Abstract
Astrocytes are crucial in neural protection after traumatic brain injury (TBI), a global health problem causing severe brain tissue damage. Astrocytic connexin 43 (Cx43), encoded by GJA1 gene, has been demonstrated to facilitate the protection of astrocytes to neural damage with unclear mechanisms. This study aims to explore the role of GJA1-20K/Cx43 axis in the astrocyte–neuron interaction after TBI and the underlying mechanisms. Primarily cultured cortical neurons isolated from embryonic C57BL/6 mice were treated by compressed nitrogen–oxygen mixed gas to simulate TBI-like damage in vitro. The transwell astrocyte–neuron co-culture system were constructed to recapitulate the interaction between the two cell types. Quantitative PCR was applied to analyze mRNA level of target genes. Western blot and immunofluorescence were conducted to detect target proteins expression. GJA1-20K overexpression significantly down-regulated the expression of phosphorylated Cx43 (p-Cx43) without affecting the total Cx43 protein level. Besides, GJA1-20K overexpression obviously enhanced the dendrite length, as well as the expression levels of function and synthesis-related factors of mitochondria in damaged neurons. GJA1-20K up-regulated functional Cx43 expression in astrocytes, which promoted mitochondria transmission from astrocytes to neurons which might be responsible to the protection of astrocyte to neurons after TBI-like damage in vitro.
Keywords: Traumatic brain injury, Astrocyte, Mitochondria transfer, GJA1-20K, Cx43
Introduction
Traumatic brain injury (TBI) is a kind of brain tissue damage caused by trauma. It is the leading cause of mortality and disability among young individuals in high-income countries, and globally the incidence of TBI is rising sharply, mainly due to increasing motor vehicle use in low- and middle-income countries (Maas et al. 2008). With the development of the society and improvement of economy, the rapid increasing of the construction industry and transportation, the number of TBI patients continues to rise. In America, TBI cases account for 30% of all traumatic deaths (Taylor et al. 2017). In patients who survived the injury, TBI also causes a variety of neurological impairments due to neuronal damage, including learning, thinking, language, emotional and behavioral cognition, and even mental disorders. The pathogenesis of TBI is complex, and the molecular targets involved are not yet clear. Therefore, it is extremely important to understand the pathological process of TBI and how to carry out effective protection and restorative treatment.
Astrocytes are the most abundant cells in the central nervous system, which is five times more than neurons. In addition to supporting and guiding neurons, astrocytes also have broad roles in neural metabolism, blood flow, and signal transmission (Iadecola and Nedergaard 2007; Attwell et al. 2010; Khakh and Sofroniew 2015). After brain injury, astrocytes proliferate and hypertrophy, and are more resistant to ischemia and hypoxia in the acute phase of brain injury, which can protect neurons (Honsa et al. 2014). In the neuroglial protective theory, it has been reported that inhibition of astrocytic mitochondria makes neurons vulnerable to cell death (Voloboueva et al. 2007).
Gap junctions (GJ) are conductive channels formed by membrane proteins termed connexins, which permit the intercellular exchange of metabolites, ions, and small molecules (Fu et al. 2017). In a previous study, astrocyte connexin 43 (Cx43), encoded by GJA1 gene, had been demonstrated to open hemichannels to drive astrocytes to release ATPs (Cotrina and Nedergaard 2009), suggesting its protective function in neural damage maybe lie in mitochondrial function. Several key phosphorylation of Cx43 at S368, S279/S282, and S262 was reported to be critical for the regulation of gap junction function and can drive the movement of Cx43 to plasma membrane (Solan and Lampe 2007). Cx43 phosphorylation was also reported with significant correlation with injury processes. For instance, the phosphorylated Cx43 (p-Cx43) was found to be greatly increased in astrocytes of the hippocampus following cerebral ischemia (Danesh-Meyer et al. 2008) and promote exosome release in TBI (Chen et al. 2018).
In TBI, it has been reported that GJA1 played a key role in neural proliferation and migration (Greer et al. 2017). GJA1-20K, the most abundant isoform produced by GJA1 alternative translation, has been reported to promote mitochondrial function in cardioprotection (Basheer et al. 2018). Here, we investigated its function after TBI and sought the mechanism to better understand the astrocyte–neuron protection and provides a potential novel target for the treatment of TBI.
