Abstract
Neurons in the penumbra (the area surrounding ischemic tissue that consists of still viable tissue but with reduced blood flow and oxygen transport) may be rescued following stroke if adequate perfusion is restored in time. It has been speculated that post-stroke angiogenesis in the penumbra can reduce damage caused by ischemia. However, the mechanism for neovasculature formation in the brain remains unclear and vascular-targeted therapies for brain ischemia remain suboptimal. Here, we show that VEGFR1 was highly upregulated in pericytes after stroke. Knockdown of VEGFR1 in pericytes led to increased infarct area and compromised post-ischemia vessel formation. Furthermore, in vitro studies confirmed a critical role for pericyte-derived VEGFR1 in both endothelial tube formation and pericyte migration. Interestingly, our results show that pericyte-derived VEGFR1 has opposite effects on Akt activity in endothelial cells and pericytes. Collectively, these results indicate that pericyte-specific expression of VEGFR1 modulates ischemia-induced vessel formation and vascular integrity in the brain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10571-021-01071-w.
Keywords: Stroke, Pericyte, Cerebrovascular, VEGFR1, Ischemia
Introduction
Stroke is a major cause of death and adult disability worldwide (Woodruff et al. 2011) and normally results from occlusion of a cerebral artery leading to focal ischemia. Neurons in the ischemic core rapidly undergo necrosis (Woodruff et al. 2011). Cells in the penumbra are exposed to ischemia, but may be rescued if adequate perfusion is restored in time. Post-stroke angiogenesis can reduce damage caused by ischemia by mitigating hypoxia and it has been speculated that the ischemic border zone is a unique area that can be rescued by angiogenic therapy (Font et al. 2010; Ergul et al. 2012). Although our understanding of the molecular regulation of neovascularization has advanced (Carmeliet and Jain 2011), the mechanism for cerebrovascular formation and functional maturation following hypoxia are unclear (Ergul et al. 2012; Zhao et al. 2015) and vascular-targeted therapies for brain ischemia remain suboptimal (Moskowitz et al. 2010; Dzietko et al. 2013).
Pericytes were first described as “contractile elements” surrounding the endothelial cells (ECs) of small blood vessels (Ribatti et al. 2011). Over a century later, our understanding of the versatile functionality of pericytes in the brain has advanced remarkably (Al Ahmad et al. 2011). Pericytes receive, orchestrate, and process signals from endothelial cells, astrocytes, and neural cells to generate diverse neurovascular functions, including regulation of capillary hemodynamics, blood–brain barrier (BBB) permeability, clearance of toxic metabolites, and angiogenesis and stem cell activity, all of which are necessary for normal brain homeostasis (Mae et al. 2011; Filosa et al. 2016; Paredes et al. 2018). However, the role of brain pericytes in the context of ischemic stroke remains largely unclear. The interaction between ECs and pericytes, which is critical for cerebrovascular integrity and proper vascular function, is disrupted after stroke (Sweeney et al. 2016). In the brain, pericytes promote establishment of the BBB, which involves the expression of BBB-associated genes and the restriction of vesicular transcytosis in the endothelium.
The vascular endothelial growth factor (VEGF) family is the predominant inducer of angiogenesis (Simons et al. 2016). In the brain, VEGFs are important regulators of angiogenesis, neuroprotection, and neurogenesis (Simons et al. 2016). VEGF-A expression is induced in the ischemic penumbra during stroke and at remote sites involved in post-ischemic brain repair. It activates VEGF receptor 2 (VEGFR2) in ECs to trigger multiple downstream signals that promote angiogenesis (Greenberg and Jin 2013). The VEGF-A/VEGFR2 pathway upregulates the expression of delta-like protein-4 (DLL4) in tip cells and then activates Notch signaling by binding to its receptor in adjacent stalk cells (Blanco and Gerhardt 2013). The activated Notch signal then downregulates the expression of VEGFR2 in stalk cells, thereby inducing stalk cell properties (Moya et al. 2012). It is well established that VEGF/VEGFR2-mediated signaling plays an important role in promoting post-ischemia neurovascular remodeling (Iadecola and Anrather 2011; Geiseler and Morland 2018). Although VEGFR2 is recognized as the predominant receptor involved in VEGF-stimulated angiogenesis, the function of VEGFR1 is less clear. VEGFR1 has several unique structural and functional characteristics (Shibuya 2013). The VEGFR1 gene encodes a full-length membrane receptor (≈200 kd) and a soluble receptor (≈110 kd), which is generated by alternative splicing of the VEGFR1 pre-mRNA and contains the extracellular ligand-binding region, but lacks the cytoplasmic signaling tyrosine kinase domain (Shibuya and Claesson-Welsh 2006). VEGFR1 binds VEGF-A with high affinity, but has weak kinase activity (Shibuya and Claesson-Welsh 2006). It is believed that VEGFR1 functions as an inert decoy by binding endogenous VEGF, thereby negatively regulating the availability of VEGF and the activation of angiogenesis through VEGFR2 (Boucher et al. 2017). Such a decoy function is attributable mainly to soluble VEGFR1. It has been reported that signaling through VEGF-A and VEGFR2 is opposed by VEGFR1 (Lee et al. 2011). Various studies have confirmed that this antagonistic function of VEGFR1 limits vascular formation (Herbert and Stainier 2011; Robciuc et al. 2016; Eilken et al. 2017) Loss of VEGFR1 function results in increased EC proliferation, impaired sprouting, and reduced formation of vessel branches (Eilken et al. 2017). PI3K/Akt is an important downstream signaling of VEGF regulating angiogenesis (Ferrara et al. 2003). The PI3K pathway is activated in endothelial cells through VEGF/VEGFR2 binding, which is important for cell migration (Lamalice et al. 2007). A large number of studies have shown that PI3K/Akt signaling plays an important role in ischemic vascular disease and tumor angiogenesis in relation to VEGF (Karar and Maity 2011; Kerr et al. 2016). However, the role for Akt as a mediator of VEGFR1 in vascular function has not been fully established.
