Abstract
In this study, blood samples and jaws were collected from 2 genotypes of blue mink (n = 289) in order to examine phenotypic expression of specific characteristics of Chediak-Higashi Syndrome (C-HS). Blood samples were subjected to differential counts to assess the proportion of abnormal polymorphonuclear leukocytes characteristic for CH-S (C-HS-leukocytes). Abnormal leukocytes with characteristic signs of C-HS were found in blood smears from all mink included in this study. Four teeth in one half of the mandible (P3, P4, M1, M2) were subjected to quantitative radiographic evaluation of alveolar bone loss and tooth loss. There was a high prevalence of destructive periodontal disease among blue mink included in this study. Mild to moderate periodontal disease (defined by less than 50% alveolar bone loss related to 1 or more teeth) affected 73.7% of young mink (age = 7 mo) and 67.9% of older animals (age ≥ 19 mo). Severe periodontal disease (defined by more than 50% bone loss related to one or more teeth) was not detected in mink aged 7 mo, but affected 15.3% of mink aged 19 mo and 39.6% of mink aged 31 mo. The positive relationship between age and periodontal disease was statistically significant (P < 0.01). The prevalence of tooth loss was found to be high among blue mink aged >19 mo (21.6%) and was also significantly related to age (P < 0.01). A significant positive interaction between alveolar bone loss and tooth loss (P < 0.01), implies that the highly prevalent tooth loss in the mink was related to and possibly caused by destructive periodontal disease. There was no significant difference in the prevalence of periodontal disease between the 2 genotypes and age was found to be the only statistical predictor of poor production results (P < 0.01) in blue mink.
Résumé
Des échantillons de sang et les mâchoires provenant de 2 génotypes de vison bleu (n = 289) ont été amassés afin d’examiner l’expression phénotypique de caractéristiques spécifiques du syndrome de Chediak-Higashi (C-HS). À partir des échantillons de sang on effectua un comptage différentiel pour évaluer la proportion de leucocytes poly-morphonucléaires anormaux caractéristiques de C-HS (leucocytes C-HS). Des leucocytes C-HS ont été trouvés sur les frottis sanguins de tous les visons inclus dans cette étude. Quatre dents d’une moitié de la mandibule (P3, P4, M1, M2) ont été soumises à une évaluation radiographique quantitative de la perte d’os alvéolaire et de la perte de dent. Une prévalence élevée de maladie parodontale destructive a été notée chez les visons bleus faisant partie de cette étude. Une maladie parodontale légère à modérée (définie comme moins de 50 % de perte d’os alvéolaire associée à une dent ou plus) affectait 73,7 % des jeunes visons (7 mois d’âge) et 67,9 % des animaux plus vieux (19 mois d’âge). Une maladie parodontale sévère (définie comme plus de 50 % de perte osseuse associée à une dent ou plus) n’était pas détectée chez les visons âgés de 7 mois, mais affectait 15,3 % des visons âgés de 19 mois et 39,6 % des visons âgés de 31 mois. L’association positive entre l’âge et une maladie parodontale était statistiquement significative (P < 0,01). La prévalence de perte de dents était élevée chez les visons bleus âgés de plus de 19 mois (21,6 %) et était significativement reliée à l’âge (P < 0,01). Une interaction positive significative entre la perte d’os alvéolaire et la perte de dents (P < 0,01), suggère que la prévalence élevée de perte de dents chez les visons était reliée et possiblement causée par une maladie parodontale destructive. Aucune différence significative dans la prévalence de maladie parodontale n’a été trouvée entre les 2 génotypes et l’âge s’est avérée être le seul indicateur statistique de pauvres résultats de production (P < 0,01) chez le vison bleu.
(Traduit par Docteur Serge Messier)
Introduction
Aleutian (aa) mink and numerous colour types (sapphire, blue iris, lavender, etc.) derived from combinations of aa with other recessive mutants are collectively termed blue, blue-grey, or Aleutian mink and are bred for their desirable fair coloured pelts. Aleutian mink are regarded as constitutionally weak mink, characterized by reduced size, poor breeding results, recurrent pyogenic infections, low resistance to the parvo virus causing Aleutian Disease (1,2), and early death. The characteristics of the blue mink are generally attributed to Chediak-Higashi syndrome (C-HS), a genetically transmitted autosomal recessive trait homologues to the C-HS trait in humans, cats, cattle, and mice (3,4).
