Abstract
The Shank family proteins are enriched at the postsynaptic density (PSD) of excitatory glutamatergic synapses. They serve as synaptic scaffolding proteins and appear to play a critical role in the formation, maintenance and functioning of synapse. Increasing evidence from genetic association and animal model studies indicates a connection of SHANK genes defects with the development of neuropsychiatric disorders. In this review, we first update the current understanding of the SHANK family genes and their encoded protein products. We then denote the literature relating their alterations to the risk of neuropsychiatric diseases. We further review evidence from animal models that provided molecular insights into the biological as well as pathogenic roles of Shank proteins in synapses, and the potential relationship to the development of abnormal neurobehavioral phenotypes.
Keywords: Autism, Neurodevelopment, Neuropsychiatric disorders, Synaptic transmission, Synaptic plasticity
Introduction
There are three SHANK genes in the human genome, which code a number of proteins particularly involved in synaptic scaffolding. These synaptic proteins can bind to a variety of other proteins enriched at the postsynaptic density (PSD) of excitatory synapses (Mossa et al. 2017; Monteiro and Feng 2017). Shank proteins are a core part of postsynaptic protein networks, thereby playing critical neurobiological roles in synapse formation, plasticity, signaling and transmission. In recent years, many genetic studies have extended strong evidence that alterations in SHANK genes are involved in the development of various psychiatric and neurological disorders, including autism spectrum disorders (ASD), Phelan–McDermid syndrome (PMS), schizophrenia (SCZ), bipolar disorders (BPD) and Alzheimer’s disease (AD; Drapeau et al. 2018; Zhang et al. 2017; Tatavarty et al. 2017; Kanani et al. 2018; Zhao et al. 2019; Alexandrov et al. 2017; MacGillavry et al. 2016). In this review, we first introduce the SHANK family genes, and their transcriptive and translational products. We then elaborate on the findings from genome-wide association studies (GWAS) that implicate their involvement in the development of a number of brain disorders. Finally, we denote findings from genetically modified animal models, which provide mechanistic insights into physiological and pathogenic roles of Shank proteins in structural and functional maintenance of synapses.
SHANK Family Genes and Their Coding Products
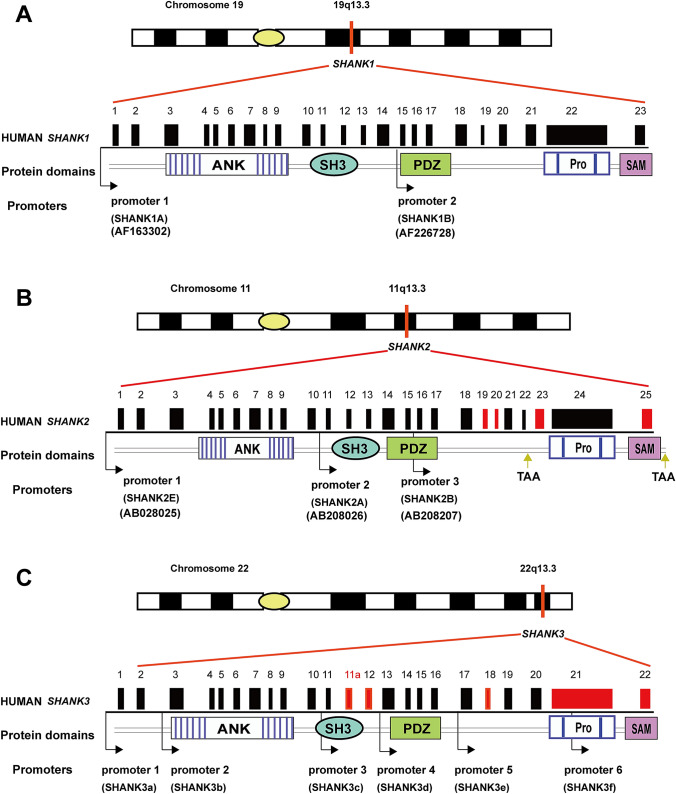
Three genes have been discovered to code Shank protein products, named as SHANK1 (also termed Synamon/SSTRIP/Spank-1), SHANK2 (also known as CortBP1/ProSAP1/Spank-3) and SHANK3 (also referred to as ProSAP2/Spank-2), respectively (Mossa et al. 2017). These genes code the corresponding final protein products, with multiple promoter activation, extensive alternative splicing of coding exons and intermediate isoform formation involved in the transcriptional and post-transcriptional processes (Mossa et al. 2017; Monteiro and Feng 2017) (Fig. 1).
Fig. 1.
SHANK family gene structure, protein domains and isoforms. Panels a, b and c show the chromosome locations, gene structures, domains and isoforms of SHANK1, SHANK2 and SHANK3, respectively. The protein-coding domains are marked and aligned to corresponding exons, including ANK, PDZ, PRO, SH3 and SAM. The positions of two promoters are indicated by black arrows. The exons in SHANK2 in red are alternatively spliced, with three identifiable promoters indicated by black arrows and two alternative stop codons (TAA) pointed by yellow arrows (b). The exons of SHANK3 in red are also alternatively spliced, with exon 11a being newly identified. The positions of six identified promoters are indicated by black arrows (c). ANK ankyrin repeat domain, PDZ postsynaptic density protein 95-discs large homologue 1-zonula occludens 1 domain, PRO proline-rich region, SAM sterile alpha motif, SH3 SRC homology 3 domain superfamily
SHANK1 is located on chromosome 19q13.3 in human, which spans ~ 55.1 kb, and contains five domains: ankyrin repeat domain (ANK, located at the N terminus), SRC homology 3 (SH3), postsynaptic density protein 95 (PSD95)-discs large homologue 1-zonula occludens 1 (PDZ), proline-rich domain (PRO) and sterile alpha motif (SAM, located at the C terminus) domains (Sheng and Kim 2000; Grabrucker et al. 2011) (Fig. 1a). It has 23 exons and 2 alternative promoters, which lead to the formation of 2 distinct transcript isoforms: Shank1A (the longest isoform, includes the above 5 domains) and Shank1B (contains PDZ, PRO and SAM domains). SHANK1 also contains alternative splicing sites that can generate different transcripts, for example, Shank1B (lacks the C-terminal SAM domain), Shank1C (lacks the N-terminal ankyrin repeat domain) and Shank1D (lacks ankyrin repeat domain, SH3 or SAM domains). Notably, Shank1 messenger appears to be expressed exclusively in the brain (Lim et al. 1999). In situ hybridization studies in rat show that the Shank1 mRNA is expressed predominately in the cerebral cortex, hippocampus and amygdala, moderately expressed in the hypothalamus and the substantia nigra, while low expression is present in the cerebellum, caudate nucleus, corpus callosum and subthalamic nucleus (Monteiro and Feng 2017; Lim et al. 1999).
SHANK2 is located on chromosome 11q13.3 in human (Fig. 1b). It spans ~ 621.8 kb and is the largest gene among the family. It contains 5 domains, with 25 exons, three alternative promoters and one stop codon. Its transcription results in three different protein isoforms, including Shank2E (containing all five protein domains), Shank2A (lacking the ankyrin repeat domain) and Shank2B (lacking the ankyrin repeat domain and the SH3 domain). Shank2 mRNA occurs in high levels in the brain and at lower levels in kidney and liver (Lim et al. 1999). Thus, Shank2 messenger is widely expressed in many regions, including the cortex, hippocampus, cerebellum, olfactory bulb and central gray (Monteiro and Feng 2017).
SHANK3 appears to be the best studied among the three family members, which is located on chromosome 22q13.3 in human (Fig. 1c). This gene spans ~ 55.1 kb in length and contains 24 exons, 6 alternative promoters and 1 alternative stop codon located in the exon 21b. Six distinct isoforms are produced following the transcriptional processing: Shank3a, Shank3b, Shank3c, Shank3d, Shank3e and Shank3f. Besides alternative promoter and mRNA splicing, the gene expression is regulated by epigenetic mechanisms such as DNA methylation and histone acetylation, resulting in differential tissue specific expression of (Monteiro and Feng 2017; Maunakea et al. 2020).
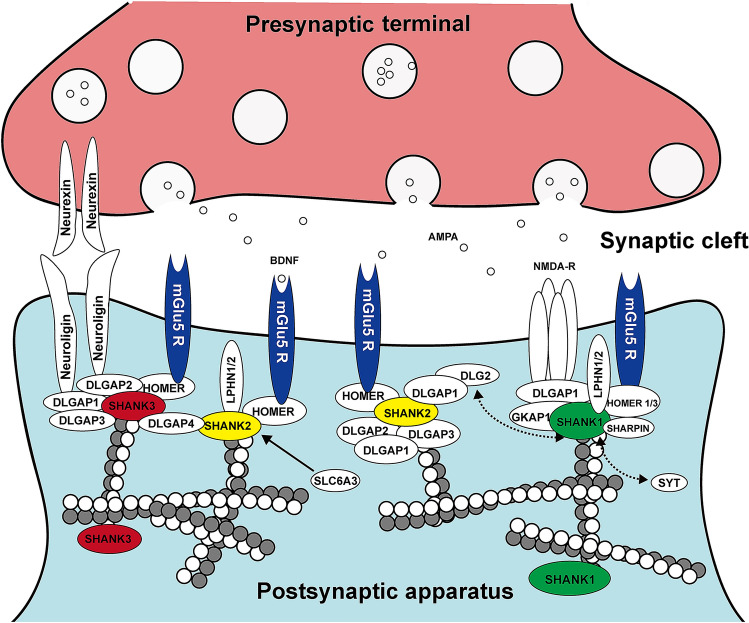
The full length structure of SHANK3 gene contains ANK, SH3, PDZ, PRO and SAM in human. However, in the mouse genomic DNA, there include another protein domain DUF535, with its function remained unclear. Shank3 mRNA expression appears to be high in the brain, heart and spleen. In the rat brain, Shank3 messenger is enriched in the hippocampal formation, thalamus, striatum and cerebellar granule cells (Lim et al. 1999; Boeckers et al. 1999). Overall, the Shank proteins are best known as synaptic scaffolding apparatus involving in the targeting, anchoring and dynamically regulating neurotransmitter receptors and signaling molecules at postsynaptic sites (MacGillavry et al. 2016). These functions appear to be executed via their domain-specific interaction with other proteins (Fig. 2). For instance, the amino-terminal ankyrin repeat domain interacts with the PSD protein SHARPIN and probably binds to the cytoskeleton through an interaction with spectrin alpha chain, non-erythrocytic 1 (SPTAN1, also known as α-fodrin) (Lim et al. 2001; Böckers et al. 2001). The N-terminal region preceding the ankyrin repeat domain may interact intra-molecularly with the six ankyrin repeats, and could restrict the access to binding partners such as SHARPIN and SPTAN1 (Mameza et al. 2013) (Fig. 2). The class I PDZ domain of SHANK3 can partnership with the SAP90/PSD95 associated protein 1 (SAPAP1, also known as GKAP1) and the glutamate receptor 1 (GluR1; also known as GRIA1) subunit of AMPA receptors (AMPARs) (Monteiro and Feng 2017; Naisbitt et al. 1999) (Fig. 2). The latter is important for dendritic spine formation and synaptic transmission. The PRO domain binds to the Homer and cortactin 10 proteins, which are important for cytoskeleton regulation, synaptic transmission and plasticity (Naisbitt et al. 1999; Hayashi et al. 2009) (Fig. 2). Moreover, the carboxy-terminal SAM domain of Shank proteins is known to self-multimerize, which is required for the localization of the proteins to the PSD (Naisbitt et al. 1999; Boeckers et al. 2005). Besides the above, Shank proteins could cofunction with many other partners to modulate neuronal and synaptic activity (Monteiro and Feng 2017; Guilmatre et al. 2014).
