Abstract
Glioma is the most common type of primary brain malignancy with high morbidity and mortality, but little is known about its pathological mechanisms. Kinesin family member 11 (KIF11) is a key driver of malignancy in glioblastoma, a grade IV glioma, but its involvement in glioma chemoresistance remains to be determined. We accessed the TCGA open datasets, collected glioma tumor tissue samples, and analyzed the expression of KIF11 in glioma patients. Meanwhile, the correlation between KIF11 and survival outcomes was determined by the Kaplan–Meier analysis. The role of KIF11 in glioma tumor cell function was assessed in an in vitro knockdown and overexpressing system. Here, we found that KIF11 was upregulated in glioma tumors and negatively correlated with overall survival outcomes via analyzing the open datasets. KIF11 was negatively correlated with TP53 expression. Furthermore, KIF11 promoted the stemness in glioma cells, accompanied by increased cell proliferation and chemoresistance. Mechanistically, we found that KIF11 promoted cell cycle progression via upregulating cyclin expression.
Keywords: KIF11, Glioma, TP53, Cancer stem cell, Cyclin
Introduction
Glioma is the most common type of malignant human brain tumors, which represents approximately 50% of all cases (Lu and Shervington 2008). According to the nuclear morphology and histological features of cellularity, glioma is divided into 4 grades by the World Health Organization: pilocytic astrocytoma (grade I), diffuse astrocytoma (grade II), grade III anaplastic astrocytoma (grade III), and glioblastoma (grade IV) (Louis et al. 2007; Wen and Kesari 2008). When compared with other cancers, glioma is relatively rare, while its mortality is disproportionately high due to its location in the brain, which limits option of adequate surgery and other therapies (Kelly 2010). Based on the location and the malignancy degree, the current strategies for glioma treatments include surgery, radiation, chemotherapy and combined therapies (Ghosh et al. 2018). However, most glioma eventually relapse and develop drug resistance, which still represents the main reason for poor effectiveness of various therapies (Shervington et al. 2006). Therefore, it is urgent to explore the mechanism that regulates the drug resistance and disease recurrence of glioma to provide effective strategies for glioma treatments.
Kinesin family motor proteins are reported to be associated with spindle formation (Yu and Feng 2010; Wordeman 2010; Chu et al. 2020). Kinesin family member 11 (KIF11) is widely studied in various cancers. In clear cell renal cell carcinoma tissues, KIF11 mRNA levels significantly increased compared with non-cancerous tissues. Furthermore, KIF11 expression was correlated with poor prognosis and clinicopathological parameters. Inhibition of KIF11 suppressed cell proliferation, cell migration, epithelial to mesenchymal transition, and promoted cell apoptosis (Jin et al. 2019). In breast cancer, KIF11 was predicted to be an oncogene and its expression was associated with the poor prognosis of breast cancer patients. Loss of KIF11 mediated by lentivirus significantly suppressed the breast cancer cell viability, migration and invasion, and enhanced cell apoptosis. Moreover, in the nude mice model, KIF11 inhibition led to reduced tumor size and weight (Zhou et al. 2019). These studies demonstrate the effects of KIF11 in promoting tumor development in clear cell renal cell carcinoma and breast cancer. However, the biological functions of KIF11 in glioma remain unclear.
In this study, we used The Cancer Genome Atlas Program (TCGA) datasets to analyze KIF11 expression and the correlation between KIF11 and survival outcomes in glioma patients. For functional assessment, we performed knockdown and overexpression of KIF11 in glioma cell lines U87 and U251, respectively. In the current study, we found that KIF11 was upregulated in glioma tumors and negatively correlated with overall survival outcomes. KIF11 was negatively correlated with TP53 expression, and promoted stemness, proliferation and chemoresistant in glioma cells. Mechanistically, KIF11 promoted cell cycle progression via upregulating cyclin expression.
Materials and Methods
TCGA Data Processing
The KIF11 expression levels in glioma patients were analyzed in UALCAN (http://ualcan.path.uab.edu/). Kaplan–Meier plots of overall survival in glioma were analyzed in cBioPortal (http://www.cbioportal.org/, Brain Lower Grade Glioma, Firehose Legacy). The correlations between KIF11, and TP53 mutation status or cyclins, respectively, were analyzed in cBioPortal (Brain Lower Grade Glioma Firehose Legacy).
