Abstract
The objective of this study was to evaluate the efficacy of commercially available disinfectants to sanitize porcine reproductive and respiratory syndrome virus (PRRSV) contaminated trailer models in cold climates (−20°C and 4°C). Disinfectants evaluated included Synergize, Aseptol 2000, Biophene, Sentramax, Virkon, Tek Trol, and DC&R. All products were applied to trailers via fumigation at 4°C. Following experimental contamination of model trailers with PRRSV MN 30-100 (5 × 105 TCID50), models were tested for the presence or absence of PRRSV-RNA by polymerase chain reaction (PCR) on swabs collected 0, 30, and 60 min after treatment. Treatments included washing only, washing plus disinfectant fumigation, washing plus fumigation, and washing plus overnight drying. The PRRSV-RNA detected across trailers ranged from 0/12 replicates in trailers treated with Synergize or allowed to dry for 8 h. These trailers were also negative for the presence of infectious PRRSV, based on the lack of sentinel pig infection (0/4 replicates). In contrast, the detection of PRRSV-positive swabs by PCR ranged from 3/12 (Aseptol) to 10/12 (Biophene). Based on these results, the efficacy of Synergize was evaluated at −20°C. In an attempt to reduce the impact of freezing on disinfectant activity, 30 mL of disinfectant was added to a 3840 mL of a 40% methanol solution, a 10% propylene glycol (PG) solution, or water alone. The PRRSV-contaminated trailers were treated with 1 of 3 disinfectant mixtures via fumigation, stored for 8 h at −20°C, allowed to thaw, and sampled as described. Trailers treated with 40% methanol or 10% PG did not freeze and were negative for PRRSV-RNA and infectious virus following thawing. In contrast, trailers treated with disinfectant and water were frozen within 60 min at −20°C, and decontamination was not successful.
Résumé
L’objectif de cette étude était d’évaluer l’efficacité de désinfectants commerciaux à décontaminer des remorques modèles contaminées par le virus du syndrome respiratoire et reproducteur porcin (PRRSV) dans des conditions climatiques froides (−20 °C et 4 °C). Les désinfectants évalués incluaient Synergize, Aseptol 2000, Biophene, Sentramax, Virkon, Tek Trol et DC&R. Tous les produits ont été appliqués aux remorques par fumigation à 4 °C. Suite à la contamination expérimentale des remorques modèles avec PRRSV MN 30-100 (5 × 105 TCID50), ces dernières furent testées pour vérifier la présence d’ARN du PRRSV par réaction d’amplification en chaîne par la polymérase (PCR) sur des écouvillons prélevés 0, 30 et 60 min après les traitements. Les traitements comprenaient un lavage seulement, un lavage plus une désinfection, un lavage plus fumigation et un lavage plus séchage durant une nuit. Le taux de détection d’ARN du PRRSV à partir des remorques était de 0/12 réplications pour les remorques traitées avec Synergize ou laissées à sécher pour 8 h. Ces remorques étaient également négatives pour la présence de PRRSV infectieux, compte tenu du fait qu’aucune infection n’est survenue chez les porcs sentinelles (0/4 réplications). La détection d’écouvillons positifs pour le PRRSV variait de 3/12 (Aseptol) à 10/12 (Biophene). Compte tenu de ces résultats, l’efficacité de Synergize a été évaluée à −20 °C. Afin de réduire l’impact du gel sur l’activité désinfectante, 30 mL de désinfectant ont été ajoutés à 3840 mL d’une solution de méthanol 40 %, de propylène glycol 10 % (PG) ou d’eau uniquement. Les remorques contaminées par le PRRSV ont été traitées avec 1 des 3 solutions de désinfectant par fumigation, laissées pendant 8 h à −20 °C puis laissées à dégeler et échantillonnées tel que décrit. Les remorques traitées avec les solutions de méthanol 40 % ou 10 % PG n’ont pas gelées et se sont révélées négatives pour la présence d’ARN du PRRSV ainsi que de virus infectieux suite au dégel des remorques. Au contraire, les remorques traitées avec le désinfectant dans l’eau étaient gelées en moins de 60 min à −20 °C et la décontamination n’était pas efficace.
