Abstract
Trans-resveratrol is a biologically active compound present in certain foods that has anti-inflammatory and anticancer properties. These beneficial effects are derived from both the immune system and cytokines. The purpose of this study was to determine the immunomodulatory effect of trans-resveratrol on the ex vivo production of inflammatory and anti-inflammatory cytokines stimulated by lipopolysaccharides (LPS). Trans-resveratrol (0, 0.01, 0.1, 1, and 10 μM) was added to blood samples from male Sprague-Dawley rats (n = 6) along with 100 U of LPS (Escherichia coli serotype, O55B5). The samples were then incubated for 4 h at 37°C and centrifuged. Finally, concentrations of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 in the plasma were analyzed using an enzyme-linked immunosorbent assay (ELISA). The production of inflammatory (TNF-α and IL-1β) and anti-inflammatory (IL-6) cytokines was suppressed by trans-resveratrol in a concentration-dependent manner. These results support the hypothesis that the immunomodulatory effect of trans-resveratrol plays an important role in disease conditions that involve an overproduction of inflammatory cytokines.
Résumé
Le trans-resvératrol est un composé biologiquement actif retrouvé dans certains aliments et qui a des propriétés anti-inflammatoires et antitumorales. Toutefois, ces effets bénéfiques nécessitent la participation du système immunitaire et des cytokines. Nous avons voulu déterminer l’effet immunomodulateur du trans-resvératrol sur la production ex vivo de certaines cytokines inflammatoires et anti-inflammatoires par une stimulation à l’aide de lipopolysaccharides (LPS). Le trans-resvératrol (0, 0,01, 0,1, 1, et 10 μM) ainsi que 100 U de LPS (E. coli sérotype O55B5) ont été ajoutés à des échantillons sanguins provenant de rats mâles Sprague-Dawley (n = 6). Ces échantillons ont été incubés pendant 4 h à 37 °C. Suite à la centrifugation, les concentrations plasmatiques du facteur de nécrose tumorale alpha (TNF-α), d’interleukines 1β (IL-1β) et 6 (IL-6) ont été déterminées par une méthode ELISA. Le trans-resvératrol a diminué la production des cytokines inflammatoires (TNF-α et IL-1β) et anti-inflammatoire (IL-6) de manière dose-dépendante. Ces résultats supportent l’hypothèse que l’effet immunomodulateur du trans-resvératrol pourrait avoir un rôle important dans les processus pathologiques impliquant une surproduction de cytokines inflammatoires.
(Traduction par les auteurs)
A wide variety of fruits and vegetables contain phenolic products with potent antioxydant properties (1). Dietary consumption of these phenolic products by animals and humans has shown a potential therapeutic application in oxidative stress-related diseases based on multiple epidemiological and animal studies (2). Trans-resveratrol (3,5,4’-trihydroxystilbene), a stilbene polyphenol, was identified as one of the biologically active compounds in grapes, peanuts, and red wine (3). Numerous in vivo and in vitro studies have demonstrated its ability to prevent cardiovascular diseases, inhibit platelet aggregation, alter eicosanoid synthesis, and modulate lipoprotein metabolism (4,5).
Trans-resveratrol may also have antioxidant and anti-inflammatory properties by modulating inflammatory cytokine production of the immune system cells (4,5). In vitro, trans-resveratrol reduces cytotoxicity of lymphocytes and natural killer cells and decreases cytokine production by CD4+ and CD8+ T cells (6). In addition, Gao et al (7) demonstrated that trans-resveratrol suppresses lymphocyte proliferation, cell-mediated cytotoxicity, and the production of tumor necrosis factor-α (TNF-α) and various interleukins by splenic lymphocytes. The latter 2 in vitro studies used concentrations of transresveratrol (5 to 6 μM), which were higher than that observed in plasma, following oral and intravenous administrations (8).
Inflammatory cytokines also promote the adhesion cancer cells to blood vessel endothelium and tissue invasion via expression of the intercellular adhesion molecule (ICAM-1) and vascular adhesion molecule 1 (VCAM-1). The antimetastatic properties of trans-resveratrol were demonstrated through the suppression of TNF-α-induced expression of ICAM-1 and VCAM-1 (9,10). Trans-resveratrol suppresses TNF-α-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis, which may provide the molecular basis for the anticarcinogenic properties of trans-resveratrol (11). Phenolic antioxidants may also inhibit inflammatory cytokine production by indirectly suppressing NF-κB binding to DNA (12). Finally, in vivo experiments have shown that TNF-α is required for tumor growth in TNF-α-deficient mice (13).
