Abstract
Prostaglandin E2 (PGE2) is a membrane-derived lipid signaling molecule important in neuronal development. Abnormal levels of PGE2, due to environmental insults prenatal development, have been linked to brain pathologies. We have previously shown that the addition of PGE2 to neuroectodermal (NE4C) stem cells affects early stages of neuronal differentiation (day 0–8) including increased stem cell motility, accelerated formation of neurospheres, and elevated calcium levels in growth cones. In this study, we further examine whether PGE2 can influence actin-dependent neuronal morphology in later stages (day 8–12) of NE4C cell differentiation. We show that exposure to PGE2 from the initiation of differentiation increased neurite length and the proportion of neurites that formed axonal loops. We also observed changes in the proportion of turning growth cones as the differentiation progressed, with a reduced likelihood of observing turning (or asymmetrical) growth cones on day 8 and increased odds on days 10 and 12. Moreover, we showed for the first time that the observed changes in cytoskeletal morphology were PGE2/PKA dependent. Interestingly, we also found that PGE2 decreased the total protein levels of the actin-bound form of spinophilin and increased levels of unbound PKA-phosphorylated ser94-spinophilin. Hence, we propose that exposure to PGE2 can destabilize the actin cytoskeleton at various stages of neuronal differentiation due to dissociation of ser94-spinophilin causing changes in neuronal morphology.
Supplementary Information
The online version of this article (10.1007/s10571-020-01029-4) contains supplementary material, which is available to authorized users.
Keywords: Lipid signaling, Prostaglandin E2, Neurodevelopment, Neuronal differentiation, Spinophilin, Autism
Introduction
Membrane phospholipids are crucial sources of lipid signaling molecules necessary for healthy brain development. Prostaglandin E2 (PGE2) is one of the major bioactive lipids within the brain. PGE2 is synthesized from arachidonic acid (AA), which is released from the phospholipid bilayer through the action of phospholipase A2 (Sang and Chen 2006). AA is then converted into PGE2 through the activity of cyclooxygenase 1 or 2 (COX-1, -2) (Park et al. 2006; Rouzer and Marnett 2009). Within the brain, COX-1 and -2 are constitutively expressed primarily in microglial and neuronal cells, respectively (Kirkby et al. 2016; Maslinska et al. 1999; Norregaard et al. 2015; Schwab et al. 2000; Hoozemans et al. 2001). PGE2 exerts its diverse role in the brain through four G protein-coupled E-prostanoid (EP 1–4) receptors (Khan et al. 2019). COX-2/PGE2 signaling is involved in important processes during brain development, including proliferation and migration (Wright and McCarthy 2009), dendritic spine formation (Burks et al. 2007), and synaptogenesis and synaptic plasticity (Yang 2009).
Growing evidence from clinical studies provides a link between abnormal signaling of the COX-2/PGE2 pathway and neurodevelopmental disorders such as Autism Spectrum Disorders (ASD). Various environmental insults including maternal infection, air pollution, or common over-the-counter medications such as acetaminophen and acetylsalicylic acid are all known to affect PGE2 levels during development (Tamiji and Crawford 2010a; Wong and Crawford 2014a) and have been associated with increased prevalence of ASD (Yoon et al. 2020; Hodges et al. 2020). In vitro and in vivo research in our lab has already provided substantial evidence for potential molecular mechanisms by which changes in the PGE2 levels (increased or reduced) can lead to abnormal neuronal development. For example, in murine neuroectodermal (NE4C) stem cells and Neuro-2a cell lines we showed that PGE2 increased proliferation, migration, and differentiation of cells, elevated intracellular calcium levels within the cytosol and growth cones, and affected the expression of various developmental genes (Wong et al. 2016; Davidson et al. 2016; Tamiji and Crawford 2010b). We also demonstrated that short-term exposure (up to 24 h) to PGE2 in differentiating NE4C cells and prenatal exposure to PGE2 in mice resulted in a cross-talk between PGE2 signaling and the Wnt canonical pathway through PKA and PI-3 K (Wong et al. 2014; Rai-Bhogal et al. 2018b). In addition, we have confirmed in mice that maternal exposure to PGE2 during pregnancy had significant changes in the expression of Wnt-target genes Mmp7, Wnt2, and Wnt3a in offspring during prenatal and early postnatal stages (Rai-Bhogal et al. 2018b). Using microarray analysis, we also found that COX-2-deficient mice had abnormal regulation of many genes involved in proper neuronal development at embryonic day 16 and 19 (Rai-Bhogal et al. 2018a). Further studies in the PGE2 injected and COX-2-deficient mice models also showed sex-dependent manifestation of autism-related behaviors (Wong et al. 2017, 2019).
Our previous study showed that PGE2 accelerates differentiation of NE4C cells, inducing earlier formation of neuronal clusters called neurospheres via a PKA-dependent mechanisms (Wong et al. 2014). This study further investigates whether PGE2/PKA signaling affects neurite outgrowth in differentiating NE4C cells. Here we demonstrate that PGE2 affects morphological features known to be influenced by actin cytoskeleton, including neurite length, growth cone turning, and the formation of axonal loops via PKA-dependent mechanisms. We also show that PGE2/PKA signaling increases the total level of actin-binding protein spinophilin and its phosphorylation at ser94. We propose that PGE2 can influence neuronal differentiation via PKA-dependent phosphorylation of actin-bound proteins.
Methods
Cell Culture
Mouse neuroectodermal NE4C stem cells were obtained from the American Tissue Culture Collection (CRL-2925, ATCC, Manassas, VA). Cells were plated on 0.01% poly-l-lysine (Sigma)-coated 60 mm culture plates (BD Falcon). The cells were maintained in Minimum Essential Media (MEM) which was supplemented with 10% Fetal Bovine Serum (FBS), 2 mM l-glutamine, and 1 × penicillin–streptomycin (invitrogen), which was changed every 2 days. Plates were kept in an incubator maintained at 5% [CO2] with 95% humidity at 37 °C.
Cell Differentiation
NE4C stem cells were passaged by dissociation using 0.05% trypsin–EDTA, followed by pelleting and resuspension in MEM + FBS (Minimum essential media and Fetal bovine serum; as described above), and then incubation overnight. Differentiation was induced with Serum-Free Media (SFM), which consisted of neurobasal media, supplemented with 1 × B-27, 2 mM l-glutamine, and 1 × penicillin–streptomycin as previously described in Wong et al (2016). Treatments were incubated in either SFM alone (control), SFM supplemented with 1 µM PGE2 (Cayman Chemical, Ann Arbor MI), 10 µM Forskolin, 10 µM KT5720, a combination of 1 µM PGE2 and 10 µM KT5720 (PGE2 + KT5720) or a combination of 10 µM Forskolin and 10 µM KT5720 (Forskolin + KT5720).
Cell Morphology Imaging and Analysis
Images were taken using a Nikon Eclipse Ti-E microscope (Tokyo, Japan) on days 8, 10, and 12 from three separate biological replicates for each day. The images were used to quantify neurite length, growth cone turning, and axonal looping on each day of differentiation. Neurite length was defined as the distance from the base of an extension to the furthest reaching filopodia. Only the longest neurite was measured for any given cell. Growth Cone Turning was assessed through the shape of each growth cone. We use a novel approach to categorizing growth cones here. Growth cones were classified as turning or not turning, based on the symmetry of each growth cone. Firstly, the midpoint of the growth cone base was determined, and a line was drawn from the midpoint to the furthest reaching filopodia to bisect the cone. The area ratio of the larger portion of the bisected growth cone to the smaller was taken. Growth cones with a ratio > 1.4 were classified as asymmetrical and therefore turning, while those with a ratio < 1.4 were classified as symmetrical, and therefore not turning. Axonal looping was measured as the number of neurites forming axonal loops out of the total number of neurites examined. While normally categorized subjectively, we classified axonal loops based on the greatest exterior angle observed along the neurite. If this angle exceeded 270°, the neurite was categorized as forming an axonal loop.
Western Blot Analysis
Cell samples were collected using the NucleoSpin RNA/Protein isolation kit (Machery-Nagel), on cells after 8, 10, and 12 days following the induction of differentiation from three biological replicates collected at different dates. For all analyses, 25 µg of protein was loaded into a 12% polyacrylamide gel (PAGE) and were electrophoresed at 100v for 1.5 h. Gels were transferred to nitrocellulose membranes.
