Abstract
Background:
Polycyclic aromatic hydrocarbons (PAHs) are endocrine disruptors resulting from incomplete combustion. Pregnancy represents a particularly vulnerable period to such exposures, given the significant influence of hormone physiology on fetal growth and pregnancy outcomes. Maternal thyroid hormones play crucial roles in fetal development and pregnancy outcomes. However, limited studies have examined gestational PAH exposure and maternal thyroid hormones during pregnancy.
Methods:
Our study included 439 women enrolled in the LIFECODES birth cohort in Boston, aiming to explore the relationship between urinary PAH metabolites and thyroid hormones throughout pregnancy. Urine samples for PAH metabolite analysis and plasma samples for thyroid hormone were measured up to four visits throughout gestation. Single pollutant analyses employed linear mixed effect models to investigate individual associations between each PAH metabolite and thyroid hormone concentration. Sensitivity analyses were conducted to assess potential susceptibility windows and fetal-sex-specific effects of PAH exposure. Mixture analyses utilized quantile g-computation to evaluate the collective impact of eight PAH metabolites on thyroid hormone concentrations. Additionally, Bayesian kernel machine regression (BKMR) was employed to explore potential non-linear associations and interactions between PAH metabolites. Subject-specific random intercepts were incorporated to address intra-individual correlation of serial measurements over time in both single pollutant and mixture analyses.
Results:
Our findings revealed positive trends in associations between PAH metabolites and thyroid hormones, both individually and collectively as a mixture. Sensitivity analyses indicated that these associations were influenced by the study visit and fetal sex. Mixture analyses suggested non-linear relationships and interactions between different PAH exposures.
Conclusions:
This comprehensive investigation underscores the critical importance of understanding the impact of PAH exposures on thyroid hormone physiology during pregnancy. The findings highlight the intricate interplay between environmental pollutants and human pregnancy physiology, emphasizing the need for targeted interventions and public health policies to mitigate adverse outcomes associated with prenatal PAH exposure.
Keywords: Polycyclic Aromatic Hydrocarbons, Thyroid, Mixture, Maternal Exposure
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) constitute a group of hydrocarbons characterized by two or more fused aromatic rings. These compounds are byproducts of incomplete combustion from various sources, including fuel and organic substances like tobacco or wood. Exposure to PAHs in the general population can occur through multiple routes, such as inhalation, dermal contact, and ingestion, particularly from consuming charcoal-broiled foods (Raymond, 1998; Tsai et al., 2001; Veltman et al., 2011; Hoseini et al., 2018; Cao et al., 2019). Common PAHs encompass both low molecular weight (LMW) and high molecular weight (HMW) varieties. Examples of LMW PAHs include Naphthalene, Fluorene, Phenanthrene, and Pyrene, while HMW PAHs include benzo[a]pyrene and benzo[a]anthracene.
Upon entering the human body, PAHs undergo a series of biotransformation processes. In Phase I metabolism, cytochrome P450 monooxygenases oxidize PAHs, forming reactive epoxide intermediates that are subsequently converted into hydroxylated metabolites (OH-PAHs) (Barbosa Jr et al., 2023). In Phase II metabolism, these hydroxy-PAH metabolites are conjugated with glucuronic acid or sulfate, facilitating detoxification and excretion through urine or feces (Estabrook et al., 1981; Pelkonen & Nebert, 1982). The measurement of PAH metabolites in urine serves as a widely utilized method to assess internal dose and estimate recent exposure to PAHs, given the short half-lives (< 24h) of urinary PAHs. Commonly detected urinary OH-PAHs are metabolites of LMW PAHs, as HMW metabolites tend to be excreted through feces and are typically undetectable in urine (Ramesh et al., 2004; Li et al., 2008; Alhamdow et al., 2019). Urinary metabolites of LMW PAHs have been integrated into the PAH analytical panel of NHANES and have been utilized in previous epidemiological studies (Cathey et al., 2020; Wang et al., 2022; Bright et al., 2023; Dai et al., 2023). Additionally, 1-hydroxypyrene (1-PYR) has been employed as an indicator of overall PAH exposure due to its moderate to high correlation with both low and high molecular weight PAHs (Merlo et al., 1998; Castano-Vinyals et al., 2004; Li et al., 2008). The health impacts of exposure to PAHs are influenced by factors such as timing of exposure (e.g., prenatal vs. postnatal), exposure duration, route, intensity, and the toxicity of specific PAH compounds. PAHs exposure can lead to issues like inflammation, DNA damage, gene mutation-induced cell damage, cardiopulmonary mortality, and potential carcinogenicity (Unwin et al., 2006; International Agency for Research on Cancer, 2010; Kim et al., 2013; Sun et al., 2021; Peng et al., 2023). Previous studies have reported immunotoxic effects of PAH exposure in both animals (Reynaud & Deschaux, 2006; O’Driscoll et al., 2018; Honda & Suzuki, 2020) and humans (Hew et al., 2015; Yu et al., 2022).
Various derivatives of PAHs, including oxygenated PAHs, have been found to enhance the transcriptional activity of thyroid receptor β (NR1A2) induced by thyroxin (Bekki et al., 2009). Moreover, these derivatives have been identified to bind to transthyretin, potentially interfering with thyroid hormone transport and activity (Sun et al., 2008; Song et al., 2012). Thyroid hormones play crucial roles in maternal health and fetal development with dysregulation being associated with spontaneous abortion, placental abruptions, preeclampsia, preterm delivery, and diminished intellectual function in offspring (Stagnaro-Green & Pearce, 2012; Nazarpour et al., 2015; Gahlawat et al., 2017; Andersen & Andersen, 2021) and early pubertal development in girls (Deng et al., 2017; Kehm et al., 2021) and boys (Wu et al., 2022; Wang et al., 2024). Prenatal exposure to PAHs has been linked to child neurodevelopmental problems through infancy to later childhood (Perera et al., 2006; Edwards et al., 2010; Jedrychowski et al., 2015; Peterson et al., 2015; Wallace et al., 2022; Sun et al., 2023). Despite the potential impact of gestational PAH exposure on pregnancy outcomes, fetal growth, and brain development, the mechanisms involved remain unclear. PAH may be impacting these phenotypes through alterations in thyroid function, but limited research has been conducted in this area.
This study aims to examine the impact of gestational PAH exposure on thyroid hormone concentrations during pregnancy. Understanding these potential links is essential for developing strategies to mitigate risks and safeguard maternal and fetal well-being during this vulnerable period. To achieve this, we employed repeated biomarker measurements at four distinct time points throughout gestation, aiming to assess the associations between urinary low molecular weight (LMW) PAH metabolites—individually and as a mixture—and plasma thyroid hormone concentrations during pregnancy.
2. Methods
2.1. Study population
Participants in the current study were a nested case-control subset of larger LIFECODES cohort (N=1648), a prospective birth cohort recruited between 2006 and 2008 at Brigham and Women’s Hospital in Boston, Massachusetts. Inclusion criteria were: (1) recruitment prior to 15 weeks of gestation; (2) ages 18–50; and (3) intention to deliver at Brigham and Women’s Hospital. Exclusion criteria included higher-order multiple gestations (triplets or greater). Further information about how participant recruitment and the criteria for eligibility have been previously described (McElrath et al., 2012; Ferguson et al., 2014). Women provided urine and blood samples at four visits during pregnancy (median 10, 18, 26, and 35 weeks gestation). Gestational age was calculated using the last menstrual period and confirmed by ultrasound according to American College of Obstetrics and Gynecology guidelines (ACOG, 2017). The study protocols were approved by the ethics and research committees of the participating institutions, and all study participants provided written informed consent.
The current analysis was conducted based on the availability of data on PAH exposure biomarkers and circulating concentrations of thyroid hormones from the nested case-control study focused on preterm birth (Cantonwine et al., 2015; Johns et al., 2017). It included 130 women who experienced preterm deliveries (before 37 weeks of gestation) and 352 randomly selected controls, representing women who delivered a single infant after a full 37-week gestation period. In this study, we further excluded individuals with preexisting thyroid disorders such as thyroid cancer, Graves’ disease, hyperthyroidism, or hypothyroidism (N = 41). Additionally, we excluded those who did not have stored plasma samples from at least one of the study visits (N = 2). Consequently, our final study group comprised 439 participants with the approximate ratio of 1:3 for preterm (N=116) to term births (N=323).
Among the 439 participants, 167 (38.0%) had measurements of urinary PAH metabolites and plasma thyroid hormone concentrations at four different time points during gestation. Meanwhile, 179 participants (40.8%) had measurements at three time points, 70 participants (15.9%) had data for two time points, and 23 participants (5.2%) had measurements at only one time point across their pregnancy.
2.2. PAH measurement
Urinary PAH metabolites were measured by isotope dilution liquid chromatography with tandem mass spectrometry (LC–MS/MS) at NSF International (Ann Arbor, MI). The LC-MS/MS method was previously described (Onyemauwa et al., 2009; Ferguson et al., 2017), and the metabolites measured included: 1-hydroxynapthalene (1-NAP); 2-hydroxynapthalene (2-NAP); 2-hydroxyfluorene (2-FLU); 1 hydroxyphenanthrene (1-PHE); 2- and 3-hydroxyphenanthrene quantitated together (2- and 3-PHE); 4-hydroxyphenanthrene (4-PHE); 9-hydroxyphenanthrene (9-PHE); and 1-hydroxypyrene (1-PYR). All PAH metabolite concentrations detected below the limit of detection (LOD) were replaced by the LOD divided by the square root of two (Hornung & Reed, 1990). To assess the distribution of PAH metabolites, we adjusted the concentrations to account for urine dilution using specific gravity (SG) as follows: PAHc = PAH [(1.015–1) / (SG – 1)], where PAHc denotes the adjusted concentration, PAH signifies the original urinary PAH concentration, SG indicates the specific gravity of the particular sample, and 1.015 corresponds to the median urinary specific gravity of all the samples (Kuiper et al., 2021). In all statistical models, we incorporated specific gravity as a covariate when working with the unadjusted PAH metabolites.
