Abstract
Excessive mitophagy plays a role in neuronal death in spinal cord injury (SCI), its molecular regulation remains largely unknown. The present study aims to determine the role of NIX, a member of a unique subfamily of death-inducing mitochondrial proteins, in the regulation of mitophagy in SCI. Here we show that NIX is highly upregulated in SCI and hypoxia, and localized to mitochondria. The mitochondria-bound NIX interacts with autophagosome-localized LC3 (Microtubule-associated protein 1 light chain 3) to form a mitochondria-NIX-LC3-autophagosome complex, resulting in excessive mitophagy in SCI. Downregulation of NIX by RNA interference restores the function of mitochondria in spinal cord neurons under hypoxia. Importantly, inhibition of NIX improves recovery of locomotor function in rats after SCI. The present study demonstrates that NIX interacts with LC3 to activate excessive mitophagy in SCI. Inhibition of NIX is therefore likely a neuroprotective strategy.
Keywords: NIX, LC3, Mitophagy, Hypoxia, Spinal cord injury
Introduction
Mitophagy is a form of autophagy that selectively removes damaged mitochondria (Youle and Narendra 2011). Although physiological levels of mitophagy are required in mitochondrial quality control in all cells, excessive mitophagy has, however, been implicated in rapid neuronal death in traumatic brain injury and cerebral ischemia (Chakrabarti et al. 2009; Shi et al. 2012; Zhao et al. 2017).
The Bcl-2/E1B-19KD-interacting protein 3 like (BNIP3L), also known as NIX, is a member of the Bcl2 family and is located in the outer member of mitochondria (Kubli et al. 2007; Zhu et al. 2013). NIX binds to the mitochondrial membrane through a transmembrane domain (TM) at its C-terminus, and directly interacts with microtubule-associated protein light chain 3 (LC3) to induce mitophagy in several cell types (Kanki 2010; Noda et al. 2010; Novak et al. 2010; Schwarten et al. 2009). Previously, we reported that NIX was upregulated in SCI in rats (Yu et al. 2013). In this study, we have tested the hypothesis that NIX mediates excessive mitophagy in SCI in rats. We show that NIX interacts with LC3 in the neuronal mitophagy, whereas downregulation of NIX by RNA interference restores the function of mitochondria in spinal cord neurons under hypoxia. We have also found that inhibition of NIX improves recovery of locomotor function in rats after SCI. Thus, our data provides significant insight into regulation of neuronal mitophagy in SCI.
Materials and Methods
Animal Model of SCI
28 Male SD rats (weight 220 ~ 250 g) were purchased from the experimental animal center of Third Military Medical University, Chongqing, China. They were randomly divided into seven groups: a sham group and 6 SCI (6 h, 12 h, 24 h, 48 h, 72 h and 96 h) groups, with four animals in each group (Yu et al. 2013). The animal protocol was approved by the Animal Care and Ethics Committee of the Third Military Medical University (SYXK-PLA-20120031), whose standards meet the Animal Care guidelines of the NIH, USA. The rats were fixed on an operating table after intraperitoneal anesthesia with sodium pentobarbital (50 mg/kg, i.p.) and the thoracic 9–11 vertebral bodies were exposed. Then the thoracic 10 spinal cord segment was exposed by spinal laminectomy. After that the Weight-drop model (Grossman et al. 1999), (Byrnes et al. 2007) was used to induce spinal cord injury. Briefly, a weight weighing 15 g fell freely from a height of 30 mm onto the exposed T10 spinal cord and retain for 2 min. In the sham group only the lamina was resected to expose the spinal cord without spinal cord injury. During the whole experiment, a heating plate was used to keep the temperature of the rats at 37 °C. Postoperative care was given to defecate and urinate until a reflex bladder was established. Penicillin (80,000 units, 4 ml/day) was intraperitoneally injected for 3 consecutive days.
Spinal Cord Neurons Culture and Hypoxia
Separation of the primary spinal cord neurons: the spinal cords were taken from the embryo fetal rats at day 14(E14) under aseptic conditions and digested with 0.25% trypsin at 37 °C for 10 min, making them into single-cell suspensions, then 200 mesh sieves were used to make the cells fully separated. After that cells were resuspended with neuronal medium which containing 1% neuronal growth supplement (ScienCell, Research Laboratories, Carlsbad, CA). After poly (D-lysine)—coated round coverslips were put into 12-well plates, the spinal cord neurons were planted onto the coverslips at a density of 2 × 105 cells/ml. Then the neurons were cultured in an CO2 Incubator (SANYO Electric Co., Ltd., Sakata, JAPAN) containing 5% CO2 and 95% air at 37 °C. For hypoxic treatment, the neurons were placed in a CO2 Incubator (Thermo Fisher Scientific, Waltham, USA) with a mixture of 1% O2, 5%CO2 and 94% N2.
Protein Extraction
Samples of spinal cords (10 mm) including the epicenter of injury were removed from rats quickly after the rats were anesthetized at different time points (sham, 6 h, 12 h, 24 h, 48 h, 72 h and 96 h) after injury. Total protein of the spinal cords was extracted using T-PER™ Tissue Protein Extraction Reagent (Thermo Fisher Scientific, Waltham, USA, 78,510). For cultured cells, total protein was extracted by lysing the cells with 1 × RIPA lysate (Beyotime biotechnology, Shanghai, China, P0013B). Halt™ protease and phosphatase inhibitor cocktail (100X) (Thermo Fisher Scientific, Waltham, USA, 78,445) were used in the protein extraction processes. Then the BCA Protein Assay (Beyotime biotechnology, Shanghai, China, P0010S) was used to determine the protein concentration according to the manufacturer’s instructions.