The lesions of affected blue mink are similar in many regards to those of humans and other animals affected by C-HS and are characterized by partial oculocutaneous albinism and abnormally large cytoplasmatic granules in neutrophilic polymorphonuclear leukocytes (5–8). Furthermore, C-HS is usually associated with neutropenia and decreased bactericidal activity of the abnormal neutrophilic leucocytes (9).
The syndrome also affects other granule containing cells, causing marked deficiency in the activity of mononuclear phagocytic cells, natural killer (NK) cells, platelets, and megacaryocytes (10–12). It is likely that these defects leave the affected mink more susceptible to most types of bacteria and thereby to the skin abscesses commonly observed in blue mink (13). Abscesses affect the skin quality significantly and are of major concern for farmers breeding blue mink. Abnormal coagulation patterns due to the deficiency in the activity of platelets is also a well described characteristic of animals carrying the C-HS trait (10). Mink affected by C-HS may also have skin or gum lacerations from which they bleed excessively and for longer than normal periods of the time.
Destructive periodontal disease may be well be known to farmers breeding blue mink and a major concern is that the condition might predispose affected mink to valvular endocarditis and generalized bacterial infections (13), but present studies on periodontal conditions in animals carrying the C-HS trait are few and based on small numbers of animals (14,15). Descriptions of early onset periodontal destruction in blue mink is in marked contrast to normal mink, where periodontal disease has been described as an age- and plaque-related disease appearing only in aged animals and rarely seen in farmed mink (16,17). There are only a few reports on the periodontal conditions of human patients with C-HS, though early onset destructive periodontal disease in relation to C-HS and other systemic diseases (acquired immunodeficiency syndrome [AIDS]) have been described and are generally assumed to be caused by defects in the host defence, leading to bacterial invasion of the periodontal tissues (14,18–21). Previous studies on mink with C-HS have demonstrated considerable variability in the extent of periodontal disease between animals and in severity between teeth, indicating that factors other than presence of systemic disease and age may also be operative, though due to the low number of animals included, these results were not conclusive (14,15).
Some blue mink seem more viable, showing development and production results similar to non-mutation genotypes and it has been suggested that the constitution and production results of blue mink strains can be improved by selective breeding. Though currently, a lack of easy and early detectable quantitative measures of the expression of C-HS in individual mink, may impair the selective breeding for more sturdy animals. In this study, phenotypic expressions of some specific characteristics of blue mink generally attributed to C-HS were subjected to quantitative assessment.
The purpose of this study was to investigate the periodontal status as identified by radiographs and visible loss of alveolar bone compared to the root length in 2 types of blue mink and to examine the possible association between this assessment and counts of abnormal polymorphonuclear leukocytes.
Material and methods
This study was comprised of 289 mink of the blue color type obtained from 5 Danish mink farms. All tested free from the Aleutian Disease Virus (ADV) antibodies in farm screening, using an additive counterimmunoelectrophoresis method (22). The mink represented 2 genotypes derived from crossing Aleutian (aa) with other colour mutants: silverblue (pp) and steelblue (psps). This study included 199 mink of sapphire genotype (aapp) and 90 mink of blue iris genotype (pspsaa). Twenty-three were young males (7 mo), the rest females. Data concerning genotype (sapphire or blue iris) and age of individual mink were obtained from all farms. The female mink were aged 7, 19, 31, or 43 mo according to the year of birth, as mink at Danish mink farms are born in May and samples were taken in November. The exact age of 50 mink was not obtained, but all were >1 y of age. Data concerning production results were collected from 4 of 5 farms included in the study.
The mink were euthanized by intraperitoneal injection with 1 mL embrutamid, mebezoniumiodid, and tetracain (T61; Intervet Danmark A.S., Skovlunde, Denmark) at the time of pelting (November). All mink were bled from a clipped claw prior to euthanasia. Blood smears were prepared immediately after bleeding. All blood smears were stained (Hemacolour; J.T. Baker, Phillipsburg, New Jersey, USA) and submitted for microscopic evaluation. The morphology of blood leukocytes was assessed visually and differential counts of a minimum of 200 blood leukocytes were performed. Differential counts included: monocytes, lymphocytes, normal polymorphonuclear lymphocytes, and C-HS leukocytes (defined as neutrophilic leukocyte containing 1 or more abnormally large lysosomes). Following pelting, heads were detached from the body and the lower jaw was exarticulated and severed in the mandibular symphysis. One randomly picked side of the mandibular bone was then x-rayed (Oralix AC X-ray machine; Phillips Electrical Ltd., London, United Kingdom, Kodak Ekta Speed radiographic film; Kodak, Rochester, New York, USA). Using these radiographs, the periodontal status of the animal was assessed by recording alveolar bone loss in the mandibular 3rd premolar (P3), 4th premolar (P4), 1st molar (M1), and 2nd molar (M2). These 4 teeth were selected due to the fact that bone loss was easier to assess than in the rest of the mandibular teeth. For each of the 4 teeth, presence of alveolar bone loss was visually assessed in the radiographs as follows (Figure 1): Healthy — no vertical or horizontal alveolar bone loss present interradicular or interdentally (B~0); mild to moderate — vertical or horizontal alveolar bone loss of up to 50% of the root length (the distance from the cemento-enamel junction to the root apex) was seen either interradicular or interdentally (B ≤1/2 distance between C and A); Severe alveolar bone loss — vertical or horizontal alveolar bone loss of more than 50% of the root length was seen either interradicular or interdentally (B >1/2 distance between C and A). One animal with loss in all 4 examined teeth was excluded from the study. On the individual level, the animal was diagnosed according to the 1 tooth of the 4 with the most severe alveolar bone loss.
