine fetal serum, 200 mM L-glutamine, and 0.1 mM of non-essential amino acids. We used a different culture plate per subcellular fraction, approximately 6.5 × 106 lymphocytes from each group were inoculated with OMP, IMP, and CP, respectively, distributed in 3 wells of the culture plate (Nunclon, Rochester, New York, USA), each well was inoculated with 10 μL of the corresponding subcellular fraction and the plates were incubated at 37°C with 5% CO2. Concanavalin A was used as a positive control for non-specific induction of cytokines. Media containing the cytokines were collected at 24, 48, 72, 96, and 120 h after inoculation and frozen until use.
Cytokine quantization
Commercial kits for mouse IFN-γ, IL-2, and IL-4 (Duoset ELISA; R&D Systems, Minneapolis, Minnesota, USA), were used for cytokine quantization as per the manufacturer instructions. The concentration used for capturing and detector antibodies was 2000 and 300 ng/mL for IL-2; 4000 and 400 ng/mL for IFN-γ; and 720 and 36 μg/mL for IL-4. The standard curve was prepared by serial dilutions of a standard stock solution: 1000 pg/ mL for IL-2 and IL-4, and 2000 pg/mL for IFN-γ.
Delayed type hypersensitivity (DTH)
Fifty microliters of OMP, IMP, CP, and phosphate buffered saline solution (PBSS) were injected, SC, into the plantar pads of the 3 mice and the induration measured at 24 and 48 h. The induration percentage was calculated as follows (15): Using a vernier calliper the indurations were measured, the amount of induration produced by PBSS was subtracted, and then the percentage of increased induration was calculated between the inoculation day and 24 to 48 h later.
Results
Antigenic proteins of B. ovis
To determine the protein profile of B. ovis subcellular fractions, SDS-PAGE and immunoblotting were performed. Figure 1 shows that the protein pattern is different for each fraction, and that the OMP had a low contamination of LPS 0.13 UE/mL as verified by the LAL test. There was high antigenic reactivity by the 3 subcellular fractions when tested against the whole cell antiserum against B. ovis (Figure 2), mainly at 42 kDa, 20 kDa, as well as a doublet at 38 kDa (OMP). The cytosol (CP) showed a polypeptide of 28 kDa that was absent in the IMP fraction, and the latter fraction showed an enriched polypeptide of 47 kDa.
Figure 1.
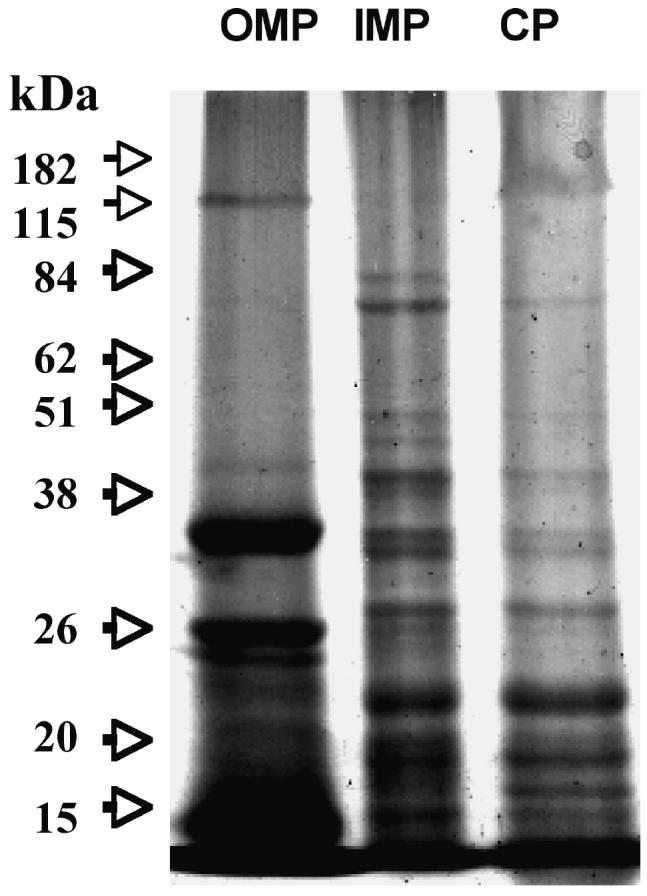
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15%) of Brucella ovis subcellular fraction proteins: Outer membrane proteins (OMP), inner membrane proteins (IMP), and cytosol (CP). Fractions were separated using sarcosyl and 15 mg of protein were used per well. Arrows indicate molecular mass markers.