Fig. 2.
Schematic drawing illustrating the potential protein–protein interaction network of the SHANK at the postsynaptic density of glutamatergic synapses. NMDA-R N-methyl-d-aspartate receptor, BDNF brain-derived neurotrophic factor, AMPA alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, DLGAP1, 2, 3 discs large associated protein 1, 2, 3, SLC6A3 solute carrier family 6 (neurotransmitter transporter, dopamine), member 3
Association of SHANK Gene Variations with Neuropsychiatric Disorders
While the SHANK genes share a very high degree of sequence and domain homology, different proteins and isoforms are differentially expressed in the central nervous system according to developmental stages, cell types and brain regions. Therefore, the neurobiological roles of Shank proteins in the brain are fairly complicated, and could be more or less differentially related to neuronal/synaptic activities and functions. Since the identification of the first mutation in SHANK genes, there has been a spectacular increase in our knowledge on the role of this family in the development of neuropsychiatric disorders. During the past several years, distinct SHANK gene variations are found to be significantly associated with the risks of different disorders (Durand et al. 2007; Toro et al. 2010; Sudhof 2008).
Autism Spectrum Disorder (ASD)
Genetic defects of SHANKs have been identified in patients with ASD in multiple reports. In 2007, Durand et al. first described a specific mutation, SHANK3 interstitial or terminal deletion or frameshift, in ASD patients (Durand et al. 2007). Accumulating genetic association studies indicate that SHANK3 variation is estimated to contribute to ~ 0.5% of ASD, which represents one of the most replicated and well-characterized genetic defects in ASD (Jaramillo et al. 2020; Bey et al. 2018). Different forms or variations of genetic defects are found in patients with ASD, including: (1) terminal microdeletion of SHANK3 in 22q13.3 or ring chromosome of 22 (Drapeau et al. 2018; Jeffries et al. 2005; Okamoto et al. 2007; Bonaglia et al. 2011; Sarasua et al. 2014); (2) point mutation (Moessner et al. 2007; Gauthier et al. 2009; Boccuto et al. 2013; Soorya et al. 2013; Cochoy et al. 2015); (3) small intragenic deletion (Marshall et al. 2008; Chen et al. 2016); (4) insertion mutation (Durand et al. 2007; Jaramillo et al. 2017, 2020; Kolevzon et al. 2011; Bonaglia et al. 2011); (5) chromosome translocations (Moessner et al. 2007; Bonaglia et al. 2001); (6) microduplication (Chen et al. 2016) and (7) frameshift variant (Kanani et al. 2018) (Table 1). Microdeletion of SHANK3 in Phelan–McDermid Syndrome (PMS) is the most common molecular defect in all types of mutations of this gene in ASD. In addition to SHANK3, mutations in SHANK1 and SHANK2 have been found in ASD patients. In 2010, Berkel et al. reported patients with ASD and mental retardation with de novo copy number variations (Berkel et al. 2010). Evidence of SHANK2 mutations causing ASD have been further identified independently in different ASD patients cohorts (Pinto et al. 2010; Wischmeijer et al. 2011; Leblond et al. 2012; Chilian et al. 2013). Mechanistically, Zaslavsky et al. showed that the SHANK2 mutations in association with ASD can lead to hyperconnectivity of human neurons (Zaslavsky et al. 2019). In comparison, there are much less reports about the involvement of SHANK1 in ASD. In 2012, Sat et al. reported an inherited deletion of 63.8 kb, encompassing SHANK1 and CLEC11A genes, segregating in six autistic individuals, four males and two females, from the same family (Sato et al. 2012). Notably, the four males were clinically menefested as high-functioning autism, while the two females carrying the same deletion only displayed anxiety and shyness. In addition, Wang et al. recently identified de novo genetic mutations of SHANK1 gene among a Chinese ASD cohort of 1543 ASD probands (Wang et al. 2016a, b). It should be noted that a recent meta-analysis study on SHANKs mutations in patients of ASD suggest that no SHANK1 duplications occur in patients with ASD (Leblond et al. 2014). Studies also show that SNP–SNP interactions in SHANK family may confer ASD risk in the Northeast Han Chinese population (Qiu et al. 2018; Bai et al. 2018), whereas there exists no correlation between SHANK1 SNPs and ASD (Qiu et al. 2019). In sum, SHANK2 and SHANK3 have genome-wide significance for their association with ASD. On the other hand, given the rare frequency of SHANK1 deleterious mutations, the clinical relevance of SHANK1 to the development of ASD remains to be ascertained.
Table 1.
Types of genetic defects in shank3 of ASD patients
| Genetic defect | SHANK3 isoforms affected | Other genes disrupted | Diagnosis | Reference | ||
|---|---|---|---|---|---|---|
| Microdeletion of SHANK3 in 22q13.3 or ring chromosome of 22 | All isoforms disrupted | More than 30 other genes | PMS | Bonaglia et al. (2011), Jeffries et al. (2005). Okamoto et al. (2007) and Drapeau et al. (2018) | ||
| Frameshift variant | Varianted copy number | |||||
| c.1231delC, p.Arg411Val | – | – | ASD | Kanani et al. (2018) | ||
| Point mutation | Rearrangement | Exon/intron/domain | ||||
| E809X, Q1243X, G1271Afs * 15, L1142Vfs * 153 | Exon 21/Pro-Rich-Domain | SHANK3 | – | ASD | Gouderet al. (2019) | |
| c.1527 G>A | Exon 12/SH3 | SHANK3a | De novo 17q12 microduplication | ASD/ID | Soorya et al. (2013) | |
| c.5008 A>T | Exon 22/SAM | SHANK3a | – | ASD/ID | Cochoy et al. (2015) | |
| c.4402 G>T | Exon 22 | – | – | ASD | Boccuto et al. (2013) | |
| c.1304 + 48 C>T | Intron 10 | – | c.421 C > G | ASD | Boccuto et al. (2013) | |
| P.Q321R | Exon 8/ANK | SHANKa-b | – | ASD | Moessner et al. (2007) | |
| c. 2265 C + 1 del G | Exon 19 | SHANKa-e | – | ASD | Gauthier et al. (2009) | |
| Deletion | Deletion length | |||||
| 106 kb | – | ACR, RABL2B, LOC150417, RPL23AP82, LOC105373100 | ASD | Chen et al. (2016) | ||
| 147 kb | – | Microduplication at 19q13.4 | ASD | Chen et al. (2016) | ||
| 277 kb | – | MAPK8IP2, ARSA, 1.4 Mb gain at 22q13.33 | ASD | Moessner et al. (2007) | ||
| 4.36 Mb | – | – | ASD | Moessner et al. (2007) | ||
| 142 kb | – | ACR, RABL2B | ASD | Durand et al. (2007) | ||
| Insertion mutation | Exon/domain | |||||
| Exon 21 | – | – | ASD | Durand et al. (2007) | ||
| Exon 11 | – | – | ASD | Kolevzon et al. (2011) | ||
| Exon 13 | – | – | ASD | Mei et al. (2016) | ||
| Chromosome translocations | Chromosome | |||||
| t(14;22)(q32.33;q13.31) 3.2 Mb deletion | – | – | ASD | Moessner et al. (2007) | ||
| t(12;22)(q24.1;q13.3) | – | – | ASD/PMS | Bonaglia et al. (2001) | ||
| Duplication | Duplication length | |||||
| 1.8 Mb | – | More than 60 other genes | ASD/ADHD | Chen et al. (2016) |
ASD autism spectrum disorder, PMS Phelan–McDermid Syndrome, ID intellectual disability, ADHD attention deficit hyperactivity disorder
Phelan–McDermid Syndrome (PMS)
PMS, also called as 22q13.3 deletion syndrome, is a rare and complex neurodevelopmental disorder characterized clinically by global developmental delay, mild dysmorphic features, motor deficits, variable degrees of intellectual disability (ID), and absent or delayed speech (Drapeau et al. 2018). The genetic changes in PMS involved the deletion of the distal long arm of chromosome 22 that could be resulted from pure terminal deletion, ring chromosomes, unbalanced translocations, mosaicisms of these anomalies and SHANK3 mutations (Bonaglia et al. 2011; Luciani et al. 2003; Phelan and McDermid 2012). The first case was a child reported in 1985, until 1994 Nesslinger et al. showed additional cases and proposed that the symptoms described in patients with chromosome 22q13.3 deletions might be a common phenotype (Watt et al. 1985; Nesslinger et al. 1994). In 2001, a PMS patient’ karyotype showed a de novo balanced translocation of t(12;22)(q24.1;q13.3) disrupting SHANK3 in exon 21, providing evidence that SHANK3 is the detrimented gene associated with the neurobehavioral deficits in PMS patients (Bonaglia et al. 2001). Indeed, small deletions of SHANK3 gene were described in many more cases, such as a short simple repeat located between exons 8 and 9, and the deletion breakpoint located in intron 8 (Toro et al. 2010; Bonaglia et al. 2006). In 2016, Zwanenburg et al. reported four PMS patients with a small deletion of 182 to 224 kb including three common genes SHANK3, ACR, and RABL2B (Zwanenburg et al. 2016). The PMS patient carrying an approximately 100 kb deletion were clinically presented as mild intellectual disability, autistic symptoms and speech delay (Britt-Marie et al. 2002). The patient carrying a terminal deletion of the last 130 kb of chromosome 22q also showed autistic features, speech delay and mild intellectual disability (Flint et al. 1995; Wong et al. 1997). Overall, it appears that SHANK3 mutation or disruption is not only a highly expressed single-gene risk factor for autism, but also a genetic causal factor for PMS (Bonaglia et al. 2011). Notably, more than 80% of patients with PMS are considered to meet the clinical criteria for ASD (De Rubeis et al. 2018). It should be further mentioned that individuals with PMS are at high risk of developing a number of neuropsychiatric conditions in adolescence or early adulthood, including catatonia, bipolar disorder and lasting regression of skills (Kohlenberg et al. 2020; Kolevzon et al. 2019).