Figure 1.
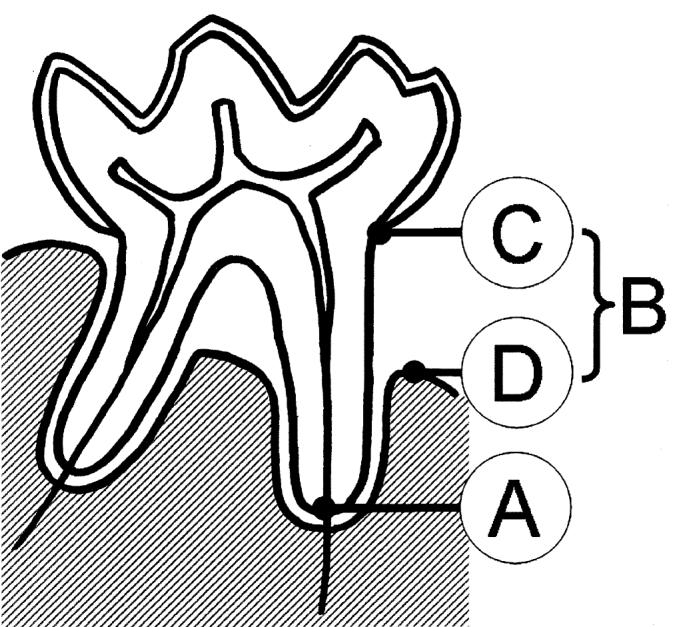
Mink tooth (molar 1 [M1]) with destructive periodontal disease illustrating reference points for bone loss measurements on radiographic images. A: apex of root, B: bone loss, C: cemento-enamel junction, D: edge of alveolar bone.
Statistical analysis
Descriptive statistics, including means, counts, and percentages, were calculated. All data analysis was performed using computer software (SAS, version 8.2; SAS Institute, Cary, North Carolina, USA) with a significance level of 0.05. Continuous variables were compared using t-tests and categorical variables by χ2-test. Ninety-five percent confidence intervals (CI) were used. The following variables were used in statistical analyses: bone loss (none, mild to moderate, or severe bone loss), tooth loss (none or tooth loss = 1 or more of the teeth missing), C-HS leukocytes (differential count of abnormal leukocytes characteristic of C-HS), age (young = 7 mo and old ≥19 mo), and production result (good ≥5 pups at weaning and poor <5 pups at weaning). Logistic regression analyses was performed on the production result and the tooth loss as dependent variable using the GENMOD procedure.
Results
Out of 289 commercially bred blue mink examined for periodontal disease identified as alveolar bone loss, 40 mink (13.8%) had normal teeth and the remaining 249 animals (86.2%) had varying degrees of periodontal disease. The degree of severity of alveolar bone and tooth loss found in individual mink and individual teeth varied. In some mink, only mild alveolar bone loss was detected in >1 teeth, in others severe alveolar bone loss and tooth loss were affecting several or all teeth.
Results of alveolar bone and tooth (P3, P4, M1, and M2) loss are presented in Table I. The intraoral distribution of the diseased teeth showed that the mandibular M2 was most prone to exhibiting bone loss of 75%, while the tooth that was the second most prone to bone loss was the mandibular M1 with only 36%. The most common tooth loss was the mandibular M2. Radiographic images of different categories of periodontal disease and healthy dentition are shown in Figure 2. The 3 mink presented in Figure 2 were selected with the purpose of demonstrating the different categories of bone destruction for each of the 4 teeth examined. In each of these mink, the 4 teeth examined were assessed with the same category, though generally this was a rare finding. Overall, we found considerable variation in the proportion of teeth per mink with destructive periodontal disease and tooth loss. The mink shown in Figure 2 had all of their teeth that were examined classed in the category of severe bone loss.