2.3. Thyroid hormone measurement
1368 plasma samples for 439 participants were available from at least one of the four study visits. Samples were assayed for thyroid-stimulating hormone (TSH), total triiodothyronine (T3), and free thyroxine (FT4) and total thyroxine (T4) at the Clinical Ligand Assay Service Satellite (CLASS) lab at the University of Michigan (Ann Arbor, MI). TSH, total T3 and total T4 were assayed using automated chemiluminescence immunoassay (Bayer ADVIA Centaur, Siemens Health Care Diagnostics, Inc.), while FT4 was measured using direct equilibrium dialysis followed by radioimmunoassay (IVD Technologies). The limits of detection (LOD) for TSH, T3, T4, and FT4 were as follows: 0.01 μIU/mL, 0.1 ng/dL, 0.3 μg/dL, and 0.1 ng/dL, respectively. When examining plasma samples, we were able to detect T4 and T3 in 100% of the cases. In contrast, 99.5% of TSH measurements were above the LOD, leaving six samples below this limit. Out of these six samples, five had FT4 measurements that were around or above the 75th percentile within our study population. To address this, we replaced the TSH values for these five samples with the TSH LOD value of 0.01 μIU/mL. For FT4, as the LOD is not biologically feasible, samples falling below this threshold were treated as missing values in our statistical analyses. Similar approaches have been used in our previous studies (Aung et al., 2017; Aker et al., 2018). As a measure of thyroid homeostasis, we calculated the ratio of T3 to T4 and included this ratio as an additional dependent variable in our analysis.
2.4. Statistical analyses
Demographic information of the study population including maternal age, education concentration, race and ethnicity, pre-pregnancy BMI, health insurance type, smoking during pregnancy, alcohol consumption during pregnancy, number of previous live births, preterm birth case status, and fetal sex were tabulated. Pearson correlations were calculated between different PAH metabolites measured across gestation. We also calculated Pearson correlations of PAHs and thyroid hormones measured at each study visit. Additionally, to assess temporal variability of PAHs at each visit, we used the ICC function from the ‘psych’ package in R, which estimates ICC and confidence intervals using variance components from a one-way analysis of variance. These parameters represent the ratio of between-individual variability to the sum of between and within-individual variability and range from zero to one, where higher values indicating higher reproducibility (Rosner, 2015). The ICCs of thyroid hormones in this cohort were reported previously [Johns et al, 2018], and range from 0.18 and 0.67, showing the lowest temporal reliability for FT4 and the highest reliability for T3. Since PAH concentrations were right-skewed, we transformed each of PAH metabolite using the natural log in statistical analysis. Sample size was 1368 from 439 participants.
2.4.1. Single pollutant analysis
We utilized linear mixed effect model (LMM) with subject-specific random intercepts to examine individual associations between each PAH metabolite and each thyroid hormone while accounting for intra-individual correlation of serial measurements collected over time. We addressed the issue of preterm birth cases being disproportionately represented by using study-specific weights based on the inverse probability of sampling preterm birth cases from the full cohort. This adjustment was made to align the relative weights of cases and controls in our study population with the proportions typically seen in a general population, as documented (Richardson et al., 2007). Covariates were primarily selected based on a priori knowledge and included in the final model after examining their impacts on the main effect estimate (>10%). Potential confounders considered were maternal age, education, race/ethnicity, pre-pregnancy BMI, marital status, tobacco use, alcohol consumption, gestational age at visit, and health insurance provider. All final models were adjusted for maternal age (continuous), gestational age (continuous), maternal pre-pregnancy BMI (continuous), maternal education (categorical; high school or less, technical school, junior college or some college, or college graduate or above), race/ethnicity (categorical; Caucasian, African-American, Asian, Hispanic, Mixed, or Unknown/Other), health insurance provider (categorical; Private or Public), and alcohol use in pregnancy (no alcohol use or some alcohol use). Additionally, we included time-varying urinary specific gravity as a covariate in the model. As a sensitivity analysis, we conducted separate multivariable linear regression models for each visit over gestation to test potential windows of susceptibility by study visit. The same covariates except for gestational age were used in the models. Furthermore, we ran linear mixed effect models stratified by fetal sex to obtain sex-specific estimates.
2.4.2. Mixture analyses
In our initial approach, we utilized quantile g-computation to investigate the collective impact of PAH on thyroid hormones, assuming linearity. Quantile g-computation employs a parametric, generalized-linear-model framework to estimate the change in the outcome associated with a simultaneous one quantile increase in all exposures within the mixture, as detailed by Keil (2020). To address the repeated measurements of exposures and outcomes in our study sample, we extended the quantile g-computation approach. This extension involved employing bootstrapping to resample subjects, rather than individual observations, ensuring the preservation of dependence within each cluster or subject across multiple measurements (Cheng et al., 2013). We conducted 500 iterations of bootstrapping, incorporating 2,500 Monte Carlo simulation iterations for each model. Participant sampling weights were additionally applied to account for study design considerations, for which a similar approach was used previously (Welch et al., 2021). We utilized the same set of covariates as employed in single pollutant analyses, and exposures were natural log transformed. Analyses were carried out using the ‘qgcomp’ package in R.
As a second approach, we employed Bayesian kernel machine regression (BKMR) to explore nonlinearity and potential interactions among PAH metabolites within the mixture. BKMR utilizes a kernel function to flexibly capture relationships between a response variable and multiple predictors (Bobb et al., 2015). We natural log-transformed the PAHs and used the same set of covariates as in the single-pollutant analyses. In implementing the BKMR model, we utilized the Markov chain Monte Carlo (MCMC) algorithm within the R package ‘bkmr.’ To ensure model convergence and maintain acceptable acceptance rates for predictor variables, we conducted 20,000 iterations for all PAHs and extended it to 50,000 iterations for FT4. There are two primary approaches to BKMR modeling: component-wise variable selection and hierarchical variable selection. While some studies have used hierarchical BKMR to adjust high correlation between pollutants, we focused on component-wise BKMR for the following reasons: (1) Gap statistics, a technique that estimates the number of clusters by comparing changes in within-cluster dispersion (Tibshirani et al., 2001), indicated ‘1’ as the optimal number of clusters in our data (Supplementary Figure 1); (2) Component-wise BKMR can also identify significant pollutants within a mixture, even in the presence of moderate correlations among pollutants (Coull et al., 2015); (3) We sought to examine all possible two-way interaction between PAH metabolites, however, hierarchical BKMR assumes no interaction between pollutants grouped together. First, we investigated non-linearity by examining individual univariate exposure-response functions. Then, to evaluate the interaction between PAH metabolites, we calculated bivariate exposure-response functions. These functions serve as indicators of interaction, signifying that there is evidence of interaction when the impact of one exposure varies across different concentrations of the other exposure. To account for repeated measures, BKMR model with random a intercept for each individual was constructed.
As a sensitivity analysis to verify the robustness of component-wise BKMR, we also conducted hierarchical BKMR modeling by grouping PAH metabolites based on their parent compounds: Naphthalene metabolites (1-NAP, 2-NAP), Fluorene metabolite (2-FLU), Phenanthrene metabolites (1-PHE, 2- and 3-PHE, 4-PHE, 9-PHE), and Pyrene metabolite (1-PYR). A PIP threshold of 0.5 was used to identify the relative importance of each group or PAH within a group. All statistical analyses were performed using R version 4.0.5.
3. Results
3.1. Characteristics of study population
The demographic characteristics of participating women (N=439) are summarized in Table 1. Briefly, most women were aged between 30 and 35 years (40.3%), predominantly Caucasian (56.9 %), attended or graduated from college (82.7%) and had private health insurance (77.2%), and 54.4% of newborns were males. Few participants reported smoking during pregnancy (6.4%) or drinking alcohol (4.1%). The majority (50.8 %) of the study sample had pre-pregnancy BMI between 18.5 and 25 kg/m2 and about 44.9% of participants were nulliparous. The distributions of maternal urinary PAH metabolite concentrations by each visit, as well as plasma thyroid hormone concentrations measured by each visit, are presented in Table 2. With adjustment for specific gravity, PAH intraclass correlation coefficients (ICCs) were the lowest for 4-PHE (0.37) and the highest for 2-FLU (0.6) (Table 3). Some PAH metabolites were moderately to strongly correlated with one another (R > 0.50), but metabolites from the same parent compound did not necessarily strongly correlate (Supplementary Figure 2a). For example, while correlations between most phenanthrene metabolites were high (0.62 < R < 0.89), one of the metabolites, 9-PHE, was only moderately correlated with another metabolite, 1-PHE (R=0.46). Additionally, naphthalene metabolites, 1-NAP and 2-NAP, showed a relatively weak correlation (0.28) with one another. The pyrene metabolite 1-PYR was correlated with most phenanthrene metabolites and a fluorene metabolite (2-FLU). Additionally, the distributions of maternal urinary PAH metabolite concentrations and thyroid hormone by fetal sex are summarized in Supplementary Table 1.
Table 1.
Summary demographics of the 439 pregnant women in the analysis
| N (%) | ||
|---|---|---|
|
| ||
| Maternal Age | Age < 25 | 55 (12.5 %) |
| 25 ≤ Age < 30 | 91 (20.7 %) | |
| 30 ≤ Age < 35 | 177 (40.3 %) | |
| 35 ≤ Age < 40 | 99 (22.6 %) | |
| 40 ≤ Age | 17 (3.9 %) | |
| Maternal Education | Missing | 10 (2.3 %) |
| Did not graduate high school | 17 (3.9 %) | |
| Graduated from high school | 49 (11.2 %) | |
| Attended or graduated from college | 363 (82.7 %) | |
| Race and Ethnicity | Caucasian | 250 (56.9 %) |
| African-American | 73 (16.6 %) | |
| Asian | 31 (7.1 %) | |
| Hispanic | 63 (14.4 %) | |
| Mixed | 12 (2.7 %) | |
| Unknown/Other | 10 (2.3 %) | |
| Pre-pregnancy BMI (kg/m 2 ) | Missing | 11 (2.5 %) |
| BMI < 18.5 | 11 (2.5 %) | |
| 18.5 ≤ BMI < 25 | 223 (50.8 %) | |
| 25 ≤ BMI < 30 | 108 (24.6 %) | |
| 30 ≤ BMI | 86 (19.6 %) | |
| Insurance | Missing | 12 (2.7 %) |
| Private | 339 (77.2 %) | |
| Public | 88 (20 %) | |
| Smoking during pregnancy | No | 411 (93.6 %) |
| Yes | 28 (6.4 %) | |
| Alcohol use during pregnancy | Missing | 9 (2.1 %) |
| No | 412 (93.8 %) | |
| Yes | 18 (4.1 %) | |
| Fetal Sex | Female | 199 (45.3 %) |
| Male | 239 (54.4 %) | |
| Preterm Birth | Yes | 116 (26.4 %) |
| No | 323 (73.6 %) | |
| Parity | 0 | 197 (44.9 %) |
| 1 | 144 (32.8 %) | |
| 2 | 64 (14.6 %) | |
| ≥ 3 | 34 (7.9 %) | |
Table 2.