Materials and Methods
Murine Astrocytes and Neuron Isolation
Cortical brain tissues of C57BL/6 fetal mice (embryonic day 18, E18) were quickly dissected followed by removing blood vessels and meninges. Then after dissociation with 0.25% trypsin–EDTA (Life technologies, Carlsbad, CA, USA) at 37 °C for 10 min, termination with DMEM-F12 supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA), as well as centrifugation at 300 g for 5 min, the collected cells were seeded in T75 flask in two different culture mediums. For neuron culture, cells were cultured in Neurobasal medium (Gibco) supplemented with 1 × B27 (Gibco), 0.5 mM GlutaMAX (Gibco), and 1% antibiotic–antimycotic solution (Corning, New York, NY, USA). After five-day culture, 5 μM Ara-C (Sigma, St. Louis, MO, USA) was added to deplete glial cells. For astrocytes culture, cells were cultured in DMEM/F12 medium (Gibco) with 10% FBS and 1% antibiotic–antimycotic solution. Meanwhile, in order to remove microglial, flasks were shaken at 400 rpm for 2 h at room temperature. All cells were placed at a 37 °C, 5% CO2 incubator. Animal studies were reviewed and approved by the People’s Hospital of Shanghai Pudong New Area Affiliated to Shanghai University of Medicine and Health Science.
In Vitro Model of TBI-Like Injury
Briefly, the neurons were seeded on the reverse side of the microporous membrane of the transwell chamber and cultured in a 37 °C 5% CO2 incubator until exhibited a state of 80–90% single-layer fusion. Then the chamber was transferred into a pressurized chamber, artificially arranged with a compressed nitrogen–oxygen mixed gas, under 1.5 MPa for 5 min. Then the chamber was placed back to the incubator and the astrocytes were seeded on the obverse side of the transwell membrane to form the astrocyte–neuron transwell co-culture system. Cell density of neurons and astrocytes was 2 × 105/mL and 6 × 105/mL, respectively. In this way, TBI-like neurite loss would be observed in pressurized neuronal cells. After pressurization, long-term effects of impulsive pressurization on neurite/axonal outgrowth, gene expression, apoptosis, etc., could be inspected (Nienaber et al. 2011). The co-cultured cells were cultured in Neurobasal medium with 1 × B27, 0.5 × N2 supplement (Gibco), 0.5 mM GlutaMAX, and 1% antibiotic–antimycotic solution. Another 48 h later, cells were harvested for further analysis. For the nocodazole treatment of astrocytes, isolated astrocytes were incubated with nocodazole (0.2 μg/mL) for 15 min before seeding.
MitoTracker Labeling
Before co-cultured, astrocyte medium was mixed with MitoTracker dyes (1:1000, Cell Signaling Technology, Danvers, MA, USA) in the presence of 750 nM p-(trifluoromethoxy)phenylhydrazone (FCCP) (Buckman et al. 2001) and incubated at 37 °C for 30 min. Labeled astrocytes could be co-cultured with neurons as described above.
Immunofluorescence
The cultured cells were fixed for 30 min with 4% paraformaldehyde, and blocked for 90 min in phosphate-buffered saline containing 5% normal bovine serum and 0.3% Triton X-100. The following primary antibodies were used: GFAP (1:500, BD Biosciences, San Jose, CA, USA) and MAP2 (1:1000, Abcam, Cambridge, CA, UK). After incubation at 4 °C overnight, samples were incubated with the corresponding Alexa-conjugated secondary antibodies (1:500, Jackson Immuno Research, West Grove, PA, USA) for 2 h. Fluorescent imaging analysis was performed using Carl Zeiss Laser Confocal Microscope LSM 780. The synaptic length was analyzed by Image J. software.
Adeno-Associated Virus (AAV) Infection
AAV9 vectors expressing GJA1-20K driven by the cytomegalovirus (CMV) promoter were produced and purified by Ultracel-10 regenerated cellulose membrane (Millipore, Billerica, MA, USA). The AAV infection was applied in 6 days after astrocytes mixed with 7-day in vitro neurons for 48 h. Astrocytes were harvested and seeded onto the upper compartment of the transwell astrocyte–neuron co-culture system.
