Abstract
The aim of this study was to evaluate the efficacy of in-feed medication with tilmicosin phosphate in order to eliminate or reduce the carriage of Actinobacillus pleuropneumoniae in the tonsils of carrier pigs. Two groups of 6 carrier animals received either a non-medicated feed (control group) or feed medicated with 400 ppm of tilmicosin phosphate (treated group) for 30 d. Three sentinel pigs were then introduced in each group and left for 29 d. The presence of A. pleuropneumoniae in tonsils was monitored using several techniques, including polymerase chain reaction (PCR). At the end of the treatment all of the control animals, but only 1 treated pig, were positive by PCR from tonsillar surface material. However, at necropsy, all control and most treated animals, as well as 1 sentinel animal, in both groups were positive by PCR from whole tonsils. In conclusion, under the experimental conditions, in-feed treatment with 400 ppm of tilmicosin phosphate significantly reduced the presence of A. pleuropneumoniae on the surface of tonsils but was unable to completely eliminate the organism from deeper tonsillar tissues and to prevent bacterial shedding by carrier animals.
Résumé
La présente étude avait par but d’évaluer l’efficacité du phosphate de tilmicosine en vue d’éliminer ou réduire la quantité d’Actinobacillus pleuropneumoniae présente dans les amygdales de porcs sains porteurs du micro-organisme. Deux groupes de 6 animaux porteurs ont reçu pendant 30 jours de l’aliment non médicamenté (groupe témoin) ou médicamenté avec du phosphate de tilmicosine 400 ppm (groupe traité). À la fin du traitement, 3 porcs sentinelles ont été introduits dans chaque groupe et laissés pendant 29 jours. Le statut des animaux envers A. pleuropneumoniae a été évalué par différentes techniques, dont l’amplification en chaîne par la polymérase (PCR). À la fin du traitement, tous les témoins mais 1 seul porc traité se sont avérés positifs par PCR à partir de grattages d’amygdale. Cependant, tous les témoins, la plupart des animaux traités et une sentinelle dans chaque groupe se sont montrés positifs par PCR à partir d’amygdales entières récoltées à l’abattage. En conclusion, le traitement avec du phosphate de tilmicosine permet une réduction significative de la présence de la bactérie à la surface des amygdales des animaux porteurs. Toutefois, dans les conditions de l’étude, il n’élimine pas complètement l’organisme présent dans les amygdales ni son excrétion par les animaux porteurs.
(traduit par les auteurs)
Porcine pleuropneumonia, caused by Actinobacillus pleuropneumoniae, is an important disease in swine production that has a significant impact on animal welfare and production economy (1). Consequences of infection may range from acute fibrinous pneumonia and pleuritis with high mortality to subclinical infection (1). Two biovars and 15 serotypes have been described for A. pleuropneumoniae (1,2), the prevalence and virulence of which vary with geographic location. Serotypes 1, 5, and 7 are the serotypes most commonly isolated from animals with clinical signs or pathological lesions in North America (3).
Actinobacillus pleuropneumoniae may be transmitted between herds through various vectors including infected animals, contaminated aerosols, and fomites. Subclinically infected pigs (carrier animals) are considered to be the leading cause of dissemination of the organism (1). In such animals, A. pleuropneumoniae is found in the upper respiratory tract, mainly in the palatine tonsils (4). To prevent the introduction of A. pleuropneumoniae into non-infected herds, animals are routinely examined for its presence. Sensitive diagnostic tools, including serological tests such as enzyme-linked immunosorbent assays (ELISA) (5–7), bacteriological isolation from tonsils following enrichment using magnetic beads (immunomagnetic separation [IMS] isolation) (8), and polymerase chain reaction (PCR) tests (9) are available for the detection of A. pleuropneumoniae. Although preventive antibiotic treatment and vaccination may be helpful in controlling the disease, it has been suggested that eradication of the organism could be the best course of action to eliminate porcine pleuropneumonia (1). Total depopulation and repopulation with A. pleuropneumoniae-free animals is considered the most effective procedure for eradication (1). In addition, medicated early weaning has been used to produce A. pleuropneumoniae-free pigs from infected sows (10). However, the costs of these methods are high (11).
