Abstract
Ebola hemorrhagic fever is a severe, usually fatal illness caused by Ebola virus, a member of the filovirus family. The use of nonhomologous immune serum in animal studies and blood from survivors in two anecdotal reports of Ebola hemorrhagic fever in humans has shown promise, but the efficacy of these treatments has not been demonstrated definitively. We have evaluated the protective efficacy of polyclonal immune serum in a mouse model of Ebola virus infection. Our results demonstrate that mice infected subcutaneously with live Ebola virus survive infection and generate high levels of anti-Ebola virus immunoglobulin G (IgG). Passive transfer of immune serum from these mice before challenge protected upto 100% of naive mice against lethal Ebola virus infection. Protection correlated with the level of anti-Ebola virus IgG titers, and passive treatment with high-titer antiserum was associated with a delay in the peak of viral replication. Transfer of immune serum to SCID mice resulted in 100% survival after lethal challenge with Ebola virus, indicating that antibodies alone can protect from lethal disease. Thus antibodies suppress or delay viral growth, provide protection against lethal Ebola virus infection, and may not require participation of other immune components for protection.
Ebola hemorrhagic fever (EHF), a severe, fatal illness caused by the Ebola virus, is characterized in humans by a rapidly progressive multisystem failure. Significant outbreaks of EHF have occurred in Zaire (1976 and 1995), Sudan (1976 and 1979), Gabon (1996), and most recently, Uganda (2000). Widespread viral replication and lytic infection of various cells in the liver, kidneys, lungs, and spleen have been found in humans and experimental models of EHF using nonhuman primates (19). Because of the high morbidity and mortality associated with EHF and the occurrence of the disease in remote and poorly staffed and equipped health care settings, there has been keen interest in the development of treatment modalities that can be used in the field. Ribavirin, an antiviral drug that is effective in the treatment of several viral hemorrhagic fevers caused by members of the families Arenaviridae (4, 16, 17) and Bunyaviridae (5, 7, 22) appears to be ineffective against filoviruses (6, 9).
Convalescent-phase human serum has been successful in Argentine hemorrhagic fever (14) and has been used in the treatment of Ebola virus infections with limited success. One laboratorian, accidentally exposed to Ebola virus, recovered after treatment with immune serum (IS) and human interferon (3). Passive immunotherapy with convalescent-phase human blood was also attempted during the EHF outbreak in Kikwit in 1995 (18). Only one of nine patients who received convalescent-phase blood died (versus 80% overall mortality in the hospital). However, in this uncontrolled trial, most of the survivors received treatment more than 9 days after symptom onset, and several of them received additional blood transfusions and better than usual medical care during their hospital stay, making it difficult to evaluate the contribution of transfusions to their recovery (20). A panel of monoclonal antibodies (MAbs) isolated from a phage library constructed from RNA isolated from bone marrow cells from survivors of the 1995 Kikwit Ebola virus outbreak was found to have a low frequency of anti-glycoprotein (GP) monoclonal antibodies (MAbs) that neutralized Ebola virus in vitro (15).
DNA vaccination studies with full-length constructs of Ebola GP and secreted glycoprotein (sGP) have demonstrated protection against lethal challenge with Ebola virus (21, 24). In these studies, high titers of anti-GP and anti-sGP immunoglobulin G (IgG) were found to correlate with protection, although small numbers of animals and insufficient assessment of vaccination-induced T-cell responses make it difficult to evaluate the contribution of antibodies (Abs) in the protection. We used a mouse model of Ebola virus infection to investigate mechanisms of Ab-mediated protection against Ebola virus. Our data demonstrate that it is possible to confer protection against fatal infection with Ebola virus by transfer of polyclonal IS. However, Ab-mediated protection appears to act by delaying viral growth, thereby providing a window of opportunity for host innate or cellular immune mechanisms to act synergistically in viral clearance. Abs may also completely inhibit viral growth and protect against lethal infection in the absence of adaptive immune responses.
MATERIALS AND METHODS
Viruses, cells, and media.
