Abstract
The purpose of this report is to validate a new protocol, the thermo-assisted drying and decontamination (TADD) system, for eliminating porcine reproductive and respiratory syndrome virus (PRRSV) from contaminated transport vehicles. Scale models of weaned pig trailers were used. The principle of TADD is to raise the interior temperature of trailers to 71°C for 30 min to promote drying and degradation of PRRSV. Trailer interiors were artificially contaminated with 5 ×105 TCID50 of PRRSV strain MN 30-100, then treated with 1 of 4 treatments: 1) TADD; 2) air only (no supplemental heat); 3) overnight (8 h) drying; and 4) washing only. Following treatment, swabs were collected from the trailer interiors at 0, 10, 20, and 30 min post-treatment and from the overnight group after 8 h. Swabs were tested for PRRSV-RNA by polymerase chain reaction (PCR). As a measure of the presence of infectious PRRSV, sentinel pigs were housed in treated trailers for 2 h post-treatment and supernatants from swabs were injected IM into naïve pigs (bioassay), the recipient pigs were then tested for PRRSV infection. All trailers were PRRSV positive by PCR immediately after washing, prior to treatment (pt). At 10 min pt, 7/10 swabs were positive from the TADD trailers; however, all swabs collected at 20 and 30 min pt were PRRSV negative by PCR, and trailer interiors were visibly dry. In contrast, 9/19, 6/10, and 6/10 swabs collected at 10, 20, and 30 min, respectively, from trailers treated with air only were positive and visibly wet. All swabs (10/10) collected from trailers treated with washing only were PRRSV positive by PCR and all swabs collected at 8 h of drying were PRRSV negative by PCR. All tests for the presence of infectious PRRSV were negative for trailers treated with TADD and overnight drying, while infectious PRRSV was detected in sentinel pigs and bioassay pigs in the other groups. Under the conditions of this study, the efficacy of the TADD system was equal to that of the overnight drying treatment, and it required a shorter period of time to complete its objective.
Résumé
Cette étude avait pour but de valider un nouveau protocole, le système de décontamination et de séchage thermo-assisté (TADD), pour éliminer le virus du syndrome respiratoire et reproducteur porcin (PRRSV) de remorques contaminées. Des modèles à l’échelle de remorques pour porcelets sevrés ont été utilisés. Le principe du TADD est d’augmenter la température intérieure des remorques jusqu’à 71 °C pour 30 min afin de favoriser le séchage et la dégradation du PRRSV. L’intérieur des remorques a été artificiellement contaminé avec 5 ×105 TCID50 de PRRSV (souche MN 30-100), puis soumis à l’un des 4 traitements suivants : 1) TADD; 2) air uniquement (pas d’apport de chaleur); 3) une nuit (8 h) de séchage; 4) lavage uniquement. L’écouvillonnage de l’intérieur des remorques a été fait 0, 10, 20 et 30 min post-traitement et pour le groupe 3 après 8 h. Les écouvillons ont été testés pour la présence d’ARN du PRRSV par réaction d’amplification en chaîne par la polymérase (PCR). Comme moyen de vérification de la présence de PRRSV infectieux, des porcs sentinelles ont été gardés dans les remorques traitées pendant 2 h post-traitement et le surnageant provenant des écouvillons a été injecté IM dans des porcs naïfs (bioessai), ces derniers étant ensuite testés pour vérifier s’ils étaient infectés par le PRRSV. Toutes les remorques étaient positives par PCR pour la présence de PRRSV immédiatement après le lavage, et avant le traitement (pt). À 10 min pt, 7/10 écouvillons pris des remorques du groupe TADD étaient positifs; toutefois, les écouvillons prélevés après 20 et 30 min étaient négatifs par PCR pour le PRRSV, et l’intérieur des remorques était apparemment sec. À l’opposé, pour les remorques traitées à l’air seulement 9/19, 6/10 et 6/10 des écouvillons prélevés après, respectivement, 10, 20 et 30 min étaient positifs et l’intérieur des remorques mouillées. Tous les écouvillons (10/10) prélevés des remorques avec lavage seulement étaient positifs par PCR pour le PRRSV et tous les écouvillons prélevés après 8 h de séchage étaient négatifs par PCR pour le PRRSV. Tous les tests pour détecter la présence de PRRSV infectieux se sont avérés négatifs pour les remorques ayant subi le traitement TADD et le séchage durant la nuit, alors que du PRRSV infectieux a été trouvé chez des porcs sentinelles et les porcs des bioessais pour les autres traitements. Parmi les conditions testées dans cette étude, l’efficacité du système TADD était la même qu’une période de séchage de 8 h, tout en étant de plus courte durée pour atteindre le même résultat.