A judicious antibiotic-based strategy may be a useful tool in eradication programs based on partial depopulation. This may lead to the elimination of the carrier state or, at least, may prevent shedding by reducing the amount of bacteria present in tonsils. Tilmicosin phosphate is a chemically modified macrolide that has proven to successfully inhibit the growth of field strains of A. pleuropneumoniae in vitro (12). Moreover, using experimentally infected animals, as well as naturally infected growing and finishing pigs, it has been shown that tilmicosin is effective in controlling acute porcine pleuropneumonia (13–15). The aim of the present study was to evaluate the ability of in-feed medication with tilmicosin to eradicate or reduce the amount of A. pleuropneumoniae in carrier pigs.
All the animals used in this study were treated in accordance with the Canadian Council on Animal Care Guidelines on Animal Use. Nineteen pigs were purchased from a herd considered negative for A. pleuropneumoniae serotypes 1 to 12 on the basis of serological monitoring using ELISA (6) during the previous 3 y. The 19 animals were at 35 d of age at the time of their introduction to the trial. On day −44, individually identified pigs were transferred to experimental farm 1 and placed in a pen with a lighting program, heating, ventilation, and other management procedures typical of modern swine farms in Canada. The facilities had been thoroughly cleaned and disinfected prior to introducing the animals. The farm contained no other animal and no other pig farm was present within 1 km. Pigs were provided water and food (nutritionally complete non-medicated pre-grower mix prepared at a local commercial mill) ad libitum. Upon arrival, animals tested negative for antibodies against A. pleuropneumoniae using an indirect ELISA (6), negative for porcine reproductive and respiratory syndrome virus (PRRSV) using an ELISA (IDEXX HerdChek PRRS 2XR ELISA kit; IDEXX Laboratories, Westbrook, Maine, USA), and negative for Mycoplasma hyopneumoniae using an M. hyopneumoniae ELISA kit (DakoCytomation, Carpinteria, California, USA).
The absence of A. pleuropneumoniae at the surface of the palatine tonsils was also evaluated by PCR in all pigs. Briefly, superficial material from palatine tonsils was obtained by brushing using cytology brushes (Cytosoft cytology brushes; Medical Packaging Corporation, Camarillo, California, USA). The brushes were placed in 1 mL of phosphate buffered saline solution (PBSS; 0.14 M NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) and immediately sent to the laboratory, where they were centrifuged at 14 000 × g for 10 min. The pellet was resuspended in 100 mL of sterile PBSS and then plated onto selective pleuropneumonia-like organisms agar (Difco, Detroit, Michigan, USA). After overnight incubation at 37°C (5% CO2), total bacterial growth was harvested with 3 mL Tris-ethelyenediamine tetraacetic acid (EDTA) buffer (TE buffer), pH 8. Total DNA was purified (Qiamp DNA MiniKit; Qiagen, Mississauga, Ontario) from 100 μL of this bacterial suspension, as per the manufacturer’s instructions. The PCRs were carried out using a kit (Adiavet A. pleuropneumoniae PCR kit; Adiagène, St-Brieuc, France), as recommended by the manufacturer. This test has been validated and proven to be sensitive (83%) and specific (100%) for A. pleuropneumoniae (9).