Weighted geometric mean (GM) and interquartile range (IQR) of SG-corrected urinary PAH metabolite and thyroid hormones by study visit of sample collection in pregnancy (NOverall=1368, NV1 =371; NV2 =352; NV3 =328; NV4 =317). Overall values were calculated using the concentrations from all four study visits. Weighted using inverse probability weighting to account for the sampling approach of the nested case-control sample.
| GM (IQR) | |||||
|---|---|---|---|---|---|
|
| |||||
| PAH | Overall | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|
| |||||
| 1-NAP (ng/L) | 810.9 (381, 1526) | 735.9 (333, 1314) | 887.4 (418, 1752) | 767.7 (376, 1524) | 864.0 (408, 1567) |
| 2-NAP (ng/L) | 2873.4 (1539, 5166) | 2678.2 (1446, 4540) | 2776.8 (1516, 4804) | 2836 (1490, 5454) | 3251.6 (1716, 5574) |
| 2-FLU (ng/L) | 217.0 (135, 299) | 233.8 (149, 331) | 225.8 (138, 322) | 180.1 (106, 285) | 229.7 (151, 298) |
| 1-PHE (ng/L) | 174.5 (111, 263) | 143.9 (90.4, 219) | 165.3 (107, 239) | 192.3 (119, 283) | 206.7 (143, 298) |
| 2-and 3-PHE (ng/L) | 147.9 (99.9, 212) | 152.5 (102, 212) | 159.2 (105, 217) | 107.4 (63.4, 173) | 179.9 (127, 236) |
| 4-PHE (ng/L) | 28.4 (17.7, 44.1) | 22.5 (14.2, 33.3) | 29.2 (18.0, 44.7) | 24.5 (15.1, 36.1) | 41.0 (28.5, 56.2) |
| 9-PHE (ng/L) | 25.3 (15.1, 36.4) | 21.9 (13.2, 30.1) | 23.4 (14.0, 34.4) | 32.5 (17.2, 53.0) | 25.2 (16.0, 34.3) |
| 1-PYR (ng/L) | 122.7 (75.5, 184) | 102.3 (63.9, 143) | 112.5 (70.0, 175) | 133.7 (83.1, 207) | 150.5 (100, 211) |
|
| |||||
| GM (IQR) | |||||
|
| |||||
| Thyroid | Overall | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|
| |||||
| FT4(ng/dL) | 1.11 (0.89, 1.38) | 1.39 (1.17, 1.64) | 1.1 (0.94, 1.31) | 1.02 (0.83, 1.21) | 0.96 (0.8, 1.17) |
| T3 (ng/dL) | 1.53 (1.29, 1.85) | 1.34 (1.11, 1.6) | 1.6 (1.36, 1.89) | 1.62 (1.35, 1.9) | 1.64 (1.38, 1.99) |
| T3/T4 | 0.15 (0.13, 0.18) | 0.13 (0.12, 0.15) | 0.15 (0.13, 0.17) | 0.16 (0.14, 0.18) | 0.16 (0.14, 0.19) |
| T4 (μg/dL) | 10.15 (9.1, 11.5) | 9.92 (8.7, 11.3) | 10.56 (9.5, 11.8) | 10.23 (9.1, 11.5) | 9.93 (8.9, 11.5) |
| TSH (μIU/mL) | 1.13 (0.82, 1.74) | 0.78 (0.55, 1.51) | 1.32 (0.99, 1.92) | 1.24 (0.9, 1.68) | 1.31 (0.97, 1.92) |
Table 3.
Intraclass correlation coefficients (ICC) and 95% confidence intervals for urinary PAH metabolites (N = 439 participants, 1368 samples)
| Uncorrected | Specific gravity-corrected | |||
|---|---|---|---|---|
|
| ||||
| PAH | ICC | 95% CI | ICC | 95% CI |
|
| ||||
| 1-NAP | 0.46 | (0.42, 0.5) | 0.38 | (0.34, 0.42) |
| 2-NAP | 0.33 | (0.29, 0.38) | 0.44 | (0.4, 0.48) |
| 2-FLU | 0.49 | (0.45, 0.53) | 0.60 | (0.56, 0.63) |
| 1-PHE | 0.42 | (0.38, 0.46) | 0.42 | (0.37, 0.46) |
| 2-and 3-PHE | 0.40 | (0.36, 0.45) | 0.41 | (0.37, 0.45) |
| 4-PHE | 0.41 | (0.37, 0.45) | 0.37 | (0.32, 0.42) |
| 9-PHE | 0.38 | (0.34, 0.42) | 0.45 | (0.41, 0.49) |
| 1-PYR | 0.49 | (0.45, 0.53) | 0.47 | (0.43, 0.51) |
3.2. Associations of single PAH exposure with Thyroid hormones
Figure 1 displays the outcomes of linear mixed-effects models with repeated measurements of PAHs and thyroid hormones gathered across four prenatal visits and detailed information is summarized in Supplementary Table 2. There were a number of notable associations between various PAH metabolites and thyroid hormones, primarily in the positive direction. These associations were particularly prominent for T3 concentrations and the T3/T4 ratio. For instance, when considering a one-interquartile range (IQR) increase in 1-PYR and 4-PHE, we observed an increase of 4.1% (95% CI: 1.3, 7.0) and 5.3% (95% CI: 2.2, 8.4) in T3, accompanied by a 3.8% (95% CI: 1.9, 5.6) and 2.9% (95% CI: 0.99, 4.7) elevation in T3/T4 ratio, respectively. 9-PHE and 2-NAP also exhibited associations with T3/T4, reflecting increases of 2.4% (95% CI: 0.82, 3.9) and 2.2% (95% CI: 0.04, 4.4) per IQR increase, respectively. 1-PHE demonstrated associations with both T3 (3.8%, 95% CI: 0.99, 6.6) and T4 (2.1% per IQR, 95% CI: 0.2, 4.0). TSH was significantly associated with 2-NAP, where an IQR increase in 2-NAP was associated with 8.5% (95% CI: 1.9, 15.1) greater TSH concentrations. FT4 did not exhibit any statistically significant associations with the PAH metabolites included in our analysis. While several point estimates for associations between PAH metabolites and TSH or T4 were negative, none were statistically significant.
Figure 1.
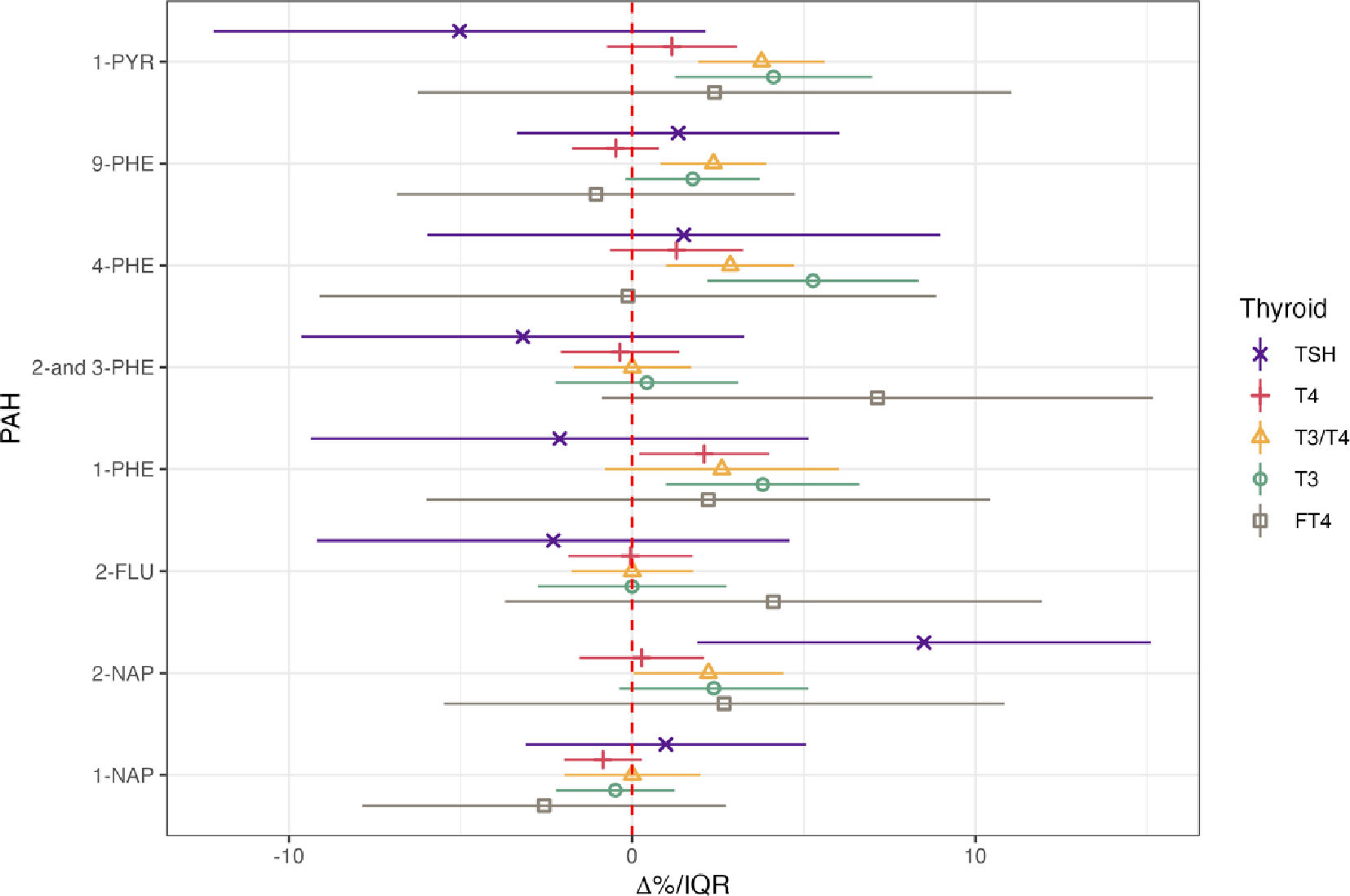
Linear mixed effect regression for thyroid hormone levels in association with urinary concentrations of PAHs (N=439 participants, 1368 samples). Forest plot depicting percent changes and 95% confidence intervals of thyroid hormones with interquartile range increase in PAH metabolites. Models were adjusted for gestational age, maternal age, education, race, insurance type, alcohol consumption, pre-pregnancy BMI, and specific gravity. PAH levels were natural log transformed.