(Traduit par Docteur Serge Messier)
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is classified in the order Nidovirales, family Arteriviridae and genus Arterivirus (1). A single-stranded enveloped RNA virus, the viability of PRRSV is reduced following exposure to increasing temperatures. Specifically, PRRSV infectivity persists for 1 to 6 d at 20°C to 21°C, 3 to 24 h at 37°C, and 6 to 20 min at 56°C (2). Over the years, PRRS has proven to be a very costly disease. In order to improve the efficacy of control and eradication measures, extensive efforts to identify potential routes of PRRSV transmission between swine farms have been attempted. Reported routes of PRRSV transmission include infected pigs, semen, contaminated fomites, insects, avian species, and aerosols (3–9). Temperature also appears to play a role in the mechanical transmission of PRRSV by fomites. Using a model to simulate producer behavior (10,11), mechanical spread of PRRSV throughout a coordinated sequence of events was more frequently observed during cold weather (< 0°C) than in warm weather (10°C to 16°C).
Recently, an evaluation of the role of the transport vehicle in the spread of PRRSV was conducted using scale models (1:150) of weaned pig trailers (12). Under the conditions of this study, it was demonstrated that PRRSV-naïve swine could become infected with PRRSV through contact with the contaminated interior of the transport models, and that the concentration of PRRSV required to infect naïve sentinel pigs was > 1 ×103 TCID50 (12). Results from these studies also suggested that PRRSV-contaminated model trailers could be consistently sanitized if allowed to dry for 12 h. However, the ability to allow trailers to dry for extended periods of time in swine production systems is cost-prohibitive, particularly in large commercial systems where sanitation programs requiring time periods greater than 2 h limit the cost-effective utilization of trailers. Therefore, protocols are needed to insure that transport trailers are dry and free of PRRSV and the risk of PRRSV transmission during transportation is reduced.
Recently, a method entitled thermo-assisted drying and decontamination (TADD) has been developed (Pig Improvement Company [PIC], Franklin, Kentucky, USA). The TADD system is based on the hypothesis that enhanced drying of PRRSV-contaminated surfaces results in the elimination of residual virus. During the TADD process, hot air is forced into the interior compartment of the trailer, increasing the surface temperature to 71°C. Based on previous data indicating the inability of PRRSV to survive beyond 6 to 20 min at 56°C (2), this temperature is then maintained for 30 min. While the principle of TADD appeared to be scientifically justified, it had not been tested. The purpose of this paper is to describe an experiment designed to validate the TADD protocol.
Materials and methods
Description of model trailers
Throughout this study, the unit of evaluation was the weaned pig trailer (12). To allow for multiple replications, the University of Minnesota Department of Biosystems and Agricultural Engineering constructed models of weaned pig trailers. These models were replicates of full-size weaned pig trailers and were built in a 1:150 scale. This scale allowed for an equivalent animal density within the model trailer (2 5-kg pigs at 0.07 m2/pig) as compared to a full-size weaned pig trailer loaded to capacity (300 5-kg pigs). Similar to the materials found in full-size trailers, the frame, roof, and exterior sidewalls of the model trailers were made of flat aluminum, the flooring consisted of polished aluminum tread-plate, and the interior walls were covered with textured styrene and insulated with foil-coated styrofoam. Each of the exterior sidewalls of the models contained openings for proper ventilation and a locking door was available on the end of each model. A comparison of the dimensions of the full-size trailer and the model can be found in Table I.