Figure 2.
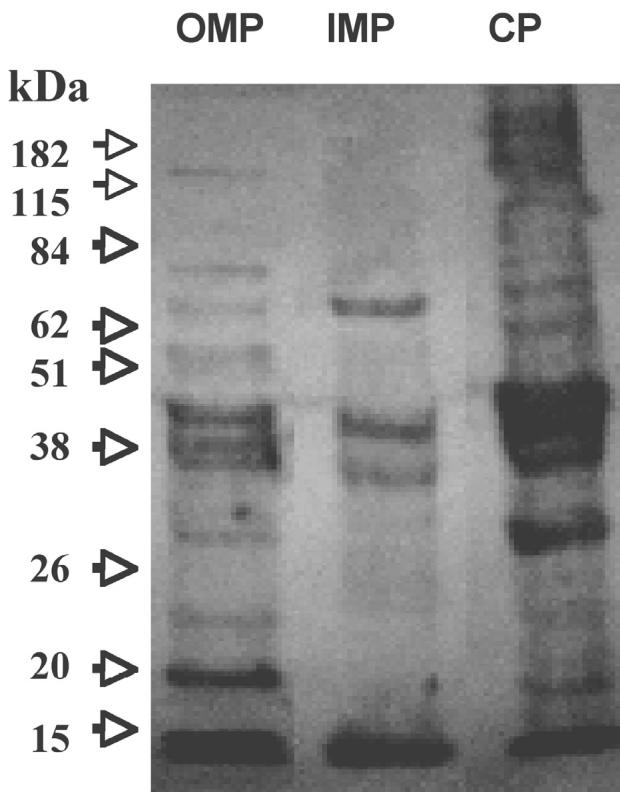
Inmunoblotting of Brucella ovis subcellular fractions: outer membrane proteins (OMP), inner membrane proteins (IMP) and cytosol (CP). Proteins from Figure 1 were transferred to a nitrocellulose membrane, antigens were detected by using rabbit serum against B. ovis 1:20 and reaction revealed using rabbit serum against immunoglobulin (Ig)G 1:1000. Arrows indicate molecular mass markers.
Cytokine response by the B. ovis subcellular fractions
Concentrations from the amount of cytokines produced by spleen derived cells after the cellular fractions, were added to mouse lymphocyte cultures, as shown in Figure 3. The OMP fraction (Figure 3A) induced a rapid and strong response from IL-2 at 24 h, with a marked reduction by 48 h. The IFN-γ showed a gradual increase from 72 h and peaked at 120 h. A low IL-4 response started at 72 h and remained low throughout the sampling period.
Figure 3.