In the analyses stratified by visit, several associations emerged that were not evident in the repeated measure analyses (Figure 2, Supplementary Table 3). For instance, 2-NAP exhibited a positive association with FT4 at visit 3, where an IQR increase in 2-NAP was associated with 22.3% (95% CI: 1.12, 43.48) greater FT4. The T3/T4 ratio displayed a 7.49% (95% CI: 1.98,12.99) increase in association with 2-and-3-PHE during visit 2, and a 3.64% and 5.48% increase in relation to 1-PHE during visits 1 and 2, with increases of 3.64% (95% CI: 0.07, 7.2) and 5.48% (95% CI: 0.11, 10.86), respectively. Furthermore, we noted that the majority of associations involving T3, T4, and T3/T4 with PAH metabolites occurred during earlier visits (visits 1 and 2) rather than in later visits (visits 3 and 4). Conversely, the positive association of TSH with 2-NAP was predominantly driven by visit 4, showing an elevation of 28.17% (95% CI: 6.08, 50.25) per IQR increase in 2-NAP. Finally, in contrast to the repeated measure analyses, we observed a somewhat stronger trend of negative associations, especially for TSH, although only the relationship between 1-PHE and TSH during visit 1 was statistically significant (−21.03% per IQR, 95% CI: −39.32, −2.74).
Figure 2.
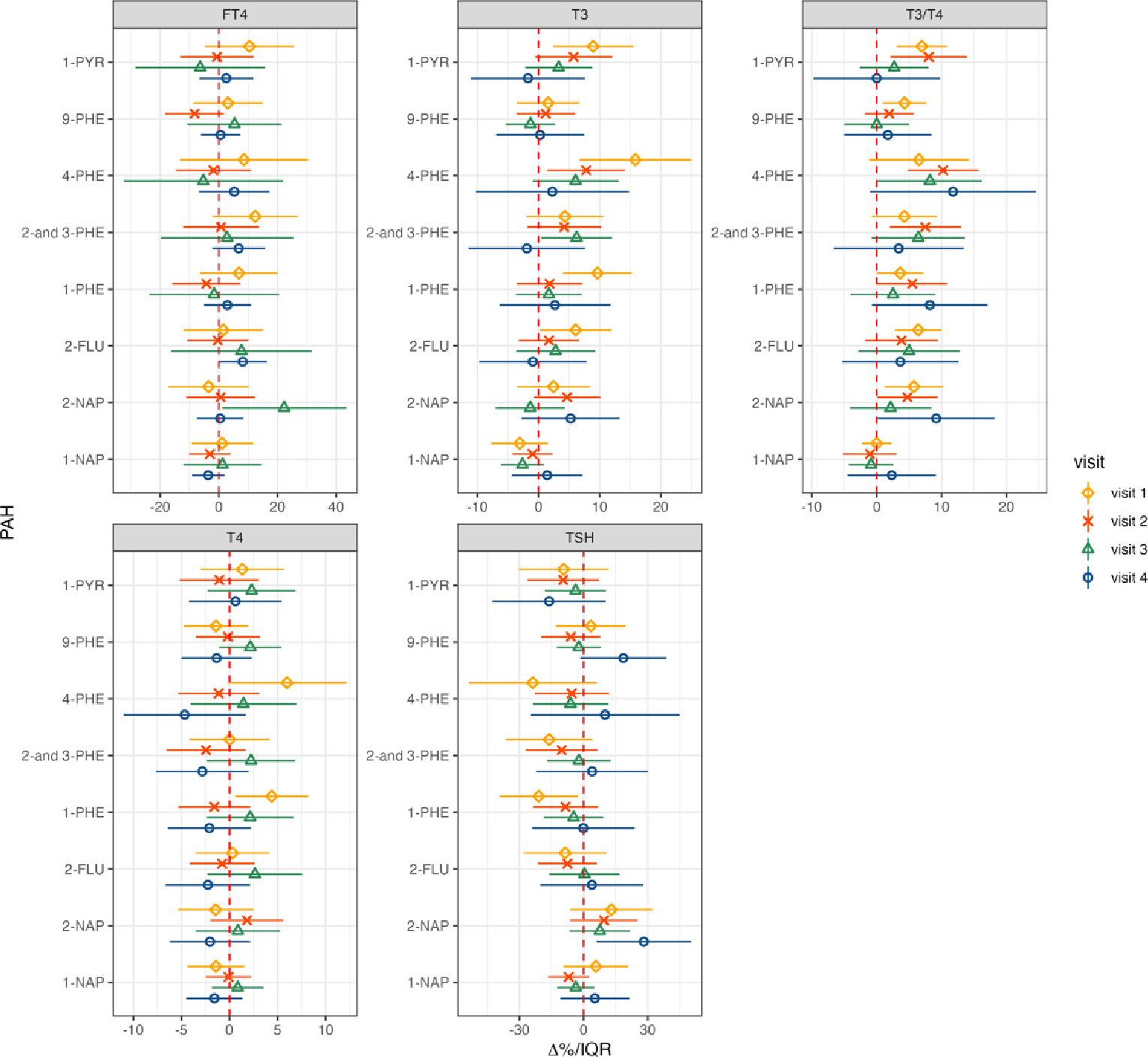
Multiple linear regressions of thyroid hormone versus urinary concentrations of PAH stratified by study visit (NV1 =371; NV2 =352; NV3 =328; NV4 =317). Forest plot depicting percent changes and 95% confidence intervals of thyroid hormones with interquartile range increase in PAH metabolites. Models were adjusted for maternal age, education, race, insurance type, alcohol consumption, pre-pregnancy BMI, and specific gravity. PAH levels were natural log transformed.
We observed distinct sex-specific effects of PAH exposure on thyroid hormones when analyzing data stratified by fetal sex (Figure 3, Supplementary Table 4). Notably, women delivering male offspring exhibited stronger positive associations of T3 with 1-PYR, 4-PHE, and 1-PHE, compared to women delivering female offspring. Specifically, IQR increase in 1-PYR among women delivering male offspring was associated with 5.01% (95%CI: 0.98, 9.04) increase in T3, while it is not associated among women who deliver female offspring (2.81 % per IQR, 95%CI: −1.15, 6.77). Likewise, the positive association between TSH and 2-NAP was predominantly seen in women delivering male offspring (10.26 % per IQR, 95%CI: 0.21, 20.31). Furthermore, we identified several positive associations between T4 and 1-PYR, 4-PHE, and 1-PHE specifically among women delivering male offspring. This contrasts with the findings from non-stratified models, where these associations were less pronounced. In contrast, the positive association between T3/T4 and 4-PHE was driven by women delivering female offspring, showing 4.4 % (95% CI: 0.95,7.85) increase per 4-PHE IQR increase.
Figure 3.