A mouse-adapted strain of Ebola virus was derived from a 1976 isolate of the Zaire subtype by serial passage through progressively older suckling mice, followed by plaque purification as described elsewhere (2). Virus was amplified to a titer of 5 × 107 PFU/ml by one passage in Vero E6 (monkey kidney) cells. Vero E6 cells were obtained from the American Type Culture Collection and propagated in modified Eagle's medium supplemented with 2% fetal bovine serum, glutamine (2 mM; Life Technologies, Gaithersburg, Md.), streptomycin (100 μg/ml; Life Technologies), and penicillin (100 U/ml; Life Technologies). All infected samples and animals were handled under maximum containment in the biosafety level 4 (BSL-4) laboratory at the Centers of Disease Control and Prevention, Atlanta, Ga. All samples from the BSL-4 laboratory were gamma irradiated (5 × 106 rads) before further processing in BSL-2 and -3 conditions.
Quantitation of virus.
Virus was titrated by a standard plaque assay on Vero E6 cells (2). Briefly, virus-containing Vero E6 supernatants or samples were serially diluted (1:10) and incubated for 1 h at 37°C on confluent monolayers of Vero E6 cells in 35-mm-diameter-well plates. Three milliliters of 2× Basal Eagle's medium (Life Technologies) supplemented with 5% fetal bovine serum, antibiotics, HEPES, and l–glutamine mixed 1:1 with 2% SeaKem agarose (Sigma, St. Louis, Mo.) was added to each well and allowed to incubate for 6 days at 37°C (until the appearance of cytopathic effect). A second layer of basal Eagle's medium-agarose medium (2 ml) containing 2% neutral red (Life Technologies) was added, and plates were reincubated at 37°C for a another 24 to 48 h for to allow for the development of plaques.
Viral antigen assay.
Levels of circulating Ebola virus antigens (Ags) in sera and organs were estimated by using a capture enzyme-linked immunosorbent assay (ELISA) as described (10). Briefly, Flexiplates (Becton Dickinson, Bedford, Mass.) were coated overnight at 4°C with mouse ascites fluid (control wells) or wells containing a mixture of seven mouse MAbs raised against viral protein VP40, GP, and nucleoprotein (NP) from Zaire 1976 and Sudan 1976 strains of Ebola virus. Tissue and serum samples were serially diluted in 5% skim milk in phosphate-buffered saline (PBS; pH 7.2) with 0.1% Tween 20 (blocking buffer) and incubated at 37°C for 1 h. Captured Ag was detected by polyclonal anti-Ebola Virus Zaire serum produced in rabbits, followed by a goat anti-rabbit horseradish peroxidase conjugate and ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] substrate (both from Kirkegaard & Perry, Gaithersburg, Md.). The optical density at 410 nm (OD410) of each sample in control wells (coated with normal mouse ascites fluid) was subtracted from the OD of the same sample in the corresponding well coated with anti-Ebola virus MAbs to derive a corrected OD. The highest dilution of sample that resulted in a corrected OD of ≥0.1 was designated the viral Ag titer of the sample. Viral Ag titers were found to have a log-linear relationship with PFU values (data not shown) for values of ≥300 PFU. We used viral Ag titers to compare levels of virus in sera and tissues.
Anti-Ebola virus IgG detection by ELISA.
Anti-Ebola virus IgG titers were determined by ELISA as previously described (11, 12). Briefly, 96-well Falcon Flexiplates (Becton Dickinson) were coated overnight (4°C) with Ebola virus-infected or uninfected (control) Vero E6 cell lysates. Sera diluted serially in blocking buffer were incubated for 1 h at 37°C, and bound IgG was detected by addition of an anti-mouse horseradish peroxidase conjugate (Biosource International, Camarillo, Calif.) followed by ABTS substrate (Kirkegaard & Perry). Corrected OD410 values were calculated as done for the Ag assay above, and IgG titers were assigned for the highest serum dilution resulting in a corrected OD of ≥0.1.
Mice.