Western Blotting
Based on protein concentration determined by the BCA Protein Assay, each sample was diluted to the same concentration with 1 × loading buffer (Bio-Rad, California, USA, 1,610,747). The method of equal quality and equal quantity was adopted when the proteins (10 μl, 30 μg) were separated by electrophoresis on 4–20% or 12% SurePAGE™ Bis–Tris Protein Gels (GenScript, Nangjing, China, M00656/M0066) and transferred onto the polyvinylidene fluoride (PVDF) membranes (Millipore, Massachusetts, USA, IPVH00010) using the Trans-Blot® Turbo™ Transfer System (Bio-Rad, California, USA). Then the PVDF membranes were blocked with a moderate amount of Quickblock™ Blocking Buffer (TBSTw) (Beyotime biotechnology, China, P0231) for 30 min at room temperature. After that one of the following primary antibodies was incubated with the PVDF membranes at 4 °C overnight: rabbit anti-NIX polyclonal antibody ab8399 (1:2000, Abcam, Cambridge, MA, USA); rabbit anti-LC3 polyclonal antibody ab48394 (1:500, Abcam, Cambridge, MA, USA); rabbit anti-GFP polyclonal antibody ab6556 (1:2000, Abcam, Cambridge, MA, USA); rabbit anti-RFP polyclonal antibody ab62341 (1:1000, Abcam, Cambridge, MA, USA); rabbit anti-COX4 polyclonal antibody A6564 (1:1000, ABclonal, Wuhan, China,); rabbit anti-TOMM20 polyclonal antibody A6774 (1:1000, ABclonal, Wuhan, China); mouse anti-β-actin monoclonal antibody AC004 (1:5000, ABclonal, Wuhan, China) or rabbit anti-α-tubulin polyclonal antibody AC007 (1:5000, ABclonal, Wuhan, China). The PVDF membranes were then washed for 5 times with TBST (Tris-Buffered Saline with 0.1% Tween-20) and incubated with an appropriate HRP-conjugated secondary antibody at room temperature for 1 h, followed by washing for 5 times with TBST. Subsequently, the membranes were incubated with the chemiluminescent reagent WBKLS0100 (Millipore, Massachusetts, USA) and the protein bands were visualized on a ChemiDoc™ Touch Imaging System (Bio-Rad, California, USA). The gray value of the protein bands was measured by the ImageJ software (NIH, Bethesda, CA) and the Image Lab software (Bio-Rad, California, USA).
Immunoprecipitation
Cultured spinal cord neurons were collected for whole-cell protein extraction and Co-IP using the Universal Magnetic Co-IP Kit AM54002 (Active Motif, California, USA) according to the manufacturer’s instructions. Antibodies including rabbit anti-GFP polyclonal antibody ab6556 (1:100, Abcam, Cambridge, MA, USA) and rabbit anti-RFP polyclonal antibody ab62341 (1:100, Abcam, Cambridge, MA, USA) were used in the antibody incubation steps. Then the protein samples were analyzed by Western blot.
Immunofluorescence
Cultured neurons on coverslips were fixed with 4% paraformaldehyde (BL539A, Biosharp, Hefei, China), penetrated with 0.3% Triton X-100 (ST795, Beyotime biotechnology, Shanghai, China), washed with PBS (phosphate buffered saline) (C20012500BT, Gibco, Thermo Fisher Scientific, Suzhou, China) and blocked with 5% BSA (AC0004, Boster biotechnology, Wuhan, China). Then one of the following antibodies was incubated with the cells at 4 °C overnight: rabbit anti-LC3 polyclonal antibody ab48394 (1:200, Abcam, Cambridge, MA, USA); mouse anti-TOMM20 monoclonal antibody ab56783 (1:50, Abcam, Cambridge, MA, USA); mouse anti-NeuN monoclonal antibody MAB377 (1:100, Millipore, Massachusetts, USA). On the second day, the slides were washed with PBS and incubated with one of the following secondary antibodies: goat anti-rabbit IgG(H + L) Alexa Fluor 488 (A0423, 1:200, Beyotime biotechnology, China), donkey anti-rabbit Alexa Fluor 555 IgG (H + L) (A0453, 1:200, Beyotime biotechnology, China), donkey anti-mouse Alexa Fluor 555 IgG(H + L) (A0460, 1:200, Beyotime biotechnology, China), rabbit anti-mouse IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 647 (A-21239, Invitrogen™, Thermo Fisher Scientific, USA). The nuclei were stained with 2-(4-Amidinophenyl)-6-indolecarbamidine –dihydrochloride (DAPI, C1005, Beyotime biotechnology, China,). Fluorescence signals were captured by a laser-scanning confocal microscope (Leica SP8, Leica Microsystems, Wetzlar, Germany). Each of the immunofluorescent experiment was repeated at least 3 times.
Immunocytochemistry
Twenty-eight days after SCI, rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg, i.p.) and fixed on an operating table, then perfused with normal saline, followed with 4% paraformaldehyde in PBS (0.1 M, pH 7.4). Spinal cord tissue with a length of about 1 cm centered on the injured area was carefully dissected out from each rat. Hematoxylin and eosin staining was performed on spinal cord tissues after paraffin embedding, sectioning and dewaxing and hydration. The sections were then observed and photographed under an optical microscope (Olympus, Tokyo, Japan).