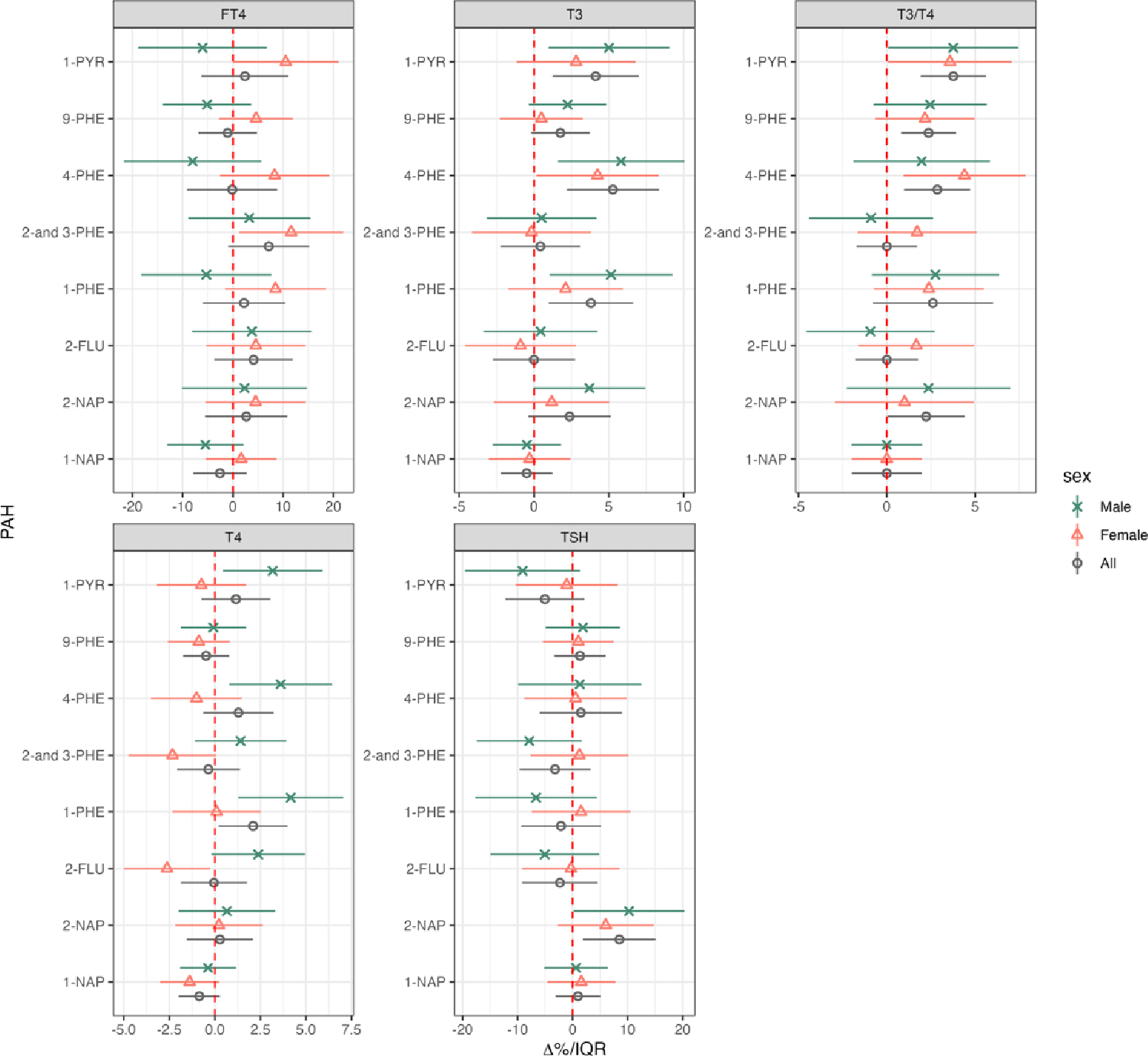
Linear mixed effect regression for thyroid hormone levels in association with urinary concentrations of PAHs stratified by fetal sex. The number of participants and sample size for each group were as follows: Nall=439 (1368), Nfemale=199 (604), Nmale=239 (762), Nmissing=1 (2). Forest plot depicting percent changes and 95% confidence intervals of thyroid hormones with interquartile range increase in PAH metabolites. Models were adjusted for gestational age, maternal age, education, race, insurance type, alcohol consumption, pre-pregnancy BMI, and specific gravity. PAH levels were natural log transformed.
3.3. Associations of PAHs mixture with Thyroid hormones
We conducted quantile g-computation to assess the collective impact of a mixture of PAHs under linearity assumption, and BKMR modeling to examine potential nonlinearity and interaction between exposure. The overall effects of this PAH mixture on thyroid hormones from quantile g-computation are summarized in Table 4. We observed positive trends of combined effect for all thyroid hormones, although the associations did not reach statistical significance. Our BKMR analysis, based on the Posterior Inclusion Probabilities (PIPs), identified 4-PHE (PIP =0.97), 2-and 3-PHE (PIP=0.94), 1-PHE (PIP=0.83), 1-NAP (PIP=0.76), and 2-FLU (PIP=0.64) as influential PAH metabolite associated with T3 concentrations (Supplementary Table 5). Based on the univariate exposure-response function (Figure 4), we observed that most of the selected PAH metabolites exhibited a positive association with T3 (Figure 4a), which was similar to our single pollutant results. We also observed a negative association with 2-and 3-PHE and a non-linear U-shaped association with 1-NAP, which were not evident in the single pollutant analyses. For our BKMR modeling of T4, 1-PHE (PIP=1), 2-FLU (PIP=0.91), 9-PHE (PIP=0.9), and 1-NAP (PIP=0.85) were identified as important predictors (Supplementary Table 5). The univariate associations of most PAH metabolites with T4 followed a similar trend to their associations with T3, with the exception of 1-NAP, which exhibited an S-shaped relationship (Figure 4b). When exploring potential pollutant interactions through the bivariate exposure-response functions for T3 and T4, we observed several interactions between the selected PAH metabolites (PIP>0.5) and the results are illustrated in Supplementary Figure 3. In our hierarchical BKMR modeling, the outcomes closely paralleled those obtained using the component-wise BKMR approach (Supplementary Table 6).
Table 4.
Percent change in Thyroid hormone concentrations associated with a one quartile increase in all exposure biomarkers within PAH mixtures estimated using quantile-g computation models. Models were adjusted for maternal age, education, race, insurance type, alcohol consumption, pre-pregnancy BMI, and specific gravity. PAH levels were natural log transformed.
| Thyroid | Beta (%) | 95% CI | p-value |
|---|---|---|---|
|
| |||
| T3 | 2.65 | (−1.03, 6.32) | 0.16 |
| T4 | 0.17 | (−2.84, 3.17) | 0.92 |
| T3/T4 | 3.33 | (−0.67, 6.67) | 0.09 |
| FT4 | 3.04 | (−4.91, 10.98) | 0.45 |
| TSH | 1.69 | (−9.03, 12.5) | 0.75 |
Figure 4.
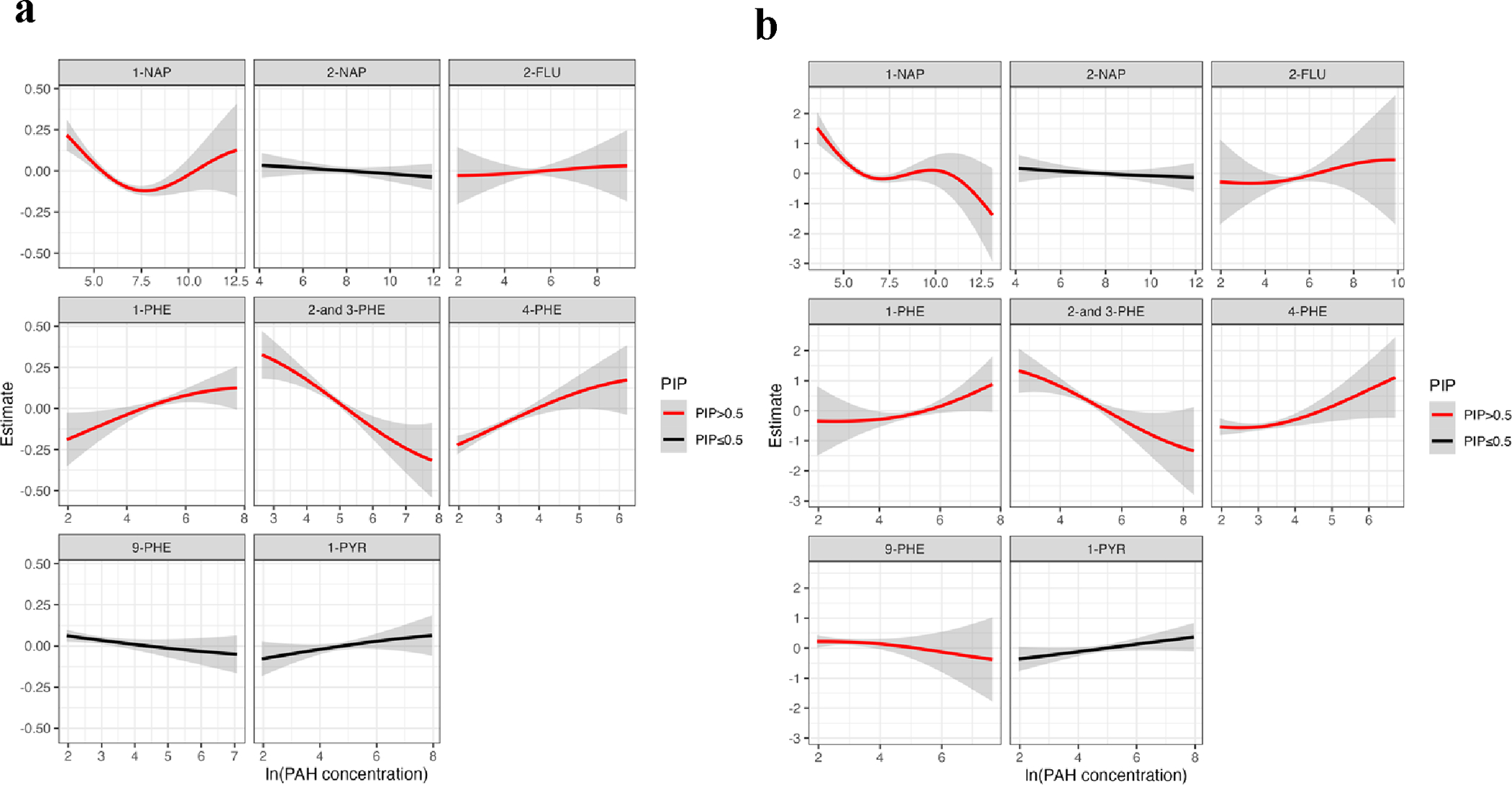
Univariate exposure–response function (95% CI) between PAH metabolites and (a) T3 Thyroid hormone (b) T4 Thyroid hormone while fixing the concentrations of other PAHs at median values. Colors indicate the selection of PAH metabolites based on PIP>0.5 of BKMR models. Models were adjusted for maternal age, gestational age, education, race, insurance type, alcohol consumption, pre-pregnancy BMI, and specific gravity. PAH levels were natural log transformed.
4. Discussion
The objective of this study was to investigate the potential associations between gestational exposure to PAH and circulating thyroid hormone concentrations in pregnant women. This was achieved by employing repeated measures of urinary PAH concentrations and plasma thyroid hormone concentrations. In our single-pollutant analysis, we consistently identified positive associations between PAH metabolites and thyroid hormones, primarily between phenanthrene metabolites and T3 concentrations and the T3/T4 ratio. Visit-specific analyses indicated that the associations of PAH exposure were notably pronounced during the first or second visits for T3, T4, and T3/T4, whereas exposure during the fourth visit exhibited a more prominent association with TSH levels. Sex-stratified analyses revealed several stronger positive associations among women carrying male fetus than women carrying female fetus. From quantile g-computation, we observed positive trends of joint associations with all thyroid hormones. Furthermore, employing BKMR models, we observed several non-linear associations and interaction between different PAHs.