Six- to eight-week-old BALB/c male and female mice were obtained from commercial suppliers (Jackson Laboratory, Bar Harbor, Maine; Harlan Sprague-Dawley, Indianapolis, Ind.). T- and B-cell-deficient BALB/cj (SCID) mice aged 6 to 8 weeks were obtained from the Jackson Laboratory. All mice were housed under pathogen-free conditions and allowed to acclimate to the BSL-4 laboratory for 3 to 4 days before use in our experiments.
Immune serum.
To generate anti-Ebola virus IS, mice were infected subcutaneously (s.c.) with 100 PFU of Ebola virus followed by intraperitoneal (i.p.) rechallenge with 104 to 106 PFU of Ebola virus 3 weeks later. One to three weeks after i.p. challenge, sera were collected, pooled, γ irradiated (5 × 106 rads), and titrated for IgG by ELISA. The pooled serum from these mice had IgG titers ranging from 20,000 to 1,600,000.
Purification of IgG from IS.
Anti-Ebola virus IgG was purified from IS with a protein G-Sepharose affinity column (Pierce, Rockford, Ill.) following the procedure recommended by the manufacturer. Purified IgG was dialyzed against PBS (pH 7.2) and filter sterilized for injection. Anti-Ebola virus titers were determined by ELISA as described above. The titers of purified IgG ranged from 20,000 to 1,600,000.
Infection procedure.
Lethal Ebola virus infections were produced by i.p. inoculation of 10 to 103 PFU mouse-adapted Ebola virus (in 0.2 ml of PBS); for nonlethal infections, 100 PFU of mouse-adapted Ebola virus (in 0.2 ml of PBS) was given s.c. as previously described (2). After infection, mice were caged in groups of five, weighed daily or on alternate days, and observed for survival for at least 21 days. For serum transfer experiments, mice were given 1 ml of γ-irradiated, pooled IS, purified IgG from IS, or normal mouse serum (NMS) i.p. 1 day before or after i.p. challenge with Ebola virus. In some experiments, blood samples were collected via retro-orbital phlebotomy for determination of Ab and viral Ag titers prior to i.p. challenge with Ebola virus.
Statistics.
Comparisons of anti-Ebola virus Ab and Ag titers between experimental groups were done by Student's t test on log-transformed data. Animal weights and survival data were compared by analysis of variance. Differences between groups for categorical data were analyzed using Fisher's exact test. P values of <0.05 were designated the cutoff for statistical significance.
RESULTS
Mice immunized s.c. with live Ebola virus have high levels of anti-Ebola IgG and are protected against lethal rechallenge.
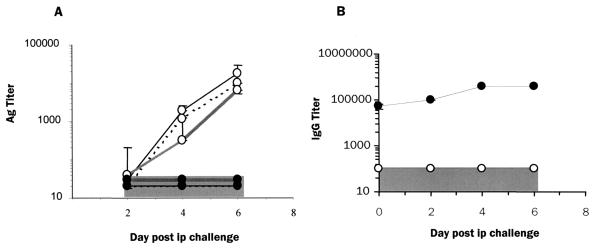
All adult BALB/c infected i.p. with the mouse-adapted Ebola virus died as reported previously (2). Signs of disease in i.p.-infected mice appeared from day 3 onward, when mice begin to lose weight and exhibit ruffled fur, huddling, and inactivity progressing to death between 6 and 8 days postinfection. In marked contrast, mice challenged s.c. showed no signs of illness, and all survived (data not shown). Furthermore, s.c.-infected mice (hereafter referred to as immune mice) were completely protected against i.p. rechallenge with doses as high as 106 PFU per mouse given 3 weeks after the primary infection (data not shown). Naive mice infected i.p. had a progressive increase in levels of viral Ag in their serum, spleen, liver, and kidneys (Fig. 1A) from day 2 postinfection until death, in contrast to immune mice, which had no detectable Ag in these tissues after i.p. challenge. Immune mice had high titers of anti-Ebola virus IgG Abs (range, 20 to 50,000) that were further elevated up to fourfold by i.p. rechallenge with Ebola virus (Fig. 1B). Anti-Ebola virus Ab titers declined from 100,000 to 100 by 32 weeks postinfection (data not shown). On Western blots, sera from immune mice detected bands representing GP, NP, VP35, VP24, and VP40 proteins, with a weaker recognition of GP compared to the other viral proteins (data not shown). In contrast, naive mice infected i.p. had undetectable levels of anti-Ebola virus IgG by ELISA until death around day 6 postinfection (Fig. 1B). These data are in agreement with patterns of Ab responses to Ebola virus reported for human disease (1, 11) in which survivors were found to have high titers of anti-Ebola virus IgG in the convalescent phase, whereas fatal disease was associated with low levels or absence of anti-Ebola virus Abs.