Here, we have investigated the function of pericyte-derived VEGFR1 in cerebral ischemia-induced angiogenesis by in vivo and in vitro assays. Using inducible genetic elements in mice we have shown that disruption of VEGFR1 in brain pericytes results in reduced post-ischemia neovascularization. We have investigated the mechanism by which the pericyte-specific expression of VEGFR1 modulates cerebral angiogenesis and vascular integrity in the ischemic brain and we describe a new mechanism involving phosphorylated VEGFR1 and Akt.
Results
Pericyte-Derived VEGFR1 Increased after pMCAO
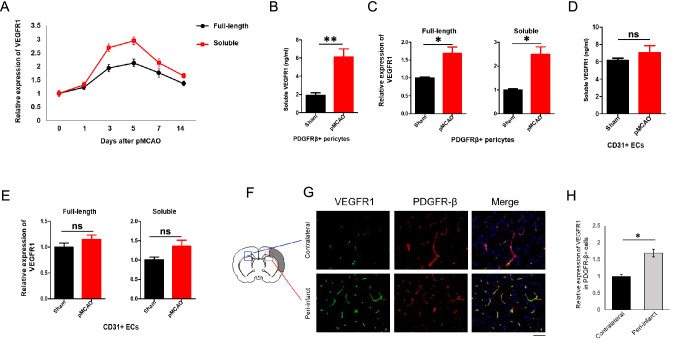
To investigate the potential role of VEGFR1 in post-stroke revascularization, in particular its role in pericytes, we examined changes in VEGFR1 expression after stroke. First, 8-week-old C57BL/6 mice were subjected to pMCAO and brain tissue from infarcted hemispheres was obtained on day 1, 3, 5, 7, and 14 after pMCAO. Quantitative reverse transcription PCR (RT-qPCR) of RNA from the ischemic tissue showed the expression of both full-length Vegfr1 and its soluble form were similarly increased at the different time points following pMCAO (Fig. 1a). Expression of both full-length and soluble Vegfr1 peaked at days 3–5 and declined from day 5 (Fig. 1a). Next, we sorted PDGFRβ (pericyte marker)-positive cells and CD31 (endothelial cell marker)-positive cells from the ischemic brains and the sham group at 7 days after pMCAO by fluorescence-activated cell sorting (Figure S1). RT-qPCR analysis of PDGFRβ + cells showed significant enrichment of full-length and soluble Vegfr1 expression relative to pericytes from the sham group (Fig. 1c). Increase of soluble VEGFR1 was also confirmed in PDGFRβ + cell culture supernatants by ELISA (Fig. 1b). RT-qPCR and ELISA showed the expression of soluble VEGFR1 to be only slightly increased in CD31-positive cells (Fig. 1d and e). We next performed immunohistochemistry to determine the level of VEGFR1 in the peri-infarct area, which usually undergoes angiogenesis post stroke. Indeed, pericytes in the peri-infarct area expressed much higher levels of VEGFR1 relative to pericytes in contralateral non-ischemic hemispheres (Fig. 1f–h). These results indicated that expression of both the soluble isoform and membrane (full-length) isoform of VEGFR1 increased in pericytes during brain ischemia, in particular in the peri-infarct area. Therefore, we hypothesized a crucial role of pericyte-derived VEGFR1 in ischemia-induced angiogenesis in the brain.
Fig. 1.
Expression of VEGFR1 in pericyte is increased after pMCAO. a qPCR analysis of full-length and soluble VEGFR1 in ischemic tissue at indicated days after pMCAO (n = 5 mice). Error bars: s.e.m. b Soluble VEGFR1 in PDGFRβ+cell culture supernatants was determined by ELISA (n = 5 mice). Error bars: s.e.m. ** P < 0.01. c qPCR analysis of full-length and soluble VEGFR1 in PDGFRβ+pericytes sorted from pMCAO or sham group at day 7 (n = 5 mice). Error bars: s.e.m. * P < 0.05. d Soluble VEGFR1 in CD31+ cell culture supernatants was determined by ELISA (n = 5 mice). Error bars: s.e.m. ns = not significant. e qPCR analysis of full-length and soluble VEGFR1 in CD31 + ECs sorted from pMCAO or sham group at day 7 (n = 5 mice). Error bars: s.e.m. ns = not significant. f Schematic of the pMCAO model. The shaded area indicates the infarct area. The framed areas indicate the peri-infarct zones in contralateral (blue) and ischemic (red) hemisphere. g VEGFR1 staining of contralateral and peri-infarct area from pMCAO brains at day 7. Scale bar, 50 μm. h Quantitation of VEGFR1 expression in the contralateral and peri-infarct area. Error bars, s.e.m. *P < 0.05
Pericyte-Derived VEGFR1 Regulates Post-Ischemia Angiogenesis
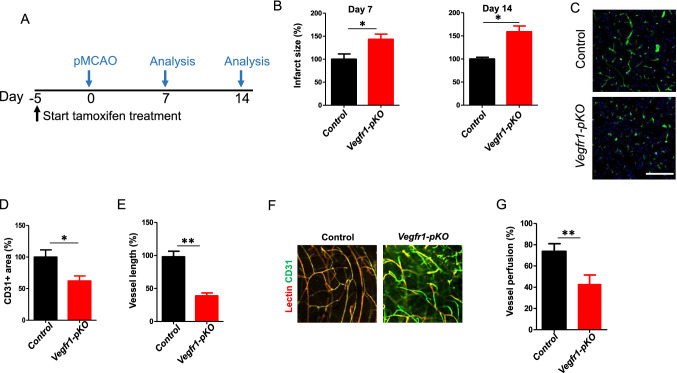