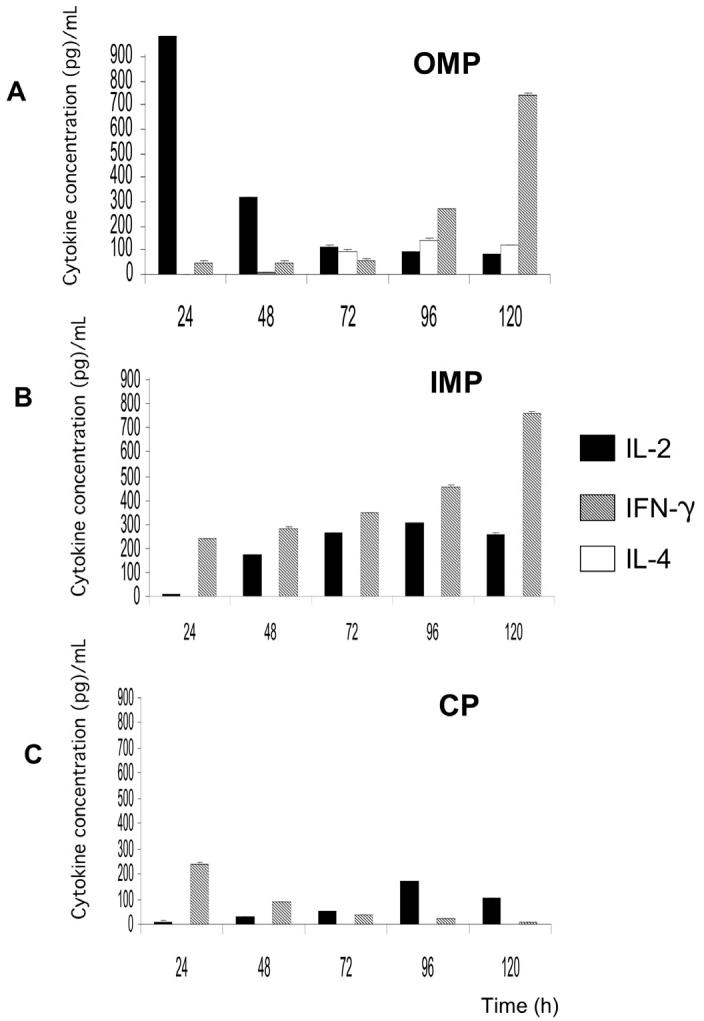
Induction of interleukin (IL)-2, IL-4, and interferon (IFN)-γ in Balb/c mice spleen cells stimulated with Brucella ovis subcellular fractions: A) outer membrane proteins (OMP); B) inner membrane proteins (IMP); and C) cytosol (CP). Culture supernatants were take out at different times and tested by enzyme-linked immunosorbent assay (ELISA)-Duoset. Standard deviations were below 10, and are not visible in the chart due to the scale.
The IMP fraction (Figure 3B) showed an increasing IFN-γ starting at 24 h an IL-2 response started at 48 h and remained at about the same level until 120 h. The IMP did not induce a detectable IL-4 response.
The CP fraction (Figure 3C) induced an initial IFN-γ response at 24 h, which dropped by 48 h and remained at low levels. The production of IL-2 was low and peaked at 96 h. The CP fraction did not induce IL-4 response and the response shown by this fraction to IL-2 and IFN-γ was poor compared with those of the other fractions. The Concanavalin A was used as a control for the cytokines, induction determined after 24 h showed activity.
Delayed type hypersensitivity
The DTH reaction elicited from the mice plantar pads by the cellular fractions was as follows: The OMP showed the highest induration (90%) at 48 h after inoculation; IMP and CP showed induration of 29.7% and 18.6% at the same time, respectively.
Discussion
The continued improvement of vaccines against B. ovis is important for the control and eradication of epididimitis in sheep (16). Live vaccines have a number of deficiencies, including diagnostic interference caused by the smooth strain vaccines in serological tests used in eradication campaigns.
One of the approaches to develop a new, safe, and efficient vaccine is the use of subcellular components, using adequate adjuvant. However, there is no information describing appropriate antigens for use in a universal subcellular vaccine against brucellosis (6). Protective antigens for this disease, defined in the mouse model, have been associated with the outer membrane of other species of Brucella (17).
Selected surface components from B. ovis R strains administered through adjuvants, protected against both rough B. ovis in rams (18) and smooth B. abortus in calves (19), independent of the adjuvant used. Zhan et al (20) demonstrated that adjuvants, such as alum and CFA, increased the magnitude of the response, compared with that induced by immunization with antigen and without adjuvant, but they had no influence on the type of immune response.
We determined the protein patterns of OMP, IMP, and CP, and demonstrated that these 3 subcellular fractions have different capacities when inducing a cellular immune response. Brucella ovis OMP-fraction profiles obtained in this study were similar to those of other studies (1,8,21).