Western Blot
Harvested cells were lysed and determined by a BCA Protein Assay kit (Sigma). Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. After blocking in 5% skim milk for 1 h at room temperature, membranes were incubated in primary antibodies targeting Cx43 (1:5000, Sigma), pCx43 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mtTFA (1:1000, Abcam), PGC-1a (1:500, Abcam), Tom20 (1:500, BD Biosciences), mtCO2 (1:1000, Abcam), Actin (1:1000, Abcam), and CJA1-20K with an HA-Tag (1:2000, Abcam) overnight at 4 °C. Membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies (Abcam). Target signals were detected by enhanced chemiluminescence (Millipore).
Quantitative Polymerase Chain Reaction (QPCR)
Total RNA of collected cells were extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA extracts were reverse transcribed and quantitated by SYBR Green assay (Promega GoTaq®qPCR Master Mix, Promega, Madison, WI, USA) on an R&D Flexa 6 qPCR system. The relative expression was calculated using the comparative cycle threshold method and analyzed by GraphPad Prism 6 software.
Statistical Analysis
All data are presented as the mean ± standard deviation (SD), and tested using Student’s t test or one-way ANOVA test with a Bonferroni post hoc test. Analysis was performed using the SPSS 17.0 and Graph Prism statistical systems, and P < 0.05 was considered statistically significant.
Results
Construction of a Transwell Astrocyte–Neuron Co-Culture System
To explore the specific mechanisms underlying the protection of astrocytes to damaged neurons in vitro, a transwell astrocyte–neuron co-culture system was established with the astrocytes on the obverse side and the damaged neurons on the reverse side of the transwell membrane (Fig. 1a). Immunofluorescence staining of transwell co-culture revealed a large amount of osmotic transfer of bottom astrocytes to the neuron layer (Fig. 1b). Observing the astrocyte transmembrane at 13, 6, 12, 24, and 36 h after co-culture, the number of astrocytes increased significantly and gradually became saturated (Fig. 1c).
Fig. 1.
Construction of a transwell astrocytes–neuron co-culture system. a Graphic presentation of the transwell astrocytes–neuron co-culture system. b Neuron monolayer immunofluorescence staining of GFAP (astrocyte), MAP2 (neurite), and DAPI (nucleus) after transwell co-culture. Scale bar = 20 μm. c Statistic graphs showing amounts of penetrated astrocytes (left) and ratio of astrocytes/neuron (right) in 3, 6, 12, 24, 36 h after transwell co-culture. Bar graphs indicate mean ± SD
Astrocyte GJA1-20K Enhanced Protection and Recovery of Damaged Neurons
To investigate the effect of astrocyte GJA1-20K in neuronal damage of TBI, we used AAV9 transfection to induce overexpression of GJA1-20K. Western blot analysis showed that GJA1-20K expression was significantly increased after transfection compared to the control group. At the same time, GJA1-20K expression increase significantly down-regulated the phosphorylation of Cx43, but did not affect the total Cx43 protein level (Fig. 2a, b). We co-cultured astrocyte overexpressing GJA1-20K with injured neuron, and measured the length of the neuron dendrites by MAP2 staining 10–14 days after in vitro culture. We found that co-culture group was significantly higher than control group, and the GJA1-20K overexpression group was also significantly higher than control group (Fig. 2c, d). These results show that co-culture of astrocytes can significantly repair injured neurons, and overexpression of GJA1-20K can significantly enhance this effect.
Fig. 2.