Clinical Specimens
40 pairs of normal or glioma tumor specimens were obtained from Cangzhou Central Hospital. The procedures were approved by the Ethical Review Committee of Cangzhou Central Hospital. Informed consent was acquired for all patients.
Cell Lines
Glioblastoma cell lines U87 and U251 were obtained from ATCC (Manassas, VA), and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY), and 100 µg/ml penicillin/streptomycin (Sigma, St. Louis, MO) at 37 °C in a wet atmosphere with 5% CO2.
Quantitative Real-Time PCR (qRT-PCR)
Cultured cells were collected for total RNA extraction by Trizol (Life Technologies, Pleasanton, CA) following the manufacture’s protocols. Complementary DNA (cDNA) was generated using Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA). For quantitative PCR, SYBR Green (Takara, Dalian, China) was used in each reaction. The threshold cycle (Ct) values were normalized against β-actin.
Western Blot
Cells were collected and lysed in lysis buffer (Beyotime, Shanghai, China) with a protease inhibitor cocktail (Roche, Upper Bavaria, Germany). The concentration of total protein was then determined by the BCA protein assay kit (Beyotime) following the manufacture’s protocol. A total of 60 μg protein was separated on a continuous sodium dodecyl sulfate–polyacrylamide gel electrophoresis (5–10%), followed by transferring onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA). The membranes with separated proteins were blocked with 5% skim milk for 45 min at room temperature, followed by incubating with primary antibodies at 4 °C overnight. The secondary antibodies were applied at room temperature for an additional hour. Next, chemiluminescent activity was detected using the ECL-Plus kit (Amersham Biosciences, Sunnyvale, CA) and was visualized by the Gel Documentation Systems (Biorad, Hercules, CA). The primary antibody against KIF11 was purchased from Sigma. Primary antibodies against CD133, GAPDH and anti-rabbit secondary antibody were from Abcam (Cambridge, MA).
Cell Counting
The KIF11-overexpressing U87 cells, the KIF11 knockdown U251 cells, and their relative control cells were seeded at a density of 5 × 104. After culturing for 7 days, the cells were harvested and stained with 0.04% trypan blue. Viable cells that exclude trypan blue were counted with a hemocytometer under the microscope. Assays for each group were repeated at least four times.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
To trace the proliferation rate or viability of glioma cells with or without 5-FU (Sigma-Aldrich) treatment, cells were seeded into 96-well culture plates at a density of 500 cells per well. Cells were carefully replaced into fresh culture medium with 10% MTT (5 mg/ml) at an indicated time point and then incubated for 3 h at 37 °C. After incubation, the medium was carefully removed and 200 µl dimethyl sulfoxide was added into each well. The absorbance at 570 nm was measured as the MTT reduction using the ELx800 absorbance microplate reader (BioTek). All assays were repeated independently at least 3 times, and each assay contained triplicates.
Colony Formation Assay
For colony formation assay, a total of 1000 single glioma cells were seeded onto 24-well ultralow attachment plates (Corning, Corning, NY). The cells were cultured for 7–14 days in culture medium additionally supplemented with 20 ng/mL endothelial growth factor (EGF, Invitrogen, Waltham, MA), 10 ng/mL FGF-2 (Invitrogen), B27 (Invitrogen), N2 (Invitrogen), and 1% sodium pyruvate. The number of tumor spheres was counted using an inverted microscope (Olympus, Tokyo, Japan).
Soft Agar Assay
For soft agar assays, a two-layer soft agar (upper 0.35% and lower 0.5%) was made following the protocol provided by the manufacturer. KIF11 overexpressing and control U87 cell suspensions and DMEM (1000 cells/ well) were mixed before adding the agar at 37 °C and then inoculated onto the upper layer of the agar. Cells were incubated for 2 weeks at 37 °C. The culture medium was changed every three days.
Cell Cycle Assay
The cell cycle distribution of KIF11-overexpressing and control U87 cells were stained using propidium iodide (PI, Sigma-Aldrich) and detected by a flow cytometer. Briefly, the cells were plated into a 6-well plate and cultured for 72 h. Once cells reached 80% confluency, the cells were collected and analyzed by PI staining. Then, the absorbance of PI was determined by FACS Caliber (BD, Franklin Lakes, NJ).