Numerous studies of Brucella antigens capable of activating a T-cell response have been carried out for vaccine and diagnostic test development purposes (22). Outer membrane proteins have been isolated and characterized from several species of Brucella; however, only the soluble OMP of B. abortus was evaluated for a cellular response. This was originally measured by lymphocyte blastogenesis (23), and is now measured by induction of several cytokines (3,5,22,24–26). Spleen cell cultures from mice injected with killed bacteria or whole bacterial extracts produced IL-2 exclusively (no IFN-γ or IL-4), in contrast to those cells from mice infected with live intracellular bacteria, which produced IL-2 and IFN-γ, but not IL-4 (20).
There is only one report about the induction of cytokine response in B. ovis. In that study the HS antigen was encapsulated into poly-ɛ-caprolactone microparticles (HS-PEC) and tested as a vaccine against B. ovis and B. abortus in mice. Subcutaneous, but not oral, administration in BALB/c mice of the HS-PEC induced high amounts of IFN-γ and IL-2, but low levels of IL-4, suggesting a predominantly Th1 cellular immune response. The use of free HS or empty PEC microparticles did not produce any protective effect (6). However, the study described here employed different B. ovis subcellular fractions (OMP, IMP, CP) to induce a cytokine response, and the response was evaluated for a longer period of time (24 to 120 h), thus extending the current knowledge of cytokine productions in response to B. ovis.
The OMP fractions in this study produced a high IL-2 response at 24 h, which diminished after 48 h; whereas IFN-γ reached its maximum value at 120 h. The OMP produced the highest DTH response. This is consistent with a classic Th1 lymphocyte response, associated with acquired cellular resistance, and DTH (25). The IL-4 was only produced by the OMP fraction, demonstrating activation by the Th2 lymphocytes and a humoral response. This response may have been due to the presence of rough lipopolysaccharide (R-LPS) residues and is supported by the fact that R-LPS epitopes on B. ovis cells are highly accessible to the immune system. Moreover, Riezu-Boj et al (27) described that the humoral response towards LPS-R is intense during B. ovis infection. In addition, circulating antibodies against R-LPS have been found during the disease, thus these molecules could play a role in antibody mediated protective immunity (17).
Based on cytokine production and a DTH response, the OMP and IMP fraction of B. ovis may be useful antigen for the development of a vaccine for B. ovis infections in sheep.
Acknowledgments
The authors thank Zoila Morales and Yolanda Medina for their technical assistant in lymphocyte culture and cytokine induction. This study was partially supported by the Cátedra de Inmunodignóstico de enfermedades bacterianas y micóticas de la Coordinación de Investigación, Facultad de Estudios Superiores Cuautitlán, UNAM-México, project PAEP 107302 of Coordinación General de Posgrado and project PAPIIT IN245802-3, UNAM, México.
References
- 1.Chin J, Turner B. Extraction of membrane antigen from Brucella ovis and an assessment of their serological activity of immunoblotting. J Gen Microbiol. 1990;136:1615–1622. doi: 10.1099/00221287-136-8-1615. [DOI] [PubMed] [Google Scholar]
- 2.Blasco JM. Brucella ovis. In: Nielsen K, Duncan R, eds. Animal Brucellosis, 1990:351–375.
- 3.Zhan YF, Cheers C. Endogenous interleukin-12 (IL-12) is involved in the resistance to Brucella abortus infection. Infect Immun. 1995;63:1387–1390. doi: 10.1128/iai.63.4.1387-1390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira SC, Harms JS, Rech EL, et al. The role of T-cells subset and cytokines in the regulation of intracellular bacterial infection. Braz J Med Biol Res. 1998;31:77–84. doi: 10.1590/s0100-879x1998000100010. [DOI] [PubMed] [Google Scholar]
- 5.Cheers C. Cell mediated immunity to murine Brucellosis and the role of cytokines. In: 50th Anniversary Meeting of Brucellosis Research Conference. Chicago, 1997.