(Traduit par Docteur Serge Messier)
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is classified in the order Nidovirales, family Arteriviridae, and genus Arterivirus (1). A single-stranded enveloped RNA virus, the viability of PRRSV is enhanced when stored at cold temperatures. Specifically, PRRSV is stable for months at temperatures of −20°C and infectivity persists for 1 wk at 4°C (2). Over the years, PRRS has proven to be a very costly disease, and in order to improve the efficacy of control and eradication measures, extensive efforts to identify potential routes of PRRSV transmission between swine farms have been attempted. Reported routes of PRRSV transmission include infected pigs, semen, contaminated fomites, insects, avian species, and aerosols (3–9). Recently, an evaluation of the role of the transport vehicle in the spread of PRRSV was conducted using scale models (1:150) of weaned pig trailers (10). Under the conditions of this study, it was demonstrated that PRRSV-naïve swine could become infected with PRRSV through contact with the contaminated interior of the transport models, and that the concentration of PRRSV required to infect naïve sentinel pigs was > 1 × 103 TCID50. This model also permitted the testing of a number of sanitation protocols for PRRSV-contaminated trailers (10,11). Results from these studies suggest that PRRSV-contaminated model trailers could be sanitized consistently if allowed to dry for 8 h (10) or if disinfected with a combination of 7% glutaraldehyde and 26% quaternary ammonium chloride (Synergize; Preserve International, Atlanta, Georgia, USA) for 90 min (11). During this latter study, the disinfectant was applied to the contaminated models via the process of fumigation at an environmental temperature of 20°C.
However, a limitation of this study was the inability to test the efficacy of the disinfectant at temperatures < 20°C. It is well established that the efficacy of a chemical disinfectant is reduced as the temperature decreases (12). In commercial swine farms in the Midwest US and Canada, newly washed livestock transport vehicles are often stored outdoors, with exposure to cold temperatures during certain seasons resulting in the freezing of residual wash water within the trailer interior. It has been speculated that such practices may prolong the viability of residual PRRSV present within the trailer interior after washing. In support of this theory, PRRSV infection of sentinel pigs has been demonstrated in contaminated trailer models stored at −20°C for 8 h then allowed to thaw prior to pig entry (10). Furthermore, prior to freezing, the models were disinfected with a 26% phenol disinfectant (TekTrol; Bio-Tek Industries, Atlanta, Georgia, USA), supporting the hypothesis that disinfectant activity is negatively influenced by cold temperatures.
Therefore, to further test this hypothesis, a study to evaluate the ability of disinfectants to sanitize PRRSV-positive transport vehicles under cold conditions was conducted. The study was conducted in 2 phases. Phase 1 consisted of the screening of different disinfectants at a temperature of 4°C; while the second phase consisted of testing selected disinfectants at −20°C. Selection for phase 2 testing was based on the results of disinfectant performance during phase 1.
Materials and methods
Description of model trailers
Throughout the study, the unit of evaluation was the weaned pig trailer (10). To allow for multiple replications, the University of Minnesota Department of Biosystems and Agricultural Engineering constructed models of weaned pig trailers. These models were replicates of full-size weaned pig trailers and were built in a 1:150 scale. This scale allowed for an equivalent animal density within the model trailer (2 5-kg pigs at 0.07 m2/pig) as compared to a full-size weaned pig trailer loaded to capacity (300 5-kg pigs). Similar to the materials found in full-size trailers, the frame, roof, and exterior sidewalls of the model trailers were made of flat aluminum, the flooring consisted of polished aluminum tread-plate, and the interior walls were covered with textured styrene and insulated with foil-coated styrofoam. Each of the exterior sidewalls of the models contained openings for proper ventilation and a locking door was available on the end of each model. A comparison of the dimensions of the full-size trailer and the model can be found in Table I.
Table I:
Dimensions and parameters of scale model trailers used in the study as compared to full-size trailers
| Parameters | Scale model | Full-size trailer |
|---|---|---|
| Materials | Aluminum | Aluminum |
| Structure | Single deck | Single deck |
| Trailer inventory | 2 pigs | 300 pigs |
| Animal density | 0.07 m2/pig | 0.07 m2/pig |
| Width of trailer | 0.28 m | 2.14 m |
| Length of trailer | 0.50 m | 9.76 m |
| Height of trailer | 0.30 m | 2.50 m |
Source of standards and strain of PRRSV
In an effort to replicate protocols of transport time and sanitation of transport vehicles, data from an international breeding stock company (Genetiporc, Alexandria, Minnesota, USA) were used throughout the study. This company sells breeding boars and gilts throughout North America and Latin America, operates approximately 15 transport vehicles, delivers approximately 1800 to 2000 truckloads of animals per year and conducts approximately 30 to 35 sanitation procedures per week. As in the other investigations of PRRSV transmission by transport (10,11), the strain of PRRSV employed in the study was MN 30-100, a field isolate recovered from a persistently infected sow (13).