There has been limited research on the relationship between PAH exposure and maternal thyroid hormones during gestation. In our recent study involving pregnant women in Puerto Rico, we observed positive associations between individual PAH metabolites and various maternal thyroid hormones, particularly with T3 concentrations and the T3/T4 ratio, which is consistent with our findings from the present study (Cathey et al., 2020). In addition, a previous investigation exploring the cross-sectional relationships between PAH and thyroid function in females during the NHANES 2007–2008 cycle reported multiple positive associations (Jain, 2016). Notably, positive associations were reported between 2-NAP, 2-PHE, and 1-PYR with T3, as well as between 1-NAP and FT4. In another study from the NHANES 2011–2012 cycle, Xing and colleagues reported positive associations between 2-FLU concentrations with T3 and T4 concentrations among females (2023). The consistent findings across these studies support the results from the present study, especially considering variations in PAH exposure levels among the three study populations. Specifically, concentrations of most PAH metabolites in our study population were higher than those observed in women in Puerto Rico, but lower than in women of reproductive age (18–40) in the NHANES 2011–2012 cycle. Detailed information on these exposure levels was summarized in our previous study (Cathey et al., 2018).
Our results also align with findings from certain animal studies. For instance, phenanthrene has been linked to a significant increase in T3 concentrations but also a decrease in the concentration of T4 in zebrafish (Wu et al., 2022). Another study reported an inverse association of phenanthrene exposure with both T3 and T4 in adult female zebrafish, with the decrease being much more pronounced for T4 compared to T3 (Zhong et al., 2023). The alteration in the T3/T4 ratio may stem from differential impacts of PAH on T3 and T4 in the opposite direction or of different magnitudes. The elevated concentrations of T3/T4 in circulation create a negative feedback loop with the hypothalamus and pituitary glands. Thus, the heightened T3/T4 concentrations alongside unchanged TSH concentrations imply potential disruption of the negative feedback loop by PAH exposure. While this convergence of evidence supports the results from our study, more research is needed, particularly to disentangle observations of individual PAH compared to the impacts of exposure to a PAH mixture.
A handful of prior experimental studies offer insights into potential mechanisms through which PAHs may impact thyroid function in pregnant women. For example, in rockfish embryos, He et al (2012) demonstrated that pyrene exposure is associated with altered thyroid development and function related genes. A previous in-vivo study reported decreased activity of Thyroid Peroxidase (TPO), an enzyme that plays a vital role in iodination of tyrosine to T3 and T4 in response to TSH, in response to pyrene exposure (Song et al., 2012). Additionally, an in-vitro study has shown that 1-NAP and 2-NAP have anti-thyroid hormone activities of T3, affecting thyroid-receptor-mediated transcription (Sun et al., 2008). Suppressed hormone activities related to PAH exposure may contribute to the overproduction of T3. Moreover, a recent animal study showed upregulated transcription of the type II iodothyronine deiodinase (dio2) gene and downregulated transcription of type III iodothyronine deiodinase (dio3) gene in phenanthrene treated zebrafish embryos (Wu et al., 2022). Dio2 is a pathway responsible for converting T4 to T3 through deiodination, while dio3 is a crucial pathway for clearance of plasma T3 to T2 (Bianco & Kim, 2006), which may also explain the increased T3 concentration in our study.
While limited information is available with respect to fetal sex-specificity, previous studies suggest the role of sex hormones on different associations of PAH exposure with thyroid hormones. Due to the intersection between the hypothalamus-pituitary–thyroid axis (HPT) and hypothalamus–pituitary– ovarian axis (HPO), the interaction between thyroid hormones and reproductive hormones is inevitable (Doufas & Mastorakos, 2000; Kjaergaard et al., 2021). For example, gonadotropin-releasing hormones (GnRHs) from HPO may interfere with the thyroid hormone axis by increasing TSH secretion (Denver, 1988; Chiba et al., 2004; Okada et al., 2004), which is associated with an increase in overall thyroid hormone synthesis. In addition, steroids may influence thyroid function by affecting the clearance of thyroid-binding globulin (TBG), a main transport protein for thyroid hormone circulation. Specifically, estrogen administration results in increased serum TBG levels, while androgen administration causes a decrease in TBG (Tahboub & Arafah, 2009). Moreover, steroid hormone treatment altered the expression of receptors for thyrotropin-releasing hormone (TRH), TSH, and thyroid hormones in the monkey uterus (Hulchiy et al., 2012). Previous experiment studies have indicated that PAHs have various estrogenic (Hayakawa et al., 2007; Zhang et al., 2016), androgenic (Vinggaard et al., 2000), or anti-androgenic effects (Vinggaard et al., 2000; Kizu et al., 2003; Bolden et al., 2017). Previous epidemiological studies also showed altered sex steroid hormone levels, including testosterone and estradiol, in relation to PAH exposures among pregnant women (Yin et al., 2017; Cathey et al., 2020), and non-pregnant women (Rafiee et al., 2023; Zhu et al., 2023). In sum, the differences by fetal sex we observed may partially be attributed to the interaction with reproductive hormones.
In our analyses, we observed stronger positive associations of PAHs with T3 and T4 in earlier visits, while positive trends of associations with TSH in later visits. Still, scarce data is available on the time-specific association between PAH and thyroid hormone, physiological changes along with thyroid hormone flux during pregnancy may provide potential explanations. For example, in early pregnancy up to midgestation, serum TBG concentration is known to increase through estrogen-induced mechanisms, resulting in increased total T3 and T4 concentration (Moleti et al., 2014; Springer et al., 2017). Since PAH has some estrogenic effects, PAHs are assumed to further affect T3 and T4 levels in earlier visits (approximately, 10 –18 weeks of gestation) by influencing estrogen levels. Additionally, early in pregnancy, human chorionic gonadotropin (hCG) - a hormone structurally similar to TSH - binds to TSH receptors (Hershman, 2004). Acting as a weak form of TSH, hCG increases T3 and T4 secretion while suppressing TSH levels (Haddow et al., 2008; Geno & Nerenz, 2022). As pregnancy progresses, the impact of hCG on suppressing TSH diminishes, which may explain the stronger PAH-TSH associations observed in later prenatal visits. However, the exact mechanisms underlying the discrepancies in the associations of PAH with thyroid by study visit and by fetal sex still remain unclear, thus further studies are necessary for verification.
To our knowledge, this study represents the first exploration into the combined impact of exposure to a PAH mixture on the thyroid function of pregnant women, while concurrently investigating the individual effects of each PAH metabolite. A notable strength is that we employed repeated measurements of both PAH and thyroid hormone concentrations up to four times in both single-pollutant and mixture analyses, utilizing a random intercept by each individual. Additionally, our research contributes to the understanding of the time-specific effects of PAH exposure during pregnancy through visit-specific analyses. Furthermore, we improve upon previous PAH studies by utilizing biomarkers instead of or in addition to other methods like air monitoring or food consumption questionnaires for exposure assessment. This allows for a more precise evaluation of individual exposure, encompassing all exposure routes beyond just inhalation or ingestion. However, certain limitations in our study warrant attention in future research. Our investigation is limited to the metabolites of four parent PAH compounds, potentially not fully encompassing the broader profile of PAH exposure. We specifically concentrated on nine PAH metabolites, drawing from the PAH analytics panel of NHANES and previous studies that have reported potential reproductive health outcomes associated with these PAHs (Bolden et al., 2017). Nevertheless, a more comprehensive examination, including other PAHs such as benzo[a]pyrene, is essential to enhance our understanding on the health effects of PAH exposure. Another limitation is the omission of thyroid-related proteins, such as albumin, TBG, or transthyretin, which play an important role in regulating and transporting thyroid hormones in the bloodstream. Future studies incorporating these proteins will enhance the comprehensive understanding of the underlying mechanisms influencing thyroid function during pregnancy.
5. Conclusion
In conclusion, our study provides insight into the impact of gestational PAH exposure on circulating thyroid hormone concentrations among pregnant women. Higher concentrations of individual PAH metabolites were associated with elevated concentrations of T3, T3/T4, and TSH, and the overall PAH mixture also suggested a cumulative positive effect. Furthermore, PAH metabolites may influence thyroid hormone concentrations in a non-linear manner and through interactions with various other PAH metabolites. This underscores the need for further investigation into the complex etiology of these associations. Given the critical roles of the thyroid in fetal growth, the onset of labor, and child neurodevelopment, the observed changes in thyroid hormone concentrations associated with PAH exposure may have implications for complications during pregnancy and beyond. Future research exploring gestational PAH exposure, the mediating influence of altered thyroid hormone concentrations, and their associations with diverse pregnancy and developmental outcomes will contribute to a more comprehensive understanding, informing targeted interventions for maternal and child health.
Supplementary Material
Acknowledgements
Support for this research was provided by grants R01ES031591 and P30ES017885 from the National Institute of Environmental Health Sciences, National Institutes of Health. KKF was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ZIA ES103321). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CRediT authorship contribution statement
Seonyoung Park: Conceptualization, Investigation, Formal analysis, Visualization, Writing - Original Draft; Ram C. Siwakoti: Methodology, Validation, Writing - Review & Editing , Kelly K. Ferguson: Methodology, Writing – review & editing , Amber L. Cathey: Conceptualization, Writing - Review & Editing , Wei Hao: Methodology, Writing - Review & Editing; David E. Cantonwine: Methodology, Writing – review & editing, Resources; Bhramar Mukherjee: Methodology, Writing – review & editing; Thomas F. McElrath: Methodology, Resources, Writing – review & editing; and John D. Meeker: Supervision, Conceptualization, Funding acquisition. All authors contributed to the article and approved the submitted version.