FIG. 1.
Subcutaneous infection with Ebola virus confers protection against lethal challenge in a mouse model. (A) Viral Ag levels in peripheral tissues and serum of naive (○) and immune (●) BALB/c mice following i.p. challenge. Three mice from each group were sacrificed on days 2, 4, and 6. Viral Ag levels measured by ELISA (see Materials and Methods) are shown for serum ( ), spleens (⋯), and livers (—). (B) Anti-Ebola virus IgG titers measured by ELISA in naive (○) and immune (●) mice following i.p. challenge (n = 3 per time point). Gray regions indicate the limit of detection in the Ab and Ag ELISAs.
), spleens (⋯), and livers (—). (B) Anti-Ebola virus IgG titers measured by ELISA in naive (○) and immune (●) mice following i.p. challenge (n = 3 per time point). Gray regions indicate the limit of detection in the Ab and Ag ELISAs.
Passive transfer of IS or IgG protects mice against lethal Ebola virus infection.
Studies of Ebola virus, infection in guinea pigs (24), nonhuman primates (9), and humans (1, 18) suggest an important role of Abs in protective immunity. However, studies using animal models to date have used nonhomologous serum in passive transfer experiments. We took advantage of the immune status of s.c.-infected mice to assess the ability of homologous IS to protect against lethal i.p. challenge with Ebola virus. Mice infected s.c. had high levels of anti-Ebola virus IgG that were boosted by i.p. challenge (Fig. 1B). Pooled serum from these mice was transferred to naive mice prior to lethal challenge with Ebola virus to assess the protective efficacy of IS. Mice that received NMS lost 15 to 25% of their original body weight during the course of infection, and all died 6 to 8 days postinfection (Fig. 2A and B). Immune serum recipients also showed signs of illness characterized by ruffling of fur and weight loss (10 to 15% of original body weight), but these changes occurred later than in NMS recipients, and a proportion of IS recipients recovered by days 12 to 14 postinfection (Fig. 2B). Fifty-six percent (18 of 32) of the mice that received IS survived i.p. infection with >30 times the 50% lethal dose of Ebola virus (Fig. 2A). In nine experiments done to evaluate the efficacy of immune serum transfer in protection against i.p. infection with Ebola virus, the survival among immune serum recipients ranged from 43 to 100%.
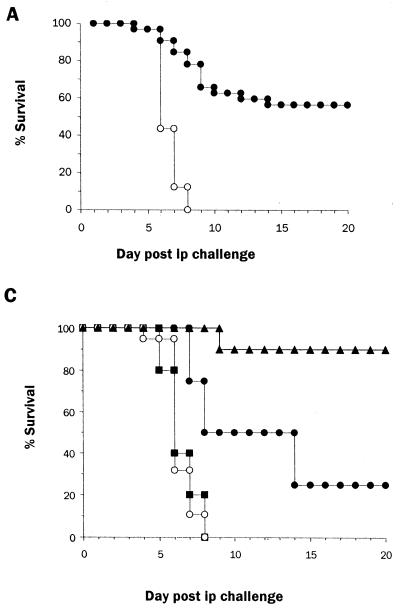
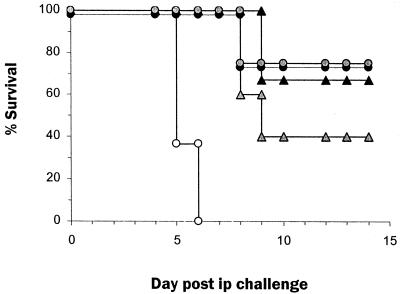
FIG. 2.