To address the role of pericyte-derived VEGFR1 in stroke, we bred Pdgfrβ-CreERT2 transgenic mice, in which tamoxifen-inducible Cre recombinase is under the control of the platelet-derived growth factor receptor β promoter, with Vegfr1-floxed mice, to obtain Pdgfrβ-CreERT2;Vegfr1flox/flox inducible conditional knockout mice. To determine Pdgfrβ-CreERT2-mediated Vegfr1 ablation in the mouse brain, Pdgfrβ-CreERT2;Vegfr1flox/flox, and Vegfr1flox/flox mice were induced with tamoxifen (intraperitoneal injection of tamoxifen, 3 mg once a day for 5, 10 or 20 days). Then PDGFRβ + cells were sorted from brains of each group. We used Western blotting and RT-qPCR to confirm that VGEFR1 was absent in brain pericytes in induced Pdgfrβ-CreERT2;Vegfr1flox/flox mice (Vegfr1-pKO) (Figure S2). Capillaries were visualized by CD31 immunohistochemistry and Vegfr1-pKO mice exhibited normal brain vascular structure before challenge compared with Vegfr1flox/flox (control) mice (Figure S3). Next, we subjected control mice and Vegfr1-pKO mice to pMCAO. All mice received tamoxifen treatment for 5 days before pMCAO to ensure Pdgfrβ-CreERT2-mediated Vegfr1 ablation in pericytes (Fig. 2a). As shown in Fig. 2b, the infarct size was significantly increased in Vegfr1-pKO mice relative to control mice at 7 and 14 days after pMCAO. Next, we analyzed the vasculature in the peri-infarct area at 7 days after pMCAO by CD31 staining. Vegfr1-pKO mice showed significant reductions in vessel area and length (Fig. 2c–e), respectively, compared with control mice. The functionality of the vessels in the peri-infarct area was evaluated by intravascular injections of biotinylated tomato lectin. The total area of tomato lectin-perfused vessels was reduced by 26% in Vegfr1-pKO mice (Fig. 2f and g). Moreover, visualization of the cerebrovascular network in the peri-infarct area by CD31 immunostaining and by tomato lectin perfusion showed that the newly formed vessels in the peri-infarct area of Vegfr1-pKO mice were distorted. The enlarged nascent vessels were accumulated with endothelial cells (Fig. 2c and f). The non-perfused vessels (tomato lectin negative and CD31 + objects) present as truncated and discontinuous (Fig. 2f). Thus, Vegfr1 deficiency in PDGFRβ + cells leads to significant reductions in brain vascular perfusion and morphological abnormality after pMCAO.
Fig. 2.
Reduced post-ischemia neovascularization in Vegfr1-pKO mice. a Experimental scheme of tamoxifen administration for the generation of Vegfr1-pKO mice. b the infarct area in Vegfr1-pKO mice relative to control at 7 days and 14 days after pMCAO. (n = 5 mice). Error bars: s.e.m. *P < 0.05. c CD31 staining of peri-infarct area at Day 7 post ischemia induction by pMCAO. Scale bar, 50 µm. d Quantification of CD31 + area at Day 7 presented as relative values in comparison to control mice. *p < 0.05, n = 5. e Vessel length. Error bars: s.e.m. **p < 0.01, n = 5. f Lectin perfusion (red) into peri-infarct vessels (green) was determined. Yellow color (green/red double-staining) indicates blood perfused vessels. Scale bar, 50 µm. g Quantification of vessel perfusion % = lectin + CD31 + area/total CD31 + area × 100, **p < 0.01, n = 5
Pericyte VEGFR1 Deficiency Impairs Brain Vascular Integrity and Pericyte Coverage
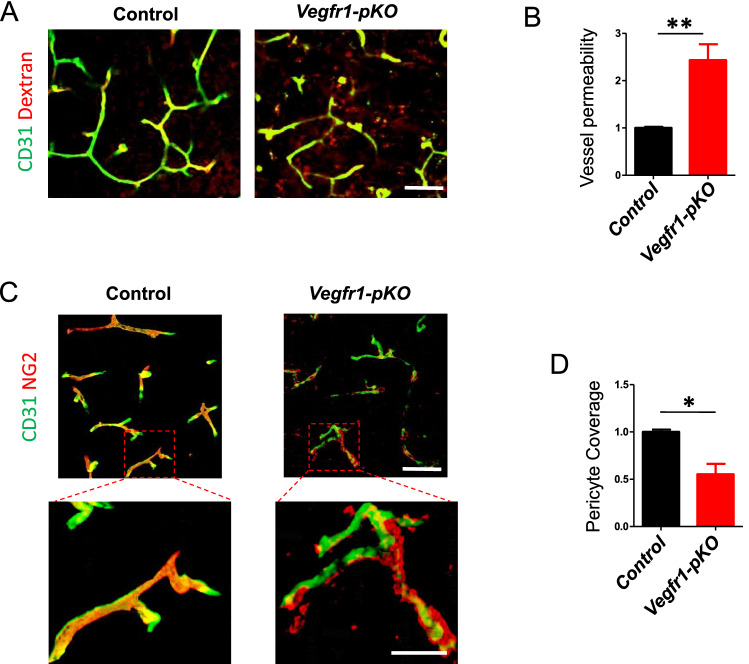
Next, we assessed the effect of Vegfr1 ablation on cerebrovascular permeability, the Vegfr1-pKO and control mice were intravenously injected with FITC-dextran (MW = 2 000 000 Da) at 7 days after pMCAO. Extravasation of FITC-dextran dye into the tissues surrounding the cerebral capillaries was much more extensive in Vegfr1-pKO mice than in controls, indicating significantly increased cerebrovascular leakage (Fig. 3a, b). The area positive for immunostaining against NG2, a typical pericyte marker, was used to segment pericyte area in the image. Pericyte coverage was analyzed by dividing NG2+ pericyte area into CD31+ endothelial area. At 7 days after pMCAO, NG2-positive pericyte coverage was significantly reduced in the brain vasculature of the peri-infarct area of Vegfr1-pKO mice relative to control mice (Fig. 3c and d). 3D reconstruction of confocal images showed clearly abnormal pericyte morphology and poor association with endothelial cells (Fig. 3c). As shown, vessel-associated NG2 immunoreactivity appeared disrupted, patchy and was missing at some vascular segments. In contrast, typical PC morphology (small cell soma, sleek longitudinal processes) was observed in the contralateral hemisphere and in control ischemic brains (Fig. 3c). These results clearly showed that disruption of VEGFR1 in cerebrovascular pericytes resulted in defective EC-pericyte contact. Moreover, ablation of VEGFR1 in pericytes led to profound morphological changes in pericytes.