Table I.
The frequency of bone loss in 4 selected teeth (premolars 3 [P3] and 4 [P4] and molars 1 [M1] and 2 [M2]) of commercially bred blue color type mink examined for periodontal disease (n = 289). Through the use of radiographs, bone loss was assessed in 4 teeth from 1 randomly chosen half of the mandible from each mink. The frequency was presented as the percentage of each individual tooth, categorized as affected by mild to moderate bone loss (up to 50% alveolar bone loss) or severe bone loss (more than 50% alveolar bone loss)
| Teeth | No bone loss | Mild to moderate bone loss | Severe bone loss | Tooth loss | Total % diseased teeth |
|---|---|---|---|---|---|
| P3 | 70.59 | 18.34 | 6.23 | 4.84 | 24.57 |
| P4 | 62.98 | 24.91 | 8.65 | 3.46 | 33.56 |
| M1 | 62.28 | 32.18 | 4.15 | 1.38 | 36.33 |
| M2 | 13.84 | 69.90 | 5.19 | 11.07 | 75.09 |
Figure 2.
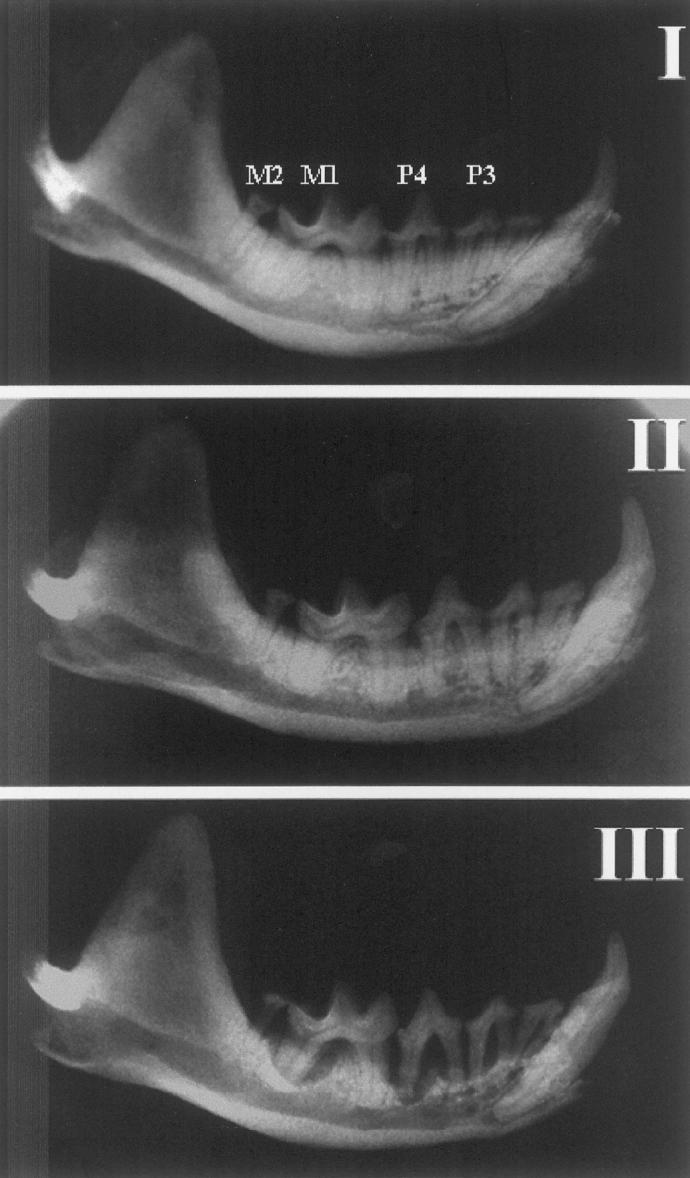
Radiographic images of the mandible in 3 blue mink obtained from same farm. I: 2-year-old female presenting no horizontal or vertical bone loss in premolar 2 (P2) to molar 2 (M2). II: 2-year-old female presenting mild horizontal bone loss in P2 to M2. III: 2-year-old female presenting severe horizontal, vertical, or both bone loss in P2 to M2.