Statistical Analysis
The data were presented as mean ± SD of replicates. Student’s t-test was utilized to determine the difference between two groups. One-way ANOVA was used for comparisons among more than two groups. Experiments were repeated independently for three times. Significant difference was defined when p value was less than 0.05.
Results
KIF11 is Upregulated in Glioma Tumor Tissues and Negatively Correlated with Survival Outcomes in Glioma
To determine the role of KIF11 in glioma tumor development, we first explored TCGA datasets and found that KIF11 was highly upregulated in glioma tumor tissues when compared with normal tissues (p < 0.001, Fig. 1a), suggesting a potential role of KIF11 in glioma tumor development. We then analyzed the expression level of KIF11 in normal and glioma patients with different race and/or genders. As shown in Fig. 1b, the expression of KIF11 was not associated with race. Similarly, the KIF11 level was comparable between glioma patients of different genders (Fig. 1c). Based on TCGA datasets, we further found that high KIF11-expression was associated with poor survival prognosis (p < 0.001, Fig. 1d), consistent with a previous report (Geng et al. 2018). However, in the UALCAN analyzed TCGA glioma patients dataset, the KIF11 levels showed no significant influence on patients survival prognosis (Fig. 1e). To confirm the findings from existing datasets, we collected a cohort of paired normal and glioma tumor tissues and detected the expression of KIF11 by qPCR. As shown in Fig. 1f, KIF11 was significantly higher in glioma tumor tissues than that in normal tissues (p < 0.001). Also, Kaplan–Meier survival analysis found that KIF11 was negatively associated with overall survival in glioma (p = 0.0375, Fig. 1g). All these data indicated that KIF11 was upregulated and could be an indicator of overall survival in glioma.
Fig. 1.
KIF11 was upregulated in glioma tumor tissues. a The KIF11 levels in normal and glioma tumor tissues were analyzed in TCGA datasets. b The KIF11 levels in normal and glioma patients with different race were analyzed in TCGA datasets. c The KIF11 levels in normal and glioma patients with different gender were analyzed in TCGA datasets. d Kaplan–Meier plots of overall survival in glioma cancer patients stratified according to their KIF11 levels in TCGA datasets. e Kaplan–Meier plots of overall survival in glioma cancer patients stratified according to their KIF11 levels in UALCAN dataset. f The KIF11 levels in paired normal and glioma tumor tissues were analyzed by qPCR. g Kaplan–Meier plots of overall survival in glioma cancer patients stratified according to their KIF11 levels. Data are shown as mean ± S.D. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant
TP53 Acts as a Negative Regulator of KIF11 Expression
The mutation of tumor suppressor gene TP53was observed in various kinds of cancers including glioma, which often leads to TP53 loss of function and a series of transcriptional changes thereby promoting tumor development (Rivlin et al. 2011). We asked whether TP53 mutation regulated KIF11 in glioma. In this regard, we analyzed the TCGA datasets and found that KIF11 was specifically higher in glioma with TP53 mutation (49 samples) than that with normal TP53 (103 samples) (p < 0.001, Fig. 2a). Through qPCR detection, we found that KIF11 was higher in U251 than in U87, glioma cell lines with/without TP53 mutation, respectively (p < 0.001, Fig. 2b). These findings suggested KIF11 was regulated by the TP53 mutation status. Next, TP53-overexpressing plasmid was constructed and transfected into U251 cells, in which the overexpression of TP53 was verified by qPCR (p < 0.001, Fig. 2c). As expected, TP53 overexpression inhibited KIF11 in U251 cells as indicated by qPCR and Western blot analysis (p < 0.001, Fig. 2d, e). Similarly, we established low TP53 expression in U87 cells by TP53 shRNA mediating knockdown (p < 0.001, Fig. 2f). In contrast to the observations in TP53 overexpressing U251 cells, TP53 knockdown in U87 cells promoted the expression of KIF11 (p < 0.001, Fig. 2g, h). All these findings suggested an inhibitory role of TP53 in regulating KIF11 expression in glioma.
Fig. 2.
