Abstract
The distribution of the immune complex vaccine virus for infectious bursal disease (IBD) in tissue was examined and the viral loads of the organs were quantitatively compared. One-day-old specific pathogen free (SPF) and maternally immune broiler chickens were injected subcutaneously with the vaccine. Lymphoid and non-lymphoid tissues were collected at various time intervals during the experiment to test for infectious bursal disease virus (IBDV)-RNA by using reverse transcriptase-polymerase chain reaction (RT-PCR). Only the bursa of Fabricius was found to be positive with unusually long viral persistence in the broiler group. The positive bursa samples were further investigated by using real-time PCR coupled with a TaqMan probe. The highest amounts of the virus were detected at its first appearance in the bursa: on day 14 post vaccination (PV) in the SPF chickens and on day 17 and day 21 PV in the maternally immune broiler group. The virus then gradually cleared, most likely due to the parallel appearance of the active immune response indicated by seroconversion.
Résumé
La distribution tissulaire du complexe immun vaccinal pour la maladie infectieuse de la bourse a été examinée et la charge virale dans les organes comparée de manière quantitative. Des poussins âgés de un jour exempts d’agents pathogènes spécifiques (SPF) et des poussins de poulet à griller maternellement immuns ont été injectés par voie sous-cutanée avec le vaccin. Des tissus lymphoïdes et non-lymphoïdes ont été prélevés à différents temps lors de l’expérience afin de détecter l’ARN du virus de la malade infectieuse de la bourse (IBDV) par réaction d’amplification en chaîne par la polymérase utilisant la transcriptase inverse (RT-PCR). Seule la bourse de Fabricius s’est avérée positive avec une persistance virale anormalement longue dans le groupe de poulet à griller. Les échantillons de bourse positifs ont été examinés en plus par épreuve de PCR en temps réel avec une sonde TaqMan. La plus grande quantité de virus a été détectée lors de sa première apparition dans la bourse : au jour 14 post-vaccination (PV) chez les poulets SPF et aux jours 17 et 21 PV chez les poulets à griller. Le virus a graduellement disparu, probablement à cause de l’apparition d’une réponse immunitaire active tel qu’indiquée par une séroconversion.
(Traduit par Docteur Serge Messier)
Introduction
Infectious bursal disease (IBD) is a common disease of chickens caused by a double-stranded RNA virus belonging to the Birnaviridae family (1). Virulent infectious bursal disease virus (IBDV) can induce severe immunosuppression in susceptible chickens, which fail to respond optimally to routinely used poultry vaccines and remain vulnerable to opportunistic field pathogens (2). Commercial chickens are usually infected early in life so it is necessary to establish protection as early as possible.
An immune complex IBD vaccine (IBDV-Icx) was developed for in ovo immunization consisting of IBDV-specific antibodies mixed with a live, attenuated IBDV strain (2512-IBDV) with intermedier plus virulence (3). This vaccine is insensitive to high levels of maternal antibodies (Mab) (4) and the potential adverse effect of the IBDV strain having considerable residual virulence in chickens with low Mab levels is prevented by the homologous antibody added to the vaccine. The efficacy of this vaccine at challenge is identical to that induced by conventional vaccines against IBD, which contain the same virus strain without antibodies (5). Moreover, Chettle and Wyeth (6) have shown advanced protection for maternally immune chickens when the IBDV-Icx is used.
During the challenge period, higher body weight gain has been recorded after vaccination with IBDV-Icx compared with conventional vaccines without antibodies (7). It has been also shown that immunosuppressive effects in specific pathogen free (SPF) chickens, caused by the highly immunogenic virus strain of the IBDV-Icx, are markedly moderated by the antibody when compared to vaccination with this virus strain alone (7).
In ovo vaccination of 18-day-old embryos is a general method for applying the IBDV-Icx that has been used successfully to vaccinate millions of commercial broiler chickens around the world. Therefore, most knowledge about IBDV-Icx was obtained using in ovo applications (8). In Hungary, very few large hatcheries have the proper equipment to apply in ovo vaccines, therefore, subcutaneous administration at 1 d of age is applied. It was reported that the use of IBDV-Icx by subcutaneous injection is safe and efficacious in SPF chickens (3) and broiler chickens (5) when given at 1 d of age. However, there is a lack of knowledge concerning the mode of action of IBDV coupled with antibodies when using different routes of administration. The aim of this study was to determine the distribution of the IBD viral RNA in the tissues of SPF and broiler chickens using reverse transcriptase-polymerase chain reaction (RT-PCR) and to compare the viral load of the positive organs using a real-time PCR assay following subcutaneous injection of IBDV-Icx.
Materials and methods
Animals
Specific pathogen free eggs (SPAFAS; Biovo, Mohács, Hungary) and broiler eggs (Cobbs; Gallus, Devecser, Hungary) were hatched at the Veterinary Research Institute, Budapest, Hungary, in standard hatcheries. The broiler parent flock was vaccinated against IBDV with a multivalent, inactivated vaccine before the laying period (Binewgodrop; Merial, Lyon, France). After hatching, chickens were housed in isolators and kept in accordance with the international animal welfare regulations reflected in Hungarian law (248/1988. [XII.31.] Korm. Rend.). Feed and water were provided ad libitum.
