Abstract
Quantities and types of ceramides and their relationships to physical properties of the horn covering the claws of clinically normal cows and cows with subclinical laminitis were investigated. Total ceramide content of the horn covering the sole and wall from cows with subclinical laminitis was 872.2 ± 146.6 μg/g and 528.6 ± 61.3 μg/g, respectively, and was significantly (P < 0.01, 0.05) lower than that from clinically normal cows. The mean moisture content in the claws from cows with subclinical laminitis (43.5% ± 4.3%) was significantly (P < 0.05) higher than that in the claws from clinically normal cows. The hardness of claws from cows with subclinical laminitis (35.2 ± 3.5) was significantly (P < 0.05) less than that of claws from clinically normal cows. Significant correlations between ceramides and moisture content (P < 0.001) and between ceramide and hardness (P < 0.001) were found in clinically normal cows and cows with subclinical laminitis. Our results indicate that decreases in ceramide contents may be related to changes in physical properties of the horn covering the claw in cows with subclinical laminitis.
Résumé
Cette étude visait à déterminer les quantités et les types de céramides, ainsi que leur relation avec les propriétés physiques de la corne recouvrant les onglons chez des vaches cliniquement normales et des vaches souffrant de fourbure sub-clinique. Le contenu total en céramide de la corne recouvrant la sole et la muraille des vaches avec fourbure sub-clinique était, respectivement, 872,2 ± 146,6 μg/g et 528,6 ± 61,3 μg/g ce qui était significativement (P < 0,01, 0,05) inférieur aux valeurs des vaches cliniquement normales. Le contenu moyen en humidité dans les onglons de vaches avec fourbure sub-clinique (43,5 ± 4,3 %) était significativement (P < 0,05) plus élevé que celui des onglons chez les vaches cliniquement normales. La dureté des onglons des vaches avec fourbure sub-clinique (35,2 ± 3,5) était significativement (P < 0,05) inférieure à celle des onglons des vaches cliniquement normale. Des corrélations significatives entre les céramides et le contenu en humidité (P < 0,001) et entre les céramides et la dureté (P < 0,001) ont été trouvées entre les vaches cliniquement normales et celles atteintes de fourbure sub-clinique. Nos résultats indiquent qu’une diminution du contenu en céramide peut être reliée à des changements dans les propriétés physiques de la corne recouvrant les onglons chez les vaches avec fourbure sub-clinique.
(Traduit par Docteur Serge Messier)
Subclinical laminitis has been proposed as the major predisposing factor in the development of ulcers on the sole, separation of the white line, and erosion of the horn covering the heel (1–5). Hemorrhage causing yellow discoloration of the horn covering the sole is regarded as a characteristic sign of subclinical laminitis, and results from the extravasation of blood from damaged vessels caused by an inflammatory response (3). Inflammatory responses associated with subclinical laminitis result in the production of inferior quality horny material over the sole, wall, and white line (1,2,6). One of the reasons for poor horn quality is abnormal deposition of keratin protein in germinal and cornified layers of the claw epidermis, caused by dysfunction of the keratinocytes (6–8). Although changes in quantity and subtypes of keratin protein in the horn covering an ulcerated bovine claw have been reported in detail (8), no information about changes in quantity and types of lipid components in subclinical laminitis is available. Lipids are components of the stratum corneum (keratinized layer) and are present in the intracellular spaces of keratinocytes (9,10).
Recently, it has been reported that ceramide, one of the major lipid components of human skin, is important for the physical properties of the stratum corneum in the epidermis. A decrease in ceramide content of human skin is closely related to skin disorders with morphological changes of the stratum corneum (9,10). Ceramides are composed of sphingosines and fatty acids (9,10). They have been shown to be predominantly associated with water barrier function (11,12) and contribute to intracellular adhesion of keratinocytes (13). Seven types of ceramides were detected in porcine (14) and human skin (15). However, in cattle, the types of ceramides and the distribution of ceramides in the horn covering the sole and wall have not been analyzed.
In this study, the quantities and types of ceramides in the horn covering the claws of clinically normal cows and cows with subclinical laminitis are described. Also, the relationship between ceramide content and physical properties of the horn covering the claw is described.