TP53 decreased KIF11 expression. a The KIF11 levels in glioma patients with mutant or wild-type TP53 status were analyzed in TCGA datasets. b The KIF11 levels in TP53 mutant U251 cells or TP53 wild-type U87 cells were analyzed by qPCR. c The mRNA expression of TP53 in U251 cells transfected with TP53 expression plasmid was determined by qPCR. d The mRNA expression of KIF11 in U251 cells transfected with TP53 expression plasmid was determined by qPCR. e The protein levels of KIF11 in U251 cells transfected with TP53 expression plasmid were determined by western blot. f The mRNA expression of TP53 in U87 cells transfected with TP53 shRNA was determined by qPCR. g The mRNA expression of KIF11 in U87 cells transfected with TP53 shRNA was determined by qPCR. h The protein levels of KIF11 in U87 cells transfected with TP53 shRNA were determined by western blot. Data are shown as mean ± S.D. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant
KIF11 Promotes Stem-Like Properties in Glioma Cells
Cancer stem cells (CSCs) are a small portion of tumor cells, which was characterized by a capacity of self-renew and a high tumorigenic ability (Bao et al. 2013). Their presence in gliomas may be responsible for tumor initiation and chemoresistance as well. To determine whether KIF11 affects the property of CSCs may be critical to understand the progress of glioma. We therefore first constructed KIF11-overexpressing U87 cells, in which KIF11 was much higher than empty-vector transfected cells by qPCR analysis (p < 0.001, Fig. 3a). Interestingly, KIF11 overexpression promoted the expression of CSC markers, such as KLF4, NANOG, OCT4 and LIN28 (p < 0.001, Fig. 3b). Consistently, spheroid assays showed that KIF11-overexpressing cells formed more spheres than control U87 cells (Fig. 3c). Furthermore, the ability of KIF11 to modulate anchorage-independent growth was determined by soft agar colony formation. As shown in Fig. 3d, the number of colonies was increased in KIF11-overexpressing cells compared to control cells (p < 0.001). Like pluripotent transcription factors shown in Fig. 3b, CD133 is a well-known glioma CSC marker, so we further detected CD133 expression in KIF11-overexpressing and control cells by qPCR and Western blot. As shown in Fig. 3e, f, CD133 was upregulated in both transcription and protein levels by KIF11 overexpression (p < 0.001). Collectively, KIF11 overexpression expedited CSC phenotypes in glioma.
Fig. 3.
KIF11 promoted glioma stem cell phenotype. a The mRNA expression of KIF11 in U87 cells transfected with KIF11 expression plasmid was determined by qPCR. b The mRNA expression of a series of stem cell markers in U87 cells transfected with KIF11 expression plasmid was determined by qPCR. c Tumor spheres formation assay of U87 cells transfected with KIF11 expression plasmid. d Soft agar colony formation assay of U87 cells transfected with KIF11 expression plasmid. e The mRNA expression of CD133 in U87 cells transfected with KIF11 expression plasmid was determined by qPCR. f The protein levels of CD133 in U87 cells transfected with KIF11 expression plasmid were determined by western blot. Data are shown as mean ± S.D. *p < 0.05; **p < 0.01; ***p < 0.001; ns not significant
KIF11 Promotes Glioma Cell Proliferation and Drug Resistance
Accumulating evidence has shown that CSCs are responsible for cell proliferation and chemoresistance. Given the role of KIF11 in regulating CSC properties, we then started to examine whether KIF11 affected the proliferation and chemoresistance of glioma cells. In terms of cell proliferation, direct cell counting and MTT assays showed that KIF11 overexpression accelerated the proliferation rate in U87 cells (p < 0.001, Fig. 4a, b). Consistently, KIF11 knockdown in U251 cells inhibited cell proliferation (p < 0.001, Fig. 4c, d). On the other hand, overexpression of KIF11 significantly increased the resistance to 5-FU in U87 cells (p < 0.001, Fig. 4e), whereas KIF11 knockdown sensitized U251 to 5-FU treatment (p < 0.001, Fig. 4f). Therefore, KIF11 promoted cell proliferation and chemoresistance in glioma.
Fig. 4.