Immune complex IBD vaccine and vaccination protocol
An immune complex vaccine (Cevac Transmune IBD Vaccine; CEVA-Phylaxia Biologicals Company, Budapest, Hungary) consisting of 102.0 EID50/0.1 mL of a live intermediate plus IBDV strain (2512-IBDV) and 20 units of hyperimmune serum against IBDV was used. Forty 1-day-old SPF chickens and 50 1-day-old broiler chickens were used. Of these chickens, 21 SPF and 24 broiler received 1 dose of IBDV-Icx by subcutaneous injection at the back of the neck. The remaining 19 SPF chickens and 26 broiler chickens were left as unvaccinated controls.
Tissue collection and preparation
Three SPF chicks were culled from each of the vaccinated and control groups at 5 h and than at days 1, 3, 6, 10, 14, and 21 post vaccination (PV). Three broiler chickens from each of the vaccinated and control groups were removed at days 7, 10, 14, 17, 21, 28, 34, and 43 PV. The chickens were weighed, blood samples were taken, and euthanized. After autopsy, part of the bursa of Fabricius, spleen, thymus, cecal tonsils, liver, and duodenum were removed from each chicken, snap-frozen in liquid nitrogen, and stored at −80°C for further analyses. For the immunofluorescence assay, organ samples were collected only from the SPF chickens, embedded in Cryomatrix (Thermo Shandon, Pittsburgh, Pennsylvania, USA), immediately frozen in liquid nitrogen, and stored at −80°C until further examination. Sera from blood samples were separated and stored at −20°C until use.
Reverse transcriptase-polymerase chain reaction
Fifty to 100 mg of tissues were processed for isolation of total RNA using reagent (TRIZOL Reagent; Gibco-BRL Life Technologies, Gaithersburg, Maryland, USA), the procedure was performed according to the protocol provided by the manufacturer. The RNA from each sample was dissolved in 20 μL of sterile RNAse-free water, quantitated spectrophotometrically, and either stored at −80°C or used immediately for RT-PCR. First-strand cDNA was synthesized in a 25 μL final volume at 37°C for 1 h using 2 μg of total RNA (Moloney Murine Leukemia Virus Reverse Transcriptase; Promega Corporation, Madison, Wisconsin, USA) and mixed primers of oligo-dT and pd(N)6 (Random Hexamer; Amersham Pharmacia, Buckinghamshire, United Kingdom) in a ratio of 4:1. After the RT reaction, a 202 base pair (bp) fragment was amplified by applying the primers specific for the VP2 gene of IBDV, designed by the sequence of 2512-IBDV strain (GenBank accession number AF457105): 202R 5′-CCGGACGACACCCTGGAG-3′ (position 182 to 200 nucleotides [nt]) and 202F 5′-AACTGGCCGGTAGGTTCTGG-3′ (position 384 to 364 nt). The PCR was conducted as follows: initial melt at 94°C for 3 min, followed by 35 cycles of a repetitive program of 30 s at 94°C, 30 s at 58°C, and 40 s at 72°C. A final elongation step at 72°C for 10 min completed the PCR procedure.
Three-week-old SPF chickens were infected with virulent IBDV and the bursae were removed 3 d later. These samples were used as positive controls for RNA extraction and RT-PCR. An IBDV-plasmid was prepared by cloning a VP2 IBDV-fragment into the phagemid (pBluescript II KS; Stratagene, La Jolla, California, USA) and used as a positive PCR control. The PCR products were visualized after electrophoresis on a 2.5% agarose gel.
Nested PCR
A nested PCR method with external primers, designated P1 and P2 (9), and internal primers, P2.3 and P5.3, was used (10). The sequences of the primer sets were as follows: P1: 5′-TCACCGTCCTCAGCTTAC-3′, P2: 5′-TCAGGATTTGGGATCAGC-3′, P2.3: 5′-CCCAGAGTCTA CACCATA-3′, P5.3: 5′-TCCTGTTGCCACTCTTTC-3′. The primers amplify a 474 bp product spanning from 703 to 1176 nt (11), which encompasses the hypervariable region of the VP2 gene. The PCR reactions were conducted following the procedures described by Liu et al (9) and Lin et al (10) for the 2 sets of primers, respectively.
Polymerase chain reaction sensitivity
The sensitivities of the RT-PCR and nested PCR assays were determined by using an IBDV strain (LIBD) replicating in a chicken embryo fibroblast (CEF). Plaque assay of 10-fold serially diluted IBDV was used to determine the viral titre (12). The RNA extraction and RT-PCR or nested PCR were performed from the serial dilutions using a pair of primers 202R/202F, in the case of RT-PCR, and P1/P2 external and P2.3/P5.3 internal primers, in the case of nested PCR. Sensitivity was calculated according to the dilution factors of the PCR assay conditions as described above and expressed in plaque forming units (PFU).
Real-time PCR
Quantification of IBDV, based on the increase of fluorescence signal during the PCR, was determined by using real-time PCR (iCycler iQ Multi-Color Real Time PCR Detection System; Bio-Rad, Hercules, California, USA) and a TaqMan probe. The following primers were designed by the sequence of 2512-IBDV strain (GenBank accession number AF457105) to amplify a 74 bp fragment of the VP2 gene: VP2F 5′-GGACACAGGGTCAGGGTCAAT-3′ (position 246 to 266 nt) VP2R 5′-GCAGTGTGTAGTGAGCACCCA-3′ (position 299 to 319 nt). The VP2-TaqMan probe was designed for the 74 bp sequence and was labelled with the reporter dye FAM at the 5′ end and the quencher dye TAMRA at the 3′ end: 5′-(FAM)-TCTTTTTCCCTGGATTCCCTGGCTCA-(TAMRA)-3′. The PCR mixture (50 μL total volume) contained 1× PCR buffer (Gibco-BRL Life Technologies), 3 mM MgCl2, 300 μM of each deoxynucleotide triphosphate (dNTP), 45 pmol of each IBDV-specific primer, 5 U of Taq DNA polymerase (Gibco-BRL Life Technologies), 1.5 pmol of TaqMan probe, and 1 μL of cDNA template. The PCR conditions were as follows: initial denaturation at 95°C for 3 min, followed by 45 cycles of a repetitive program of 30 s denaturation at 95°C, 30 s annealing at 60°C, and 30 s extension at 72°C.