A seeder-pig challenge-exposure model of infection (16) was used to generate carriers of A. pleuropneumoniae. Briefly, after 5 d of acclimation (day −39), 3 pigs (seeder pigs) were randomly selected from the 19 animals and intranasally infected with 107 colony forming units (CFU) of A. pleuropneumoniae serotype 1 field strain FMV 91-6514 (sensitive to tilmicosin; MIC 16 μg/mL), then placed back in contact with the remaining animals. Within 10 d all of the pigs showed clinical signs typical of porcine pleuropneumonia, including dyspnea, coughing, fever, and prostration, which persisted for 3 to 4 d for most pigs. Seven animals, including 1 seeder pig, died within 3 d post exposure. At necropsy they presented typical lesions of porcine pleuropneumonia. Macroscopic examination of their lungs revealed necrotic zones analogous to those caused by A. pleuropneumoniae. For bacteriological confirmation, lung samples were taken from 3 dead pigs and sent to the diagnostic laboratory, where pure cultures of A. pleuropneumoniae serotype 1 were obtained. The severity of the clinical signs observed was unexpected, since in a previous study using the same infection model, the challenge strain FMV 91-6514 had been found to produce mild clinical signs (17). However, that study had used pigs from a different genetic line obtained from a conventional herd. Since such herds are often subclinically infected with 1 or more serotypes of A. pleuropneumoniae (1), previous exposure of these animals to the organism is likely to have occurred. In the present study, pigs were, in fact, free of the pathogen, as confirmed by the negative results of PCR and serology tests at the beginning of the trial. Hence, the genetic line and/or origin of the animals might have had an impact in their susceptibility to the strain used in this trial.
The number of surviving animals was insufficient for the needs of the experimental design. Therefore, at day −25, 10 additional contemporary pigs were purchased from the same herd and placed with the first group of animals. At that time, none of the surviving pigs presented any clinical signs. However, within 10 d, all 10 newly introduced pigs showed signs of porcine pleuropneumonia, indicating that carrier animals were still shedding bacteria. In addition, the recrudescence of the disease led to the death of 6 other animals (3 of which belonged to the newly introduced group).
On day −3, all of the 16 animals that recovered had become carriers of A. pleuropneumoniae, as demonstrated by PCR from tonsillar superficial material (Table I). Of these animals, 12 pigs infected using the model system were selected and grouped in pairs on the basis of the correspondence of the severity of the clinical signs observed during infection, their body weight, and the time of their introduction to the trial. The 4 remaining pigs were humanely euthanized. Each pig in each pair was randomly assigned to a control or a treated group. Control pigs were moved to a pen similar in dimensions and characteristics to the one described above, but located at experimental farm 2, 20 km away from farm 1 and more than 1 km from any other pig farm. Treated pigs were moved a similar distance and then relocated in a similar clean pen in farm 1.
Table I.
Number of carrier pigs found positive for Actinobacillus pleuropneumoniae by polymerase chain reaction (PCR), either from tonsillar brushing or from whole tonsils, and by immunomagnetic selective isolation (IMS) at different times during the trial in both the control and treated groupsa
| Time (days)
|
||||||
|---|---|---|---|---|---|---|
| −3
|
30
|
59
|
||||
| Method | Treated | Control | Treated | Control | Treated | Control |
| PCR from tonsillar brushing | 6 | 6 | 1b | 6b | 1c | 4 |
| PCR from whole tonsils | nd | nd | nd | nd | 5 | 6 |
| IMS isolation | nd | nd | nd | nd | 5 | 4 |
nd — Not done
Each group was composed of 6 animals
Significant difference (χ2, P < 0.05)
Only 5 animals were tested by PCR from tonsillar brushing at day 59
From days 0 to 30 all animals in both groups were fed the same pregrower ration, except that for treated pigs it was medicated with 400 ppm of tilmicosin phosphate (Pulmotil; Elanco, Division of Eli Lilly Canada, Guelph, Ontario). Composite samples of medicated and non-medicated food were collected and assayed for tilmicosin (13–15). The antibiotic concentration was 376 ppm (94% of claim) in the medicated feed and less than 10 ppm in the non-medicated feed. No other medication was administered to the pigs during the entire study.