Disinfectants
Disinfectants selected for phase 1 of the trial included Synergize (as described), TekTrol (as described), Sentramax (22% quaternary ammonium chloride; Bio-Sentry, Stone Mountain, Georgia, USA), Biophene (19.8% phenol; Bio-Sentry), Aseptol 2000 (9.2% quaternary ammonium chloride and 12.75% glutaraldehyde; SEC Repro, Quebec), Virkon (20% peroxygen; Antec International, Suffolk, United Kingdom), and DC&R (19.2% 2-hydroxymethyl-2-nitro-1,3 propanediol, 2.28% formaldehyde, and 3.08% quaternary ammonium chloride; Loveland Industries, Greeley, Colorado, USA).
Experimental design for phase 1: Screening of disinfectants at 4°C
This phase was conducted on the University of Minnesota Swine Disease Eradication Center (SDEC) research farm during a week where the mean daytime temperature was predicted to range from 0°C to 4°C. Four model trailers were employed throughout the study. A 25-m3 nursery room was chosen to house the trailer models, which were placed in adjacent pens (2 trailers/pen). To initiate each replicate, the walls, ceilings and floors of each trailer were contaminated with PRRSV MN 30-100 at a concentration of 5 × 105 TCID50 that had been prepared in 5-mL aliquots of minimum essential medium (MEM) using a hand-operated multi-use power mister (Chapin Manufacturing, Batavia, New York, USA). This high concentration of PRRSV was selected to exceed the previously determined concentration of PRRSV necessary to infect naïve sentinel pigs housed in the model trailers (10) to thoroughly test disinfectant efficacy. Prior to contamination, the floors of trailers were covered with wood shavings, a common practice in the North American seed stock industry (S. Dee, personal experience 1987–present).
Following contamination, trailers were assigned 1 of 9 treatments. A total of 12 replicates were conducted for each treatment, allowing for the detection of a 30% reduction in the proportion of infected trailers at a target alpha level of 0.05 and an 80% study power.
Treatment 1 (washing only)
Consisted of a manual scraping of the interior of contaminated trailers to remove soiled bedding (bedding removal) followed by the washing of the trailer interior. Scraping of the trailer’s interior was done using a hand-held plastic scraper. To insure that mechanical spread of PRRSV did not occur between treated trailers and control trailers, the blade of the scraper was immersed in 70% ethanol, rinsed with sterile water, and swabbed between trailers. Trailers were washed for 72 s using a commercial power-washer (model number TB5030A; American Made Cleaners, Beresford, South Dakota, USA) that provided 21°C water delivered at a pressure of 20 500 kPa (10,11). The 72-s wash time was based on records from the seed stock company reference described earlier indicating that the average time required to wash a full-size weaned pig trailer was approximately 2 h (R. Witt, Genetiporc, personal communication April 2002). Due to the 1:150 differential in size between the full-size trailer and the model trailer, the time used to wash a model trailers was calculated to be 72 s.
Treatments 2 to 8 (disinfecting via fumigation)
Consisted of the application of the previously described products. Disinfectants were delivered at manufacturer ’s recommended concentrations and applied to the trailer interior using a hurricane fogger (Curtis Dyna-Fog, Westfield, Indiana, USA). To mimic commercial transport protocols, the amount of disinfectant prepared was based on the volume of the nursery room (“garage”) that housed the 4 trailers. During fumigation, models were positioned 1 m with doors fully opened to maximize contact of the fumigant with the trailer interior. Following the release of all prepared disinfectant (10 min of release time/product), trailers were moved outside onto a concrete pad for a 60-minute contact period. Each disinfectant was evaluated 12 times, and then a new product was tested. To minimize the carry-over of residual disinfectant between replicates and between products, trailers were washed and hand dried using individual paper towels, and the nursery room airspace was purged for 1 h by means of the mechanical ventilation system.
Treatment 9 (overnight drying)
Consisted of the removal of bedding and washing the contaminated trailers, as described in treatments 1 to 8, followed by an 8-h (overnight) period of drying in a separate nursery room heated to 20°C. No disinfecting was conducted.