There is evidence to suggest that the anti-inflammatory and anticarcinogenic properties of trans-resveratrol involve alterations of immune cell functions and cytokine production by monocytes and macrophages (6,7,9–11,13). In this study, we investigated the immunomodulatory effect of trans-resveratrol on the ex vivo production of inflammatory and anti-inflammatory cytokines by mononuclear blood cells stimulated with lipopolysaccharide (LPS), while respecting known plasma concentrations of trans-resveratrol following the oral administration of a pharmacologically effective dose.
Six Sprague-Dawley rats (specific pathogen free, barrier facility; Charles River Laboratories, Wilmington, Massachusetts, USA), each weighing between 300 and 350 g, were used for this study. Animals were housed in a standard laboratory animal environment (fresh air: 15 changes/h, temperature: 21 ± 3°C, humidity: 30% to 70% and light-dark cycle: 12:12 h). Animals were kept in pairs in polycarbonate cages (Ancare, Bellmore, New York, USA) on hardwood sawdust bedding (Beta chip; Northeastern Products Company, Warrenburg, New York, USA) and acclimatized for at least 3 d prior to the initiation of the study. Rats received tap water and a standard laboratory rodent diet (Charles River Rodent Chow 5075; St-Constant, Quebec) ad libitum. The experimental protocol was approved by the Institutional Animal Care and Use Committee prior to animal use and was in accordance with the guidelines of the Canadian Council on Animal Care (14).
Animals were anesthetized (pentobarbital 80 mg/kg) and 10 mL of blood was drawn directly from the heart of each animal. The ex vivo production of cytokines was performed based on a validated method published in the literature (15). Briefly, blood samples (2 mL) from each animal were incubated with 0, 0.01, 0.1, 1, and 10 μM of resveratrol (Sigma Chemical Company, Oakville, Ontario) in 2 mL of saline with low-dose LPS (100 U of Escherichia coli serotype O55B5; Sigma Chemical Company). Trans-resveratrol concentrations were chosen according to previous findings following oral and intravenous administrations (8). Samples were incubated for 4 h at 37°C on a rocking platform. Tubes were then centrifuged for 10 min at 4°C at 2500 × g. Plasma supernatants were collected and stored at −80°C pending analysis.
Concentrations of TNF-α, IL-1β, and IL-6 were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (ELISA kits; Pierce/Endogen, Rockford, Illinois, USA). The analytical ranges of TNF-α, IL-1β, and IL-6 were 30 to 2500, 25.6 to 2500, and 31.5 to 2000 pg/mL, respectively. The specificity (defined in kit insert) and reproducibility of the TNF-α after a 1/4 dilution was excellent (98.0% to 106.6%). On the other hand, binding of IL-1β and IL-6 to various proteins in blood resulted in a low recovery (< 70%). As a result, only the unbound fraction of IL-1β and IL-6 was measured by the ELISA kit in standard curve, quality-control, and study samples.
The relationship between the concentration levels of trans-resveratrol and the production of LPS-induced concentrations of cytokines were fitted with the following Imax model (ADAPT-II‚ Release 4.0 [16]):
Where CYTO(C) is the concentration of the cytokine at any given concentration of trans-resveratrol (0, 0.01, 0.1, 1, or 10 μM), CYTOBASE is the concentration of the cytokine at baseline (following the incubation of 0 μM of trans-resveratrol), Imax is the maximum inhibition of cytokines, (RES) is the concentration of trans-resveratrol incubated, and IC50 is the effective concentration to achieve 50% of Imax. The effective concentration to achieve 90% of Imax (IC90) was derived from the IC50 parameter (IC50/0.9). Data were fitted (Adapt-II) using a weighting factor of Wi = 1/σ i2 where the variance σi2 was calculated for each observation (16). Statistical comparisons of cytokine levels for different concentrations of trans-resveratrol (0, 0.01, 0.1, 1, or 10 μM) were performed using a Student’s t-test. The alpha level was adjusted based on the number of comparisons performed simultaneously (α = 0.05/5). Statistical analyses were performed using the computer software (SYSTAT, Version 8.0 for Windows; Systat, Cary, North Carolina, USA).