On day 30, 3 sentinel pigs were introduced in both the control and treated groups and left in contact with the carrier animals for 29 d. Sentinel animals were purchased from the same high health status herd. Seven days before introduction sentinels had tested negative by serology for A. pleuropneumoniae serotypes 1 to 12, M. hyopneumoniae, and PRRSV (data not shown). None of the sentinel pigs in either group showed any clinical signs until the end of the trial. This suggests that the animals were shedding lower numbers of bacteria at this later stage, although they were highly contagious in the first part of the trial. This hypothesis should be tested using a higher number of sentinels.
Body weight (BW) and girth circumference (GC) were measured on all pigs located on farm 1 on trial days −3, 30, and 59. The BW of pigs located on farm 2 was estimated from their GC using the prediction equation BW = −82.57 + 1.67 × GC, parameters that were obtained by an ordinary least square regression from data collected from pigs on farm 1. Food consumption was recorded on a pen basis during the whole experiment. The calculated food intake of the treated pigs was equivalent to an antibiotic dosage of 22 mg/kg BW: (400 ppm × 5.5%) at the beginning of trial (day 0) and a dosage of 16 mg/kg BW, and (400 ppm × 4%) at the end of treatment (day 30), for a mean of approximately 19 mg/kg BW. Temporal variation of mean pig weight was similar between treated and untreated carrier pigs (χ2, P > 0.05; data not shown). Despite the severity of the clinical signs the experimental animals had suffered, they exhibited food intake and daily weight gain rates similar to those of healthy growing pigs (data not shown). Controversial reports have been published (18–21) on the effects that clinical and subclinical infection with A. pleuropneumoniae may have on production performance. While our results suggest that there is no association between infection with A. pleuropneumoniae and performance, the number of animals used in this study is insufficient to adequately evaluate the impact of pathogen load on productivity.
Blood samples and tonsillar superficial material were also collected from carrier pigs at trial days −3 and 19; from sentinel pigs at days 37, 44, and 51; and from both carrier and sentinel pigs at days 30 and 59. A saline boiled extract was prepared from the homologous strain FMV 91-6514 and used as an antigen to test the animals by indirect ELISA (6). For carrier pigs, ELISA titers increased from low values (mean OD ≤ 0.1) before infection (day −44) to higher values at trial day −3, then remained, in essence, unchanged until the end of the trial (Figure 1). Before, during, and after treatment the titers were lower for treated pigs in comparison to control animals, although the difference was not significant. Sentinel pigs in both groups showed low serological titers throughout the trial (Figure 1). Additionally, 10 contemporary pigs from the same specific pathogen free (SPF) farm where trial animals were purchased showed low titers for A. pleuropneumoniae at day 59 (OD ≤ 0.1, data not shown). The PCR results from tonsillar surface material showed that at day 30, all 6 control animals but only 1 treated animal were positive for A. pleuropneumoniae (Table I). Moreover, the effect of the treatment persisted until the end of the trial even if rations were no longer medicated since day 30, given that on day 59 A. pleuropneumoniae was found by PCR on the tonsillar surface of only 1 treated carrier pig while the presence of the organism was demonstrated in all control animals (Table I). In contrast, none of the sentinel pigs in either group were positive by PCR from tonsillar surface material.
Figure 1.
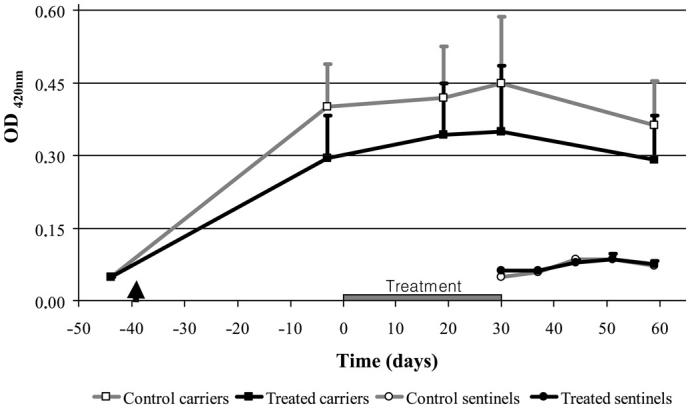
Temporal variation of serological titers against Actinobacillus pleuropneumoniae serotype 1 strain FMV 91-6514 as measured by an indirect enzyme-linked immunosorbent assay (ELISA) in both control and treated carrier pigs, as well as in sentinel animals, within both groups. The arrowhead indicates the time at which seeder pigs were placed in contact with the naive pigs to generate carrier animals. Treated carrier pigs received feed medicated with tilmicosin phosphate at 400 ppm from day 0 to day 30. Naive sentinel animals were introduced in both groups after the end of the tilmicosin treatment. OD — optical density.