The percentage of mink categorized as having mild to moderate or severe bone loss and the percentage affected by tooth loss in each of the genotypes (sapphire and blue iris) are presented in Table II. In evaluating the pattern of severity of alveolar bone loss, 47 mink (16.3%) were categorized as having severe bone loss in at least 1 of the 4 teeth, and 202 mink (69.9%) were categorized as having mild bone loss in at least 1 tooth. There was a statistically significant relationship between age and bone loss (P < 0.01) and between age and tooth loss (P < 0.01). A high prevalence of mild to moderate bone loss was affecting 73.7% of the mink aged 7 mo. Severe bone and tooth loss was not detected in the young age group, but there was a rise in the proportion of animals with severe bone loss with increasing age, affecting 15.3% of mink aged 19 mo and 39.1% of mink aged 31 mo. This dramatic rise in severe bone loss was paralleled by a similar rise in tooth loss (Figure 3). The association between periodontal disease and tooth loss, expressed as a significant positive interaction between bone loss and tooth loss (P < 0.01), implied that the highly prevalent tooth loss in these mink was related to and possibly caused by destructive periodontal disease. No statistical difference could be detected in the prevalence of bone loss and tooth loss between the genotypes sapphire and blue iris (Table II).
Table II.
The frequency of periodontal disease in 2 genotypes of blue color type minks examined (n = 289). Through the use of radiographs, periodontal disease was assessed in 1 randomly chosen half of the mandible from each mink by determining the alveolar bone loss and tooth loss of 4 selected teeth (premolar 3 [P3] and 4 [P4] and molar 1 [M1] and 2 [M2]). The frequency is presented as the percentage of mink categorized as affected by mild to moderate bone loss (defined by up to 50% alveolar bone loss related to 1 or more teeth) or severe bone loss (defined by more than 50% alveolar bone loss related to 1 or more teeth) versus non-affected animals. The percentage of blue mink with tooth loss (related to 1 or more teeth) in each genotype is also presented
| Genotype | No bone loss | Mild to moderate bone loss | Severe bone loss | Tooth loss |
|---|---|---|---|---|
| Blue iris (n = 90) | 17.8 | 61.1 | 21.1 | 13.3 |
| Sapphire (n = 199) | 12.0 | 73.9 | 14.1 | 14.6 |
| P (genotype by bone loss) | — | 0.091 | — | — |
| P (genotype by tooth loss) | — | — | — | 0.780 |
Figure 3.
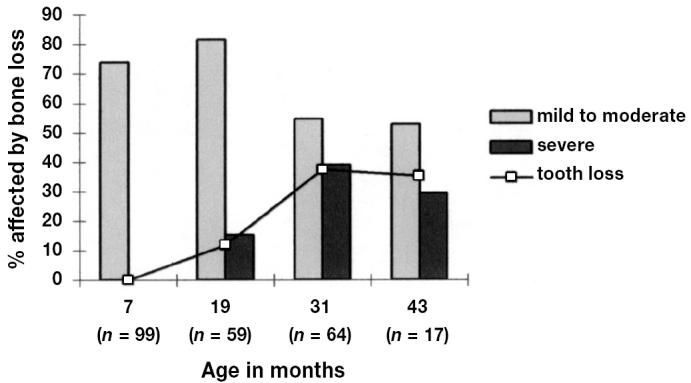
Periodontal disease related to 4 age groups of blue mink (n = 239) was determined by assessing loss of alveolar bone on radiographs of one half of the mandible and is presented as percentage of mink categorized as affected by mild to moderate or severe bone loss. Percentage of blue mink with tooth loss in each age group is also presented.
There was a statistically significant difference in the prevalence of alveolar bone loss and tooth loss between mink supplied from different farms, expressed as a significant interaction between farm identification and bone loss (P < 0.01) and farm identification and tooth loss (P < 0.01). This result may reflect a difference in the age distribution in mink supplied by the farms (Table III). Farm 1 supplied only young animals (7 mo) and these mink showed a 70% prevalence of mild to moderate bone loss and no severe bone loss or tooth loss. Similarly, farm 3 supplied only older mink and the prevalence of bone loss and tooth loss was relatively higher than in other farms. In the remaining 3 farms, the age distribution was almost identical (39.5%, 33.3%, and 33.3% young animals from farms 2, 4, and 5, respectively). Due to collinearity, it was not possible to test the difference statistically. However, mink from farms 2 and 4 had a 2 to 3 times higher rates of severe bone loss and 3 to 4 times higher rates tooth loss than farm 5 (Table III).
Table III.
