Abstract
Ischemia is characterized by a transient, insufficient, or permanent interruption of blood flow to a tissue, which leads to an inadequate glucose and oxygen supply. The nervous tissue is highly active, and it closely depends on glucose and oxygen to satisfy its metabolic demand. Therefore, ischemic conditions promote cell death and lead to a secondary wave of cell damage that progressively spreads to the neighborhood areas, called penumbra. Brain ischemia is one of the main causes of deaths and summed with retinal ischemia comprises one of the principal reasons of disability. Although several studies have been performed to investigate the mechanisms of damage to find protective/preventive interventions, an effective treatment does not exist yet. Adenosine is a well-described neuromodulator in the central nervous system (CNS), and acts through four subtypes of G-protein-coupled receptors. Adenosine receptors, especially A1 and A2A receptors, are the main targets of caffeine in daily consumption doses. Accordingly, caffeine has been greatly studied in the context of CNS pathologies. In fact, adenosine system, as well as caffeine, is involved in neuroprotection effects in different pathological situations. Therefore, the present review focuses on the role of adenosine/caffeine in CNS, brain and retina, ischemic events.
Keywords: Brain, Retina, Ischemia, Adenosine receptors, Caffeine, Neuroprotection
Introduction
Hypoxia–ischemia (HI) is characterized by a local or systemic, transient or permanent, interruption of blood flow, and oxygen supply, leading to an inability to meet cellular energy demands. When the CNS is affected, the cell death caused by ischemia provokes brain injury and neurological disabilities. This pathological condition can affect both developing and mature CNS, with long-term consequences and few preventive/therapeutic interventions. In addition, all the main retinopathies that cause blindness in the world, such as age-related macular disease (AMD), glaucoma, and diabetic retinopathy (Bourne et al. 2017), display an ischemic component at some point of the disease, resulting in a worsening of visual impairment. So, ischemia is also a main problem in the ophthalmology field.
Since there is no effective treatment for ischemia, and concerning its negative outcomes, the importance to understand the mechanisms of cell death and possible neuroprotective interventions becomes evident. Ischemia induces several alterations in cellular physiology, starting with a decrease in ATP production, that affects all ATP-dependent cellular functions, followed by the release of neurotransmitters, such as glutamate, leading to excitotoxicity and cell death (Nicholls et al. 1987; Lipton 1999; Reid et al. 2003; Kostandy 2012; Mayor and Tymianski 2018). Moreover, there is also an increase in the extracellular availability of adenosine, both from ATP hydrolysis and by reversal of adenosine transporters (Melani et al. 2014b; Pedata et al. 2016). A number of studies have demonstrated a role for adenosine receptors in hypoxic and ischemic conditions. Adenosine receptors are G protein-coupled receptors named A1 and A3, which classically inhibit adenylyl cyclase (AC) by activating Gi protein, while A2A and A2B increase AC activity through Gs/Golf (Borea et al. 2018). A1 and A2A are the most abundant adenosine receptors in the CNS, and several studies have shown their involvement in cell protection mechanisms. These receptors are non-selectively inhibited by low–moderate concentrations of caffeine that can be achieved by daily doses of coffee (Fredholm et al. 2017). Caffeine is considered a mild stimulant to the CNS, and it can also be found in several other sources of foods and drinks consumed worldwide by the majority of adults (Heckman et al. 2010; Mitchell et al. 2014). Therefore, the present review will focus on studies that shed light into caffeine, as well as adenosine, as a promising therapeutic tool for ischemia. Moreover, it also brings data from epidemiology, health system costs, the mechanisms involved in ischemia-induced cell death, available treatments, and the present challenges. However, it is important to note that there is a robust amount of data on the roles of adenosine and caffeine on neurophysiology and neuroprotection, apart from ischemic context, that goes beyond the proposal of this review, but it is essentially connected to the subject and can be satisfactorily appreciated by some fulfilling readings (Cunha 2005, 2016; Costenla et al. 2010; Gomes et al. 2011; Dos Santos-Rodrigues et al. 2015; Kolahdouzan and Hamadeh 2017; Liu et al. 2019; Lopes et al. 2019).
Epidemiology of Ischemic Events
Adult Stroke
Among neurological diseases, stroke accounts for the largest proportion of deaths (67.3%), disability-adjusted life years (DALYs—47.3%), and it is the third overall leading cause of death worldwide after heart disease and cancer (Moskowitz et al. 2010; GBD 2017; Lallukka et al. 2018).
Stroke is classified into two main categories: ischemic, when blood flow is interrupted by a clot/thrombus, accounting for 87% of the cases; or hemorrhagic, when there is a rupture of a blood vessel resulting in leakage to adjacent tissue (Ovbiagele and Nguyen-huynh 2011; Bejot et al. 2016; Lee et al. 2018). As the nervous tissue has a high energy demand, the oxygen and substrate deprivation lead to irreversible damage detectable within minutes. Thus, it results in brain damage and neurological disabilities that can be reflected in impaired behaviors associated with memory, learning and locomotion (Janardhan and Qureshi 2004; Li et al. 2013; Lee et al. 2018).
There are several risk factors associated with the incidence of stroke, with hypertension being the most prevalent among modifiable ones, linked to 35% of the cases. Other risk factors include smoking, obesity, poor diet, sedentary lifestyle, diabetes mellitus, high alcohol consumption, psychosocial factors, cardiac cause, and ratio of apolipoprotein B and apolipoprotein A1 (O’Donnell et al. 2010; Soler and Ruiz 2010; Bejot et al. 2016). Many of these are considerably easy to overcome, so preventive strategies should be used to reduce the risk and the cost of treatment. Non-modifiable factors connected to the pathology are as follows: age, as the incidence increases with aging (Wolf et al. 1992; Rosamond et al. 2008; Romero et al. 2008); gender, overall stroke incidence is lower in women, even though these numbers change when incidence and mortality are analyzed at older ages (Rothwell et al. 2005; Löfmark and Hammarström 2007; Reeves et al. 2008); genetics/heredity; and ethnicity (Soler and Ruiz 2010).
The economic burden of the disease is extremely relevant, as patients may need permanent care depending on the severity of the stroke (Table 1). In 40% of the cases, patients acquire moderate to severe impairment and need special care, while 10% need constant care in long-term care facilities (Rajsic et al. 2019). Table 1 also summarizes the current ischemic stroke treatment, which is based on two fronts approved by the United States Food and Drug Administration, along with its limitations.
Table 1.
Cost, treatment/limitations for stroke and perinatal ischemia
| Condition | Cost | Treatment | Treatment limitations |
|---|---|---|---|
| Stroke |
US: $140.000/patient/lifetime (Johnson et al. 2016); $11.145/patient/1st year (Mozaffarian et al. 2015; Rajsic et al. 2019); US economic burden estimated to be $240.7 billion by 2030 (Ovbiagele et al. 2013); Europe: € 27 billion/year (formal care), € 11.1 billion/year (informal care) (Gustavsson et al. 2011; Rajsic et al. 2019); Brazil: $2.315–35.092/patient (Safanelli et al. 2019; Vieira et al. 2019) |
Intravenously administered recombinant-tPA) (Marko et al. 2020; Vermeij et al. 2018; Wardlaw et al. 2014) | Narrow safety window, blood pressure has to be ≤ 180/110 mmHg, with or without antihypertensive treatment; must show no evidence of bleeding in the brain or bleeding diathesis (Zivin 2009); Shows ineffective results in 10% for carotid occlusion or 30% for MCAO (Derex and Cho 2017) |
| Intra-arterial thrombectomy (ITA) (Goyal et al. 2016; Vermeij et al. 2018) | More invasive, risk of vessel trauma and distal embolization; Effectiveness depends on circulation return within 6 h; Recommended for proximal anterior circulation (Derex and Cho 2017; Furlan et al. 2005; Goyal et al. 2016) | ||
| Cell therapy (Stilley et al. 2004; Rabinovich et al. 2005; Kondziolka et al. 2005; Honmou et al. 2011; Bhasin et al. 2013; Jiang et al. 2013; Lemmens and Steinberg 2013; Hao et al. 2014) | Ongoing clinical trials. Still need further experiments and results in order to understand its benefits and possible side effects | ||
| Perinatal Ischemia |
US: $50.000 for survivors of hypothermia treatment (Massaro et al. 2016); $900.000/lifetime for those who develop cerebral palsy (Eunson 2015) |
Therapeutic hypothermia (Simbruner et al. 2010; Higgins et al. 2011) |
Risk of mortality and neurological outcomes persist in 50% of the neonates affected by HI (Edwards et al. 2010; Jacobs et al. 2013); Lower efficacy for newborns who suffered serious insults (Higgins et al. 2011); Benefits may be limited to those born at term or late preterm (Perlman 2006; Rees et al. 2011; Jacobs et al. 2013); Treatment should be started within 6 h after birth (Higgins et al. 2011; Jacobs et al. 2013) |
Perinatal Hypoxia–Ischemia
Concerning prenatal developmental, HI may occur due to a mechanical process, placental insufficiency, prolonged labor or folds in umbilical cord (De Haan et al. 2006; Martinez-Biarge et al. 2012), besides events of preeclampsia and maternal bleeding (Paolo 2012). There are also other risk factors, such as anemia, hypotension, multiple births, smoking, and drug abuse (Pundik et al. 2006). Pre- and perinatal lesions alter CNS development, causing different outcomes according to the kind of insult, the developmental period, the intensity, and the affected area. HI events, in addition to causing newborns to die, are also the main triggering factor for encephalopathy (Kurinczuk et al. 2010) and permanent brain damage in children (Johnston et al. 2009; Volpe 2012). Perinatal hypoxic-ischemic encephalopathy (HIE) affects 1–3 of every 1000 babies born at term (Yang and Lai 2011). Of these children, 15–20% die in the postnatal period, characterizing HIE as one of the most significant causes of neonatal mortality. Of those who survive, 25% develop permanent neurophysiological consequences (Vannucci 2000; Chen et al. 2009). In spite of the advances in neonatal medicine, the proportion of infants diagnosed with neurological deficits after suffering perinatal insults remains stable (McIntyre et al. 2013).
In premature (or underweight) newborns, the numbers are even more alarming, since the incidence of perinatal asphyxia corresponds to around 60%, and 20–50% of the babies who have undergone HI events exhibit deficits later, such as difficulty in concentration, cognitive delay (Filloux et al. 1996; Gross et al. 2005), visual, motor and perceptual disorders, hyperactivity (Vannucci 2000; Perlman 2006) and, in even more severe cases, epilepsy and cerebral palsy (Nelson et al. 2003).
Cognitive damage, although strongly associated with neuromotor deficits, can be seen in children who have suffered HIE, in the presence or absence of motor impairments (Van Handel et al. 2007; Schreglmann et al. 2020). These sequelae can mark the school phase due to learning delays (Robertson and Perlman 2006) and the impairments may persist throughout adolescence, with an intense reduction in episodic memory (Gadian 2000), poor performance in executive functions, and visual and verbal memory (Mañeru et al. 2001).
Neonatal care represents a major burden for health systems around the world. Considering neonatal intensive care units (NICUs) in the US, there is an estimative of 77.9 admissions per 1000 live births in the period between 2007 and 2012 (Harrison and Goodman 2015). The damage caused by HIE is associated with high morbidity and mortality, which requires the highest level NICU care and interventions. In this context, HIE newborns have been considered to be part of a small group of patients who consume the major amount of NICU expenses (Bayne 2018). Information for expenditures and limitations of treatment for perinatal ischemia is summarized in Table 1.
In order to obtain therapies for the prevention of mortality and treatment of disabilities, the exploration of certain key factors involved in these damages is essential. Studies in animal models have revealed potential candidates for therapeutic intervention based on mechanisms anti-excitotoxicity, anti-oxidation, anti-inflammation, and anti-apoptosis (Greco et al. 2020).
Retinopathies with Ischemic Components
Ischemia may be considered a key factor in the pathophysiology of visual diseases, including retinopathies. Retina has been classified as one of the most energetically demanding tissues, being even more metabolically active than the brain (Ames 1992; Yu and Cringle 2001; Wong-Riley 2010). The acute or chronic occlusion of retinal microvasculature may impair retinal perfusion causing permanent visual loss, such as verified in glaucoma, DR, and AMD (Schmidt et al. 2008; Kaur et al. 2008; Szabadfi et al. 2010).
Glaucoma
Glaucoma is an optic neuropathy whose main clinical sign is the increase in intraocular pressure (IOP) and the main outcome is progressive and irreversible visual loss. It is estimated to affect more than 60 million people worldwide (Quigley and Broman 2006) and this number is expected to increase to 111.8 million in 2040 (Tham et al. 2014). It is classified as a prevalent neurodegenerative disease (Jiang et al. 2020) and the most important cause of irreversible blindness (Tham et al. 2014).
Glaucoma has been considered a multifactorial disease with genetic and environmental components, with aging being the most important risk factor (Doucette et al. 2015). Although its pathophysiology has not been completely defined so far, some mechanisms are proposed to explain the causes underlying retinal ganglion cell death and optic nerve damage (Doucette et al. 2015). Ischemic conditions may be created by an increase in IOP which exerts pressure on retinal vasculature (Harris et al. 2001; Osborne et al. 2001). Besides, glutamate excitotoxicity (Casson 2006), oxidative stress (Ko et al. 2005; Tezel 2006; Gericke et al. 2019), and inflammation (Fontaine et al. 2002; Wong et al. 2015; Gericke et al. 2019) have also a role in glaucoma pathogeny.
As current available interventions to treat glaucoma have several limitations, the development of new therapeutic agents is of great relevance concerning the economic burden represented by glaucoma treatment (Table 2).
Table 2.
Cost, treatment/limitations for the three most prevalent causes of vision loss
| Condition | Cost | Treatment | Limitations |
|---|---|---|---|
| Glaucoma |
US: $8157/patient/year (no vision loss) up to $18.670 (with vision impairment) (Feldman et al., 2020); Europe: €969/person/year (Traverso et al. 2005) |
Topic beta-blockers |
All treatments target IOP, although it is estimated that half of glaucoma patients do not have an increase in IOP (Shields 2008); Direct medical costs as medication, laser and surgical interventions can not be afforded by a significant part of the population, especially in developing countries (Zhao et al. 2018) |
| Cholinergic, alpha-adrenergic, carbonic anhydrase inhibitors (Mantravadi and Vadhar 2015; Foundation 2017) | |||
|
Prostaglandin analogs | |||
| Laser therapy (Garg and Gazzard 2018) | |||
|
Surgical procedures | |||
| Diabetic retinopathy |
US: $493 million (medical costs in 2004) (Rein et al. 2006) Sweden: €433/patient/year (Heintz et al. 2010); Germany: €671–2933/patient/year (Kähm et al. 2018) |
Laser photocoagulation (Stewart 2016) |
Target an advanced stage of the disease, uncomfortable administration, high costs, long-term side effects, and ultimately do not cure the disease (Al-Shabrawey et al. 2013; Wong et al. 2016); Clinical studies with pharmacological candidates are still inconclusive or were discontinued due to side effects |
| Intravitreal injections of anti-VEGF (Stewart 2016) | |||
| Control of glycemia diet or medication (Stewart 2016) | |||
| Candidates for the treatment of DR: aldose reductase inhibitors, anti-inflammatory drugs, carbonic anhydrase inhibitors, fenofibrate, hyaluronidase, PKCβ1/2 inhibitors, and renin-angiotensin system blockers (Tarr et al. 2012; Ebneter and Zinkernagel 2016) | |||
| Age-related macular degeneration | US: $7.133/patient/year (direct medical costs); $30.000/patient/year (additional indirect costs) (Brown et al. 2016) | Intravitreal injection of agents that indirectly block VEGF actions (Amoaku et al. 2015; Khanna et al. 2019); |
Anti-VEGF drugs have been less beneficial than expected (Schlottmann et al. 2017); Corticosteroid treatment increases risk for cataract and ocular hypertension (Kuppermann et al. 2015; Rezar-Dreindl et al. 2017) |
| Corticosteroid implants as an adjunctive therapy (Campochiaro et al. 2012; Bailey et al. 2017; Vakalis et al. 2015; Bonfiglio et al. 2017) | |||
| Combination of anti-VEGF, corticosteroids and photodynamic therapy with the use of verteporfin (Kawczyk-Krupka et al. 2015) |
Diabetic Retinopathy
Considering people in working age, DR is the leading cause of vision loss and blindness (Ding and Wong 2012; Yau et al. 2012). In general, one-third of the patients with DM may present DR (Nam Han Cho et al. 2017), being more prevalent among patients with type 1 DM (Tarr et al. 2012). For the next years, the number of people affected by DR is expected to dramatically increase, which reflects the high incidence of DM, obesity, and also population aging (Saaddine et al. 2008; Ting et al. 2016).
Retinal damage derived from chronic hyperglycemia in DM is complex, but the central event is attributed to oxidative stress (Brownlee 2001; Arden and Sivaprasad 2011; Mendonca et al. 2020). Hyperglycemia-induced alterations cause endothelial cell dysfunction, breakdown of blood–retinal barrier and increase in vascular permeability, leading to edema (Zhang et al. 2014; Stitt et al. 2016). Moreover, the production of trophic factors is reduced, which is associated with capillary degeneration (Brownlee 2001; Arden and Sivaprasad 2011). As a result, the tissue responds to ischemic-induced signaling, triggering events of neovascularization and generating an abnormal retinal vasculature, which characterizes the proliferative stage of the disease (Al-Shabrawey et al. 2013). Furthermore, the present view of DR involves neuroinflammation (Karlstetter et al. 2015; Yu et al. 2015), neurodegeneration (Kadłubowska et al. 2016; Simó et al. 2018), and excitotoxicity (Kokona et al. 2016; Ola et al. 2019) events that may precede vascular alterations.
The impacts of DR on visual function represent a relevant challenge in public health, especially concerning care expenditures and treatments (Table 2). Besides the expenses directly related to healthcare, a significant economic impact of DR is linked to the insertion or permanency of these subjects in the job market (Rein et al. 2006). Thus, further studies are needed to develop new effective therapeutic treatments.
Age-Related Macular Degeneration
AMD is a progressive degenerative disease that primarily impairs the central retina and leads to irreversible vision loss. It is currently considered a major cause of blindness in elderly people (Smith et al. 2001), affecting 170 million people in the world (Pennington and DeAngelis 2016). Studies project that the number of patients diagnosed with AMD may expand to 288 million by 2040 (Wong et al. 2014). The high number of cases is directly attributed to the increase in life expectancy, particularly in developed countries.
AMD consists of a multifactorial disease whose etiology comprises genetic and environmental elements. A series of genes have already been identified (Al-Zamil and Yassin 2017) as well as lifestyle risk factors, such as light exposure (Chalam et al. 2011), diet (Chapman et al. 2019), and tobacco smoking (Smith et al. 2001).
The disease can also be classified into two types: dry and wet AMD, although specific pharmacological options are only available for the treatment of wet AMD (Supuran 2019) (Table 2), but there are no preventive strategies or cure (Hernández-Zimbrón et al. 2018). The burden related to AMD is highly underestimated (Brown et al. 2005); however, it is clear that vision loss negatively affects not only one’s health but also their contribution and interaction with others and consequently impacts society (Table 2).
Mechanisms of Cell Death Provoked by Ischemia
The development of new, and efficient, treatment depends on the profound understanding of cell death phenomenon during the time of ischemia itself and reperfusion period (Dirnagl et al. 1999). Ischemia refers to a pathological lack of blood supply to a given tissue, so its maintenance is drastically impaired (Fig. 1). When tissue perfusion is low, cells are deprived of oxygen and metabolic substrates, and excretes begin to accumulate (Osborne et al. 2004; Kalogeris et al. 2016). Once a tissue becomes ischemic, a metabolic dysfunction is triggered. There is a decrease in glycolysis and oxidative phosphorylation, reducing ATP production, which, in turn, leads to failure in ionic pumps and ionic imbalance (Lipton 1999; Kalogeris et al. 2016). Reduction in sodium–potassium pump activity decreases the removal of intracellular sodium, affecting membrane potential maintenance, and consequently depolarizing cell membrane. Another result of cytoplasmic sodium accumulation is the passive influx of chloride, which also attracts water into the cell, causing cell swelling, and eventually, cell lysis, accompanied by cell content extravasation (Edinger and Thompson 2004; Duprez et al. 2009; Galluzzi et al. 2012). This kind of acute death is known as necrotic cell death and occurs mainly at the core of the ischemic region. Voltage-gated calcium channels (VGCC) are activated by this depolarization, increasing cytoplasm calcium concentrations and triggering neurotransmitter release (Mayor and Tymianski 2018).
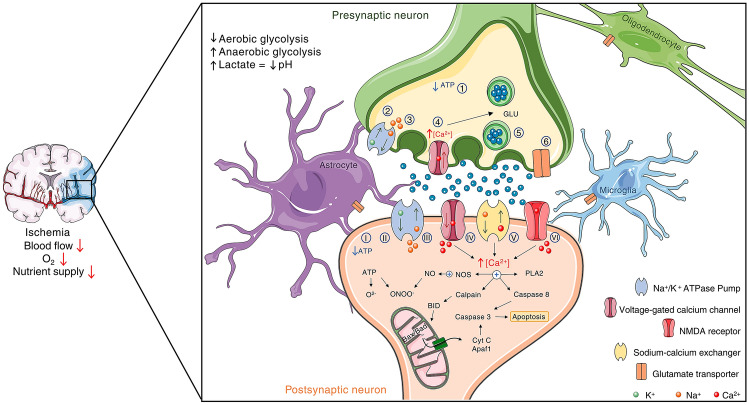
Fig. 1.