In addition to the above studies demonstrated the link of SHANK3 alterations to PMS, a few reports showed changes in SHANK2 gene in individuals with developmental deficits. Thus, a de novo 3.5 Mb deletion of SHANK2 from 11q13.2 to 11q13.4 was related to global developmental delay in a case study (Marcou et al. 2017). Furthermore, Fischetto et al. identified a paternal microduplication (400 kb) of SHANK2 in a boy with mild development delay (DD) by single nucleotide polymorphism (SNP) array analysis (Fischetto et al. 2017).
Schizophrenia (SCZ)
There are many studies reported alterations in the SHANK genes in patients with SCZ. In 2007, Failla et al. reported a patient with SCZ exhibited a 22q13.3-qter duplication encompassing SHANK3 (Failla et al. 2007). The patient developed initial behavioral abnormalities (agitation, social closer and passivity) at the age of 13, followed by auditory hallucinations, loss of self-control, dysfunction of self-awareness with temporary amnesia, disorientation, aggressiveness and sleep disturbances. Her symptoms of apathy, poor personal hygiene and adaptive difficulties met the diagnostic criteria for borderline intellectual dysfunction and disorganized SCZ. In 2010, Gauthier et al. reported two de novo mutations (R1117X and R536W) in 2 families by sequencing SHANK3 in 185 SCZ patients, with 1 mutation being found in three brothers diagnosed with SCZ (Gauthier et al. 2010). In 2015, Zhang et al. reported that circulating microRNA-7 (miR-7) level is significantly increased in SCZ patients, which included 50 patients and 50 healthy controls (Zhang et al. 2017). They also showed that the levels of Shank3 mRNA and protein are reduced by overexpression of miR-7, and increased by miR-7 knockdown. Further morede Sena Cortabitarte et al. investigated the relationship between SHANK3 and SCZ, indicating that the p.G1011V variant would be a promising candidate variant for follow-up studies in larger samples and functional investigation (Ana et al. 2017). The role of SHANK2 in SCZ was first reported by Peykov et al. (2015); they sequenced SHANK2 in a cohort of 481 SCZ patients and observed a significant increase in the number of rare missense variants in these individuals as compared with controls. They also detected four non-synonymous variants in the SCZ cohort (i.e., S610Y, R958S, P1119T and A1731S) relevant to functional impairments to various degrees (Peykov et al. 2015). Specially, A1731S overexpression reduced the number of SHANK2-Basoon-positive synapses. Then, in 2016, whole-genome sequencing in numerous families showed that at least three members diagnosed as schizophrenia were caused by mutations of SHANK2 (Homann et al. 2016). Additionally, Lenertz et al. analyzed 199 SCZ patients and 206 healthy controls, and found that the T allele of variant rs3810280 located in SHANK1 promoter led to serious damage to auditory working memory, which was replicated in the other patients at-risk for SCZ (Lennertz et al. 2012). Moreover, Fromer et al. reported a de novo SHANK1 frameshift mutation in a SCZ patient (Fromer et al. 2014). Taken together, emerging evidence points to a potential association between the SHANK gene family and the risk of SCZ, while future investigations are needed to include more patients.
Bipolar Disorder (BPD)
A substantial number of studies support that SHANK3 mutations are related to the risk of BPD. In 1996, Sovner et al. first reported a patient diagnosed with atypical BPD, whose genetic phenotype was a 22q13 deletion syndrome involving SHANK3. Following this report, a series of case studies have shown that patients with the PMS often present a high incidence of BPD. In 2012, Verhoeven et al. described two patients with PMS and BPD in the same family (Verhoeven et al. 2012). The younger brother displayed severe intellectual disability (ID), hyperactivity together with temper tantrums, repetitive behaviors, lack of language communication, loss of interest in daily activities and increase of his social withdrawal. The older brother presented with a mild ID, recurrent major depressive episodes and unstable pattern of mood. In 2012, Denayer et al. reported four patients with PMS and BPD (Denayer et al. 2012). These patients displayed ASD, severe to profound ID, disruptive behaviors, anxiety and self-absorbing behaviors. In 2012, Vucurovic et al. also found seven patients with PMS that displayed psychiatric features of BPD, with this mental status related to SHANK3 complex multiple deletions (Vucurovic et al. 2012). In other clinical and preclinical studies, SHANK3 changes could be correlated with manic or depressive episodes of BPD (Crisafulli et al. 2013; Ortiz et al. 2015). In 2014, Noor et al. identified a 276 kb duplication encompassing several genes in a BPD case, including seven exons of SHANK2 (Noor et al. 2014). More recently, four point mutations in SHANK2 (c.3979G>A; c.2900A>G; c.4461C>T; c.4926G>A) have been identified in BPD patients (Yang and Jiang 2018). To date, there is no report regarding SHANK1 mutation screening in patients with BPD. However, there is one study reporting that psychostimulant-induced hyperactivity is reduced rather than enhanced in shank1 knockout mice. This finding was considered to speak against the observed behavioral phenotype of this gene in BPD (Sungur et al. 2018). In brief summary, mutations in SHANK3 and SHANK2 are associated with the risk of BPD based on genetic studies, whereas whether this situation exists for shank1 remains unclear.
Alzheimer’s Disease (AD)
AD is a common age-related neurodegenerative disease causing dementia in the elderly. The pathological hallmark of AD includes extracellular deposition of β-amyloid (Aβ) and intraneuronal accumulation of phosphorylated tau (pTau) (Sheng et al. 2012). A pathogenic role of Aβ products, especially the soluble Aβ oligomers, has been proposed for AD. Thus, it is suggested that the Aβ oligomers impair synaptic transmission and cause synaptic dysfunction and degeneration (Haass and Selkoe 2007; Crews and Masliah 2010). Studies have been carried out to understand the interplay between the Shank family and AD pathogenesis. Roselli et al. reported that Aβ can disrupt two scaffold proteins, Homer1b and Shank1 (Roselli et al. 2009). Specifically, Aβ treatment decreased levels of these proteins, and induced ultrastructural changes in the postsynaptic sites. Pham et al. observed a reduction in the levels of Shank1 and Shank3 in the brain of APP transgenic mice, with a decrease of Shank1 levels also found in the frontal cortex of patients with AD (Pham et al. 2010). Gong et al. reported a significant increase in the levels of Shank2 in synaptosomes isolated from the middle frontal gyrus in patients with AD as compared with controls (Gong et al. 2009). This group also showed a decrease in the level of Shank3 and an increase of Shank2 protein in AD relative to control, while no significant difference in Shank1 were found between the AD and control groups. In 2012, a study reported a reduced level of Aβ (479 mg/L) in the cerebrospinal fluid in a bipolar disorder patient with dementia carrying a SHANK3 deletion mutation (Vucurovic et al. 2012). Lately, Zhao et al. found that microRNA-34a could mediate a down-regulation of Shank3 in sporadic AD cases (Vucurovic et al. 2012). Together, there is no report thus far indicating distinct SHANKs changes in AD. However, experimental studies point to some connections between Shank protein dysregulation and the molecular neuropathology of AD.
Temporal Lobe Epilepsy (TLE)
To date, there are a few studies on SHANK genes involved in the pathogenesis of temporal lobe epilepsy. In 2013, a study by Han et al. demonstrated that Shank3 overexpression could induce seizures in vivo (Han et al. 2013). To explore the underlying mechanism, Wang et al. carried out a kinome-wide siRNA screen and identified multiple kinases that potentially regulate Shank3 protein stability and serve as druggable target (Wang et al. 2019a, b, c). Specifically, ERK2 was found to bind, phosphorylate and promote the poly-ubiquitination-dependent degradation of Shank3. In 2016, Zhang et al. investigated Shank3 expression of in patients with intractable TLE and in pilocarpine-induced rat model of epilepsy, Shank3 protein levels and immunolabeling were increased in the neocortex of TLE patients and epileptic animals relative to controls (Zhang et al. 2016). Additionally, our group found that following seizure-induction, Shank1/2/3 mRNA was downregulated at acute stage, upregulated at latent stage, and returned to the basal level at chronic stage (Fu et al. 2020). We have also characterized an involvement of DNA methylation in the regulation of shank3 in epilepsy (Fu et al. 2020). Further, inhibition of Shank3 expression could significantly mitigate seizure severity at the acute stage of pilocarpine induced-epilepsy (own unpublished observation). Studies are currently carried out to explore the transcriptional mechanism relative to epileptogenesis in animal models and in clinical setting.
SHANK Gene Modified Animal Models of Neuropsychiatric Disorders
As elaborated above, SHANK gene alterations appear to be widely linked to the risks of multiple neuropsychiatric disorders according to GWAS data from human populations. The genetic variation profiles and the clinical manifestations appear to be also remarkably heterogeneous among the affected families and individuals. Clearly, the SHANK gene family plays pleiotropic neurobiological roles in the nervous system, which warrant thorough mechanistic investigations. In this regard, a number of animal models carrying specific Shank mutations have been established, with researches on these models extended valuable insights into the biological functions of this family as well as mechanistic underpinning of related brain disorders. In this section, we further denote major findings from studies on animals carrying Shank mutations, especially in the context of molecular and functional deficits related to neurobehavioral abnormalities.