The addition of LPS to whole blood resulted in an important increase in the inflammatory cytokine TNF-α (normal values <1 pg/mL [7]). Basal concentrations of TNF-α were below the level of quantification. Concentrations of 1 and 10 μM of trans-resveratrol, with the addition of LPS, resulted in a marked decrease in TNF-α concentrations, with a complete suppression observed at 10 μM of trans-resveratrol (Figure 1). Mean concentrations of the anti-inflammatory cytokine TNF-α following incubation with 0, 0.01, and 0.1 μM of trans-resveratrol were significantly higher than that observed at 1 μM. Mean concentrations of TNF-α following incubation of 0, 0.01, 0.1, and 1 μM of trans-resveratrol were significantly higher than those observed at 10 μM (complete suppression). The LPS-induced concentrations of the inflammatory cytokine IL-1β were very low in all samples compared to those observed for TNF-α and basal concentrations were below the level of quantification. Complete suppression of IL-1β (< 25.6 pg/mL) was observed when transresveratrol was incubated at concentrations equal to or higher than 0.1 μM. Considering the important variability in mean values of IL-1β and the small sample size used in the study (n = 6), no statistically significant differences were observed.
Figure 1.
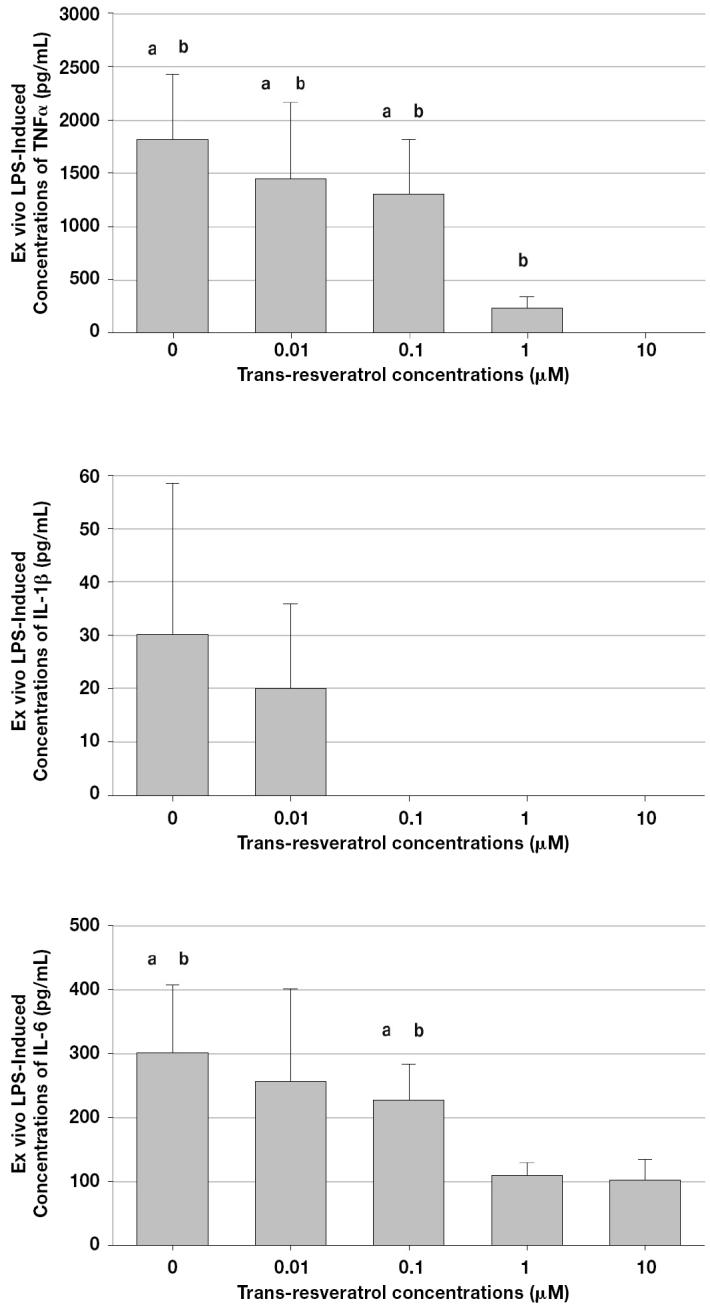
Mean (± s) immunomodulatory effect of trans-resveratrol on ex vivo whole blood lipopolysaccharide (LPS)-induced inflammatory (tumor necrosis factor [TNF]- α and interleukin [IL]-1β) and anti-inflammatory (IL-6) cytokines. Concentrations of TNF-α (top), IL-1β (middle), and IL-6 (bottom) decreased following incubation with trans-resveratrol concentrations (aP < 0.05/5 versus 1 μM, bP < 0.05/5 versus 10 μM).