The frequency of periodontal disease in commercially bred blue color type mink (n = 289) from 5 Danish mink farms. The percentage of young mink (7 mo) among the mink obtained from each farm is presented. Through the use of radiographs, periodontal disease was assessed in 1 randomly chosen half of the mandible from each mink, by determining the alveolar bone loss and tooth loss of 4 selected teeth (premolar 3 [P3] and 4 [P4] and molar 1 [M1] and 2 [M2]). The frequency is presented as the percentage of animals categorized as affected by mild to moderate bone loss (defined by less than 50% alveolar bone loss related to 1 or more teeth) or severe bone loss (defined by more than 50% alveolar bone loss related to 1 or more teeth) versus non-affected animals. The percentage of blue mink with tooth loss (related to 1 or more teeth) in each genotype is also presented
| Farm identification | n | Percentage of mink aged 7 mo | Mild to moderate bone loss | Severe bone loss | Tooth loss |
|---|---|---|---|---|---|
| 1 | 24 | 100 | 70.8 | 0 | 0 |
| 2 | 65 | 39.5 | 52.3 | 29.2 | 18.5 |
| 3 | 50 | 0 | 90 | 8 | 24 |
| 4 | 75 | 33.3 | 66.7 | 21.3 | 17.3 |
| 5 | 75 | 33.3 | 74.7 | 10.7 | 5.3 |
| P (frequency by farm) | — | — | 0.001 | 0.001 | 0.006 |
| P (frequency by age) | — | — | 0.001 | 0.001 | 0.001 |
The blood morphology and reproduction results were compared to the presence of periodontal disease and tooth loss (Table IV). Statistically significant differences were found in mean C-HS leukocyte counts between mink with bone loss and those without (P = 0.02) and between mink with tooth loss and those without (P = 0.02).
Table IV.
The results of blood counts and reproduction results from commercially bred blue color type mink (n = 289) from 5 Danish mink farms were compared to the presence of periodontal disease and tooth loss. Blood morphology was assessed visually by differential counts of a minimum of 200 blood leucocytes and percentages of C-HS leukocytes (defined as neutrophilic leukocyte containing 1 or more abnormally large lysosomes) were recorded. Through the use of radiographs, periodontal disease was assessed in 1 randomly chosen half of the mandible from each mink, by determining the alveolar bone loss and tooth loss of 4 selected teeth (premolar 3 [P3] and 4 [P4] and molar 1 [M1] and 2 [M2]). The animals were recorded as affected by periodontal disease and or tooth loss or not affected. Reproduction results were obtained from farm records and presented as the average number of pups at weaning. The P-values indicate significant differences in leucocytecounts between the groups compared
| Reproduction results | Mean C-HS leucocyte count | Standard deviation | P | |
|---|---|---|---|---|
| Periodontal disease | 7.9 | 8.6 | 5.7 | 0.002 |
| No periodontal disease | 8.9 | 11.1 | 6.6 | — |
| Tooth loss | 5.8 | 7.2 | 4.4 | 0.018 |
| No tooth loss | 8.4 | 9.2 | 6.1 | — |
| High production result | 8.8 | 9.8 | 5.3 | 0.002 |
| Low production result | 6.4 | 7.5 | 5.7 | — |
For the mink, where production results data were recorded (n = 189), individual production results varied from 0 to 10 pups with an average production result of 4.3 pups. A non-significant, but slightly negative, correlation between C-HS leukocyte counts and production results (r = 0.03) of the blue mink was observed. There was a statistically significant difference (P < 0.01) in mean C-HS leukocyte counts between the group of blue mink with good production results (average = 7.5; CI: 6.4 to 8.5) and the group with low production results (average = 9.8; CI: 8.8 to 10.8). The results from the logistic regression revealed significant associations between the production result and the age of the mink (P < 0.01), but production results were not statistically associated with the C-HS leukocyte counts, dental damage, age, or farm. Logistic regression demonstrated that loss of 1 or more teeth was statistically associated with age (P < 0.01) and periodontal disease in remaining teeth (P < 0.01), and negatively associated with the C-HS leukocyte counts (P < 0.01).
Discussion
The prevalence of mild to moderate periodontal disease, defined by alveolar bone loss of less than 50% of the root length in 1 or more teeth, was found to be high among blue mink in all farms and all age groups included in this study. This result corresponds with previous studies of dental status in blue mink showing a high prevalence of periodontal disease (14,15). Severe periodontal disease, defined by alveolar bone loss of more than 50% of the root length in 1 or more teeth, tooth loss, or both were not identified in sexually immature animals (approximately 7 mo of age), but there was a significant rise in the number of teeth lost and severe bone loss in animals of increased age. Since severe periodontal disease affected the main proportion of breeding mink ≥19 mo, but was relatively infrequent among younger mink, a breeding strategy that included pelting of blue mink after their first breeding could decrease the prevalence of severe periodontal disease, thereby improving the health status and welfare of the population of blue mink. The relationship between destructive periodontal disease and tooth loss, expressed as a significant positive interaction between bone loss and tooth loss, implied that the highly prevalent tooth loss in these mink was related to and possibly caused by destructive periodontal disease.