Table I.
Dimensions and parameters of scale model trailers used in the study as compared to full-size trailers
| Parameters | Scale model | Full-size trailer |
|---|---|---|
| Materials | Aluminum | Aluminum |
| Structure | Single deck | Single deck |
| Trailer inventory | 2 pigs | 300 pigs |
| Animal density | 0.07 m2/pig | 0.07 m2/pig |
| Width of trailer | 0.28 m | 2.14 m |
| Length of trailer | 0.50 m | 9.76 m |
| Height of trailer | 0.30 m | 2.50 m |
Experimental design for phase 1
The study was conducted on the University of Minnesota Swine Disease Eradication Center (SDEC) research farm. Four model trailers were employed throughout the study. A 25-m3 nursery room was chosen to house the trailer models, which were placed in adjacent pens (1 trailer/pen). To initiate each replicate, the walls, ceilings, and floors of each trailer were contaminated with PRRSV isolate MN 30-100. As in the other investigations of PRRSV transmission by transport trailers (12), the strain of PRRSV employed in the study was MN 30-100, a field isolate recovered from a persistently infected sow (13). Trailers were contaminated using a concentration of 5 ×105 TCID50 that had been prepared in 5-mL aliquots of minimum essential medium (MEM) using a hand-operated multi-use power mister (Chapin Manufacturing, Batavia, New York, USA). This high concentration of PRRSV was selected to exceed the previously determined concentration of PRRSV necessary to infect naïve sentinel pigs housed in the model trailers (12) to thoroughly test the efficacy of the TADD protocol. Prior to contamination, the floors of trailers were covered with wood shavings, a common practice in the North American seed stock industry. Following contamination, trailers were assigned 1 of 4 treatments. A total of 10 replicates were conducted for each treatment, allowing for the detection of a 35% reduction in the proportion of infected trailers at a target alpha level of 0.05 and an 80% study power.
Treatment 1 (washing only)
Consisted of a manual scraping of the interior of contaminated trailers to remove soiled bedding (bedding removal) followed by the washing of the trailer interior. Scraping of the trailer’s interior was done using a hand-held plastic scraper. To insure that mechanical spread of PRRSV did not occur between treated trailers and control trailers, the blade of the scraper was immersed in 70% ethanol, rinsed with sterile water, and swabbed between trailers. Trailers were washed for 72 s using a commercial power-washer (model number TB5030A; American Made Cleaners, Beresford, South Dakota, USA) that provided 21°C water delivered at a pressure of 20 500 kPa (12). The 72-s wash time was based on records from a US seed stock company reference indicating that the average time required to wash a full-size weaned pig trailer was approximately 2 h (12). Due to the 1:150 differential in size between the full-size trailer and the model trailer, the time used to wash a model trailers was calculated to be 72 s.
Treatment 2 (TADD)
Consisted of heating the interior of the PRRSV-contaminated trailer models to 71°C for 30 min. After trailers were contaminated, scraped, and washed as described, a 50 000 BTU/hour propane heater (Dyna-Glo; CFM RMC International, Skokie, Illinois, USA) was placed 15 cm from the rear of the trailer (Figure 1) and heating was initiated. Monitoring of trailer temperature was conducted using a hand-held laser thermometer (Raytek ST-30; Raytek Corporation, Santa Cruz, California, USA). To ascertain when the surface temperature reached 71°C and to insure that this temperature was maintained throughout each replicate, temperature data were collected every 15 min from 8 selected points in the model. Locations sampled included points 2.5-cm and 50-cm caudal to the rear opening of the model on the floor, both walls, and the ceiling. Once all points reached 71°C, diagnostic sampling was initiated. The temperature and humidity in the room and the air velocity from the heater were also measured using a Kestrel weather meter (Nielsen-Kellerman, Chester, Pennsylvania, USA).
Figure 1.
Placement of propane heater in relation to the porcine reproductive and respiratory syndrome virus (PRRSV)-contaminated model trailer during the thermo-assisted drying and decontamination (TADD) protocol.