Deleterious effects of ischemia on presynaptic and postsynaptic neurons. The interruption or reduction of blood flow is associated with the decrease of O2 levels and nutrient supply. Neurons respond to these effects by decreasing aerobic glycolysis, while increasing anaerobic glycolytic process, leading to the accumulation of lactate and to a pH reduction. As ATP levels decrease (1 and I), the failure of Na+/K+/ATP pumps may occur (2 and II), which cause electrolyte imbalance (3 and III), depolarization and opening of Ca2+ voltage-dependent membrane channels (4 and IV). In the presynaptic neuron, these alterations increase neurotransmitter release, especially glutamate (5). The reversal of EAAT transporters contributes to the increase in glutamate availability in the synaptic cleft as well (6). ATP deficiency also impacts neurons by generating reactive oxygen/nitrogen species (e.g., superoxide and peroxynitrite). The depolarization mediated by intracellular sodium increase (III) stimulates voltage-gated Ca2+ channel (IV). Intracellular Ca2+ level is also elevated through the reversal of Na+/Ca2+ exchanger (V). Ca2+ overload also affects the postsynaptic neuron as a result of NMDA receptor hyperactivation (VI), triggering glutamate excitotoxicity. Thus, intracellular Ca2+ accumulation leads to the activation of different death pathways such as the one mediated by NOS, Calpain, Caspase, and phospholipase A2 (PLA2). It is important to note that other pathways contributing to cell death are not described in the scheme for summarization purposes. For the clarity of the scheme Bax/Bad are show in mitochondria matrix
Excitotoxicity
Glutamate is the major excitatory neurotransmitter in the CNS, and during ischemic events, a massive glutamate release occurs mainly through two different modes. Initially, glutamate is released by exocytosis, a calcium-dependent mechanism, and then by reversal of the glutamate transporters, a calcium-independent mechanism (Nicholls et al. 1987; Reid et al. 2003; Kostandy 2012). Both ATP and glutamate are also released through hemichannels (Pedata et al. 2016). Independent of the mechanism of release, glutamate further depolarizes glutamate receptors-containing cells, creating a positive feedback (Verkhratsky and Shmigol 1996; De Flora et al. 1998). The depolarization also promotes the release of magnesium from NMDA receptors (Zeevalk and Nicklas 1992), making them even more responsive to glutamate, and further intensifying the depolarization, by sodium and calcium influx. Calcium entry through NMDA receptors, VGCC and sodium–calcium exchangers can trigger signaling pathways that promote cell death through apoptosis (Figs. 1 and 2). Caspase-8 and calpains mediate BH3-interacting domain death agonist cleavage, which translocates into mitochondria, where it interacts with another set of pro-apoptotic proteins, like Bax, Bak, and Bad. This signaling promotes changes in mitochondrial permeability, triggering the release of mitochondrial proteins like cytochrome c (Cyt C), contributing to apoptosome formation through interaction with apoptotic protease activating factor 1 (Apaf-1) (Orrenius et al. 2015; Datta et al. 2020). The mechanism leads to effector caspase activation (e.g., caspase 3), leading to protein cleavage and DNA fragmentation, a hallmark of apoptosis (Fig. 1). Briefly, other apoptotic pathways are also provoked by ischemia, such as activation of death receptors by molecules, like tumor necrosis factor-alpha (TNF-α) and first apoptosis signal ligand (FasL). These induce apoptosis through the activation of procaspase-8, leading to executioner caspase-3 cleavage, which triggers cell signaling involving p53 translocation to nucleus and induces transcription of pro-apoptotic genes like Bax and Puma (Datta et al. 2020).
Fig. 2.
Temporal profile of glutamate release/excitotoxicity (green), oxidative stress (blue), inflammation (orange), and cell death (purple) after ischemia induction. Glutamate release begins a few minutes after the onset of ischemia, reaching a peak within an hour if the noxious event lasts for that long. Extracellular glutamate content gradually decreases as soon as reperfusion takes place, and the time to restore basal levels is related to the severity of ischemia. Excitotoxic and necrotic cell death occurs rapidly at the ischemic core but programmed cell death and infarct volume are still ongoing for some days until it is not detectable anymore in weeks. At the onset of reperfusion, with the reestablishment of oxygen supply, reactive oxygen species production dramatically increases, reaching a peak close to 24 h after ischemia, when it starts to decline. Inflammation is the latest event, with microglial and macrophage activation, adhesion molecules expression, neutrophil infiltration, astroglial response, and cytokine release taking place within some hours after ischemia. Since inflammation is a multifactorial process, different phenomena occur in maximal intensity in a larger time window, with some events still rising up to 7 days, decreasing thereafter. The curves for non-treated conditions were based on data that explore the temporal pattern of mentioned parameters in the same study. The effect of adenosinergic intervention was based on data from studies mentioned throughout the text that explored at least one of the illustrated events in ischemia and demonstrated protective effects at one or more of the time periods shown
Oxidative Stress
Oxidative stress is another hallmark of an event of ischemia/reperfusion. Data suggest that reactive oxidative species (ROS) production begins at early reperfusion (Selakovic et al. 2011; Nakano et al. 2017; Godinho et al. 2018; Kapoor et al. 2019) (Fig. 2), when ATP is hydrolyzed to hypoxanthine, and ultimately converted to uric acid and superoxide ions in a calpain-dependent way. Superoxide ions can form hydroxyl radicals by Harber–Weiss reaction or interact with nitric oxide, induced by NMDA receptor activation during ischemia, generating peroxynitrite and nitrosyl radicals (Chan 1996; Love 1999; Osborne et al. 2004; Kostandy 2012). Furthermore, calcium interference on mitochondrial function causes an accumulation of free electrons, which will be accepted by the oxygen in the early phase of reperfusion, generating superoxide anions (Won et al. 2002). Regardless of the mechanism in which oxidative stress is generated, it triggers cell damage in the form of lipid peroxidation, DNA fragmentation or protein degradation (Czerska et al. 2015).
Inflammatory Contribution
Inflammation-induced cell death after ischemia is also a well-established late-component of the pathology (Stevens et al. 2002; Fang et al. 2006; Weston et al. 2007; Kriz and Lalancette-Hébert 2009; Moxon-Emre and Schlichter 2010; Perego et al. 2011; Shrivastava et al. 2013; Kawabori and Yenari 2015; Cotrina et al. 2017; Zhang et al. 2019; Kapoor et al. 2019) (Fig. 2). Within the lesion site, the expression of chemokines and adhesion molecules increases, recruiting immune cells from the bloodstream (e.g., T cells and macrophages). Local microglia are activated and secrete inflammatory mediators that can potentially worsen the tissue damage in late phases of ischemia/reperfusion, increasing infarct size. TNF-α and interleukin 1 beta (IL-1β), important inflammatory mediators, are increased within ischemic lesion regions, contributing to cell death in the brain and retina. Corroborating these data, blockade of these inflammatory mediators reduces the magnitude of the damage (Stoll et al. 2002; Osborne et al. 2004; Kawabori and Yenari 2015).
Role of Adenosine in Ischemic Conditions
Adenosine is a nucleoside that functions as a neuromodulator in the CNS, regulating the release of neurotransmitters, synaptic plasticity, sleep–wake cycle, and cell death (Sheth et al. 2014). Adenosine acts through four types of G-protein-coupled receptors already cloned and classified into A1, A2A, A2B, and A3. The A1 and A3 receptors are classically coupled to Gi/o protein, inhibiting AC activity and the production of cAMP (Fig. 3a). On the other hand, A2A and A2B receptors are classically coupled to Gs/olf protein, activating AC and increasing cAMP levels, which will, in turn, act on a series of effector proteins (Sheth et al. 2014).
Fig. 3.
Intracellular pathways coupled to adenosine receptors and CNS distribution. a There are four types of adenosine receptors named A1, A2A, A2B, and A3. The A1 and A3 receptors activate Gi/o protein, while A2A and A2B receptors are coupled to Gs/olf protein inhibiting and stimulating, respectively, adenylyl cyclase. Thus, adenosine receptors regulate cAMP levels, which impacts on protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac) activity. A series of other effector proteins may also be modulated. Moreover, adenosine receptors can stimulate the phospholipase C (PLC) pathway. A1 receptors regulate PLC via beta/gamma complex (Biber et al. 1997; Dickenson and Hill 1998), whereas A2A receptors act through Gq protein (Ribeiro et al. 2016; Socodato et al. 2011). Both A2B and A3 receptors can also stimulate PLC (Abbracchio et al. 1995; Kohno et al. 1996; Pilitsis and Kimelberg 1998). b The distribution of adenosine receptors varies dramatically within the CNS. High densities of A1 receptors are expressed in the cortex, hippocampus, and cerebellum, while A2A receptors are more abundant in the striatum and olfactory bulb. In contrast, A3 and A2B receptors are diffusely distributed in all brain regions in smaller amounts when compared to A1 and A2A receptors (Sheth et al. 2014). A1, A2A, A2B, and A3 receptors are also found in retinal cells, a structure that is part of the CNS, of different animals (Dos Santos-Rodrigues et al. 2015; Brito et al. 2016; Grillo et al. 2019; Portugal et al. 2021)
Data from different experimental approaches may raise doubts concerning adenosine affinity for its receptors. In the most common view, A1 and A2A are considered high-affinity receptors and A2B and A3 are low-affinity receptors (Beukers et al. 2000; Effendi et al. 2020; De Filippo et al. 2020). Indeed, the observation that adenosine could have high or low affinity for A2 receptors led to the distinction of A2A (high affinity) and A2B (low affinity) receptors (Bruns et al. 1986). However, Fredholm and colleagues (Fredholm et al. 2011; Fredholm 2014) have reported the difficulty in measuring adenosine affinity and pointed out that a reliable method to estimate this information is to assess the potency of each receptor. This way, A1, A2A, and A3 might be equipotent, while A2B is supposed to require higher concentrations of adenosine to elicit the same response (Fredholm 2014). Interestingly, high amounts of adenosine are only released in pathological conditions, such as hypoxia, which also causes A2B receptors upregulation (Vecchio et al. 2019).
Many studies in the literature describe the increase in extracellular adenosine availability during an ischemic event (Pedata et al. 1993; Frenguelli et al. 2007; Melani et al. 2012; Chu et al. 2013). The transient release of adenosine also increases during the period of cerebral ischemia and remains elevated during the reperfusion process (Ganesana and Jill Venton 2018). At the beginning of ischemia, adenosine arises from the hydrolysis of the released ATP and, later, cells release adenosine through their nucleoside transporters (Melani et al. 2012). Moreover, ischemia increases the expression of ecto-5´nucleotidase (CD73) in astrocytes, and induces its expression in microglia, enhancing extracellular adenosine formation (Braun et al. 1997). Within minutes, the concentration of adenosine in the extracellular medium reaches 1 mM, high enough to activate all P1-type receptors (adenosine receptors) (Melani et al. 2012), which are abundantly expressed in the CNS (Fig. 3b).
Interestingly, increased adenosine levels reduce neuronal damage and decrease the infarct area in rodent models of ischemia (Deleo et al. 1988; Dux et al. 1990; Lin and Phillis 1992; Mori et al. 1992; Park and Rudolphi 1994; Gidday et al. 1995; Matsumoto et al. 1996; Miller et al. 1996; Jiang et al. 1997; Newman et al. 1998; Tatlisumak et al. 1998; Kitagawa et al. 2002). Treatment with a daily dose of exogenous adenosine, initiated 24 h after cerebral ischemia and maintained for 7 days, contributes to decreased cell death and sensorimotor functional recovery in the CA1 area of hippocampus of rats (Seydyousefi et al. 2019). Accordingly, the knockout (KO) for ecto-5′nucleotidase (CD73) shows increase in ischemic damage (Petrovic-Djergovic et al. 2012). The signaling involved in adenosine protection in HI is still unclear, but the anti-apoptotic effect of adenosine in human umbilical vein endothelial cells (HUVECs) is reduced by the blockade of MAP kinase pathway (MEK/ERK1/2), nitric oxide synthase (NOS), and protein kinase A (PKA) (Feliu et al. 2019). Moreover, adenosinergic agents also represent a potential pathway for neuroprotection in immature neurons (Shalak and Perlman 2004; Perlman 2006).
Protective Role of Adenosine Through A1 Receptor in Ischemia
The activation of A1 receptors has been correlated with protective effects in ischemic situations both in mature and immature CNS (Melani et al. 2014b; Pedata et al. 2016) An important mechanism related to this effect refers to its capacity to regulate neuronal excitability by restricting calcium influx and, consequently, inhibiting the release of neurotransmitters, such as glutamate (Corradetti et al. 1984; Dunwiddie 1984; Andiné et al. 1990; Goda et al. 1998; Latini et al. 1999b; Sebastiäo et al. 2001; Tanaka et al. 2001; Marcoli et al. 2003; Arrigoni et al. 2005; Batti and O’Connor 2010). In fact, it has been shown that ischemia-induced synaptic depression is greatly inhibited in hippocampal slices of A1 KO mouse, reinforcing the idea of protection through modulation of synaptic activity (Johansson et al. 2001; Kawamura et al. 2019). Indeed, treatment with A1 receptor agonists (CPA or CHA) reduces lactate dehydrogenase (LDH) release induced by HI in cultures of cerebellar granule (Logan and Sweeney 1997), and hippocampal and cortex neurons (Daval and Nicolas 1994). In addition, A1 receptors antagonist, DPCPX, could reverse this effect in granule cells (Logan and Sweeney 1997). A recent study has shown that the presence of CPA, an A1 full agonist, or the partial agonists 2′-dCCPA and 3′-dCCPA, during the entire experimental procedure, in hippocampal slices, protects the neurons from oxygen glucose deprivation (OGD)-induced irreversible depression and increases cell viability of SH-SY5Y human neuroblastoma cells in culture after OGD (Martire et al. 2019). The same protective profile was observed in vitro, using primary cultures of neurons prepared from turtle brain homogenates subjected to anoxic condition (Milton et al. 2007). In this case, treatment with the selective A1 receptor agonist, CCPA, prevents cell death and anoxia-induced ROS production, but A1 antagonist (DPCPX) exacerbates the injury (Milton et al. 2007).
The cell signaling involved in A1 receptor protection against ischemic death is also an important research field. In primary cortical neurons in culture, the increase in cell viability by treatment with paeoniflorin, before and during OGD, occurs via A1 receptor activation and depends on Akt and ERK1/2 phosphorylation (Zhong et al. 2015). On the other hand, there is evidence that incubation with a high concentration of an A1 receptor agonist (500 nM CPA) induces neuronal damage in the CA1 region of hippocampal slices, which is prevented by DPCPX (Stockwell et al. 2016). The authors suggest a mechanism of adenosine-induced persistent synaptic depression, which includes AMPA subunits internalization through dephosphorylation.
Similar effects are also found by using in vivo models of ischemia. In general, acute pre-treatment with A1 agonists preserves the morphology of neurons, spatial memory, and learning capacity; increases neuron survival and neurological scores; and reduces mortality in gerbils (Héron et al. 1994; Von Lubitz et al. 1994a, 1996). Accordingly, in young A1 KO mice (P10), larger infarct area has been reported after unilateral HI (Winerdal et al. 2016). Administration of A1 agonist CCPA 24 h before middle cerebral artery occlusion (MCAO) protocol is also protective, reducing infarct area, TNF-α levels, and lipid peroxidation and increasing superoxide dismutase (SOD) and glutathione (GSH) levels (Hu et al. 2012). Administration of A1 agonist CPA 1 h before ischemia also reduces lipid peroxidation when analyzed at 3 h and 3 days after ischemia (Sufianova et al. 2014). Atef et al. (2018) investigated the signaling activated by A1 receptor in ischemia. The incubation of A1 agonist CHA at the onset of reperfusion drastically diminishes pyknotic nuclei in hippocampal neurons induced by bilateral carotid occlusion. The receptor agonist promotes reduction of reactive microglia, glutamate, TNF-α, inducible NOS (iNOS), interleukin 6 (IL-6), Thiobarbituric acid reactive substances (TBARS), c-fos, Cyt C, and caspase-3, all increased by ischemia. Meanwhile, it increases interleukin 10 (IL-10) and nuclear factor erythroid 2-related factor 2 (Nrf2) and elicits better performances in behavior tasks. Ischemia also increases phospho-ERK1/2 and diacylglycerol levels but those were further increased by CHA, which also potentiates the reduction in cAMP promoted by ischemia (Atef et al. 2018). A more recent study shows that pretreatment with A1 agonist CCPA for 30 min reduces the brain infarction area after 90 min of MCAO, and this effect correlates to the increase in glycogen synthase kinase 3 beta (Gsk3b) phosphorylation (Geng et al. 2020). In another study, Cui et al. (2016) have shown that a blocker of dynamin-related protein 1 reduces stroke volume and improves neurological score of mice submitted to MCAO, depending on A1 receptor, and involving increase in levels of extracellular adenosine through regulation of the ecto-5′ nucleotidase (CD39) expression in astrocytes via cAMP/PKA/cAMP-response element binding protein (CREB) phosphorylation. The protection afforded by electroacupuncture, which increases adenosine levels and reduces infarct volume in a model of MCAO also depends on A1 receptor (Dai et al. 2017). Treatment with A1 receptor agonists, soon after ischemia, is also effective in protecting neurons, improving neurological scores and reducing mortality (von Lubitz et al. 1988; von Lubitz and Marangos 1990). Accordingly, acute pre-treatment with A1 antagonists CPX or 8-CPT significantly worsens the outcome and enhances neuronal destruction induced by global ischemia (Boissard et al. 1992; Von Lubitz et al. 1994a; Phillis 1995; Olsson et al. 2004).
However, while studies using an acute treatment with A1 receptor antagonists show aggravation in ischemic damage, chronic treatment, previous to ischemia, has a protective effect. Exposure to A1 antagonist CPX—1 mg/kg, i.p. for 15 days, up to 24 h before the ischemic event, reduces neuronal damage (Von Lubitz et al. 1994a). Such effect could be attributed to the fact that prolonged inhibition of a receptor induces its upregulation, a common neurochemical plasticity response observed in the CNS that also applies to A1 receptor (Jacobson et al. 1996; Hettinger-Smith et al. 1996; Brito et al. 2012). Curiously, adult A1 KO mice show no effect on cell death in hippocampus, cortex, and striatum after a 12-min global ischemia followed by 4 days of reperfusion, raising the question if compensatory mechanisms could be active in animals lacking A1 receptor, which predominantly seem to promote the survival of the CNS cells in ischemic conditions (Olsson et al. 2004).
Thus, A1 receptor consists of an interesting target of studies in the context of ischemic damage. Its essential effect represented by the reduction of neuronal excitability has proven to be beneficial in mature and immature brain lesions. Despite that, it is important to highlight that chronic treatments with A1 antagonists may trigger compensatory mechanisms as receptor upregulation, which may be relevant as a protective strategy.
The Modulatory Effect of A2A Receptors in Ischemic Conditions
Classically, A1 and A2A adenosine receptors elicit opposite intracellular responses. Accordingly, many studies demonstrate that A2A receptor antagonism, as well as A1 activation, is protective against ischemic damage. A2A selective antagonist, administered just before ischemia, protects hippocampal neurons in a global prosencephalic ischemia model (Phillis 1995; Von Lubitz et al. 1995). Similarly, treatment with an A2A selective antagonist (ZM241385) before ischemia reduces neuronal damage in hippocampal cells and improves animal performance in Morris water maze (Higashi et al. 2002). The beneficial effect provided by blockade of A2A receptors in ischemic events is reinforced by studies showing that A2A receptor KO protects from cerebral ischemic damage (Chen et al. 1999; Gui et al. 2009). When administered after the ischemic event (which in fact has greater clinical relevance), an A2A antagonist also has protective effects. The use of SCH58261 after the ischemic event reduces neuronal damage in neonate and adult rats (Bona et al. 1997; Monopoli et al. 1998; Melani et al. 2003, 2006b). In newborn piglets, the A2A inhibition-induced protection involves an increase in Na+/K+ ATPase pump activity, and prevention of the ischemia-induced phosphorylation of NMDA receptor subunit GluN1 at ser897 and of dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP32) at thr34. The protection also includes the reduction in ischemia-induced nitrative and oxidative stress (Yang et al. 2013). Mohamed and collaborators (Mohamed et al. 2016) have analyzed in more detail the intracellular pathways triggered by A2A antagonism in ischemia. Intrahippocampal injections at the end of a 45-min ischemic event decrease protein levels of phospho-ERK (p-ERK), NFκB, TNF-α, IL-6, iNOS, caspase-3, Cyt C, p-CREB, and c-fos, all increased by ischemia/reperfusion (Mohamed et al. 2016). Moreover, the authors report a decrease in glutamate and TBARS, alongside increases in IL-10 and nuclear Nrf2 with the antagonist treatment. However, exposure to A2A receptor antagonist (CSC), 2 h after stroke onset, has no protective effect in lesion volume, which could be due to the time window of effectiveness or dose (Fronz et al. 2014). Furthermore, prolonged use of SCH58261, starting 5 min and twice/day after tMCAO, does not change infarct volume when analyzed 7 days later, suggesting a time window of apparent protection that remains to be fully understood (Melani et al. 2015). Finally, A2A receptor KO in younger (P7) rats intensifies the damage, caused by the occlusion of the left common carotid, and the performance in behavioral tests, such as rotarod (Ådén et al. 2003), which raises the question whether the effect of A2A receptor blockade depends on the maturity of the tissue and/or differentiation of specific features during development to achieve neuroprotection.
To understand the role of adenosine A2A receptor in ischemia-induced cell death, experiments with agonists have also been performed. In the gerbil, A2A agonist (APEC), administered systemically and chronically for 13 days before the ischemic insult, has beneficial effects on the survival of hippocampal neurons and animals (Von Lubitz et al. 1995). Systemic administration of A2A agonist ATL-146e or CGS 21680, just before reperfusion onset, protects from motor dysfunction and cell viability in spinal cord ischemia–reperfusion and infarct size, oxidative stress, and memory impairment in global cerebral ischemia, respectively (Reece et al. 2006; Grewal et al. 2019). The treatment with low doses (0.01–0.1 mg/kg i.p.) of this same agonist for seven days (and twice a day), after transient cerebral ischemia, decreases gliosis, the infarct area in the cortex, but not in the striatum, as well as the myelin disorganization in the striatum (Melani et al. 2014a). The possible explanation for the apparent contradiction (activation of A2A receptor and protection) is the modulation of functions in non-neuronal cells-glial, endothelial, and immune cells that leads to several benefic effects (described in the next topic).