Fig. 3.
Impaired vascular integrity and pericyte coverage in pericyte-specific VEGFR1 deficiency. a Dextran (red) was injected to mice before euthanization 7 days post pMCAO. Brain tissue from control and Vegfr1-pKO mice was stained for CD31 (green) to label the blood vessels. Scale bar, 50 μm. b Quantification of the ratio of dextran outside vs inside of blood vessel. Error bars: s.e.m. **p < 0.01, n = 5. c Representative confocal microscopy three dimensional reconstruction images of brain sections showing NG2-positive pericyte coverage (red) and CD31-positive endothelial cells (green) in the peri-infarct regions of control and Vegfr1-pKO mice. Higher magnification showed disrupted pericytes-endothelial cells association in Vegfr1-pKO mice. scale bars, 50 μm; inset, 20 μm. d Quantification of pericyte coverage of blood vessels. n = 4 per group
Pericytes Production of sVEGFR1 Inhibits Brain Endothelial VEGFR2 Signaling
Various studies have established the antagonistic function of VEGFR1 and its expression in ECs limits vascular growth (Boucher et al. 2017; Lee et al. 2011; Herbert and Stainier 2011). However, the role of pericyte-derived VEGFR1 in brain angiogenesis is not known. As shown in Figs. 1 and 2, VEGFR1 expression is upregulated in pericytes during the repair stage after ischemia and VEGFR1 ablation resulted in increased infarct size and chaotic vessel formation. We consequently hypothesized that pericyte-derived soluble VEGFR1 could affect endothelial sprouting in ischemic brain by functionally antagonizing VEGFR2 signaling. Therefore, we next investigated the effect of VEGFR1 ablation on VEGF/VEGFRs activation after stroke. PDGFβ + cells and CD31 + cells were sorted from control and Vegfr1-pKO mice, respectively, at 7 days after pMCAO. Phosphorylation of VEGFR2 was significantly enhanced at Y-1214 in CD31 + endothelial cells, while the level of total VEGFR2 did not significantly change (Fig. 4a, b), indicating a lack of VEGFR1 inhibition on VEGFR2. On the other hand, the level of phosphorylated VEGFR2 in pericytes was slightly but not significantly altered (Fig. 4f, g). Moreover, as AKT signaling is a known downstream pathway of VEGFR2 that regulates vessel formation, the phosphorylation of AKT was also determined. The level of p-Akt was increased in endothelial cells, confirming enhanced VEGFR2 signaling in Vegfr1-pKO mice (Fig. 4a, e). Interestingly, p-Akt was significantly decreased in PDGFRβ + cells (Fig. 4f, j). Suggesting, pericyte-derived VEGFR1 has opposite effects on Akt activity in ECs and pericytes. We therefore hypothesized that pericyte-derived VEGFR1 plays different roles in ECs and pericytes per se that coordinately regulates ischemia-induced revascularization in the brain.
Fig. 4.
VEGFR1 deletion exert differential effects on VAkt activation in ECs and pericytes. PDGFβ + cells and CD31 + cells were sorted from control and Vegfr1-pKO mice at 7 days after pMCAO, respectively. a–e Analyses of VEGFR2 (Y-1214) and Akt (Ser473) phosphorylation and VEGFR1 (full-length, soluble) expression in ECs (n = 5 mice). Error bars: s.e.m. * P < 0.05, **p < 0.01, ns = not significant. f–j Analyses of VEGFR2 (Y-1214) and Akt (Ser473) phosphorylation and VEGFR1 (full-length, soluble) expression in pericytes (n = 5 mice). Error bars: s.e.m. *P < 0.05, **p < 0.01, ns = not significant
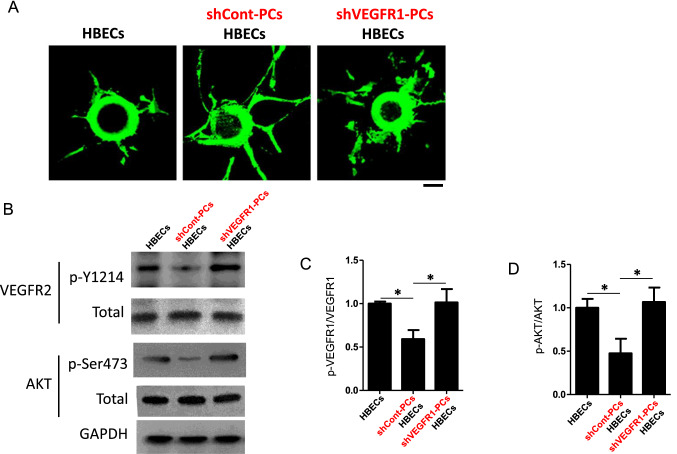
To further investigate the role of pericyte-derived VEGFR1 in stroke, we used a shRNA lentivirus (Sigma SHCLNV-NM_002019 MISSION® shRNA Lentiviral Transduction Particles) to suppress Vegfr1 expression in HBPCs (Human Brain Vascular Pericytes, Sciencell). We assessed whether Vegfr1-silenced HBPCs affected brain endothelial cell sprouting using an in vitro co-culture assay. In this assay, microbeads were coated with parental HBECs (Human Brain Microvascular Endothelial Cells, Sciencell) and then embedded in a gel matrix. HBPCs transfected with control shRNA (shCont-PCs) or shRNA for VEGFR1 (shVEGFR1-PCs) were seeded on the top of the gel. In this co-culture, the parental HBPCs served as feeders to introduce secreted soluble factors to HBECs through the gel. As shown in Fig. 5, shCont-PCs had significantly enhanced HBEC sprouting compared with HBECs cultured alone (Fig. 5a), suggesting the indispensable role of pericytes in endothelial sprouting. However, Vegfr1-silenced HBPCs remarkably impaired HBEC tube formation and presented as discontinuous and disordered structure (Fig. 5a). To quantify VEGFR2 activation in HBECs, HBECs were harvested after 5 days of culture in the gel. The levels of total and phosphorylated VEGFR2 in HBECs were examined by Western blotting. Phosphorylation of VEGFR2 in HBECs was decreased by shCont-PCs compared with HBECs cultured alone and was significantly induced by shVGFER1-PC co-culture (Fig. 5b, c). Furthermore, co-culture of VEGFR1-silenced HBPCs with HBECs induced Akt phosphorylation (Fig. 5b, d). Together, these findings demonstrate that brain pericytes promote endothelial sprouting through paracrine VEGFR1 signaling. The pericyte-derived VEGFR1-mediated VEGFR2-Akt signaling is required for brain endothelial sprouting.