Treatment 3 (air only)
Consisted of the movement of air without supplemental heat, into the trailer interior using a 1550-rpm split-capacity blower placed 15-cm from the trailer opening. The application of the airflow and diagnostic sampling were initiated immediately after washing. The temperature of the trailer interior, the environmental temperature and humidity, and air velocity were recorded, as previously described.
Treatment 4 (overnight drying)
Consisted of bedding removal and washing of contaminated trailers, as described in treatments 1 to 3. This was followed by an 8-h (overnight) period of drying. No disinfecting was conducted. Temperature and humidity were recorded as previously described.
Controls
Ten control replicates for each treatment were also included in the design to validate that the methods used in treating the trailers did not result in accidental contamination of the models. This consisted of sham-inoculating bedded trailers using MEM that was void of PRRSV. Trailers were then scraped, washed, and treated as previously described.
Diagnostic monitoring
To evaluate the effect of the treatments on the sanitation of the models, the interior of each trailer (0.14 cm2) was swabbed 10, 20, and 30 min following the initiation of each treatment. Specifically, TADD sampling was initiated when a temperature of 71°C was reached in trailers treated with air only or washing only. Trailers allowed to dry overnight were sampled 8 h post-treatment. Prior to sampling, swabs were moistened with MEM, drawn over the walls, floor, and ceiling using a zigzag pattern, and placed in sterile plastic tubes (Falcon, Franklin Lakes, New Jersey, USA) containing 2-mL of MEM and frozen at −70°C. Swabs were tested for the presence of PRRSV-RNA by using polymerase chain reaction (PCR) (TaqMan PCR; Perkin-Elmer Applied Biosystems, Foster City, California, USA) (14). After each replicate of each treatment was completed, trailers were re-washed, hand-dried with disposable paper towels, and swabbed to verify that the trailers were free of residual PRRSV-RNA.
Data analysis
The treatments were compared with washing only as the base treatment and drying for 8 h being the gold standard for treatment. It was expected that drying would be 100% efficacious and washing would have no effect on the likelihood of finding a positive test. A Kruskal-Wallis non-parametric analysis of variance (ANOVA) was used to test for differences between treatments.
Experimental design for phase 2: Assessment of infectious PRRSV in trailer models post-treatment
Besides testing for the presence of PRRSV-RNA, treated trailers were tested for the presence of infectious PRRSV through the placement of sentinel pigs in trailers following treatment. This was also conducted at the SDEC research farm. Animals were obtained from a source documented as PRRSV-naïve based on 10 y of clinical, diagnostic, and production data. All pigs were 3 wk old and were blood tested to insure a PRRSV-naïve status upon arrival to the study site. Sera were tested for PRRSV-antibodies by enzyme-linked immunosorbent assay (ELISA) (IDEXX ELISA; IDEXX Laboratories, Westbrook, Maine, USA). During this phase, the contamination and treatment protocols of model trailers, as previously described, were repeated and a total of 3 replicates per treatment were conducted. Following completion of each replicate, 1 PRRSV-naïve sentinel pig was housed in a treated trailer for a 2-h “transport” period. This length of time was based on data from the director of transportation of the reference seedstock company, indicating that the mean period of time required for a shipment of pigs to leave the site 1; breeding, gestation, and farrowing farm, and arrive at site 2; nursery, within the state of Minnesota was 2 h (12). Pigs were placed into the trailers at 60 min post-treatment to allow for TADD-treated trailers to properly cool. Following the 2-h period, each pig was removed from a trailer and tested for PRRSV-RNA by using PCR on day 3 and 7 post-exposure. Pigs were placed in individual pens, allocated to separate rooms according to treatment and control groups. Nose-to-nose contact between pigs was prevented at all times. Biosecurity measures were put in place to prevent the spread of PRRSV between rooms (15). These protocols included changing disposable boots, gloves, and coveralls between rooms, and 5-s immersion of boots in 6.5% sodium hypochlorite boot baths upon entering each room. Between replicates, trailers were washed, disinfected, dried, and swabbed to document the absence of residual PRRSV between replicates.