Samples of horn covering the claw were obtained from Holstein-Friesian cows from a slaughterhouse. Cows were evaluated to determine overall health of the claw and were identified as having sound hooves (n = 13) or as suffering from subclinical laminitis (n = 8). The diagnosis of subclinical laminitis was made on the basis whether the horn covering the sole had extensive yellow discoloration and evidence of hemorrhaging (3). Lameness and abnormal stance were not detected in any of the 21 cows prior to slaughter.
Samples of horn were obtained from hind lateral claw from clinically normal cows and cows with subclinical laminitis. Six zones were designated from the horn covering the claw (3): Zone 1 — bulb; zone 2 — sole-bulb junction; zone 3 — apex of the sole; zone 4 — white area at the toe; zone 5 — abaxial wall 2 cm from the sole; and zone 6 — dorsal wall 2 cm from the sole.
Lipid extraction from the samples of horn covering the claw was performed according to the methods by Imokawa et al (16). Briefly, 500 mg samples of bovine horn (from the claw) were reduced to fine shavings using a rasp. Lipid extraction was performed at room temperature for 1 h in a 15-fold solution of chloroform:methanol (2:1). Ceramides from samples of horn (from the claw) were separated by thin-layer chromatography (TLC) and were dissolved by treating twice with chloroform:methanol:acetic acid (190:9:1). After passing the solvent through the chromatography column, the chromatograms were air-dried, sprayed with 10% CuSO3-8%H3PO4 aqueous solution, and charred on a 180°C hot plate. The charred lipids were quantified using computer software (Image software; National Institute of Health, Maryland, USA). Ceramides were quantified by determining the amount of ceramides on a TLC chart of appropriate commercial standards and expressed as μg ceramide/g horn (from the claw). Hydroxyl fatty acid ceramide and nonhydroxyl fatty acid ceramide were used as standards for ceramides I, IIa, IIb, III, IV, V + VI, and VII (16). Reproducibility of this method was confirmed by using triplicate samples from the same animal, and deviation of values was within 5%. Zone 2 was used to analyze moisture content and hardness of the horn covering the claw. Moisture contents of the horn covering the claw were determined as previously reported (17). Samples of horn (from the claw) were dried at 180°C for 5 h in order to calculate their moisture contents. Zone 2 hardness was measured 3 times with a durometer (type D; Rex Gauge Company, Illinois, USA).
Statistical analysis of the data was performed using an analysis of variance (ANOVA) and Tukey’s t-test (Figure 2 and Table I), and Spearman’s correlation coefficient test (Figure 3) using computer software (StatView; SAS Company, Cary, North Carolina, USA). Values were regarded as significant if P < 0.05. Values are expressed as means ± standard deviation (s).
Figure 2.
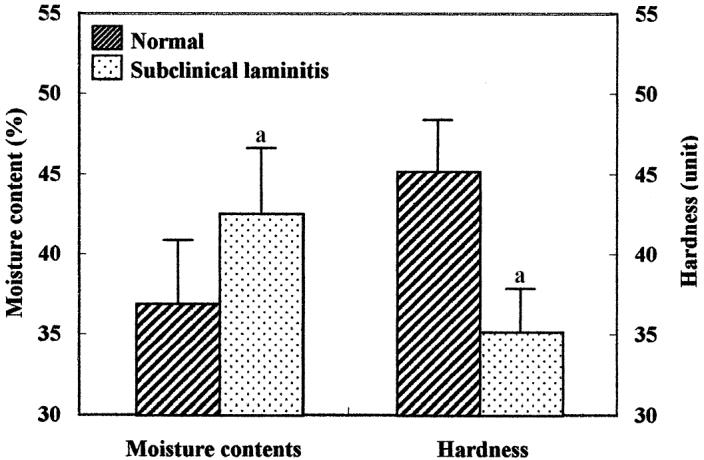
Moisture contents and hardness of the horn covering the sole (zone 2) from clinically normal cows and cows with subclinical laminitis. Values are means ± s.
a Significantly (P < 0.05) different from value in both groups.
Table 1.