Fig. 5.
Pericytes regulate brain endothelial tube formation through paracrine VEGFR1 pathway. a Endothelial sprouts extending from HBECs-coated beads in 3D fibrin gel culture were imaged at day 5. Scale bar, 50 µm. b–d Western blot analysis of VEGFR2 (Y-1214) and Akt (Ser473) phosphorylation in HBECs that cultured in indicated conditions. HBECs were harvested and sorted after 5 days culturing in the gel. n = 3. Error bars: s.e.m. *P < 0.05
Intracellular VEGFR1 Signaling is required for Pericyte Migration and BBB Formation
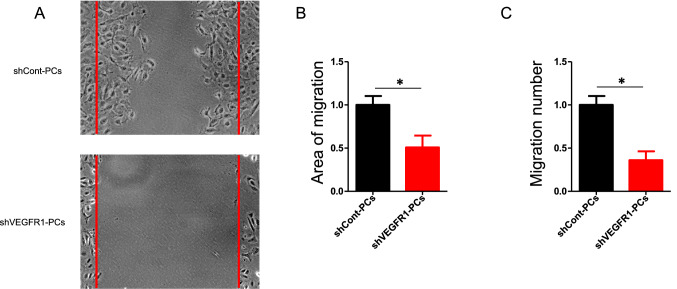
Next, we assessed direct effects of VEGFR1 signaling on pericyte function and signal transduction. Consistent with our in vivo observations, morphological abnormalities were found in VEGFR1-silenced HBPCs. F-actin and smooth muscle actin-α staining showed that the cytoskeleton was reorganized by silencing VEGFR1 (Figure S4). We performed lateral scratch wound assays with control HBPCs and sh-Vegfr1-silenced HBPCs. Silencing of VEGFR1 expression significantly decreased pericyte motility (Fig. 6a, b). To further validate the defect in pericyte recruitment to endothelial cells, we performed a Transwell assay, in which HBECs were plated in the bottom chamber of the Transwell and HBPCs were seeded in the top chamber. Knockdown of VEGFR1 in pericytes results in reduced migration. These results strengthen the assertion that loss of VEGFR1 signaling in pericytes impairs pericyte migration and EC-pericyte interaction (Fig. 6c). Taken together, the cellular experiments identified VEGFR1-dependent control of pericyte migration. VEGFR1-depleted HBPCs showed a strong phenotype of decreased migration and impaired association with ECs demonstrating that VEGFR1 signaling is indispensable in pericytes. Emerging evidence indicates that membrane VEGFR1 is upregulated during vascular reperfusion stages in ischemic tissue (Imoukhuede et al. 2013). Given unaltered VEGFR2 expression in pericytes post ischemia (Fig. 4f, g), we investigated the crucial role of intracellular VEGFR1 signal transduction in brain pericytes. HBPCs were with incubated VEGFR1-specific ligand placental growth factor (PLGF) to promote VEGFR1 internalization. As we expected, immunocytochemical analysis demonstrated prominent nuclear localization of VEGFR1 in cultured pericytes upon PLGF stimulation (Fig. 7a). As shown in Fig. 2f, pAKT level was declined in pericytes after pMCAO in vivo. Correspondingly, we observed that shVEGFR1 silencing resulted in decreased Akt phosphorylation in HBPCs, compared with shControl (Fig. 7b). These data indicate that VEGFR1 in pericytes influences Akt signaling in a VEGFR2-independent manner. The membrane-localized VEGFR1 mediates intracellular signaling pathway to induce brain pericyte migration.
Fig. 6.
VEGFR1 is required for pericyte migration. a shCont-PCs and shVEGFR1-PCs were cultured on fibronectin until reaching confluence. A uniform linear scrape wound was made across the cell layer. Three independent experiments were performed. b Area of migration analyzed 24 h after making scratches (n = 5). Error bars: s.e.m. *P < 0.05. c shCont-PCs or shVEGFR1-PCs were seeded in the upper chambers of transwell inserts, parental HBECs were seeded in the lower chambers. After 4 h, cells on the lower side of the filter were stained with 0.5% crystal violet and scored in five independent fields (n = 3). Error bars: s.e.m. *P < 0.05
Fig. 7.
Membrane-localized VEGFR1 regulates Akt signaling in pericytes. a Immunocytochemical analysis of VEGFR1 localization identified prominent nuclear VEGFR1 in cultured pericytes upon PlGF stimulation. b–c Western blot analysis of Akt (Ser473) phosphorylation in shCont-PCs and shVEGFR1-PCs. n = 3. Error bars: s.e.m. **p < 0.01
Discussion
Although the role of pericytes in cerebrovascular integrity has been investigated, the molecular and cellular mechanisms underlying pericyte-mediated angiogenesis are not clearly understood. The present study reveals a crucial role for pericyte VEGFR1 signaling in cerebrovascular formation and maintenance. We show that pericyte VEGFR1 signaling is required for post-ischemia neovascularization and vascular integrity and pericyte coverage. Furthermore, we provide evidence for pericyte regulation of brain endothelial tube formation through a paracrine VEGFR1 pathway. Meanwhile, membrane-localized VEGFR1-mediated intracellular signaling pathways regulate pericyte migration.