Controls
Three protocol control replicates were also conducted. This involved the use of sham-inoculated (MEM only) trailers to insure that accidental contamination of equipment and pigs did not occur.
Swine bioassay
As another means to validate the presence or absence of viable PRRSV in trailer interiors, supernatants from swabs collected from trailers in all 4 treatment groups were tested using swine bioassay (14). The swine bioassay procedure consisted of administering the sample in question to a naïve pig via intramuscular injection, followed by an assessment on whether there was a change in the PRRSV status of the naïve sentinels. To prepare the sample, 1-mL aliquots of supernatants from trailer swabs collected at 30 min post-treatment were pooled 10:1 by treatment (4 total pools, 1 pool/treatment group). Each of the 4 treatment pools was divided in half and a 5-mL aliquot of the supernatant was injected into an individual pig (2 pigs/treatment pool). Pigs were then monitored for changes in their PRRSV status. Two sham-inoculated (MEM only) negative controls were conducted as well.
Results
The study period consisted of 4 d and all of the treatments were conducted on all of the days. Testing of TADD-treated trailers was consistently initiated when the interior of the models reached 71°C, approximately 12 to 15 min following the initial application of heated air. Across the TADD replicates, the mean temperature recorded across all sampling points was 73.2°C (range 68.3°C to 81.7°C). The results from trailer interior swabs collected at 0, 10, 20, and 30 min post-treatment are summarized in Figure 2. Porcine reproductive and respiratory syndrome virus RNA was detected in all 10 replicates from all treatment groups at 0 min post-treatment. At 10 min post-treatment, PRRSV-RNA was detected in 7/10 TADD-treated trailers, 9/10 air only trailers, and 10/10 washing only trailers. At 20 min post-treatment, all swabs collected from the TADD-treated trailers were PRRSV negative by PCR and the trailer interiors were visibly dry. In contrast, the number of swabs that were PRRSV positive by PCR across the remaining treatments ranged from 6/10 (air only) to 10/10 (washing only). Trailer interiors were visibly wet in both cases. All swabs collected from TADD-treated trailers were PRRSV negative by PCR at 30 min post-treatment, while 6/10 and 10/10 swabs were PRRSV positive by PCR in the trailers treated by air only and washing only, respectively, at the same time post-treatment. As before, trailer interiors were visibly dry after treatment with the TADD system, in contrast to wet trailer interiors following the air only and washing only treatments. All swabs collected from trailers allowed to dry for 8 h and protocol controls were PRRSV negative by PCR. As in the TADD-treated trailers, the interiors were visibly dry. All swabs collected from trailers that had been re-washed and hand dried with disposable paper towels between replicates were PRRSV negative by PCR.
Figure 2.
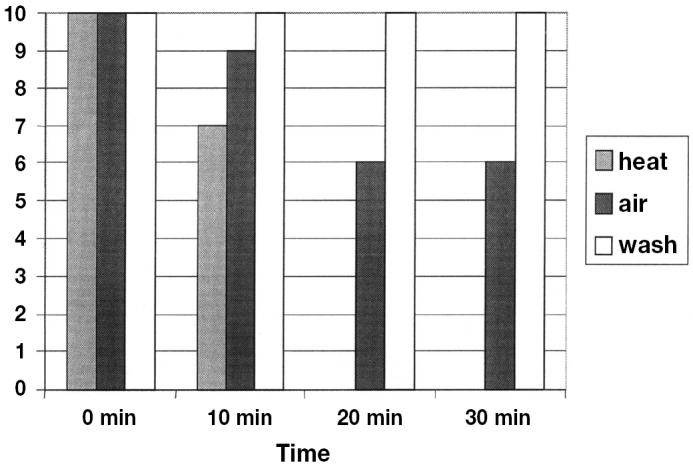
Summary of polymerase chain reaction (PCR) data recovered from trailer interiors according to treatments applied for 30 min. The Y-axis represents the number of PRRSV positive swabs detected out of a total of 10 replicates per treatment while the X-axis summarizes data from the individual treatments according to sampling time. Due to the fact that swabbing did not occur in the overnight drying treatment until 8 h post-treatment, these data are not included.