Protection of mice following passive transfer of IS correlates with anti-Ebola virus IgG titers. Pooled IS from s.c.-infected mice was irradiated, titered for anti-Ebola virus IgG (mean titer, 25,600), and administered i.p. (1 ml/mouse) 1 day before an i.p. challenge with a mouse-adapted Ebola virus (10 PFU/mouse). Irradiated NMS was administered (1 ml/mouse i.p.) to age-matched mice as controls. Circulating IgG titers estimated 1 day after serum transfer ranged from 100 to 6,400 in IS recipients. (A) Survival of mice that received NMS (n = 32; ○) or IS (n = 32; ●) before challenge with mouse-adapted Ebola virus. (B) Weight change in NMS (n = 32; ○) and IS (n = 20; ●) recipients that survived Ebola virus challenge during the course of infection. Each point represents the mean and standard error of the calculated percent prechallenge (day 0) weight for each mouse. (C) Survival of mice that received NMS (n = 19; ○) or IS resulting in IgG titers of 1:100 to 1:400 (n = 6; ■), 1:1,600 (n = 4; ●), or 1:6,400 (n = 9; ▴).
The variability in survival among IS recipients was further investigated by determination of anti-Ebola virus IgG titers resulting from serum transfer. Survival after IS transfer correlated with anti-Ebola IgG titers. Thus, serum titers below 100 to 400 resulted in no survivors among six mice (Fig. 2C), whereas titers of 1,600 resulted in a delay in death (mean time to death, 10.5 days in IS recipients versus 7 days in NMS recipients) and a 25% survival (one of four mice surviving) (Fig. 2C). In a representative experiment (Fig. 2C), posttransfer serum titers of ≥6,400 provided the highest protection, 89% (8 of 9 mice), with the only death occurring on day 9. Further, in a different experiment, all of five IS recipients with anti-Ebola virus IgG titers of >6,400, survived a lethal challenge with 103 PFU (30,000 times the 50% lethal dose) of Ebola virus. IS recipients that survived i.p. Ebola virus infection remained healthy and had no detectable viral Ags by day 25 postinfection (data not shown), and these survivors had anti-Ebola virus IgG titers (25,600 to 409,600) that were higher than those before challenge (100 to 6,400). IS recipients that survived the first i.p. infection were able to resist a subsequent lethal challenge with Ebola virus on day 30 postinfection (data not shown), indicating the development of protective immunity as seen with s.c. challenge with Ebola virus (Fig. 1). In repeated experiments we found that the titer required for protection against lethal infection with Ebola varied between different batches of pooled IS; however, in each experiment we were able to determine a titer above which complete protection was achieved.
To further confirm that the protection associated with IS is Ab mediated, we tested the protective efficacy of IgG purified from IS in naive mice. As shown in Fig. 3, 40% (two of five) mice that received purified IgG resulting in a circulating titer of <100,000 survived, compared to 75% (three of four) of IS recipients with anti-Ebola virus Ab titers of ≥100,000. The percentage of survivors increased with increasing posttransfer titers. Thus, 66% (two of three) of mice with titers of >400,000 survived, compared to 75% (three of four) of IS recipients with similar titers. Nevertheless, these data indicate that at equivalent titers, IgG provided levels protection similar to those associated with IS.
FIG. 3.
Transfer of purified IgG from IS protects mice from lethal Ebola virus infection. Shown is the survival of mice that received NMS (n = 12; ○) IS, with resulting titers of ≤1:100,000 (n = 4; ●) or ≥1:200,000 (n = 4; ●), or purified IgG with titers of ≤1:100,000 (n = 5;  ) or ≥1:200,000 (n = 3; ▴).
) or ≥1:200,000 (n = 3; ▴).
The ability of IS to protect mice after infection was also tested. IS provided complete protection from lethal Ebola virus infection (survival of all of six IS recipients, versus none of six NMS recipients) when given 1 day postinfection. In this experiment, all IS recipients had posttransfer serum anti-Ebola virus IgG titers of ≥6,400.
Ab-associated protection does not require complete suppression of viral replication.