Recuperative therapeutic strategies for stroke are focused on revascularization, neuroprotection, and neuroregeneration, but most of the strategies that have been clinically tested fail to show benefit in humans (Stankowski and Gupta 2011; Liman and Endres 2012). Ischemia-induced angiogenesis is an essential event to improve capillary blood flow surrounding an ischemic infarct that promotes regenerative processes and neuroplasticity. Unfortunately, however, this process is still incompletely understood and therefore not exploited for therapeutic purposes (Arenillas et al. 2007). Angiogenesis, the formation of new capillaries from existing blood vessels, requires multiple angiogenic factors to work together with spatio-temporal precision to correctly pattern functional blood vessels (Ucuzian et al. 2010; Vanhollebeke et al. 2015). To form mature and functional blood vessels, pericytes are required to stabilize capillaries. It has been established that pericytes are not simply an important component of the BBB, but also have a central role in the regulation of angiogenesis and maintenance of brain vessel integrity, but their exact role in brain vascularization remains unclear (Winkler et al. 2011; ElAli et al. 2014). Platelet-derived growth factor B (PDGF-B) and its receptor (PDGFRβ) are critical for the recruitment of brain pericytes onto newly formed vessels during angiogenesis (Hellstrom et al. 1999). Analysis of human brain samples showed that PDGF-B and its receptor are expressed on microvessels following stroke (Krupinski et al. 1997). PDGFRβ expression was significantly increased in microvessels, mainly on pericytes, in the infracted area 48 h after MCAO. Co-delivery of PDGF-BB with VEGF was reported to recruit pericytes and enhance vessel maturation (Hellberg et al. 2010). ANG1 also reduced VEGF-induced vascular leakage, whereas TGF-β promoted the interaction between endothelial cells and pericytes (Fuxe et al. 2011). However, this does not conclusively show that pericytes regulate vascular formation in the brain as previously indicated.
Inactivation of VEGFR1 in pericytes only modestly affects pericyte number during development, and pericytes limit local VEGF signaling by expressing VEGFR1 (Herbert and Stainier 2011). However, our data indicate that pericytes express high levels of VEGFR1 for a period of time after stroke, which may be particularly relevant for the regulation of post-stroke angiogenesis. Consistent with previous reports, pericytes regulate endothelial sprouting by expressing soluble VEGFR1, which limits local VEGF signaling. More importantly, the role of membrane VEGFR1 in pericytes is indispensable for pericyte recruitment in ischemia-induced angiogenesis, which is crucial for the integrity of cerebral vascular function.
The role of pericyte VEGFR1 identified in this study may be exploited for effective tissue reperfusion in ischemic brains, which has important implications for the treatment of stroke.
Materials and Methods
Animals
To generate the pericyte-specific Flt1/Vegfr1 knockout, we bred Pdgfrβ-CreERT2 transgenic mice, in which a tamoxifen-inducible Cre recombinase is under the control of the platelet-derived growth factor receptor β promoter, with Vegfr1-floxed mice to obtain Pdgfrβ-CreERT2, Vegfr1flox/flox inducible conditional knockout mice. Pdgfrβ-CreERT2, Vegfr1flox/flox (Vegfr-pKO), or Vegfr1flox/flox (control) mice were treated with tamoxifen (intraperitoneal injection of tamoxifen, 3 mg once a day for the number of days indicated). Eight-week-old wild-type, control, or Vegfr1-pKO male mice underwent either sham or permanent MCAO. The mice were housed under specific pathogen-free (SPF) conditions with a 12-h light–dark cycle. All operations were performed under anesthesia induced by 4% isoflurane in a 70% nitrogen/30% oxygen gas mixture via an anesthesia machine (MIDMARK, Versailles, Ohio USA). Body temperature was maintained at 37 ± 0.5 °C using a heating pad. Our animal study protocols were approved by and conducted in accordance with the Animal Ethics Committee of the Army Medical University (Third Military Medical University, Chongqing, China) and ARRIVE (Animal Research: Reporting In Vivo Experiments).
Permanent Middle Cerebral Artery Occlusion (pMCAO)
Focal cerebral ischemia was induced by distal middle cerebral artery occlusion (MCAO) through a small burr hole. Briefly, mice were placed on an operating table at 37.5 °C under a dissecting microscope, and an incision was made between the left lateral part of the orbit and the left ear. The parotid gland and the temporal muscle were pushed in the distal direction with a pair of anatomical forceps. A small craniotomy was made with a 1 mm burr above the anterior distal branch of the MCA using a high-speed microdrill. The inner layer of the skull was removed with fine forceps, and the dura and arachnoid were carefully opened. The MCA was bipolarly electrocauterized, applying forceps coupled to an electrosurgical unit. The muscle and soft tissue were replaced, and the skin was closed using 4–0 nylon sutures. One milliliter of physiological 0.9% NaCl was given subcutaneously. The duration of anesthesia was 6 to 8 h. Mice were kept for 20 h in a recovery room at 28 °C before they were transferred to the normal animal facility unit.
Quantitative Reverse Transcription PCR (RT-qPCR)
Total RNA was isolated from cells (in vitro) and brains using TRIzol Reagent for real-time RT-PCR (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer’s instructions. Any genomic DNA potentially present in the RNA was removed by incubation with ribonuclease-free deoxyribonuclease I. RNA quality was verified using a bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and RNA was quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) with the ABI PRISM 7700 Sequence Detection System. Relative gene expression values were evaluated with the 2-ΔΔCt method using Gapdh as a housekeeping gene. The primers used were as follows: mouse mFlt1/Vegfr1 (Forward primer GTCACAGATGTGCCGAATGG, Reverse primer TGAGCGTGATCAGCTCCAGG) and mouse soluble Flt1/Vegfr1 (Forward primer GTCACAGATGTGCCGAATGG, Reverse primer TGACTTTGTGTGGTACAATC).
Flow Cytometry
Flow cytometric analysis was performed using a FACS Calibur (BD Biosciences) and fluorescence-conjugated mouse mAbs for CD31 and PDGFRβ. FITC-conjugated or PE-conjugated immunoglobulins were used as controls.