Adenovirus Infection
Adenoviral vector encoding Mito-RFP (Hanbio Technology, Shanghai, China), GFP-LC3 (Hanbio Technology), GFP-NIX shRNA (Hanbio Technology), GFP-NIXΔTM (Hanbio Technology), Ad-GFP-control (Hanbio Technology), Ad-RFP-control (Hanbio Technology), RFP-NIX (Obio Technology, Shanghai, China) and Ad-RFP-control (Obio Technology) were, respectively, added to cultured spinal cord neurons at a multiplicity of infection (MOI) of 30 for 4 h. The target sequence of NIX for RNAi was 5′- GCAGCAATGGCAACGGTAATG -3′. The target sequence of the transmembrane domain of NIX was 5′-TTCCTGAAGGTCTTCATCCCATCTCTCTTCCTCTCTCACGTGTTGGCTTTGGGGCTGGGCATCTATATC-3'.
Treatment with Ad-NIXshRNA In Vivo
The Animal model of SCI was established as described above. In the sham group, only the lamina was resected to expose the spinal cord without spinal cord injury. Immediately post-SCI, rats were fixed in a stereotaxic apparatus. In the SCI + Ad-NIXshRNA group, an amount of 1.5 μl (1.26 × 1010 PFU/mL) adenovirus Ad-NIXshRNA was slowly injected into the damaged areas using a microsyringe, in the SCI + Ad-control group the same amount of adenovirus Ad-control were injected into the damaged areas. As to the sham group, rats were injected with 1.5 μl of PBS alone. (The injection time and retaining time was 2 min).
Behavioral Test
Two colleagues blinded to the experiments used the Basso, Beattie, and Bresnahan (BBB scales) (Basso et al. 1995) to assess the locomotor activity of every rat 1 day before operation and weekly (1,2,3,4) after the operation.
Statistical Analysis
Results were presented as mean ± standard deviation (SD). Statistical differences among groups were analyzed by t test or one-way ANOVA using GraphPad Prism (Version 7.0, GraphPad Software Inc., La Jolla, California, USA). A difference was considered significant when P value < 0.05.
Results
Upregulation of NIX and LC3-II after SCI and Hypoxia
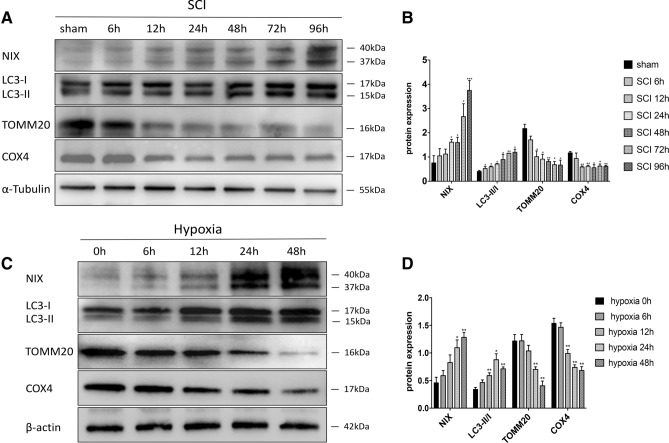
We first determined the expression of NIX and LC3 in the spinal cord tissue of SD rats after SCI. Western blot analysis showed that NIX began to increase at 6 h after injury and reached its peak at 96 h. Levels of LC3-I did not change significantly from sham group to SCI 96 h group, but the expression of the LC3-II gradually increased, and the ratios of LC3-II/LC3-I were significantly increased from 6 h compared with sham group. At the same time, Translocase Of Outer Mitochondrial Membrane 20 (TOMM20), a marker of the mitochondrial outer membrane (Wurm et al. 2011), and cyclooxegenase IV (COXIV), a transmembrane protein localized to the mitochondria (Wurm et al. 2011), declined significantly after 12 h of injury (Fig. 1a, b).
Fig. 1.
Upregulation of NIX and LC3-II after SCI and hypoxia. a, c Western blots showing altered expression of NIX, LC3-II/I, TOMM20, COX4. α-Tubulin and β-actin were used as the loading control, respectively. b Quantitative analysis of NIX, ratio of LC3-II/I, TOMM20, COX4 protein levels after SCI. Data were presented as the mean ± SD (n = 3 from three independent experiments; *p < 0.05; **p < 0.01; ***p < 0.001 vs. the sham group, one-way ANOVA). (D)Quantitative analysis of NIX, ratio of LC3-II/I, TOMM20, COX4 protein levels after exposed to hypoxia. Data were presented as the mean ± SD (n = 3 from three independent experiments; *p < 0.05; **p < 0.01vs. the normoxia group, one-way ANOVA)
We then determined the expression levels of NIX by Western blot on cultured spinal cord neurons exposed to normoxic or hypoxic conditions for 6 h, 12 h, 24 h and 48 h. NIX was barely expressed in the case of normoxic conditions. Along with the increment of hypoxia time, the expression of NIX increased and reached a peak at 48 h. Meanwhile, the expression of LC3-II began to increase at 12 h and the ratio of LC3-II/LC3-I reached its peak at 24 h after exposure to hypoxia. Tomm20 and COX4 began to decline significantly 24 h after hypoxia (Fig. 1c/d).