Ab-mediated protection in this model could be operating either by complete inhibition of viral replication or by simply delaying the rate of viral growth. To investigate these possibilities, we determined the relationship between viral Ag titers and survival in IS recipients. Two of nine IS recipients had high levels of viral Ags in serum (range, 640 to 2,560 [Fig. 4]) similar to those in all of nine NMS recipients (range, 640 to 2,560) and died by day 7 postinfection. These IS recipients also had lower anti-Ebola virus Ab titers (1:400), consistent with earlier results (Fig. 2C). The surviving seven IS recipients had undetectable serum Ag titers on day 4 postinfection. In three of these mice, the titers peaked on day 8 postinfection (mean titer, 3,680; range, 160 to 10,240) and returned to undetectable levels by day 15 (Fig. 4), whereas the remaining four survivors had no detectable viral Ag in serum at any time point tested. The delayed peak of viral antigenemia suggests that anti-Ebola virus Ab inhibits or retards viral growth in at least a proportion (three of seven [43%]) of mice, and the failure to detect viral Ag indicates complete neutralization of the virus in the remaining four survivors.
FIG. 4.
Recipients of IS have delayed viral Ag kinetics compared to recipients of NMS following Ebola virus infection. Viral Ag levels were measured by ELISA on days 4, 8, and 15 after i.p. infection with 10 PFU of Ebola virus. All of nine NMS recipients (○) died by day 8. Two of nine IS recipients with anti-Ebola virus Ab titers of 1:400 ( ) also died by day 8. The remaining seven IS recipients, with Ab titers of 1:1,600 to 1:6,400 (●), survived beyond 25 days. The gray region indicates the limit of detection of Ag ELISAs.
) also died by day 8. The remaining seven IS recipients, with Ab titers of 1:1,600 to 1:6,400 (●), survived beyond 25 days. The gray region indicates the limit of detection of Ag ELISAs.
Immunodeficient mice are protected against lethal Ebola virus infection by IS transfer.
The experiments above with transfer of IS in immunocompetent mice suggested that nonhumoral adaptive immune responses may participate in protection against Ebola virus infection. We next investigated the ability of IS alone to protect against lethal Ebola virus infection by transferring IS to T- and B-cell-deficient (SCID) mice. All SCID mice that received IS had high titers of anti-Ebola virus IgG (1:400,000) 1 day after the transfer. All of five SCID mice that received IS survived lethal challenge, whereas none of five recipients of NMS survived (Fig. 5). None of the IS recipients showed any sign of illness during the 21-day observation period. Thus, IS alone had the ability to confer protection against lethal Ebola virus infection in the absence of the adaptive host immune system.
FIG. 5.
IS with high titers of anti-Ebola virus IgG alone is sufficient to protect immunodeficient mice from lethal Ebola virus infection. The graph shows the survival of SCID mice administered NMS (n = 5; ○) or IS with resulting titers of ≥1:400,000 (n = 5; ●) and were challenged with a lethal dose of Ebola virus (10 PFU/mouse i.p.) 1 day after serum transfer.
DISCUSSION
The mouse model of Ebola virus infection used in our experiments has some features that resemble human infections with Ebola virus. Mice infected s.c. clear the virus and generate high titers of anti-Ebola virus IgG (Fig. 1), as do human survivors (1, 11). In contrast, the disease course in mice infected i.p. resembles that in humans who die, who have high levels of Ebola virus in the circulation and low levels or absence of anti-Ebola IgG at the time of death (1). This dichotomy in the outcome of Ebola virus infection is not seen in other animal models. In guinea pigs and nonhuman primates, inoculation with adapted or naturally lethal live virus results in death irrespective of the route of infection (19). Thus, other animal models do not allow the development of high Ab titers. These advantages over other animal models and similarities with human cases encouraged us to use a mouse model to investigate the role of Abs in protection against Ebola virus infection.