ELISA
Cells [primary human brain microvascular endothelial cells (HBECs) and primary human brain pericytes (HBPCs)] were washed with PBS (−) and cultured at 5 × 105 cells/ml in complete culture medium for 48 h. The concentration of secreted soluble VEGFR1 in spent culture supernatant was determined quantitatively with a Quantikine ELISA kit specific for s-VEGFR1 (R&D Systems) based on the sandwich immunoassay technique. Medium samples were diluted and added to a 96-well plate coated with a monoclonal Ab specific for VEGFR1/sFlt1 and were incubated for 2 h. Any unbound sFlt1 was removed by washing with a buffer and then an enzyme-linked polyclonal Ab specific for VEGFR1/sFlt1 was added. After incubation, unbound Ab enzyme conjugate was washed off and the samples were then incubated with a substrate solution. Enzyme-catalyzed chromogen formation was quantified by measuring the visible absorbance at 450 nm. Using a calibration curve plotted with recombinant human sFlt1, the concentrations of expressed sFlt1 (in pg/ml of spent culture supernatant from 5 × 105 cells) were calculated from the absorbance value.
Vascular Length and Area
To assess reparative angiogenesis, a subset of animals (n = 5) was euthanized and brain sections were prepared 7 days after pMCAO. Cryosections of the brains were incubated with an anti-CD31 antibody (1:200) overnight at 4 °C and then with Alexa Fluor 555 anti-rat immunoglobulin G (1:500; ThermoFisher) for 1 h and analyzed by fluorescence microscopy. The length of CD31-positive objects per field and the CD31-positive area per field were determined in five random fields for each slide using Image-J® software. All data are presented as the mean ± SD.
Western Blotting Analyses
For western blotting (WB) assays, cells were washed twice with ice-cold PBS and harvested with gentle scraping. The cells were then lysed in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS and 0.5% sodium deoxycholate) supplemented with a mixture of proteases and phosphatase inhibitors.
The following antibodies were used for immunoblotting: rabbit anti-phospho-Akt Ser473 (1:1,000, Cell Signaling 4060), rabbit anti-Akt (1:1,000, Cell Signaling 9272), anti-phospho-VEGFR2 Y-1214 (1:500, Abcam ab131241), rabbit anti-VEGFR2 (1:1,000, Cell Signaling 2479), rabbit anti-VEGFR1 (1:500, Abcam ab32152), and rabbit anti-GAPDH (1:10,000, Cell Signaling 2118).
FITC-Dextran Assay
Mice at 7 days after pMCAO were positioned on a heating-plate and the tail vein was cannulated using polyethylene tubing (PE-10: BD Medical Technology) with a 30-gauge disposable needle (Sigma-Aldrich) connected to a syringe filled with 5 mg fluorescein isothiocyanate (FITC)-conjugated dextran (2000-kDa; Sigma-Aldrich) dissolved in 500 μl phosphate-buffered saline (PBS). The fluorescein tracer was slowly injected via the tail vein catheter.
shRNA-Mediated Knockdown of Vegfr1 Expression
The Mission shRNA gene kit against human FLT1/VEGFR1 was purchased from Sigma-Aldrich (SigmaSHCLNV-NM_002019 MISSION® shRNA Lentiviral Transduction Particles human). A control vector, SHC002 (a non-targeting shRNA that activates the RNAi pathway without targeting any known human gene) was also purchased (Sigma-Aldrich). Lentiviral stocks encoding the shRNA of Flt1/Vegfr1 were prepared by transient cotransfection of 293 T cells (ATCC, USA) with the shRNA-encoding transfer vector and the two packaging vectors at a stipulated ratio of 4:3:1, respectively. Forty-eight hours post transfection, lentiviruses released into the medium were collected, harvested by centrifugation (500 × g for 10 min at 4 °C), and filtered through a 0.45 μm filter to ensure removal of cell debris and floating cells. Subconfluent monolayers of HBPCs were transduced with the shRNA-encoding lentivirus stocks in the presence of polybrene (8 μg/ml). Efficacies of the individual shRNAs in knocking down Flt1/Vegfr1 expression were evaluated by Western blotting performed on whole cell extracts.
Migration Assay
Twenty-four-well Transwell inserts with an 8 μm pore size were coated with a thin layer of collagen (Type I, rat tail, BD Biosciences, Bedford, MA, USA). HBPCs were cultured in Pericyte Growth medium (PromoCell, Germany) with 10% fetal bovine serum (Lonza, China) and growth factors for 12 h before switching to medium with no growth factors or serum for 4 h before the start of the experiment. HBECs were washed twice with EBM-2, trypsinized, and plated into the lower chamber at a ratio of one cell (in insert) to five cells (in chamber cell number). Cells were photographed at 10 × magnification, and four fields each from duplicate samples were counted to quantify migration.
F-Actin Staining
For F-actin staining, cells were plated on 12-mm-diameter coverslips and incubated for 24 h, washed twice with phosphate-buffered saline (PBS), and fixed in 4% paraformaldehyde for 10 min at room temperature. After two additional washes with PBS, cells were permeabilized with 0.1% Triton X-100 for 2–5 min and washed again with PBS. Rhodamine conjugates of phalloidin (Thermo Fisher Scientific) were used to stain F-actin. The cells were stained for 40 min at room temperature. The fluorescence images of F-actin and nuclei were visualized by confocal microscopy (NIKON, Japan).
In vitro Endothelial Sprouting Assay
Parental HBECs were coated on cytodex 3 microcarrier beads (GE Healthcare Life Sciences). HBEC-coated beads were then resuspended in a 2 mg/ml fibrinogen solution (Sigma-Aldrich) in PBS at a density of 250 beads/ml. Fibrinogen/bead suspension (0.5 ml) was then added to each well of a 4-well chamber slide (Nunc® Lab-Tek® II) containing 0.625 U of thrombin (Sigma-Aldrich). After gels had solidified, 0.8 ml of EGM-2 medium containing 2 × 105 shRNA-transduced HBPCs as a feeder layer was added onto the gel in each well. The 3D culture was maintained in a CO2 incubator at 37 °C for 5 days to observe endothelial sprouting. To visualize and count the sprouts, the fibrin gel 3D culture was fixed and stained with UEA lectin (Vectorlabs, FL-1061) for 2 h.