References
- ACOG. (2017). Committee Opinion no 700: methods for estimating the due date. Obstet Gynecol, 129(5), e150–e154. [DOI] [PubMed] [Google Scholar]
- Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, & Meeker JD (2018). Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environment international, 113, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhamdow A, Lindh C, Albin M, Gustavsson P, Tinnerberg H, & Broberg K (2019). Cardiovascular disease-related serum proteins in workers occupationally exposed to polycyclic aromatic hydrocarbons. Toxicological Sciences, 171(1), 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, & Andersen S (2021). Hyperthyroidism in pregnancy: evidence and hypothesis in fetal programming and development. Endocrine Connections, 10(2), R77–R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, Johns LE, Ferguson KK, Mukherjee B, McElrath TF, & Meeker JD (2017). Thyroid hormone parameters during pregnancy in relation to urinary bisphenol A concentrations: a repeated measures study. Environment international, 104, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa F Jr, Rocha BA, Souza MC, Bocato MZ, Azevedo LF, Adeyemi JA, Santana A, & Campiglia AD (2023). Polycyclic aromatic hydrocarbons (PAHs): Updated aspects of their determination, kinetics in the human body, and toxicity. Journal of Toxicology and Environmental Health, Part B, 26(1), 28–65. [DOI] [PubMed] [Google Scholar]
- Bekki K, Takigami H, Suzuki G, Tang N, & Hayakawa K (2009). Evaluation of toxic activities of polycyclic aromatic hydrocarbon derivatives using in vitro bioassays. Journal of Health Science, 55(4), 601–610. [Google Scholar]
- Bianco AC, & Kim BW (2006). Deiodinases: implications of the local control of thyroid hormone action. The Journal of clinical investigation, 116(10), 2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, & Coull BA (2015). Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics, 16(3), 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden AL, Rochester JR, Schultz K, & Kwiatkowski CF (2017). Polycyclic aromatic hydrocarbons and female reproductive health: a scoping review. Reproductive Toxicology, 73, 61–74. [DOI] [PubMed] [Google Scholar]
- Bright A, Li F, Movahed M, Shi H, & Xue B (2023). Chronic exposure to low-molecular-weight polycyclic aromatic hydrocarbons promotes lipid accumulation and metabolic inflammation. Biomolecules, 13(2), 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF, & Meeker JD (2015). Urinary bisphenol A levels during pregnancy and risk of preterm birth. Environmental health perspectives, 123(9), 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wen Y, Zhou Y, & Chen W (2019). Effects of environmental and lifestyle exposure on urinary polycyclic aromatic hydrocarbon metabolites: a cross-sectional study of urban adults in China. Environmental Epidemiology, 3, 50–51. [DOI] [PubMed] [Google Scholar]
- Castano-Vinyals G, D’errico A, Malats N, & Kogevinas M (2004). Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occupational and environmental medicine, 61(4), e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, McElrath TF, Cantonwine DE, Pace G, Alshawabkeh A, Cordero JF, & Meeker JD (2018). Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environmental Pollution, 232, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey AL, Watkins DJ, Rosario ZY, Vega CMV, Loch-Caruso R, Alshawabkeh AN, Cordero JF, & Meeker JD (2020). Polycyclic aromatic hydrocarbon exposure results in altered CRH, reproductive, and thyroid hormone concentrations during human pregnancy. Science of The Total Environment, 749, 141581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yu Z, & Huang JZ (2013). The cluster bootstrap consistency in generalized estimating equations. Journal of Multivariate Analysis, 115, 33–47. [Google Scholar]
- Chiba H, Amano M, Yamada H, Fujimoto Y, Ojima D, Okuzawa K, Yamanome T, Yamamori K, & Iwata M (2004). Involvement of gonadotropin-releasing hormone in thyroxine release in three different forms of teleost fish: barfin founder, masu salmon and goldfish. Fish Physiology and Biochemistry, 30, 267–273. [Google Scholar]
- Coull BA, Bobb JF, Wellenius GA, Kioumourtzoglou M-A, Mittleman MA, Koutrakis P, & Godleski JJ (2015). Part 1. Statistical learning methods for the effects of multiple air pollution constituents. Res Rep Health Eff Inst, 183(183), 1–2. [PubMed] [Google Scholar]
- Dai Y, Xu X, Huo X, & Faas MM (2023). Effects of polycyclic aromatic hydrocarbons (PAHs) on pregnancy, placenta, and placental trophoblasts. Ecotoxicology and Environmental Safety, 262, 115314. [DOI] [PubMed] [Google Scholar]
- Deng X, Liu Q, Liu S, Li W, Chen Y, Wen Y, & Luo Y (2017). Determination of polycyclic aromatic hydrocarbons in girls and association between polycyclic aromatic hydrocarbons exposure and puberty timing. Wei Sheng yan jiu= Journal of Hygiene Research, 46(5), 743–748. [PubMed] [Google Scholar]
- Denver RJ (1988). Several hypothalamic peptides stimulate in vitro thyrotropin secretion by pituitaries of anuran amphibians. General and Comparative Endocrinology, 72(3), 383–393. [DOI] [PubMed] [Google Scholar]
- Doufas AG, & Mastorakos G (2000). The hypothalamic-pituitary-thyroid axis and the female reproductive system. Annals of the New York Academy of Sciences, 900(1), 65–76. [DOI] [PubMed] [Google Scholar]
- Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, Flak E, Li Z, Wang S, & Rauh V (2010). Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environmental health perspectives, 118(9), 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R, Saeki Y, Chacos N, Capdevila J, & Prough R (1981). Polycyclic hydrocarbon metabolism: a plethora of phenomena. Advances in Enzyme Regulation, 19, 3–17. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, & Meeker JD (2014). Environmental phthalate exposure and preterm birth. JAMA pediatrics, 168(1), 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Pace GG, Weller D, Zeng L, Pennathur S, Cantonwine DE, & Meeker JD (2017). Urinary polycyclic aromatic hydrocarbon metabolite associations with biomarkers of inflammation, angiogenesis, and oxidative stress in pregnant women. Environmental science & technology, 51(8), 4652–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahlawat P, Singh A, Nanda S, & Kharb S (2017). Thyroid dysfunction in early pregnancy and spontaneous abortion. Biomedical and Biotechnology Research Journal (BBRJ), 1(1), 81–84. [Google Scholar]
- Geno KA, & Nerenz RD (2022). Evaluating thyroid function in pregnant women. Critical reviews in clinical laboratory sciences, 59(7), 460–479. [DOI] [PubMed] [Google Scholar]
- Haddow JE, McClain MR, Lambert-Messerlian G, Palomaki GE, Canick JA, Cleary-Goldman J, Malone FD, Porter TF, Nyberg DA, & Bernstein P (2008). Variability in thyroid-stimulating hormone suppression by human chronic gonadotropin during early pregnancy. The Journal of Clinical Endocrinology & Metabolism, 93(9), 3341–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Onoda Y, Tachikawa C, Hosoi S, Yoshita M, Chung SW, Kizu R, Toriba A, Kameda T, & Tang N (2007). Estrogenic/antiestrogenic activities of polycyclic aromatic hydrocarbons and their monohydroxylated derivatives by yeast two-hybrid assay. Journal of Health Science, 53(5), 562–570. [Google Scholar]
- He C, Zuo Z, Shi X, Sun L, & Wang C (2012). Pyrene exposure influences the thyroid development of Sebastiscus marmoratus embryos. Aquatic Toxicology, 124, 28–33. [DOI] [PubMed] [Google Scholar]
- Hershman JM (2004). Physiological and pathological aspects of the effect of human chorionic gonadotropin on the thyroid. Best Practice & Research Clinical Endocrinology & Metabolism, 18(2), 249–265. [DOI] [PubMed] [Google Scholar]
- Hew K, Walker A, Kohli A, Garcia M, Syed A, McDonald-Hyman C, Noth E, Mann J, Pratt B, & Balmes J (2015). Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clinical & Experimental Allergy, 45(1), 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, & Suzuki N (2020). Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. International Journal of Environmental Research and Public Health, 17(4), 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene, 5(1), 46–51. [Google Scholar]
- Hoseini M, Nabizadeh R, Delgado-Saborit JM, Rafiee A, Yaghmaeian K, Parmy S, Faridi S, Hassanvand MS, Yunesian M, & Naddafi K (2018). Environmental and lifestyle factors affecting exposure to polycyclic aromatic hydrocarbons in the general population in a Middle Eastern area. Environmental Pollution, 240, 781–792. [DOI] [PubMed] [Google Scholar]
- Hulchiy M, Zhang H, Cline JM, Hirschberg AL, & Sahlin L (2012). Receptors for thyrotropin-releasing hormone, thyroid-stimulating hormone, and thyroid hormones in the macaque uterus: effects of long-term sex hormone treatment. Menopause, 19(11), 1253–1259. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, I. (2010). Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures (Vol. 92). IARC Press, International Agency for Research on Cancer. [Google Scholar]
- Jain RB (2016). Association between polycyclic aromatic hydrocarbons and thyroid function among males and females: data from NHANES 2007–2008. International Journal of Environmental Health Research, 26(4), 405–419. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R, & Sowa A (2015). Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environmental Science and Pollution Research, 22, 3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Cantonwine DE, Mukherjee B, Meeker JD, & McElrath TF (2018). Subclinical changes in maternal thyroid function parameters in pregnancy and fetal growth. The Journal of Clinical Endocrinology & Metabolism, 103(4), 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Seely EW, & Meeker JD (2017). Longitudinal profiles of thyroid hormone parameters in pregnancy and associations with preterm birth. PLoS One, 12(1), e0169542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehm RD, Oskar S, Tehranifar P, Zeinomar N, Rundle AG, Herbstman JB, Perera F, Miller RL, & Terry MB (2021). Associations of prenatal exposure to polycyclic aromatic hydrocarbons with pubertal timing and body composition in adolescent girls: Implications for breast cancer risk. Environmental research, 196, 110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, & White AJ (2020). A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environmental health perspectives, 128(4), 047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Jahan SA, Kabir E, & Brown RJ (2013). A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environment international, 60, 71–80. [DOI] [PubMed] [Google Scholar]