GJA1-20K enhanced protection and recovery of damaged neurons. a Western blotting analysis of total and phosphorylated Cx43 after transfection of GJA1-20K in astrocytes. GJA1-20K were transfected with a C-terminal HA tag. As GJA1-20K shares the same amino acids with Cx43 C-terminus, Cx43 N-terminus antibodies and HA antibodies were used to target Cx43 and GJA1-20K, respectively. b Graph-scale quantification analysis of WB results, compared with vehicle control group. **P < 0.01, ***P < 0.001 (c) MAP2 immunofluorescence staining of separated cultured and co-cultured astrocytes and neurons in transwell system. Single-cultured normal and damaged neurons, and damaged neurons co-cultured with vehicle/GJA1-20K astrocytes were assessed. Scale bar = 20 μm. d Statistics of dendrite length of MARP2 staining results. **P < 0.01, versus normal group; #P < 0.05, ##P < 0.01, n.s., not significant, versus single-cultured damaged group. Bar graphs indicate mean ± SD
GJA1-20K Promoted Mitochondria Delivery from Astrocytes to Neurons
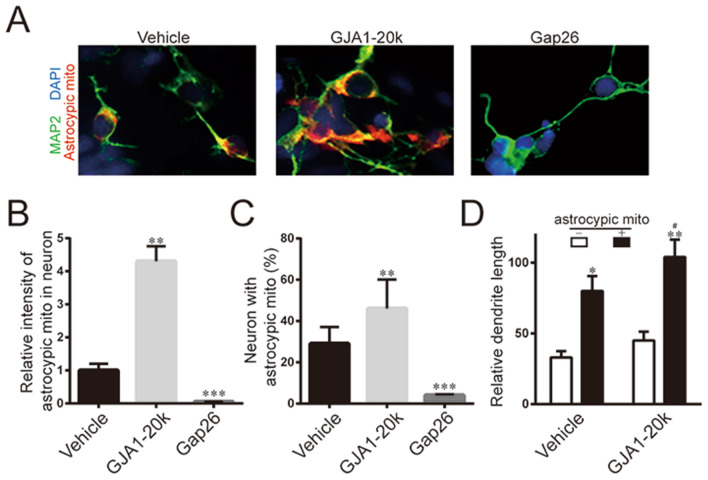
In previous study, GJA1-20K has been reported to facilitate mitochondrial interaction with microtubules (Basheer et al. 2017), so we tracked the mitochondria in astrocytes. We co-cultured MitoTracker pre-labeled astrocytes with damaged neurons, and then using immunofluorescence staining of neuron to find out mitochondrial transport in neuronal repair. We observed a significant mitochondrial transport in the protective repair of neurons by astrocytes, meanwhile overexpression of GJA1-20K significantly enhanced mitochondrial transmission of astrocytes–neuron. To achieve Cx43 knockdown, Gap26, a specific Cx43 hemichannel blocker acting on the level of its first extracellular loop was applied (0.25 mg/mL) 1 day after astrocyte–neuron mixture, which almost completely blocked mitochondrial transmission (Fig. 3a).
Fig. 3.
GJA1-20K promoted mitochondria transmission from astrocytes to neurons. a Immunofluorescence staining of neuron–astrocyte co-cultures with or without Gap26 treatment. Astrocytes were pre-labeled with MitoTracker before transwell co-culture. Gap26 were introduced to block Cx43. b Quantification analysis of immunofluorescence intensity of transferred astrocyte mitochondria. **P < 0.01, ***P < 0.01, versus vehicle group. (C) Statistics of neurons with transferred astrocyte mitochondria. **P < 0.01, ***P < 0.01, versus vehicle group. d Statistics of relative dendrite length of neurons with or without delivered astrocyte mitochondria. *P < 0.05, **P < 0.01, versus neurons without astrocytic mitochondria; #P < 0.05, versus vehicle group. Bar graphs indicate mean ± SD
After addition of Gap26, the fluorescence intensity and number of mitochondrial markers derived from astrocytes in neurons were significantly lower than those in the overexpression group (Fig. 3b, c). At the same time, the dendrite length of neurons receiving astrocytes mitochondria was significantly higher than that of neurons not receiving astrocytes mitochondria, and the overexpression of GJA1-20K further enhanced the repair of dendrite length (Fig. 3d). These results indicated that GJA1-mediated mitochondrial transport was an important process in the repair of damaged neurons by astrocytes.
Astrocyte GJA1-20K Induced Stability and Activity of Neuronal Mitochondria
We further investigated changes in mitochondrial activity and structurally important factors in injured neuron after co-culture. Western blot analysis showed that expression of two important activators of mitochondrial biosynthesis in neuron, mtTFA and PGC-1a, was significantly increased after co-cultured with astrocytes, and overexpression of GJA1-20K enhanced this effect (Fig. 4a). Meanwhile, Tom20, which is involved in mitochondrial transport function, and mtCO2, which characterizes mitochondrial function, also showed significant rebound after co-culture and was further enhanced by overexpression of GJA1-20K (Fig. 4b). QPCR assays for the above four factors showed interesting changes in mRNA level. MtTFA and PGC-1a were consistent with that in protein levels, while mRNA levels of Tom20 and mtCO2 had no significant difference (Fig. 4c). Those results indicated that in the synthesis of mitochondrial biosynthesis, astrocytes promoted transcription of mitochondrial synthesis-related genes in damaged neurons, while in mitochondrial transport, the regulation of astrocytes on damaged neuron-related proteins occurred at the stage of translation or post-translational modification, or it enhanced direct delivery of active mitochondria without stimulating the expression of involved genes in neurons.