Intraperitoneal infection of mice with the mouse-adapted Ebola virus resulted in a predictable and progressive rise in viral Ag leading to death in 6 to 8 days. However, IS transfer in mice before and 1 day after Ebola virus challenge protected them from lethality. Our data are supported by a recent study by Wilson et al. that demonstrated that a panel of MAbs that recognized epitopes from Ebola virus GP1 and sGP provide protection from lethal challenge with Ebola virus in mice at levels similar to our experiments (23). However, in this study it was not clear whether protection in recipients was mediated by immediate neutralization of challenged virus or viral replication occurred initially and was followed by clearance.
Our study points to a complex role of Abs in protection against Ebola virus. The protection observed is clearly mediated by the IgG fraction of serum (Fig. 3). Our data also demonstrate directly that there is complete clearance of virus by day 15 postinfection (Fig. 4). The protective efficacy of Abs appears to be titer dependent. Although there was significant variability between the protective titer of different batches of IS in our experiments, in general, serum transfer resulting in titers of ≥6,400 provided at least partial protection, and posttransfer titers of ≥400,000 resulted in complete protection, even in the absence of adaptive host immunity.
Control of the virus in animals that receive IS could be attained either by complete suppression of viral replication, e.g., by binding and neutralization of the virus, or by a partial inhibition of replication, allowing other immune mechanisms to clear the virus. The observation that the peak of viral antigenemia occurs later in IS recipients that survive indicates that Abs limit but do not completely block viral replication in a proportion of animals, although all these mice cleared the virus by day 15 postinfection. In the remaining survivors, however, there was no detectable viral Ag at any of the sampling time points. In these mice, viral replication appears to be completely suppressed by the transferred IS. Thus, it appears that Abs can induce protection by both means, complete neutralization and inhibition of viral replication. It is possible that the mechanism that dominates is determined by the Ab titer in the recipient. Recipients with the highest titers can clear the virus even in the absence of adaptive immunity and may neutralize virions early in the course of infection, as demonstrated by the survival of SCID mice, while those with lower (but protective) titers have a delayed kinetics of viral growth allowing other factors, such as adaptive immune responses, to clear the virus. Interestingly, mice protected by passive immunotherapy, on exposure to the virus, attained protection against subsequent lethal rechallenge with Ebola virus (data not shown).
In the mouse model, high-titered homologous IS provides significant protection against lethal infection with Ebola virus, but the mechanisms underlying Ab-mediated protection, particularly the relationship between Ab-mediated immunity and viral clearance by adaptive immune responses, need further exploration. Our data suggest that the use of convalescent-phase or immune serum as potential therapy for Ebola virus infections warrants further investigation.
ACKNOWLEDGMENTS
We thank T. Ksiazek for antibodies and advice on the antigen and antibody ELISAs for Ebola virus, G. Reynolds and L. Pezzanite for assistance in animal handling, C. J. Peters and Kaja Murali Krishna for critical comments and helpful suggestions, and J. O'Connor for editorial assistance in the preparation of the manuscript.
REFERENCES
- 1.Baize S, Leroy E M, Georges-Courbot M C, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch S P, McCormick J B, Georges A J. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 2.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever, J. Infect Dis. 1998;178:651–661. doi: 10.1086/515386. . (Erratum, 178:1553.) [DOI] [PubMed] [Google Scholar]