Controls
Twelve replications of a control were also included in the design. The purpose of the control was to validate that the methods used in treating the trailers did not result in accidental contamination of the models. This consisted of sham-inoculating bedded trailers using MEM that was void of PRRSV. Trailers were then scraped, washed, and fumigated with sterile saline using the fogger, followed by a 30-min contact period.
Diagnostic monitoring
To evaluate the effect of the treatments on the sanitation of the models, the interior of each trailer (0.14 m2) was swabbed immediately after washing (0 min), 30, and 60 min post-treatment. Trailers were allowed to dry and were sampled 8 h post-treatment. Prior to sampling, swabs were moistened with MEM; drawn over the walls, floor, and ceiling using a zigzag pattern; placed in sterile plastic tubes (Falcon, Franklin Lakes, New Jersey, USA) containing 2-mL of MEM; and frozen at −70°C. Following collection of all required samples, swabs were tested for the presence of PRRSV-RNA by qualitative PCR (TaqMan PCR; Perkin-Elmer Applied Biosystems, Foster City, California, USA) (14). After each replicate of each treatment was completed, trailers were re-washed, hand-dried with disposable paper towels, and swabbed to verify that trailers were free of residual PRRSV-RNA.
Data analysis
The treatments were compared with washing only as the base treatment and drying for 8 h being the gold standard for treatment. It was expected that drying would be 100% efficacious and washing would have no effect on the likelihood of finding a positive test. A Kruskal-Wallis non-parametric analysis of variance (ANOVA) was used to test for differences between treatments.
Assessment of infectious PRRSV in trailer models post-treatment
In addition to testing for the presence of PRRSV-RNA, treated trailers were tested for the presence of infectious PRRSV through the placement of sentinel pigs in trailers following treatment. This was conducted at the Swine Disease Eradication Center research farm. Animals were obtained from a source documented as PRRSV-naïve based on 10 y of clinical, diagnostic, and production data from the farm’s historical database. All pigs were 3 wk old and were blood tested to insure a PRRSV-naïve status upon arrival to the study site. Sera were tested for PRRSV-antibodies by using an ELISA (IDEXX 2X-R ELISA; IDEXX Laboratories, Westbrook, Maine, USA) and PRRSV-RNA by using a PCR. During this phase, the contamination and treatment protocols of model trailers described in phase 1 were repeated and a total of 4 replicates per treatment were conducted. Following completion of each replicate, 1 PRRSV-naïve sentinel pig was housed in a treated trailer for a 2-h “transport” period. This length of time was based on data from the director of transportation of the reference seedstock company that indicated that the mean period of time required for a shipment of pigs to leave the site 1; breeding, gestation, and farrowing farm, and arrive at site 2; nursery, within the state of Minnesota was 2 h (R. Witt, Genetiporc, personal communication April 2002). Pigs were placed into the trailers at 60 min post-treatment.
Four replicate controls were also conducted. The protocol involved the use of sham-inoculated (MEM only) trailers that were scraped, washed, and treated with saline using the fogger to insure that accidental contamination of equipment and pigs did not occur.
Following the 2-h period, on day 3 and 7 postexposure, each pig was removed from a trailer and tested for PRRSV-RNA by PCR and, on day 14 postexposure, for PRRSV antibodies by ELISA (IDEXX 2X-R ELISA). Pigs were placed in individual pens and allocated to separate rooms, according to treatment and control groups. Nose-to-nose contact between pigs was prevented at all times. Biosecurity measures were put in place to prevent the spread of PRRSV between rooms (15). These protocols included changing disposable boots, gloves, and coveralls between rooms, and 5-s immersion of boots in 6.5% sodium hypochlorite boot baths upon entering each room. Between replicates, trailers were washed, disinfected, dried, and swabbed to document the absence of residual PRRSV between replicates.