Animal Models with Shank1 Mutations
There have been studies using mice in which exons 14-15 of Shank1 are deleted (Shank1 Δex14–15), resulting in the destruction of the PDZ region (Hung et al. 2008; Silverman et al. 2011; Wohr et al. 2011).These mice display mild anxiety-related behavior during the light–dark test, reduced exploratory locomotion in a novel open field and reduced ultrasonic vocalizations (USV). The animals also show reduced motor coordination and contextual fear conditioning, but present enhanced spatial learning or working memory. Levels of the postsynaptic proteins guanylate kinase-associated protein (GKAP) and Homer are reduced in the brain, along with reduced spine density, smaller and thinner PSD in CA1 region of the hippocampus. Moreover, studies have demonstrated early communication deficits and repetitive behaviors in the Shank1 knockout mouse model of ASD (Sungur et al. 2014, 2016). Notably, in Shank1 mutant mice with a targeted replacement of exons 14 and 15, the animals exhibit normal sociability, but insufficient object exploration and deficit in object recognition (Sungur et al. 2017). The findings from this mouse line also suggest that Shank1 is involved in neocortical-dependent associative learning (Collins and Galvez 2018) (Table 2).
Table 2.
Molecular and behavioral phenotypes of shanks mutant animals
| Gene | Animal species | Exons targeted | Disorders associated | Behavioural features | References | |||
|---|---|---|---|---|---|---|---|---|
| Repetitive behaviours | Social behaviors | Learning and Memory | Other behaviors | |||||
| SHANK1 | Mouse | Exon 7 (Δex7) | ASD | Increased self-grooming in females, increased locomotor activity and behaviours, reduced digging | Impaired social interaction | Impaired novel objective recognition memory | Increased anxiety-like behaviors | Schmeisser et al. (2012) |
| Mouse | Exons 14-15 (Δex14-15) | ASD | Increased repetitive self-grooming, reduced motor coordination | Deficit in social communicative behavior | Impaired fear conditioning, enhanced spatial learning, impaired retention of spatial memory | Increased anxiety-like behaviors in the light–dark test, reduced exploratory locomotion | Hung et al. (2008), Silverman et al. (2011) and Wohr et al. (2011) | |
| Mouse | Exons 14-15 (Δex14-15) | ASD | Not mentioned | Delay in early development of ultrasonic communication, deficit in social communication | Not mentioned | Not mentioned | Sungur et al. (2016) | |
| Mouse | Exons 14-15 (Δex14-15) | BPD | Reduced psychostimulant-induced hyperactivity | Not mentioned | Not mentioned | Not mentioned | Sungur et al. (2018) | |
| Mouse | Exons 14-15 (Δex14-15) | ASD | Not mentioned | No significant changes in social behaviors | Insufficient object exploration, deficit in object recognition | Not mentioned | Sungur et al. (2017) | |
| Mouse | Shank1 KO | – | Not mentioned | Not mentioned | Decline of neocortical dependent associative learning and memory consolidation | Not mentioned | Collins and Galvez (2018) | |
| Mouse | Shank1 KO | ASD | Not mentioned | Deficit in social communication | Not mentioned | Not mentioned | Sungur et al. (2016) | |
| SHANK2 | Mouse | Exons 6-7 (Δex6-7) | ASD | Enhance jumping, decreased digging behaviours, increased grooming in a novel object recognition arena | Reduced interaction, normal levels of social novelty recognition and olfactory | Impaired spatial learning and memory, normal levels of novel object recognition memory | Impaired nesting behavior, hyperactivity, increased anxiety-like behaviour | Won et al. (2012) and Lim et al. (2017) |
| Mouse | Exon 7 (Δex7) | ASD | Not mentioned | Social deficits | Deficits in spatial learning and memory | Not mentioned | Lim et al. (2017) | |
| Mouse | L7-cre; Exons 6-7 (Δex6-7) | ASD | No evidence of hyperactivity, task-specific repetitive behaviour | ASD-like social impairments | Impairments in motor learning | Intact baseline motor performance | Peter et al. (2016) | |
| Mouse | Exons 4-7 (Δex4-7) | ASD | No lesions | Normal initiation of social interaction in identical test, perturbed recognition of social novelty | Not mentioned | No anxiety-like behaviour | Peca et al. (2011) | |
| Mouse | Exon 24 (Δe24) | BPD | Hyperactivity | Deficits in social behaviors | Impaired in spatial learning, deficits in cognitive behaviors | Enhanced reward-seeking behavior, mania-like behaviors, circadian rhythms change | Pappas et al. (2017) | |
| Mouse | Pv-Cre; Exons 6-7 (Δex6-7) | ASD Seizure | Moderate hyperactivity, enhanced self-grooming in novel environment | Normal levels of social interaction | Normal learning and memory | Normal anxiety-like behavior | Lee et al. (2018) | |
| Mouse | Viaat-Cre; Exons 6-7 (Δex6-7) | ASD | Hyperactivity | Social interaction deficits, mild social communication deficits | Not mentioned | Anxiety-like behaviors | Kim et al. (2018) | |
| Mouse | CaMKII-Cre; Exons 6-7 (Δex6-7) | ASD | Repetitive self-grooming, mild hyperactivity | Social communication deficits | Not mentioned | Normal anxiety-like behaviors | Kim et al. (2018) | |
| Mouse | Exons 6-7 (Δex6-7) | ASD | Not mentioned | Not mentioned | Not mentioned | Impaired basal tactile perception and acute pain response, decreased sensitivity to chronic pain and inflammatory pain | Ko et al. (2016) | |
| Mouse | Exons 4-9 (Δex4-9B) | ASD | Excessive self-grooming, inflexible behavior | Reduced social sniffing, reduced reciprocal social interactions | Impaired novel object recognition | – | Bozdagi et al. (2010) and Yang et al. (2012) | |
| SHANK3 | Mouse | Exons 4-9 (Δex4-9J) | ASD | Excessive self-grooming, increased head pokes | Reduced interest, decreased bidirectional social interactions | Impaired short and long memory | – | Wang et al. (2011) |
| Mouse | Exons 4-9 (Δex4-9) | ASD/PMS | Increased repetitive grooming, | Abnormal social interaction | Impaired novel and spatial object recognition learning and memory | Normal locomotor activity and locomotor habituation | Jaramillo et al. (2016) | |
| Mouse | Exon 9 (Δex9) | – | Increased self-grooming | Normal | Impaired spatial memory | Increased rearing in a novel environment, absence of anxiety-like behavior | Lee et al. (2015) | |
| Mouse | Exon 11 (Δex11) | ASD | Increased self-grooming | Impaired social interaction | Impaired spatial memory | No anxiety-like behaviour, exhibit restricted interest and an avoidance phenotype when exposed to inanimate objects, exhibit only minor deficits in motor coordination, increased aggression and hyposensitivity to pain | Schmeisser et al. (2012) and Vicidomini et al. (2017) | |
| Mouse | Exons 12-13 (SHANKE13) | ASD | Increased repetitive grooming | Social interaction deficits | Impaired spatial learning | Reduced rearing activity | Jaramillo et al. (2017) | |
| Mouse | Exons 13-16 (Δex13-16) | ASD/PMS | Increased self-grooming | Normal social interaction | Deficits in discrimination learning | Normal locomotor activity | Copping et al. (2017) | |
| Mouse | Exons 13-16 (Δex13-16) | ASD | Excessive self-injurious grooming, causing skin lesion | Dysfunctional social interaction behavior, decreased reciprocal interaction in interacting dyadic test, decreased anogenital sniffing, decreased frequency of nose-to-nose interaction | Not mentioned | Increased anxiety-like behavior, reduced exploratory locomotion | Peca et al. (2011), Guo et al. (2019) and Wang et al. (2017) | |
| Mouse | Glutamatergic Shank3 (exons 14–16) Δex14–16 | – | Enhanced repetitive self-grooming, normal digging | Enhanced direct social interaction, normal social approach and social novelty recognition | Not mentioned | Normal locomotor activity and enhanced anxiolytic-like behavior | Yoo et al. (2019a, b) | |
| Mouse | GABAergic Shank3 (exons 14–16) Δex14–16 | ASD | Enhanced self-grooming and suppressed digging and enhanced rearing | Enhanced direct social interaction and suppressed social communication | Not mentioned | Show hypoactivity in a novel environment, increased anxiety-like behaviors in the light–dark test, differential influences on other types of anxiety-like behaviors | Yoo et al. (2018) | |
| Mouse | Global Shank3 Exons 14-16 Δex14–16 | ASD | Enhanced self-grooming but digging and climbing behavior was suppressed | Enhanced direct social interaction and suppressed social communication | Not mentioned | Show hypoactivity in a novel environment, increased anxiety-like behaviors in the light–dark test, differential influences on other types of anxiety-like behaviors | Yoo et al. (2018) | |
| Exon 21 (Δex21) | ASD | Increased grooming in older mice | Minimal social interaction, normal communication | Impaired in spatial learning, impaired spatial memory during the probe trial | Impaired motor coordination, hypersensitivity to heat, avoidance toward inanimate objects, absence of anxiety-like behavior, aberrant locomotor response to novelty | Kouser et al. (2013), Qin et al. (2018) and Ma et al. (2018) | ||
| Exon 21 (lnsG3680) | ASD | Robust repetitive/compulsive grooming behavior, skin lesion between 4 and 6 months of age | Social interaction deficits | Impaired motor learning, normal basic working memory | Increased anxiety-like behavior, reduced locomotion, impaired coordination capability, reduced PPI | Zhou et al. (2016) | ||
| Exon 21 (R1117X) | SCZ | Allogrooming/barbering, skin lesion between 4 and 6 months of age | Social interaction deficits, social dominance behavior | Impaired motor learning, normal basic working memory | Increased anxiety-like behavior, reduced locomotion, impaired coordination capability, impaired PPI | Zhou et al. (2016) | ||
| Exons 4-22 (Δex4-22) | ASD | Excessive self-grooming causing skin lesion | Normal | Impaired spatial memory and learning | Reduced locomotion, reduced startle reactivity | Wang et al. (2016a, b) | ||
| Mouse | Exons 4-22 (Δex4-22) | PMS | Excessive Self-grooming | Normal social interaction | Contextual learning impaired, working memory not altered | Developmental delays, response delays to auditory startle, reduced spontaneous locomotion, impaired motor coordination and balance sensory abilities (sound discrimination, strong visual and olfactory function), increased freezing under fear condition, increased novelty-induced anxiety | Drapeau et al. (2018) | |