Total protein isolation was performed using the NucleoSpin RNA/Protein Kit (Macherey–Nagel), on cells after days 8, 10, and 12 following the induction of differentiation. PAGE gel electrophoresis was used to separate the isolated samples and then transferred to 0.45 µM nitrocellulose membranes (Bio-Rad). Membranes were blocked in 5% milk in 1× Tris buffer saline 0.05% Tween 20 (TBS-T) for 1 h at room temperature and then probed with primary antibodies diluted in 2% milk to the same concentration across all runs. Protein samples were probed with anti-p-ser94 spinophilin [RU499, 1:1000; obtained from the Greengard laboratory; (Hsieh-Wilson et al. 2003)], mouse monoclonal anti-B-Actin (Abcam; 1:10,000; ab8245, Cambridge, MA, USA), and mouse monoclonal anti-GAPDH (Abcam, 1:10000; ab8245, Cambridge, MA, USA) or anti-spinophilin (Cell Signalling, 1:1000, mAb #14136, Danvers, MA, USA). Membranes were rinsed 5 times in 1 × TBS-T before probing with appropriate HRP-tagged secondary antibodies. Goat-anti-rabbit (Abcam, 1:10000, ab6276, Cambridge, MA, USA) was incubated overnight, and Goat-anti-mouse (1:10000; catalog No. ab6789) was incubated for 2 h. Membranes were then washed three times for 5 min in TBS-T and then incubated with ECL Prime Western Blotting Detection Reagent (Bio-Rad). Membranes were imaged with the Geliance 600 Imaging System (Perkin Elmer). For quantification, protein signal intensity was first normalized to GAPDH signal intensity. The relative protein expression was then normalized to the control cell treatment (protein expression in control cells = 1).
Statistical Analysis
Statistical analyses were performed using the core open source software R (R Core Team 2013). All continuous data including neurite length, axonal loop formation, and western blot analysis were analyzed by use of a one- or two-way ANOVA followed by Tukey’s HSD post hoc comparisons to determine morphological differences and differences in protein expression levels, and visually displayed using a violin plot to depict the probability distribution of data within a given treatment. Significance was determined as p < 0.05 for all tests. Numerical data in figures are presented as mean ± standard deviation (SD). Data for growth cone turning were analyzed through generalized logistical modeling and data in the tables are presented as Odds ratios (95% confidence interval). In all figures, n represents the number of individual measurements made for a given treatment. All measurements were taken from three independent biological replicates. Total sample size was calculated based on morphology analysis using G*Power 3 software (Faul et al. 2007). Effect size was set to 0.25 and a sample size of 269 was determined to reach a power of 0.8. Images were taken such that a minimum of 68 measurements could be performed for each test. However, all cells were measured for each image taken.
Results
Prostaglandin E2 Increased Neurite Extension Length Via PKA-Dependent Mechanism
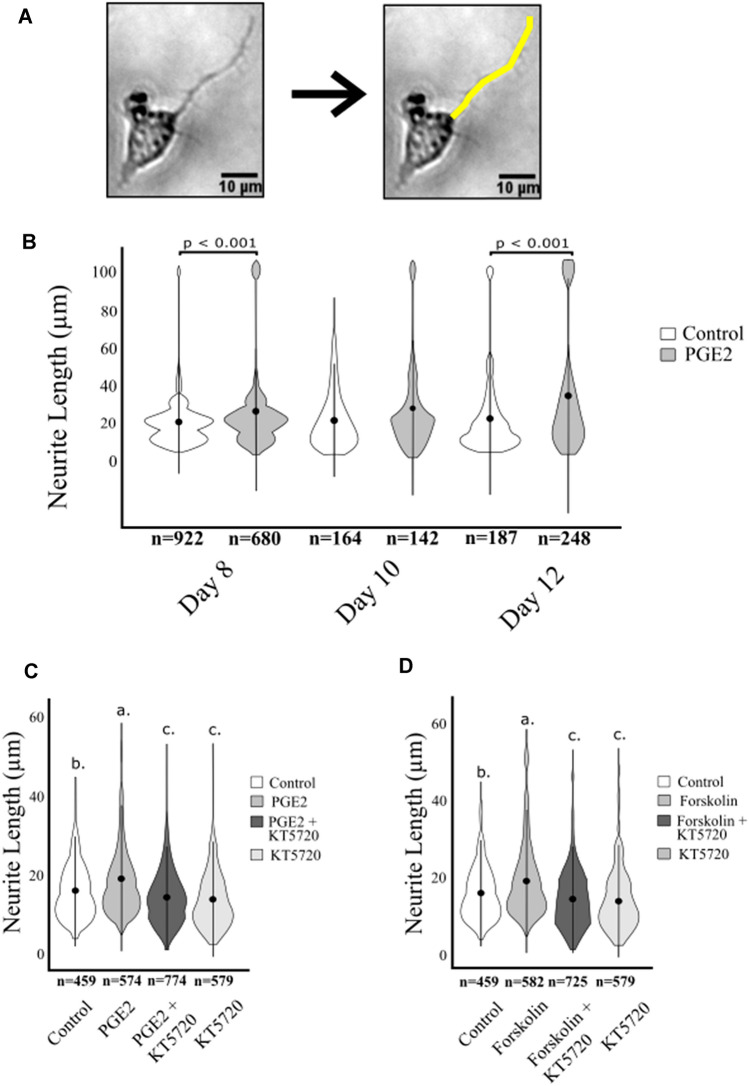
We previously established that neuroectodermal (NE4C) stem cells go through specific stages of neuronal differentiation, including proliferation (day 2), aggregation, and inward migration (day 4–6) and formation of neuronal clusters (neurospheres) followed by neurite outgrowth (days 8–12) (Wong et al. 2016; Davidson et al. 2016). We showed that PGE2 increased proliferation of NE4C stem cells and accelerated their differentiation into neuronal-lineage cells, including formation of neurospheres and expression of differentiation marker Oct4 prior to day 8 of differentiation (Wong et al. 2016). In this study, we further examined the effect of PGE2 on neurite outgrowth on days 8–12 and investigated whether the effects were PKA dependent. Neurite length was quantified by measuring the total distance between the base of the neurite, to the furthest filopodial extension (Fig. 1a and “Methods”).
Fig. 1.
Average neurite length of differentiated NE4C cells. NE4C cells were differentiated over 12 days and neurite length was quantified in µM (± SD). N values shown represent the number of neurites measured for each treatment. a Representative images of neurite length measurement in a differentiated cell on day 12. b Treatment with PGE2 across days 8, 10, and 12 shows that PGE2 increases neurite length on days 8 and 12 compared to control cells. Significance is indicated as ***(p < 0.001). c, d Cells treated with PGE2 (c) and Forskolin (d) were treated with KT5720 to determine PKA dependence. Significant differences are indicated with different letters. Cells treated with PGE2 showed similar trends to those treated with Forskolin on day 8. The effects of both treatments were attenuated by the addition of KT5720
A two-way ANOVA was performed, comparing neurite length between treatments (control and PGE2), across days (8, 10, and 12). We found a significant interaction between day and condition (F2,2437 = 4.056, n = 2343 p < 0.05). There were also significant differences for the day main effect (F2,2437 = 17.782, p < 0.001), with overall differences observed between day 8 and day 12 (p < 0.001) and between day 10 and day 12 (p < 0.01). However, no significant difference was found between days 8 and 10 (p = 0.167), suggesting a period of growth occurring predominantly between days 10 and 12. We also found differences for the main effect of treatment on neurite length (F1,2437 = 48.635, p < 0.001).