The data show an important neuroprotective function triggered by the inhibition/absence of A2A receptors in neurons in different models of cerebral ischemia. Interestingly, in some cases, the protective action can also be achieved after ischemia, which makes A2A receptor inhibition a promising tool in both prevention and treatment. In addition, the time frame of pharmacological intervention is crucial for the protective effect, as well as the period of development. In any case, the evidence mostly places the inhibition of A2A receptors as a common denominator of neuroprotection.
Protective Mechanisms Through A1 and A2A Receptors Related to Glial and Other Cells
The protective effect of A2A receptors inhibition can also be attributed to the regulation of synaptic transmission and glutamate release (Cunha et al. 1994; Latini et al. 1999a; Melani et al. 2003; Pugliese et al. 2009; Lopes et al. 2011; Maraula et al. 2013; Effendi et al. 2020). This effect seems to occur through modulation of A1 receptor activity, at least in the hippocampus (Lopes et al. 2002). It is known that glial cells play an important role in the regulation of glutamate availability and excitotoxicity. Interestingly, in astrocytes, A2A receptors inhibit glutamate uptake by excitatory amino acid transporter (EAAT)-2 while stimulating EAAT-2-independent release via PKA activation (Nishizaki et al. 2002). Acute (30 min) or chronic (24 h) activation of the A2A receptor with CGS 21680 reduces d-aspartate uptake in astrocyte cultures, probably by decrease of glutamate transporters EAAT1 and EAAT2 mRNA expression (Matos et al. 2012). Cultures of rat astrocytes subjected to OGD for 150 min show great cell death after 24 h of reoxygenation. Death is inhibited by guanosine through a mechanism that depends on the activation of the A1 receptor and the MAPK and protein kinase C (PKC) pathway. Activation of these pathways prevents the OGD-promoted reduction of EAAT2 glutamate transporters in the membrane, restoring glutamate uptake and, consequently, restricting cell death (Dal-Cim et al. 2019). This evidence is in agreement with the increase in the amount of EAAT2 by overexpression of A1 receptors in astrocytes (Wu et al. 2011; Hou et al. 2020). Recently, it has been demonstrated in mouse astrocyte cultures subjected to OGD that the formation of A1–A2A heterodimers reduces the expression of EAAT2 through the transcription factor YY1 and repression of PPARγ transcription. Interestingly, the effect is blocked by the pharmacological activation or inhibition of the A1 and A2A receptor, respectively (Hou et al. 2020). Thus, A2A receptors activation reduces the ability of glial cells to decrease glutamate availability, which could be harmful in ischemic events. In agreement, there is an increase in the expression of EAAT2 in astrocytes genetically devoid of A2A receptors (Matos et al. 2015; Hou et al. 2020). In addition, A2A receptor inhibition reduces reactive astrogliosis in slices of hippocampal rats submitted to OGD (Pugliese et al. 2009). It remains to be evaluated whether reactive astrogliosis depends on the modulation of glutamate transporters. New evidence points out that the astrocytic Lrp4 protein contributes to cell death induced by photothrombosis, ischemic stroke, and OGD, since Lrp4 KO animals exhibit lower cell death when compared to controls (Ye et al. 2018). The authors demonstrated that the absence of the protein reduces reactive astrogliosis and increases the release of ATP and astrocytic adenosine in ischemic conditions, which contributes to the reduction of neuronal death through activation of the P2X7 and A2A receptors (Ye et al. 2018). In fact, astrocytes are a considerable source of adenosine release in ischemic conditions (Martín et al. 2007; Takahashi et al. 2010).
Ischemia, ATP and glutamate per se can also induce microglial activation (Pforte et al. 2005; Davalos et al. 2005; Melani et al. 2006a; Lai et al. 2011). Furthermore, A2A receptor, stimulated by adenosine released during ischemia, activates microglia (Orr et al. 2009). Reactive microglia releases high concentrations of glutamate (Takeuchi et al. 2006; Socodato et al. 2015) and ATP (Imura et al. 2013), contributing to a positive feedback loop of microglial activation and enhancing excitotoxicity. Accordingly, glutamate release after ischemia can be attenuated by treatment with A2A antagonist (SCH 58261) in vivo and in rat and human cortex slices (Marcoli et al. 2003, 2004; Melani et al. 2003). Furthermore, A2A antagonism prevents the ischemia-promoted increase in p-p38 and TNF-α in microglia and in p-JNK in oligodendrocytes, which would lead to a disorganization of myelin (Melani et al. 2006b, 2009; Mohamed et al. 2016). Another interesting point is that NGF plays a neuroprotective role in cerebral ischemia. Astrocytes, together with microglia, are the main responsible for NGF secretion, which is stimulated by A1 and A2A receptors, respectively (Heese et al. 1997; Ciccarelli et al. 1999; Liu et al. 2019). The relation of NGF production/secretion and A1 or A2A receptors in ischemic events is still unclear. The activation of A2A receptor is also related to the production and release of neurotrophic factors, such as glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) (Gomes et al. 2006, 2013; Tebano et al. 2008; Sebastião and Ribeiro 2009; Jeon et al. 2011; Vaz et al. 2015), which could help maintain/restore function and neuronal integrity. However, the release of these neurotrophic factors may not compensate for several other malefic alterations triggered by A2A receptor activation during ischemia. Activation of A2A receptor in microglial cells also induces cyclooxygenase 2 (COX-2) content, and prostaglandin E2 release (Fiebich et al. 1996), nuclear translocation of hypoxia inducible factor (HIF-1α) and transcription activation of vascular endothelial growth factor (VEGF) and iNOS (Merighi et al. 2015).
Besides glial cells, other cell types can contribute to protection induced by A2A inhibition. The specific inactivation of A2A receptors in endothelial cells have recently shown to be beneficial in a model of embolic MCAO. The cell-specific knockout decreases infarct volume and improves neurological outcome (Zhou et al. 2019). Furthermore, the KO shows reduced protein levels of adhesion molecules, such as VCAM and ICAM, neutrophil and monocytes infiltration, reduced blood–brain barrier leakage, and consequently reduced edema. The mechanism seems to involve less activation of the NLRP3 inflammasome in the endothelial KO. Taken together, these data indicate that a plethora of changes triggered by A2A receptor inhibition during or soon after ischemia result in CNS protection.
Furthermore, the best window for treatment with A2A receptor inhibitors, and therefore, the outcome efficiency after ischemia, can be challenging due to the action in non-neuronal cells. The activation of A2A receptor in immune cells could contribute to the protection after ischemia/reperfusion, as it reduces infiltration of those cells into the ischemic site, and release of inflammation signal, which aggravates injury (Haskó et al. 2008; Antonioli et al. 2014; Melani et al. 2014a). Studies indicate that adenosine, by activating A2A, A2B, and A3 receptors, restrains the production of macrophage pro-inflammatory mediators, such as TNFα, IL-6, IL-12, NO, and macrophage inflammatory protein (MIP)-1α (Antonioli et al. 2019). In vitro, human dermal microvascular endothelial cells (HDMECs) and rabbit DMECs show less apoptotic levels after hypoxia when treated with A2A agonist (CGS-21680) before the onset of hypoxia and again before reoxygenation (Cao et al. 2017, 2019). Thus, these results show that A2A receptors may also account for protection by preserving the vascular integrity and can hinder the best protocol of treatment with the A2A inhibitors.
Beneficial Effects of A2B Receptors in Ischemia
The role of A2B receptors has been less explored but, interestingly, due to the low affinity for adenosine and the relative paucity in the brain, A2B receptors appear to be activated, and may be biologically operative, mainly under noxious situations, such as hypoxic or ischemic conditions, when adenosine levels increase (Koeppen et al. 2011; Popoli and Pepponi 2012). In the stratum radiatum of the CA1, the A2B receptor is found in non-astrocytic cells, and the number and labelling density increase after cerebral ischemic preconditioning (Zhou et al. 2004). In A2B KO mice, basal levels of TNF-α and adhesion molecules, such as ICAM-1, P-selectin, and E-selectin, are increased (Yang et al. 2006). In addition, A2B receptors demonstrate an important function in endothelial cells to control vascular leakage and neutrophil infiltration induced by hypoxia in several organs (Eckle et al. 2008). However, in the brain, although the genetic absence of A2B receptors in bone marrow increases vascular permeability, A2B receptor agonist or antagonist treatment has no effect (Eckle et al. 2008). Additionally, hypoxia upregulates A2B receptors, together with HIF-1α and IL-6, in primary microglial cells (Merighi et al. 2017). Interestingly, the role of A2B receptors in tissue-type plasminogen activator (tPA) treatment, one of the frontlines to treat stroke in humans, was recently evaluated. An inconvenient side effect of tissue-type plasminogen activator is the possibility to induce hemorrhagic transformation. Treatment with an A2B agonist (BAY 60-6583) after ischemia reduces infarct volume in the presence or not of tPA and counteracts the blood–brain barrier damage induced by tPA (Li et al. 2017). These data could open the possibility to include A2B agonists as adjuvants in tPA treatment after stroke.
A2B receptors were also studied in the context of protection mediated by propofol in ischemia. This anesthetic could reduce microglial proliferation, and the levels of nitric oxide, TNF-α, and IL-1β, all increased by transient MCAO. An A2B antagonist (MRS agar) blocks the beneficial effects of propofol, suggesting an interesting protective effect of propofol in ischemia through A2B activation (Yu et al. 2019). Docosahexaenoic acid protects hippocampal slices from OGD-promoted cell death through A2B receptors activation (Molz et al. 2015). In the same model, A2B antagonists (MRS1754 or PSB603) delay OGD-induced anoxic depolarization, restoring field excitatory postsynaptic potentials (fEPSPs), decreasing the apoptotic marker cytochrome c, and improving neuronal survival (Fusco et al. 2018).
Therefore, different from A1 and A2A receptors, which are widely distributed in the CNS, A2B receptors may play a restricted role in adverse conditions, such as ischemia. As a consequence, A2B antagonists may elicit the protection of ischemic neurons. Moreover, as A2B modulation may impact blood circulation, pharmacological strategies based on this receptor should benefit the scheme of pharmacological intervention in cases of stroke.
The Action of A3 Receptors in Ischemic Events
The A3 receptors also appear to be involved in the process of cell survival and death, depending on the level of receptor activation and pathophysiological conditions, such as the ischemic process (Abbracchio and Cattabeni 1999; Borea et al. 2009). Pretreatment with a selective A3 agonist (Cl-IB-MECA) increases cell viability of primary cortical cultures exposed to OGD, as well as attenuates ischemia-induced TUNEL labeling and cerebral infarct volume, and increases locomotor activity (Chen et al. 2006). Treatment with IB-MECA after ischemia reduces infarct size, reactive gliosis, and microglia infiltration when evaluated 7 days later (Von Lubitz et al. 2001). The reduction of microglial infiltration by IB-MECA after ischemic events may depend on direct inhibition of chemotaxis and down-regulation of Rho GTPases (Choi et al. 2011). In agreement, KO for the A3 receptor exhibits greater ischemic (Chen et al. 2006) or hypoxic damage (Fedorova et al. 2003). In vitro studies with hippocampal slices, prepared from young rats (P12–P16), submitted to 15-min OGD, result in depression of fEPSPs that was persistent only at CA3 region, but not at CA1, and application of A3 receptor antagonist (VUF5574 or MRS1191) prevented the persistent depression (Dennis et al. 2011). The authors suggest that A3 activation can partially contribute to OGD-induced AMPA receptors internalization in the CA3 region, potentially protecting it from following excitotoxicity. In hippocampus slices from adult rats, A3 antagonists prevent sustained depression induced by OGD at CA1 region (Pugliese et al. 2007). Moreover, in a model of global ischemia of the anterior brain in the gerbil, chronic administration of IB-MECA (100 μg/kg i.p. daily for 10 days before ischemia), reduces neuron loss in the hippocampus (Von Lubitz et al. 1994b). In human astrocytoma cells, low concentration of Cl-IB-MECA reduces hypoxia-induced apoptosis, as well as cell death is exacerbated in A3 KO astrocytes (Björklund et al. 2008).
Although, high concentrations of adenosine or 2-Cl-IB-MECA seem to be toxic to oligodendrocyte cultures prepared from optic nerve, by causing ROS production, mitochondrial membrane depolarization and caspase dependent cell death, which are blocked by MRS 1220, an A3 receptor antagonist (González-Fernández et al. 2014). Furthermore, MRS 1220 reduces OGD-induced cell death in isolated optic nerve, also restoring myelin basic protein levels.
The evidence strengthens the idea that A3 receptor activation triggers a protective mechanism in ischemic events, similar to A1 receptor stimulation. The neuroprotective effect seems to depend directly on the activation of the receptor in neuronal cells; however, an indirect effect via other cell types cannot be ruled out.
Role of Adenosine Receptors in Ischemic Retina
In the mature retina, A2A receptor inhibition or A1 receptor activation has also beneficial effects in ischemic conditions. The increased availability of adenosine, using an adenosine deaminase blocker, or an A1 receptor agonist, both applied just before ischemia, preserves the tissue integrity, and the electrical activity impaired by ischemia (Larsen and Osborne 1996). Interestingly, A1 receptors blockade also impairs the histological protective effect provided retinal ischemic preconditioning (Sakamoto et al., 2004). In addition, the A2A inhibition protects both structure and tissue functionality after ischemic events of 5, 30, or 60 min, whereas A1 blockade does not exert the same effect (Li et al. 1999). In a model of ischemia induced by increased IOP and reperfusion for 7 days, the A2A antagonist KW6002 also reduces the inflammatory response and the apoptotic levels in the rat retina (Boia et al. 2017). In the same model, an A2A antagonist (SCH 58261) reduces microglial reactivity, IL-1β levels, and TUNEL staining (Madeira et al. 2016a). Intriguingly, selective A2A activation before ischemia alleviates the thinning of the inner retina (Konno et al. 2006).
In both in vitro and in vivo models of the retina, A3 receptor selective agonist provides protection against excitotoxic stimuli and ischemia–reperfusion injury, increasing the survival of retinal cells, including ganglion cells (Galvao et al. 2015). This protective effect could occur through receptor desensitization (Pugliese et al. 2007).
Caffeine as a Possible Neuroprotector in Ischemia
Caffeine and Coffee Consumption
Caffeine (1,3,7-trimethylxanthine) is an alkaloid that belongs to the class of xanthines, being the most consumed psychostimulant in the world. The worldwide consumption of caffeine occurs through different sources, such as coffee, teas, chocolates, soft drinks, energy drinks, and medicines (Heckman et al. 2010; Yoon and Danesh-Meyer 2019). However, the main source of this stimulant in Western society is through the consumption of coffee, where its concentration can vary between 40 to 180 mg/150 mL. In Western countries, the daily intake of caffeine reaches 70–80% of the population (Heckman et al. 2010; Mitchell et al. 2014), increases with age, and the consumption, considering all sources, can vary from 135 to 213 mg/day (Drewnowski and Rehm 2016). Brazil is the second largest consumer of coffee in the world, and the consumption of caffeine by adults, from all sources, can reach 300 mg/day (Heckman et al. 2010; Sousa and Da Costa 2015).
Molecular Mechanisms and Caffeine Metabolism
The biological effects triggered by caffeine concentration reached by average daily consumption are related to its antagonism of adenosine receptors, more specifically A1 and A2A receptors (Rivera-Oliver and Díaz-Ríos 2014). Besides adenosine, other molecular targets can be modulated by caffeine only at high/toxic concentrations, which are unlikely to be reached in humans by any form of normal use of caffeine-containing beverages. Comparing to the concentration range that selectively inhibits adenosine receptors, caffeine can inhibit phosphodiesterase (in a ten times higher concentration), GABAA receptors (40 times higher), and mobilize calcium from intracellular stores (100 times higher) probably by its action on ryanodine receptors (Fredholm 1979; Fredholm et al. 1999; Gupta et al. 2018). Thus, the vast majority of the effects described in animal models and human studies using caffeine are exclusively related to inhibition of adenosine receptors (see Box 1 for information about caffeine dose translation).
Box 1: How to Translate Caffeine Dose from Animal Models to Humans.
Several studies have been researching the role of caffeine in different pathologies using animal models. Concerning that, it is important to bear in mind that caffeine dose cannot be directly compared between animals and humans because of the difference in the body surface area (BSA). Reagan-Shaw et al. (2007) call the attention to the usage of appropriate normalizations to extrapolate animal dose to humans. The Food and Drug Administration (FDA) recommends the usage of a factor (Km) to convert animal dose to human equivalent dose (HED) using the following formula:
For example, the treatment of a mouse with 30 mg/kg of caffeine corresponds to a HED of 2.43 mg/kg, since the values of Km for adult human (with 60 kg) and mouse (Table 1) are, respectively, 37 and 3 (Reagan-Shaw et al. 2007). Therefore, for an adult with 60 kg it corresponds to 146 mg of caffeine, which is a low dose for humans. However, the Km for rats is 6, so a 30 mg/kg treatment corresponds to a HED of 4.86 or 292 mg of caffeine, a higher HED. The HED obtained in the rat, but not in the mouse example is in the range considered, by The American College of Obstetricians and Gynecologists, unsafe for pregnant women. Therefore, researchers must be careful about which dose they should choose depending on the consumption range they may plan to stimulate in humans.
After ingestion, caffeine is rapidly and completely (99%) absorbed by the gastrointestinal tract in humans, reaching a plasma peak between 15 and 120 min. For doses of 5–8 mg/kg, the plasma caffeine concentration can vary between 8 and 10 mg/L (Arnaud 1993). Due to its hydrophobic profile, caffeine is able to cross all biological barriers, such as hemato-intestinal, hemato-placental, blood–brain barrier, and blood–retinal barrier (Arnaud 1993; Cappelletti et al. 2015). The half-life of caffeine in humans varies between 2.5 and 4.5 h for doses less than 10 mg/kg (Fredholm et al. 1999). Caffeine metabolism occurs in the liver and is carried out mainly by the cytochrome P450 1A2 enzyme system (CYP1A2), even though xanthine oxidase and acetyltransferase 2 (NAT-2) also contribute to this function (Nehlig 2018). However, the functionality of CYP1A2 is reduced in different animals, neonates and premature babies, which dramatically increases the half-life of caffeine in these individuals (Arnaud 1993; Fredholm et al. 1999; Nehlig 2018). Thus, metabolic rate is another important factor that influences caffeine effect in animal models. For doses lower than 10 mg/kg, the half-life of caffeine ranges from 0.7 to 1.2 h in rats and mice, 1–4 h in rabbits, 3–5 h in monkeys (Bonati et al. 1984; Arnaud 1993; Xu et al. 2010).
The metabolism of caffeine that occurs in the liver produces, among other components, three dimethylxanthines: paraxanthine, theobromine, and theophylline. Among the three, paraxanthine is produced in a greater proportion (84%), followed by theobromine (12%) and theophylline (4%) (Cappelletti et al. 2015). These metabolites have physiological actions (Ribeiro and Sebastião 2010). Interestingly, it has been observed that after chronic caffeine consumption, the concentration of theophylline in the brain of mice seems to remain higher than their own peripheral concentrations, higher than the concentrations of other metabolites and higher than the concentration of caffeine itself. These findings suggest that caffeine metabolism in the CNS may be different (Johansson et al. 1996), which could impact the outcome of treatment with caffeine. Therefore, more studies aiming to understand the role of these metabolites in ischemic events could help reach an efficient protocol of therapy.
Implications of Caffeine Exposure During Development
Caffeine and its metabolites can accumulate during pregnancy since clearance and excretion are reduced due to decreased CYP1A2 activity (Stavric 1988; Nehlig 2018). The ability of caffeine to freely cross the placental barrier, coupled to the fact that its metabolism is immature during embryonic and postnatal development, can lead to a high concentration of this compound in the body of these fetuses/neonates and compromise the correct development of different systems. In fact, there are a number of studies that relate the administration of high doses of caffeine during embryonic development in animal models with teratogenic effects (Tye et al. 1993; Sahir et al. 2000; Momoi et al. 2008; Li et al. 2012; Ma et al. 2012, 2014; Tan et al. 2012; Xu et al. 2012). In humans, epidemiological surveys have shown an increased risk of low birth weight (Momoi et al. 2008; Sengpiel et al. 2013), fetal growth restriction (Klebanoff et al. 2002; Bracken et al. 2003; Bakker et al. 2010) and miscarriage as caffeine intake increases. In some cases even the consumption of one cup of coffee (100 mg caffeine) per day increases the risk (Konje and Cade 2008; Weng et al. 2008; Bakker et al. 2010; Chen et al. 2014; Li et al. 2015; Rhee et al. 2015). However, The American College of Obstetricians and Gynecologists states that less than 200 mg per day of caffeine consumption does not appear to be a major contributing factor in miscarriage or preterm birth, whereas for fetal growth restriction it is undetermined (Counseling 2019). In addition, caffeine is used in the treatment of apnea of prematurity, which decreases the risks of patent ductus arteriosus, brain injury, retinopathy of prematurity (ROP), and postnatal steroid use (Abdel-Hady 2015; Kua and Lee 2017; Kumar and Lipshultz 2019). Nevertheless, the best therapeutic window, dose, and duration of therapy remain to be determined (Abdel-Hady 2015; Kumar and Lipshultz 2019).