Experimental design for phase 2: Testing of selected disinfectants at −20°C
Following completion of phase 1, all disinfectants that were PRRSV-negative by PCR on all 12 replicates at 60 min post-treatment were selected for further testing of efficacy under freezing (−20°C) conditions. To assess the effect that prolonging the process of freezing would have on disinfectant activity, selected disinfectants were diluted to their manufacturer’s recommended concentrations in 3840 mL of 1 of 3 mixtures: water alone, a 10% propylene glycol (PG) and water solution, or a 40% methanol solution. This latter product (SPLASH windshield washer fluid [WWF], Fox Packaging Companies, St. Paul, Minnesota, USA) was certified not to freeze at temperatures of −25°C. To initiate each replicate, trailers were contaminated and washed as in phase 1, and then treated with 1 of the aforementioned 3 disinfectant mixtures. To determine whether water alone, WWF, or the 10% PG solution had any virucidal properties, trailers were treated with each mixture in the absence of disinfectant. All mixtures were applied via fumigation as described in phase 1. Trailers were then stored overnight (8 h) in a −20°C freezer (Frigidaire chest freezer, model number FFC15K1CW1; Electrolux Home Products, Cleveland, Ohio, USA). The next day, trailers were allowed to thaw at 20°C and tested for the presence of PRRSV-RNA by PCR and infectious PRRSV by sentinels, as previously described. As in phase 1, a wash only and an overnight drying treatment were included in phase 2. A total of 20 replicates were conducted for each treatment, allowing for the detection of a 50% reduction in the proportion of infected trailers at a target alpha level of 0.05 and an 80% study power. Four replicates of sentinel pig exposure were conducted per treatment.
Controls
Positive controls consisted of testing the efficacy of all mixtures outside of the trailer model. To do this, 45 mL aliquots of water alone, WWF, and 10% PG (with and without disinfectant) were placed in plastic containers and spiked with 5 mL (5 × 105 TCID50) of PRRSV MN 30-100. Containers were frozen for 8 h at −20°C, and thawed. A 5-mL aliquot of each mixture was removed and submitted for PCR testing. Negative controls consisted of sham-inoculated mixtures. A total of 6 replications were conducted for each control mixture.
Data analysis
The treatments were compared with washing only as the base treatment and drying for 8 h being the gold standard for treatment. It was expected that drying would be 100% efficacious and that washing would have no effect on the likelihood of finding a positive test. A Kruskal-Wallis non-parametric ANOVA was used to test for differences between treatments.
Results
Phase 1
During the week that the study was conducted, the average daily temperature ranged from 4°C to 6°C (mean 4.5°C) with a relative humidity ranging from 66% to 100% (mean 78%). As recommended, all disinfectants were applied at a 1:128 concentration except for Virkon, which was applied at a 1% concentration, according to label recommendations. The results from trailer interior swabs collected at 0, 30, and 60 min post-treatment are summarized in Figure 1. Porcine reproductive and respiratory syndrome virus-RNA was detected in all 12 replicates from all treatment groups at 0 min posttreatment. At 30 min post-treatment, PRRSV-RNA was detected across all treatments, ranging from 2/12 positive (Synergize) to 12/12 positive (Biophene). At 60 min post-treatment, all swabs collected from the trailers treated with Synergize were negative for PRRSV by PCR. In contrast, the number of PRRSV positive swabs by PCR across the remaining disinfectant treatments ranged from a low of 3/12 (Aseptol 2000) to a high of 10/12 (Biophene) at 60 min post-treatment. All swabs were PRRSV positive by PCR 60 min post-treatment in the wash only group. All swabs collected from trailers allowed to dry for 8 h and the controls were negative for PRRSV by PCR. All swabs collected from trailers that had been re-washed and hand dried with disposable paper towels between replicates were PRRSV negative by PCR.
Figure 1.
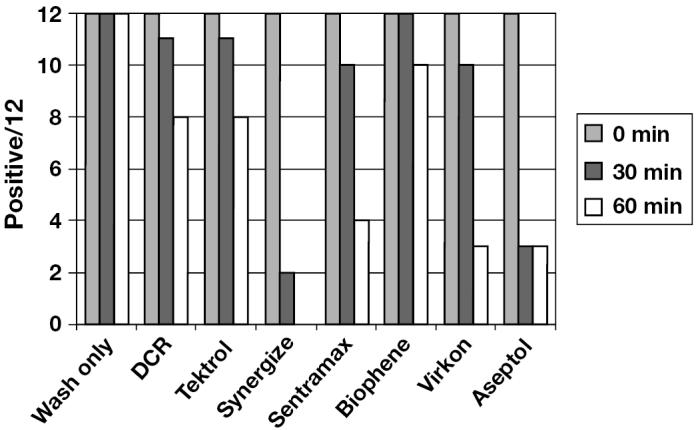
Summary of polymerase chain reaction (PCR) results from phase 1. The Y-axis represents the number of PCR positive swabs out of the 12 replicates of each treatment, while the X-axis summarizes the various treatments.