Given the significance of the interaction, as well as the significance of the treatment effect, further pairwise comparisons within each day were performed. On day 8, neurites in cells treated with PGE2 were significantly longer than in control cells, with lengths of 23.42 µm and 18.92 µm, respectively (Fig. 1b; p < 0.001). On day 10 of differentiation, cells treated with PGE2 had neurites 24.89 µm long, compared to control cells (19.81 µm) but the difference did not reach significance (p = 0.119). By day 12, neurites of cells treated with PGE2 measured 31.82 µm long, which was significantly longer than the neurites in control cells at 21.15 µm (p < 0.001). It appears that the effects of PGE2 on neurite length are dependent on the day of differentiation. We observed that in the PGE2-treated cells the rate of neurite length increase per day (see “Methods”) followed different trajectories as compared to the control cells. Between days 8 and 10, the rate of neurite length increase was 0.45 µm per day in the control and it was 1.5 faster in the PGE2 cells at 0.74 µm per day. However, between days 10 and 12 the increase in neurite length rate was 0.67 µm per day in the control cells, whereas in PGE2 cells the rate of increase was 5 time greater at 3.47 µm per day (Fig. 1b). Overall, we observed that the PGE2 treatment increased the neurite length across differentiation. Moreover, the neurite length in control cells increased at a relatively constant rate during differentiation, whereas in PGE2-treated cells the neurite length increases at a higher rate in later days of neuronal differentiation.
In this study, we investigated whether PGE2-PKA mechanisms regulated neurite length extension using the PKA blocker KT5720 (Fig. 1c). A one-way ANOVA was performed to identify differences between the treatments (Control, PGE2, PGE2 + KT5720, and KT5720) on day 8 of differentiation and significant differences were found between treatments (F3,2382 = 52.95, n = 2386, p < 0.001). Based on main effect significance, further pairwise comparisons between treatments were performed. We showed that neurite length was significantly higher in PGE2-treated cells at 19.05 µm compared to control cells at 15.89 µm (p < 0.001). Moreover, as compared to the length in PGE2-treated cells, the addition of KT5720 significantly reduced the neurite length to 14.39 µm in PGE2 + KT5720-treated cells (p < 0.001), and KT5720-treated cells at 13.79 µm (p < 0.001) suggesting a PGE2/PKA effect on neurite length. Both cells treated with KT5720 alone or a combination of PGE2 + KT5720 had neurite lengths that were significantly reduced relative to control cells (p < 0.001).
To confirm that the neurite elongation observed in the PGE2-treated cells was PKA dependent, we examined differentiated NE4C cells with the addition of Forskolin (Fig. 1d). A one-way ANOVA was performed comparing treatments (Control, Forskolin, Forskolin + KT5720, and KT5720) and a significant difference was found between treatments (F3,2341 = 29.85, n = 2345, p < 0.001). We found that cells treated with Forskolin had neurites that were 18.96 µm, which were significantly longer than neurites in control cells at 15.89 µm (p < 0.001). Further cells treated with Forskolin had longer neurites than those treated with Forskolin + KT5720 with neurites 14.79 µm long (p < 0.001), or KT5720 alone with neurites 13.79 µm long (p < 0.001), mimicking results seen in PGE2-treated cells and showing the influence of PKA in neurite growth (Fig. 1d). We also found that cells treated with KT5720 alone were significantly shorter that the control cells (p < 0.01), and not significantly different than cells treated with Forsk. + KT5720 (p = 0.277) again suggesting a basal involvement of PKA in neurite elongation. Additional experiments with another PKA inhibitor H89 show the same overall outcomes and are presented in the supplementary Figure S1.
Prostaglandin E2 Affects Growth Cone Symmetry
Growth cone turning is considered an essential behavior for navigating axons (Gomez and Letourneau 2014). Growth cones often oscillate between symmetrical and asymmetrical shapes, which is an indicator of the navigational state and turning properties (Goodhill et al. 2015). In this study, we evaluated whether growth cone turning was affected by PGE2/PKA mechanisms during differentiation by examining the proportion of growth cones that were symmetrical vs. asymmetrical. Asymmetrical growth cones were assigned to a “turning” group and those that were symmetrical were assigned to a “non-turning” group (Fig. 2 and “Methods”).
Fig. 2.
Measurement of turning growth cones. NE4C cells were imaged and the symmetry of each growth cone was used to classify each as turning (asymmetrical) or non-turning (symmetrical)
Generalized logistic modeling was used to examine the odds of observing a turning (asymmetric) growth cone following treatment with PGE2 on days 8, 10, and 12 (see “Methods”). Our model converged at an AIC of 629.4285 and our intercept (control cells on day 8) was found to be significant with an odds ratio of 2.00 (p < 0.05) (Table 1A). PGE2 exposure significantly reduced the odds of observing turning growth cones with an odds ratio of 0.23 (p < 0.001). Examining the effect of day of differentiation, we saw no significant change in odds for control cells on day 10 and a decrease in the odds of observing turning growth cones on day 12 with odds ratios of 2.21 (p = 0.790) and 0.68 (p < 0.01), respectively. Interestingly, in contrast to the decrease in the odds of observing turning growth cones in PGE2 exposed cells on day 8, the odds significantly increased in cells treated with PGE2 on days 10 and 12 with odds ratios of 10.13 (p < 0.01) and 64.34 (p < 0.001), respectively.
Table 1.
Exposure to PGE2 affects the odds that a growth cone will be classified as turning across differentiation
| A. Odds of observing turning growth cones between PGE2- and control-treated cells between days 8 and 12 | ||
|---|---|---|
| Treatment—day | OR (95% CI) | p-Value |
| Control—day 8 | 2.00 (2.72–1.47) | 2.36E−02* |
| Control—day 10 | 2.21 (3.24–1.51) | 7.90E−01 |
| Control—day 12 | 0.68 (1.00–0.463) | 5.43E−03** |
| PGE2—day 8 | 0.23 (0.373–0.143) | 6.86E−06*** |
| PGE2—day 10 | 10.13 (17.79–5.77) | 3.96E−03** |
| PGE2—day 12 | 36.32 (64.37–20.50) | 3.99E−07*** |
| B. Odds of observing turning growth cones on day 8 for control cells and cells treated with PGE2, PGE2 + KT570, or KT5720 | ||
|---|---|---|
| Treatment | OR (95% CI) | p-Value |
| Control | 2.18 (2.82–1.69) | 2.44E−03** |
| PGE2 | 0.55 (0.78–0.39) | 9.15E−05*** |
| PGE2 + KT5720 | 3.05 (4.15–2.25) | 2.74E−01 |
| KT5720 | 1.68 (2.41–1.17) | 4.69E−01 |
| C. Odds of observing turning growth cones on day 8 for control cells, and cells treated with Forskolin, Forskolin + KT5720, or KT5720 alone | ||
|---|---|---|
| Treatment | OR (95% CI) | p-Value |
| Control | 2.18 (2.82–1.96) | 2.44E−03** |
| Forskolin | 0.43 (0.62–0.30) | 6.71E−06*** |
| Forskolin + KT5720 | 1.90 (2.59–1.40) | 6.59E−01 |
| KT5720 | 1.68 (2.41–1.17) | 4.69E−01 |
Multinomial logistic regression was performed to determine the odds of observing a turning (asymmetrical) growth cone compared to a non-turning (symmetrical) growth cone. Data are presented as the Odds ratio (OR) of observing turning growth cones in each treatment
We further examined the contribution of PKA to the effects on growth cone turning on day 8, using KT5720. We used generalized logistic modeling to determine if any of our treatments (Control, PGE2, PGE2 + KT5720, or KT5720) affected the odds of observing turning growth cones (Table 1B). Our model converged with an AIC of 485 and our intercept (control cells) had a significant odds ratio of 2.18 (p < 0.01). As expected, PGE2 exposure reduced the odds of observing turning growth cones with an odds ratio of 0.55 (p < 0.001). The addition of KT5720 rescued growth cone turning. There was no significant effect of PGE2 + KT5720 or KT5720 alone on the odds of observing turning growth cones, with odds ratios of 3.05 (p = 0.27) and 1.68 (p = 0.47), respectively. This suggests that growth cone turning is suppressed by PGE2-PKA mechanisms.