Fig. 4.
Astrocyte GJA1-20K induced stability and activity of neuronal mitochondria via transwell co-culture. a Western blotting analysis of two crucial activators of mitochondria biosynthesis, mtTFA and PGC-1a. Quantification was shown in the lower panel. b Western blotting analysis of Tom20 and mtCO2. Quantification was shown in the lower panel. c qPCR analysis of mRNA levels of above four protein factors. Bar graphs indicate mean ± SD. *P < 0.05, **P < 0.01, versus single-cultured damaged neurons; #P < 0.05, versus vehicle transfected group
Discussion
TBI has become a severe public health problem worldwide (Langlois et al. 2006). Over past two decades, neuroprotection has gradually become a key consideration in the treatment of TBI. Therefore, it is necessary to understand the mechanism of astrocytic neuroprotective function in TBI. In our research, we used a astrocyte–neuron co-culture model to simulate TBI, which has been proven to be a good method to study its pathophysiology (Mcintosh et al. 1989). After neural damage, astrocytic mitochondria can be released into extracellular to trigger protective neuroglial crosstalk (Falchi et al. 2013; Hayakawa et al. 2016).
GJs, formed by connexin hemichannels, are specialized sites of cell–cell contact that allow the passage of ions, intracellular metabolites, and messenger molecules, as well as provide adhesion sites between adjacent cells (Contreras et al. 2004; Evans et al. 2006; Hoang et al. 2010; Saez et al. 2005) Among GJ-associated proteins, GJA1 encoded Cx43 is widely expressed in all organ (Beyer et al. 1987). Unlike full-length GJA1-43k, GJA1-20K is enriched at the interface between mitochondria and microtubules and has a strong tropism for mitochondria function (Fu et al. 2017). Recently, studies on the protection of mitochondrial function and cell damage have shown that functional GJA1 (non-phosphorylated GJA1) on mitochondrial membrane is important for mitochondrial morphology and function stabilization and post-injury repair (Pecoraro et al. 2018; Kim et al. 2017). However, these studies have not further explained how GJA1 enters mitochondria. A recent study reported that endogenous GJA1-20K is increased after oxidative damage in cells and can be transferred to mitochondria via its C-terminal tubulin (Tubulin), promoting the production of functional GJA1 and stabilizing the mitochondrial membrane, to reduce oxidative stress caused by endoplasmic reticulum–mitochondrial dysfunction (Basheer et al. 2017, 2018; Sun et al. 2014; Contreras et al. 2004). Unfortunately, the above studies have not yet penetrated into the specific mechanisms by which regulated mitochondria are transmitted between cells. In our study, we have demonstrated that GJA1-20K can stabilize cell mitochondria by up-regulating functional GJA1 and promote mitochondrial movement.
Based on our transwell co-culture system, we inferred that long actin-based membranous cellular extensions, termed tunneling nanotubes (TNTs), might be involved in mitochondrial transportation. Current research on post-injury protection with TNTs is increasing, but it is currently focused on stem cells (Sinclair et al. 2016; Bose et al. 2018), instead of astrocyte–neuron protection. Based on our results, we believed that the transfer of mitochondria from the stem cells to the damaged cells via the TNTs channel could be enhanced by overexpression of GJA1-20K. In other words, intercellular mitochondria need to pass through the TNTs-GJA1 channel to enter the target cells and activate their biological activities. Our study has shown that GJA1-20K-related TNTs mediate astrocytic mitochondrial intercellular delivery and play a crucial role in the process of neuroprotection.