| Mouse | Exons 4-22 (Δex4-22) | PMS | Excessive self-grooming | Normal social interaction | Contextual learning impaired, working memory not altered | Developmental delays, response delays to auditory startle, reduced spontaneous locomotion, impaired motor coordination and balance sensory abilities (sound discrimination, strong visual and olfactory function), increased freezing under fear condition, increased novelty-induced anxiety | Drapeau et al. (2018) | |
| Mouse | Shank3B KO | ASD | Exhibited repetitive grooming | Deficits in reciprocal social interactions | Impaired learning and memory | Reduced open field activity, deficits in sensory responses, show anxiety-related behaviors | Dhamne et al. (2017) | |
| Mouse | Shank3B KO | ASD | Not mentioned | Not mentioned | Show learning deficits | Enhanced pitch discrimination | Rendall et al. (2019) | |
| Mouse | Shank3B KO | ASD | Increased repetitive grooming | Normal social communication, impaired social interactions | Not mentioned | No anxiety-like behaviors | Balaan et al. (2019) | |
| Mouse | Exon 8 (Q321R) | ASD | Increased repetitive grooming | Normal social interaction and social communication | Normal learning and memory | Normal locomotor activity, increased anxiety-like behavior, decreased seizure susceptibility | Yoo et al. (2019a, b) | |
| Rat | Exon 6 (Δex6) | PMS | Not mentioned | Not mentioned | Deficits in long-term social recognition memory | No anxiety-like behaviors, deficits in attention | Harony-Nicolas et al. (2017) | |
| Rat | Exon 6 (Δex6) | PMS | Not mentioned | Not mentioned | Not mentioned | Exhibit degraded cortical responses to sound | Engineer et al. (2018) | |
| Rat | Shank3 knock-out | ASD/PMS | Not mentioned | Behavioral responses to pro-social 50-kHz USV are reduced, deficits in a number of key juvenile social play interactions | Not mentioned | Norman motor activity in a novel open field arena, reduced ultrasonic vocalizations | Berg et al. (2018) | |
| Macaques | Exon 21 (lnsG3680) | ASD | Increased repetitive behaviours | Social interaction deficits | Impaired spatial learning | Motor deficits | Zhou et al. (2019) | |
ASD autism spectrum disorder, PMS Phelan–McDermid Syndrome, SCZ schizophrenia, PPI pre-pulse inhibition, BPD bipolar disorder
Animal Models with Shank2 Mutations
Studies have been carried on a few Shank2 mutant mouse lines (Table 2). One group of investigators developed Shank2 exons 6 and 7 deletion mice (Shank2 Δex6-7) (Won et al. 2012; Lim et al. 2017). The animals exhibit increased self-grooming, increased anxiety-like behavior and locomotor activity, reduced social interaction by USV, impaired spatial learning and memory. Another group generated Shank2 mutant mice with the exon 7 deleted (Shank2 Δex7), the mutant mice display social deficits and impaired spatial learning and memory (Lim et al. 2017). Moreover, Lim et al. demonstrated that enhancing inhibitory synaptic function can reverse spatial memory deficits in Shank2 mutant mice (Lim et al. 2017). In 2010, Berkel et al. generated shank2 mutant mice with a 120 kb deletion selectively disrupting exon 7, and found that the mutation did not result in autism-like behavior nor intellectual disability (Berkel et al. 2010). In addition, Shank2 Δe24−/− mice are anhedonic, display significantly increased locomotor activity, abnormal reward-seeking behavior, have perturbations in circadian rhythms, and show deficits in social and cognitive behaviors. The major behavioral features in these mice are considered to be reminiscent of bipolar disorder (Pappas et al. 2017).
Many molecular changes related to synapses have been reported in Shank2 mutant mice. InΔex6-7 mice, the protein level of triheteromeric subunit GluN1 of the N-methyl-d-aspartate (NMDA) glutamate receptor is increased in the hippocampus, while the level of GluA1 subunit of amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) is reduced (Won et al. 2012). In shank2 Δex7 mice, synaptic proteins of the glutamate receptor sbunits, GluN1, GluN2A and GluA2, in striatum, and GluN1 and GluN2B in hippocampus, are increased (Schmeisser et al. 2012). The Shank2 Δex6-7 mice display impaired basal tactile perception, acute pain response as well as decreased sensitivity to chronic pain and inflammatory pain (Ko et al. 2016). Reduced spine density in hippocampal CA1 is also observed in Shank2Δex7 mice. The structure of PSD is not apparently changed in the latter two Shank2mutant mouse lines.
A series of studies have demonstrated that specific cell type deletions of Shank2 in mice can result in diverse synaptic and behavioral abnormalities. Male mice lacking Shank2 in excitatory neurons (CaMKII-Cre;Shank2fl/fl) exhibit social interaction deficits and mild social communication deficits, hyperactivity, and anxiety-like behaviors. Notably, male mice lacking Shank2 in GABAergic inhibitory neurons (Viaat-Cre;shank2fl/fl) also show social repetitive self-grooming, communication deficits, and mild hyperactivity. These behavioral changes were associated with distinct changes of hippocampal and striatal neural circuits in the two mouse lines (Kim et al. 2018). Mice with Shank2 deletion in parvalbumin neurons exhibit moderate hyperactivity, enhanced self-grooming and suppressed seizure susceptibility, whereas the social behaviors, learning and memory and even anxiety level in the animals appear normal (Seungjoon et al. 2018). In addition, mice with Shank2-deficient in cerebellar Purkinje cells display abnormal task-specific repetitive behavior, impairments in motor learning and ASD-like social impairment (Peter et al. 2016).
A recent study found a novel Shank2 transcriptional variant—exon 4′, located between exons 4 and 5 (Lee et al. 2020). Exon 4′ appears to be a modifying domain participating in genetic compensation for ASD patients, and may bring out phenotypic heterogeneity in Shank2 Δex6-7 mice model (Lee et al. 2020). Wegener et al. also demonstrated genetic interactions in the Shank2 mutant mice, by comparing Shank2 Δex7 mice with Shank2 Δex6-7 mice (Wegener et al. 2018).
Animal Models with Shank3 Mutations and Knockout (KO)
Multiple lines of Shank3 mutant mice and, more recently, rats and macaques that carry global, conditional and point mutations or knockout (KO) in shank3, have been generated (Berg et al. 2018; Harony-Nicolas et al. 2017; Zhou et al. 2019) (Table 2). Molecular and behavioral characterizations have provided much information about normal and disease-related functions of Shank3. Overall, these animals display diverse synaptic, neuronal, brain connectivity and behavioral abnormalities. Studies on these animals have extended substantial understanding into how Shank3 mutations lead to various phenotypic abnormalities (Drapeau et al. 2018; Schmeisser et al. 2012; Peca et al. 2011; Bozdagi et al. 2010; Wang et al. 2011, 2017; Kouser et al. 2013; Lee et al. 2015; Jaramillo et al. 2015; Wang et al. 2016a, b; Copping et al. 2017; Guo et al. 2019; Yoo et al. 2018, 2019a, b; Dhamne et al. 2017; Kabitzke et al. 2018; Vicidomini et al. 2016; Wang et al. 2019a, b, c; Qin et al. 2018; Wang et al. 2019a, b, c).
The Shank3 mutation mouse models include those with deletions of exons 4-9 (Shank3Δex4-9, Shank3Δex4-9B and Shank3Δex4-9J), exons 4-7 (Shank3 Δex4-7), exons 12-13 (Δex12-13), exons 13-16 (Shank3Δex13-16), exons 14-16 (Shank3Δex14-16), exon 6 (Shank3 Δex6), exon 8 (Shank3 Δex8), exon 9 (Shank3 Δex9), exon 11 (Shank3 Δex11), exon 21 (Shank3 Δex21), exons 4-22 (Shank3 Δex4-22) and so on (Drapeau et al. 2018; Schmeisser et al. 2012; Berg et al. 2018; Peca et al. 2011; Bozdagi et al. 2010; Wang et al. 2011, 2017; Kouser et al. 2013; Lee et al. 2015; Jaramillo et al. 2015; Wang et al. 2016a, b; Copping et al. 2017; Guo et al. 2019; Yoo et al. 2018, 2019a, b; Dhamne et al. 2017; Kabitzke et al. 2018; Vicidomini et al. 2016; Engineer et al. 2018). Repetitive behaviors and increased self-grooming are observed in all Shank3 mutant lines, most of which also show social interaction deficits. Anxiety-like behaviors and abnormal locomotor activity, and learning and memory deficits, are commonly found in these Shank3 mutant animals. In behavioral phenotyping experiments, self-injurious grooming causing skin lesions is observed in some Shank3 hile normal social behaviors are found in other Shank3 mutant lines (Δex4-7, Δex13-16, Δex9, glutamatergic exons 14-16, Δex21, Δex4-22 and Shank3BKO) (Drapeau et al. 2018; Peca et al. 2011; Kouser et al. 2013; Lee et al. 2015; Wang et al. 2016a, b; Copping et al. 2017; Yoo et al. 2018, 2019a, b; Chantell et al. 2018; Yoo et al. 2019a, b). Normal learning and memory are observed in the Shank3 (Δex8) mice (Yoo et al. 2019a, b), while no anxiety-like behaviors are found in the Shank3 (Δex4-7, Δex9, Δex21 and Shank33B KO) mouse (Peca et al. 2011; Kouser et al. 2013; Lee et al. 2015; Chantell et al. 2018) and Δex6 rat (Berg et al. 2018). The locomotor activity appears normal in the Shank3 (Δex4-9, Δex8, Δex13-16 and glutamatergic exons 14-16) mice (Jaramillo et al. 2011; Copping et al. 2017; Yoo et al. 2018, 2019a, b) and in the Shank3 KO rat (Berg et al. 2018).
The Shank3 Δex4-22 mice present normal levels of social interest, but they persist in unsuccessful efforts to engage the social partner and exhibit non-social behaviors that include repetitive self-grooming behavior during social encounter event (Wang et al. 2016a, b). Although the Shank3 Δex9 mice do not show autistic-like behavior, they show mildly impaired spatial memory and increased rearing in a novel environment (Lee et al. 2015). In 2013, Han et al. reported that Shank3 transgenic mice with an N-terminal EGFP-tag into this gene exhibited manic-like behaviors, which appears to recapitulate the phenotype of human SHANK3 duplication (Han et al. 2013). In addition, Zhou et al. generated two Shank3 mutant mouse lines (lnsG3680 and R1117X mutation) (Yang et al. 2016). The mice with the ASD-linked lnsG3680 mutation exhibit stronger repetitive/compulsive grooming behavior. The mice with the SCZ-linked R1117X mutation manifest stronger allogrooming and social dominance-like behavior.