Oligonucleotide primers for 18S rRNA, as a normalizer were used to amplify a 186 bp product (13): 18S sense 5′-CGGCTACCACATCCAAGGAA-3′ (position 370 to 389 nt) and 18S anti-sense 5′-GCTGGAATTACCGCGGCT-3′ (position 539 to 556 nt). To quantify the relative abundance of 18S rRNA, TaqMan probe was used (13): 5′-(FAM)-TGCTGGCACCAGACTTGCCCTC-(TAMRA)-3′. The PCR mixture (50 μL total volume) contained 1× PCR buffer (Gibco-BRL Life Technologies), 3 mM MgCl2, 300 μM of each dNTP, 300 nM of each 18S-specific primer, 5 U of Taq DNA polymerase (Gibco-BRL), 50 nM of 18S-TaqMan probe, and 1 μL of cDNA template. The PCR conditions were as follows: initial melt at 95°C for 3 min, followed by 40 cycles of a repetitive program of 15 s at 95°C, and 60 s at 62°C. The IBDV and 18S amplifications were performed in separate tubes.
A bursa cDNA sample from a chicken vaccinated with IBDV was 10-fold serially diluted from 10−1 to 10−5 for 18S rRNA and 5-fold serially diluted in 4 steps, beginning from the 10-fold diluted sample for IBDV (10×, 50×, 250×, etc.) to achieve calibration curves.
Correlation coefficient and PCR efficiency calculated by using computer software (iCycler Software; Bio-Rad) in order to show the accuracy and linearity of the measurements. Results were expressed in the threshold cycle value (CT), the cycle at which the change in the emission of fluorescence of the reporter dye passes a significance threshold as calculated by the computer software (iCycler Software; Bio-Rad). The higher the initial amount of DNA of interest, the sooner accumulated product is detected in the PCR process and the lower the CT value. Standardization to normalize for differences in the amount of total RNA added to each reaction was made according to the comparative CT method (ΔΔCT method). For real-time PCR, each sample was repeated in triplicates. Each experiment contained no template controls, negative control bursa of Fabricius sample from the uninoculated group, positive control bursa of Fabricius samples from virulent IBDV vaccinated chickens, test samples, and dilution series.
Immunofluorescence assay (IFA)
Sections were stained by using standard immunofluorescence techniques (14) with some modifications. In brief, cryostat sections of 5 to 7 μm thick were picked up onto poly-L-Lysin (Sigma Diagnostics, St. Louis, Missouri, USA) treated slides (Multitest Slides; ICN Biomedical, Costa Mesa, California, USA). They were air-dired and fixed in pure acetone for 10 min. After rehydration in phosphate-buffered saline solution (PBSS) contaning 4% (v/v) inactivated horse serum for blocking non-specific binding sites (pH = 7.1), the sections were incubated with a previously determined dilution of polyclonal (rabbit anti-VP2 of IBDV; 1:50) and mouse monoclonal (anti-μ; 1:200) antibodies (70 μL/section) for 1 h at 37°C in a humidified chamber. Slides were rinsed 3 times in washing buffer (PBSS + 0.05% TWEEN 20) and were subsequently incubated with swine anti- rabbit IgG conjugated with fluorescein isothiocyanate (FITC; DAKO, Glostrup, Denmark) and/or rabbit anti-mouse FITC conjugate, as described above, at a dilution recommended by the manufacturer. At the completion of the procedure, contrast staining was performed in 0.01% Evans Blue. After rinsing in distilled water, the slides were dried and mounted (Supermount Permanent Aqueous Mounting Medium; BioGenex, San Ramon, California, USA) and viewed by using an epifluorescence microscope (Nikon Optophot; Nikon, Melville, New York, USA).
Enzyme-linked immunosorbent assay (ELISA)
Antibody titres against IBDV were determined by using an ELISA kit (FlockChek Infectious Bursal Disease Antibody Test Kit; IDEXX Laboratories, Westbrook, Maine, USA) following the protocol provided by the manufacturer. Titers were calculated according to the manufacturer’s instructions and serum samples with titers greater than 396 were considered positive. Results were expressed as geometric mean antibody titers (GMT) calculated from serum samples of 3 chickens.
Results
Reverse transcriptase-polymerase chain reaction and nested PCR
The detection limit of the RT-PCR and nested PCR assays for the detection of IBDV were determined. After RT-PCR amplification of the serially 10-fold diluted IBDV strain, a distinct fragment of 202 bp was observed in the dilution containing 104 PFU viruses. The nested PCR showed a distinct fragment of 472 bp in the dilution containing 101 PFU IBDV (data not shown).