At the end of the trial (day 59) all carrier and sentinel pigs were humanely killed and their palatine tonsils collected. Gloves and scalpels were changed between pigs to prevent cross-contamination. The IMS isolation of A. pleuropneumoniae was performed from whole tonsils, as previously described (8), by using immunomagnetic beads (Dynal Biotech, Oslo, Norway) coated with rabbit immunoglobulin (Ig)G anti-A. pleuropneumoniae serotype 1. Colonies suspected of being A. pleuropneumoniae positive were tested for β-nicotinamide adenine dinucleotide (NAD) dependence and Christie, Atkins, and Murch-Peterson (CAMP) reaction. The NAD dependent and CAMP positive colonies were serotyped using standard techniques (3). Whole tonsils were processed for PCR (Adiavet A. pleuropneumoniae), as previously described (9).
The presence of A. pleuropneumoniae was demonstrated by PCR from whole tonsils in 5 out of 6 treated pigs, and in all 6 control carrier pigs. Furthermore, the organism was isolated by IMS isolation from the same 5 treated carrier animals. On the other hand, 1 treated carrier pig was negative by both PCR and IMS isolation (Table I). Actinobacillus pleuropneumoniae could not be isolated by means of IMS isolation from any of the sentinel animals. In contrast, 1 sentinel pig in each group was positive by PCR from whole tonsils, which is consistent with the higher sensitivity of this technique (9).
Serology is a good method to confirm previous exposure to A. pleuropneumoniae, but it cannot be used to determine if animals are still carriers of the organism. On the other hand, PCR from surface material obtained after brushing of the palatine tonsils is an interesting, non-invasive technique that was used successfully in this study to identify A. pleuropneumoniae carrier pigs. However, negative results obtained using this method should be interpreted with caution, since in subclinically infected carrier animals A. pleuropneumoniae could be absent from the sampled superficial material but still present in deeper tonsillar tissues. In fact, limitations of PCR from tonsillar surface material were manifest in this study, since many animals found negative by this technique tested positive for A. pleuropneumoniae by IMS isolation and PCR from whole tonsils collected at necropsy. These 2 latter techniques showed good agreement between themselves (Table II) and, as already suggested (9), they may be considered valuable tools for the confirmation of the presence or absence of the pathogen in healthy animals.
Table II.
Agreementa between immunomagnetic separation (IMS) isolation and polymerase chain reaction (PCR) from whole tonsils obtained at necropsy (day 59) from carrier animals in both treated and control groups
| IMS isolation
|
|||
|---|---|---|---|
| PCR from whole tonsils | Positive | Negative | Total |
| Positive | 9 | 2 | 11 |
| Negative | 0 | 5 | 5 |
| Total | 9 | 7 | 16 |
Cohen’s κ coefficient = 0.83
Results obtained by PCR from whole tonsils and IMS isolation show that, under the conditions of this study, the elimination of A. pleuropneumoniae from tonsils of subclinically infected pigs cannot be completely achieved by an in-feed tilmicosin treatment. Furthermore, the presence of 1 positive sentinel pig in the treated group suggests that, despite the treatment, A. pleuropneumoniae was still transmissible. Nevertheless, in-feed medication with tilmicosin phosphate might still be considered as a potential strategy to reduce the spread of the organism from carrier to naive pigs. In fact, the negative status of 1 treated carrier pig at the end of the present trial by all the 3 methods of evaluation used suggests that by following a tilmicosin-based treatment carrier pigs can eventually become free of the bacterium. Additionally, A. pleuropneumoniae could not be found on the surface of the tonsils of most tilmicosin-treated pigs, in contrast to all of the control animals. This suggests that tilmicosin treatment is able to restrict the pathogen to deeper tonsillar tissues. This fact might have an impact in reducing the shedding of the bacteria. Further experiments using larger numbers of sentinel animals should be performed to evaluate this hypothesis.