KIF11 promoted glioma cancer proliferation and drug resistance. a Cell viability of U87 cells transfected with KIF11 expression plasmid was determined by cell count assay. b Cell viability of U87 cells transfected with KIF11 expression plasmid was determined by MTT assay. c Cell viability of U251 cells transfected with KIF11 shRNA was determined by cell count assay. d Cell viability of U251 cells transfected with KIF11 shRNA was determined by MTT assay. e Cell viability of U87 cells transfected with or without KIF11 expressing plasmid and treated with a gradient concentration of 5-FU was determined by MTT assay. f Cell viability of U251 cells transfected with or without KIF11 shRNA and treated with a gradient concentration of 5-FU was determined by MTT assay. Data are shown as mean ± S.D. *p < 0.05; **p < 0.01; ***p < 0.001; ns not significant
KIF11 Promotes Cell Cycle-Associated Gene Expression
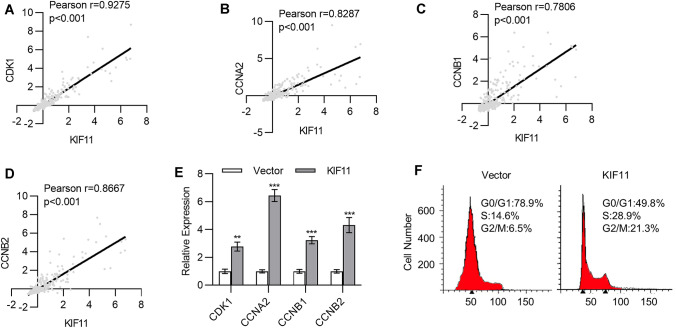
KIF11, as a kinesin, is required for the formation of the bipolar spindle in mitosis and cell cycle regulation. To answer how KIF11 regulated cancer cell characteristics in glioma, we started to analyze the gene expression associated with KIF11 using cBioportal in TCGA datasets (TCGA, Brain Lower Grade Glioma, Firehose Legacy). Among the top 100 positively correlated genes, KIF11 was positively associated with a series of cell cycle-associated genes. As shown in Fig. 5a–d, KIF11 was positively associated with a series of cyclins, such as CDK1, CCNA2, CCNB1 and CCNB2 (p < 0.001, Fig. 5a, d). Furthermore, through qPCR analysis, we found that KIF11 overexpression in U87 cells increased cyclin expression, including CDK1, CCNA2, CCNB1 and CCNB2 (p < 0.001, Fig. 5e). To this end, we speculated that KIF11 might promote cell cycle progression by enhancing cyclin expression. We next detected the cell cycle by PI staining and found that KIF11 overexpression increased the proportion cells in S phase and G2/M phase (Fig. 5f), suggesting promoted cell division. All these findings indicate that KIF11 modulated cell cycle progression by increasing cell cycle-associated gene expression.
Fig. 5.
KIF11 promoted cell cycle-associated gene expression. a–d The correlation of KIF11 with CDK1 (a), CCNA2 (b), CCNB1 (c), and CCNB2 (d) in glioma patients was analyzed in TCGA data set. e The mRNA expression of CDK1, CCNA2, CCNB1, and CCNB2 in U87 cells transfected with KIF11 expression plasmid was determined by qPCR. f Cell cycle analysis of the U87 cells transfected with KIF11 expression plasmid. Data are shown as mean ± S.D. *p < 0.05; **p < 0.01; ***p < 0.001; ns not significant
Discussion
The mortality of glioma remains high mainly due to recurrence and drug resistance (Roy et al. 2015). Therefore, exploring the mechanism that regulates the drug resistance and disease recurrence of glioma would be critical to provide effective strategies for glioma treatment. Although previous studies demonstrated that KIF11 was an important driver of glioblastoma proliferation and invasion, and CSCs were sensitive to KIF11 inhibition (Venere et al. 2015; Valensin et al. 2009). It remains unclear whether KIF11 inhibition contributes to CSC-mediated chemoresistance. In this study, we found that KIF11 was upregulated in glioma tumors and negatively associated with the overall survival of glioma patients in different cohorts. Furthermore, KIF11 was upregulated in TP53 mutant patients or glioma cells. In addition, KIF11 promoted cell cycle progression by upregulating cyclin expression. Importantly, we demonstrated the effect of KIF11 in regulating tumor stemness and drug resistance in glioma.