Overexpression of NIX Induces an Upregulation of LC3-II in Cultured Spinal Cord Neurons
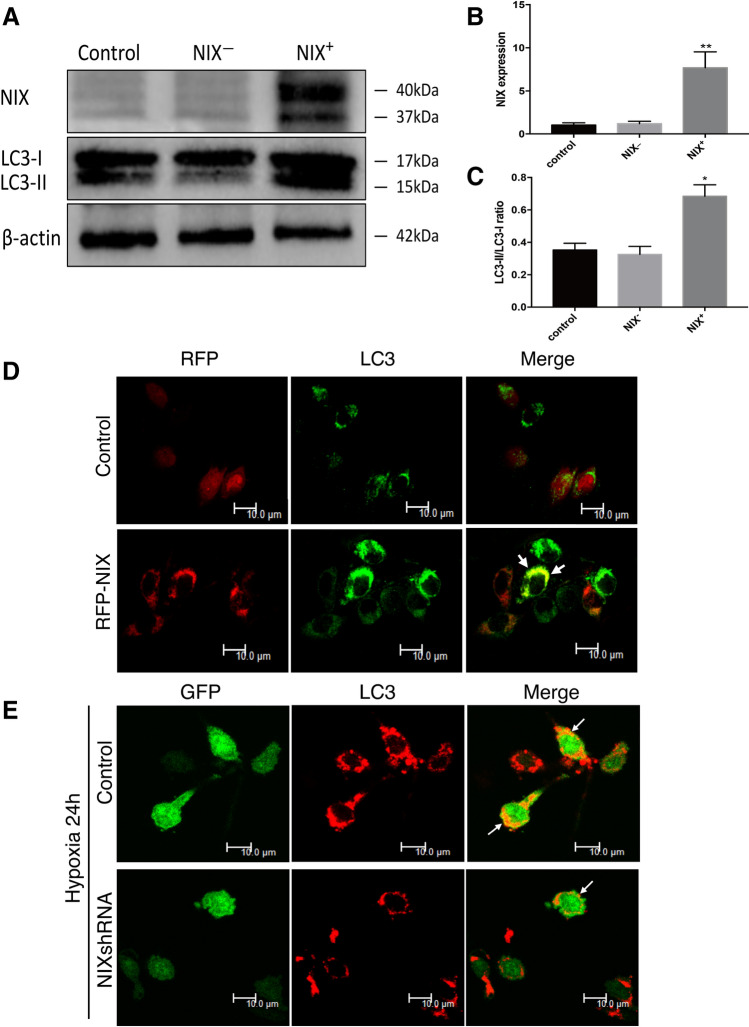
Since upregulation of NIX correlated with that of LC3-II, we were interested to determine whether the expression of NIX was sufficient to induce upregulation of LC3-II and autophagy. We constructed adenoviral vectors, respectively, expressing the RFP-NIX and a GFP-NIXshRNA, and tested the vectors in cultured spinal cord neurons. Forty-eight hour after transfection of the adenoviral NIX vector, protein samples were prepared and analyzed for the changes of NIX and LC3 by Western blot. The empty vector was used as a control. As shown in Fig. 2a–c, NIX in the overexpression group was significantly increased compared with the other two groups as expected. Upregulation of NIX resulted in a significant increase in the ratio of LC3-II/LC3-I. The numbers of green fluorescence punctuate of LC3 was significantly increased in the RFP-NIX overexpression group compared with the control group, and NIX co-localized with LC3 (Fig. 2d). When the neurons were exposed to hypoxia for 24 h, inhibition of the expression of NIX remarkably reduced the red fluorescence punctuates of LC3 compared with the control group (Fig. 2e).
Fig. 2.
Overexpression of NIX induces an upregulation of LC3-II in cultured spinal cord neurons. a Western blots showing altered expression of NIX and LC3-II/I after neurons were transfected with RFP-NIX (overexpressing full length of NIX) or NIXshRNA (knockdown NIX by shRNA). β-actin was used as the loading control. b, c Quantitative analysis of NIX and LC3-II/I protein levels after treatment. (Mean ± SD, n = 3 from three independent experiments; *p < 0.05; **p < 0.01; vs. the control group, t test). d Immunofluorescence images of expression of LC3 (green) after neurons were transfected with Ad-RFP-NIX or Ad-RFP-control for 48 h. Scale bar: 10 μm. e Immunofluorescent images of expression of LC3 (red) after treatment with Ad-GFP-NIXshRNA or Ad-GFP-control for 24 h then exposed to hypoxia for 24 h. Scale bar:10 μm. GFP, Green fluorescent protein; RFP, Red fluorescent protein; Ad, Adenovirus. shRNA, Short hairpin RNA
NIX Interacts with LC3 to Form a Mitochondria-NIX-LC3-Autophagosome Complex in Spinal Cord Neurons
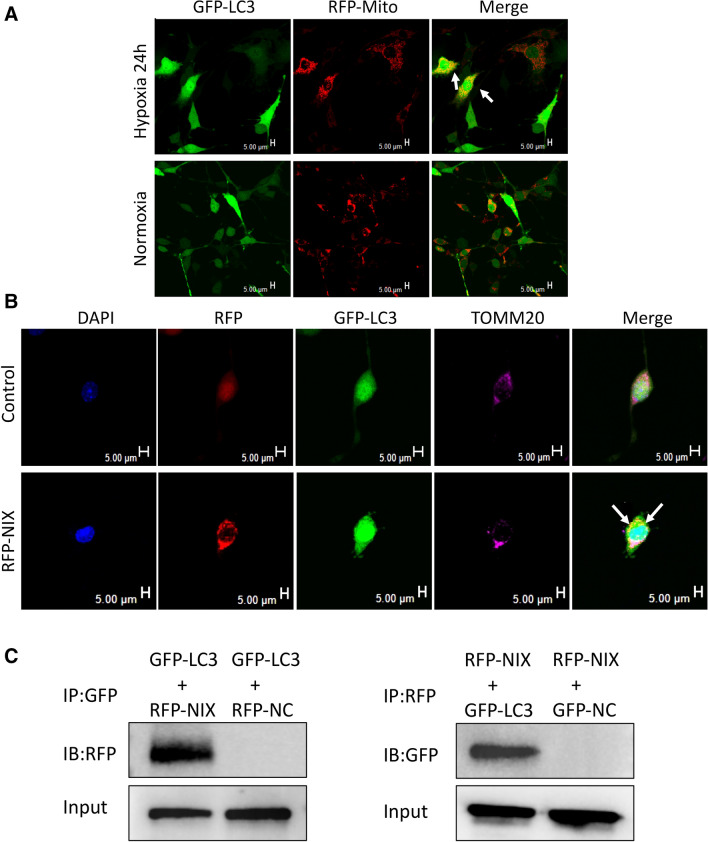
As it was demonstrated that the overexpression of NIX was sufficient to induce autophagy in cultured spinal cord neurons, we hypothesized that there would be a link between NIX and LC3. To test this hypothesis, we transfected cultured spinal cord neurons with adenoviral vectors encoding GFP-LC3 and Mito-RFP (a marker of mitochondria), at normoxia for 24 h. The experimental group was then exposed to hypoxia for 24 h, while the control group was kept at normoxic condition for 24 h. When the cultured spinal cord neurons were exposed to hypoxia mitochondria began to collapse and to colocalized with the LC3-labeled autophagosomes (Fig. 3a), suggesting that hypoxia-induced mitochondrial degeneration and mitophagy. Next, we infected the cultured spinal cord neurons with adenoviruses encoding RFP-NIX and GFP-LC3 for 48 h in normoxic conditions. The expression of NIX resulted in colocalization of GFP-LC3-labeled autophagosomes and TOMM20-positive mitochondria as revealed by confocal microscopy (Fig. 3b). To explore how mitophagy was regulated by NIX we performed co-immunoprecipitation to identify the interactions between NIX and LC3. We detected a clear band of NIX using GFP-LC3 as a bait to pull down NIX and a lighter band when NIX worked as a bait protein to pull down GFP-LC3 (Fig. 3c). Taking these findings together, we demonstrated that NIX interacts with LC3 to form a mitochondria-NIX-LC3-autophagosome leading to mitophagy in spinal cord neurons.