- Kizu R, Okamura K, Toriba A, Kakishima H, Mizokami A, Burnstein KL, & Hayakawa K (2003). A role of aryl hydrocarbon receptor in the antiandrogenic effects of polycyclic aromatic hydrocarbons in LNCaP human prostate carcinoma cells. Archives of toxicology, 77, 335–343. [DOI] [PubMed] [Google Scholar]
- Kjaergaard AD, Marouli E, Papadopoulou A, Deloukas P, Kuś A, Sterenborg R, Teumer A, Burgess S, Åsvold BO, & Chasman DI (2021). Thyroid function, sex hormones and sexual function: a Mendelian randomization study. European journal of epidemiology, 36, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JR, O’Brien KM, Ferguson KK, & Buckley JP (2021). Urinary specific gravity measures in the US population: Implications for the adjustment of non-persistent chemical urinary biomarker data. Environment international, 156, 106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, & Patterson DG Jr (2008). Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environmental research, 107(3), 320–331. [DOI] [PubMed] [Google Scholar]
- McElrath TF, Lim K-H, Pare E, Rich-Edwards J, Pucci D, Troisi R, & Parry S (2012). Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. American journal of obstetrics and gynecology, 207(5), 407. e401–407. e407. [DOI] [PubMed] [Google Scholar]
- Merlo F, Andreassen A, Weston A, Pan C, Haugen A, Valerio F, Reggiardo G, Fontana V, Garte S, & Puntoni R (1998). Urinary excretion of 1-hydroxypyrene as a marker for exposure to urban air levels of polycyclic aromatic hydrocarbons. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 7(2), 147–155. [PubMed] [Google Scholar]
- Moleti M, Trimarchi F, & Vermiglio F (2014). Thyroid physiology in pregnancy. Endocrine Practice, 20(6), 589–596. [DOI] [PubMed] [Google Scholar]
- Nazarpour S, Tehrani FR, Simbar M, & Azizi F (2015). Thyroid dysfunction and pregnancy outcomes. Iranian journal of reproductive medicine, 13(7), 387. [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll CA, Gallo ME, Hoffmann EJ, Fechner JH, Schauer JJ, Bradfield CA, & Mezrich JD (2018). Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS One, 13(12), e0209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada R, Yamamoto K, Koda A, Ito Y, Hayashi H, Tanaka S, Hanaoka Y, & Kikuyama S (2004). Development of radioimmunoassay for bullfrog thyroid-stimulating hormone (TSH): effects of hypothalamic releasing hormones on the release of TSH from the pituitary in vitro. General and Comparative Endocrinology, 135(1), 42–50. [DOI] [PubMed] [Google Scholar]
- Onyemauwa F, Rappaport SM, Sobus JR, Gajdošová D, Wu R. a., & Waidyanatha S (2009). Using liquid chromatography–tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. Journal of Chromatography B, 877(11–12), 1117–1125. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, & Nebert DW (1982). Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacological reviews, 34(2), 189–222. [PubMed] [Google Scholar]
- Peng B, Dong Q, Li F, Wang T, Qiu X, & Zhu T (2023). A systematic review of polycyclic aromatic hydrocarbon derivatives: Occurrences, levels, biotransformation, exposure biomarkers, and toxicity. Environmental science & technology, 57(41), 15314–15335. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai W-Y, Tang D, Diaz D, Hoepner L, Barr D, Tu Y-H, & Camann D (2006). Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental health perspectives, 114(8), 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, & Semanek D (2015). Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA psychiatry, 72(6), 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee A, Hoseini M, Akbari S, & Mahabee-Gittens EM (2023). Exposure to Polycyclic Aromatic Hydrocarbons and adverse reproductive outcomes in women: current status and future perspectives. Reviews on Environmental Health(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, & Weyand EH (2004). Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. International journal of toxicology, 23(5), 301–333. [DOI] [PubMed] [Google Scholar]
- Raymond CVJ (1998). Estimating the lung deposition of particulate polycyclic aromatic hydrocarbons associated with multimodal urban aerosols. Inhalation toxicology, 10(3), 183–204. [Google Scholar]
- Reynaud S, & Deschaux P (2006). The effects of polycyclic aromatic hydrocarbons on the immune system of fish: a review. Aquatic Toxicology, 77(2), 229–238. [DOI] [PubMed] [Google Scholar]
- Richardson DB, Rzehak P, Klenk J, & Weiland SK (2007). Analyses of case-control data for additional outcomes. Epidemiology, 441–445. [DOI] [PubMed] [Google Scholar]
- Rosner B (2015). Fundamentals of biostatistics. Cengage learning. [Google Scholar]
- Song M, Kim Y-J, Park Y-K, & Ryu J-C (2012). Changes in thyroid peroxidase activity in response to various chemicals. Journal of Environmental Monitoring, 14(8), 2121–2126. [DOI] [PubMed] [Google Scholar]
- Springer D, Jiskra J, Limanova Z, Zima T, & Potlukova E (2017). Thyroid in pregnancy: From physiology to screening. Critical reviews in clinical laboratory sciences, 54(2), 102–116. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A, & Pearce E (2012). Thyroid disorders in pregnancy. Nature Reviews Endocrinology, 8(11), 650–658. [DOI] [PubMed] [Google Scholar]
- Sun B, Wallace ER, Ni Y, Loftus CT, Szpiro A, Day D, Barrett ES, Nguyen RH, Kannan K, & Robinson M (2023). Prenatal exposure to polycyclic aromatic hydrocarbons and cognition in early childhood. Environment international, 178, 108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Shen O-X, Xu X-L, Song L, & Wang X-R (2008). Carbaryl, 1-naphthol and 2-naphthol inhibit the beta-1 thyroid hormone receptor-mediated transcription in vitro. Toxicology, 249(2–3), 238–242. [DOI] [PubMed] [Google Scholar]
- Sun K, Song Y, He F, Jing M, Tang J, & Liu R (2021). A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Science of The Total Environment, 773, 145403. [DOI] [PubMed] [Google Scholar]
- Tahboub R, & Arafah BM (2009). Sex steroids and the thyroid. Best Practice & Research Clinical Endocrinology & Metabolism, 23(6), 769–780. [DOI] [PubMed] [Google Scholar]
- Tibshirani R, Walther G, & Hastie T (2001). Estimating the number of clusters in a data set via the gap statistic. Journal of the royal statistical society: series B (statistical methodology), 63(2), 411–423. [Google Scholar]
- Tsai P-J, Shieh H-Y, Lee W-J, & Lai S-O (2001). Health-risk assessment for workers exposed to polycyclic aromatic hydrocarbons (PAHs) in a carbon black manufacturing industry. Science of The Total Environment, 278(1–3), 137–150. [DOI] [PubMed] [Google Scholar]
- Unwin J, Cocker J, Scobbie E, & Chambers H (2006). An assessment of occupational exposure to polycyclic aromatic hydrocarbons in the UK. Annals of Occupational Hygiene, 50(4), 395–403. [DOI] [PubMed] [Google Scholar]
- Veltman K, Huijbregts MA, Rye H, & Hertwich EG (2011). Including impacts of particulate emissions on marine ecosystems in life cycle assessment: The case of offshore oil and gas production. Integrated environmental assessment and management, 7(4), 678–686. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Hnida C, & Larsen JC (2000). Environmental polycyclic aromatic hydrocarbons affect androgen receptor activation in vitro. Toxicology, 145(2–3), 173–183. [DOI] [PubMed] [Google Scholar]
- Wallace ER, Ni Y, Loftus CT, Sullivan A, Masterson E, Szpiro AA, Day DB, Robinson M, Kannan K, & Tylavsky FA (2022). Prenatal urinary metabolites of polycyclic aromatic hydrocarbons and toddler cognition, language, and behavior. Environment international, 159, 107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li A, & Xu Q (2022). The association between urinary polycyclic aromatic hydrocarbons metabolites and Type 2 diabetes mellitus. International Journal of Environmental Research and Public Health, 19(13), 7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu W, Bravo MA, Liu S, Xi X, Zhou Y, Zhang Q, & Liu Q (2024). Prepubertal exposure to polycyclic aromatic hydrocarbons are associated with early pubertal development onset in boys: A longitudinal study. Journal of hazardous materials, 470, 134160. [DOI] [PubMed] [Google Scholar]
- Welch BM, Keil AP, Bommarito PA, van t’Erve TJ, Deterding LJ, Williams JG, Lih FB, Cantonwine DE, McElrath TF, & Ferguson KK (2021). Longitudinal exposure to consumer product chemicals and changes in plasma oxylipins in pregnant women. Environment international, 157, 106787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhong L, Ru H, Yao F, Ni Z, & Li Y (2022). Thyroid disruption and growth inhibition of zebrafish embryos/larvae by phenanthrene treatment at environmentally relevant concentrations. Aquatic Toxicology, 243, 106053. [DOI] [PubMed] [Google Scholar]
- Xing W, Gu W, Liang M, Wang Z, Fan D, Zhang B, & Wang L (2023). Sex-specific effect of urinary metabolites of polycyclic aromatic hydrocarbons on thyroid profiles: results from NHANES 2011–2012. Environmental Science and Pollution Research, 30(16), 47168–47181. [DOI] [PubMed] [Google Scholar]
- Yin S, Tang M, Chen F, Li T, & Liu W (2017). Environmental exposure to polycyclic aromatic hydrocarbons (PAHs): The correlation with and impact on reproductive hormones in umbilical cord serum. Environmental Pollution, 220, 1429–1437. [DOI] [PubMed] [Google Scholar]
- Yu Y. y., Jin H, & Lu Q (2022). Effect of polycyclic aromatic hydrocarbons on immunity. Journal of Translational Autoimmunity, 100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dong S, Wang H, Tao S, & Kiyama R (2016). Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environmental Pollution, 213, 809–824. [DOI] [PubMed] [Google Scholar]
- Zhong L, Wu L, Ru H, Wei N, Yao F, Zhang H, Ni Z, Duan X, & Li Y (2023). Sex-specific thyroid disruption caused by phenanthrene in adult zebrafish (Danio rerio). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 263, 109484. [DOI] [PubMed] [Google Scholar]
- Zhu X, Meng Y, Ju Y, Yang Y, Zhang S. e., Miao L, & Liu Z (2023). Association of the urinary polycyclic aromatic hydrocarbons with sex hormones stratified by menopausal status older than 20 years: a mixture analysis. Environmental Science and Pollution Research, 30(20), 57717–57727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