We used Forskolin to further verify the PGE2/PKA effect on growth cone turning. We used generalized logistic modeling to compare the effect of our treatments (Control, Forskolin, Forskolin + KT5720, and KT5720) on the odds of observing turning growth cones (Table 1C). Our model converged with an AIC of 469 and our intercept (control cells) had a significant odds ratio of 2.18 (p < 0.01). Similar to PGE2 exposed cells, there was a decrease in the odds of observing turning growth cones in Forskolin-treated cells with an odds ratio of 0.43 (p < 0.001). We again observed that the addition of KT5720 rescued growth cone turning. Treatment of cells with Forskolin + KT5720 or KT5720 alone did not significantly affect the odds of observing turning growth cones with odds ratios of 1.90 (p = 0.66) and 1.91 (p = 0.72), respectively. These findings further confirm that PGE2/PKA signaling can reduce growth cone turning in differentiating NE4C cells.
Prostaglandin E2 Increases the Incidence of Axonal Looping in Differentiating Cells
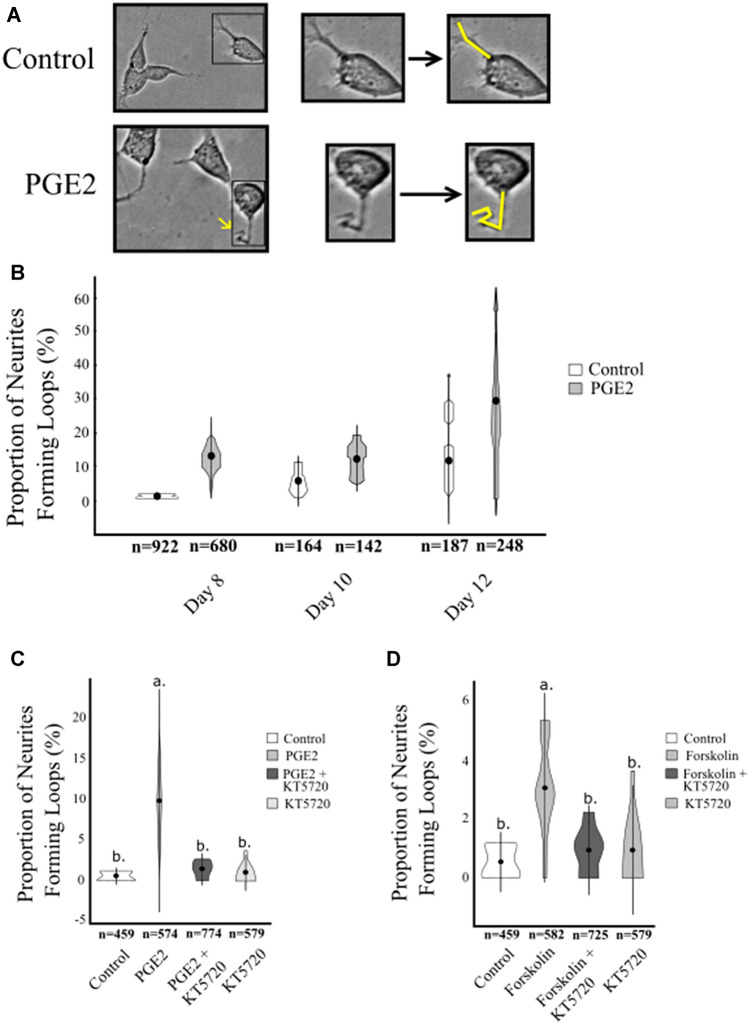
Axonal outgrowth and guidance are key to critical neurodevelopmental processes in vivo including early cell migration and synapse formation (Raper and Mason 2010; Stoeckli 2018). Aberrations in axonal outgrowth and navigation can lead to self-fasciculation, in which axons turn inwards and adhere to themselves (Mondal et al. 2014). This process can be visually observed through the presence of axonal loops and has been reported in culture cells (Davis et al. 2017; Smit et al. 2017). In this study, we examined whether PGE2 can influence axonal loop formation during differentiation on days 8–12. We report the percentage of extensions forming loops out of the total number of neurites quantified. Axonal loops are defined as a neurite of any length that turned more than 270◦ regardless of if the neurite continued to elongate (Fig. 3a).
Fig. 3.
Percentage of axonal loops formed out of total neurites. NE4C cells were imaged during days 8, 10, and 12 during differentiation and the proportion of neurites forming loops of all cells measured was determined. Results are shown as a percentage of neurites forming loops out of the total number of neurites measured (± SD). N values, representing the total number of neurites measured for each condition are shown below the x-axis. a Representative images of control cells and PGE2-treated cells on day 8 of differentiation showing an example of typical neurites and neurites forming a loop, respectively. b Cells treated with PGE2 had a higher percentage of neurites form loops on day 12 compared to control cells. c Cells treated with PGE2 formed significantly more loops than control treated cells as well as cells treated with KT5720. d Cells treated with forskolin showed a similar trend to those treated with PGE2, forming significantly more loops than control cells or cells treated with KT5720
We performed a two-way ANOVA comparing the mean percentage of loops observed turning between control and PGE2-treated cells, across days 8, 10, and 12 of differentiation (see “Methods”). We observed no significant interaction between treatment and days (F2,85 = 1.699, n = 2343, p < 0.189). However, the main effect of day was significant (F2,85 = 18.366, p < 0.001), with no significant difference between days 8 and 10 (p = 0.982) but with significant differences between 10 and 12 (p < 0.001) and 8 and 12 (p < 0.001) (Fig. 3b). There was also a significant main effect of treatment observed (F1,85 = 29.474, p < 0.001), indicating that exposure to PGE2 can induce the formation of axonal loops in vitro.
To determine if the effect of PGE2 on the formation of axonal loops was PKA dependent, we used KT5720. We performed a one-way ANOVA comparing the average proportion of axonal loops formed on day 8 between treatments (Control, PGE2, PGE2 + KT5720, KT5720) and found a significant effect between treatments (F3,43 = 17.89, n = 2386 p < 0.001) (Fig. 3c). The proportion of neurites forming loops in PGE2-treated cells was 9.7% compared to 0.5% in control cells (p < 0.001). Compared to PGE2-treated cells, the addition of KT5720 reduced the number of axonal loops to 1.4% in PGE2 + KT5720-treated cells (p < 0.001) and 0.9% in cells treated with KT5720 alone (p < 0.001) indicating that the increased formation of axonal loops by PGE2 exposure was influenced by PKA. Additionally, there was no significant difference between control cells, and cells treated with PGE2 + KT5720 (p = 0.9328) or cells treated with KT5720 alone (p = 0.9912). These findings show that PGE2/PKA signaling can contribute to axonal loop formation.
We used Forskolin to further confirm whether the increase in axonal loop formation seen with PGE2 was PKA dependent. The proportion of neurites forming axonal loops was compared between treatments (Control, Forskolin, Forskolin + KT5720, and KT5720) on day 8 using one-way ANOVA and a significant difference between treatments was found (F3,44 = 12.85, n = 2345, p < 0.001). The proportion of neurites forming axonal loops in Forskolin-treated cells was 3%, which was significantly higher than 0.5% in control cells (p < 0.001), 0.9% in Forskolin + KT5720-treated cells (p < 0.001), and 0.9% in KT5720-treated cells (p < 0.001), producing a similar response to cells treated with PGE2 (Fig. 3d). Additionally, we observed no significant difference in axonal loop formation between control cells, and Forskolin + KT5720-treated cells (p = 0.7971) or KT5720-treated cells (p = 0.7883), verifying that the increase in axonal looping we had observed was PKA dependent.
Prostaglandin E2 Increases the Level of Phosphorylated Spinophilin at ser94 Through PKA
Many changes in neuronal morphology that occur during differentiation, including neurite outgrowth, and axonal pathfinding greatly rely on modification of actin cytoskeleton and actin stabilizing proteins such as spinophilin (Davis et al. 2017; Geraldo and Gordon-Weeks 2009; Miller and Suter 2018). Previous in vivo studies have shown that the total expression of spinophilin within the preoptic areas of young rats is increased by the direct injection of PGE2 (Burks et al. 2007). Other studies have also demonstrated that the phosphorylation of spinophilin by PKA at ser94 reduces its binding affinity to actin (Bielas et al. 2007; Hsieh-Wilson et al. 2003). In this study, we examined for the first time whether the phosphorylation of spinophilin at ser94 can be regulated by a PGE2/PKA-dependent mechanism during neuronal differentiation.