Results of detection of specific RNA for IBDV VP2 gene by RT-PCR are shown in Tables I and II. In the IBDV-Icx vaccinated SPF group (Table I), the 202 bp fragment was detected in the bursa of Fabricius on day 14 and day 21 PV. In the IBDV-Icx vaccinated broiler group (Table II), 2 out of 3 bursa samples on day 17 PV, 1 out of 2 on day 21 PV, and all bursa samples on the subsequent sampling days were IBDV positive, until the end of the trial period on day 43 PV. Infectious bursal disease virus was not detected in the spleen, thymus, cecal tonsils, liver, or duodenum in any of the vaccinated groups. Furthermore, every tissue from the uninoculated control groups remained consistently negative at each sampling interval (data not shown).
Table I.
Virus detection by reverse transcriptase-polymerase chain reaction (RT-PCR) and immunofluorescence assay (IFA) in various organs and serological response detected by enzyme-linked immunosorbent assay (ELISA) in serum samples from specific pathogen free (SPF) chickens inoculated with immune complex infectious bursal disease vaccine (IBDV-Icx)
| RT-PCR
|
IFA | ELISA | ||||||
|---|---|---|---|---|---|---|---|---|
| Hours and days PV | BF | SP | TH | CT | L | D | BF | Serum |
| 5h | 0/1a | 0/2 | 0/3 | nd | 0/3 | 0/3 | 0/1 | 1.78b |
| 1 | 0/3 | 0/2 | 0/3 | nd | 0/3 | 0/3 | 0/1 | 2.88 |
| 3 | 0/3 | 0/3 | 0/3 | nd | 0/3 | 0/2 | 0/1 | 2.82 |
| 6 | 0/3 | 0/3 | 0/3 | 0/1 | nd | nd | 0/1 | 2.09 |
| 10 | 0/3 | 0/3 | 0/3 | 0/1 | 0/3 | 0/3 | 0/1 | 3.24 |
| 14 | 3/3 | 0/2 | 0/3 | 0/2 | nd | nd | 1/1 | 832.00 |
| 21 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/2 | 1/1 | 794.00 |
nd — Not determined; PV — post vaccination; BF — bursa of Fabricius; SP — spleen; TH — thymus;
CT — cecal tonsils; L — liver; D — duodenum
Number positive/total tested
Results are expressed as geometric mean antibody titers (GMT) of 3 serum samples
Table II.
Virus detection by reverse transcriptase-polymerase chain reaction (RT-PCR) in various organs and serological response detected by enzyme-linked immunosorbent assay (ELISA) in serum samples from maternally immune broiler chickens inoculated with immune complex infectious bursal disease vaccine (IBDV-Icx)
| RT-PCR
|
ELISA | ||||||
|---|---|---|---|---|---|---|---|
| Days PV | BF | SP | TH | CT | L | D | Serumb |
| 14 | 0/3a | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 354.8c |
| 17 | 2/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 343.1 |
| 21 | 1/2 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 316.2 |
| 28 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 2290.9 |
| 34 | 3/3 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2089.3 |
| 43 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3090.3 |
PV — post vaccination; BF — bursa of Fabricius; SP — spleen; TH — thymus; CT — cecal tonsils;
L — liver; D — duodenum
Number positive/total tested
ELISA results from day 7 and day 10 PV are indicated in the text
Results are expressed as geometric mean antibody titers (GMT) of 3 serum samples
All bursa samples and the randomly tested spleen, thymus, and cecal tonsil samples that were found to be negative by RT-PCR in both previous experiments, were tested by nested PCR. Every sample negative by RT-PCR also proved to be negative by nested PCR (data not shown).
Real-time PCR analysis of the bursa of Fabricius samples
For real-time analysis, the tissue samples used were previously found to be positive by RT-PCR. Thus, the following samples were included: bursa of Fabricius samples from the IBDV-Icx vaccinated SPF group from day 14 and day 21 PV, and bursa of Fabricius samples from the IBDV-Icx vaccinated broiler group from days 17, 21, 28, 34, and 43 PV. Bursae from the virulent IBDV infected SPF chickens were used as positive controls. The bursa of Fabricius samples, from day 22, from the uninoculated control group and a no-template control were used as negative controls.
Calibration curves were constructed and linearity was achieved for 18S- and IBDV-specific primer sets in 5 and 4 orders of magnitude, respectively, under the conditions we used. Reproducibility of the replicate measurements was sufficient, as shown by the standard deviation values (Tables III A and B). Regression analysis of the CT values generated by the dilution series obtained the following values: 0.999 correlation coefficient and 92.8% of PCR efficiency for 18S rRNA and 0.992 correlation coefficient and 100.7% of PCR efficiency for IBDV. The slopes of the regression lines were 3.508 and 3.306 for 18S rRNA and IBDV, respectively.
Table III.