Quantities (means and s) and types of ceramides in the claw horns from normal cows and cows with subclinical laminitis (μg/g)
| Sole
|
Wall
|
||||||
|---|---|---|---|---|---|---|---|
| Type of ceramides | 1 | 2 | 3 | 4 | 5 | 6 | |
| Normal | CER I | 41.2 ± 13.5 | 21.3 ± 5.4 | 13.2 ± 4.3 | 15.3 ± 3.4 | 0 | 0 |
| CER IIa | 286.6 ± 34.5 | 315.2 ± 34.2 | 307.7 ± 44.5 | 325.5 ± 77.4 | 280.6 ± 48.3b | 250.1 ± 27.4 | |
| CER IIb | 166.3 ± 20.5 | 211.7 ± 51.5 | 217.2 ± 41.3 | 206.4 ± 38.1 | 206.2 ± 55.1b | 250.8 ± 18.7b | |
| CER III | 226.5 ± 28.1 | 340.4 ± 29.5 | 254.1 ± 37.6 | 234.3 ± 51.4 | 0 | 0 | |
| CER IV | 21.7 ± 3.2 | 26.1 ± 4.8 | 12.4 ± 4.5 | 20.1 ± 4.3 | 0 | 0 | |
| CER V + VI | 122.3 ± 15.6 | 218.4 ± 33.5 | 197.3 ± 40.5 | 203.5 ± 41.9 | 66.3 ± 12.4 | 136.2 ± 22.5b | |
| CER VII | 327.1 ± 40.2 | 300.4 ± 46.1 | 311.4 ± 47.3 | 237.1 ± 33.7 | 0 | 0 | |
| Total ceramides | 1346.4 ± 221.3 | 638.6 ± 82.2 | |||||
| Subclinical-laminitis | CER I | 32.2 ± 11.3 | 18.6 ± 6.6 | 14.1 ± 2.6 | 13.2 ± 2.6 | 0 | 0 |
| CER IIa | 221.1 ± 18.6b | 252.7 ± 18.6b | 240.6 ± 28.5b | 236.8 ± 28.1b | 244.6 ± 33.5b | 198.2 ± 16.4b | |
| CER IIb | 96.2 ± 18.2b | 122.6 ± 17.8b | 130.9 ± 16.2b | 104.6 ± 12.1b | 172.6 ± 35.2b | 180.8 ± 9.8b | |
| CER III | 172.5 ± 17.6b | 288.3 ± 20.2b | 200.6 ± 22.9b | 146.0 ± 23.6b | 0 | 0 | |
| CER IV | 18.8 ± 4.6 | 20.1 ± 5.2 | 10.5 ± 9.8 | 17.2 ± 4.9 | 0 | 0 | |
| CER V + VI | 110.3 ± 15.2 | 188.6 ± 24.8 | 177.6 ± 36.1 | 168.1 ± 40.2 | 55.8 ± 16.2 | 124.9 ± 16.5b | |
| CER VII | 226.6 ± 36.5b | 210.1 ± 37.6b | 207.2 ± 35.6b | 187.9 ± 22.1b | 0 | 0 | |
| Total ceramides | 872.2 ± 144.6a | 528.6 ± 61.3b | |||||
P < 0.01
P < 0.05; Normal-subclinical laminitis
Figure 3.
Interrelationship between total ceramide, moisture content, and hardness of the horn covering the sole (zone 2) from clinically normal cows and cows with subclinical laminitis.
A typical pattern of TLC for ceramides extracted from 6 parts of the normal horn (from the claw) is shown in Figure 1. Ceramides I, IIa, IIb, III, IV, V + VI, and VII were detected in samples of horn covering the sole, and ceramides IIa, IIb, and V + VI were detected in samples of horn covering the wall of the hoof. Total ceramide content of the horn covering the sole and wall from cows with subclinical laminitis was 872.2 ± 144.6 μg/g and 528.6 ± 61.3 μg/g, respectively, and was significantly (P < 0.01 and P < 0.05) lower than that of clinically normal cows (Table I).
Figure 1.