Fig. 3.
NIX interacts with LC3 to form a mitochondria-NIX-LC3-autophagosome complex in spinal cord neurons. a Colocalization of mitochondrial and GFP-LC3 under normoxia or hypoxia for 24 h; Scale bar, 5 μm. b The formation of mitochondria-NIX-LC3-autophagosome complex was detected by immunofluorescent (white arrow, colocalization of GFP-LC3, RFP-NIX and TOMM20, Scale bar, 5 μm). c Co-immunoprecipitation of GFP-LC3 and RFP-NIX
Inhibition of NIX by shRNA Protects Neurons Against Hypoxic Damage
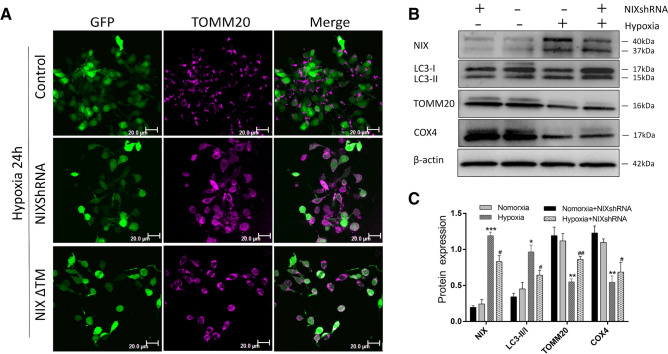
To further explore the role of NIX in neuronal mitophagy, we used Ad-NIXshRNA and Ad-NIXΔTM to infect cultured spinal cord neurons at normoxic conditions for 24 h. The neurons were further exposed to hypoxia for another 24 h and then collected for morphological and biochemical analyses. Compared with the control group, inhibition of NIX by both RNA interference or deletion of its transmembrane domain (dominant-negative) protected mitochondria from hypoxia-induced fractionation and degeneration, although the NIXΔTM was less effective than NIXshRNA in the protection (Fig. 4a). Western blot analysis revealed that the ratio of LC3-II/LC3-I and protein expression level of NIX in the NIXshRNA treated group was decreased significantly compared with the hypoxia alone group. The levels of TOMM20 and COX4 under hypoxia were increased when NIX was inhibited by NIXshRNA (Fig. 4b, c). Taken these data together, inhibition of NIX protected the amounts of mitochondria against hypoxic damage in neurons.
Fig. 4.