The LPS-induced concentrations of the anti-inflammatory cytokine IL-6 were above the limit of quantitation (> 31.5 pg/mL) in all samples after the incubation of trans-resveratrol except for basal concentrations, which were below the level of quantification. Mean concentration of IL-6 following incubation with 0 and 0.1 μM of trans-resveratrol were significantly higher than those observed at 1 and 10 μM. The mean concentration of IL-6 following incubation of 0.01 μM of trans-resveratrol was highly variable and not significantly different than those observed at the 1 and 10 μM concentration levels. Previous in vivo and in vitro studies have shown that IL-6 can inhibit the production of LPS-induced TNF-α and IL-1β (17,18); however, considering that the production of IL-6 decreased with increasing concentrations of trans-resveratrol, it is unlikely that IL-6 contributed to the overall suppression of inflammatory cytokines.
The Imax model fitted the inhibitory effect of trans-resveratrol on the production of TNF-α, IL-1β, and IL-6 adequately, with r2 values of 0.852, 0.809, and 0.819, respectively (Table I). The maximum inhibitory effect of trans-resveratrol (Imax %) on TNF-α, IL-1β, and IL-6 corresponded to a 102.0%, 88.3%, and 65.4% decrease from baseline. Mean IC50 parameters of trans-resveratrol for TNF-α, IL-1β, and IL-6 were very low (0.25, 0.49, and 0.33 μM, respectively).
Table 1.
Mean (± s) immunomodulatory effect (Imax model) of trans-resveratrol on the production of cytokines
| Cytokines | CYTOBase (pg/mL) | Imax (pg/mL) | Imax (%) | IC50 (mM) | IC90 (mM) | r2 |
|---|---|---|---|---|---|---|
| TNF-α | 1174 ± 310 | 1203 ± 310 | 102.0 ± 1.0 | 0.25 ± 0.09 | 0.28 ± 0.10 | 0.852 |
| IL-1β | 30.4 ± 9.5 | 27.9 ± 12.2 | 88.3 ± 12.2 | 0.49 ± 0.87 | 0.54 ± 0.97 | 0.809 |
| IL-6 | 293 ± 119 | 204 ± 123 | 65.4 ± 16.4 | 0.33 ± 0.40 | 0.36 ± 0.44 | 0.819 |
TNF-α — tumor necrosis factor-α; IL-1β — interleukin 1β; IL-6 — interleukin 6; CYTOBase — concentration level of cytokine at baseline; Imax — maximum inhibition of cytokine; IC50 — the effective concentration to achieve 50% of Imax; IC90 — the effective concentration to achieve 90% of Imax; r2 — coefficient of correlation
Recent studies have demonstrated that the anti-inflammatory and anticarcinogenic properties of trans-resveratrol involve immune cell functions and cytokine production by monocytes and macrophages (6,7,9–11,13). In this study, we determined that trans-resveratrol modified inflammatory cytokine production by blood cells stimulated ex vivo with LPS. The quantification of cytokine concentrations was performed using a validated ELISA method (15). We have previously demonstrated that trans-resveratrol plasma concentrations following oral administration in male Sprague-Dawley rats ranged from between 0.1 and 10 μM (8). Therefore, a physiological range of concentrations were used to evaluate the immunomodulatory effect of trans-resveratrol.