CSCs were commonly believed to be closely associated with drug resistance and poor response to various treatment strategies (Vinogradov and Wei 2012; Liu et al. 2020). Self-renewal and differentiation are the two important characters of stem cells. Stem cells thus possess the indefinite lifetime and could reproduce for long periods, making it prone to accumulation of mutations, eventually resulting in genetic instability (Jilkine and Gutenkunst 2014). The genetic instability and diverse tumor cell subpopulation-derived tumor heterogeneity are the main contributing factors of poor response to treatment strategies of various tumors. Several studies have demonstrated the presence of CSC subpopulation in glioma with variable differentiation status and self-renewal capacity (Sakariassen et al. 2007). Moreover, CSC-enriched glioma cells exhibited decreased sensitivity to irradiation treatment, because CSCs rapidly responded to radiation-induced DNA damage (Bao et al. 2006). Besides, numerous studies demonstrated the effect of tumor stemness in drug resistance (Abdullah and Chow 2013). Currently, temozolomide (TMZ) is the choice of chemotherapeutic agent for gliomas. However, TMZ showed no effect on the self-renewal of CD133+ glioma stem cells (Liu et al. 2006; Clement et al. 2007). The HEDGEHOG-GLI1 signaling is required for glioma stemness and the cyclopamine or lentiviral-mediated interference of this signaling altered the tumorigenicity of gliomas (Clement et al. 2007), indicating that the drug resistance of glioma may be regulated by the HEDGEHOG-GLI1 signaling-mediated stemness. In our study, we found that KIF11 promoted the expression levels of glioma stem cell markers (KLF4, NANOG, OCT4 and LIN28), sphere formation of glioma cells, soft agar colony formation and the expression of the stemness marker CD133. Meanwhile, KIF11 promoted glioma cancer proliferation and drug resistance to 5-FU. These results demonstrated that KIF11 promoted chemoresistance-associated tumor stemness in glioma.
Given that KIF11 is a kinesin spindle protein that plays an important role in cell division, it is conceivable that KIF11 may promote cell cycle progression to enhance cell proliferation. Cell cycle is defined as a series of events including DNA replication and cell division (Collins et al. 1997). During the cell cycle, various protein phosphorylation controls critical events, which requires the kinase family. The activation of kinases requires the association with the “cyclin” subunit. Therefore, the dysregulation of cyclins would influence cell cycle progression, which is widely believed to be associated with tumor development. Cancer is the disease of uncontrolled cell proliferation, which is regulated by the cell cycle machinery. Cyclin D1 was observed to be highly expressed in invasive areas of glioma (Grignon et al. 1998), and its overexpression in human glioma cell line U87 enhanced cell invasion via regulating cell motility and matrix metalloproteinase activity (Arato-Ohshima and Sawa 1999). Cyclin D1 was also shown to be a target of miR-15b in glioma, where miR-15b suppressed cell proliferation and induced cell apoptosis via mediating the disruption of cyclin D1 (Sun et al. 2014). Moreover, other cyclins were also reported to be associated with glioma (Chen et al. 2019, 2017; Deshmukh et al. 2018). In our study, we analyzed the expression levels of various cyclins (CDK1, CCNA2, CCNB1 and CCNB2) in TCGA database and found that KIF11 was positively associated with their expression. In U87 cells, the overexpression of KIF11 enhanced cyclin expression, and increased the proportion of S phase and G2/M phase cells, suggesting that KIF11 modulates cell cycle progression by increasing cell cycle-associated gene expression, cyclins in particular.
Mutations in the tumor suppressor gene TP53 that result in its loss of function have been observed in gastric cancer, breast cancer and hepatocellular carcinoma (Norberg et al. 2001; Uchino et al. 1993; Oda et al. 1992). In glioblastoma, around 30% tumors harbored TP53 mutations (Zheng et al. 2008). Tp53-dependent cell cycle arrest has been involved in mediating the sensitivity of chemotherapy (Moreno et al. 2007; Varna et al. 2009). Moreover, TP53 has been indicated as a master regulator of CSC self-renewal, differentiation and tumorigenic potential in glioblastoma (Zheng et al. 2008). It is conceivable that TP53 mutation may be involved in KIF11-upregulated stemness, cell cycle progression and chemoresistance. In our study, we found that KIF11 was negatively correlated with TP53 expression, indicating a potential interaction between them. However, the mechanism underlying KIF11 and TP53 interaction remains to be explored in future work.
Conclusion
In conclusion, we explored the biological function of KIF11 in glioma and found that KIF11 was upregulated in glioma tumors as compared with normal tissues. KIF11 negatively correlated with the overall survival of glioma patients. It should be noted that the sample size of the normal tissues sample in the TGCA analysis is relatively small in this study. The functional assay demonstrated that KIF11 promoted stemness, proliferation and drug resistance in glioma cells and was negatively correlated with TP53 expression. Mechanistically, KIF11 promoted cell cycle progression via upregulating cyclin expression.