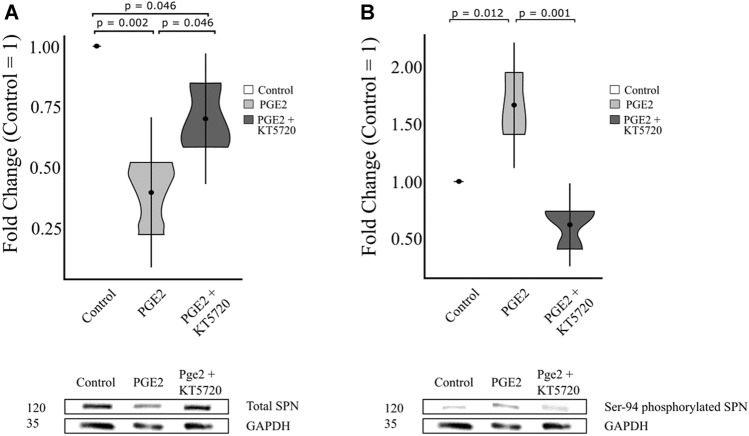
Western blot analysis was used to quantify relative amounts of total spinophilin between treatments (Control, PGE2, PGE2 + KT5720) on day 8 of differentiation (Fig. 4). A one-way ANOVA showed that there was a significant difference between treatments (F2,6 = 19.47, n = 9, p < 0.01). Compared to the basal level of total spinophilin in control cells (considered a fold change of 1.0), the expression level decreased to a fold change of 0.40 in PGE2-treated cells (p < 0.01) (Fig. 4a). Treatment with PGE2 + KT5720 resulted in a 0.70-fold change compared to the control cells (p < 0.05), which was significantly higher than cells treated with PGE2 alone (p < 0.05) indicating that the reduction of total spinophilin level was influenced by PGE2-PKA.
Fig. 4.
PGE2-PKA effect on total and ser94 phosphorylated spinophilin expression. Protein was isolated from cells on day 8 following the induction of differentiation after treatment with PGE2 or PGE2 + KT5720 and the a total spinophilin protein and b P-Ser94 phosphorylated spinophilin were quantified relative to control cells through western blotting. Results are shown as fold change to control cells (± SD)
To further understand the potential regulation of spinophilin through PGE2, we quantified the expression of p-ser94 spinophilin using western blot (Fig. 4b). A one-way ANOVA found a significant difference in p-ser94 spinophilin expression level between treatments (Control, PGE2, PGE2 + KT5720) on day 8 of differentiation (F2,6 = 23.43, n = 9, p < 0.05). The expression of p-ser94 spinophilin was significantly higher in PGE2-treated cells showing a 1.66-fold increase compared to control cells (p < 0.05). The expression of p-ser94 spinophilin was lower in PGE2 + KT5720-treated cells with a fold change of 0.62 relative to control cells, and significantly lower than in PGE2-treated cells (p < 0.001), verifying that the increase in the p-ser94 spinophilin levels was PGE2/PKA dependent.
Overall, we show that in differentiating NE4C cells, PGE2 treatment reduced expression level of total spinophilin and increased the expression of p-ser94 spinophilin both influenced by PKA-dependent mechanisms. The increased levels of p-ser94 spinophilin observed here likely affects it affinity to actin cytoskeleton, which was previously reported and will be further discussed.
Discussion
This study provides evidence for the first time that the PGE2/PKA signaling can influence actin-dependent neuronal morphology such as neurite length, and growth cone turning and looping as well as the level and phosphorylation of the actin-binding protein spinophilin. We used NE4C cells as a model system to show that as the differentiation progresses exposure to PGE2 promoted neurite outgrowth, increases growth cone turning, and promotes axonal self-fasciculation. We also show that PGE2 decreases the level of total actin-bound spinophilin and increases the level of its unbound form phosphorylated at ser94.
We found a PKA-dependent increase in neurite length in cells treated with PGE2 throughout differentiation. The presence of neurites is one of the defining characteristics that neuronal cells have arrested proliferation and become differentiated cells (Craig and Banker 1994). The process of neurite elongation is driven by the polymerization of the actin cytoskeleton and the coordination of the actin and microtubule cytoskeletons to drive the leading edge of the neurite’s growth cone (Dent and Gertler 2003). Neurite length in vitro can be a good representation of abnormalities with dendrite and axonal outgrowth in vivo (Radio and Mundy 2008). We have previously shown that 24 h exposure to PGE2 on day 12 of differentiation in NE4C stem cells increased neurite length (Davidson et al. 2016). Increased neurite length was also observed in neuronal motor cell lines following 48 h exposure to PGE2 as well as in sensory-like neuronal cells exposed to PGE2 for 24 h (Nango et al. 2017; Mitani et al. 2016). In this study, we provide additional evidence that exposure of NE4C cells to PGE2 from the initiation of differentiation significantly increased neurite length during neuronal differentiation in a PKA-dependent manner. We observed that the rate of neurite length was greatly influenced by PGE2 during later differentiation stages after day 10 when compared to the control cells. This may speak to a critical period of the effect of PGE2 during differentiation. A study by Park et al. (2015) found that levels of PGE2 increased throughout neuronal differentiation of primary rat neural stem cells. A separate group found that PGE2-induced neuritogenesis was abolished by the inhibition of PKA, providing further evidence of a PGE2-PKA effect on neuronal differentiation (Mitani et al. 2016). It is feasible to speculate that prenatal exposure to environmental risk factors known to influence PGE2 levels (Tamiji and Crawford 2010a; Wong and Crawford 2014a; Wong et al. 2015) may also affect progression of neuronal development during the critical times.
We also observed decreases in the proportion of growth cones turning, as classified by the symmetry of each growth cone, which is indicative of abnormal axonal pathfinding. The establishment of proper neuronal circuitry throughout development depends on precise axonal pathfinding. The motility of growth cones enables them to respond to attractive and repulsive cues as neurites navigate to their intended targets (Gasperini et al. 2017; Craig 2018). The structure of a growth cone can be separated into a central region filled with organelles and microtubules and a peripheral region or the leading edge which consists primarily of actin fibers (Miller and Suter 2018; Craig 2018). While growth cone turning is dependent on the spatio-temporal coordination of the assembly and disassembly of actin and microtubule cytoskeletons the shape of a growth cone is primarily determined by actin at the leading edge of a growth cone (Dent et al. 2011; Lowery and Van Vactor 2009; Gordon-Weeks 2004). Both attractive and repulsive growth cone turning affect the shape of a growth cone. Prior to attractive turning, there is a rapid accumulation of F-actin at the site where the growth cone will turn (Lin and Forscher 1993; Gallo and Letourneau 2000). In contrast, localized increases in retrograde actin flow, leading to localized growth cone collapses, occur during repulsive growth cone turning (Luo et al. 1993; Fan and Raper 1995). While overall disruptions in actin have been found to disrupt neurite outgrowth, actin disruption localized to the growth cone increased growth cone collapse, decreasing overall growth cone area (Zhou and Cohan 2001). We postulate that the marked decline in the asymmetry (turning) of growth cones as a result of exposure to PGE2 is a strong indicator that there was a dysregulation in actin polymerization dynamics. Moreover, we determined that these morphological changes are driven by PKA. Our findings show that during the process of neuronal differentiation PGE2/PKA not only increases neurite length but also affects axonal turning, both likely via dysregulation of actin dynamics.