Sentinel pig testing indicated the absence of infectious PRRSV in TADD-trailers and overnight drying trailers (0/3 positive replicates per treatment). Swine bioassay testing of pooled swab supernatants also indicated the absence of infectious PRRSV-RNA in samples collected from trailers in both groups (0/2 positive replicates per treatment). In contrast, sentinel pigs became infected with PRRSV in 2/3 and 3/3 replicates following housing in air only and washing only treated trailers, respectively. Infectious PRRSV was also detected by swine bioassay in pigs inoculated with supernatants from swabs taken from air only (2/2 pigs) and washing only (2/2 pigs) treated trailers.
The mean air velocity recorded during the TADD protocol was 7.5 m/s (range 7.0 to 8.0 m/s), while that recorded during the air only treatment was 17.5 m/s (range 17.0 to 18.0 m/s). The average temperature of the air delivered by the split capacity blower was 14.5°C (range 14.0°C to 15.5°C). The environmental temperature throughout the study period and relative humidity averaged 7.7°C (range 4.5°C to 14.3°C) and 79% (range 53% to 100%), respectively.
Data analysis
At 0 and 10 min post-treatment, no significant differences were seen between treatment groups. At 20 and 30 min post-treatment, the number of PRRSV positive samples detected in TADD-treated trailers was significantly less (P ≤ 0.05) than the washing only and the air only treatment groups. No significant differences were observed between TADD-treated trailers and trailers allowed to dry overnight (P > 0.05).
Discussion
The objective of this study was to evaluate the ability of the TADD system to sanitize PRRSV-contaminated model trailers, and to compare it to other methods. As in a previous study (12), this study utilized scale models of weaned pig trailers to enhance repeatability of selected treatments. Outcomes measured included the presence or absence of PRRSV-RNA and infectious PRRSV on swabs collected from the trailer interior post-treatment and evaluation of the PRRS status of naïve sentinels that were housed in treated trailers. Results suggest that under the conditions of the study, the efficacy of the TADD system at 30 min post-treatment was equal to that of 8 h of overnight drying. Porcine reproductive and respiratory syndrome virus RNA was not detected by PCR in any of the replicates where trailer models were treated either with the TADD system or those allowed to dry for an 8-h period. In contrast, trailers treated with air only or washing only were not as effective, based on the detection of both PRRSV-RNA and infectious PRRSV by the aforementioned methods throughout the sampling period.
As with all scientific studies, this study contained several acknowledged limitations. The primary limitation is that this study was not conducted in full-size trailers carrying large loads of pigs. The models used in the study are based on weaned pig trailers and their construction/design does not mimic a trailer that transports market animals, variables that could certainly impact the level of contamination in the trailer interior and the ease of cleaning. Therefore, the TADD system requires further evaluation in full-size trailers, both of the weaned pig and market swine designs. A high concentration of PRRSV was also used to contaminate the trailers, and it is not known if this level of contamination is representative of actual transportation conditions. The entire interior of the models were contaminated, and this may not be representative of field conditions. Also, the size of the swab was not proportional to the size of the model trailer, and this may have impacted the recovery of PRRSVRNA. However, it has been previously determined that sentinel pigs can be infected with PRRSV in the model trailers when models are contaminated with concentrations of ≥ 1 ×103 TCID50. Therefore, in order to test the efficacy of the TADD system, a high concentration was desired. The study was also conducted using a specific age of pig; it was not possible to conduct the study using market age animals or adult breeding swine. It was not possible to quantify the amount of PRRSV-RNA present in samples found positive by PCR; however, there did appear to be sufficient quantity of virus to infect sentinel pigs. Therefore, a future objective may be to sample and quantify the actual concentration of PRRSV in commercial trailers since quantitative PCR assays are becoming available in certain diagnostic laboratories. Furthermore, the results of this study cannot be extrapolated to other swine pathogens, such as transmissible gastroenteritis virus or Salmonella spp., and further testing is required using alternative agents before such claims can be made.