Funding
This work was supported by Hebei Provincial Health Commission (20191770).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Ethical Review Committee of Cangzhou Central Hospital.
Informed Consent
Each informed consent was acquired.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdullah LN, Chow EK (2013) Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med 2(1):3. 10.1186/2001-1326-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arato-Ohshima T, Sawa H (1999) Over-expression of cyclin D1 induces glioma invasion by increasing matrix metalloproteinase activity and cell motility. Int J Cancer 83(3):387–392. 10.1002/(sici)1097-0215(19991029)83:3%3c387::aid-ijc15%3e3.0.co;2-o [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120):756–760. 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH (2013) Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol Chapter 61:14–25. 10.1002/0471141755.ph1425s61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DG, Zhu B, Lv SQ, Zhu H, Tang J, Huang C, Li Q, Zhou P, Wang DL, Li GH (2017) Inhibition of EGR1 inhibits glioma proliferation by targeting CCND1 promoter. J Exp Clin Cancer Res 36(1):186. 10.1186/s13046-017-0656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lin L, Xian N, Zheng Z (2019) Annexin A2 regulates glioma cell proliferation through the STAT3cyclin D1 pathway. Oncol Rep 42(1):399–413. 10.3892/or.2019.7155 [DOI] [PubMed] [Google Scholar]
- Chu C, Zhong G, Li H (2020) Structure and function of subcortical periodic cytoskeleton throughout the nervous system. STEMedicine 1(1):e9. 10.37175/stemedicine.v1i1.9 [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17(2):165–172. 10.1016/j.cub.2006.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K, Jacks T, Pavletich NP (1997) The cell cycle and cancer. Proc Natl Acad Sci USA 94(7):2776–2778. 10.1073/pnas.94.7.2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh RS, Sharma S, Das S (2018) Cyclin F-dependent degradation of RBPJ inhibits IDH1(R132H)-mediated tumorigenesis. Cancer Res 78(22):6386–6398. 10.1158/0008-5472.Can-18-1772 [DOI] [PubMed] [Google Scholar]
- Geng RX, Li N, Xu Y, Liu JH, Yuan FE, Sun Q, Liu BH, Chen QX (2018) Identification of core biomarkers associated with outcome in glioma: evidence from bioinformatics analysis. Dis Markers 2018:3215958. 10.1155/2018/3215958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Nandi S, Bhattacharjee S (2018) Combination therapy to checkmate glioblastoma: clinical challenges and advances. Clin Transl Med 7(1):33. 10.1186/s40169-018-0211-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignon Y, Duyckaerts C, Bennecib M, Hauw JJ (1998) Cytoarchitectonic alterations in the supramarginal gyrus of late onset Alzheimer’s disease. Acta Neuropathol 95(4):395–406. 10.1007/s004010050816 [DOI] [PubMed] [Google Scholar]
- Jilkine A, Gutenkunst RN (2014) Effect of dedifferentiation on time to mutation acquisition in stem cell-driven cancers. PLoS Comput Biol 10(3):e1003481. 10.1371/journal.pcbi.1003481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Dai Y, Wang Y, Zhang S, Liu G (2019) High kinesin family member 11 expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Clin Pathol 72(5):354–362. 10.1136/jclinpath-2018-205390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ (2010) Gliomas: survival, origin and early detection. Surg Neurol Int 1:96. 10.4103/2152-7806.74243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5:67. 10.1186/1476-4598-5-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yin S, Brobbey C, Gan W (2020) Ubiquitination in cancer stem cell: roles and targeted cancer therapy. STEMedicine 1(3):e37. 10.37175/stemedicine.v1i3.37 [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109. 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Shervington A (2008) Chemoresistance in gliomas. Mol Cell Biochem 312(1–2):71–80. 10.1007/s11010-008-9722-8 [DOI] [PubMed] [Google Scholar]