In this study, we also determined that PGE2-PKA signaling affected axonal loop formation during differentiation of NE4C cells. The formation of axonal loops is indicative of the inability of dendrites to self-avoid, a key aspect of axonal pathfinding (Amthor and Oyster 1995; Sdrulla and Linden 2006). Issues with self-avoidance can ultimately result in nervous system circuit abnormalities as dendrites cannot form connections properly. Such circuit abnormalities are believed to be related to neurodevelopmental disorders including retinal dysplasia (Weiner et al. 2004), tuberous sclerosis (Choi et al. 2008), and most commonly in ASD (Minshew and Williams 2007). Abnormal calcium levels may help to explain the higher proportion of axonal loops observed here in PGE2-treated cells. In fact, we have previously shown that exposure to PGE2 increases baseline and amplitude of intracellular calcium levels within growth cones of differentiating NE4C cells (Davidson et al. 2016). Calcium signals are well-established mediators of growth cone turning and outgrowth, which were both dysregulated following PGE2 exposure in this study (Cohan et al. 1987; Zheng 2000). In addition to the contribution of calcium to abnormal growth cone turning, which we observed in this study, intracellular calcium levels have a key role in neurite adhesion, with many cell adhesion proteins inducing increases in calcium (Sheng et al. 2013; Doherty et al. 2000). Previous research suggests that cross-talk between PKA and calcium is important for processes in cell migration including the restructuring of the actin cytoskeleton (Howe 2011). The increase in axonal loop formation in PGE2-treated cells may speak to a dysregulation of both PKA- and calcium-dependent migration mechanisms ultimately leading to a dysregulation of actin. Literature provides evidence that increases in axonal loop formation can result in abnormalities in neuronal projections through cortical layers, affecting the organization of neurons within minicolumns of the cortex (DeFelipe 2005). We speculate that there may be abnormalities in neuronal projection and minicolumn organization in increased PGE2 models in vivo.
We also observed a decline in the total levels of the actin-binding protein spinophilin in cells exposed to PGE2 during differentiation. Spinophilin was independently isolated from rat brain by two groups characterizing a strong binding affinity to both F-actin filaments (Nakanishi et al. 1997) as well as the dendritic spine-enriched protein phosphatase 1 (PP1) (Allen et al. 1997). Spinophilin functions as a link between excitatory synaptic activity and changes in dendritic spine morphology and density, with early studies demonstrating that spinophilin is involved in the stabilization of the actin cytoskeleton in both dendritic spines and filopodia (Satoh et al. 1998; Yan et al. 1999). Studies in spinophilin knock-out mice found an increase in spine density and a retraction of filopodia (Feng et al. 2000). The connection of PGE2 and total spinophilin expression has already been shown in rodent in vivo models. There was a reduction in the expression of total spinophilin at postnatal day 14 (PN14) in cerebellum of rats injected daily with COX-2 inhibitors between PN7 and PN13 (Dean et al. 2012a, b). The same group also found an increase in total spinophilin expression following postnatal injection of PGE2 into the preoptic area (Wright and McCarthy 2009; Burks et al. 2007). Using the anti-ser94-spinophilin antibody provided by Hsieh-Wilson et al. (2003), our current study is the first to show that despite the decrease in the total expression of spinophilin due to PGE2/PKA signaling there was also increased levels of ser94 phosphorylated spinophilin. Spinophilin contains several sequences for phosphorylation by PKA with the two major sites identified being Ser94 and Ser177 (Hsieh-Wilson et al. 2003). Phosphorylation of spinophilin at ser94 decreases the stoichiometry of the spinophilin to F-actin interaction by as much as 53% (Hsieh-Wilson et al. 2003). Higher proportions of bound (unphosphorylated) spinophilin are associated with greater actin stability, which is thought to be the result of the actin-capping activity of bound spinophilin in which spinophilin binds to the barbed ends of polymerizing actin, thereby preventing its polymerization (Schuler and Peti 2008). Proper regulation of actin-capping is necessary for normal neurite elongation, and axonal pathfinding, both of which were affected in our increased PGE2 model. Interestingly, Hsieh-Wilson et al. (2003) showed that unphosphorylated actin-bound spinophilin was localized to the post-synaptic density (PSD), whereas phosphorylated Ser94 and Ser177 unbound spinophilin was found in the cytosol. This shift in the subcellular localization of spinophilin was influenced by PKA. Our findings show for the first time that the PGE2-PKA signaling can upregulate the levels of Ser94 unbound spinophilin and affect actin dynamics such as axonal looping. We suggest that abnormal PGE2 levels during prenatal stages of development due to environmental influences can contribute to modification of dendritic spines function through change actin dynamics resulting from the phosphorylation of spinophilin at ser94.
Examining our results in their entirety, we propose a model in which PGE2 can regulate actin-dependent cellular morphology through PKA-dependent signaling (Fig. 5). Arachidonic acid is normally released from the cell membrane in response to environmental cues and is metabolized into PGE2. Through its EP receptors, PGE2 can regulate the activity of PKA (Jiang and Dingledine 2013; Regan 2003). It has been shown that the phosphorylation of spinophilin at ser94 reduces the affinity of spinophilin to actin, causing a reduction in the stability of actin and actin-microtubule crosslinking (Xu et al. 2008; Uematsu et al. 2005). The PGE2-regulated changes in cytoskeletal stability in turn caused the abnormal actin-dependent morphologies observed in this study, including increases in neurite length and the formation of axonal loops, as well as decreases in the odds of observing turning growth cones.
Fig. 5.
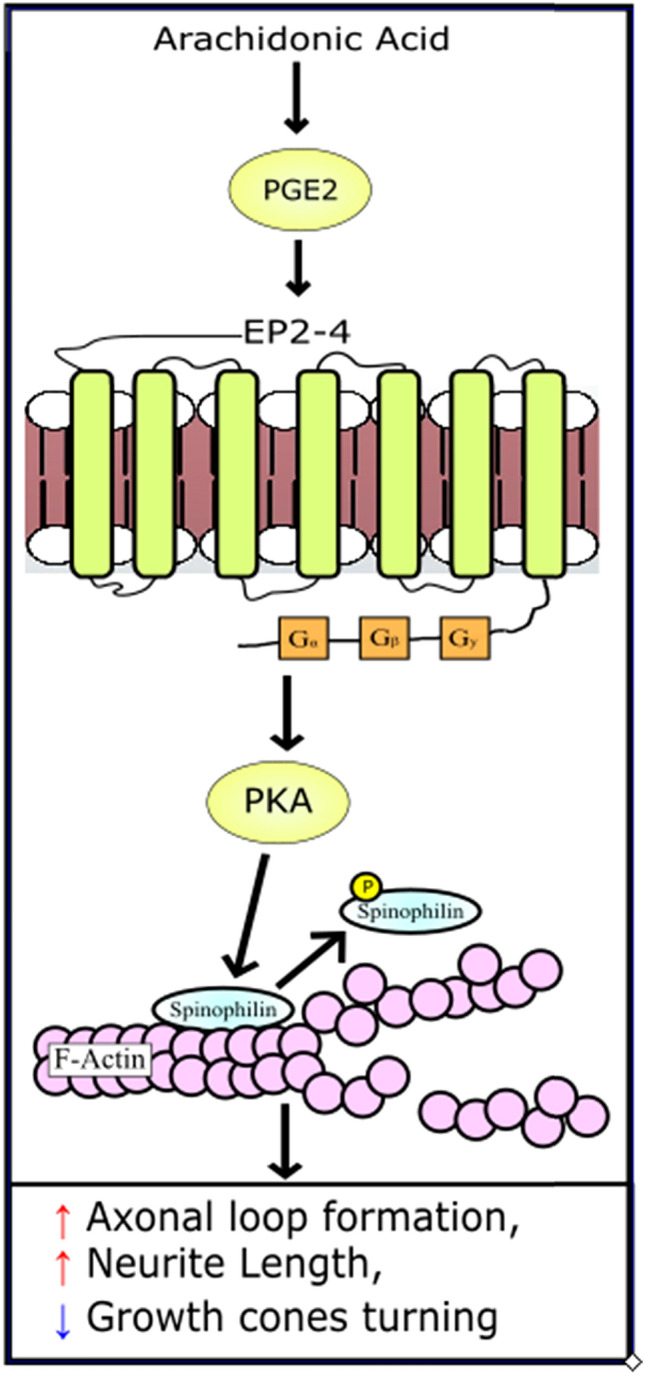
Proposed model of PGE2 effect on spinophilin. PGE2 is synthesized from arachidonic acid which is released from the cell membrane in response to various environmental factors. Literature has demonstrated that PGE2 can increase the activity of PKA through E-Prostanoid receptors. Here we have demonstrated that through PGE2, PKA can phosphorylate spinophilin. We propose that this results in dysregulation of actin stability, leading to morphologies including increased axonal loop formation and neurite length, and a decrease in growth cone turning
As PGE2 levels can be influenced by various environmental risk factors during prenatal development, this model may help to elucidate the connection between abnormal lipid signaling and the etiology of some neuropathologies (Wong et al. 2015; Wong and Crawford 2014b). The regulation of cytoskeletal dynamics is important throughout the course of development. We have provided first evidence that increases in the levels of the lipid signaling molecule PGE2 can affect these dynamics in vitro. We also provide evidence that it is PGE2-PKA signaling that contribute to certain neuronal defects due to abnormalities in cytoskeletal dynamics. We speculate that changes in PGE2 levels during critical times in development may contribute to some neuronal pathologies. These findings provide a first step in examining these changes in vivo, currently ongoing in our lab in mouse models of ASD.