Inhibition of NIX by shRNA protects neurons against hypoxic damage. a Immunofluorescence analysis of mitochondria in neurons transfected with Ad-NIXshRNA, Ad-NIXΔTM or Ad-control at 24 h after hypoxia (TOMM20, magenta). Scale bar, 20 μm. b Western blot analysis of NIX, LC3, TOMM20, and COX4 in spinal cord neurons infected with NIX shRNA before hypoxia. c Quantitative analysis of relative levels of proteins using β-actin as a loading control (mean ± SD; n = 3 from three independent experiments; *p < 0.05; **p < 0.01, ***p < 0.001vs. the normoxia group; #p < 0.05; ##p < 0.01 vs. the hypoxia group, one-way ANOVA). Ad, Adenovirus. shRNA, Short hairpin RNA. NIXΔTM, NIX without the transmembrane domain
The Effect of Phosphorylation on Mitophagy
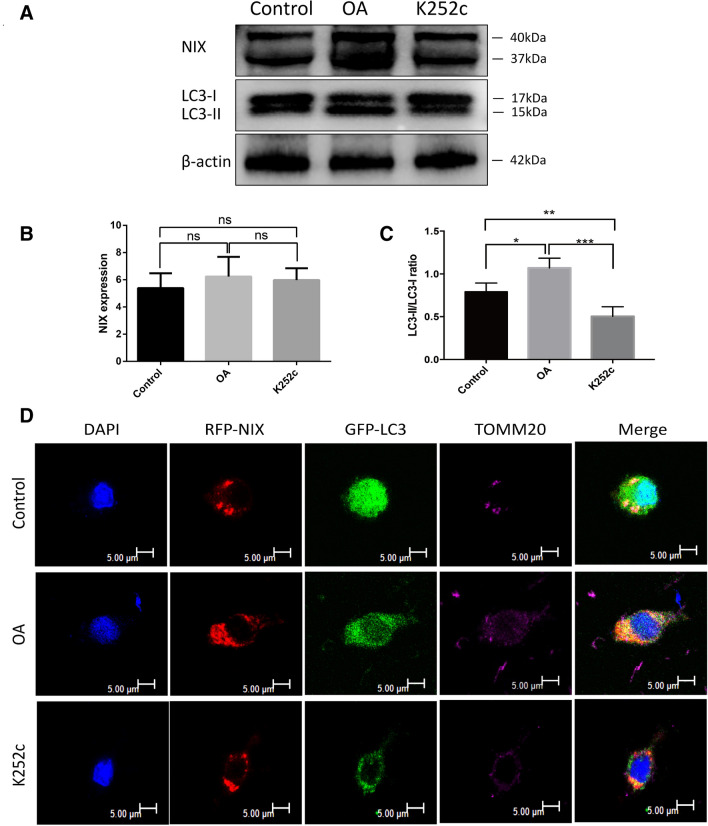
We next tested if serine/threonine phosphatase or serine/threonine kinase inhibitor would affect mitophagy. Cultured spinal cord neurons were transfected with the NIX-expressing adenoviral vector for 4 h and cultured for 42 h, then treated with the serine/threonine phosphatase inhibitor okadaic acid (OA, 50 nM), the serine/threonine kinase inhibitor K252c (16uM) (Zhu et al. 2013) or equal amount of PBS in the control group for 6 h. The cells were collected for Western blot analysis to determine the ratio of LC3-II/LC3-I. As shown in Fig. 5a–c, there is no difference in the expression of NIX among the groups but the ratio of LC3-II/LC3-I in the OA treated group is the highest of the three and the K252c treated group is lower than that of the control group. To further confirm the results, we used confocal microscopy to observe the effects of OA and K252c on mitophagy (Fig. 5d). We detected extensive colocation of NIX and LC3 on Tomm20-positve mitochondria and a large number of mitophagosomes in the OA treated group. On the contrary, the K252c treated group resulted in fewer mitophagosomes than the control group. Collectively, these data supported that enhancing phosphorylation of NIX promoted mitophagy.
Fig. 5.
Effect of OA and K252c on mitophagy. a Western blots analysis was used to determine the ratio of LC3-II/I after the neurons were treated with OA, K252c or PBS for 6 h, β-actin was used as the loading control. b, c Quantitative analysis of NIX, LC3-II/I protein levels after treatment. (Mean ± SD, n = 3 from three independent experiments; *p < 0.05; **p < 0.01; ***p < 0.001, t test). d Colocalization of GFP-LC3, RFP-NIX, and TOMM20 (magenta) was detected by immunofluorescence, Scale bar, 5 μm. OA: Okadaic acid
Inhibition of NIX by shRNA Protects Spinal Cord Neurons and Improves Locomotion Recovery in Rats After Spinal Cord Injury
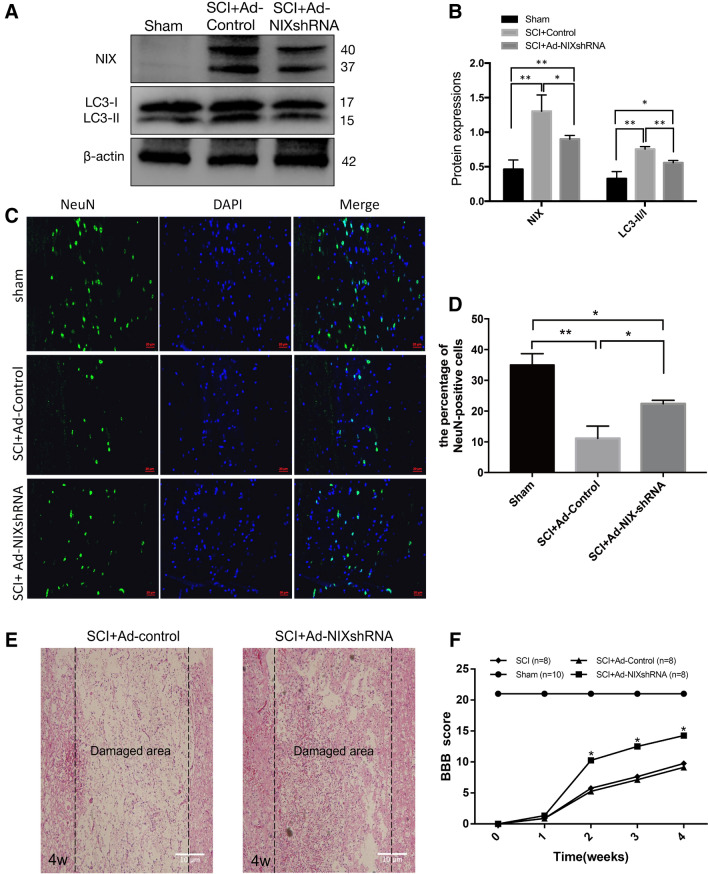
Forty rats were randomly divided into four groups: sham, SCI, SCI + Ad-control, and SCI + Ad-NIXshRNA group (10 in each group). Two investigators who blinded to the experimental groups used the BBB scale (Basso et al. 1995) to assess the locomotor activity of each rat 1 day before the operation and every week after surgery until the 4th week. After 28 days of treatment, the animals were sacrificed and the spinal cords were removed for subsequent experiments. Western blot analysis was used to determine the expression level of NIX. As expected, the expression of NIX and LC3-II in the Ad-NIXshRNA treated group was significantly lower than that of the Ad-control treated group (Fig. 6a, b).