The threshold cycle (CT) values for the replicate measurements of 18S rRNA (A) and infectious bursal disease virus (IBDV) (B) standard curves
| A
| |||||
|---|---|---|---|---|---|
| CT values for cDNA dilutions
|
|||||
| Replicates of 18S rRNA standard curve | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 |
| 1 | 13.5 | 17.1 | 20.6 | 24.1 | 27.7 |
| 2 | 13.6 | 17.0 | 20.7 | 24.4 | 27.0 |
| 3 | 13.6 | 17.3 | 20.9 | 24.6 | 27.8 |
| Mean | 13.57 | 17.13 | 20.73 | 24.37 | 27.50 |
| s | 0.06 | 0.15 | 0.15 | 0.25 | 0.44 |
| B
| |||||
| CT values for cDNA dilutions
|
|||||
| Replicates of IBDV standard curve | 10× | 50× | 250× | 1250× | |
| 1 | 24.4 | 26.7 | 29.6 | 30.8 | |
| 2 | 24.4 | 26.7 | 29.4 | 31.2 | |
| 3 | 23.9 | 27.0 | 29.5 | 31.2 | |
| Mean | 24.23 | 26.80 | 29.50 | 31.07 | |
| s | 0.29 | 0.17 | 0.10 | 0.23 | |
S — standard deviation
To control for variation in sampling and RNA preparation the CT values for IBDV-specific product were standardized for each sample of interest by the CT values for 18S rRNA, as a housekeeping gene. The 18S rRNA was a proper normalizer for these experiments as its expression levels remained constant during the infection period: 10.4 and 10.8 CT (on day 14 and day 21 PV, respectively) in the SPF chickens and between 10.5 and 11.9 CT (during the period between day 17 and day 43 PV) in the broiler chickens. On each sampling day, 3 bursa samples were processed (except on day 21 PV in the broiler chicken experiment when 2 bursa samples were used) and each sample was used in triplicate, for real-time PCR analysis. The means of the triplicates were calculated and differences between IBDV-specific and 18S-specific CT values (ΔCT) were determined for each sample. As a reference (baseline), the non-vaccinated bursa sample was chosen and its ΔCT was applied to determine the ΔΔCT values and than the relative viral load levels (2−ΔΔCT) for each sample. Finally, results were expressed as N-times increase in the viral load compared to the uninoculated control reference level.
In the broiler chicken experiment, 1 out of 3 bursa of Fabricius at day 17 PV and 1 out of 2 bursa of Fabricius at day 21 PV remained negative since the IBDV-specific ΔCT values were comparable to the ΔCT of the uninoculated negative control (20.67, 20.60, and 21.2, respectively). These 2 samples were negative by RT-PCR as well (Table II), and they were not included in the calculations as, due to the lack of virus replication, these samples were regarded as not informative enough for the purpose of quantitative studies. In the SPF chicken group, all of the bursa of Fabricius were infected by the IBDV-Icx vaccine virus. In this group, the relative viral load in the bursa samples decreased between day 14 and day 21 PV with values from 622 to 148 times above the uninoculated control value as a baseline (Figure 1). In the broiler chicken experiment, the highest viral load at day 17 and day 21 PV decreased by day 28 and further by day 34 and day 43 PV (Figure 1). The highest viral load (14263.1 times above the baseline) was measured in the positive control bursa (data not shown).
Figure 1.

Relative quantification of infectious bursal disease virus (IBDV) RNA in bursa of Fabricius of specific pathogen free (SPF) chickens and broiler chickens vaccinated with immune complex IBD vaccine (IBDV-Icx). Results are expressed as N-times increase in the viral load compared to the uninoculated control reference level. Mean values were calculated from 2 to 3 bursa samples collected on the same day and measured 3 times. The standard deviations are indicated. D — day; PV — post vaccination.
Immunofluorescence assay
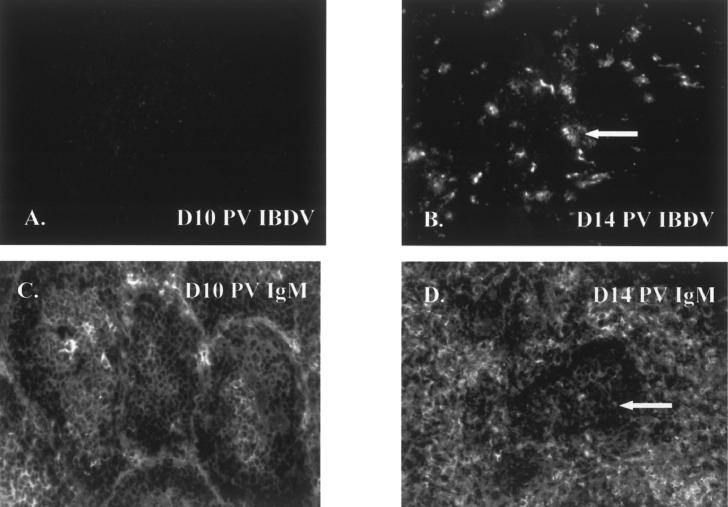
Infectious bursal disease virus-specific antigens, indicating IBDV replication, were seen in the bursa of Fabricius sections from IBDVI-cx vaccinated SPF chickens and collected at day 14 and day 21 PV (Table I, Figure 2B). In bursa of Fabricius samples from other time points, barely visible, scattered, very small spots were present, which were qualified as non-specific background reaction (Figure 2A). Tissue samples collected any time point of the experiment from the thymus, spleen, and cecal tonsils proved to be IBDV-antigen negative (data not shown).
Figure 2.
Immunofluorescence assay (IFA) staining of bursa of Fabricius sections stained with antibodies against infectious bursal disease virus (IBDV) (A,B) or IgM (C,D) from specific pathogen free (SPF) chickens vaccinated with immune complex IBD vaccine (IBDV-Icx). At day 10 post vaccination (PV), the IBDV antigen was not detectable (A) and the bursa tissue showed normal follicular structure (C). By day 14 PV, IBDV-specific antigens were indicative of IBDV replication (B) together with destroyed follicular structure, lymphocyte depletion, and inflammatory signs in the interfollicular tissue (D). Arrows show IBDV-specific antigens (B) and lymphocyte depletion (D). Magnification: 200×.