Sentinel pig testing indicated the absence of infectious PRRSV following treatment with Synergize (0/4 positive), Aseptol 2000 (0/4 positive), and overnight drying (0/4 positive). At least 1 sentinel pig became infected with PRRSV during the 2-h holding period in trailers treated with the other products, including 3 positive replicates in the wash only group.
Data analysis
All samples at 0 min were found to be positive. At 30 min only 2 disinfectants (Synergize, Aseptol 2000) had a significant effect (P < 0.05). At 60 min only 1 disinfectant showed no positives (Synergize), though 4 disinfectants (Synergize, Aseptol 2000, Sentramax, Virkon) showed a significant decrease in rates of positives (P < 0.05). However, due to the limited sample size, the 4 disinfectants were not significantly different from each other, or from drying (Figure 1).
Phase 2
Based on the previously described selection criterion (12/12 swabs were PRRSV negative by PCR at 60 min post-treatment), Synergize was the only product selected for testing in phase 2. Thirty milliliters was added to 3840 mL of the various mixtures (WWF, 10% PG, or water alone), providing a 1:128 concentration. Trailers that were treated with mixtures containing water alone or water plus disinfectant were visibly frozen within 60 min following placement in the −20°C freezer. In contrast, residual liquid in the interior of trailers treated with mixtures containing 10% PG remained in a semi-solid (slush) consistency, and freezing was completely prevented in trailers treated with mixtures containing WWF. Similar observations were made in the control containers.
The serum PCR results are summarized in Figure 2. All 20 swabs collected from trailers treated with disinfectant plus WWF or disinfectant plus 10% PG were negative for PRRSV by PCR, and there was no evidence of infection in sentinel pigs. Similar results were seen in trailers that were allowed to dry overnight. However, PRRSVRNA (17/20 replicates) and infectious PRRSV (2/4 replicates) were present following treatment with WWF only (no disinfectant added). Similarly, PRRSV-RNA was detected in 18/20 trailers treated with 10% PG alone; along with evidence of PRRSV infection in 2/4 sentinel pigs. In contrast, trailers treated with water alone plus disinfectant demonstrated evidence of PRRSV-RNA (4/20 replicates) and infectious PRRSV (1/4 replicates). Finally, treatment with water alone (no disinfectant added) resulted in the detection of PRRSV-RNA in 19/20 replicates, as well as 3/4 sentinel pig replicates.
Figure 2.
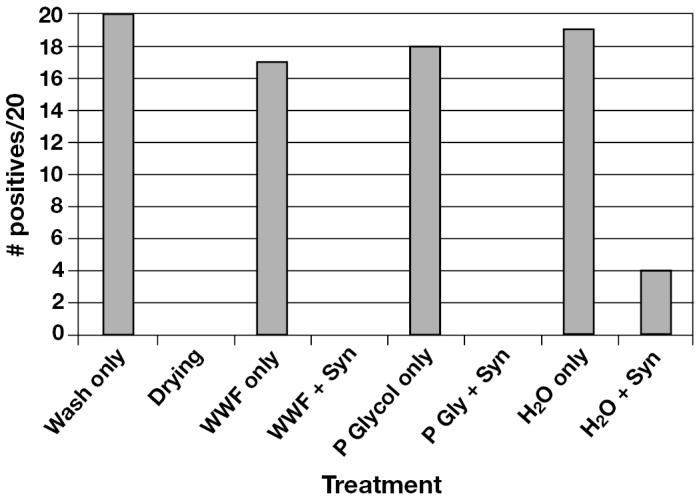
Summary of polymerase chain reaction (PCR) data from phase 2. The Y-axis represents the number of PCR-positive swabs detected out of a total of 20 replicates per treatment, while the X-axis summarizes the individual treatments.
WWF — windshield washer fluid (40% methanol); Syn — Synergize; P Glycol — 10% propylene glycol
Data analysis
Only treatments involving Synergize showed a significant decrease in the probability of positive swabs (P < 0.05), as compared to treatments without Synergize. Treatments using Synergize did not have a significantly higher level of positives than the gold standard of drying.