Fig. 6.
Knockdown of NIX by shRNA has a neuroprotective role in rats after SCI. a Western blot analysis the expression of NIX in sham, Ad-control–treated, and Ad-NIXshRNA–treated rat at 28 days after SCI. b Quantitative analysis the levels of NIX and LC3-II/I. β-actin was used as a loading control (mean ± SD, n = 3 from three independent experiments; *p < 0.05; **p < 0.01, t test). c Immunofluorescent staining of NeuN in sham, Ad-control–treated, and Ad-NIXshRNA–treated rat at 28 days after SCI, Scale bar, 20 μm. d Number of NeuN-positive cells in sham, Ad-control–treated, and Ad-NIXshRNA–treated rat at 28 days after SCI (mean ± SD, n = 3 from three independent experiments; *p < 0.05; **p < 0.01; t test). e Representative images of the histological analysis of the spinal cord tissue sections in Ad-control– treated and Ad-NIXshRNA–treated rat at 28 days after SCI; Scale bar, 10 μm. (F) Statistical analysis of the BBB test in Sham, SCI, Ad-control–treated, and Ad-NIXshRNA–treated rat at different time point after SCI
NeuN is a neuronal nuclear antigen that is commonly used as a biomarker of neurons (Mullen et al. 1992). The numbers of NeuN-positive cells were compared between the Ad-NIXshRNA treated rats and the Ad-control treated rats to estimate survival of neurons. The NeuN-positive cells in the Ad-NIXshRNA treatment group were significantly more than those in the control group although less than the sham group (Fig. 6c, d).
Then we observed pathological changes of the two groups. In the Ad-control treated rats the structure of the spinal cord tissue was disorganized with massive neuronal necrosis and cavernous formation. In the Ad-NIXshRNA treated rats, however, the structure and cellularity were much improved. The number of neurons that survived around the damaged area increased in the Ad-NIXshRNA treated rats (Fig. 6e). These findings suggest that knockdown of NIX is protective neurons after spinal cord injury.
As shown in Fig. 6f, there was no obvious recovery in all groups during the first 1 week but at 14 days after SCI the Ad-NIXshRNA-treated group performed significantly better on the BBB test than the other two groups.
Discussion
Previously we observed a robust increase of double-membraned autophagosomes that contained damaged mitochondria post-SCI, and concluded that mitophagy was involved in the death of spinal cord neurons in SCI (Yu et al. 2013). In the present study we show that the expression levels of NIX and LC3-II are dramatically upregulated after SCI and in hypoxia. We further show that forced expression of NIX is sufficient to induce upregulation of LC3-II and activate excessive mitophagy leading to significant loss of mitochondria as evidenced by the declined expression of mitochondrial markers TOMM20 and COX4. The fact that overexpression of NIX triggers excessive mitophagy while downregulation of NIX inhibits it supports strongly a regulatory role of NIX in excessive mitophagy after spinal cord injury. This role is further supported by the evidence that NIX is localized to the outer membrane of mitochondria and interacts with LC3 to form a mitochondrial-NIX-LC3 complex (Fig. 3). This appears to be a plausible mechanism by which NIX mediates excessive mitophagy leading to significant reduction of mitochondria after spinal cord injury or in cultured spinal cord neurons exposed to hypoxia. Although a physical level of mitophagy is required for mitochondrial quality control, excessive mitophagy has been suggested to contribute to autophagic cell death in acute neurodegenerative diseases such as spinal cord injury and stroke (Shi et al. 2014). Inhibition of NIX could therefore reduce excessive mitochondrial loss and be neuroprotective.
While upregulation of NIX is observed in mostly in acute ischemic diseases, for example, stroke, myocardial infarction and hemorrhagic shock, deregulation of NIX is also implicated in a variety of chronic diseases such as cancers and chronic neurodegenerative diseases (Ashrafi and Schwarz 2013). A recent study shows that NIX is a substrate of PARK2 in the process of promoting mitophagy in Parkinson's disease (Gao et al. 2015). Physiologically, in addition to mitochondrial quality control, NIX has been reported to be indispensable for mitochondrial clearance during reticulocyte maturation.
NIX and BNIP3 are members of a unique subfamily of death-inducing mitochondrial proteins. It has been reported that BNIP3 induces excessive mitophagy and causes delayed neuronal cell death in stroke (Shi et al. 2014). In this study, we provide evidence for a role of NIX in activating excessive mitophagy in spinal cord neurons after SCI. Consistent with this, studies have found that forced expression of NIX can lead to perinatal cardiomyopathy in mice, while the lack of NIX can protect mice from Gq -induced cardiomyopathy (Diwan et al. 2008). Whether NIX plays a protective or detrimental role may depend on the mitophagy levels induced by NIX in different disease processes. For SCI, our data show that inhibiting mitophagy by downregulating NIX protects mitochondria from excessive mitophagy and reduce neuronecrosis. The result is consistent with a recent study showing that inhibition of autophagy with estradiol promotes locomotor recovery after SCI in rats (Lin et al. 2016).
Mitophagy is involved in different species and cell lines. The pathways that mediate mitophagy have been extensively studied (Twig et al. 2008) (Zhang and Ney 2009) (Maiuri et al. 2007). At present, there exist three models of NIX-dependent mitophagy. Firstly, NIX may induce mitophagy by triggering depolarization of the mitochondria (Twig et al. 2008). Secondly, NIX is able to act as an autophagy receptor to recruit autophagy components and induce mitophagy (Zhang and Ney 2009). Finally, NIX can activate autophagy by increasing the amount of free Beclin 1 in cells (Maiuri et al. 2007). In this study, we have discovered that the mitochondria-localized NIX interacts with the autophagosome-localized LC3 to form a mitochondria-NIX-LC3-autophagosome complex, which results in excessive mitophagy and neuronal cell death post-SCI. Whether NIX induces neuronal mitophagy through other mechanisms needs to be further studied.