When analyzing the results of this study it should be stressed that they are biased towards a worst-case scenario. Carrier pigs used in this study were, in fact, convalescent animals that recovered from a severe episode of pleuropneumonia. In contrast, in several subclinically infected herds, carrier pigs never show clinical signs of pleuropneumonia (1). Since such animals are likely to be less heavily colonized by A. pleuropneumoniae, in-feed medication with tilmicosin might be more effective in eliminating or reducing the presence of the pathogen in these herds. In addition, most A. pleuropneumoniae field strains show a minimum inhibitory concentrations (MIC) to tilmicosin of about 8 μg/mL (22), a value that is half the MIC of strain FMV 91-6514 used in this study. It may therefore be hypothesized that in-feed administered tilmicosin phosphate might be more effective in eliminating more susceptible strains. In conclusion, treatment of pig carriers of A. pleuropneumoniae with in-feed tilmicosin phosphate at 400 ppm for a period of 30 d significantly reduces the presence of the bacterium at the surface of the palatine tonsils, but is unable to completely eliminate the organism from deeper tissues.
Acknowledgments
The authors are grateful to Réal Boutin for the identification of the specific pathogen free farm. The authors thank Diane Côté for outstanding technical assistance and Julie Trudel and Laurent Jacod for their help with sampling. This work was supported by Elanco, Division of Eli Lilly Canada.
Footnotes
Dr. Broes’ current address is Génétiporc, Saint-Bernard, Quebec.
References
- 1.Taylor DJ. Actinobacillus pleuropneumoniae. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. Ames, Iowa: Iowa State University Press, 1999:343–354.
- 2.Blackall PJ, Klaasen HL, van den Bosch H, Kuhnert P, Frey J. Proposal of a new serovar of Actinobacillus pleuropneumoniae: serovar 15. Vet Microbiol. 2002;84:47–52. doi: 10.1016/s0378-1135(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 3.Mittal KR, Higgins R, Lariviere S, Nadeau M. Serological characterization of Actinobacillus pleuropneumoniae strains isolated from pigs in Quebec. Vet Microbiol. 1992;32:135–148. doi: 10.1016/0378-1135(92)90101-x. [DOI] [PubMed] [Google Scholar]
- 4.Sidibe M, Messier S, Lariviere S, Gottschalk M, Mittal KR. Detection of Actinobacillus pleuropneumoniae in the porcine upper respiratory tract as a complement to serological tests. Can J Vet Res. 1993;57:204–208. [PMC free article] [PubMed] [Google Scholar]
- 5.Inzana TJ, Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987;55:1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottschalk M, Altman E, Charland N, De Lasalle F, Dubreuil JD. Evaluation of a saline boiled extract, capsular polysaccharides and long-chain lipopolysaccharides of Actinobacillus pleuropneumoniae serotype 1 as antigens for the serodiagnosis of swine pleuropneumonia. Vet Microbiol. 1994;42:91–104. doi: 10.1016/0378-1135(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 7.Fenwick BW, Osburn BI. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect Immun. 1986;54:575–582. doi: 10.1128/iai.54.2.575-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagne A, Lacouture S, Broes A, D’Allaire S, Gottschalk M. Development of an immunomagnetic method for selective isolation of Actinobacillus pleuropneumoniae serotype 1 from tonsils. J Clin Microbiol. 