Findings from the animal models support that Shank3 mutations can affect the structure and function of excitatory synapses. The loss of Shank3 leads to an increase in metabotropic glutamate receptor 5 (mGluR5) in both synaptosome and postsynaptic density-enriched fractions in the hippocampus (Kouser et al. 2013). Co-localization studies show that mGluR5 and PSD-95 are increased in the striatum in Shank3Δex4-22 mice (Wang et al. 2016a, b). GluA2, GluN2A, and GluN2B, SAPAP3/GKAP3, Homer1, and PSD-93 are reduced in Shank3Δex13-16 mice (Peca et al. 2011). Wang et al. reported that Shank3 mutation preferentially affects glutamatergic synaptic transmission in the striatopallidal medium spiny neurons (MSNs), while enhancing this transmission via a Gq-coupled human M3 muscarinic receptor (hM3Dq) can rescue the repetitive grooming behavior (Wang et al. 2017). In 2017, Jaramillo et al. generated Shank3 mutant mice with a transcriptional stop cassette prior to exon 13 (Shank3E13). These mice showed impaired hippocampal LTP and striatal glutamatergic synaptic transmission, displayed increased repetitive grooming and decreased rearing, spatial memory deficits and impaired social interaction (Jaramillo et al. 2017). In addition, conditional knockout of Shank3 in the anterior cingulate cortex (ACC) resulted in excitatory synaptic dysfunction. The animals exhibited apparent social interaction deficits, which can be reversed by restoration of Shank3 expression in the ACC, or systemic administration with an α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor-positive modulator (Guo et al. 2019).
Lately, whole genomic and neuronal subtype specific Shank3 deletion mouse models are generated (Drapeau et al. 2018; Yoo et al. 2018, 2019a, b). The synaptic and behavioral deficits seen in global Shank3Δ14–16, GABAergic Shank3Δ14–16 and glutamatergic Shank3Δ14–16 deletion mice are characterized in detail. For instance, neuronal excitability is abnormally increased in layer 2/3 pyramidal neurons in the medial prefrontal cortex (mPFC) in mouse with a glutamatergic Shank3 and global Shank3 deletion models. Direct social interaction and repetitive self-grooming are abnormally increased in the above three lines. One study also examined the changes of adult genetic reversal to rescue phenotypes in a Shank3 mutant mice, indicating that re-expression of Shank3 in adult mice leads to improvement in synaptic structure and function in the striatum, along with rescue of behavioral abnormalities including social interaction deficit and repetitive grooming, whereas anxiety and motor coordination deficit could not be recovered (Mei et al. 2016). Another study showed that early restoration of Shank3 expression in the knockout mice could ameliorate the ASD-like behavioral phenotypes (Jaramillo et al. 2020).
In the past, Shank genes editing has been mainly focused on rodents, but recently Zhou et al. created genetically engineered non-human primate model for the first time. Primate models of psychiatric disorders are expected to better recapitulate the behavioral phenotypes of autism spectrum and other disorders in humans, and may be more suited for the evaluation of therapeutics. The cynomolgus macaques with Shank3 mutation showed increased repetitive behaviour, motor deficits and sleep disturbances as well as social and learning impairments, resembling the clinical features of human autism (Zhou et al. 2019).
We attempt to summarize the molecular and behavioral phenotypes of shanks mutant animals, with the major features from different models included in Table 2. Overall, all Shank3 mutant animals manifest repetitive and increased self-grooming behaviors, most of them showed abnormal social communication and interaction, impaired learning and memory, reduced locomotion activity and increased anxiety-like behavior. However, it should be noted that different or different extents of behavioral deficits can exist in Shank3 mutant lines, even in the same Shank3 mutants as shown in different studies. Therefore, much additional work is needed to understand the role of Shank proteins in context of the involvement of neural pathways and circuits.
Conclusions and Future Perspectives
SHANKs genes can generate multiple protein isoforms that are distinctively expressed in the brain dependent on developmental stages, cell types and anatomical locations. Growing evidence from genome association studies strongly suggests a connection between SHANK gene alterations and some neuropsychiatric disorders, especially ASD and SCZ, with mutations in SHANK2 and SHANK3 may be a potential monogenic cause for ASD. More researches on SHANK association with TLE are expected to uncover some of the neurobiological role of the proteins in modulating excitatory synaptic circuitries involved in epileptogenesis. In this regard, it is worth noting that most neuropsychiatric disorders are caused by the absence or haploinsufficiency of SHANK genes, whereas SHANK overexpression is observed in temporal lobe epilepsy.
Animals carrying different shank mutations show distinct and shared molecular and behavioral characteristics. All mutants appear to alter excitatory synapses and their neurotransmission. Various mutations of the same gene may cause synaptic and circuitry defects resulting in different neurobehavioral phenotypes and clinical features. Again, more studies are needed to understand underlying neurocircuitry changes. Albeit numerous questions remain unresolved, studies on SHANK family in human and animal models have provided novel molecular bases for neuropsychiatric disorders.
Abbreviations
- PSD
Postsynaptic density
- ASD
Autism spectrum disorders
- PMS
Phelan–McDermid Syndrome
- AD
Alzheimer’s disease
- SCZ
Schizophrenia
- BPD
Bipolar disorders
- ID
Intellectual disability
- DD
Developmental delay
- GWAS
Genome-Wide Association Studies
- SNP
Single nucleotide polymorphism
- NMDA
N-Methyl-d-aspartate
- ANK
Ankyrin repeat domain
- PDZ
Postsynaptic density protein 95-discs large homologue 1-zonula occludens 1 domain
- PRO
Proline-rich region
- SAM
Sterile alpha motif
- SH3
SRC homology 3 domain superfamily
- BDNF
Brain-derived neurotrophic factor
- AMPA
Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- DLGAP1, 2, 3
Discs large associated protein 1, 2, 3
- SLC6A3
Solute carrier family 6 (neurotransmitter transporter, dopamine), member 3
Author Contributions
L-W and D-L drafted the manuscript; W-BX and B-XZ draw the figures; Z-HL designed the outline and revised the manuscript; X-XY proof-edited and finalized the manuscript; BX provided financial support. All of the authors have approved of the publication of the manuscript.
Funding
This work has been supported through funding from the National Natural Science Foundation, China (Grant Number 81671299), The Hunan Natural Science Foundation (Grant Number 2018JJ2648).
Data Availability
All data and material files are available upon request.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical Approval
All procedures were followed in accordance with the Ethical Standards of the Responsible Committee on Animal and Human Experimentation (The Ethics Review Committee of Xiangya Hospital, Numbers 201603296 and 201603297) and with the Helsinki Declaration of 1964 and later versions.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lily Wan and Du Liu have contributed equally to this work.
Contributor Information
Zhao-Hui Luo, Email: luozhaohui_xy@126.com.
Bo Xiao, Email: xiaobo_xy@126.com.
References
- Alexandrov PN, ZhaoY VJ, Lin C, Lukiw WJ (2017) Deficits in the proline-rich synapse-associated Shank3 protein in multiple neuropsychiatric disorders. Front Neurol 8:670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ana DSC, Degenhardt F, Strohmaier J et al (2017) Investigation of SHANK3 in schizophrenia. Am J Med Genet B 174:390–398 [DOI] [PubMed] [Google Scholar]
- Bai Y, Qiu S, Li Y et al (2018) Genetic association between SHANK2 polymorphisms and susceptibility to autism spectrum disorder. IUBMB Life 70:763–776 [DOI] [PubMed] [Google Scholar]
- Balaan C, Corley MJ, Eulalio T et al (2019) Juvenile Shank3b deficient mice present with behavioral phenotype relevant to autism spectrum disorder. Behav Brain Res 356:137–147 [DOI] [PMC free article] [PubMed]
- Berg EL, Copping NA, Rivera JK et al (2018) Developmental social communication deficits in the Shank3 rat model of Phelan–McDermid syndrome and autism spectrum disorder. Autism Res Off J Int Soc Autism Res 11:587–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel S, Marshall CR, Weiss B et al (2010) Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 42:489–491 [DOI] [PubMed] [Google Scholar]
- Bey AL, Wang X, YanH KN, Passman RL, Yang Y et al (2018) Brain region-specific disruption of Shank3 in mice reveals a dissociation for cortical and striatal circuits in autism-related behaviors. Transl Psychiatry 8:94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuto L, Lauri M, Sarasua SM, Skinner CD, Buccella D, Dwivedi A et al (2013) Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur J Hum Genet 21:310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böckers TM, Mameza MG, Kreutz MR et al (2001) Synaptic scaffolding proteins in rat brain: ankyrin b repeats of the multidomain Shank protein family interact with the cytoskeletal protein α-fodrin. J Biol Chem 276:40104–40112 [DOI] [PubMed] [Google Scholar]
- Boeckers TM, Kreutz MR, Winter C et al (1999) Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci 19:6506–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckers TM, Liedtke T, Spilker C et al (2005) C-Terminal synaptic targeting elements for postsynaptic density proteins ProSAP1/ Shank2 and ProSAP2/Shank3. J Neurochem 92:519–524 [DOI] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Beri S et al (2011) Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet 7:e1002173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Borgatti R et al (2001) Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet 69:261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Mani E et al (2006) Identification of a recurrent breakpoint within the SHANK3 gene in the 22q13.3 deletion syndrome. J Med Genet 43:822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Scattoni ML et al (2010) Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 1:15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt-Marie A, Jacqueline SG, Ran A et al (2002) FISH-mapping of a 100-kb terminal 22q13 deletion. Hum Genet 110:439–443 [DOI] [PubMed] [Google Scholar]
- Chantell B, Corley MJ, Tiffany E et al (2018) Juvenile Shank3b deficient mice present with behavioral phenotype relevant to autism spectrum disorder. Behav Brain Res 356:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Chen HI, Liao HM et al (2016) Clinical and molecular characterization of three genomic rearrangements at chromosome 22q13.3 associated with autism spectrum disorder. Psychiatr Genet 27:23–33 [DOI] [PubMed] [Google Scholar]
- Chilian B, Abdollahpour H, Bierhals T et al (2013) Dysfunction of SHANK2 and CHRNA7 in a patient with intellectual disability and language impairment supports genetic epistasis of the two loci. Clin Genet 84:560–565 [DOI] [PubMed] [Google Scholar]