- Moreno CS, Matyunina L, Dickerson EB, Schubert N, Bowen NJ, Logani S, Benigno BB, McDonald JF (2007) Evidence that p53-mediated cell-cycle-arrest inhibits chemotherapeutic treatment of ovarian carcinomas. PLoS ONE 2(5):e441. 10.1371/journal.pone.0000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg T, Klaar S, Karf G, Nordgren H, Holmberg L, Bergh J (2001) Increased p53 mutation frequency during tumor progression–results from a breast cancer cohort. Cancer Res 61(22):8317–8321 [PubMed] [Google Scholar]
- Oda T, Tsuda H, Scarpa A, Sakamoto M, Hirohashi S (1992) p53 gene mutation spectrum in hepatocellular carcinoma. Cancer Res 52(22):6358–6364 [PubMed] [Google Scholar]
- Rivlin N, Brosh R, Oren M, Rotter V (2011) Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer 2(4):466–474. 10.1177/1947601911408889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Lahiri D, Maji T, Biswas J (2015) Recurrent glioblastoma: where we stand. South Asian J Cancer 4(4):163–173. 10.4103/2278-330x.175953 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sakariassen PO, Immervoll H, Chekenya M (2007) Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia 9(11):882–892. 10.1593/neo.07658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shervington A, Cruickshanks N, Wright H, Atkinson-Dell R, Lea R, Roberts G, Shervington L (2006) Glioma: what is the role of c-Myc, hsp90 and telomerase? Mol Cell Biochem 283(1–2):1–9. 10.1007/s11010-006-2495-z [DOI] [PubMed] [Google Scholar]
- Sun G, Shi L, Yan S, Wan Z, Jiang N, Fu L, Li M, Guo J (2014) MiR-15b targets cyclin D1 to regulate proliferation and apoptosis in glioma cells. Biomed Res Int 2014:687826. 10.1155/2014/687826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, Noguchi M, Ochiai A, Saito T, Kobayashi M, Hirohashi S (1993) p53 mutation in gastric cancer: a genetic model for carcinogenesis is common to gastric and colorectal cancer. Int J Cancer 54(5):759–764. 10.1002/ijc.2910540509 [DOI] [PubMed] [Google Scholar]
- Valensin S, Ghiron C, Lamanna C, Kremer A, Rossi M, Ferruzzi P, Nievo M, Bakker A (2009) KIF11 inhibition for glioblastoma treatment: reason to hope or a struggle with the brain? BMC Cancer 9:196. 10.1186/1471-2407-9-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varna M, Lehmann-Che J, Turpin E, Marangoni E, El-Bouchtaoui M, Jeanne M, Grigoriu C, Ratajczak P, Leboeuf C, Plassa LF, Ferreira I, Poupon MF, Janin A, de The H, Bertheau P (2009) p53 dependent cell-cycle arrest triggered by chemotherapy in xenografted breast tumors. Int J Cancer 124(4):991–997. 10.1002/ijc.24049 [DOI] [PubMed] [Google Scholar]
- Venere M, Horbinski C, Crish JF, Jin X, Vasanji A, Major J, Burrows AC, Chang C, Prokop J, Wu Q, Sims PA, Canoll P, Summers MK, Rosenfeld SS, Rich JN (2015) The mitotic kinesin KIF11 is a driver of invasion, proliferation, and self-renewal in glioblastoma. Sci Transl Med 7(304):304ra143. 10.1126/scitranslmed.aac6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Wei X (2012) Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond) 7(4):597–615. 10.2217/nnm.12.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359(5):492–507. 10.1056/NEJMra0708126 [DOI] [PubMed] [Google Scholar]
- Wordeman L (2010) How kinesin motor proteins drive mitotic spindle function: lessons from molecular assays. Semin Cell Dev Biol 21(3):260–268. 10.1016/j.semcdb.2010.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Feng YM (2010) The role of kinesin family proteins in tumorigenesis and progression: potential biomarkers and molecular targets for cancer therapy. Cancer 116(22):5150–5160. 10.1002/cncr.25461 [DOI] [PubMed] [Google Scholar]
- Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, DePinho RA (2008) p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 455(7216):1129–1133. 10.1038/nature07443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chen WR, Yang LC, Wang J, Sun JY, Zhang WW, He ZY, Wu SG (2019) KIF11 functions as an oncogene and is associated with poor outcomes from breast cancer. Cancer Res Treat 51(3):1207–1221. 10.4143/crt.2018.460 [DOI] [PMC free article] [PubMed] [Google Scholar]