- 6.Murillo M, Grilló MJ, Reñé J, et al. A Brucella ovis antigenic bearing poly-ɛ-caprolactone microparticle confer protection against experimental brucellosis in mice. Vaccine. 2001;19:4099–4106. doi: 10.1016/s0264-410x(01)00177-3. [DOI] [PubMed] [Google Scholar]
- 7.Morton RJ, Simons KR, Confer AW. Major outer proteins of Pasteurella haemolytica serovars 1-15: comparison of separation techniques and surface-exposed proteins on selected serovars. Vet Microbiol. 1996;51:319–330. doi: 10.1016/0378-1135(96)00010-7. [DOI] [PubMed] [Google Scholar]
- 8.Filip C, Fletcher G, Wulff JL, Earthart CF. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium–lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young NS, Levin J, Prendergast RA. An invertebrate coagulation system activated by endotoxin: Evidence for enzymatic mechanism. J Clin Invest. 1972;51:1790–1794. doi: 10.1172/JCI106980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 11.Bradford MA. A rapid and sensible method for the quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacterophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Towbin H, Stachelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salas TE, Núñez del Arco AL, Mejia PS. Diagnóstico de Brucella ovis. In: Diagnóstico de Brucelosis Animal. INIFAP, IICA, OPS. 2001:140–145.
- 15.Chatelain R, Varkila K, Coffman RL. IL-4 induces a Th2 response in Leishmania major infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 16.Bowden RA, Estein SM, Zygmum MS, Dubray G, Cloeckaert A. Identification of protective outer membrane antigens of Brucella ovis by passive immunization of mice with monoclonal antibodies. Microbes Infect. 2000;2:481–488. doi: 10.1016/s1286-4579(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 17.Bowden RA, Cloeckaert A, Zygmum SM, Bernad S, Dubray G. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species by enzyme-linked immunosorbent assay and flow cytometry. Infect Immun. 1995;63:3945–3952. doi: 10.1128/iai.63.10.3945-3952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blasco JM, Gamazo C, Winter AJ, et al. Evaluation of whole cell and subcellular vaccines against Brucella ovis in rams. Vet Immunol Immunopathol. 1993;37:257–279. doi: 10.1016/0165-2427(93)90198-d. [DOI] [PubMed] [Google Scholar]
- 19.Adams G, Fitch T, Allen C. Derivation and evaluation of the rough brucellosis vaccine in cattle. In: III Foro Internacional de Brucelosis. Acapulco, México, 1998.
- 20.Zhan YF, Kelso A, Cheers C. Differencial activation of Brucellareactive CD4+ T-cells by Brucella infection or immunization with antigenic extracts. Infect Immun. 1995;63:969–975. doi: 10.1128/iai.63.3.969-975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cloeckaert A, Kerkhops P, Limet JN. Antibody response to Brucella outer membrane proteins in bovine brucellosis: Immunoblot analysis and competitive enzyme-linked immunoabsorbent assay using monoclonal antibodies. J Clin Microbiol. 1992;20:3168–3174. doi: 10.1128/jcm.30.12.3168-3174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan YF, Yang JL, Cheers C. Cytokine response of T-Cell Subsets from Brucella abortus Infected mice to soluble Brucella proteins. Infect Immun. 1993;61:2841–2847. doi: 10.1128/iai.61.7.2841-2847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin CL, Verstrate DR, Winter AJ. Immune response of cattle to Brucella abortus outer membrane proteins measured by lymphocyte blastogenesis. Vet Immunol Immunopathol. 1985;9:383–396. doi: 10.1016/0165-2427(85)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes DM, Baldwin CL. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect Immun. 1995;63:1130–1133. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan YF, Kelso A, Cheers C. Cytokine production in the murine response to Brucella infection or immunization with antigenic extracts. Immunology. 1993;80:458–461. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan YF, Cheers C. Differential induction of macrophage — derived cytokines by live and dead intracellular bacteria in vitro. Infect Immun. 1995;63:720–723. doi: 10.1128/iai.63.2.720-723.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riezu-Boj JL, Moriyon I, Blasco JM, Gamazo C, Díaz R, Winter AJ. Analysis of immunoblot of the antibody response of sheep infected by smooth and rough Brucella to outer membrane proteins extracted by hot saline. Infect Immun. 1990;58:489–494. [Google Scholar]