Discussion
The objective of this study was to evaluate the ability of commercially available disinfectants to sanitize PRRSV-contaminated model trailers at temperatures of 4°C and −20°C. As in previous studies (10,11), this study utilized scale models of weaned pig trailers to enhance replication of selected treatments. Outcomes measured included the presence or absence of PRRSV-RNA, as detected on swabs collected from the trailer interior post-treatment, and the evaluation of PRRS status of naïve sentinels that were housed in treated trailers. Results suggest that under the conditions of the study, the efficacy of the disinfectants at the temperatures tested was variable. In phase 1, PRRSV-RNA was not detected by PCR in any of the replicates where trailer models were treated either with Synergize and those allowed to dry for an 8-h period. Due to limited sample sizes, it was not possible to statistically differentiate between Synergize, Sentramax, Aseptol 2000, and Virkon. However, it must be pointed out that infectious PRRSV was found in models treated with Sentramax and Virkon, but not in trailers treated with Synergize and Aseptol 2000.
With regard to phase 2, treatment of trailers with Synergize in combination with WWF or in combination with 10% PG resulted in no detectable evidence of PRRSV-RNA or infectious PRRSV. In contrast, both outcomes were observed in trailer models treated with Synergize and water. This may have been due to the addition of WWF or PG, which reduced or prevented freezing of the trailers, thereby prolonging the activity of the disinfectant. In contrast, trailers treated with a mixture of Synergize and water were visibly frozen within 60 min and the activity of the disinfectant appeared to be reduced under these conditions, based on the detection of both PRRSV-RNA and infectious PRRSV. Finally, the efficacy of overnight drying for eliminating PRRSV from contaminated trailers was again demonstrated and the results supported previously published data (10,11).
As with all scientific studies, this study contained several acknowledged limitations. The primary limitation in phase 1 was inadequate sample size to statistically differentiate the efficacy of 4 of the treatments, while in phase 2, only 1 disinfectant was tested based on the selection criterion. Most importantly, it must be remembered that this study was not conducted in full-size trailers carrying large loads of pigs. The models used in the study are based on weaned pig trailers and their construction/design does not mimic a trailer that transports market animals, variables that could certainly impact the level of contamination in the trailer interior and the ease of cleaning. In both phases, the disinfectants were applied via fumigation, and this method may not be an efficacious means of delivering disinfectants in full size trailers. Also, detergents were not employed and the use of such products may have affected the results. Therefore, these protocols need further evaluation in full-size trailers, both of the weaned pig and market swine designs. A high concentration of PRRSV was used to contaminate the trailers and it is not known if this level of contamination is representative of actual transport conditions, nor is it known whether the delivery method (hand sprayer) for the purpose of vehicle contamination is representative of swine aerosols. The entire interior (ceilings, walls, floors) of the models were contaminated, and this may not be representative of field conditions where pigs are less likely to be able to contaminate all surfaces. Also, the size of the swab was not proportional to the size of the model trailer, and this may have had an impact on the recovery of PRRSV-RNA. However, it has been previously determined that sentinel pigs can be infected with PRRSV in the model trailers when models are contaminated with concentrations of ≥1 × 103 TCID50. Therefore, in order to test the efficacy of the decontamination protocol, a high concentration was desired. The study was also conducted using a specific age of pig; it was not possible to conduct the study using market age animals or adult breeding swine. Due to budgetary constraints, it was not possible to quantify the amount of PRRSV-RNA present in samples positive by PCR or do further assessments to determine the infectious dose of PRRSV that remained in the trailer post-treatment. However, in phase 2, there did appear to be sufficient quantity of virus to infect some of the sentinel pigs. Therefore, a future objective may be to sample and quantify the actual concentration of PRRSV in commercial trailers since quantitative PCR assays are becoming available in certain diagnostic laboratories. Furthermore, the results of this study cannot be extrapolated to other swine pathogens, such as transmissible gastroenteritis virus or Mycoplasma hyopneumoniae, and further testing is required using alternative agents before such claims can be made. Finally, it must be remembered that the WWF product used in the study contained 40% methanol (wood alcohol), and a 100% methanol concentration can cause blindness in humans when ingested orally. Prior to initiating the study, the primary author consulted with his personal physician regarding the risk that the 40% product may provide to study personnel during the fumigation process. It was the opinion of the health care professional that, while the fumes generated during the process may serve as a mild topical irritant, the concern was negligible. Based on personal experience during the trial, the author tends to agree with this assessment, yet it is important to raise the awareness of this possible risk factor.