The structure of the bursa of Fabricius was intact until day 10 PV, as detected by staining for antibody IFA against IgM (Figure 2C), but signs of virus replication, such as depletion of lymphocytes, atrophy of the follicles, and inflammatory signs in the interfollicular tissue, were detected at day 14 PV (Figure 2D) and onward (data not shown).
Enzyme-linked immunosorbent assay
An active immune response to IBDV-Icx vaccination could be observed in both vaccinated groups, as monitored by using an ELISA (Tables I and II). The SPF chickens seroconverted by day 14 PV (Table I) when the geometric mean titer (GMT) was 832 and remained at this level until day 21 PV. Serum samples from the uninoculated SPF chicken group remained negative (data not shown). Maternal antibody GMTs of 1820 at day 7 PV and 759 at day 10 PV were detected in the vaccinated broiler chicken group and declined to 355 by day 14 PV, below the baseline value of 396 given by the manufacturer (Table II). Two weeks later, on day 28 PV, IBDV-specific antibodies appeared and further increased by day 43 PV, until the end of the experiment. In the uninoculated broiler chicken group, maternal antibody levels declined below the baseline value by day 14 PV in the absence of an active antibody response (data not shown).
Discussion
The tissue distribution of the subcutaneously applied IBDV-Icx virus and the quantitative analysis of the viral load in the IBDVpositive organs were studied in this paper. The vaccine virus was detected in the bursa of Fabricius in SPF chickens from day 14 PV until the end of the experiment, at day 21 PV. Appearance of IBDV as late as day 14 PV was unexpected, comparing to the results obtained from in ovo IBDV-Icx experiments. Corley et al (15) demonstrated the IBDV-Icx virus proteins in the bursa of Fabricius in SPF chickens at day 6 post in ovo vaccination (PIOV) using antigen capture ELISA (ACCE). Jeurissen et al (4) detected the IBDV-Icx virus in the bursa of Fabricius by immunohistochemistry from day 10 until day 19 PIOV, when only a few small spots of the virus were seen. Furthermore, in our experiment no virus was found in any other tissues we tested, while IBDV-Icx antigen was detected in the spleen and thymus by others (4,15). To confirm our results, IFA and a more sensitive method, the nested PCR, were used to test all bursa of Fabricius samples.
Results coincided with those obtained by RT-PCR, namely, IBDV was detected only in the bursa of Fabricius on day 14 and day 21 PV (Table I, Figure 2). As the results of the IFA, nested PCR, and RT-PCR were the same in the case of bursa of Fabricius, similar results were expected for the spleen, thymus, and cecal tonsil samples, therefore, these were tested only randomly and all proved to be negative.
Sensitivity of the PCR assays utilized in our study was similar to those published by Phong et al (16) and Wu et al (17). The discrepancy between results in the literature (earlier detection in the bursa of Fabricius and positive signals in the thymus and spleen) and our results (later detection and lack of signal in other lymphoid tissues) could be due to the different route and timing of vaccine delivery. Sharma (18) found differences between vaccination in ovo or at hatch, namely, tissue distribution, was more extensive and virus levels in tissues were higher if the vaccine was given in embryonated eggs. Komine et al (19) demonstrated that vaccine efficiency and virus detection after vaccination depend on the mode of application. Based on the results of Jochemsen and Jeurissen (20) an in ovo applied antigen is taken up by the embryo orally and transferred into the gastrointestinal system and the lungs, unlike in the situation following subcutaneous application. Therefore, it can be assumed that different types of cells, lymphoid tissues, and/or mechanisms may take part in the immune response against the same antigen resulting in different pattern of virus replication. It should also be taken into consideration that Icx vaccines of different companies may contain different amounts of specific antibodies (in view of the confidentiality of the composition). Kumar and Charan (21) have shown that IBDV coated with different units of antibodies showed different degrees of replication. Consequently, the amount of antibodies in the IBDV-Icx could exert an influence on the onset and degree of virus replication.
In the maternally immune broiler chickens, the virus was detected between day 17 and day 43 PV, until the end of the trial period. In this group, a few days delay was observed in the first detection when compared to the results of the SPF chickens, probably due to the inhibiting effect of Mabs. The decline of Mab was previously found to correlate with timing of bursal destruction caused by the IBDV-Icx (22). In this study, decline of ELISA titers by day 14 PV could allow the onset of virus replication around this time and the first detection 3 d later. Corley et al (23) examined the distribution of IBDV-Icx virus in tissues in the presence of high levels of Mab and detected it as early as day 3 PIOV in the bursa of Fabricius by nested PCR (unfortunately, it is not clear whether the thymus and spleen were tested or not). On the other hand, ACCE was not able to show IBDV antigen in any tissue tested (23). Nested PCR was used in this experiment and virus was undetectable before day 18 in the bursa of Fabricius, furthermore, any other tissue tested randomly remained negative. However, persistence of the vaccine virus in the broiler chicken group was unusually long. In the broiler chickens, only one study reported similar length of persistence of a variant, non-attenuated IBDV strain given to 1-day-old maternally immune chickens and detected after between 2 and 6 wk by using RT-PCR (24). In SPF chickens, IBDV persisted for about 4 wk in the bursa of Fabricius after inoculation at 2 wk of age with a variant IBDV strain (25) or with a bursa of Fabricius-derived IBDV strain (26).