Apnea of Prematurity and Caffeine
Clinical Aspects of the Apnea of Prematurity
An apneic episode is characterized by respiratory failure that lasts more than fifteen seconds and it is accompanied by hypoxia, bradycardia, cyanosis, or pallor. It is one of the most common diagnoses in the NICU and requests the attention of the medical community. Its occurrence is inversely proportional to gestational age, and it can be classified as central, obstructive, or mixed (Martin and Wilson 2012; Eichenwald 2016). The understanding of the pathogenesis of the apnea of prematurity has revealed central (e.g., decreased central chemosensitivity, hypoxic ventilatory depression) and peripheral (e.g., dysregulation of carotid body activity, excessive bradycardic response) mechanisms involved in these events and it has guided the search for therapeutic interventions not only to increase survival but also to avoid long-term consequences that may include neurodevelopmental disorders (Martin and Wilson 2012). Usually, these children request air supply to survive, and exposure to higher oxygen tension can lead to ROP. In premature children, the exposure to high oxygen tension, compared to in uterus conditions, inhibits retinal normal vessel growth, creating avascular/ischemic zones (Schmidt et al. 2007; Liegl et al. 2016; Hartnett 2017). As the newborn develops, tissue metabolic demand increases, triggering signaling pathways to promote neovascularization, and consequently, formation of disorganized and nonfunctional vessels. ROP is a leading cause of infant blindness worldwide (Gilbert 2008; Blencowe et al. 2013; Quimson 2015; Bashinsky 2017), and the treatment for the disease includes photocoagulation and use of VEGF inhibitors (Liegl et al. 2016).
Therapeutic Agents and Caffeine Function
The procedures to treat apnea include options, such as nasal continuous positive airway pressure (NCPAP), which reduces frequency and severity of apnea by decreasing the risk of obstructive apnea; blood transfusion in the attempt to reduce apnea by increasing respiratory drive, oxygen carrying capacity, and tissue oxygenation, a short-lived method linked to anemia occurrence; and the xanthine therapy, which is the standard method, normally by using caffeine citrate due to its longer half-life (Eichenwald 2016).
Xanthines exhibit respiratory effects as they improve ventilation and increase carbon dioxide sensitivity by blockade of adenosine receptors. Although caffeine had been used for thirty years, the first study evaluating the long-term efficacy and safety of caffeine therapy for apnea of prematurity was developed by Schmidt and colleagues and published in 2007 (Schmidt et al. 2007). Previously, this group has demonstrated that caffeine reduces the incidence of bronchopulmonary dysplasia (Schmidt et al. 2006). Then, Schmidt and co-authors (2007) have observed that, at eighteen to twenty-one months old, caffeine significantly enhances the rate of survival without developing neurological problems (Schmidt et al. 2007). They show a reduction in the severity of eye disease, cerebral palsy, and cognitive delay, as well as a better psychomotor development in the caffeine-treated group. The authors discuss that these results could be achieved because treatment with caffeine reduces important variables: time with respiratory support, the need of postnatal corticosteroids, the surgery to close a patent ductus arteriosus, and the rate of bronchopulmonary dysplasia. However, the strongest intermediate variable is the reduced time for any positive airway pressure, because it can compromise the lungs, which in turn can evolve into a bronchopulmonary dysplasia, a risk factor for the development of neurological issues (Schmidt et al. 2007). Therefore, caffeine levels higher than 7.9 mg/kg body weight per day have been reported as being safe and effective in apnea of prematurity treatment in neonates born before 28 weeks of gestation (Francart et al. 2013).
Caffeine and Retinopathy of Prematurity
Caffeine treatment for infants with apnea of prematurity also reduces severity of ROP. As an ischemic retinopathy, adenosine is also released as a consequence of ischemia, and the role of the nucleoside has been investigated in animal models of ROP, described as oxygen-induced retinopathy (OIR). Genetic inactivation of A2A or treatment with an antagonist of the receptor, KW6002, reduces vaso-obliteration induced by OIR and inhibits irregular retinal angiogenesis both in young and adult animals (Liu Xiao-Ling et al. 2010; Zhou et al. 2018). KO of A1 receptor also has positive effects on vaso-obliteration in a OIR model, reducing normal vessel growth, even though it does not reduce neovascularization into the vitreous (Zhang et al. 2015). Zhang et al. (2017) have also evaluated the effect of caffeine (1 g/L) through nursing mothers, in OIR model (from P7–P12), during different time windows: P0–7 (pre-treatment), P0–17 (continuous treatment), P7–12 (hyperoxic phase), and P12–17 (hypoxic phase). Caffeine exposure reduces vaso-obliteration and creation of avascular zones when treatment occurs during the entire period, or even restricted to hyperoxic phase. Furthermore, neovascularization is reduced by treating during any time window except for pre-treatment. Importantly, caffeine treatment does not interfere in normal postnatal vascular development (Zhang et al. 2017). Further analyzes show that the effect of caffeine (the decrease of avascular zones) at P12 is totally dependent on A2A, while the effect on avascular zones and neovascularization at P17 is only partially correlated to the receptor. In accordance, caffeine (10 mg/kg i.p.), as a single application 15 min before protocol of hyperoxia (80% oxygen) for 24 or 48 h, reduces oxidative stress markers, like lipid peroxidation, heme oxygenase-1 (HO-1), and H2O2 formation, and reduces gene expression of Nrf2, glutamate-cysteine ligase and increases gene expression of SOD3 in brain homogenates (Endesfelder et al. 2017). Additionally, the authors observe reduction of inflammatory markers such as iNOS, IL-1β, TNF-1α, and interferon gamma, a reduction of apoptotic mediators, like nuclear poly (ADP ribose) polymerase 1 (PARP-1), apoptosis inducing factor (AIF) and caspase-3, and a reduction of the matrix metalloproteinase 2 activity, which could contribute to neurotoxicity and inflammation. Caffeine citrate (20 mg/kg i.p. at P0 and maintenance doses of 5 mg/kg/day from P1–13) and ketorolac (COX inhibitor; topical ocular administration once a day from P5–7) reduce severe OIR performed from P0–14 and analyzed at P14, or 7 days later (enabling recovery) (Aranda et al. 2016). Therefore, the data obtained by animal model studies corroborate the idea of caffeine as a good therapeutic tool to reduce retinal damage in ROP. Most recently, it was shown that treatment with caffeine (30 mg/kg, single in ovo injection, 48 h before ischemia) protects chick embryo retinal cells in an ex vivo model of acute ischemia (OGD). The protective effect is dependent on CREB phosphorylation and BDNF signaling. Such effect could be mimicked by DPCPX, an antagonist of adenosine A1 receptors, indicating the presumably mechanism of action for caffeine (Pereira-Figueiredo et al. 2020). It is important to note that other pharmacological interventions have also been investigated as potential treatment for ROP, such as Omega-3 fatty acid, insulin-like growth factor 1 inducers, vitamin A, cyclooxygenase inhibitors, inositol, and propranolol (Beharry et al. 2016; Aranda et al. 2019).
Caffeine in the Immature Ischemic Brain
Caffeine Dose and Neuroprotection
Studies in animal models have also been positively correlating low–moderate doses of caffeine treatment with cell survival in the immature brain, exposed to ischemic events in different developmental windows. A protective effect for caffeine is achieved in a close time window, at least less than 6 h, after ischemia. Interestingly, even a single injection of caffeine (5 mg/kg i.p.), directly after HI, also reduces infarct zone and cerebral atrophy in rats submitted to ischemia at P10 and analyzed at P24 (Winerdal et al. 2017). Cognitive function also seems to be affected by a single dose of caffeine (10 mg/kg i.p.) after induction of ischemia at P7, and evaluated months later, as a better performance was observed in Morris water maze at P90–95 rats (Alexander et al. 2013). Treatment of P7 rats with caffeine citrate (20 mg/kg/day i.p) just before ischemia, and during the following 3 days, reduces TUNEL staining in hippocampus and parietal cortex analyzed at P11 (Kilicdag et al. 2014). Using a similar protocol of treatment, caffeine citrate (20 mg/kg i.p.), given just after ischemia and 24 h later, also restores standard behavior, besides cortical and hippocampal volume, in adult rats submitted to ischemia at P6 (Potter et al. 2018). Recently, Di Martino and colleagues have shown that a single dose of caffeine (5 mg/kg i.p.) right after HI in rats at P10 reduces global damage score, apoptotic cell number, microglial activation, and inflammatory gene expression. The protection did not occur if caffeine was administered at 6, 12, or 24 h after HI (Di Martino et al. 2020). Therefore, the available data from animal models indicate a protective role for low–moderate doses of caffeine when administered in a close time window, at least less than 6 h after ischemia.
Beneficial outcomes with caffeine treatment in the drinking water of dams are also observed. Low dose of caffeine (0.3 g/L in the drinking water of the dams), from P0 to P21, reduces brain damage induced by HI performed at P7, and evaluated by brain weight at P21 (Bona et al. 1995). On the other hand, a high dose of caffeine (0.8 g/L) has no protective effect (Bona et al. 1995).
White Matter Brain Injury
Few studies explore the caffeine protective effects in the white matter of the ischemic immature brain. Caffeine exposure (0.3 g/L in drinking water through the dam) as soon as P2–P12 reduces periventricular white matter injury (PWMI) induced by chronic hypoxia (P3–P12) (Back et al. 2006). PWMI is known to affect very low birth weight infants, and it is the leading cause of neurological disability in survivors of prematurity (Volpe 2003; Ferriero 2004). Accordingly, treatment of P7 rats with caffeine citrate (20 mg/kg/day i.p) just before ischemia, and during the following 3 days, decreases white matter damage in subcortical regions (Fa-Lin et al. 2015).
Effect of Caffeine in the Adult Brain and Retina in Ischemic Conditions
Brain Ischemia and Role of Caffeine
The preventive/therapeutic potential of caffeine for ischemia has also been investigated in CNS of adult animals. It has been reported a correlation between coffee consumption and lower risks of stroke (Lopez-Garcia et al. 2009; Larsson et al. 2011; Kim et al. 2012; Liebeskind et al. 2016). The correlation is not direct to caffeine, and the potential of other coffee compounds cannot be ruled out (Cossenza 2018). Even though, evidence using adult animal models corroborates the idea that the main chemical agent involved in this protection is caffeine. Exposure to caffeine in water (0.2%) for 4 weeks before a 5-min bilateral occlusion in adult gerbils, evaluating 7 days after ischemia, reduces loss of pyramidal cells in the CA1 region of the hippocampus (Rudolphi et al. 1989). Resonance images and histopathological analysis of adult rodents reveal differences between chronic (three times a day by gavage, 20 mg/kg per dose for the first week, and 30 mg/kg per dose in the third week, last dose at 24 h before ischemia) and acute (10 mg/kg i.v. 30 min before ischemia) effects of caffeine on ischemic neuronal injury of rats subjected to forebrain ischemia. While chronic treatment reduces neuronal injury, acute treatment has no effect (Sutherland et al. 1991).
Caffeine Effect in Ischemic Retinopathies
Interesting data concerning ischemia in the mature retina are also reported. Recently, the effect of caffeine (100 µM) was evaluated in an in vitro model of diabetic macular edema, the major cause of vision loss in diabetic retinopathy. The xanthine reduces permeability, induced by hyperglycemia/hypoxia, in monolayer culture of human retinal pigment epithelial cells (ARPE-19) by restoring tight junctions and reducing apoptotic rates (Maugeri et al. 2017). Treatment with caffeine in drinking water (1 g/L) for 2 weeks, before the ischemia induction until the end of the experiment, reduces apoptotic levels and pro-inflammatory cytokines when analyzed 7 days after the transient IOP raise, even though exacerbates 48 h after IOP (Boia et al. 2017). Using photocoagulation of trabecular meshwork of limbal veins to produce ocular hypertension (OTH), to mimic glaucoma symptoms, the same group, using the same treatment protocol, shows that caffeine could diminish inflammation and ganglion cell loss 7 days, but not 3 days, after OTH (Madeira et al. 2016b). In humans, a 20-year follow-up involving 121.172 people found no association between caffeinated coffee consumption and the risk of developing primary open-angle glaucoma (POAG). However, for those with family history of glaucoma and high IOP, the association seems to exist, as coffee drinkers show higher chances of developing the pathology (Kang et al. 2008). But, the risk may not be due to caffeine’s effects on IOP, since ocular application of the compound does not contribute to elevate IOP in a small five patients study with POAG/OTH (Chandra et al. 2011). However, it does show an acute IOP-elevating effect in a study with seventeen healthy patients (Redondo et al. 2020). It seems that this acute effect is dependent on the level of routine consumption, being more expressive in low caffeine consumers as demonstrated in a study involving forty patients (Vera et al. 2019). The positive association of caffeinated coffee consumption and risk of exfoliation glaucoma or exfoliation glaucoma suspect, compared to abstainers, is also reported in another follow-up involving more than 120.000 people for more than twenty years (Pasquale et al. 2012), as for higher IOP in coffee consumers with open-angle glaucoma in a smaller study involving 3654 patients (Chandrasekaran et al. 2005).
Concluding Remarks
Ischemia provokes cell death in developing and mature CNS, promoting neurological disabilities and ophthalmological deficits. The neural damage occurs as a consequence of energy deficit and ionic imbalance, which leads to excitotoxicity, oxidative stress, and inflammation. Several studies have been investigating the protective potential of adenosine receptors since the concentration of adenosine increases soon after the ischemic event. Taken together, the data from adult CNS indicate that adenosine release during ischemia is protective mainly via activation of A1 receptors. Even the upregulation of A1 receptors, previous to an ischemic event, reduces the tissue damage, possibly by increasing the availability of receptors to be activated by released adenosine during ischemia (Rudolphi et al. 1989; Von Lubitz et al. 1994a). The main protective mechanism mediated by A1 receptor seems to be the inhibition of neurotransmitter release, especially glutamate, attenuating the ischemia-induced excitotoxicity. A few studies focus on the signaling pathways involved in the beneficial role of A1 receptors. The stimulation of A1 receptors reduces oxidative stress, TNFα production, and increases phosphorylation of ERK and GSK3β (Fig. 4).
Fig. 4.
Effects of adenosine receptor modulation in ischemic events. a The increase of extracellular adenosine availability during ischemia allows the activation of all adenosine receptors in different cell types. In astrocytes, stimulation (positive symbol, +) of A1 receptors (green), or inhibition (─┤) of A2A receptors (red), reduces EAAT1/2 exacerbating the augment in extracellular glutamate and contributing to excitotoxicity. Astrocytes and microglia experience an increase in CD39 and CD73 content in ischemic events. In the case of A1 receptors, the regulation of EAATs occurs through MAPK/PKC pathway. The activation of microglial A2A receptors induces intracellular pathways related to an inflammatory response. Furthermore, in endothelial cells, A2B (purple) and A3 (yellow) receptors stimulation, or A2A receptor inhibition, reduces VCAM/ICAM content, immune cells infiltration, BBB breakdown, and edema. The inhibition of A2A receptors in oligodendrocyte and microglia cells reduces, respectively, p-JNK and p-p38 as well as TNF-α. Canonical pathway is represented in postsynaptic neuron. The right panel depicts the pre- and postsynaptic terminals and the effect of adenosine receptors agonists or antagonists during ischemia. b The activation of presynaptic A1 receptors decreases glutamate release through a direct mechanism or through the inhibition of voltage-gated Ca2+ channels (VGCC). Antagonists of A2A presynaptic receptors also inhibit glutamate release. In the postsynaptic neurons, A1 agonism or A2A antagonism triggers multiple intracellular pathways promoting antioxidant and anti-inflammatory responses, decreasing oxidative stress and cell death
There is a substantial amount of data indicating that A2A receptor inhibition is also protective in ischemic CNS. The absence of A2A (KO) in adults renders a greater resistance against ischemia-provoked cell death (Chen et al. 1999; Gui et al. 2009). The main protective mechanism provided by the inhibition of A2A receptors seems to be the modulation of synaptic transmission. In addition, it has been described that the blockade of A2A receptors also promotes the modulation of glutamate availability by astrocytes, the control of inflammatory signals in microglia, as well as the maintenance of myelin organization, empowering the protective outcome (Fig. 4). The protection could also involve endothelial cells since the absence of A2A receptors in these cells renders several protective changes in the context of ischemia (Zhou et al. 2019). A few studies have focused on the signaling pathways that support this beneficial role. Moreover, the inhibition of A2A receptors diminishes p-ERK, NFκB, TNF-α, IL-6, iNOS, caspase-3, Cyt C, p-JNK, p-p38, p-CREB, c-fos, and ROS, all increased by ischemia/reperfusion (Fig. 4). Finally, it seems that the effect can also depend on the CNS area since activation of A2A can reduce damage induced by ischemia specifically in the spinal cord and cortex (Reece et al. 2006; Melani et al. 2014a).
Although there are fewer data concerning A2B and A3 receptors effect, the stimulation of these scarce receptors has been associated with a protective outcome acting on different types of cells, endothelial, microglia, and neurons (Fig. 4). Nonetheless, in vivo intervention with A3 receptor agonists could be challenging due to a hypotensive response.
It is relevant to highlight that most of the studies using selective pharmacological tools to modulate adenosine system in ischemia were performed in adult animals. A few available data from adenosine role in the immature context points to a different protective mechanism from adults. For instance, the absence of A1 receptors (KO) can prevent hypoxia-induced ventriculomegaly, a distinctive trace of periventricular leukomalacia, commonly associated with brain damage in premature infants (Turner et al. 2003); treatment with adenosine A1 agonist, after HI, does not feature neuroprotective results (Ådén et al. 2001); and A2A KO aggravates neuronal damage in immature brain after HI (Ådén et al. 2003). What is the source for this different response? The signaling underlying cell survival, in developing or mature neurons, can differ in crucial aspects, especially involving calcium transients and NMDA receptor activity (Cunha 2005). These differences probably account for the existence of some conflicting data about the role of adenosine receptors in brain ischemia. In addition, some studies show that the coupling to G protein/intracellular pathways of A2A receptors may change during development. Socodato and colleagues have shown that A2A receptor activation leads to cell death through coupling to PLC-protein kinase C in a narrow window of an early period of retinal development (Socodato et al. 2011). Although A1 receptor expression/content is upregulated by A2A receptor (Pereira et al. 2010; Brito et al. 2012), which could explain the findings, the study discards this possibility by showing that A1 receptor blockade has no effect. Therefore, the mechanisms involved in the different resistance to ischemia from immature to mature brain still represents a field to be explored.
Considering that the main molecular targets of caffeine, at least in a non-toxic dose, are A1 and A2A receptors, together with the great amount of data correlating the adenosine system with neuroprotection, many studies have been investigating the potential of caffeine to alleviate ischemic damage. Since caffeine has been used in the apnea of prematurity for more than thirty years, due to its bronchodilator effect, the majority of studies have explored the outcome in the immature CNS. A considerable number of studies show that low–moderate doses of caffeine attenuate the ischemia-induced injury in immature CNS, indicating that the inhibition of A2A receptor could be more efficient to save CNS cells than stimulation of A1/A2B/A3 receptors by released adenosine or selective agonists. It is not well established the reason why the blockade of A2A prevails when compared to activation of A1 receptor by adenosine released during ischemia. It probably involves different neurochemical aspects, such as adenosine/caffeine metabolization, strict control of extracellular adenosine availability, control of A1 by A2A receptor in heterodimers, and upregulation/downregulation after treatment, among others. Besides that, due to the activity of A2A receptors in different cell types during ischemia, caffeine could target not only neurons but almost every other CNS cell type (endothelial, microglial, astrocytes, and oligodendrocytes), perhaps resulting in a stronger prosurvival outcome (Fig. 4). Despite all that, the therapeutic time window of caffeine administration seems to be narrow and close to the ischemic event. Accordingly, a single exposure to caffeine soon after ischemia reduces infarct area and ameliorates cognitive function evaluated later in the adult.
The data from mature retina studies indicate that pretreatment with A1 agonist or post-treatment with A2A antagonists reduces the damage provoked by ischemia. Using animal models of glaucoma, caffeine exposure, before ischemia and for additional two weeks, decreases ischemic injury. However, caffeine treatment in a later period can worsen ischemic deterioration. The analysis of the possible correlation of human consumption and glaucoma in studies that include a higher number of subjects, evaluating longer periods, show positive correlation of coffee drinkers with the (a) chance to develop the pathology in those with family history of glaucoma and high intraocular pressure; (b) higher intraocular pressure in open-angle glaucoma patients; and (c) risk of exfoliation glaucoma, even though no association of coffee consumption with the risk of developing POAG was found.
Finally, in the mature brain, there are only two studies with animal models demonstrating that chronic, but not acute, caffeine treatment reduces the damage promoted by ischemia (Rudolphi et al. 1989; Sutherland et al. 1991). More intriguingly, one of these studies suggests that the effect depends on the upregulation of A1 receptor, even though it was not tested (Rudolphi et al. 1989). Thus, despite the great number of studies in immature CNS, it is still unclear whether caffeine protects the mature brain from ischemia and the gap of information is even wider concerning the signaling pathways involved. Even in the immature CNS, the cellular mechanisms involved in the protective role of caffeine have not been largely explored. Although different experimental paradigms strengthen the neuroprotective role of caffeine in the context of CNS ischemia, further studies are still required to successfully translate the current knowledge to human therapies.
Acknowledgements
Nascimento AA thanks Coordination of Superior Level Staff Improvement (CAPES) for doctoral fellowships, Cunha-Rodrigues MC thanks National Council of Scientific and Technological Development (CNPq) for postdoctoral fellowship, and Pereira-Figueiredo D thanks for Research Support of the State of Rio de Janeiro (FAPERJ) for postdoctoral fellowship. Calaza KC thanks CNPq and FAPERJ for the individual research fellowships.