Despite these limitations, the study had many recognizable strengths. It continued to add to the knowledge base regarding decontamination of PRRSV-contaminated transport vehicles. It also utilized supplemental diagnostic tests (sentinel pig exposure and swine bioassay) to validate the data from the PCR testing and to provide a more accurate indication if infectious PRRSV remained within trailers post-treatment. The results once again re-enforce the value of drying commercial livestock vehicles for inactivation of PRRSV and, as before, the use of scale models allowed for frequent replication of each treatment, an essential component of all transmission studies. While it was true that the trailer size and pig numbers were small, the models provided equivalent animal densities to that of a full-size trailer. Finally, whenever possible, industry standards for transport times, and wash water temperatures and pressures were used to replicate real-world situations.
In conclusion, based on the information generated through these experiments it is hoped that swine producers and practitioners will continue to understand the merit of sanitizing livestock transport vehicles, particularly when PRRSV is involved, and that these data will support the use of protocols that require drying as the basis for the decontamination process.
Acknowledgments
Funding for this study was made available by Pig Improvement Company (PIC), Franklin, Kentucky, USA.
References
- 1.Cavanagh N. Nidovirales: A new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 2.Benfield DA, Nelson E, Collins JE, et al. Characterization of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) J Vet Diagn Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- 3.Dee SA, Joo HS, Pijoan C. Controlling the spread of PRRS virus in the breeding herd through management of the gilt pool. Swine Health Prod. 1994;3:64–69. [Google Scholar]
- 4.Christopher-Hennings J, Nelson EA, Hines RJ, et al. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995;7:456–464. doi: 10.1177/104063879500700406. [DOI] [PubMed] [Google Scholar]
- 5.Otake S, Dee SA, Rossow KD, Joo HS, Deen J, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus by fomites. Swine Health Prod. 2002;10:59–65. [PubMed] [Google Scholar]
- 6.Otake S, Dee SA, Rossow KD, Moon RD, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by mosquitoes. Can J Vet Res. 2002;66:191–195. [PMC free article] [PubMed] [Google Scholar]
- 7.Otake S, Dee SA, Rossow KD, Moon RD, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica) Vet Rec. 2003;152:73–76. doi: 10.1136/vr.152.3.73. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman JJ, Yoon KJ, Pirtle EC, Sanderson TJ, McGinely MJ. Studies of porcine reproductive and respiratory syndrome virus in avian species. Vet Microbiol. 1997;55:329–336. doi: 10.1016/s0378-1135(96)01320-x. [DOI] [PubMed] [Google Scholar]
- 9.Torremorell M, Pijoan C, Janni K, Walker R, Joo HS. Airborne transmission of Actinobacillus pleuropneumoniae and porcine reproductive and respiratory syndrome virus in nursery pigs. Amer J Vet Res. 1997;58:828–832. [PubMed] [Google Scholar]
- 10.Dee SA, Deen J, Rossow KD, et al. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during warm weather. Can J Vet Res. 2003;67:12–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Dee SA, Deen J, Rossow KD, et al. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during cold weather. Can J Vet Res. 2002;66:232–239. [PMC free article] [PubMed] [Google Scholar]
- 12.Dee SA, Deen J, Otake S, Pijoan C. An assessment of transport vehicles as a source of porcine reproductive and respiratory syndrome virus transmission to susceptible pigs. Can J Vet Res. 2004;68:124–133. [PMC free article] [PubMed] [Google Scholar]
- 13.Bierk MD, Dee SA, Rossow KD, et al. Diagnostic investigation of chronic PRRS virus infection in a breeding herd of pigs. Vet Rec. 2001;148:687–690. doi: 10.1136/vr.148.22.687. [DOI] [PubMed] [Google Scholar]
- 14.Molitor TW, Tune KA, Shin J, Collins J, Kapur V. Applications of TaqMan™ PCR in the detection of porcine reproductive and respiratory syndrome virus. Proc AD Leman Swine Conf 1997: 173–175.
- 15.Dee SA, Deen J, Pijoan C. An evaluation of 4 intervention strategies to prevent mechanical transmission of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:19–26. [PMC free article] [PubMed] [Google Scholar]