For Akt silencing in the 3D culture, HBECs were transfected with siRNA for either Akt isoform at 48 h after R-Ras38V transduction and subsequently coated onto microcarrier beads 24 h later. Expression of the GSK-3β S9A mutant in the 3D culture was performed in a similar time frame after R-Ras38V transduction.
Statistical Analysis
Results are expressed as the mean ± SD. One-way ANOVA with Dunnett’s post hoc test was performed using GraphPad Prism version 4.03 for Windows. p < 0.05 was considered significant.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
C.X.G., Q.Z, F.F.L., and Q.W.Y. conceived this study and designed the experiments. C.X.G. established the pMCAO models. C.X.G., Q.Z., X.Y.X., J.J.Y., G.Q.Y, and H.J.C. performed the experiments and analyzed the data. J.L., C.M.D., R.X., and Z.M.Q. performed immunofluorescence analysis. Z.Y.M., K.Z., and F.X.W. gave scientific advice. C.X.G, Q.Z., F.F.L, and Q.W.Y. wrote the paper.
Funding
This work was supported by the National Natural Science Fund for Distinguished Young Scholars (81525008) and the National Natural Science Fund for Outstanding Young Scholars (81822015).
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
The study complies with current ethical consideration.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chang-Xiong Gong and Qin Zhang have contributed equally to this paper.
Contributor Information
Fangfei Li, Email: lifangfei@tmmu.edu.cn.
Qing-Wu Yang, Email: yangqwmlys@hotmail.com.
References
- Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO (2011) Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab 31(2):693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenillas JF, Sobrino T, Castillo J, Davalos A (2007) The role of angiogenesis in damage and recovery from ischemic stroke. Curr Treat Options Cardiovasc Med 9(3):205–212 [DOI] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H (2013) VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med 3(1):a006569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JM, Clark RP, Chong DC, Citrin KM, Wylie LA, Bautch VL (2017) Dynamic alterations in decoy VEGF receptor-1 stability regulate angiogenesis. Nat Commun 8:15699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM (2013) Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res 4(2):189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Dieguez-Hurtado R, Schmidt I, Nakayama M, Jeong HW, Arf H, Adams S, Ferrara N, Adams RH (2017) Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun 8(1):1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElAli A, Theriault P, Rivest S (2014) The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci 15(4):6453–6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Alhusban A, Fagan SC (2012) Angiogenesis: a harmonized target for recovery after stroke. Stroke 43(8):2270–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676 [DOI] [PubMed] [Google Scholar]
- Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ (2016) Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 323:96–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font MA, Arboix A, Krupinski J (2010) Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev 6(3):238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe J, Tabruyn S, Colton K, Zaid H, Adams A, Baluk P, Lashnits E, Morisada T, Le T, O’Brien S, Epstein DM, Koh GY, McDonald DM (2011) Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation. Am J Pathol 178(6):2897–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiseler SJ, Morland C (2018) The janus face of VEGF in stroke. Int J Mol Sci 19(5):1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Jin K (2013) Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci 70(10):1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg C, Ostman A, Heldin CH (2010) PDGF and vessel maturation. Recent Results Cancer Res 180:103–114 [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126(14):3047–3055 [DOI] [PubMed] [Google Scholar]
- Herbert SP, Stainier DY (2011) Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 12(9):551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J (2011) Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 14(11):1363–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoukhuede PI, Dokum AO, Annex BH, Popel AS (2013) Endothelial cell-by-cell profiling reveals the temporal dynamics of VEGFR1 and VEGFR2 membrane localization after murine hindlimb ischemia. Am J Physiol Heart and Circ Physiol 304(8):H1085–H1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karar J, Maity A (2011) PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 4:51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BA, West XZ, Kim YW, Zhao Y, Tischenko M, Cull RM, Phares TW, Peng XD, Bernier-Latmani J, Petrova TV, Adams RH, Hay N, Naga Prasad SV, Byzova TV (2016) Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun 7:10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, Kaluza J (1997) A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke 28(3):564–573 [DOI] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J (2007) Endothelial cell migration during angiogenesis. Circ Res 100(6):782–794 [DOI] [PubMed] [Google Scholar]
- Lee HK, Chauhan SK, Kay E, Dana R (2011) Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7-dependent pathway. Blood 117(21):5762–5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman TG, Endres M (2012) New vessels after stroke: postischemic neovascularization and regeneration. Cerebrovasc Dis 33(5):492–499 [DOI] [PubMed] [Google Scholar]
- Mae M, Armulik A, Betsholtz C (2011) Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr Pharm Des 17(26):2750–2754 [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67(2):181–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A (2012) Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell 22(3):501–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes I, Himmels P, Ruiz de Almodovar C (2018) Neurovascular communication during CNS development. Dev Cell 45(1):10–32 [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E (2011) The role of pericytes in angiogenesis. Int J Dev Biol 55(3):261–268 [DOI] [PubMed] [Google Scholar]
- Robciuc MR, Kivela R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppanen VM, Kakela R, Eklund L, Wasserman DH, Groen AK, Alitalo K (2016) VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab 23(4):712–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M (2013) Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153(1):13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L (2006) Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 312(5):549–560 [DOI] [PubMed] [Google Scholar]
- Simons M, Gordon E, Claesson-Welsh L (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17(10):611–625 [DOI] [PubMed] [Google Scholar]
- Stankowski JN, Gupta R (2011) Therapeutic targets for neuroprotection in acute ischemic stroke: lost in translation? Antioxid Redox Signal 14(10):1841–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, Zlokovic BV (2016) Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19(6):771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucuzian AA, Gassman AA, East AT, Greisler HP (2010) Molecular mediators of angiogenesis. J Burn Care Res 31(1):158–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY (2015) Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. Elife. 10.7554/eLife.06489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV (2011) Central nervous system pericytes in health and disease. Nat Neurosci 14(11):1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV (2011) Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener 6(1):11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163(5):1064–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.