Abbreviations
- AC
Adenylyl cyclase
- AMD
Age-related macular disease
- BDNF
Brain-derived neurotrophic factor
- CNS
Central nervous system
- COX-2
Cyclooxygenase 2
- CREB
CAMP-response element binding protein
- Cyt C
Cytochrome c
- DALYs
Disability-adjusted life years
- DM
Diabetes mellitus
- DR
Diabetic retinopathy
- EAAT
Excitatory amino acid transporter
- FasL
First apoptosis signal ligand
- GDNF
Glial cell-derived neurotrophic factor
- GSH
Glutathione
- Gsk3β
Glycogen synthase kinase 3 beta
- HI
Hypoxia–ischemia
- HIE
Hypoxic-ischemic encephalopathy
- IL-10
Interleukin 10
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- iNOS
Inducible nitric oxide synthase
- IOP
Intraocular pressure
- LDH
Lactate dehydrogenase
- MCAO
Middle cerebral artery occlusion
- NGF
Nerve growth factor
- NICUs
Neonatal intensive care units
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- Nrf2
Nuclear factor erythroid 2-related factor 2
- OGD
Oxygen glucose deprivation
- OIR
Oxygen-induced retinopathy
- OTH
Ocular hypertension
- PKA
Protein kinase A
- PKC
Protein kinase C
- POAG
Primary open-angle glaucoma
- ROP
Retinopathy of prematurity
- ROS
Reactive oxidative species
- SOD
Superoxide dismutase
- TBARS
Thiobarbituric acid reactive substances
- TIA
Transient ischemic attack
- TNF-α
Tumor necrosis factor-alpha
- tPA
Tissue-type plasminogen activator
- VEGF
Vascular endothelial growth factor
- VGCC
Voltage-gated calcium channels
Author Contributions
KCC had the original idea, all the authors performed literature search and participated in the writing of the original draft. KCC and RB critically revised the work.
Funding
This work was supported by grants from National Council of Scientific and Technological Development (CNPq), National Institute of Science and Technology—National Institute for Translational Neuroscience (INCT/INNT), Coordination of Superior Level Staff Improvement (CAPES), and Foundation for Research Support of the State of Rio de Janeiro (FAPERJ).
Declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
R. Brito and K. C. Calaza have equally contributed.
References
- Abbracchio MP, Cattabeni F (1999) Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann N Y Acad Sci 890:79–92. 10.1111/j.1749-6632.1999.tb07983.x [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Brambilla R, Ceruti S et al (1995) G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol 48:1038–1045 [PubMed] [Google Scholar]
- Abdel-Hady H (2015) Caffeine therapy in preterm infants. World J Clin Pediatr 4:81. 10.5409/wjcp.v4.i4.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ådén U, Leverin AL, Hagberg H, Fredholm BB (2001) Adenosine A1 receptor agonism in the immature rat brain and heart. Eur J Pharmacol 426:185–192. 10.1016/S0014-2999(01)01220-1 [DOI] [PubMed] [Google Scholar]
- Ådén U, Halldner L, Lagercrantz H et al (2003) Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke 34:739–744. 10.1161/01.STR.0000060204.67672.8B [DOI] [PubMed] [Google Scholar]
- Alexander M, Smith AL, Rosenkrantz TS, Holly Fitch R (2013) Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci 3:177–190. 10.3390/brainsci3010177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabrawey M, Elsherbiny M, Nussbaum J et al (2013) Targeting neovascularization in ischemic retinopathy: recent advances. Expert Rev Ophthalmol 8:267–286. 10.1586/eop.13.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zamil WM, Yassin SA (2017) Recent developments in age-related macular degeneration: a review. Clin Interv Aging 12:1313–1330. 10.2147/CIA.S143508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A 3rd (1992) Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can J Physiol Pharmacol 70(Suppl):S158–S164. 10.1139/y92-257 [DOI] [PubMed] [Google Scholar]
- Amoaku WM, Chakravarthy U, Gale R et al (2015) Defining response to anti-VEGF therapies in neovascular AMD. Eye 29:721–731. 10.1038/eye.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiné P, Rudolphi KA, Fredholm BB, Hagberg H (1990) Effect of propentofylline (HWA 285) on extracellular purines and excitatory amino acids in CA1 of rat hippocampus during transient ischaemia. Br J Pharmacol 100:814–818. 10.1111/j.1476-5381.1990.tb14097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonioli L, Csóka B, Fornai M et al (2014) Adenosine and inflammation: what’s new on the horizon? Drug Discov Today 19:1051–1068. 10.1016/j.drudis.2014.02.010 [DOI] [PubMed] [Google Scholar]
- Antonioli L, Fornai M, Blandizzi C et al (2019) Adenosine signaling and the immune system: when a lot could be too much. Immunol Lett 205:9–15. 10.1016/j.imlet.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Aranda JV, Cai CL, Ahmad T et al (2016) Pharmacologic synergism of ocular ketorolac and systemic caffeine citrate in rat oxygen-induced retinopathy. Pediatr Res 80:554–565. 10.1038/pr.2016.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda JV, Qu J, Valencia GB, Beharry KD (2019) Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin Perinatol 43:360–366. 10.1053/j.semperi.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Arden GB, Sivaprasad S (2011) Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev 7:291–304. 10.2174/157339911797415620 [DOI] [PubMed] [Google Scholar]
- Aref AA, Gedde SJ, Budenz DL (2017) Glaucoma drainage implant surgery. Dev Ophthalmol 59:43–52. 10.1159/000458485 [DOI] [PubMed] [Google Scholar]
- Arnaud MJ (1993) Metabolism of caffeine and other components of coffee. Caffeine, coffe and health. Raven Press, New York, pp 43–95 [Google Scholar]
- Arrigoni E, Crocker AJ, Saper CB et al (2005) Deletion of presynaptic adenosine A1 receptors impairs the recovery of synaptic transmission after hypoxia. Neuroscience 132:575–580. 10.1016/j.neuroscience.2004.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atef RM, Agha AM, Abdel-Rhaman ARA, Nassar NN (2018) The Ying and Yang of adenosine A1 and A2A receptors on ERK1/2 activation in a rat model of global cerebral ischemia reperfusion injury. Mol Neurobiol 55:1284–1298. 10.1007/s12035-017-0401-1 [DOI] [PubMed] [Google Scholar]
- Back SA, Craig A, Luo NL et al (2006) Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol 60:696–705. 10.1002/ana.21008 [DOI] [PubMed] [Google Scholar]
- Bailey C, Chakravarthy U, Lotery A et al (2017) Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye 31:1707–1715. 10.1038/eye.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker R, Steegers EAP, Obradov A et al (2010) Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study. Am J Clin Nutr 91:1691–1698. 10.3945/ajcn.2009.28792 [DOI] [PubMed] [Google Scholar]
- Bashinsky AL (2017) Retinopathy of prematurity. N C Med J 78:124–128. 10.18043/ncm.78.2.124 [DOI] [PubMed] [Google Scholar]
- Batti L, O’Connor JJ (2010) Tumor necrosis factor-alpha impairs the recovery of synaptic transmission from hypoxia in rat hippocampal slices. J Neuroimmunol 218:21–27. 10.1016/j.jneuroim.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Bayne LE (2018) Big data in neonatal health care: big reach, big reward? Crit Care Nurs Clin North Am 30:481–497. 10.1016/j.cnc.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Beharry KD, Valencia GB, Lazzaro DR, Aranda JV (2016) Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin Perinatol 40:189–202. 10.1053/j.semperi.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejot Y, Daubail B, Giroud M (2016) Epidemiology of stroke and transient ischemic attacks: current knowledge and perspectives. Rev Neurol (Paris) 172:59–68. 10.1016/j.neurol.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Beukers MW, den Dulk H, van Tilburg EW et al (2000) Why are A(2B) receptors low-affinity adenosine receptors? Mutation of Asn273 to Tyr increases affinity of human A(2B) receptor for 2-(1-hexynyl)adenosine. Mol Pharmacol 58:1349–1356. 10.1124/mol.58.6.1349 [DOI] [PubMed] [Google Scholar]
- Bhasin A, Srivastava MVP, Mohanty S et al (2013) Stem cell therapy: a clinical trial of stroke. Clin Neurol Neurosurg 115:1003–1008. 10.1016/j.clineuro.2012.10.015 [DOI] [PubMed] [Google Scholar]
- Biber K, Klotz KN, Berger M et al (1997) Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J Neurosci 17:4956–4964. 10.1523/jneurosci.17-13-04956.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund O, Shang M, Tonazzini I et al (2008) Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol 596:6–13. 10.1016/j.ejphar.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Blencowe H, Lawn JE, Vazquez T et al (2013) Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 74:35–49. 10.1038/pr.2013.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boia R, Elvas F, Madeira MH et al (2017) Treatment with A2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell Death Dis 8:e3065. 10.1038/cddis.2017.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissard CG, Lindner MD, Gribkoff VK (1992) Hypoxia produces cell death in the rat hippocampus in the presence of an A1 adenosine receptor antagonist: an anatomical and behavioral study. Neuroscience 48:807–812. 10.1016/0306-4522(92)90268-7 [DOI] [PubMed] [Google Scholar]
- Bona E, Adén U, Fredholm BB, Hagberg H (1995) The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res 38:312–318. 10.1203/00006450-199509000-00007 [DOI] [PubMed] [Google Scholar]
- Bona E, Aden U, Gilland E et al (1997) Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology 36:1327–1338 [DOI] [PubMed] [Google Scholar]
- Bonati M, Latini R, Tognoni G et al (1984) Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat and mouse. Drug Metab Rev 15:1355–1383. 10.3109/03602538409029964 [DOI] [PubMed] [Google Scholar]
- Bonfiglio V, Reibaldi M, Fallico M et al (2017) Widening use of dexamethasone implant for the treatment of macular edema. Drug Des Devel Ther 11:2359–2372. 10.2147/DDDT.S138922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borea PA, Gessi S, Bar-Yehuda S, Fishman P (2009) A3 adenosine receptor: pharmacology and role in disease. Handb Exp Pharmacol. 10.1007/978-3-540-89615-9_10 [DOI] [PubMed] [Google Scholar]
- Borea PA, Gessi S, Merighi S et al (2018) Pharmacology of adenosine receptors: the state of the art. Physiol Rev 98:1591–1625. 10.1152/physrev.00049.2017 [DOI] [PubMed] [Google Scholar]
- Bourne RRA, Flaxman SR, Braithwaite T et al (2017) Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health 5:e888–e897. 10.1016/S2214-109X(17)30293-0 [DOI] [PubMed] [Google Scholar]
- Bracken MB, Triche EW, Belanger K et al (2003) Association of maternal caffeine consumption with decrements in fetal growth. Am J Epidemiol 157:456–466. 10.1093/aje/kwf220 [DOI] [PubMed] [Google Scholar]
- Braun N, Lenz C, Gillardon F et al (1997) Focal cerebral ischemia enhances glial expression of ecto-5’-nucleotidase. Brain Res 766:213–226. 10.1016/s0006-8993(97)00559-3 [DOI] [PubMed] [Google Scholar]
- Brito R, Pereira MR, Paes-De-Carvalho R, Calaza KDC (2012) Expression of A1 adenosine receptors in the developing avian retina: in vivo modulation by A2A receptors and endogenous adenosine. J Neurochem 123:239–249. 10.1111/j.1471-4159.2012.07909.x [DOI] [PubMed] [Google Scholar]
- Brito R, Pereira-Figueiredo D, Socodato R et al (2016) Caffeine exposure alters adenosine system and neurochemical markers during retinal development. J Neurochem. 10.1111/jnc.13683 [DOI] [PubMed] [Google Scholar]
- Brown MM, Brown GC, Stein JD et al (2005) Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol 40:277–287. 10.1016/S0008-4182(05)80070-5 [DOI] [PubMed] [Google Scholar]
- Brown MM, Brown GC, Lieske HB et al (2016) Societal costs associated with neovascular age-related macular degeneration in the United States. Retina 36:285–298. 10.1097/IAE.0000000000000717 [DOI] [PubMed] [Google Scholar]
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820. 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA (1986) Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol 29:331–346 [PubMed] [Google Scholar]
- Campochiaro PA, Brown DM, Pearson A et al (2012) Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 119:2125–2132. 10.1016/j.ophtha.2012.04.030 [DOI] [PubMed] [Google Scholar]
- Cao J, Li W, Cui C et al (2017) Hypoxic postconditioning attenuates apoptosis via activation of adenosine A2a receptors on dermal microvascular endothelial cells of human flaps. J Surg Res 217:144–152. 10.1016/j.jss.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Cao J, Lin H, Li W et al (2019) Ischemia postconditioning protects dermal microvascular endothelial cells of rabbit epigastric skin flaps against apoptosis via adenosine A2a receptors. J Plast Surg Hand Surg 53:76–82. 10.1080/2000656X.2018.1550417 [DOI] [PubMed] [Google Scholar]
- Cappelletti S, Piacentino D, Sani G, Aromatario M (2015) Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol 13:71–88. 10.2174/1570159X13666141210215655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson RJ (2006) Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin Exp Ophthalmol 34:54–63. 10.1111/j.1442-9071.2006.01146.x [DOI] [PubMed] [Google Scholar]
- Chalam KV, Khetpal V, Rusovici R, Balaiya S (2011) A review: role of ultraviolet radiation in age-related macular degeneration. Eye Contact Lens 37:225–232. 10.1097/ICL.0b013e31821fbd3e [DOI] [PubMed] [Google Scholar]
- Chan PH (1996) Role of oxidants in ischemic brain damage. Stroke 27:1124–1129. 10.1161/01.str.27.6.1124 [DOI] [PubMed] [Google Scholar]
- Chandra P, Gaur A, Varma S (2011) Effect of caffeine on the intraocular pressure in patients with primary open angle glaucoma. Clin Ophthalmol 5:1623–1629. 10.2147/OPTH.S25291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran S, Rochtchina E, Mitchell P (2005) Effects of caffeine on intraocular pressure: the Blue Mountains Eye Study. J Glaucoma 14:504–507. 10.1097/01.ijg.0000184832.08783.be [DOI] [PubMed] [Google Scholar]
- Chapman NA, Jacobs RJ, Braakhuis AJ (2019) Role of diet and food intake in age-related macular degeneration: a systematic review. Clin Exp Ophthalmol 47:106–127. 10.1111/ceo.13343 [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J et al (1999) A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19:9192–9200. 10.1523/jneurosci.3724-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-J, Harvey BK, Shen H et al (2006) Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res 84:1848–1855. 10.1002/jnr.21071 [DOI] [PubMed] [Google Scholar]
- Chen YY, Wu ML, Kao MH et al (2009) Perinatal outcome of recurrent pre-eclampsia versus pre-eclampsia in nulliparas. J Obstet Gynaecol Res 35:1042–1046. 10.1111/j.1447-0756.2009.01057.x [DOI] [PubMed] [Google Scholar]
- Chen LW, Wu Y, Neelakantan N et al (2014) Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Med 12:1–12. 10.1186/s12916-014-0174-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee JC, Ju C et al (2011) A3 adenosine receptor agonist reduces brain ischemic injury and inhibits inflammatory cell migration in rats. Am J Pathol 179:2042–2052. 10.1016/j.ajpath.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Xiong W, Zhang DL et al (2013) Regulation of adenosine levels during cerebral ischemia. Acta Pharmacol Sin 34:60–66. 10.1038/aps.2012.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Bruno V et al (1999) Activation of A(1) adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia 27:275–281 [PubMed] [Google Scholar]
- Corradetti R, Lo Conte G, Moroni F et al (1984) Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur J Pharmacol 104:19–26. 10.1016/0014-2999(84)90364-9 [DOI] [PubMed] [Google Scholar]
- Cossenza M (2018) Coffe is more than caffeine. J Caffeine Adenosine Res 8(3):83–85. 10.1089/caff.2018.0014 [Google Scholar]
- Costenla AR, Cunha RA, de Mendonça A (2010) Caffeine, adenosine receptors, and synaptic plasticity. J Alzheimer’s Dis 20:S25–S34. 10.3233/JAD-2010-091384 [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lou N, Tome-Garcia J et al (2017) Direct comparison of microglial dynamics and inflammatory profile in photothrombotic and arterial occlusion evoked stroke. Neuroscience 343:483–494. 10.1016/j.neuroscience.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counseling P (2019) ACOG Committee Opinion No. 762: prepregnancy counseling. Obstet Gynecol 133:E78–E89. 10.1097/AOG.0000000000003013 [DOI] [PubMed] [Google Scholar]
- Cui M, Ding H, Chen F et al (2016) Mdivi-1 protects against ischemic brain injury via elevating extracellular adenosine in a cAMP/CREB-CD39-dependent manner. Mol Neurobiol 53:240–253. 10.1007/s12035-014-9002-4 [DOI] [PubMed] [Google Scholar]
- Cunha RA (2005) Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signal 1:111–134. 10.1007/s11302-005-0649-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem 139:1019–1055. 10.1111/jnc.13724 [DOI] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, van der Ploeg I et al (1994) Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res 649:208–216. 10.1016/0006-8993(94)91066-9 [DOI] [PubMed] [Google Scholar]
- Czerska M, Mikołajewska K, Zieliński M et al (2015) Today’s oxidative stress markers. Med Pr 66:393–405. 10.13075/mp.5893.00137 [DOI] [PubMed] [Google Scholar]
- Dai QX, Geng WJ, Zhuang XX et al (2017) Electroacupuncture-induced neuroprotection against focal cerebral ischemia in the rat is mediated by adenosine A1 receptors. Neural Regen Res 12:228–234. 10.4103/1673-5374.200806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Cim T, Poluceno GG, Lanznaster D et al (2019) Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen/glucose deprivation in cortical astrocyte cultures: involvement of A1 and A2A adenosine receptors and PI3K, MEK, and PKC pathways. Purinergic Signal 15:465–476. 10.1007/s11302-019-09679-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Sarmah D, Mounica L et al (2020) Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl Stroke Res. 10.1007/s12975-020-00806-z [DOI] [PubMed] [Google Scholar]
- Daval JL, Nicolas F (1994) Opposite effects of cyclohexyladenosine and theophylline on hypoxic damage in cultured neurons. Neurosci Lett 175:114–116. 10.1016/0304-3940(94)91092-8 [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G et al (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- De Filippo E, Hinz S, Pellizzari V et al (2020) A(2A) and A(2B) adenosine receptors: the extracellular loop 2 determines high (A(2A)) or low affinity (A(2B)) for adenosine. Biochem Pharmacol 172:113718. 10.1016/j.bcp.2019.113718 [DOI] [PubMed] [Google Scholar]
- De Flora A, Franco L, Guida L et al (1998) Ectocellular CD38-catalyzed synthesis and intracellular Ca(2+)-mobilizing activity of cyclic ADP-ribose. Cell Biochem Biophys 28:45–62. 10.1007/BF02738309 [DOI] [PubMed] [Google Scholar]
- De Haan M, Wyatt JS, Roth S et al (2006) Brain and cognitive-behavioural development after asphyxia at term birth. Dev Sci 9:350–358. 10.1111/j.1467-7687.2006.00499.x [DOI] [PubMed] [Google Scholar]
- Deleo J, Schubert P, Kreutzberg GW (1988) Protection against ischemic brain damage using propentofylline in gerbils. Stroke 19:1535–1539. 10.1161/01.STR.19.12.1535 [DOI] [PubMed] [Google Scholar]
- Dennis SH, Jaafari N, Cimarosti H et al (2011) Oxygen/glucose deprivation induces a reduction in synaptic AMPA receptors on hippocampal CA3 neurons mediated by mGluR1 and adenosine A3 receptors. J Neurosci 31:11941–11952. 10.1523/JNEUROSCI.1183-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derex L, Cho T-H (2017) Mechanical thrombectomy in acute ischemic stroke. Rev Neurol (Paris) 173:106–113. 10.1016/j.neurol.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Di Martino E, Bocchetta E, Tsuji S et al (2020) Defining a time window for neuroprotection and glia modulation by caffeine after neonatal hypoxia-ischaemia. Mol Neurobiol 57:2194–2205. 10.1007/s12035-020-01867-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson JM, Hill SJ (1998) Involvement of G-protein βγ subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol 355:85–93. 10.1016/S0014-2999(98)00468-3 [DOI] [PubMed] [Google Scholar]
- Ding J, Wong TY (2012) Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 12:346–354. 10.1007/s11892-012-0283-6 [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–397. 10.1016/S0166-2236(99)01401-0 [DOI] [PubMed] [Google Scholar]
- Dos Santos-Rodrigues A, Pereira MR, Brito R et al (2015) Adenosine transporters and receptors: key elements for retinal function and neuroprotection. Vitam Horm 98:487–523. 10.1016/bs.vh.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Doucette LP, Rasnitsyn A, Seifi M, Walter MA (2015) The interactions of genes, age, and environment in glaucoma pathogenesis. Surv Ophthalmol 60:310–326. 10.1016/j.survophthal.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Rehm CD (2016) Sources of caffeine in diets of US children and adults: trends by beverage type and purchase location. Nutrients. 10.3390/nu8030154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV (1984) Interactions between the effects of adenosine and calcium on synaptic responses in rat hippocampus in vitro. J Physiol 350:545–559. 10.1113/jphysiol.1984.sp015217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P (2009) Major cell death pathways at a glance. Microbes Infect 11:1050–1062. 10.1016/j.micinf.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Dux E, Fastbom J, Ungerstedt U et al (1990) Protective effect of adenosine and a novel xanthine derivative propentofylline on the cell damage after bilateral carotid occlusion in the gerbil hippocampus. Brain Res 516:248–256. 10.1016/0006-8993(90)90925-2 [DOI] [PubMed] [Google Scholar]
- Ebneter A, Zinkernagel MS (2016) Novelties in diabetic retinopathy. Endocr Dev 31:84–96. 10.1159/000439391 [DOI] [PubMed] [Google Scholar]
- Eckle T, Faigle M, Grenz A et al (2008) A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111:2024–2035. 10.1182/blood-2007-10-117044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16:663–669. 10.1016/j.ceb.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Edwards AD, Brocklehurst P, Gunn AJ et al (2010) Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340:409. 10.1136/bmj.c363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effendi WI, Nagano T, Kobayashi K, Nishimura Y (2020) Focusing on adenosine receptors as a potential targeted therapy in human diseases. Cells 9:785. 10.3390/cells9030785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenwald EC, Committee on Fetus and Newborn, American Academy of Pediatrics (2016) Apnea of prematurity. Pediatrics. 10.1542/peds.2015-3757 [Google Scholar]