Supplementary Information
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 104 kb)
Acknowledgements
We would also like to thank Dr. Greengard for donating the anti p-ser94 spinophilin antibodies (Hsieh-Wilson et al. 2003).
Funding
This study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Data Availability
All data are stored on the York university servers which can be accessed upon request.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflicts of interest or competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allen PB, Ouimet CC, Greengard P (1997) Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci 94(18):9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor FR, Oyster CW (1995) Spatial organization of retinal information about the direction of image motion. Proc Natl Acad Sci 92(9):4002–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas SL, Serneo FF, Chechlacz M, Deerinck TJ, Perkins GA, Allen PB, Ellisman MH, Gleeson JG (2007) Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell 129(3):579–591. 10.1016/j.cell.2007.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks SR, Wright CL, McCarthy MM (2007) Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons. Neuroscience 146(3):1117–1127. 10.1016/j.neuroscience.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X (2008) Tuberous sclerosis complex proteins control axon formation. Genes Dev 22(18):2485–2495. 10.1101/gad.1685008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan CS, Connor JA, Kater SB (1987) Electrically and chemically mediated increases in intracellular calcium in neuronal growth cones. J Neurosci 7(11):3588–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EM (2018) Model for coordination of microtubule and actin dynamics in growth cone turning. Front Cell Neurosci 12:394. 10.3389/fncel.2018.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Banker G (1994) Neuronal polarity. Ann Rev Neurosci 17(1):267–310. 10.1146/annurev.ne.17.030194.001411 [DOI] [PubMed] [Google Scholar]
- Davidson JM, Wong CT, Rai-Bhogal R, Li H, Crawford DA (2016) Prostaglandin E2 elevates calcium in differentiated neuroectodermal stem cells. Mol Cell Neurosci 74:71–77. 10.1016/j.mcn.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Davis O, Merrison-Hort R, Soffe SR, Borisyuk R (2017) Studying the role of axon fasciculation during development in a computational model of the Xenopus tadpole spinal cord. Sci Rep 7(1):13551. 10.1038/s41598-017-13804-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM (2012a) Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci 35(8):1218–1229. 10.1111/j.1460-9568.2012.08032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean SL, Wright CL, Hoffman JF, Wang M, Alger BE, McCarthy MM (2012b) Prostaglandin E2 stimulates estradiol synthesis in the cerebellum postnatally with associated effects on Purkinje neuron dendritic arbor and electrophysiological properties. Endocrinology 153(11):5415–5427. 10.1210/en.2012-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J (2005) Reflections on the structure of the cortical minicolumn. Neocortical modularity and the cell minicolumn. Nova Biomedical, New York, pp 57–92 [Google Scholar]
- Dent EW, Gertler FB (2003) Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40(2):209–227. 10.1016/s0896-6273(03)00633-0 [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB (2011) The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Williams G, Williams EJ (2000) CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol Cell Neurosci 16(4):283–295. 10.1006/mcne.2000.0907 [DOI] [PubMed] [Google Scholar]
- Fan J, Raper JA (1995) Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron 14(2):263–274. 10.1016/0896-6273(95)90284-8 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P (2000) Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA 97(16):9287–9292. 10.1073/pnas.97.16.9287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC (2000) Neurotrophins and the dynamic regulation of the neuronal cytoskeleton. J Neurobiol 44(2):159–173. 10.1002/1097-4695(200008)44:2%3c159::aid-neu6%3e3.0.co;2-h [DOI] [PubMed] [Google Scholar]
- Gasperini RJ, Pavez M, Thompson AC, Mitchell CB, Hardy H, Young KM, Chilton JK, Foa L (2017) How does calcium interact with the cytoskeleton to regulate growth cone motility during axon pathfinding? Mol Cell Neurosci 84:29–35. 10.1016/j.mcn.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Geraldo S, Gordon-Weeks PR (2009) Cytoskeletal dynamics in growth-cone steering. J Cell Sci 122(Pt 20):3595–3604. 10.1242/jcs.042309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Letourneau PC (2014) Actin dynamics in growth cone motility and navigation. J Neurochem 129(2):221–234. 10.1111/jnc.12506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhill GJ, Faville RA, Sutherland DJ, Bicknell BA, Thompson AW, Pujic Z, Sun B, Kita EM, Scott EK (2015) The dynamics of growth cone morphology. BMC Biol 13:10. 10.1186/s12915-015-0115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Weeks PR (2004) Microtubules and growth cone function. J Neurobiol 58(1):70–83. 10.1002/neu.10266 [DOI] [PubMed] [Google Scholar]
- Hodges H, Fealko C, Soares N (2020) Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr 9(Suppl 1):S55-s65. 10.21037/tp.2019.09.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, Rozemuller AJ, Janssen I, De Groot CJ, Veerhuis R, Eikelenboom P (2001) Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol 101(1):2–8 [DOI] [PubMed] [Google Scholar]
- Howe AK (2011) Cross-talk between calcium and protein kinase A in the regulation of cell migration. Curr Opin Cell Biol 23(5):554–561. 10.1016/j.ceb.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh-Wilson LC, Benfenati F, Snyder GL, Allen PB, Nairn AC, Greengard P (2003) Phosphorylation of spinophilin modulates its interaction with actin filaments. J Biol Chem 278(2):1186–1194. 10.1074/jbc.M205754200 [DOI] [PubMed] [Google Scholar]
- Jiang J, Dingledine R (2013) Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci 34(7):413–423. 10.1016/j.tips.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan GA, Bhagat S, Alam MI (2019) PGE(2) -induced migration of human brain endothelial cell is mediated though protein kinase A in cooperation of EP receptors. J Leukoc Biol 105(4):705–717. 10.1002/JLB.2A0918-361R [DOI] [PubMed] [Google Scholar]
- Kirkby NS, Chan MV, Zaiss AK, Garcia-Vaz E, Jiao J, Berglund LM, Verdu EF, Ahmetaj-Shala B, Wallace JL, Herschman HR, Gomez MF, Mitchell JA (2016) Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-kappaB and NFAT transcriptional pathways. Proc Natl Acad Sci USA 113(2):434–439. 10.1073/pnas.1517642113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Forscher P (1993) Cytoskeletal remodeling during growth cone-target interactions. J Cell Biol 121(6):1369–1383. 10.1083/jcb.121.6.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D (2009) The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 10(5):332–343. 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA (1993) Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75(2):217–227. 10.1016/0092-8674(93)80064-l [DOI] [PubMed] [Google Scholar]
- Maslinska D, Kaliszek A, Opertowska J, Toborowicz J, Deregowski K, Szukiewicz D (1999) Constitutive expression of cyclooxygenase-2 (COX-2) in developing brain. A. Choroid plexus in human fetuses. Folia Neuropathol 37(4):287–291 [PubMed] [Google Scholar]