- Cochoy DM, Kolevzon A, Kajiwara Y, Schoen M, Pascual LM, Lurie S et al (2015) Phenotypic and functional analysis of SHANK3 stop mutations identified in individuals with ASD and/or ID. Mol Autism 6:23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Galvez R (2018) Neocortical SHANK1 regulation of forebrain dependent associative learning. Neurobiol Learn Mem 155:173–179 [DOI] [PubMed] [Google Scholar]
- Copping NA, Berg EL, Foley GM et al (2017) Touchscreen learning deficits and normal social approach behavior in the Shank3B model of Phelan–McDermid Syndrome and autism. Neuroscience 345:155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet 19:R12-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli C, Chiesa A, Han C et al (2013) Case–control association study of 36 single-nucleotide polymorphisms within 10 candidate genes for major depression and bipolar disorder. Psychiatry Res 209:121–123 [DOI] [PubMed] [Google Scholar]
- De Rubeis S, Siper PM, Durkin A et al (2018) Delineation of the genetic and clinical spectrum of Phelan–McDermid syndrome caused by SHANK3 point mutations. Mol Autism 9:31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denayer A, Van Esch H, Ravel TD et al (2012) Neuropsychopathology in 7 patients with the 22q13 deletion syndrome: presence of bipolar disorder and progressive loss of skills. Mol Syndromol 3:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamne SC, Silverman JL, Supe CE (2017) Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism 8:26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Riad M, Kajiwara Y, Buxbaum JD (2018) Behavioral phenotyping of an improved mouse model of Phelan–McDermid Syndrome with a complete deletion of the Shank3 gene. eNeuro 5:ENEURO.0046-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F et al (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39:25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Rahebi KC, Borland MS, Buell EP, Kilgard MP (2018) Shank3-Deficient rats exhibit degraded cortical responses to sound: Shank3 rats exhibit degraded cortical responses. Autism Res 11:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failla P, Romano C, Alberti A (2007) Schizophrenia in a patient with subtelomeric duplication of chromosome 22q. Clin Genet 71:599–601 [DOI] [PubMed] [Google Scholar]
- Flint J, Wilkie AO, Buckle VJ, Winter RM, Holland AJ, McDermid HE (1995) The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nat Genet 9:132–140 [DOI] [PubMed] [Google Scholar]
- Fischetto R, Palumbo O, Ortolani F et al (2017) Clinical and molecular characterization of a second family with the 12q14 microdeletion syndrome and review of the literature. Am J Med Genet A 173:1922–1930 [DOI] [PubMed] [Google Scholar]
- Fromer MPA, Kavanagh DH, Williams HJ et al (2014) De novo mutations in schizophrenia implicate synaptic networks. Nature 506:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YJ, Liu D, Guo JL et al (2020) Dynamic change of shanks gene mRNA expression and DNA methylation in epileptic rat model and human patients. Mol Neurobiol 57:3712–3726 [DOI] [PubMed] [Google Scholar]
- Gauthier J, Champagne N, Lafreniere RG et al (2010) De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci USA 107:7863–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Spiegelman D, Piton A et al (2009) Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B 150B:421–424 [DOI] [PubMed] [Google Scholar]
- Gong Y, Lippa CF, Zhu J, Lin Q, Rosso AL (2009) Disruption of glutamate receptors at Shank-postsynaptic platform in Alzheimer’s disease. Brain Res 1292:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouder L, Vitrac A, Goubran-Botros H et al (2019) Altered spinogenesis in iPSC-derived cortical neurons from patients with autism carrying de novo SHANK3 mutations. Sci Rep 9:94 [DOI] [PMC free article] [PubMed]
- Grabrucker AM, Schmeisser MJ, Schoen M, Boeckers TM (2011) Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol 21:594–603 [DOI] [PubMed] [Google Scholar]
- Guo B, Chen J, Chen Q et al (2019) Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci 22:1223–1234 [DOI] [PubMed] [Google Scholar]
- Guilmatre A, Huguet G, Delorme R et al (2014) The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol 74:113–122 [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–112 [DOI] [PubMed] [Google Scholar]
- Han K, Holder JL, Schaaf CP et al (2013) SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 503:72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harony-Nicolas H, Kay M, du Hoffmann J et al (2017) Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. eLife 6:e18904 [DOI] [PMC free article] [PubMed]
- Hayashi MK, Tang C, Verpelli C et al (2009) The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann OR, Misura K, Lamas E et al (2016) Whole-genome sequencing in multiplex families with psychoses reveals mutations in the SHANK2 and SMARCA1 genes segregating with illness. Mol Psychiatry 21:1690–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C et al (2008) Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci 28:1697–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo TC, Speed HE, Xuan Z, Reimers JM, Liu S, Powell CM (2015) Altered striatal synaptic function and abnormal behaviour in Shank3 Exon 4–9 deletion mouse model of autism. Autism Res Off J Int Soc Autism Res 9:350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo TC, Speed HE, Xuan Z et al (2016) Altered striatal synaptic function and abnormal behaviour in Shank3 Exon4-9 deletion mouse model of autism. Autism Res 9:350–375 [DOI] [PMC free article] [PubMed]
- Jaramillo TC, Speed HE, Xuan Z et al (2017) Novel Shank3 mutant exhibits behaviors with face validity for autism and altered striatal and hippocampal function. Autism Res 10:42–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo TC, Xuan Z, Reimers JM, Escamilla CO, Liu S, Powell CM (2020) Early restoration of Shank3 expression in Shank3 knockout mice prevents core ASD-like behavioural phenotypes. eNeuro 7:ENEURO03320319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries AR, Curran S, Elmslie F et al (2005) Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A 137:139–147 [DOI] [PubMed] [Google Scholar]
- Kabitzke PA, Brunner D, He D et al (2018) Comprehensive analysis of two Shank3 and the Cacna1c mouse models of autism spectrum disorder. Genes Brain Behav 17:4 [DOI] [PubMed] [Google Scholar]
- Kanani F, Study D, Balasubramanian M (2018) SHANK3 variant as a cause of nonsyndromal autism in an 11-year-old boy and a review of published literature. Clin Dysmorphol 27:1 [DOI] [PubMed] [Google Scholar]
- Kim R, Kim J, Chung C, Ha S, Kim E (2018) Cell-type-specific Shank2 deletion in mice leads to differential synaptic and behavioral phenotypes. J Neurosci 38:2684–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlenberg TM, Trelles MP, McLarney B, Betancur C, Thurm A, Kolevzon A (2020) Psychiatric illness and regression in individuals with Phelan–McDermid syndrome. J Neurodev Disord 12:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HG, Oh SB, Zhuo M, Kaang BK (2016) Reduced acute nociception and chronic pain in Shank2−/− mice. Mol Pain 12:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Cai G, Soorya L et al (2011) Analysis of a purported SHANK3 mutation in a boy with autism: clinical impact of rare variant research in neurodevelopmental disabilities. Brain Res 1380:98–105 [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Delaby E, Berry-Kravis E, Buxbaum JD, Betancur C (2019) Neuropsychiatric decompensation in adolescents and adults with Phelan–McDermid syndrome: a systematic review of the literature. Mol Autism 10:50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouser M, Speed HE, Dewey CM et al (2013) Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J Neurosci 33:18448–18468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CS, Heinrich J, Delorme R et al (2012) Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet 8:e1002521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CS, Nava C, Polge A et al (2014) Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments. PLoS Genet 10:e1004580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Chung C, Ha S et al (2015) Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front Cell Neurosci 9:94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee E, Kim R et al (2018) Shank2 deletion in parvalbumin neurons leads to moderate hyperactivity, enhanced self-grooming and suppressed seizure susceptibility in mice. Front Mol Neurosci 11:209 [DOI] [PMC free article] [PubMed]
- Lee YS, Yu NK, Chun JW et al (2020) Identification of a novel Shank2 transcriptional variant in Shank2 knockout mouse model of autism spectrum disorder. Mol Brain 13:54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennertz L, Wagner M, Wolwer W et al (2012) A promoter variant of SHANK1 affects auditory working memory in schizophrenia patients and in subjects clinically at risk for psychosis. Eur Arch Psychiatry Clin Neurosci 262:117–124 [DOI] [PubMed] [Google Scholar]
- Lim CS, Kim H, YuNK KSJ, Kaang BK (2017) Enhancing inhibitory synaptic function reverses spatial memory deficits in Shank2 mutant mice. Neuropharmacology 112:104–112 [DOI] [PubMed] [Google Scholar]
- Lim S, Naisbitt S, Yoon J et al (1999) Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem 274:29510–29518 [DOI] [PubMed] [Google Scholar]
- Lim S, Sala C, Yoon J et al (2001) A novel postsynaptic density protein that directly interacts with the shank family of proteins. Mol Cell Neurosci 17:385–397 [DOI] [PubMed] [Google Scholar]
- Luciani JJ, Mas PD, Depetris D et al (2003) Telomeric 22q13 deletions resulting from rings, simple deletions, and translocations: cytogenetic, molecular, and clinical analyses of 32 new observations. J Med Genet 40:690–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, Kerr JM, Kassner J, Frost NA, Blanpied TA (2016) Shank-cortactin interactions control actin dynamics to maintain flexibility of neuronal spines and synapses. Eur J Neurosci 43:179–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameza MG, Dvoretskova E, Bamann M et al (2013) SHANK3 gene mutations associated with autism facilitate ligand binding to the shank3 ankyrin repeat region. J Biol Chem 288:26697–26708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB et al (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcou CA, Studinski Jones AL, Murphree ML, Kirmani S, Hoppman NL (2017) De novo 11q deletion including SHANK2 in a patient with global developmental delay. Am J Med Genet A 173:801–805 [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Nagarajan RP, Bilenky M et al (2020) Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 466:253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Monteiro P, Zhou Y et al (2016) Adult restoration of Shank3 expression rescues selective autistic like phenotypes. Nature 530:481–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P, Feng GP (2017) SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci 18:147–157 [DOI] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J et al (2007) Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet 81:1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossa A, Giona F, Pagano J, Sala C, Verpelli C (2017) SHANK genes in autism: defining therapeutic targets. Prog Neuropsychopharmacol Biol Psychiatry 84:416–423 [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC et al (1999) Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 23:569–582 [DOI] [PubMed] [Google Scholar]