- Endesfelder S, Weichelt U, Strauß E et al (2017) Neuroprotection by caffeine in hyperoxia-induced neonatal brain injury. Int J Mol Sci 18:1–24. 10.3390/ijms18010187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eunson P (2015) The long-term health, social, and financial burden of hypoxic-ischaemic encephalopathy. Dev Med Child Neurol 57:48–50. 10.1111/dmcn.12727 [DOI] [PubMed] [Google Scholar]
- Fa-Lin X, Hui-Qing C, Cai-Hong W et al (2015) Effects of caffeine citrate on myelin basic protein in neonatal rats with hypoxicischemic brain damage. Chin J Contemp Pediatr 17:984–988. 10.7499/j.issn.1008-8830.2015.09.020 [PubMed] [Google Scholar]
- Fang SH, Wei EQ, Zhou Y et al (2006) Increased expression of cysteinyl leukotriene receptor-1 in the brain mediates neuronal damage and astrogliosis after focal cerebral ischemia in rats. Neuroscience 140:969–979. 10.1016/j.neuroscience.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Fedorova IM, Jacobson MA, Basile A, Jacobson KA (2003) Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol Neurobiol 23:431–447. 10.1023/A:1023601007518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RM, Cioffi GA, Liebmann JM, Weinreb RN (2020) Current knowledge and attitudes concerning cost-effectiveness in glaucoma pharmacotherapy: a glaucoma specialists focus group study. Clin Ophthalmol 14:729–739. 10.2147/OPTH.S236030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliu C, Peyret H, Poitevin G et al (2019) Complementary role of P2 and adenosine receptors in ATP induced-anti-apoptotic effects against hypoxic injury of HUVECs. Int J Mol Sci. 10.3390/ijms20061446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM (2004) Neonatal brain injury. N Engl J Med 351:1985–1995. 10.1056/NEJMra041996 [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Biber K, Lieb K et al (1996) Cyclooxygenase-2 expression in rat microglia is induced by adenosine A2a-receptors. Glia 18:152–160. 10.1002/(SICI)1098-1136(199610)18:2%3c152::AID-GLIA7%3e3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- Filloux FM, Adair J, Narang N (1996) The temporal evolution of striatal dopamine receptor binding and mRNA expression following hypoxia-ischemia in the neonatal rat. Dev Brain Res 94:81–91. 10.1016/0165-3806(96)00053-3 [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N et al (2002) Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci 22:1–7. 10.1523/jneurosci.22-07-j0001.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundation EGS (2017) European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th edition—chapter 3: treatment principles and options supported by the EGS Foundation: part 1: foreword; introduction; glossary; chapter 3 treatment principles and options. Br J Ophthalmol 101:130–195. 10.1136/bjophthalmol-2016-EGSguideline.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francart SJ, Allen MK, Stegall-Zanation J (2013) Apnea of prematurity: caffeine dose optimization. J Pediatr Pharmacol Ther 18:45–52. 10.5863/1551-6776-18.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB (1979) Are methylxanthine effects due to antagonism of endogenous adenosine? Trends Pharmacol Sci 1:129–132. 10.1016/0165-6147(79)90046-4 [Google Scholar]
- Fredholm BB (2014) Adenosine—a physiological or pathophysiological agent? J Mol Med (Berl) 92:201–206. 10.1007/s00109-013-1101-6 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J et al (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–133 [PubMed] [Google Scholar]
- Fredholm BB, Yang J, Wang Y (2017) Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol Aspects Med 55:20–25. 10.1016/j.mam.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA et al (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63:1–34. 10.1124/pr.110.003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N (2007) Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem 101:1400–1413. 10.1111/j.1471-4159.2006.04425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronz U, Deten A, Baumann F et al (2014) Continuous adenosine A2A receptor antagonism after focal cerebral ischemia in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 387:165–173. 10.1007/s00210-013-0931-7 [DOI] [PubMed] [Google Scholar]
- Furlan AJ, Higashida R, Katzan I et al (2005) Intra-Arterial thrombolysis in acute ischemic stroke. In: Lyden PD (ed) thrombolytic therapy for acute stroke. Current clinical neurology. Humana Press, Totowa. 10.1385/1-59259-933-8:159 [Google Scholar]
- Fusco I, Ugolini F, Lana D et al (2018) The selective antagonism of adenosine A2B receptors reduces the synaptic failure and neuronal death induced by oxygen and glucose deprivation in rat CA1 hippocampus in vitro. Front Pharmacol 9:1–18. 10.3389/fphar.2018.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadian DG (2000) Developmental amnesia associated with early hypoxic-ischaemic injury. Brain 123:499–507. 10.1093/brain/123.3.499 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM et al (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120. 10.1038/cdd.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao J, Elvas F, Martins T et al (2015) Adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration. Exp Eye Res 140:65–74. 10.1016/j.exer.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Ganesana M, Jill Venton B (2018) Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLoS ONE 13:1–18. 10.1371/journal.pone.0196932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Gazzard G (2018) Selective laser trabeculoplasty: past, present, and future review-article. Eye 32:863–876. 10.1038/eye.2017.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Neurological Disorders Collaborator Group (2017) Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16(11):877–897. 10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W, Cai L, Han K et al (2020) Electroacupuncture pretreatment alleviates cerebral ischemia-reperfusion injury by increasing GSK-3 β phosphorylation level via adenosine A1 receptor. Biomed Res Int. 10.1155/2020/6848450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, Mann C, Zadeh JK et al (2019) Elevated intraocular pressure causes abnormal reactivity of mouse retinal arterioles. Oxid Med Cell Longev 2019:9736047. 10.1155/2019/9736047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM, Fitzgibbons JC, Shah AR et al (1995) Reduction in cerebral ischemic injury in the newborn rat by potentiation of endogenous adenosine. Pediatr Res 38:306–311. 10.1203/00006450-199509000-00006 [DOI] [PubMed] [Google Scholar]
- Gilbert C (2008) Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 84:77–82. 10.1016/j.earlhumdev.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Goda H, Ooboshi H, Nakane H et al (1998) Modulation of ischemia-evoked release of excitatory and inhibitory amino acids by adenosine A1 receptor agonist. Eur J Pharmacol 357:149–155. 10.1016/S0014-2999(98)00559-7 [DOI] [PubMed] [Google Scholar]
- Godinho J, de Sa-Nakanishi AB, Moreira LS et al (2018) Ethyl-acetate fraction of Trichilia catigua protects against oxidative stress and neuroinflammation after cerebral ischemia/reperfusion. J Ethnopharmacol 221:109–118. 10.1016/j.jep.2018.04.018 [DOI] [PubMed] [Google Scholar]
- Gomes CARV, Vaz SH, Ribeiro JA, Sebastião AM (2006) Glial cell line-derived neurotrophic factor (GDNF) enhances dopamine release from striatal nerve endings in an adenosine A2A receptor-dependent manner. Brain Res 1113:129–136. 10.1016/j.brainres.2006.07.025 [DOI] [PubMed] [Google Scholar]
- Gomes CV, Kaster MP, Tomé AR et al (2011) Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808:1380–1399. 10.1016/j.bbamem.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Gomes C, Ferreira R, George J et al (2013) Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflamm 10:1–13. 10.1186/1742-2094-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández E, Sánchez-Gómez MV, Pérez-Samartín A et al (2014) A3 Adenosine receptors mediate oligodendrocyte death and ischemic damage to optic nerve. Glia 62:199–216. 10.1002/glia.22599 [DOI] [PubMed] [Google Scholar]
- Goyal M, Menon BK, van Zwam WH et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England) 387:1723–1731. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- Greco P, Nencini G, Piva I et al (2020) Pathophysiology of hypoxic–ischemic encephalopathy: a review of the past and a view on the future. Acta Neurol Belg 120:277–288. 10.1007/s13760-020-01308-3 [DOI] [PubMed] [Google Scholar]
- Grewal AK, Singh N, Singh TG (2019) Neuroprotective effect of pharmacological postconditioning on cerebral ischaemia–reperfusion-induced injury in mice. J Pharm Pharmacol 71:956–970. 10.1111/jphp.13073 [DOI] [PubMed] [Google Scholar]
- Grillo SL, McDevitt DS, Voas MG et al (2019) Adenosine receptor expression in the adult zebrafish retina. Purinergic Signal 15:327–342. 10.1007/s11302-019-09667-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Andersson K, Chen Y et al (2005) Effect of perinatal asphyxia on tyrosine hydroxylase and D2 and D1 dopamine receptor mRNA levels expressed during early postnatal development in rat brain. Mol Brain Res 134:275–281. 10.1016/j.molbrainres.2004.10.030 [DOI] [PubMed] [Google Scholar]
- Gui L, Duan W, Tian H et al (2009) Adenosine A2Areceptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res 1297:185–193. 10.1016/j.brainres.2009.08.050 [DOI] [PubMed] [Google Scholar]
- Gupta RC, Srivastava A, Lall R (2018) Toxicity potential of nutraceuticals. Methods Mol Biol 1800:367–394. 10.1007/978-1-4939-7899-1_18 [DOI] [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F et al (2011) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:718–779. 10.1016/j.euroneuro.2011.08.008 [DOI] [PubMed] [Google Scholar]
- Hao L, Zou Z, Tian H et al (2014) Stem cell-based therapies for ischemic stroke. Biomed Res Int 2014:468748. 10.1155/2014/468748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Jonescu-Cuypers C, Martin B et al (2001) Simultaneous management of blood flow and IOP in glaucoma. Acta Ophthalmol Scand 79:336–341. 10.1034/j.1600-0420.2001.079004336.x [DOI] [PubMed] [Google Scholar]
- Harrison W, Goodman D (2015) Epidemiologic trends in neonatal intensive care, 2007–2012. JAMA Pediatr 169:855–862. 10.1001/jamapediatrics.2015.1305 [DOI] [PubMed] [Google Scholar]
- Hartnett ME (2017) Advances in understanding and management of retinopathy of prematurity. Surv Ophthalmol 62:257–276. 10.1016/j.survophthal.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770. 10.1038/nrd2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman MA, Weil J, de Mejia EG (2010) Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75:77–87. 10.1111/j.1750-3841.2010.01561.x [DOI] [PubMed] [Google Scholar]
- Heese K, Fiebich BL, Bauer J, Otten U (1997) Nerve growth factor (NGF) expression in rat microglia is induced by adenosine A2a-receptors. Neurosci Lett 231:83–86. 10.1016/s0304-3940(97)00545-4 [DOI] [PubMed] [Google Scholar]
- Heintz E, Wirehn AB, Peebo BB et al (2010) Prevalence and healthcare costs of diabetic retinopathy: a population-based register study in Sweden. Diabetologia 53:2147–2154. 10.1007/s00125-010-1836-3 [DOI] [PubMed] [Google Scholar]
- Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-De La Paz L et al (2018) Age-related macular degeneration: new paradigms for treatment and management of AMD. Oxid Med Cell Longev. 10.1155/2018/8374647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héron A, Lekieffre D, Le Peillet E et al (1994) Effects of an A1 adenosine receptor agonist on the neurochemical, behavioral and histological consequences of ischemia. Brain Res 641:217–224. 10.1016/0006-8993(94)90149-X [DOI] [PubMed] [Google Scholar]
- Hettinger-Smith BD, Leid M, Murray TF (1996) Chronic exposure to adenosine receptor agonists and antagonists reciprocally regulates the A1 adenosine receptor-adenylyl cyclase system in cerebellar granule cells. J Neurochem 67:1921–1930. 10.1046/j.1471-4159.1996.67051921.x [DOI] [PubMed] [Google Scholar]
- Higashi H, Meno JR, Marwaha AS, Winn HR (2002) Hippocampal injury and neurobehavioral deficits following hyperglycemic cerebral ischemia: Effect of theophylline and ZM 241385. J Neurosurg 96:117–126. 10.3171/jns.2002.96.1.0117 [DOI] [PubMed] [Google Scholar]
- Higgins RD, Raju T, Edwards AD et al (2011) Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 159:851-858.e1. 10.1016/j.jpeds.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Houkin K, Matsunaga T et al (2011) Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134:1790–1807. 10.1093/brain/awr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Li Y, Huang Y et al (2020) Adenosine receptor A1–A2a heteromers regulate EAAT2 expression and glutamate uptake via YY1-induced repression of PPARγ transcription. PPAR Res 2020:2410264. 10.1155/2020/2410264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Dong H, Zhang H et al (2012) Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res 1459:81–90. 10.1016/j.brainres.2012.04.017 [DOI] [PubMed] [Google Scholar]
- Imura Y, Morizawa Y, Komatsu R et al (2013) Microglia release ATP by exocytosis. Glia 61:1320–1330. 10.1002/glia.22517 [DOI] [PubMed] [Google Scholar]
- Jacobs SE, Berg M, Hunt R et al (2013) Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB (1996) Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci 17:108–113. 10.1016/0165-6147(96)10002-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhan V, Qureshi AI (2004) Mechanisms of ischemic brain injury. Curr Cardiol Rep 6:117–123. 10.1007/s11886-004-0009-8 [DOI] [PubMed] [Google Scholar]
- Jeon SJ, Rhee SY, Ryu JH et al (2011) Activation of adenosine A2A receptor up-regulates BDNF expression in rat primary cortical neurons. Neurochem Res 36:2259–2269. 10.1007/s11064-011-0550-y [DOI] [PubMed] [Google Scholar]
- Jiang N, Kowaluk EA, Lee CH et al (1997) Adenosine kinase inhibition protects brain against transient focal ischemia in rats. Eur J Pharmacol 320:131–137. 10.1016/S0014-2999(96)00905-3 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhu W, Zhu J et al (2013) Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant 22:2291–2298. 10.3727/096368912X658818 [DOI] [PubMed] [Google Scholar]
- Jiang S, Kametani M, Chen DF (2020) Adaptive immunity: new aspects of pathogenesis underlying neurodegeneration in glaucoma and optic neuropathy. Front Immunol 11:1–5. 10.3389/fimmu.2020.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Georgiev V, Kuosmanen T, Fredholm BB (1996) Long-term treatment with some methylxanthines decreases the susceptibility to bicuculline- and pentylenetetrazol-induced seizures in mice. Relationship to c-fos expression and receptor binding. Eur J Neurosci 8:2447–2458. 10.1111/j.1460-9568.1996.tb01539.x [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV et al (2001) Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA 98:9407–9412. 10.1073/pnas.161292398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BH, Bonafede MM, Watson C (2016) Short- and longer-term health-care resource utilization and costs associated with acute ischemic stroke. Clinicoecon Outcomes Res 8:53–61. 10.2147/CEOR.S95662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Ishida A, Ishida WN et al (2009) Plasticity and injury in the developing brain. Brain Dev 31:1–10. 10.1016/j.braindev.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadłubowska J, Malaguarnera L, Wąż P, Zorena K (2016) Neurodegeneration and neuroinflammation in diabetic retinopathy: potential approaches to delay neuronal loss. Curr Neuropharmacol 14:831–839. 10.2174/1570159x14666160614095559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähm K, Laxy M, Schneider U et al (2018) Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care 41:971–978. 10.2337/dc17-1763 [DOI] [PubMed] [Google Scholar]
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2016) Ischemia/Reperfusion. Compr Physiol 7:113–170. 10.1002/cphy.c160006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Willett WC, Rosner BA et al (2008) Caffeine consumption and the risk of primary open-angle glaucoma: a prospective cohort study. Invest Ophthalmol Vis Sci 49:1924–1931. 10.1167/iovs.07-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Sharma S, Sandhir R, Nehru B (2019) Temporal changes in physiological and molecular markers in various brain regions following transient global ischemia in rats. Mol Biol Rep 46:6215–6230. 10.1007/s11033-019-05060-7 [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Scholz R, Rutar M et al (2015) Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 45:30–57. 10.1016/j.preteyeres.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Kaur C, Foulds WS, Ling E-A (2008) Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol 2:879–889. 10.2147/opth.s3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabori M, Yenari MA (2015) Inflammatory responses in brain ischemia. Curr Med Chem 22:1258–1277. 10.2174/0929867322666150209154036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Ruskin DN, Masino SA (2019) Adenosine A1 receptor-mediated protection of mouse hippocampal synaptic transmission against oxygen and/or glucose deprivation: a comparative study. J Neurophysiol 122:721–728. 10.1152/jn.00813.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawczyk-Krupka A, Bugaj AM, Potempa M et al (2015) Vascular-targeted photodynamic therapy in the treatment of neovascular age-related macular degeneration: clinical perspectives. Photodiagn Photodyn Ther 12:161–175. 10.1016/j.pdpdt.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Khanna S, Komati R, Eichenbaum DA et al (2019) Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol 4:1–8. 10.1136/bmjophth-2019-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilicdag H, Daglioglu YK, Erdogan S, Zorludemir S (2014) Effects of caffeine on neuronal apoptosis in neonatal hypoxic-ischemic brain injury. J Matern Neonatal Med 27:1470–1475. 10.3109/14767058.2013.878694 [DOI] [PubMed] [Google Scholar]
- Kim B, Nam Y, Kim J et al (2012) Coffee consumption and stroke risk: a meta-analysis of epidemiologic studies. Korean J Fam Med 33:356–365. 10.4082/kjfm.2012.33.6.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Mori A, Shimada J et al (2002) Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol Res 24:317–323. 10.1179/016164102101199819 [DOI] [PubMed] [Google Scholar]
- Klebanoff MA, Levine RJ, Clemens JD, Wilkins DG (2002) Maternal serum caffeine metabolites and small-for-gestational age birth. Am J Epidemiol 155:32–37. 10.1093/aje/155.1.32 [DOI] [PubMed] [Google Scholar]
- Ko ML, Peng PH, Ma MC et al (2005) Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med 39:365–373. 10.1016/j.freeradbiomed.2005.03.025 [DOI] [PubMed] [Google Scholar]
- Koeppen M, Eckle T, Eltzschig HK (2011) Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol 61:145–186. 10.1016/B978-0-12-385526-8.00006-0 [DOI] [PubMed] [Google Scholar]
- Kohno Y, Ji XD, Mawhorter SD et al (1996) Activation of A3 adenosine receptors on human eosinophils elevates intracellular calcium. Blood 88:3569–3574. 10.1182/blood.v88.9.3569.bloodjournal8893569 [PMC free article] [PubMed] [Google Scholar]
- Kokona D, Georgiou PC, Kounenidakis M et al (2016) Endogenous and synthetic cannabinoids as therapeutics in retinal disease. Neural Plas. 10.1155/2016/8373020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahdouzan M, Hamadeh MJ (2017) The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Ther 23:272–290. 10.1111/cns.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L et al (2005) Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg 103:38–45. 10.3171/jns.2005.103.1.0038 [DOI] [PubMed] [Google Scholar]
- Konje JC, Cade JE (2008) Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ 337:1334–1338. 10.1136/bmj.a2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno T, Sato A, Uchibori T et al (2006) Adenosine A2A receptor mediated protective effect of 2-(6-cyano-1-hexyn-1-yl)adenosine on retinal ischaemia/reperfusion damage in rats. Br J Ophthalmol 90:900–905. 10.1136/bjo.2006.091496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostandy BB (2012) The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci 33:223–237. 10.1007/s10072-011-0828-5 [DOI] [PubMed] [Google Scholar]
- Kriz J, Lalancette-Hébert M (2009) Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol 117:497–509. 10.1007/s00401-009-0496-1 [DOI] [PubMed] [Google Scholar]
- Kua KP, Lee SWH (2017) Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br J Clin Pharmacol 83:180–191. 10.1111/bcp.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Lipshultz (2019) Caffeine and clinical outcomes in premature neonates. Children 6:118. 10.3390/children6110118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppermann BD, Goldstein M, Maturi RK et al (2015) Dexamethasone intravitreal implant as adjunctive therapy to ranibizumab in neovascular age-related macular degeneration: a multicenter randomized controlled trial. Ophthalmologica 234:40–54. 10.1159/000381865 [DOI] [PubMed] [Google Scholar]
- Kurinczuk JJ, White-Koning M, Badawi N (2010) Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 86:329–338. 10.1016/j.earlhumdev.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Lai AY, Dhami KS, Dibal CD, Todd KG (2011) Neonatal rat microglia derived from different brain regions have distinct activation responses. Neuron Glia Biol 7:5–16. 10.1017/S1740925X12000154 [DOI] [PubMed] [Google Scholar]
- Lallukka T, Ervasti J, Lundstrom E et al (2018) Trends in diagnosis-specific work disability before and after stroke: a longitudinal population-based study in Sweden. J Am Heart Assoc. 10.1161/JAHA.117.006991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AK, Osborne NN (1996) Involvement of adenosine in retinal ischemia. Studies on the rat. Invest Ophthalmol Vis Sci 37:2603–2611 [PubMed] [Google Scholar]
- Larsson SC, Virtamo J, Wolk A (2011) Coffee consumption and risk of stroke in women. Stroke 42:908–912. 10.1161/STROKEAHA.110.603787 [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Corradetti R et al (1999a) Effect of A(2A) adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices. Br J Pharmacol 128:1035–1044. 10.1038/sj.bjp.0702888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Pedata F, Corradetti R (1999b) Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br J Pharmacol 127:729–739. 10.1038/sj.bjp.0702591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RHC, Lee MHH, Wu CYC et al (2018) Cerebral ischemia and neuroregeneration. Neural Regen Res 13:373–385. 10.4103/1673-5374.228711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens R, Steinberg GK (2013) Stem cell therapy for acute cerebral injury: what do we know and what will the future bring? Curr Opin Neurol 26:617–625. 10.1097/WCO.0000000000000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Rosenbaum PS, Jennings NM et al (1999) Differing roles of adenosine receptor subtypes in retinal ischemia-reperfusion injury in the rat. Exp Eye Res 68:9–17. 10.1006/exer.1998.0573 [DOI] [PubMed] [Google Scholar]