Combined with our previous studies on the involvement of GJ in brain injury that abnormal expression of GJA5 and GJA1 in neurons is significantly correlated with brain damage factors, such as oxidative stress and increased inflammatory products (Chen et al. 2017), we indicated that this series of changes may be related to activation of the ERK1/2 pathway caused competitive inhibition of the PKA pathway lead to increased stress of endogenous GJA1-20K and the subsequent protection of mitochondria. On the other hand, after TBI, the increase of endogenous GJA1-20K alone is not sufficient to produce enough cytoprotective factors. Those possibilities are related to the intrinsic relationship between neuroprotective function and nerve damage by astrocytes, and also suggest that mitochondria through TNTs-GJA1 channel intercellular delivery is critical for the clinical diagnosis and prognosis of TBI. In our future study, we will combine clinical analysis with the relationship between the changes of TNTs-GJA1 functional channels and the severity and prognosis of TBI, focusing on the identification of astrocyte-derived mitochondria via TNTs-GJA1 channel for neuronal protection and its relationship with TBI processes, and hope to find new target and new strategies for the treatment of TBI.
Conclusion
The present study experimentally constructs a transwell astrocyte–neuron co-culture system to recapitulate the interaction between astrocytes and neurons after TBI-like damage in vitro. GJA1-20K overexpression up-regulates functional Cx43 expression in astrocytes, which promotes mitochondria transmission from astrocytes to neurons which might be responsible to the protection of astrocyte to neurons after TBI-like damage in vitro. It sheds new light on the therapeutic potential of astrocyte GJA1-20K in TBI treatment.
Funding
The study was supported by Shanghai Natural Science Foundation (19ZR1447400); Key Project of Jiangxi Science and Technology Department (20192BBGL70022); National Natural Science Foundation of China (81960236); Key scientific research projects of Jiangxi Provincial Department of Education (GJJ190022); Scientific research plan of Jiangxi health and Family Planning Commission (20203139); and Younth Key Project of Jiangxi Natural Science Foundation (20202ACBL216005).
Data Availability
Data could be available upon reasonable request.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Animals
Animal studies were reviewed and approved by the People’s Hospital of Shanghai Pudong New Area Affiliated to Shanghai University of Medicine and Health Science.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dabin Ren, Ping Zheng and Shufeng Zou have been contributed equally to this work.
Contributor Information
Jiugeng Feng, Email: fengjg1979@sina.com.
Chunlong Zhong, Email: drchunlongzhong@126.com.
Wei Chen, Email: weichen445@163.com.
References
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468(7321):232–243. 10.1038/nature09613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer WA, Fu Y, Shimura D, Xiao S, Agvanian S, Hernandez DM, Hitzeman TC, Hong T, Shaw RM (2018) Stress response protein GJA1–20k promotes mitochondrial biogenesis, metabolic quiescence, and cardioprotection against ischemia/reperfusion injury. JCI Insight. 10.1172/jci.insight.121900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer WA, Xiao S, Epifantseva I, Fu Y, Kleber AG, Hong T, Shaw RM (2017) GJA1-20k arranges actin to guide Cx43 delivery to cardiac intercalated discs. Circ Res 121(9):1069–1080. 10.1161/circresaha.117.311955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, Goodenough DA (1987) Connexin43: a protein from rat-heart homologous to a gap junction protein from liver. J Cell Biol 105(6):2621–2629. 10.1083/jcb.105.6.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A, Basu R, Maulik M, Das Sarma J (2018) Loss of Cx43-mediated functional gap junction communication in meningeal fibroblasts following mouse hepatitis virus infection. Mol Neurobiol 55(8):6558–6571. 10.1007/s12035-017-0861-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JF, Hernandez H, Kress GJ, Votyakova TV, Pal S, Reynolds IJ (2001) MitoTracker labeling in primary neuronal and astrocytic cultures: influence of mitochondrial membrane potential and oxidants. J Neurosci Methods 104(2):165–176. 10.1016/s0165-0270(00)00340-x [DOI] [PubMed] [Google Scholar]
- Chen W, Guo Y, Yang W, Chen L, Ren D, Wu C, He B, Zheng P, Tong W (2018) Phosphorylation of connexin 43 induced by traumatic brain injury promotes exosome release. J Neurophysiol 119(1):305–311. 10.1152/jn.00654.2017 [DOI] [PubMed] [Google Scholar]