1998;36:251–254. doi: 10.1128/jcm.36.1.251-254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fittipaldi N, Broes A, Harel J, Kobisch M, Gottschalk M. Evaluation and field validation of PCR tests for detection of Actinobacillus pleuropneumoniae in subclinically infected pigs. J Clin Microbiol. 2003;41:5085–5093. doi: 10.1128/JCM.41.11.5085-5093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dritz SS, Chengappa MM, Nelssen JL, et al. Growth and microbial flora of nonmedicated, segregated, early weaned pigs from a commercial swine operation. J Am Vet Med Assoc. 1996;208:711–715. [PubMed] [Google Scholar]
- 11.Dufresne L. Financial evaluation of disease eradication. Adv Pork Prod. 2002;13:143–162. [Google Scholar]
- 12.Salmon SA, Watts JL, Case CA, Hoffman LJ, Wegener HC, Yancey RJ., Jr Comparison of MICs of ceftiofur and other antimicrobial agents against bacterial pathogens of swine from the United States, Canada, and Denmark. J Clin Microbiol. 1995;33:2435–2444. doi: 10.1128/jcm.33.9.2435-2444.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore GM, Basson RP, Tonkinson LV. Clinical field trials with tilmicosin phosphate in feed for the control of naturally acquired pneumonia caused by Actinobacillus pleuropneumoniae and Pasteurella multocida in swine. Am J Vet Res. 1996;57:224–228. [PubMed] [Google Scholar]
- 14.Moore GM, Mowrey DH, Tonkinson LV, Lechtenberg KF, Schneider JH. Efficacy dose determination study of tilmicosin phosphate in feed for control of pneumonia caused by Actinobacillus pleuropneumoniae in swine. Am J Vet Res. 1996;57:220–223. [PubMed] [Google Scholar]
- 15.Paradis MA, Vessie GH, Merrill JK, et al. Efficacy of tilmicosin in the control of experimentally induced Actinobacillus pleuropneumoniae infection in swine. Can J Vet Res. 2004;68:7–11. [PMC free article] [PubMed] [Google Scholar]
- 16.Lechtenberg KF, Shryock TR, Moore G. Characterization of an Actinobacillus pleuropneumoniae seeder pig challenge-exposure model. Am J Vet Res. 1994;55:1703–1709. [PubMed] [Google Scholar]
- 17.Paradis MA, Vessie GH, Merrill JK, et al. Evaluation of the efficacy of tilmicosin phosphate in feed (Pulmotil) for the prevention of Actinobacillus pleuropneumoniae challenge infection in swine. Proc 15th Int Pig Vet Soc 1998:196.
- 18.Andreasen M, Mousing J, Thomsen LK. No overall relationship between average daily weight gain and the serological response to Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae in eight chronically infected Danish swine herds. Prev Vet Med. 2001;49:19–28. doi: 10.1016/s0167-5877(01)00174-x. [DOI] [PubMed] [Google Scholar]
- 19.Rohrbach BW, Hall RF, Hitchcock JP. Effect of subclinical infection with Actinobacillus pleuropneumoniae in commingled feeder swine. J Am Vet Med Assoc. 1993;202:1095–1098. [PubMed] [Google Scholar]
- 20.Assavacheep P, Persson M, Luengyosluechakul S, et al. Actinobacillus pleuropneumoniae in Thai pig herds. Prevalence of serum antibodies and relation to performance. J Vet Med B Infect Dis Vet Public Health. 2003;50:390–395. doi: 10.1046/j.1439-0450.2003.00688.x. [DOI] [PubMed] [Google Scholar]
- 21.Straw BE, Shin S, Callihan D, Petersen M. Antibody production and tissue irritation in swine vaccinated with Actinobacillus bacterins containing various adjuvants. J Am Vet Med Assoc. 1990;196:600–604. [PubMed] [Google Scholar]
- 22.Paradis MA, Higgins R, Lariviere S, De Lasalle F, Wilson J, Dick P. In-vitro antimicrobial activity of tilmicosin against field swine pathogens. Proc 17th Int Pig Vet Soc 2002:41.