- Li XD, He RR, Qin Y et al (2012) Caffeine interferes embryonic development through over-stimulating serotonergic system in chicken embryo. Food Chem Toxicol 50:1848–1853. 10.1016/j.fct.2012.03.037 [DOI] [PubMed] [Google Scholar]
- Li W, Huang R, Shetty RA et al (2013) Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis 59:18–25. 10.1016/j.nbd.2013.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao H, Song JM et al (2015) A meta-analysis of risk of pregnancy loss and caffeine and coffee consumption during pregnancy. Int J Gynecol Obstet 130:116–122. 10.1016/j.ijgo.2015.03.033 [DOI] [PubMed] [Google Scholar]
- Li Q, Han X, Lan X et al (2017) Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol Dis 108:173–182. 10.1016/j.nbd.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS, Sanossian N, Fu KA et al (2016) The coffee paradox in stroke: increased consumption linked with fewer strokes. Nutr Neurosci 19:406–413. 10.1179/1476830515Y.0000000035 [DOI] [PubMed] [Google Scholar]
- Liegl R, Hellstrom A, Smith LE (2016) Retinopathy of prematurity: the need for prevention. Eye Brain 8:91–102. 10.2147/EB.S99038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Phillis JW (1992) Deoxycoformycin and oxypurinol: protection against focal ischemic brain injury in the rat. Brain Res 571:272–280. 10.1016/0006-8993(92)90665-V [DOI] [PubMed] [Google Scholar]
- Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568. 10.1016/j.shpsa.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Liu YJ, Chen J, Li X et al (2019) Research progress on adenosine in central nervous system diseases. CNS Neurosci Ther 25:899–910. 10.1111/cns.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Xiao-Ling XL, Zhou R, Pan QQ et al (2010) Genetic inactivation of the adenosine A2Areceptor attenuates pathologic but not developmental angiogenesis in the mouse retina. Invest Ophthalmol Vis Sci 51:6625–6632. 10.1167/iovs.09-4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfmark U, Hammarström A (2007) Evidence for age-dependent education-related differences in men and women with first-ever stroke. Results from a community-based incidence study in northern Sweden. Neuroepidemiology 28:135–141. 10.1159/000102141 [DOI] [PubMed] [Google Scholar]
- Logan M, Sweeney MI (1997) Adenosine A1 receptor activation preferentially protects cultured cerebellar neurons versus astrocytes against hypoxia-induced death. Mol Chem Neuropathol 31:119–133. 10.1007/BF02815237 [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Kull B et al (2002) Adenosine A2A receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience 112:319–329. 10.1016/S0306-4522(02)00080-5 [DOI] [PubMed] [Google Scholar]
- Lopes JP, Pliássova A, Cunha RA (2019) The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem Pharmacol 166:313–321. 10.1016/j.bcp.2019.06.008 [DOI] [PubMed] [Google Scholar]
- Lopes LV, Sebastiao AM, Ribeiro JA (2011) Adenosine and related drugs in brain diseases: present and future in clinical trials. Curr Top Med Chem 11:1087–1101. 10.2174/156802611795347591 [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM et al (2009) Coffee consumption and risk of stroke in women. Circulation 119:1116–1123. 10.1161/CIRCULATIONAHA.108.826164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S (1999) Oxidative stress in brain ischemia. Brain Pathol 9:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZL, Qin Y, Wang G et al (2012) Exploring the caffeine-induced teratogenicity on neurodevelopment using early chick embryo. PLoS ONE 7:1–8. 10.1371/journal.pone.0034278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZL, Wang G, Cheng X et al (2014) Excess caffeine exposure impairs eye development during chick embryogenesis. J Cell Mol Med 18:1134–1143. 10.1111/jcmm.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MH, Boia R, Elvas F et al (2016a) Selective A2Areceptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Transl Res 169:112–128. 10.1016/j.trsl.2015.11.005 [DOI] [PubMed] [Google Scholar]
- Madeira MH, Ortin-Martinez A, Nadal-Nícolas F et al (2016b) Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci Rep 6:1–13. 10.1038/srep27532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañeru C, Junqué C, Botet F et al (2001) Neuropsychological long-term sequelae of perinatal asphyxia. Brain Inj 15:1029–1039. 10.1080/02699050110074178 [DOI] [PubMed] [Google Scholar]
- Mantravadi AV, Vadhar N (2015) Glaucoma. Prim Care 42:437–449. 10.1016/j.pop.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Maraula G, Traini C, Mello T et al (2013) Effects of oxygen and glucose deprivation on synaptic transmission in rat dentate gyrus: role of A2A adenosine receptors. Neuropharmacology 67:511–520. 10.1016/j.neuropharm.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Marcoli M, Raiteri L, Bonfanti A et al (2003) Sensitivity to selective adenosine A1 and A2A receptor antagonists of the release of glutamate induced by ischemia in rat cerebrocortical slices. Neuropharmacology 45:201–210. 10.1016/S0028-3908(03)00156-4 [DOI] [PubMed] [Google Scholar]
- Marcoli M, Bonfanti A, Roccatagliata P et al (2004) Glutamate efflux from human cerebrocortical slices during ischemia: vesicular-like mode of glutamate release and sensitivity to A2A adenosine receptor blockade. Neuropharmacology 47:884–891. 10.1016/j.neuropharm.2004.06.022 [DOI] [PubMed] [Google Scholar]
- Marko M, Posekany A, Szabo S et al (2020) Trends of r-tPA (recombinant tissue-type plasminogen activator) treatment and treatment-influencing factors in acute ischemic stroke. Stroke 51:1240–1247. 10.1161/STROKEAHA.119.027921 [DOI] [PubMed] [Google Scholar]
- Martín ED, Fernández M, Perea G et al (2007) Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia 55:36–45. 10.1002/glia.20431 [DOI] [PubMed] [Google Scholar]
- Martin RJ, Wilson CG (2012) Apnea of prematurity. Compr Physiol 2(4):2923–2931. 10.1002/cphy.c100021 [DOI] [PubMed] [Google Scholar]
- Martinez-Biarge M, Madero R, Gonzlez A et al (2012) Perinatal morbidity and risk of hypoxic-ischemic encephalopathy associated with intrapartum sentinel events. Am J Obstet Gynecol 206:148.e1-148.e7. 10.1016/j.ajog.2011.09.031 [DOI] [PubMed] [Google Scholar]
- Martire A, Lambertucci C, Pepponi R et al (2019) Neuroprotective potential of adenosine A 1 receptor partial agonists in experimental models of cerebral ischemia. J Neurochem 149:211–230. 10.1111/jnc.14660 [DOI] [PubMed] [Google Scholar]
- Massaro AN, Murthy K, Zaniletti I et al (2016) Intercenter cost variation for perinatal hypoxic-ischemic encephalopathy in the era of therapeutic hypothermia. J Pediatr 173:76-83.e1. 10.1016/j.jpeds.2016.02.033 [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Dos S-R et al (2012) Adenosine A 2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia 60:702–716. 10.1002/glia.22290 [DOI] [PubMed] [Google Scholar]
- Matos M, Shen H-Y, Augusto E et al (2015) Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol Psychiatry 78:763–774. 10.1016/j.biopsych.2015.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Sakaki T, Kohmura E et al (1996) Amelioration of ischemic brain damage by the preischemic administration of propentofylline (HWA285) in a rat focal ischemia. Brain Res 723:228–230. 10.1016/0006-8993(96)00258-2 [DOI] [PubMed] [Google Scholar]
- Maugeri G, D’Amico AG, Rasà DM et al (2017) Caffeine prevents blood retinal barrier damage in a model, in vitro, of diabetic macular edema. J Cell Biochem 118:2371–2379. 10.1002/jcb.25899 [DOI] [PubMed] [Google Scholar]
- Mayor D, Tymianski M (2018) Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology 134:178–188. 10.1016/j.neuropharm.2017.11.050 [DOI] [PubMed] [Google Scholar]
- McIntyre S, Taitz D, Keogh J et al (2013) A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol 55:499–508. 10.1111/dmcn.12017 [DOI] [PubMed] [Google Scholar]
- Melani A, Pantoni L, Bordoni F et al (2003) The selective A2Areceptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res 959:243–250. 10.1016/S0006-8993(02)03753-8 [DOI] [PubMed] [Google Scholar]
- Melani A, Amadio S, Gianfriddo M et al (2006a) P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 26:974–982. 10.1038/sj.jcbfm.9600250 [DOI] [PubMed] [Google Scholar]
- Melani A, Gianfriddo M, Vannucchi MG et al (2006b) The selective A2Areceptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res 1073–1074:470–480. 10.1016/j.brainres.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Melani A, Cipriani S, Vannucchi MG et al (2009) Selective adenosine A2a receptor antagonism reduces JNK activation in oligodendrocytes after cerebral ischaemia. Brain 132:1480–1495. 10.1093/brain/awp076 [DOI] [PubMed] [Google Scholar]
- Melani A, Corti F, Stephan H et al (2012) Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Exp Neurol 233:193–204. 10.1016/j.expneurol.2011.09.036 [DOI] [PubMed] [Google Scholar]
- Melani A, Corti F, Cellai L et al (2014a) Low doses of the selective adenosine A2Areceptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res 1551:59–72. 10.1016/j.brainres.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Melani A, Pugliese AM, Pedata F (2014b) Adenosine receptors in cerebral ischemia. Int Rev Neurobiol 119:309–348. 10.1016/B978-0-12-801022-8.00013-1 [DOI] [PubMed] [Google Scholar]
- Melani A, Dettori I, Corti F et al (2015) Time-course of protection by the selective A2A receptor antagonist SCH58261 after transient focal cerebral ischemia. Neurol Sci 36:1441–1448. 10.1007/s10072-015-2160-y [DOI] [PubMed] [Google Scholar]
- Mendonca H, Carpi-Santos R, Da Costa CK, Blanco Martinez A (2020) Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res 15:625–635. 10.4103/1673-5374.266910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Borea PA, Stefanelli A et al (2015) A2a and a2b adenosine receptors affect HIF-1α signaling in activated primary microglial cells. Glia 63:1933–1952. 10.1002/glia.22861 [DOI] [PubMed] [Google Scholar]
- Merighi S, Bencivenni S, Vincenzi F et al (2017) A2B adenosine receptors stimulate IL-6 production in primary murine microglia through p38 MAPK kinase pathway. Pharmacol Res 117:9–19. 10.1016/j.phrs.2016.11.024 [DOI] [PubMed] [Google Scholar]
- Miller LP, Jelovich LA, Yao L et al (1996) Pre-and peristroke treatment with the adenosine kinase inhibitor, 5′-deoxyiodotubercidin, significantly reduces infarct volume after temporary occlusion of the middle cerebral artery in rats. Neurosci Lett 220:73–76. 10.1016/S0304-3940(96)13234-1 [DOI] [PubMed] [Google Scholar]
- Milton SL, Nayak G, Kesaraju S et al (2007) Suppression of reactive oxygen species production enhances neuronal survival in vitro and in vivo in the anoxia-tolerant turtle Trachemys scripta. J Neurochem 101:993–1001. 10.1111/j.1471-4159.2007.04466.x [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Knight CA, Hockenberry J et al (2014) Beverage caffeine intakes in the U.S. Food Chem Toxicol 63:136–142. 10.1016/j.fct.2013.10.042 [DOI] [PubMed] [Google Scholar]
- Mohamed RA, Agha AM, Abdel-Rahman AA, Nassar NN (2016) Role of adenosine A2A receptor in cerebral ischemia reperfusion injury: signaling to phosphorylated extracellular signal-regulated protein kinase (pERK1/2). Neuroscience 314:145–159. 10.1016/j.neuroscience.2015.11.059 [DOI] [PubMed] [Google Scholar]
- Molz S, Olescowicz G, Kraus JR et al (2015) Purine receptors are required for DHA-mediated neuroprotection against oxygen and glucose deprivation in hippocampal slices. Purinergic Signal 11:117–126. 10.1007/s11302-014-9438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momoi N, Tinney JP, Liu LJ et al (2008) Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. Am J Physiol Heart Circ Physiol 294:H2248–H2256. 10.1152/ajpheart.91469.2007 [DOI] [PubMed] [Google Scholar]
- Monopoli A, Lozza G, Forlani A et al (1998) Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. NeuroReport 9:3955–3959. 10.1097/00001756-199812010-00034 [DOI] [PubMed] [Google Scholar]
- Mori M, Nishizaki T, Okada Y (1992) Protective effect of adenosine on the anoxic damage of hippocampal slice. Neuroscience 46:301–307. 10.1016/0306-4522(92)90052-4 [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67:181–198. 10.1016/j.neuron.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon-Emre I, Schlichter LC (2010) Evolution of inflammation and white matter injury in a model of transient focal ischemia. J Neuropathol Exp Neurol 69:1–15. 10.1097/NEN.0b013e3181c3ce6c [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS et al (2015) Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131:e29-322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Yamashita T, Li Q et al (2017) Time-dependent change of in vivo optical imaging of oxidative stress in a mouse stroke model. J Neurosci Res 95:2030–2039. 10.1002/jnr.24047 [DOI] [PubMed] [Google Scholar]
- Nam Han Cho et al. (2017) International Diabetes Federation Atlas eighth edition 2017. ISBN: 978-2-930229-87-4
- Nehlig A (2018) Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev 70:384–411. 10.1124/pr.117.014407 [DOI] [PubMed] [Google Scholar]
- Nelson KB, Grether JK, Dambrosia JM et al (2003) Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res 53:600–607. 10.1203/01.PDR.0000056802.22454.AB [DOI] [PubMed] [Google Scholar]
- Newman GC, Hospod FE, Trowbridge SD et al (1998) Restoring adenine nucleotides in a brain slice model of cerebral reperfusion. J Cereb Blood Flow Metab 18:675–685. 10.1097/00004647-199806000-00010 [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Sihra TS, Sanchez-Prieto J (1987) Calcium-dependent and-independent release of glutamate from synaptosomes monitored by continuous fluorometry. J Neurochem 49:50–57. 10.1111/j.1471-4159.1987.tb03393.x [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Nagai K, Nomura T et al (2002) A new neuromodulatory pathway with a glial contribution mediated via A2a adenosine receptors. Glia 39:133–147. 10.1002/glia.10100 [DOI] [PubMed] [Google Scholar]
- O’Donnell MJ, Xavier D, Liu L et al (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet (London, England) 376:112–123. 10.1016/S0140-6736(10)60834-3 [DOI] [PubMed] [Google Scholar]
- Ola MS, Alhomida AS, LaNoue KF (2019) Gabapentin attenuates oxidative stress and apoptosis in the diabetic rat retina. Neurotox Res 36:81–90. 10.1007/s12640-019-00018-w [DOI] [PubMed] [Google Scholar]
- Olsson T, Cronberg T, Rytter A et al (2004) Deletion of the adenosine A1 receptor gene does not alter neuronal damage following ischaemia in vivo or in vitro. Eur J Neurosci 20:1197–1204. 10.1111/j.1460-9568.2004.03564.x [DOI] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li X-J et al (2009) Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci 12:872–878. 10.1038/nn.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B (2015) Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun 460:72–81. 10.1016/j.bbrc.2015.01.137 [DOI] [PubMed] [Google Scholar]
- Osborne NN, Melena J, Chidlow G, Wood JPM (2001) A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol 85:1252–1259. 10.1136/bjo.85.10.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JPM et al (2004) Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 23:91–147. 10.1016/j.preteyeres.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Nguyen-huynh MN (2011) Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 10.1007/s13311-011-0053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B, Goldstein LB, Higashida RT et al (2013) Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 44:2361–2375. 10.1161/STR.0b013e31829734f2 [DOI] [PubMed] [Google Scholar]
- Paolo T (2012) The high-risk newborns. J Matern Neonatal Med 25:6–7. 10.3109/14767058.2012.664893 [DOI] [PubMed] [Google Scholar]
- Park CK, Rudolphi KA (1994) Antiischemic effects of propentofylline (HWA 285) against focal cerebral infarction in rats. Neurosci Lett 178:235–238. 10.1016/0304-3940(94)90767-6 [DOI] [PubMed] [Google Scholar]
- Pasquale LR, Wiggs JL, Willett WC, Kang JH (2012) The relationship between caffeine and coffee consumption and exfoliation glaucoma or glaucoma suspect: a prospective study in two cohorts. Invest Ophthalmol Vis Sci 53:6427–6433. 10.1167/iovs.12-10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedata F, Latini S, Pugliese AM, Pepeu G (1993) Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J Neurochem 61:284–289. 10.1111/j.1471-4159.1993.tb03566.x [DOI] [PubMed] [Google Scholar]
- Pedata F, Dettori I, Coppi E et al (2016) Purinergic signalling in brain ischemia. Neuropharmacology 104:105–130. 10.1016/j.neuropharm.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Pennington KL, DeAngelis MM (2016) Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis 3:1–20. 10.1186/s40662-016-0063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG (2011) Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflamm 8:174. 10.1186/1742-2094-8-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MR, Hang VR, Vardiero E et al (2010) Modulation of A1 adenosine receptor expression by cell aggregation and long-term activation of A2a receptors in cultures of avian retinal cells: involvement of the cyclic AMP/PKA pathway. J Neurochem 113:661–673. 10.1111/j.1471-4159.2010.06641.x [DOI] [PubMed] [Google Scholar]
- Pereira-Figueiredo D, Brito R, Araújo DSM et al (2020) Caffeine exposure ameliorates acute ischemic cell death in avian developing retina. Purinergic Signal 16:41–59. 10.1007/s11302-020-09687-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman JM (2006) Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther 28:1353–1365. 10.1016/j.clinthera.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Petrovic-Djergovic D, Hyman MC, Ray JJ et al (2012) Tissue-resident ecto-5′ nucleotidase (CD73) regulates leukocyte trafficking in the ischemic brain. J Immunol 188:2387–2398. 10.4049/jimmunol.1003671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pforte C, Henrich-Noack P, Baldauf K, Reymann KG (2005) Increase in proliferation and gliogenesis but decrease of early neurogenesis in the rat forebrain shortly after transient global ischemia. Neuroscience 136:1133–1146. 10.1016/j.neuroscience.2005.08.043 [DOI] [PubMed] [Google Scholar]
- Phillis JW (1995) The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res 705:79–84. 10.1016/0006-8993(95)01153-6 [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Kimelberg HK (1998) Adenosine receptor mediated stimulation of intracellular calcium in acutely isolated astrocytes. Brain Res 798:294–303. 10.1016/S0006-8993(98)00430-2 [DOI] [PubMed] [Google Scholar]
- Popoli P, Pepponi R (2012) Potential therapeutic relevance of adenosine A2B and A2A receptors in the central nervous system. CNS Neurol Disord 11:664–674. 10.2174/187152712803581100 [DOI] [PubMed] [Google Scholar]
- Portugal CC, da Encarnação TG, Sagrillo MA, Pereira MR, Relvas JB, Socodato R, Paes-de-Carvalho R (2021) Activation of adenosine A3 receptors regulates vitamin C transport and redox balance in neurons. Free Radic Biol Med 163:43–55. 10.1016/j.freeradbiomed.2020.11.039 [DOI] [PubMed] [Google Scholar]
- Potter M, Rosenkrantz T, Fitch RH (2018) Behavioral and neuroanatomical outcomes in a rat model of preterm hypoxic-ischemic brain injury: effects of caffeine and hypothermia. Int J Dev Neurosci 70:46–55. 10.1016/j.ijdevneu.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Coppi E, Volpini R et al (2007) Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration. Biochem Pharmacol 74:768–779. 10.1016/j.bcp.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese AM, Traini C, Cipriani S et al (2009) The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices. Br J Pharmacol 157:818–830. 10.1111/j.1476-5381.2009.00218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundik S, Robinson S, Lust WD et al (2006) Regional metabolic status of the E-18 rat fetal brain following transient hypoxia/ischemia. Metab Brain Dis 21:309–317. 10.1007/s11011-006-9031-4 [DOI] [PubMed] [Google Scholar]
- Quigley H, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267. 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quimson SK (2015) Retinopathy of prematurity: pathogenesis and current treatment options. Neonatal Netw 34:284–287. 10.1891/0730-0832.34.5.284 [DOI] [PubMed] [Google Scholar]
- Rabinovich SS, Seledtsov VI, Banul NV et al (2005) Cell therapy of brain stroke. Bull Exp Biol Med 139:126–128. 10.1007/s10517-005-0229-y [DOI] [PubMed] [Google Scholar]
- Rajsic S, Gothe H, Borba HH et al (2019) Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ 20:107–134. 10.1007/s10198-018-0984-0 [DOI] [PubMed] [Google Scholar]
- Razeghinejad MR, Spaeth GL (2011) A history of the surgical management of glaucoma. Optom Vis Sci 88:E39-47. 10.1097/OPX.0b013e3181fe2226 [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N (2007) Dose translation from animal to human studies revisited. FASEB J 22:659–661. 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- Redondo B, Vera J, Molina R, Jiménez R (2020) Short-term effects of caffeine intake on anterior chamber angle and intraocular pressure in low caffeine consumers. Graefe’s Arch Clin Exp Ophthalmol 258:613–619. 10.1007/s00417-019-04556-z [DOI] [PubMed] [Google Scholar]
- Reece TB, Kron IL, Okonkwo DO et al (2006) Functional and cytoarchitectural spinal cord protection by ATL-146e after ischemia/reperfusion is mediated by adenosine receptor agonism. J Vasc Surg 44:392–397. 10.1016/j.jvs.2006.04.032 [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D (2011) The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci 29:551–563. 10.1016/j.ijdevneu.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G et al (2008) Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7:915–926. 10.1016/S1474-4422(08)70193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Bekkers JM, Clements JD (2003) Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci 26:683–687. 10.1016/j.tins.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Rein DB, Zhang P, Wirth KE et al (2006) The economic burden of major adult visual disorders in the United States. Arch Ophthalmol 124:1754–1760. 10.1001/archopht.124.12.1754 [DOI] [PubMed] [Google Scholar]
- Rezar-Dreindl S, Eibenberger K, Buehl W et al (2017) Role of additional dexamethasone for the management of persistent or recurrent neovascular agerelated macular degeneration under ranibizumab treatment. Retina 37:962–970. 10.1097/IAE.0000000000001264 [DOI] [PubMed] [Google Scholar]