There was a statistically significant difference in the prevalence of bone loss and tooth loss in mink between farms. This significant difference may be caused by differences in the age distribution of the mink supplied from each farm, though comparisons between the 3 farms with almost identical age distribution showed considerable variance in the occurrence of bone loss and tooth loss. The sampling procedure chosen for this study did not allow for statistical assessment of such farm variation, which may be attributable to individual differences in management or breeding strategies.
The average production result for blue mink included in this study (4.3 pups) was somewhat lower than the average production result of Danish farm minks on 5.3 pups (23). Our results indicated a statistically significant difference in mean C-HS leukocyte counts between mink with good production results and mink with poor production results. This can be explained by age differences in the group of mink with good production results and the group with poor production results, since production results were found to be statistically associated with the age of the mink.
Leukocytes are an important component of periodontal health. Abnormal leukocytes with dysfunctional giant granules are an important component of disease processes related to C-HS (24–29). Abnormal leukocytes with characteristic signs of C-HS were found in blood smears from all of the mink included in this study. A statistically significant difference was detected in mean C-HS leukocyte counts between the mink with and without alveolar bone and also between mink with and without tooth loss, but the logistic regression did not reveal a significant association between the production results and the C-HS leukocyte counts. Due to possible confounding effects, it is uncertain whether the difference in C-HS leukocyte counts reflects the different age distributions in the 2 groups, rather than an actual relationship between the number of C-HS leukocytes in peripheral blood and development of periodontal disease.
This study confirms that destructive periodontal disease is a highly prevalent disease problem among farmed blue mink. The high prevalence of periodontal disease undoubtedly impairs the welfare of blue mink. In this study, increasing age proved to be the only strong predictor of periodontal disease in blue mink, though considerable variation within blue mink of same age groups implied that factors other than the mere presence of the C-HS trait and ageing has an effect on the development of periodontal disease in individual blue mink, further research would be required to identify and define such factors.
Acknowledgments
The authors thank the mink farmers involved for contributing the material that this study is based on and for their assistance during sampling procedures. The authors also thank B. Kruse, J. Holm, and K. Eske for their skilled technical assistance.
References
- 1.Eklund CM, Hadlow WJ, Kennedy RC, Boyle CC, Jackson TA. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968;118:510–526. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- 2.Helmbolt CF, Jungherr EL. The pathology of Aleutian disease in mink. Am J Vet Res. 1958;19:212. [PubMed] [Google Scholar]
- 3.Windhorst DB, Padgett G. The Chediak-Higashi syndrome and the homologous trait in animals. J Invest Dermatol. 1973;60:529–537. doi: 10.1111/1523-1747.ep12703609. [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Kaplan J. Complementation analysis of Chediak-Higashi syndrome: the same gene may be responsible for the defect in all patients and species. Somat Cell Mol Genet. 1993;19:459–468. doi: 10.1007/BF01233251. [DOI] [PubMed] [Google Scholar]
- 5.Leader RW, Padgett GA, Gorham JR. Studies of abnormal blood leococyte bodies in the mink. Blood. 1963;22:477. [PubMed] [Google Scholar]
- 6.Padgett GA, Reiquam CW, Gorham JR, Henson JB, O’Mary CC. Comparative studies of the Chediak-Higashi syndrome. Am J Pathol. 1967;51:553–571. [PMC free article] [PubMed] [Google Scholar]
- 7.Padgett GA, Reiquam CW, Henson JB, Gorham JR. Comparative studies of susceptibility to infection in the Chediak-Higashi syndrome. J Pathol Bacteriol. 1968;95:509–522. doi: 10.1002/path.1700950224. [DOI] [PubMed] [Google Scholar]
- 8.Bell TG, Padgett GA, Patterson WR, Meyers KM, Gorham JR. Prolonged bleeding time in Aleutian mink associated with a cyclo-oxygenase-independent aggregation defect and nucleotide deficit in blood platelets. Am J Vet Res. 1980;41:910–914. [PubMed] [Google Scholar]
- 9.Clark RA, Kimball HR, Padgett GA. Granulocyte chemotaxis in the Chediak-Higashi syndrome of mink. Blood. 1972;39:644–649. [PubMed] [Google Scholar]
- 10.Meyers KM, Holmsen H, Seachord CL, Hopkins G, Gorham J. Characterization of platelets from normal mink and mink with the Chediak-Higashi syndrome. Am J Hematol. 1979;7:137–146. doi: 10.1002/ajh.2830070206. [DOI] [PubMed] [Google Scholar]
- 11.Menard M, Meyers KM. Storage pool deficiency in cattle with the Chediak-Higashi syndrome results from an absence of dense granule precursors in their megakaryocytes. Blood. 1988;72:1726–1734. [PubMed] [Google Scholar]
- 12.Lodmell DL, Bergman RK, Bloom ME, Ewalt LC, Hadlow WJ, Race RE. Impaired phagocytosis by the mononuclear phagocytic system in sapphire mink affected with Aleutian disease. Proc Soc Exp Biol Med. 1990;195:75–78. doi: 10.3181/00379727-195-43121. [DOI] [PubMed] [Google Scholar]
- 13.Hunter DB, Lemieux N. Diseases. In: Hunter B, ed. Mink... biology, health and disease. Guelph: Graphic and Print Services, 1996:16–21.