A relative quantification of viral load was made in the IBDVpositive bursa of Fabricius samples originating from different time points after vaccination. Previous studies used real-time PCR to measure IBDV RNA levels or to detect mutations in IBDV strains (27–30). In the present study, the sequence-specific TaqMan probe was used for the 18S rRNA and for IBDV, in separate tubes. The widely used 18S rRNA was chosen as an internal control because of its abundance and stability during the infection. For example, the average CT values for 18S in the broiler experiment were 10.9, 11.0, and 11.1 at day 17, day 28, and day 34 PV, respectively, indicating the consistency throughout the infection. However, some individual samples showed fluctuations in the CT values (the biggest difference between 2 samples was 2.8 CT representing about 7 times difference in dilution) originating from sampling and RNA purification. To minimize these variations, the differences for 18S rRNA were used to correct the CT values for IBDV. In both experiments, the highest virus amounts were detected in those bursa samples, which proved to be RT-PCR positive at the earliest time, at day 14 PV in the SPF chickens and at day 17 to day 21 PV in the broiler chicken group (Table II, Figure 1). These levels gradually declined during the trial period. As both groups sero-converted due to vaccination, this decline could be explained by the active immune response that restricted the virus replication and induced gradual clearance of the virus from the bursa. In the SPF chicken group, the relative virus level reduced to one quarter during 1 wk. In the broiler chicken group, viral levels decreased from about 600 to 10 during the 4-week trial period (Figure 1). Moody et al (27) showed that IBDV completely cleared from the blood by the 4th d after infection, however, the virus persists for a longer time in the bursa of Fabricius, the target organ for virus replication (31,32). The detectable levels of vaccine virus were measured by the end of both experiments in the bursa of Fabricius indicating even longer persistence than shown.
In conclusion, the subcutaneous IBDV-Icx vaccination resulted in delayed vaccine virus replication in the bursa of Fabricius compared to in ovo vaccination, and it also caused long persistence of IBDV-Icx virus in maternally immune broiler chickens. Gradually decreased viral RNA levels were measured in the positive organs of both SPF chicken and broiler chicken groups throughout the experimental period. The applied real-time PCR procedure has been proven to be an accurate, sensitive, and reproducible quantitative tool to compare the relative viral levels in the bursa.
Acknowledgments
The authors thank Dr. Béla Lomniczi and Dr. Gábor M. Kovács (Veterinary Medical Research Institute, Hungarian Academy of Sciences) for their suggestions and Dr. József Tóvári (National Institute of Oncology, Department of Tumor Progression) for his technical help. This work was supported by the National Research and Development Program (NKFP) 4/040/2001 and Ministry of Agriculture and Rural Development (FVM) KF-139/10 in Hungary.
References
- 1.Kibenge FSB, Dhillon AS, Russel RG. Biochemistry and immunology of infectious bursal disease virus. J Gen Virol. 1988;69:1757–1775. doi: 10.1099/0022-1317-69-8-1757. [DOI] [PubMed] [Google Scholar]
- 2.Sharma JM. Embryo vaccination of specific-pathogen-free chickens with infectious bursal disease virus: tissue distribution of the vaccine virus and protection of hatched chickens against disease. Avian Dis. 1986;30:776–780. [PubMed] [Google Scholar]
- 3.Whitfill CE, Haddad EE, Ricks CA, et al. Determination of optimum formulation of a novel infectious bursal disease virus (IBDV) vaccine constructed by mixing bursal disease antibody with IBDV. Avian Dis. 1995;39:687–699. [PubMed] [Google Scholar]
- 4.Jeurissen SHM, Janse EM, Lehrbach PR, Haddad EE, Avakian A, Whitfill CE. The working mechanism of an immune complex vaccine that protects chickens against infectious bursal disease. Immunol. 1998;95:494–500. doi: 10.1046/j.1365-2567.1998.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad EE, Whitfill CE, Avakian AP, et al. Efficacy of a novel infectious bursal disease virus immune complex vaccine in broiler chickens. Avian Dis. 1997;41:882–889. [PubMed] [Google Scholar]
- 6.Chettle NJ, Wyeth PJ. Protection against challenge with very virulent infectious bursal disease vaccine using various vaccines and different regimes. Proceedings of International Symposium on Infectious Bursal Disease and Chicken Anaemia, Rauischholzhausen, Germany. 1994:280–285.