- Rhee J, Kim R, Kim Y et al (2015) Maternal caffeine consumption during pregnancy and risk of low birth weight: a dose-response meta-analysis of observational studies. PLoS ONE 10:1–18. 10.1371/journal.pone.0132334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM (2010) Caffeine and adenosine. J Alzheimer’s Dis. 10.3233/JAD-2010-1379 [DOI] [PubMed] [Google Scholar]
- Ribeiro FF, Neves-Tomé R, Assaife-Lopes N et al (2016) Axonal elongation and dendritic branching is enhanced by adenosine A2A receptors activation in cerebral cortical neurons. Brain Struct Funct 221:2777–2799. 10.1007/s00429-015-1072-1 [DOI] [PubMed] [Google Scholar]
- Rivera-Oliver M, Díaz-Ríos M (2014) Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: a review. Life Sci 101:1–9. 10.1016/j.lfs.2014.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CMT, Perlman M (2006) Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr Child Health (Oxford) 11:278–282. 10.1093/pch/11.5.278 [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Morris J, Pikula A (2008) Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis 2:287–303. 10.1177/1753944708093847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K et al (2008) Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117:e25-146. 10.1161/CIRCULATIONAHA.107.187998 [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Silver LE et al (2005) Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet (London, England) 366:1773–1783. 10.1016/S0140-6736(05)67702-1 [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Keil M, Fastbom J, Fredholm BB (1989) Ischaemic damage in gerbil hippocampus is reduced following upregulation of adenosine (A1) receptors by caffeine treatment. Neurosci Lett 103:275–280. 10.1016/0304-3940(89)90112-2 [DOI] [PubMed] [Google Scholar]
- Saaddine JB, Honeycutt AA, Narayan KMV et al (2008) Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005–2050. Arch Ophthalmol (Chicago, Ill 1960) 126:1740–1747. 10.1001/archopht.126.12.1740 [DOI] [PubMed] [Google Scholar]
- Safanelli J, Vieira LGDR, de Araujo T et al (2019) The cost of stroke in a public hospital in Brazil: a one-year prospective study. Arq Neuropsiquiatr 77:404–411. 10.1590/0004-282X20190059 [DOI] [PubMed] [Google Scholar]
- Sahir N, Bahi N, Evrard P, Gressens P (2000) Caffeine induces in vivo premature appearance of telencephalic vesicles. Dev Brain Res 121:213–217. 10.1016/S0165-3806(00)00037-7 [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yonoki Y, Kuwagata M, Saito M, Nakahara T, Ishii K (2004) Histological protection against ischemia-reperfusion injury by early ischemic preconditioning in rat retina. Brain Res 1015(1–2):154–160. 10.1016/j.brainres.2004.04.074 [DOI] [PubMed] [Google Scholar]
- Schlottmann PG, Alezzandrini AA, Zas M et al (2017) New treatment modalities for neovascular age-related macular degeneration. Asia-Pac J Ophthalmol 6:514–519. 10.22608/APO.2017258 [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P et al (2006) Caffeine therapy for apnea of prematurity. N Engl J Med 354:2112–2121. 10.1056/NEJMoa054065 [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P et al (2007) Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357:1893–1902. 10.1056/NEJMoa073679 [DOI] [PubMed] [Google Scholar]
- Schmidt K-G, Bergert H, Funk R (2008) Neurodegenerative diseases of the retina and potential for protection and recovery. Curr Neuropharmacol 6:164–178. 10.2174/157015908784533851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreglmann M, Ground A, Vollmer B, Johnson MJ (2020) Systematic review: long-term cognitive and behavioural outcomes of neonatal hypoxic–ischaemic encephalopathy in children without cerebral palsy. Acta Paediatr Int J Paediatr 109:20–30. 10.1111/apa.14821 [DOI] [PubMed] [Google Scholar]
- Sebastiäo AM, De Mendonça A, Moreira T, Alexandre Ribeiro J (2001) Activation of synaptic NMDA receptors by action potential-dependent release of transmitter during hypoxia impairs recovery of synaptic transmission on reoxygenation. J Neurosci 21:8564–8571. 10.1523/jneurosci.21-21-08564.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA (2009) Triggering neurotrophic factor actions through adenosine A2A receptor activation: implications for neuroprotection. Br J Pharmacol 158:15–22. 10.1111/j.1476-5381.2009.00157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selakovic V, Korenic A, Radenovic L (2011) Spatial and temporal patterns of oxidative stress in the brain of gerbils submitted to different duration of global cerebral ischemia. Int J Dev Neurosci 29:645–654. 10.1016/j.ijdevneu.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Sengpiel V, Elind E, Bacelis J et al (2013) Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med. 10.1186/1741-7015-11-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydyousefi M, Moghanlou AE, Metz GAS et al (2019) Exogenous adenosine facilitates neuroprotection and functional recovery following cerebral ischemia in rats. Brain Res Bull 153:250–256. 10.1016/j.brainresbull.2019.09.010 [DOI] [PubMed] [Google Scholar]
- Shalak L, Perlman JM (2004) Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev 80:125–141. 10.1016/j.earlhumdev.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Sheth S, Brito R, Mukherjea D et al (2014) Adenosine receptors: expression, function and regulation. Int J Mol Sci 15:2024–2052. 10.3390/ijms15022024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields MB (2008) Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol 19:85–88. 10.1097/ICU.0b013e3282f3919b [DOI] [PubMed] [Google Scholar]
- Shrivastava K, Llovera G, Recasens M et al (2013) Temporal expression of cytokines and signal transducer and activator of transcription factor 3 activation after neonatal hypoxia/ischemia in mice. Dev Neurosci 35:212–225. 10.1159/000348432 [DOI] [PubMed] [Google Scholar]
- Simbruner G, Mittal RA, Rohlmann F, Muche R (2010) Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 126:e771–e778. 10.1542/peds.2009-2441 [DOI] [PubMed] [Google Scholar]
- Simó R, Stitt AW, Gardner TW (2018) Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61:1902–1912. 10.1007/s00125-018-4692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W, Assink J, Klein R et al (2001) Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 108:697–704. 10.1016/S0161-6420(00)00580-7 [DOI] [PubMed] [Google Scholar]
- Socodato R, Brito R, Calaza KC, Paes-De-Carvalho R (2011) Developmental regulation of neuronal survival by adenosine in the in vitro and in vivo avian retina depends on a shift of signaling pathways leading to CREB phosphorylation or dephosphorylation. J Neurochem 116:227–239. 10.1111/j.1471-4159.2010.07096.x [DOI] [PubMed] [Google Scholar]
- Socodato R, Portugal CC, Domith I et al (2015) c-Src function is necessary and sufficient for triggering microglial cell activation. Glia 63:497–511. 10.1002/glia.22767 [DOI] [PubMed] [Google Scholar]
- Soler EP, Ruiz VC (2010) Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev 6:138–149. 10.2174/157340310791658785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AG, Da Costa THM (2015) Usual coffee intake in Brazil: results from the National Dietary Survey 2008–9. Br J Nutr 113:1615–1620. 10.1017/S0007114515000835 [DOI] [PubMed] [Google Scholar]
- Stavric B (1988) Methylxanthines: toxicity to humans. 1. Theophylline. Food Chem Toxicol 26:541–565. 10.1016/0278-6915(88)90007-5 [DOI] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J et al (2002) The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res 932:110–119. 10.1016/S0006-8993(02)02292-8 [DOI] [PubMed] [Google Scholar]
- Stewart MW (2016) Treatment of diabetic retinopathy: recent advances and unresolved challenges. World J Diabetes 7:333. 10.4239/wjd.v7.i16.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilley CS, Ryan CM, Kondziolka D et al (2004) Changes in cognitive function after neuronal cell transplantation for basal ganglia stroke. Neurology 63:1320–1322. 10.1212/01.wnl.0000140700.44904.53 [DOI] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M et al (2016) The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51:156–186. 10.1016/j.preteyeres.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Stockwell J, Chen Z, Niazi M et al (2016) Protein phosphatase role in adenosine A1 receptor-induced AMPA receptor trafficking and rat hippocampal neuronal damage in hypoxia/reperfusion injury. Neuropharmacology 102:254–265. 10.1016/j.neuropharm.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M (2002) Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol 513:87–113. 10.1007/978-1-4615-0123-7_3 [DOI] [PubMed] [Google Scholar]
- Sufianova GZ, Sufianov AA, Shapkin AG (2014) Effect of cyclopentyladenosine on lipid peroxidation during focal cerebral ischemia. Bull Exp Biol Med 157:228–230. 10.1007/s10517-014-2531-z [DOI] [PubMed] [Google Scholar]
- Supuran CT (2019) Agents for the prevention and treatment of age-related macular degeneration and macular edema: a literature and patent review. Expert Opin Ther Pat 29:761–767. 10.1080/13543776.2019.1671353 [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Peeling J, Lesiuk HJ et al (1991) The effects of caffeine on ischemic neuronal injury as determined by magnetic resonance imaging and histopathology. Neuroscience 42:171–182. 10.1016/0306-4522(91)90157-J [DOI] [PubMed] [Google Scholar]
- Szabadfi K, Mester L, Reglodi D et al (2010) Novel neuroprotective strategies in ischemic retinal lesions. Int J Mol Sci 11:544–561. 10.3390/ijms11020544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Otsuguro K, Ohta T, Ito S (2010) Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br J Pharmacol 161:1806–1816. 10.1111/j.1476-5381.2010.01002.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J et al (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281:21362–21368. 10.1074/jbc.M600504200 [DOI] [PubMed] [Google Scholar]
- Tan Y, Liu J, Deng Y et al (2012) Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicol Lett 214:279–287. 10.1016/j.toxlet.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Tanaka E, Yasumoto S, Hattori G et al (2001) Mechanisms underlying the depression of evoked fast EPSCs following in vitro ischemia in rat hippocampal CA1 neurons. J Neurophysiol 86:1095–1103. 10.1152/jn.2001.86.3.1095 [DOI] [PubMed] [Google Scholar]
- Tarr JM, Kaul K, Wolanska K et al (2012) Retinopathy in diabetes. Adv Exp Med Biol 771:88–106. 10.1007/978-1-4614-5441-0_10 [DOI] [PubMed] [Google Scholar]
- Tatlisumak T, Takano K, Carano RAD et al (1998) Delayed treatment with an adenosine kinase inhibitor, GP683, attenuates infarct size in rats with temporary middle cerebral artery occlusion. Stroke 29:1952–1958. 10.1161/01.STR.29.9.1952 [DOI] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Potenza RL et al (2008) Adenosine A2A receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J Neurochem 104:279–286. 10.1111/j.1471-4159.2007.05046.x [DOI] [PubMed] [Google Scholar]
- Tezel G (2006) Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 25:490–513. 10.1016/j.preteyeres.2006.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY et al (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Ting DS, Cheung GC, Wong TY (2016) Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 44(4):260–277. 10.1111/ceo.12696 [DOI] [PubMed] [Google Scholar]
- Traverso CE, Walt JG, Kelly SP et al (2005) Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol 89:1245–1249. 10.1136/bjo.2005.067355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CP, Seli M, Ment L et al (2003) A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci USA 100:11718–11722. 10.1073/pnas.1931975100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye K, Pollard I, Karlsson L et al (1993) Caffeine exposure in utero increases the incidence of apnea in adult rats. Reprod Toxicol 7:449–452. 10.1016/0890-6238(93)90089-p [DOI] [PubMed] [Google Scholar]
- Vakalis N, Echiadis G, Pervena A et al (2015) Intravitreal combination of dexamethasone sodium phosphate and bevacizumab in the treatment of exudative AMD. Sci Rep. 10.1038/srep08627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel M, Swaab H, De Vries LS, Jongmans MJ (2007) Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr 166:645–654. 10.1007/s00431-007-0437-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC (2000) Hypoxic-ischemic encephalopathy. Am J Perinatol 17:113–120. 10.1055/s-2000-9293 [DOI] [PubMed] [Google Scholar]
- Vaz SH, Lérias SR, Parreira S et al (2015) Adenosine A2A receptor activation is determinant for BDNF actions upon GABA and glutamate release from rat hippocampal synaptosomes. Purinergic Signal 11:607–612. 10.1007/s11302-015-9476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio EA, White PJ, May LT (2019) The adenosine A2B G protein-coupled receptor: recent advances and therapeutic implications. Pharmacol Ther 198:20–33. 10.1016/j.pharmthera.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Vera J, Redondo B, Molina R et al (2019) Effects of caffeine on intraocular pressure are subject to tolerance: a comparative study between low and high caffeine consumers. Psychopharmacology 236:811–819. 10.1007/s00213-018-5114-2 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Shmigol A (1996) Calcium-induced calcium release in neurones. Cell Calcium 19:1–14. 10.1016/S0143-4160(96)90009-3 [DOI] [PubMed] [Google Scholar]
- Vermeij J-D, Westendorp WF, van de Beek D, Nederkoorn PJ (2018) Post-stroke infections and preventive antibiotics in stroke: update of clinical evidence. Int J Stroke 13:913–920. 10.1177/1747493018798557 [DOI] [PubMed] [Google Scholar]
- Vieira LGDR, Safanelli J, De AT et al (2019) The cost of stroke in private hospitals in Brazil: a one-year prospective study. Arq Neuropsiquiatr 77:393–403. 10.1590/0004-282x20190056 [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2003) Cerebral white matter injury of the premature infant-more common than you think. Pediatrics 112:176–180. 10.1542/peds.112.1.176 [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2012) Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol 72:156–166. 10.1002/ana.23647 [DOI] [PubMed] [Google Scholar]
- von Lubitz DKJE, Marangos PJ (1990) Cerebral ischemia in gerbils: postischemic administration of cyclohexyl adenosine and 8-sulfophenyl-theophylline. J Mol Neurosci 2:53–59. 10.1007/BF02896926 [DOI] [PubMed] [Google Scholar]
- von Lubitz DK, Dambrosia JM, Kempski O, Redmond DJ (1988) Cyclohexyl adenosine protects against neuronal death following ischemia in the CA1 region of gerbil hippocampus. Stroke 19:1133–1139. 10.1161/01.str.19.9.1133 [DOI] [PubMed] [Google Scholar]
- Von Lubitz DK, Lin RC, Melman N et al (1994a) Chronic administration of selective adenosine A1 receptor agonist or antagonist in cerebral ischemia. Eur J Pharmacol 256:161–167. 10.1016/0014-2999(94)90241-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Lin RCS, Popik P et al (1994b) Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol 263:59–67. 10.1016/0014-2999(94)90523-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DK, Lin RC, Jacobson KA (1995) Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol 287:295–302. 10.1016/0014-2999(95)00498-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DK, Beenhakker M, Lin RC et al (1996) Reduction of postischemic brain damage and memory deficits following treatment with the selective adenosine A1 receptor agonist. Eur J Pharmacol 302:43–48. 10.3171/2009.11.JNS081052.Molecular [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DK, Simpson KL, Lin RC (2001) Right thing at a wrong time? Adenosine A3 receptors and cerebroprotection in stroke. Ann N Y Acad Sci 939:85–96. 10.1111/j.1749-6632.2001.tb03615.x [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Murray V, Berge E, del Zoppo GJ (2014) Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 10.1002/14651858.CD000213.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X, Odouli R, Li DK (2008) Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol 198:279.e1-279.e8. 10.1016/j.ajog.2007.10.803 [DOI] [PubMed] [Google Scholar]
- Weston RM, Jones NM, Jarrott B, Callaway JK (2007) Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J Cereb Blood Flow Metab 27:100–114. 10.1038/sj.jcbfm.9600324 [DOI] [PubMed] [Google Scholar]
- Winerdal M, Winerdal ME, Wang Y-Q et al (2016) Adenosine A1 receptors contribute to immune regulation after neonatal hypoxic ischemic brain injury. Purinergic Signal 12:89–101. 10.1007/s11302-015-9482-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winerdal M, Urmaliya V, Winerdal ME et al (2017) Single dose caffeine protects the neonatal mouse brain against hypoxia ischemia. PLoS ONE 12:1–7. 10.1371/journal.pone.0170545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, D’Agostino RB, O’Neal MA et al (1992) Secular trends in stroke incidence and mortality. The Framingham Study. Stroke 23:1551–1555. 10.1161/01.str.23.11.1551 [DOI] [PubMed] [Google Scholar]
- Won SJ, Kim DY, Gwag BJ (2002) Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol 35:67–86. 10.5483/bmbrep.2002.35.1.067 [DOI] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106–e116. 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- Wong MS, Huang P, Li W et al (2015) T-Helper1/T-Helper2 cytokine imbalance in the iris of patients with glaucoma. PLoS ONE 10:1–16. 10.1371/journal.pone.0122184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Cheung CMG, Larsen M et al (2016) Diabetic retinopathy. Nat Rev Dis Prim 2:16012. 10.1038/nrdp.2016.12 [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT (2010) Energy metabolism of the visual system. Eye Brain 2:99–116. 10.2147/EB.S9078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lee MR, Kim T et al (2011) Regulation of ethanol-sensitive EAAT2 expression through adenosine A1 receptor in astrocytes. Biochem Biophys Res Commun 406:47–52. 10.1016/j.bbrc.2011.01.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu Y-H, Chen J-F, Schwarzschild MA (2010) Neuroprotection by caffeine: time course and role of its metabolites in the MPTP model of Parkinson’s disease. Neuroscience 167:475–481. 10.1016/j.neuroscience.2010.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhang B, Liang G et al (2012) Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS ONE. 10.1371/journal.pone.0044497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Lai MC (2011) Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol. 10.1155/2011/609813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG et al (2006) The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 116:1913–1923. 10.1172/JCI27933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Wang B, Kwansa H et al (2013) Adenosine A2A receptor contributes to ischemic brain damage in newborn piglet. J Cereb Blood Flow Metab 33:1612–1620. 10.1038/jcbfm.2013.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JWY, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XC, Hu JX, Li L et al (2018) Astrocytic Lrp4 (Low-density lipoprotein receptor–related protein 4) contributes to ischemia-induced brain injury by regulating ATP release and adenosine-A2AR (Adenosine A2A Receptor) signaling. Stroke 49:165–174. 10.1161/STROKEAHA.117.018115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JJ, Danesh-Meyer HV (2019) Caffeine and the eye. Surv Ophthalmol 64:334–344. 10.1016/j.survophthal.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ (2001) Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res 20:175–208. 10.1016/s1350-9462(00)00027-6 [DOI] [PubMed] [Google Scholar]
- Yu Y, Chen H, Su SB (2015) Neuroinflammatory responses in diabetic retinopathy. J Neuroinflamm 12:1–15. 10.1186/s12974-015-0368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang X, Kang F et al (2019) Propofol attenuates inflammatory damage on neurons following cerebral infarction by inhibiting excessive activation of microglia. Int J Mol Med 43:452–460. 10.3892/ijmm.2018.3974 [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Nicklas WJ (1992) Evidence that the loss of the voltage-dependent Mg2+ block at the N-methyl-d-aspartate receptor underlies receptor activation during inhibition of neuronal metabolism. J Neurochem 59:1211–1220. 10.1111/j.1471-4159.1992.tb08430.x [DOI] [PubMed] [Google Scholar]
- Zhang X, Zeng H, Bao S et al (2014) Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci 4:1–14. 10.1186/2045-3701-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li H, Li B et al (2015) Adenosine A1 receptors selectively modulate oxygen-induced retinopathy at the hyperoxic and hypoxic phases by distinct cellular mechanisms. Invest Ophthalmol Vis Sci 56:8108–8119. 10.1167/iovs.15-17202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou R, Li B et al (2017) Caffeine preferentially protects against oxygen-induced retinopathy. FASEB J 31:3334–3348. 10.1096/fj.201601285R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao J, Wang R et al (2019) Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci Ther 25:1329–1342. 10.1111/cns.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao PY, Rahmathullah R, Stagg BC et al (2018) A worldwide price comparison of glaucoma medications, laser trabeculoplasty, and trabeculectomy surgery. JAMA Ophthalmol 136:1271–1279. 10.1001/jamaophthalmol.2018.3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Song WL, Xu YC et al (2015) Paeoniflorin ameliorates ischemic neuronal damage in vitro via adenosine A1receptor-mediated transactivation of epidermal growth factor receptor. Acta Pharmacol Sin 36:298–310. 10.1038/aps.2014.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A-M, Li W-B, Li Q-J et al (2004) A short cerebral ischemic preconditioning up-regulates adenosine receptors in the hippocampal CA1 region of rats. Neurosci Res 48:397–404. 10.1016/j.neures.2003.12.010 [DOI] [PubMed] [Google Scholar]
- Zhou R, Zhang S, Gu X et al (2018) Adenosine A2A receptor antagonists act at the hyperoxic phase to confer protection against retinopathy. Mol Med 24:1–13. 10.1186/s10020-018-0038-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zeng X, Li G et al (2019) Inactivation of endothelial adenosine A(2A) receptors protects mice from cerebral ischaemia-induced brain injury. Br J Pharmacol 176:2250–2263. 10.1111/bph.14673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin JA (2009) Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug Administration (FDA). Ann Neurol 66:6–10. 10.1002/ana.21750 [DOI] [PubMed] [Google Scholar]