- 14.Lavine WS, Page RC, Padgett GA. Host response in chronic periodontal disease. V. The dental and periodontal status of mink and mice affected by Chediak-Higashi syndrome. J Periodontol. 1976;47:621–635. doi: 10.1902/jop.1976.47.11.621. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson GT. Increased susceptibility to periodontitis in mink affected by a lysosomal disease. J Periodontal Res. 1969;4:259–267. doi: 10.1111/j.1600-0765.1969.tb01977.x. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder HE, Page RC. Minks. Periodontitis in man and other animals. New York: Karger, 1982:116–126.
- 17.Kennedy AH. Diseases affecting the mouth and teeth. The Mink in Health and Disease. Toronto: Fur Trade Journal of Canada, 1951:27–32.
- 18.Blume RS, Wolff SM. The Chediak-Higashi syndrome: studies in four patients and a review of the literature. Medicine (Baltimore) 1972;51:247–280. [PubMed] [Google Scholar]
- 19.Delcourt-Debruyne EM, Boutigny HR, Hildebrand HF. Features of severe periodontal disease in a teenager with Chediak-Higashi syndrome. J Periodontol. 2000;71:816–824. doi: 10.1902/jop.2000.71.5.816. [DOI] [PubMed] [Google Scholar]
- 20.Shibutani T, Gen K, Shibata M, Horiguchi Y, Kondo N, Iwayama Y. Long-term follow-up of periodontitis in a patient with Chediak-Higashi syndrome. A case report. J Periodontol. 2000;71:1024–1028. doi: 10.1902/jop.2000.71.6.1024. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton RE, Jr, Giansanti JS. The Chediak-Higashi syndrome. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1974;37:754–761. doi: 10.1016/0030-4220(74)90141-8. [DOI] [PubMed] [Google Scholar]
- 22.Uttenthal A. Screening for antibodies against Aleutian disease virus (ADV) in mink. Elucidation of dubious results by additive counterimmunoelectrophoresis. Appl Theor Electrophor. 1992;3:83–84. [PubMed] [Google Scholar]
- 23.Boergesen E. Avlsresultat 2003. Dansk Pelsdyravl. 2003;10:50–51. [Google Scholar]
- 24.Schroeder HE, Münzel-Pedrazzoli S, Page RC. Correlated morphometric and bichemical analysis of gingival tissue in early chronic gingivitis in man. Arch Oral Biol. 1973;18:899. doi: 10.1016/0003-9969(73)90060-5. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder HE, Graf-de Beer M, Attström A. Initial gingivitis in dogs. J Periodont Res. 1975;10:115. doi: 10.1111/j.1600-0765.1975.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 26.Lindhe J, Schroeder HE, Page RC, Munzel-Pedrazzoli S, Hugoson A. Clinical and stereologic analysis of the course of early gingivitis in dogs. J Periodontal Res. 1974;9:314–330. doi: 10.1111/j.1600-0765.1974.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 27.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249. [PubMed] [Google Scholar]
- 28.Page RC, Schroeder HE. Spontaneous chronic periodontitis in adult dogs. A clinical and histopathological survey. J Periodontol. 1981;52:60–73. doi: 10.1902/jop.1981.52.2.60. [DOI] [PubMed] [Google Scholar]
- 29.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]