It is striking that all known outer mitochondrial membrane (OMM)-localized mitophagy receptors contain a transmembrane domain with conserved serine/threonine preceding the LIR motif, indicating that LIR phospho-regulation mechanisms may represent a potential target for adjusting mitophagy receptor function (Hamacher-Brady and Brady 2016). Interestingly, phosphorylation of BNIP3 at serine 17 and serine 24 strengthens its banding with LC3B and GATE-16 (Zhu et al. 2013), while phosphorylation of NIX at serine 34 and serine 35 enhances its interaction with LC3 (Rogov et al. 2017). The phosphorylation of NIX at serine 81 is known to be necessary for its activity in inducing mitophagy (Yuan et al. 2017). Our data show that inhibition of serine/threonine phosphatases with okadaic acid enhances mitophagy while the serine/threonine kinase inhibitor K252c has the opposite effect. It is currently not known which kinases are involved in regulating the phosphorylation state of BNIP3 and NIX LIR motifs. Whether there exist other phosphatases or protein kinases that may have the same effects remains to be discovered.
Our data together suggests that NIX activates excessive neuronal mitophagy and induces mitochondrial degeneration and neuron cell death after spinal cord injury. The NIX-induced cell death pathway is a potential target for neuroprotective strategies.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81572210 and 81372106 to Z.Z.), and from the Brain Canada and ALS Canada (to J.K).
Author contributions
PN: conceptualization, performed the experiments, wrote the original manuscript; HW: methodology, data curation and validation; DY and HW: formal analysis, software, validation; BN: resources and project administration; JK and ZZ: funding acquisition, supervision, revised the manuscript.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
The animal protocol was approved by the Animal Care and Ethics Committee of the Third Military Medical University, whose standards meet the Animal Care guidelines of the NIH, USA.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiming Kong, Email: Jiming.Kong@umanitoba.ca.
Zhengfeng Zhang, Email: zhangz3@126.com.
References
- Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21 [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden AI (2007) Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain 130:2977–2992 [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Eng J, Ivanov N, Garden GA, La Spada AR (2009) Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Mol Brain 2:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan A, Wansapura J, Syed FM, Matkovich SJ, Lorenz JN, Dorn GW 2nd (2008) Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation 117:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Chen D, Si J, Hu Q, Qin Z, Fang M, Wang G (2015) The mitochondrial protein BNIP3L is the substrate of PARK2 and mediates mitophagy in PINK1/PARK2 pathway. Hum Mol Genet 24:2528–2538 [DOI] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR (1999) Alterations in AMPA receptor subunit expression after experimental spinal cord contusion injury. J Neurosci 19:5711–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR (2016) Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci 73:775–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki T (2010) Nix, a receptor protein for mitophagy in mammals. Autophagy 6:433–435 [DOI] [PubMed] [Google Scholar]
- Kubli DA, Ycaza JE, Gustafsson AB (2007) Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J 405:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Chen B, Huang KL, Dai YS, Teng HL (2016) Inhibition of autophagy by estradiol promotes locomotor recovery after spinal cord injury in rats. Neurosci Bull 32:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G (2007) Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26:2527–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211 [DOI] [PubMed] [Google Scholar]
- Noda NN, Ohsumi Y, Inagaki F (2010) Atg8-family interacting motif crucial for selective autophagy. FEBS Lett 584:1379–1385 [DOI] [PubMed] [Google Scholar]
- Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Lohr F, Popovic D, Occhipinti A, Reichert AS, Terzic J, Dotsch V, Ney PA, Dikic I (2010) Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep 11:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogov VV, Suzuki H, Marinkovic M, Lang V, Kato R, Kawasaki M, Buljubasic M, Sprung M, Rogova N, Wakatsuki S, Hamacher-Brady A, Dotsch V, Dikic I, Brady NR, Novak I (2017) Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins. Sci Rep 7:1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarten M, Mohrluder J, Ma P, Stoldt M, Thielmann Y, Stangler T, Hersch N, Hoffmann B, Merkel R, Willbold D (2009) Nix directly binds to GABARAP: a possible crosstalk between apoptosis and autophagy. Autophagy 5:690–698 [DOI] [PubMed] [Google Scholar]
- Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS NeurosciTher 18:250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Zhu S, Li V, Gibson SB, Xu X, Kong J (2014) BNIP3 interacting with LC3 triggers excessive mitophagy in delayed neuronal death in stroke. CNS NeurosciTher 20:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm CA, Neumann D, Lauterbach MA, Harke B, Egner A, Hell SW, Jakobs S (2011) Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc Natl AcadSci USA 108:13546–13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Li M, Ni B, Kong J, Zhang Z (2013) Induction of neuronal mitophagy in acute spinal cord injury in rats. Neurotox Res 24:512–522 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zheng Y, Zhang X, Chen Y, Wu X, Wu J, Shen Z, Jiang L, Wang L, Yang W, Luo J, Qin Z, Hu W, Chen Z (2017) BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13:1754–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ney PA (2009) Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 16:939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Chen Z, Qi J, Duan S, Huang Z, Zhang C, Wu L, Zeng M, Zhang B, Wang N, Mao H, Zhang A, Xing C, Yuan Y (2017) Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function. Oncotarget 8:20988–21000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Massen S, Terenzio M, Lang V, Chen-Lindner S, Eils R, Novak I, Dikic I, Hamacher-Brady A, Brady NR (2013) Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J BiolChem 288:1099–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]