- Miller KE, Suter DM (2018) An integrated cytoskeletal model of neurite outgrowth. Front Cell Neurosci 12:447. 10.3389/fncel.2018.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL (2007) The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol 64(7):945–950. 10.1001/archneur.64.7.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani K, Sekiguchi F, Maeda T, Tanaka Y, Yoshida S, Kawabata A (2016) The prostaglandin E2/EP4 receptor/cyclic AMP/T-type Ca(2+) channel pathway mediates neuritogenesis in sensory neuron-like ND7/23 cells. J Pharmacol Sci 130(3):177–180. 10.1016/j.jphs.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Mondal A, Black B, Kim YT, Mohanty S (2014) Loop formation and self-fasciculation of cortical axon using photonic guidance at long working distance. Sci Rep 4:6902. 10.1038/srep06902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Obaishi H, Satoh A, Wada M, Mandai K, Satoh K, Nishioka H, Matsuura Y, Mizoguchi A, Takai Y (1997) Neurabin: a novel neural tissue–specific actin filament–binding protein involved in neurite formation. J Cell Biol 139(4):951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nango H, Kosuge Y, Miyagishi H, Sugawa K, Ito Y, Ishige K (2017) Prostaglandin E2 facilitates neurite outgrowth in a motor neuron-like cell line, NSC-34. J Pharmacol Sci 135(2):64–71. 10.1016/j.jphs.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Norregaard R, Kwon TH, Frokiaer J (2015) Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res Clin Pract 34(4):194–200. 10.1016/j.krcp.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Pillinger MH, Abramson SB (2006) Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol (Orlando, Fla) 119(3):229–240. 10.1016/j.clim.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Park SY, Ma W, Yoon SN, Kang MJ, Han JS (2015) Phospholipase D1 increases Bcl-2 expression during neuronal differentiation of rat neural stem cells. Mol Neurobiol 51(3):1089–1102. 10.1007/s12035-014-8773-y [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna [Google Scholar]
- Radio NM, Mundy WR (2008) Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. NeuroToxicology 29(3):361–376. 10.1016/j.neuro.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Rai-Bhogal R, Ahmad E, Li H, Crawford DA (2018a) Microarray analysis of gene expression in the cyclooxygenase knockout mice—a connection to autism spectrum disorder. Eur J Neurosci 47(6):750–766. 10.1111/ejn.13781 [DOI] [PubMed] [Google Scholar]
- Rai-Bhogal R, Wong C, Kissoondoyal A, Davidson J, Li H, Crawford DA (2018b) Maternal exposure to prostaglandin E(2) modifies expression of Wnt genes in mouse brain—an autism connection. Biochem Biophys Rep 14:43–53. 10.1016/j.bbrep.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Mason C (2010) Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol 2(9):a001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JW (2003) EP2 and EP4 prostanoid receptor signaling. Life Sci 74(2):143–153. 10.1016/j.lfs.2003.09.031 [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ (2009) Cyclooxygenases: structural and functional insights. J Lipid Res 50(Supplement):S29–S34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Chen C (2006) Lipid signaling and synaptic plasticity. Neuroscientist 12(5):425–434. 10.1177/1073858406290794 [DOI] [PubMed] [Google Scholar]
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A (1998) Neurabin-II/spinophilin an actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem 273(6):3470–3475 [DOI] [PubMed] [Google Scholar]
- Schuler H, Peti W (2008) Structure-function analysis of the filamentous actin binding domain of the neuronal scaffolding protein spinophilin. FEBS J 275(1):59–68. 10.1111/j.1742-4658.2007.06171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Nguyen TD, Postler E, Meyermann R, Schluesener HJ (2000) Selective accumulation of cyclooxygenase-1-expressing microglial cells/macrophages in lesions of human focal cerebral ischemia. Acta Neuropathol 99(6):609–614 [DOI] [PubMed] [Google Scholar]
- Sdrulla AD, Linden DJ (2006) Dynamic imaging of cerebellar Purkinje cells reveals a population of filopodia which cross-link dendrites during early postnatal development. Cerebellum 5(2):105–115 [DOI] [PubMed] [Google Scholar]
- Sheng L, Leshchyns’ka I, Sytnyk V (2013) Cell adhesion and intracellular calcium signaling in neurons. Cell Commun Signal 11:94. 10.1186/1478-811x-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit D, Fouquet C, Pincet F, Zapotocky M, Trembleau A (2017) Axon tension regulates fasciculation/defasciculation through the control of axon shaft zippering. eLife. 10.7554/eLife.19907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET (2018) Understanding axon guidance: are we nearly there yet? (Cambridge, England). Development. 10.1242/dev.151415 [DOI] [PubMed] [Google Scholar]
- Tamiji J, Crawford DA (2010a) The neurobiology of lipid metabolism in autism spectrum disorders. Neuro-Signals 18(2):98–112. 10.1159/000323189 [DOI] [PubMed] [Google Scholar]
- Tamiji J, Crawford DA (2010b) Prostaglandin E(2) and misoprostol induce neurite retraction in neuro-2a cells. Biochem Biophys Res Commun 398(3):450–456. 10.1016/j.bbrc.2010.06.098 [DOI] [PubMed] [Google Scholar]
- Uematsu K, Futter M, Hsieh-Wilson LC, Higashi H, Maeda H, Nairn AC, Greengard P, Nishi A (2005) Regulation of spinophilin Ser94 phosphorylation in neostriatal neurons involves both DARPP-32-dependent and independent pathways. J Neurochem 95(6):1642–1652. 10.1111/j.1471-4159.2005.03491.x [DOI] [PubMed] [Google Scholar]
- Weiner JA, Koo SJ, Nicolas S, Fraboulet S, Pfaff SL, Pourquié O, Sanes JR (2004) Axon fasciculation defects and retinal dysplasias in mice lacking the immunoglobulin superfamily adhesion molecule BEN/ALCAM/SC1. Mol Cell Neurosci 27(1):59–69. 10.1016/j.mcn.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Wong C, Crawford DA (2014a) Lipid signalling in the pathology of autism spectrum disorders. In: Patel VB, Preedy VR, Martin CR (eds) Comprehensive guide to autism. Springer, New York, NY [Google Scholar]
- Wong C, Crawford DA (2014b) Lipid signalling in the pathology of autism spectrum disorders. Compr Guide Autism 18:1259–1283 [Google Scholar]
- Wong CT, Ahmad E, Li H, Crawford DA (2014) Prostaglandin E2 alters Wnt-dependent migration and proliferation in neuroectodermal stem cells: implications for autism spectrum disorders. Cell Commun Signal 12:19. 10.1186/1478-811x-12-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CT, Wais J, Crawford DA (2015) Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur J Neurosci 42(10):2742–2760 [DOI] [PubMed] [Google Scholar]
- Wong CT, Ussyshkin N, Ahmad E, Rai-Bhogal R, Li H, Crawford DA (2016) Prostaglandin E2 promotes neural proliferation and differentiation and regulates Wnt target gene expression. J Neurosci Res 94(8):759–775. 10.1002/jnr.23759 [DOI] [PubMed] [Google Scholar]
- Wong C, Bestard-Lorigados I, Rai-Bhogal R, Crawford DA (2017) Abnormal prostaglandin E2 signalling results in autism-associated behaviours in novel mouse models. In: Paper presented at the Society for Neuroscience, Washington DC, USA
- Wong CT, Bestard-Lorigados I, Crawford DA (2019) Autism-related behaviors in the cyclooxygenase-2-deficient mouse model. Genes Brain Behav 18(1):e12506. 10.1111/gbb.12506 [DOI] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM (2009) Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J Neurosci 29(42):13274–13282. 10.1523/JNEUROSCI.3603-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chen Y, Lu R, Cottingham C, Jiao K, Wang Q (2008) Protein kinase A phosphorylation of spinophilin modulates its interaction with the alpha 2A-adrenergic receptor (AR) and alters temporal properties of alpha 2AAR internalization. J Biol Chem 283(21):14516–14523. 10.1074/jbc.M710340200 [DOI] [PubMed] [Google Scholar]
- Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P (1999) Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP–32 and spinophilin. Nat Neurosci 2(1):13–17 [DOI] [PubMed] [Google Scholar]
- Yang HW (2009) COX-2 regulation of prostaglandins in synaptic signaling. Sheng li ke xue jin zhan [Progress in physiology] 40(4):317–320 [PubMed] [Google Scholar]
- Yoon SH, Choi J, Lee WJ, Do JT (2020) Genetic and epigenetic etiology underlying autism spectrum disorder. J Clin Med. 10.3390/jcm9040966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ (2000) Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature 403(6765):89–93. 10.1038/47501 [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Cohan CS (2001) Growth cone collapse through coincident loss of actin bundles and leading edge actin without actin depolymerization. J Cell Biol 153(5):1071–1084. 10.1083/jcb.153.5.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 104 kb)
Data Availability Statement
All data are stored on the York university servers which can be accessed upon request.