- Chen W, Guo YJ, Yang WJ, Zheng P, Zeng JS, Tong WS (2017) Connexin40 correlates with oxidative stress in brains of traumatic brain injury rats. Restor Neurol Neurosci 35(2):217–224. 10.3233/Rnn-160705 [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MVL, Saez JC (2004) Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Rev 47(1–3):290–303. 10.1016/j.brainresrev.2004.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M (2009) Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signall 5(2):223–232. 10.1007/s11302-009-9138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Huang R, Nicholson LF, Green CR (2008) Connexin43 antisense oligodeoxynucleotide treatment down-regulates the inflammatory response in an in vitro interphase organotypic culture model of optic nerve ischaemia. J Clin Neurosci 15(11):1253–1263. 10.1016/j.jocn.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L (2006) The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J 397:1–14. 10.1042/Bj20060175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi AM, Sogos V, Saba F, Piras M, Congiu T, Piludu M (2013) Astrocytes shed large membrane vesicles that contain mitochondria, lipid droplets and ATP. Histochem Cell Biol 139(2):221–231. 10.1007/s00418-012-1045-x [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhang S-S, Xiao S, Basheer WA, Baum R, Epifantseva I, Hong T, Shaw RM (2017) Cx43 isoform GJA1-20k promotes microtubule dependent mitochondrial transport. Front Physiol. 10.3389/fphys.2017.00905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer K, Chen J, Brickler T, Gourdie R, Theus MH (2017) Modulation of gap junction-associated Cx43 in neural stem/progenitor cells following traumatic brain injury. Brain Res Bull 134:38–46. 10.1016/j.brainresbull.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555. 10.1038/nature18928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang QV, Qian HH, Ripps H (2010) Functional analysis of hemichannels and gap-junctional channels formed by connexins 43 and 46. Mol Vis 16(145–50):1343–1352 [PMC free article] [PubMed] [Google Scholar]
- Honsa P, Pivonkova H, Harantova L, Butenko O, Kriska J, Dzamba D, Rusnakova V, Valihrach L, Kubista M, Anderova M (2014) Increased expression of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in reactive astrocytes following ischemia. Glia 62(12):2004–2021 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10(11):1369–1376. 10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18(7):942–952. 10.1038/nn.4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SN, Kwon HJ, Im SW, Son YH, Akindehin S, Jung YS, Lee SJ, Rhyu IJ, Kim Y, Seong JK, Lee J, Yoo HC, Granneman JG, Lee YH (2017) Connexin 43 is required for the maintenance of mitochondrial integrity in brown adipose tissue. Sci Rep. 10.1038/s41598-017-07658-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM (2006) The epidemiology and impact of traumatic brain injury. J Head Trauma Rehabil. 10.1097/00001199-200609000-00001 [DOI] [PubMed] [Google Scholar]
- Maas AIR, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7(8):728–741. 10.1016/s1474-4422(08)70164-9 [DOI] [PubMed] [Google Scholar]
- Mcintosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL (1989) Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28(1):233–244. 10.1016/0306-4522(89)90247-9 [DOI] [PubMed] [Google Scholar]
- Nienaber M, Lee JS, Feng R, Lim JY (2011) Impulsive pressurization of neuronal cells for traumatic brain injury study. J Vis Exp. 10.3791/2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro M, Pinto A, Popolo A (2018) Inhibition of Connexin 43 translocation on mitochondria accelerates CoCl2-induced apoptotic response in a chemical model of hypoxia. Toxicol In Vitro 47:120–128. 10.1016/j.tiv.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MVL (2005) Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta Biomembr 1711(2):215–224. 10.1016/j.bbamem.2005.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair KA, Yerkovich ST, Hopkins PMA, Chambers DC (2016) Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther. 10.1186/s13287-016-0354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD (2007) Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol 217(1–3):35–41. 10.1007/s00232-007-9035-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L-Q, Gao J-L, Cui C-M, Cui Y, Jing X-B, Zhao M-M, Wang Y-C, Tian Y-X, Wang K-J, Cui J-Z (2014) Astrocytic p-connexin 43 regulates neuronal autophagy in the hippocampus following traumatic brain injury in rats. Mol Med Rep 9(1):77–82. 10.3892/mmr.2013.1787 [DOI] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, Xu LK (2017) Traumatic brain injury-related emergency department visits, hospitalizations, and deaths: United States, 2007 and 2013. MMWR Surveill Summ 66(9):1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Suh SW, Swanson RA, Giffard RG (2007) Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J Neurochem 102(4):1383–1394. 10.1111/j.1471-4159.2007.04634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data could be available upon reasonable request.