The incubation of LPS without trans-resveratrol produced very high concentrations of TNF-α (mean = 1854 pg/mL), as compared to those observed for IL-1β (mean: 30 pg/mL). Trans-resveratrol suppressed the production of TNF-α and IL-1β in a concentration-dependent manner, with very low values of IC50 (0.25 and 0.49 μM, respectively). Complete suppression of TNF-α and IL-1β was observed at 10 and 0.1 mM, respectively. Therefore, at concentrations comparable to those required for other biological effects (5), trans-resveratrol completely suppressed the production of the inflammatory cytokines TNF-α and IL-1β. These results demonstrate a clear immunomodulatory role for trans-resveratrol.
We also investigated the immunomodulatory effect of trans-resveratrol on IL-6 production since the stimulation of monocytes and macrophages may also result in the production of anti-inflammatory cytokines (7). The maximum inhibitory effect of trans-resveratrol (Imax %) on the production of IL-6 was decreased by 65.4% (IC50 of 0.33 μM) compared to the baseline. These results suggest that the inhibitory effect of trans-resveratrol on the production of the anti-inflammatory cytokine IL-6 was not as potent as those observed for the inflammatory cytokines TNF-α and IL-1β.
In conclusion, our results clearly demonstrate that physiological non-toxic concentrations of trans-resveratrol suppressed the production of inflammatory cytokines. These results suggest that trans-resveratrol may have therapeutic potential in cases where the overproduction of inflammatory cytokine contributes to the pathogenesis of various diseases processes, such as inflammation, cancer cell proliferation, and oxidative stress. Correlations between the plasma concentrations of trans-resveratrol following dietary or commercially available forms and the production of inflammatory cytokines remains to be determined in vivo.
References
- 1.Rice-Evans C, Miller NJ, Paganga G. Antioxydant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 2.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 3.Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vit. 1992;43:49–52. [Google Scholar]
- 4.Soleas G, Diamandis E, Goldberg D. Resveratrol, a molecule whose time has come? And gone? Clin Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 5.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–73. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 6.Falchetti R, Fuggetta MP, Lanzilli G, Tricarico M, Ravagnan G. Effects of resveratrol on human immune cell function. Life Sci. 2001;70:81–96. doi: 10.1016/s0024-3205(01)01367-4. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Xu YX, Janakiraman N, Chapman RA, Gautam SC. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol. 2001;2:1299–1308. doi: 10.1016/s0006-2952(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 8.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Therap. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 9.Bertelli AAE, Baccalini R, Battaglia E, Falchi M, Ferrero ME. Resveratrol inhibits TNF-α-induced endothelial cell activation. Therapy. 2001;56:613–616. [PubMed] [Google Scholar]
- 10.Asensi M, Medina I, Ortega A, et al. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Rad Biol Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 11.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-α-induced activation of nuclear transcription factors NF-κB, activation protein-1, and apoptosis. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Kinneer K, YE J, Chen BJ. Inhibition of nuclear factor kappaB by phenolic antioxidants signaling and inflammatory cytokine expression. Mol Pharmacol. 2003;64:211–219. doi: 10.1124/mol.64.2.211. [DOI] [PubMed] [Google Scholar]
- 13.Saganuma M, Okabe W, Marino W, Sakai A, Suekoa E, Fujiki H. Essential role of tumor necrosis factor-α (TNF-α) in tumor promotion as revealed by TNF-α-deficient mice. Cancer Res. 1999;59:4516–4518. [PubMed] [Google Scholar]
- 14.CCAC Guidelines to the Care and Use of Experimental Animals. CCAC, 150 Albert Street, Ottawa, Ontario. Available at www.ccac.ca Last accessed February 28, 2005.
- 15.Suzuki K, Koyama T, Kobayashi S, et al. Novel method for detection of ex vivo tumor necrosis factor alpha production by monocytes. J Clin Lab Anal. 2002;16:273–278. doi: 10.1002/jcla.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Argenio DZ, Schumitzky A. ADAPT II User’s Guide. Pharmacokinetic/Pharmacodynamic Systems Analysis Software Biomedical Simulations Resource, University of Southern California, Los Angeles, California, 1997.
- 17.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 19901;75:40–47. [PubMed]
- 18.Aderka D, Le JM, Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989;143:3517–3523. [PubMed] [Google Scholar]