- Nesslinger NJ, Gorski JL, Kurczynski TW et al (1994) Clinical, cytogenetic, and molecular characterization of seven patients with deletions of chromosome 22q13.3. Am J Hum Genet 54:464–472 [PMC free article] [PubMed] [Google Scholar]
- Noor A, Lionel AC, Cohen-Woods S et al (2014) Copy number variant study of bipolar disorder in Canadian and UK populations implicates synaptic genes. Am J Med Genet B 165B:303–313 [DOI] [PubMed] [Google Scholar]
- Okamoto N, Kubota T, Nakamura Y et al (2007) 22q13 Microduplication in two patients with common clinical manifestations: a recognizable syndrome? Am J Med Genet A 143A:2804–2809 [DOI] [PubMed] [Google Scholar]
- Ortiz A, Bradler K, Garnham J, Slaney C, Alda M (2015) Nonlinear dynamics of mood regulation in bipolar disorder. Bipolar Disord 17:139–149 [DOI] [PubMed] [Google Scholar]
- Pappas AL, Bey AL, Wang X, Rossi M, Jiang YH (2017) Deficiency of Shank2 causes mania-like behavior that responds to mood stabilizers. JCI Insight 2:e92052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT et al (2011) Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472:437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter SA, Ten Brinke MM, Stedehouder J et al (2016) Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat Commun 1:12627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peykov S, Berkel S, Schoen M et al (2015) Identification and functional characterization of rare SHANK2 variants in schizophrenia. Mol Psychiatry 20:1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham E, Crews L, Ubhi K et al (2010) Progressive accumulation of amyloid-beta oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J 277(14):3051–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan K, McDermid HE (2012) The 22q13.3 deletion syndrome (Phelan–McDermid syndrome). Mol Syndromol 2:186–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L et al (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466:368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Ma K, Wang ZJ, Hu Z, Yan Z (2018) Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat Neurosci 21:564–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Li Y, Bai Y et al (2019) SHANK1 polymorphisms and SNP–SNP interactions among SHANK family: a possible cue for recognition to autism spectrum disorder in infant age. Autism Res 12:375–383 [DOI] [PubMed] [Google Scholar]
- Qiu S, Li Y, Li Y et al (2018) Association between SHANK3 polymorphisms and susceptibility to autism spectrum disorder. Gene 651:100–105 [DOI] [PubMed] [Google Scholar]
- Rendall AR, Perrino PA, Buscarello AN, Fitch RH (2019) Shank3B mutant mice display pitch discrimination enhancements and learning deficits. Int J Dev Neurosci 72:13–21 [DOI] [PubMed]
- Roselli F, Hutzler P, Wegerich Y, Livrea P, Almeida OF (2009) Disassembly of shank and homer synaptic clusters is driven by soluble beta-amyloid(1–40) through divergent NMDAR-dependent signalling pathways. PLoS ONE 4:e6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasua SM, Boccuto L, Sharp JL et al (2014) Clinical and genomic evaluation of 201 patients with Phelan–McDermid syndrome. Hum Genet 133:847–859 [DOI] [PubMed] [Google Scholar]
- Sato D, LionelAC LCS et al (2012) SHANK1 deletions in males with autism spectrum disorder. Am J Hum Genet 90:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser MJ, Ey E, Wegener S et al (2012) Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 486:256–260 [DOI] [PubMed] [Google Scholar]
- Seungjoon L, Eunee L, Ryunhee K et al (2018) Shank2 deletion in parvalbumin neurons leads to moderate hyperactivity, enhanced self-grooming and suppressed seizure susceptibility in mice. Front Mol Neurosci 11:209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E (2000) The Shank family of scaffold proteins. J Cell Sci 113:1851–1856 [DOI] [PubMed] [Google Scholar]
- Sheng M, Sabatini BL, Sudhof TC (2012) Synapses and Alzheimer’s disease. Cold Spring Harb Perspect Biol 4(5):a005777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL et al (2011) Sociability and motor functions in Shank1 mutant mice. Brain Res 1380:120–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soorya L, Kolevzon A, Zweifach J et al (2013) Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism 4:18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungur AO, Jochner MCE, Hani H, Ayse K, Holger G, Rainer KWS, Markus W (2017) Aberrant cognitive phenotypes and altered hippocampal BDNF expression related to epigenetic modifications in mice lacking the post-synaptic scaffolding protein SHANK1: Implications for autism spectrum disorder. Hippocampus 27:906–919 [DOI] [PubMed] [Google Scholar]
- Sungur AÖ, Redecker TM, Andres E et al (2018) Reduced efficacy of d-amphetamine and 3,4-methylenedioxymethamphetamine in inducing hyperactivity in mice lacking the postsynaptic scaffolding protein SHANK1. Front Mol Neurosci 11:419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungur AO, Schwarting RK, Wohr M (2016) Early communication deficits in the Shank1 knockout mouse model for autism spectrum disorder: developmental aspects and effects of social context. Autism Res 9:696–709 [DOI] [PubMed] [Google Scholar]
- Sungur AO, Vorckel KJ, Schwarting RK, Wohr M (2014) Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: developmental aspects and effects of social context. J Neurosci Methods 234:92–100 [DOI] [PubMed] [Google Scholar]
- Tatavarty V, Pacheco AT, Kuhnle CG, Lin H, Turrigiano GG (2017) Autism-associated Shank3 is essential for homeostatic compensation in rodent V1. Neuron 106:769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Konyukh M, Delorme R et al (2010) Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet 26:363–372 [DOI] [PubMed] [Google Scholar]
- Verhoeven WM, Egger JI, Willemsen MH, Leijer GJD, Kleefstra T et al (2012) Phelan–McDermid syndrome in two adult brothers: atypical bipolar disorder as its psychopathological phenotype? Neuropsychiatr Dis Treat 8:175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicidomini C, Ponzoni L, Lim D et al (2016) Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice. Mol Psychiatry 22:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucurovic K, Landais E, Delahaigue C et al (2012) Bipolar affective disorder and early dementia onset in a male patient with SHANK3 deletion. Eur J Med Genet 55:625–629 [DOI] [PubMed] [Google Scholar]
- Wang T, Guo H, Xiong B et al (2016a) De novo genic mutations among a Chinese autism spectrum disorder cohort. Nat Commun 7:13316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Adamski CJ, Bondar VV et al (2019a) A kinome-wide RNAi screen identifies ERK2 as a druggable regulator of Shank3 stability. Mol Psychiatry 25:2504–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pang K, Han K et al (2019b) An autism-linked missense mutation in SHANK3 reveals the modularity of Shank3 function. Mol Psychiatry 25:2534–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li C, Chen Q et al (2017) Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J Clin Investig 127:1978–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bey AL, Katz BM et al (2016b) Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat Commun 7:11459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM et al (2011) Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet 20:3093–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Zhong P, Ma K et al (2019c) Amelioration of autism-like social deficits by targeting histone methyltransferases EHMT1/2 in Shank3-deficient mice. Mol Psychiatry 25:2517–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt JL, Olson IA, Johnston AW, Ross HS, Couzin DA, Stephen GSA (1985) Familial pericentric inversion of chromosome 22 with a recombinant subject illustrating a ‘pure’ partial monosomy syndrome. J Med Genet 22:283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener S, Buschler A, Stempel AV et al (2018) Defective synapse maturation and enhanced synaptic plasticity in Shank2 Deltaex7(−/−) mice. eNeuro 5:ENEURO0398-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischmeijer A, Magini P, Giorda R et al (2011) Olfactory receptor-related duplicons mediate a microdeletion at 11q13.2q13.4 associated with a syndromic phenotype. Mol Syndromol 1:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Hung AY, Sheng M, Crawley JN (2011) Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS ONE 6:e20631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Ning Y, Flint J et al (1997) Molecular characterization of a 130-kb terminal microdeletion at 22q in a child with mild mental retardation. Am J Hum Genet 60:113–120 [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY et al (2012) Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 486:261–265 [DOI] [PubMed] [Google Scholar]
- Yang YWX, Jiang YH (2018) SHANK2 harbors potentially pathogenic mutations associated with bipolar disorder. Bipolar Disord 20:63–141 [Google Scholar]
- Yang Z, Kaiser T, Monteiro P et al (2016) Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron 89:147–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo T, Cho H, Lee J, Park H, Kim E (2018) GABA neuronal deletion of Shank3 exons 14–16 in mice suppresses striatal excitatory synaptic input and induces social and locomotor abnormalities. Front Cell Neurosci 12:341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo T, Cho H, Park H, Lee J, Kim E (2019a) Shank3 exons 14–16 deletion in glutamatergic neurons leads to social and repetitive behavioral deficits associated with increased cortical layer 2/3 neuronal excitability. Front Cell Neurosci 13:458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YE, Yoo T, Lee S et al (2019b) Shank3 mice carrying the human Q321R mutation display enhanced self-grooming, abnormal electroencephalogram patterns, and suppressed neuronal excitability and seizure susceptibility. Front Mol Neurosci 12:155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavsky K, Zhang WB, McCready FP et al (2019) SHANK2 mutations associated with autism spectrum disorder cause hyperconnectivity of human neurons. Nat Neurosci 22:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sun XY, Zhang LY (2017) MicroRNA-7/Shank3 axis involved in schizophrenia pathogenesis. J Clin Neurosci 22:1254–1257 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao BB, Xiong Y et al (2016) Expression of SHANK3 in the temporal neocortex of patients with intractable temporal epilepsy and epilepsy rat models. Cell Mol Neurobiol 37:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Jaber VR, LeBeauf A, Sharfman NM, Lukiw WJ (2019) MicroRNA-34a (miRNA-34a) mediated down-regulation of the post-synaptic cytoskeletal element SHANK3 in sporadic Alzheimer’s disease (AD). Front Neurol 10:28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kaiser T, Monteiro P et al (2016) Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron 89:147–162 [DOI] [PMC free article] [PubMed]
- Zhou Y, Sharma J, Ke Q et al (2019) Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature 570:326–331 [DOI] [PubMed] [Google Scholar]
- Zwanenburg RJ, Ruiter SA, Van Den Heuvel ER, Flapper BC, Van Ravenswaaij-Arts CM (2016) Developmental phenotype in Phelan–McDermid (22q13.3 deletion) syndrome: a systematic and prospective study in 34 children. J Neurodev Disord 8:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material files are available upon request.