- 7.Kelemen M, Forgách K, Iván J, et al. Pathological and immunological study of an in ovo complex vaccine against infectious bursal disease. Acta Vet Hung. 2000;48:443–454. doi: 10.1556/004.48.2000.4.7. [DOI] [PubMed] [Google Scholar]
- 8.Ricks CA, Avakian A, Bryan T, et al. In ovo vaccination technology. Adv Vet Med. 1999;41:495–515. [PubMed] [Google Scholar]
- 9.Liu HJ, Giambrone JJ, Dormitorio T. Detection of genetic variations in serotype 1 isolates of infectious bursal disease virus using polymerase chain reaction and restriction endonuclease analysis. J Virol Meth. 1994;48:281–291. doi: 10.1016/0166-0934(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Kato A, Otaki Y, Nakamura T, Sasmaz E, Ueda S. Sequence comparisons of highly virulent infectious bursal disease virus prevalent in Japan. Avian Dis. 1993;37:315–323. [PubMed] [Google Scholar]
- 11.Kibenge FS, Jackwood DJ, Mercado CC. Nucleotide sequence analysis of genome segment A of infectious bursal disease virus. J Gen Virol. 1990;71:569–577. doi: 10.1099/0022-1317-71-3-569. [DOI] [PubMed] [Google Scholar]
- 12.Skeeles JK, Lukert PD, Fletcher OJ, Leonard JD. Immunization studies with a cell-culture-adapted infectious bursal disease virus. Avian Dis. 1979;23:456–465. [Google Scholar]
- 13.Pivarcsi A, Bodai L, Réthi B, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–730. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 14.Allan GM, McNulty MS, Connor TJ, McCracken RM, McFerran JB. Rapid diagnosis of infectious bursal disease infection by immunofluorescence on clinical material. Avian Pathol. 1984;13:419–427. doi: 10.1080/03079458408418544. [DOI] [PubMed] [Google Scholar]
- 15.Corley MM, Giambrone JJ, Dormitorio TV. Detection of infectious bursal disease vaccine viruses in lymphoid tissues after in ovo vaccination of specific-pathogen-free embryos. Avian Dis. 2001;45:897–905. [PubMed] [Google Scholar]
- 16.Phong SF, Hair-Bejo M, Omar AR, Aini I. Sequence analysis of Malaysian infectious bursal disease virus isolate and the use of reverse transcriptase nested polymerase chain reaction enzymelinked immunosorbent assay for the detection of VP2 hypervariable region. Avian Dis. 2003;47:154–162. doi: 10.1637/0005-2086(2003)047[0154:SAOMIB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Wu CC, Lin TL, Zhang HG, Davis VS, Boyle JA. Molecular detection of infectious bursal disease virus by polymerase chain reaction. Avian Dis. 1992;36:221–226. [PubMed] [Google Scholar]
- 18.Sharma JM. Embryo vaccination of specific-pathogen-free chickens with infectious bursal disease virus: tissue distribution of the vaccine virus and protection of hatched chickens against disease. Avian Dis. 1986;30:776–780. [PubMed] [Google Scholar]
- 19.Komine K, Ohta H, Fujii H, Watanabe Y, Kamata S, Sugiyama M. Efficacy of subcutaneous application of live infectious bursal disease vaccine in young chickens with maternally derived antibody. J Vet Med Sci. 1995;57:647–653. doi: 10.1292/jvms.57.647. [DOI] [PubMed] [Google Scholar]
- 20.Jochemsen P, Jeurissen SHM. The localization and uptake of in ovo injected soluble and particulate substances in the chicken. Poultry Sci. 2002;81:1811–1817. doi: 10.1093/ps/81.12.1811. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, Charan S. Virus enhancement following infection with antibody-coated infectious bursal disease virus (IBDV) in chickens. Ind J Exp Biol. 2001;39:1314–1317. [PubMed] [Google Scholar]
- 22.Iván J, Nagy N, Magyar A, Kacskovics I, Mészáros J. Functional restoration of the bursa of Fabricius following in ovo infectious bursal disease vaccination. Vet Immunol Immunopathol. 2001;79:235–248. doi: 10.1016/s0165-2427(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 23.Corley MM, Giambrone JJ, Dormitorio TV. Evaluation of the immune response and detection of infectious bursal disease viruses by reverse transcriptase-polymerase chain reaction and enzyme-linked immunosorben assay after in ovo vaccination of commercial broilers. Avian Dis. 2002;46:803–809. doi: 10.1637/0005-2086(2002)046[0803:EOTIRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Elankumaran S, Heckert RA, Moura L. Pathogenesis and tissue distribution of a variant strain of infectious bursal disease virus in commercial broiler chickens. Avian Dis. 2002;46:169–176. doi: 10.1637/0005-2086(2002)046[0169:PATDOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Henderson KS, Jackwood DJ. Comparision of the dot blot hybridization assay with antigen detection assays for the diagnosis of infectious bursal disease virus infections. Avian Dis. 1990;34:744–748. [PubMed] [Google Scholar]
- 26.Abdel-Alim GA, Saif YM. Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Dis. 2001;45:646–654. [PubMed] [Google Scholar]
- 27.Moody A, Sellers S, Bumstead N. Measuring infectious bursal disease virus RNA in blood by multiplex real-time quantitative RT-PCR. J Virol Meth. 2000;85:55–64. doi: 10.1016/s0166-0934(99)00156-1. [DOI] [PubMed] [Google Scholar]
- 28.Jackwood DJ, Sommer SE. Identification of infectious bursal disease virus quasispecies in commercial vaccines and field isolates of this double-stranded RNA virus. Virology. 2002;304:105–113. doi: 10.1006/viro.2002.1724. [DOI] [PubMed] [Google Scholar]
- 29.Jackwood DJ, Spalding BD, Sommer SE. Real-time reverse transcriptase-polymerase chain reaction detection and analysis of nucleotide sequences coding for a neutralizing epitope on infectious bursal disease viruses. Avian Dis. 2003;47:738–744. doi: 10.1637/6092. [DOI] [PubMed] [Google Scholar]
- 30.Rautenschlein S, Yeh H-Y, Njenga MK, Sharma JM. Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Arch Virol. 2002;147:285–304. doi: 10.1007/s705-002-8320-2. [DOI] [PubMed] [Google Scholar]
- 31.Sharma JM, Kim I-J, Rautenschlein S, Yeh H-Y. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev Comp Immunol. 2000;24:223–235. doi: 10.1016/s0145-305x(99)00074-9. [DOI] [PubMed] [Google Scholar]
- 32.Van den Berg TP, Eterradossi N, Toquin D, Meulemans G. Infectious bursal disease (Gumboro disease) Rev Sci Tech Off Int Epiz. 2000;19:527–543. [PubMed] [Google Scholar]