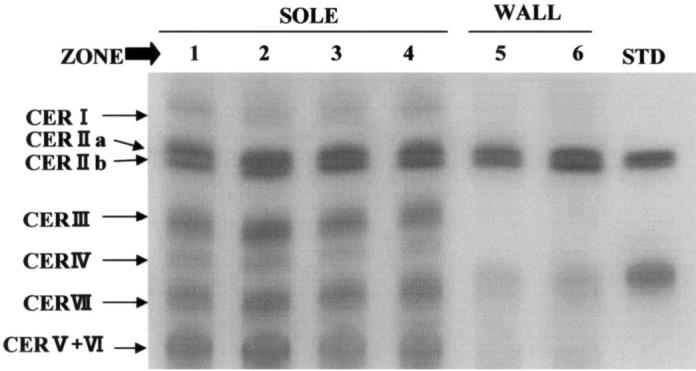
Typical pattern of thin-layer chromatograms of ceramides extracted from the horn covering the bovine claw. CER — ceramide. Zone of the horn covering the sole and wall of hoof: Zone 1 — bulb; zone 2 — sole-bulb junction; zone 3 — sole; zone 4 — toe; zone 5 and zone 6 — wall.
This is the first report on types of ceramides within the horn covering the bovine claw. It has been reported that 7 types of ceramides have been detected in the stratum corneum (keratinized layer) of human skin (16). In this study, the patterns of chromatograms derived from ceramides from the horn covering the sole were similar to those of human skin reported by Imokawa et al (16). Keratinocytes in the stratum germinativum (stratum basal) are important for production of the horn covering the claw and cell metabolism in cows with laminitis is markedly inhibited (8). Defective keratin deposition in the stratum germinativum and stratum corneum was detected in cows with subclinical laminitis and was caused by abnormal function of keratinocytes (8). Since ceramides are synthesized by keratinocytes in the stratum germinativum (18), it is possible that the decrease in ceramide contents in the horn covering the claws of cows with subclinical laminitis is also caused by changes in keratinocyte function under the condition of an inflammatory response. Total ceramide content of clinically normal samples of the horn covering the sole was 1346.4 ± 221.3 μg/g and was significantly (P < 0.01) higher than that of horn covering the wall, which was 638.6 ± 82.6 μg/g. The differences in the types of ceramides in the horn from the sole and wall that was observed in the present study is not understood.
Moisture content and hardness of the horn covering the sole are shown in Figure 2. The mean moisture content in the horn covering the sole of cows with subclinical laminitis (43.5% ± 4.3%) was significantly (P < 0.05) higher than that in clinically normal cows (37.2% ± 3.6%). The hardness of the claws from cows with subclinical laminitis (35.2 ± 3.5 duorometer points) was significantly (P < 0.05) less than that from clinically normal cows (46.2 ± 3.2 duorometer points). Interrelationships among ceramides, moisture content, and hardness of horn from the claw from clinically normal cows and cows with subclinical laminitis are shown in Figure 3. Significant (P < 0.001) negative correlations between ceramides and moisture content and between moisture and hardness were found in both clinically normal cows and cows with subclinical laminitis. In contrast, a significant (P < 0.001) positive correlation between ceramides and hardness was found in both clinically normal cows and cows with subclinical laminitis.
Moisture content is related to the microscopic structure and biochemical composition of the horn and is closely related to hardness of the horn covering the claw (19). Maclean et al (19) found a significantly higher moisture content in laminitic horn and concluded that the increased water content was likely responsible for the softer horn, which is prone to damage in animals affected by laminitis. Ceramides have been shown to be predominantly associated with water barrier function (11,12) and contribute to intracellular adhesion of keratinocytes (13). We hypothesized that failure of the water barrier function of the horn covering the claw, caused by a reduction in ceramides in cows with subclinical laminitis, is associated with a higher moisture content and lowered hardness of the horn from cows with subclinical laminitis. A strong and intact horn (stratum corneum) is required to protect the claw’s soft tissues and stratum germinativum from physical loads and to maintain the biomechanical function of the claw (19,20). Thus, a decrease in ceramides in the horn covering the claw of a cow with subclinical laminitis can lead to changes in the physical properties of the horn (from the claw) that may predispose the horn to ulcers on the soles, separation of the white line, and erosion of the horn covering the heel.
In summary, 7 types of ceramides from the horn covering the sole and 3 types of ceramide from the horn covering the wall were described and it was found that ceramide contents are reduced in the horn covering the claw of cows with subclinical laminitis. A decrease in ceramide content in cows with subclinical laminitis may be associated with changes in physical properties of the horn, thus predisposing the horn covering the claw to lesions.