Despite these limitations, the study had recognizable strengths. It continued to add to the base of knowledge about sanitizing PRRSV-contaminated transport trailers and, in contrast to previous work (10,11), this study evaluated disinfectant activity under conditions not previously addressed. It verified once more that Synergize is an effective disinfectant against PRRSV. This may be due to the combination of the quaternary ammonium chloride that acts on the viral envelope, thereby permitting the glutaraldehyde to enter the cell and exert its effects on the viral nucleic acid (16). Supplemental diagnostic tests (sentinel pig exposure) were also used to validate the data from the PCR testing and to provide a more accurate indication if infectious PRRSV remained within trailers post-treatment. The results reinforce the value of drying commercial livestock vehicles for inactivation of PRRSV and, as before, the use of scale models allowed for frequent replication of each treatment, an essential component of all transmission studies. While it was true that the trailer size and pig numbers were small, the models provided equivalent animal densities to that of a full-size trailer. Finally, whenever possible, industry standards for transport times, and wash water temperatures, pressures, as well as disinfecting products and practices were used to replicate real-world situations, including the presence of organic debris after washing. While the use of detergents may reduce the frequency of this event, these products were not included in this study.
In conclusion, based on the information generated through these experiments it is hoped that swine producers and practitioners will continue to understand the merit of sanitizing livestock transport vehicles, particularly when PRRSV is involved, and that the these data will assist them in the selection of efficacious products and protocols.
Acknowledgments
Funds for this study were made available through the Boehringer- Ingelheim PRRS research initiative.
References
- 1.Cavanagh N. Nidovirales: A new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 2.Benfield DA, Nelson E, Collins JE, et al. Characterization of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) J Vet Diagn Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- 3.Dee SA, Joo HS, Pijoan C. Controlling the spread of PRRS virus in the breeding herd through management of the gilt pool. Swine Health Prod. 1994;3:64–69. [Google Scholar]
- 4.Christopher-Hennings J, Nelson EA, Hines RJ, et al. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995;7:456–464. doi: 10.1177/104063879500700406. [DOI] [PubMed] [Google Scholar]
- 5.Otake S, Dee SA, Rossow KD, Joo HS, Deen J, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus by fomites. Swine Health Prod. 2002;10:59–65. [PubMed] [Google Scholar]
- 6.Otake S, Dee SA, Rossow KD, Moon RD, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by mosquitoes. Can J Vet Res. 2002;66:191–195. [PMC free article] [PubMed] [Google Scholar]
- 7.Otake S, Dee SA, Rossow KD, Moon RD, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica) Vet Rec. 2003;152:73–76. doi: 10.1136/vr.152.3.73. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman JJ, Yoon KJ, Pirtle EC, Sanderson TJ, McGinely MJ. Studies of porcine reproductive and respiratory syndrome virus in avian species. Vet Microbiol. 1997;55:329–336. doi: 10.1016/s0378-1135(96)01320-x. [DOI] [PubMed] [Google Scholar]
- 9.Torremorell M, Pijoan C, Janni K, Walker R, Joo HS. Airborne transmission of Actinobacillus pleuropneumoniae and porcine reproductive and respiratory syndrome virus in nursery pigs. Am J Vet Res. 1997;58:828–832. [PubMed] [Google Scholar]
- 10.Dee SA, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res 2004;(in press). [PMC free article] [PubMed]
- 11.Dee SA, Deen J, Burns D, Douthit G, Pijoan C. An assessment of sanitation protocols for commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:208–214. [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips JE. Physical methods of veterinary disinfection and sterilization. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. Blackwell Scientific Publications Ltd, 1st ed, Oxford 1987:117–143.
- 13.Bierk MD, Dee SA, Rossow KD, et al. Diagnostic investigation of chronic PRRS virus infection in a breeding herd of pigs. Vet Rec. 2001;148:687–690. doi: 10.1136/vr.148.22.687. [DOI] [PubMed] [Google Scholar]
- 14.Molitor TW, Tune KA, Shin J, Collins J, Kapur V. Applications of TaqManTM PCR in the detection of porcine reproductive and respiratory syndrome virus. Proc AD Leman Swine Conf 1997: 173–175.
- 15.Dee SA, Deen J, Pijoan C. Evaluation of 4 intervention strategies to prevent mechanical transmission of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:19–26. [PMC free article] [PubMed] [Google Scholar]
- 16.Russell AD, Hugo WB. Chemical disinfectants. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. Blackwell Scientific Publications Ltd, 1st ed, Oxford 1987:12–42.