- 3.Emond R T, Evans B, Bowen E T, Lloyd G. A case of Ebola virus infection. Br Med J. 1977;2:541–544. doi: 10.1136/bmj.2.6086.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enria D A, Briggiler A M, Levis S, Vallejos D, Maiztegui J I, Canonico P G. Tolerance and antiviral effect of ribavirin in patients with Argentine hemorrhagic fever. Antiviral Res. 1987;7:353–359. doi: 10.1016/0166-3542(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 5.Fisher-Hoch S P, Khan J A, Rehman S, Mirza S, Khurshid M, McCormick J B. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet. 1995;346:472–475. doi: 10.1016/s0140-6736(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 6.Huggins J W. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev Infect Dis. 1989;11(Suppl. 4):S750–S761. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 7.Huggins J W, Hsiang C M, Cosgriff T M, Guang M Y, Smith J I, Wu Z O, LeDuc J W, Zheng Z M, Meegan J M, Wang Q N. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164:1119–1127. doi: 10.1093/infdis/164.6.1119. [DOI] [PubMed] [Google Scholar]
- 8.Jahrling P B, Geisbert J, Swearengen J R, Jaax G P, Lewis T, Huggins J W, Schmidt J J, LeDuc J W, Peters C J. Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch Virol Suppl. 1996;11:135–140. doi: 10.1007/978-3-7091-7482-1_12. [DOI] [PubMed] [Google Scholar]
- 9.Jahrling P B, Geisbert T W, Geisbert J B, Swearengen J R, Bray M, Jaax N K, Huggins J W, LeDuc J W, Peters C J. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(Suppl. 1):S224–S234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 10.Ksiazek T G, Rollin P E, Jahrling P B, Johnson E, Dalgard D W, Peters C J. Enzyme immunosorbent assay for Ebola virus antigens in tissues of infected primates. J Clin Microbiol. 1992;30:947–950. doi: 10.1128/jcm.30.4.947-950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ksiazek T G, Rollin P E, Williams A J, Bressler D S, Martin M L, Swanepoel R, Burt F J, Leman P A, Khan A S, Rowe A K, Mukunu R, Sanchez A, Peters C J. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl. 1):S177–S187. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 12.Ksiazek T G, West C P, Rollin P E, Jahrling P B, Peters C J. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis. 1999;179(Suppl. 1):S192–S198. doi: 10.1086/514313. [DOI] [PubMed] [Google Scholar]
- 13.Kudoyarova-Zubavichene N M, Sergeyev N N, Chepurnov A A, Netesov S V. Preparation and use of hyperimmune serum for prophylaxis and therapy of Ebola virus infections. J Infect Dis. 1999;179(Suppl. 1):S218–S223. doi: 10.1086/514294. [DOI] [PubMed] [Google Scholar]
- 14.Maiztegui J I, Fernandez N J, de Damilano A J. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama T, Rodriguez L L, Jahrling P B, Sanchez A, Khan A S, Nichol S T, Peters C J, Parren P W, Burton D R. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J Virol. 1999;73:6024–6030. doi: 10.1128/jvi.73.7.6024-6030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick J B, King I J, Webb P A, Scribner C L, Craven R B, Johnson K M, Elliott L H, Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 17.McKee K T, Jr, Huggins J W, Trahan C J, Mahlandt B G. Ribavirin prophylaxis and therapy for experimental Argentine hemorrhagic fever. Antimicrob Agents Chemother. 1988;32:1304–1309. doi: 10.1128/aac.32.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, Colebunders R, Muyembe-Tamfum J J. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(Suppl. 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 19.Peters C J, Sanchez A, Rollin P E, et al. Filoviridae: Marburg and Ebola viruses. In: Fields B N, Knipe D N, Howley P M, et al., editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1161–75. [Google Scholar]
- 20.Sadek R F, Khan A S, Stevens G, Peters C J, Ksiazek T G. Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995: determinants of survival. J Infect Dis. 1999;179(Suppl. 1):S24–S27. doi: 10.1086/514311. [DOI] [PubMed] [Google Scholar]
- 21.Vanderzanden L, Bray M, Fuller D, Roberts T, Custer D, Spik K, Jahrling P, Huggins J, Schmaljohn A, Schmaljohn C. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology. 1998;246:134–144. doi: 10.1006/viro.1998.9176. [DOI] [PubMed] [Google Scholar]
- 22.van de Wal B W, Joubert J R, van Eeden P J, King J B. A nosocomial outbreak of Crimean-Congo haemorrhagic fever at Tygerberg Hospital. Part IV. Preventive and prophylactic measures. S Afr Med J. 1985;68:729–732. [PubMed] [Google Scholar]
- 23.Wilson J A, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn A L, Hart M K. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Sanchez A, Yang Z, Zaki S R, Nabel E G, Nichol S T, Nabel G J. Immunization for Ebola virus infection. Nat Med. 1998;4:37–42. doi: 10.1038/nm0198-037. [DOI] [PubMed] [Google Scholar]