References
- 1.Peterse DJ. Laminitis and interdigital dermatitis and heel horn erosion. Vet Clin North Am Food Anim Pract. 1985;1:83–90. doi: 10.1016/s0749-0720(15)31352-9. [DOI] [PubMed] [Google Scholar]
- 2.Bradley HK, Shannon D, Neilson DR. Subclinical laminitis in dairy heifers. Vet Rec. 1989;125:177–197. doi: 10.1136/vr.125.8.177. [DOI] [PubMed] [Google Scholar]
- 3.Greenough PR, Vermunt JJ. Evaluation of subclinical laminitis in a dairy herd and observations on associated nutritional and management factors. Vet Rec. 1991;128:11–17. doi: 10.1136/vr.128.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Enevoldsen C, Grohn YT, Thysen I. Sole ulcers in dairy cattle: association with season, cow characteristics, disease, and production. J Dairy Sci. 1991;74:1284–1298. doi: 10.3168/jds.S0022-0302(91)78284-2. [DOI] [PubMed] [Google Scholar]
- 5.Enevoldsen C, Grohn YT, Thysen I. Heel erosion and other interdigital disorders in dairy cows: association with season, cow characteristics, disease, and production. J Dairy Sci. 1991;74:1299–1309. doi: 10.3168/jds.S0022-0302(91)78285-4. [DOI] [PubMed] [Google Scholar]
- 6.Kempson SA, Logue DN. Ultrastructural observations of hoof horn from dairy cows: the structure of the white line during the first lactation. Vet Rec. 1993;132:499–502. doi: 10.1136/vr.132.20.499. [DOI] [PubMed] [Google Scholar]
- 7.Kempson SA, Logue DN. Ultrastructural observations of hoof horn from dairy cows: changes in the white line. Vet Rec. 1993;132:524–527. doi: 10.1136/vr.132.21.524. [DOI] [PubMed] [Google Scholar]
- 8.Hendry KA, MacCallum AJ, Knight CH, Wilde CJ. Laminitis in the dairy cow: a cell biological approach. J Dairy Res. 1997;64:475–486. [PubMed] [Google Scholar]
- 9.Elias PM, Meonon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 10.Golden GM, Guzec DB, Kennedy AH, McKie JE, Potts RO. Stratum corneum lipid phase transition and water barrier properties. Biochemistry. 1989;26:2382–2388. doi: 10.1021/bi00382a045. [DOI] [PubMed] [Google Scholar]
- 11.Werner Y, Lindberg M. Transepidermal water loss in dry and clinically normal skin in patient with atopic dermatitis. Acta Dermatol Venereol. 1990;65:102–105. [PubMed] [Google Scholar]
- 12.Werner Y. The water content of the stratum corneum in patient with atopic dermatitis: Measurement with the Corneometer CM 420. Acta Dermatol Venereol. 1986;66:281–284. [PubMed] [Google Scholar]
- 13.Rawlings A, Watkinson A, Harding C, et al. Changes in stratum corneum lipid structure and water barrier function during mechanical stress. 17th International Federation Societies of Cosmetic Chemists. Symposium, Vienna, Austria. 1992:14pp.
- 14.Wertz PW, Downing DT. Ceramides of pig epidermis: structure determination. J Lipid Res. 1983;24:759–765. [PubMed] [Google Scholar]
- 15.Stewart ME, Downing DT. A new 6-hydoroxy-4-sphingenincontaining ceramide in human skin. J Lipid Res. 1999;40:1434–1439. [PubMed] [Google Scholar]
- 16.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiological factor in atopic dry skin? J Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi H, Nagahata H. Relationship between serum biotin concentration and moisture content of the sole horn in cows with clinical laminitis or sound hooves. Vet Rec. 2001;148:209–210. doi: 10.1136/vr.148.7.209. [DOI] [PubMed] [Google Scholar]
- 18.Uchida Y, Hara M, Nishio H, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–2082. [PubMed] [Google Scholar]
- 19.Maclean CW. The long-term effects of laminitis in dairy cows. Vet Rec. 1971;89:34–37. doi: 10.1136/vr.89.2.34. [DOI] [PubMed] [Google Scholar]
- 20.Baggot DG, Bunch KJ, Grill GR. Variation in some inorganic components components and physical properties of claw keratin associated with claw disease in the British Friesian cow. Br Vet J. 1988;144:534–542. doi: 10.1016/0007-1935(88)90023-1. [DOI] [PubMed] [Google Scholar]



