Abstract
Background:
Organophosphate, pyrethroid, and neonicotinoid insecticides have resulted in adrenal and gonadal hormone disruption in animal and in vitro studies; limited epidemiologic evidence exists in humans. We assessed relationships of urinary insecticide metabolite concentrations with adrenal and gonadal hormones in adolescents living in Ecuadorean agricultural communities.
Methods:
In 2016, we examined 522 Ecuadorian adolescents (11–17y, 50.7% female, 22% Indigenous; ESPINA study). We measured urinary insecticide metabolites, blood acetylcholinesterase activity (AChE), and salivary testosterone, dehydroepiandrosterone (DHEA), 17β-estradiol, and cortisol. We used general linear models to assess linear (β = % hormone difference per 50% increase of metabolite concentration) and curvilinear relationships (β2 = hormone difference per unit increase in squared ln-metabolite) between ln-metabolite or AChE and ln-hormone concentrations, stratified by sex, adjusting for anthropometric, demographic, and awakening response variables. Bayesian Kernel Machine Regression was used to assess non-linear associations and interactions.
Results:
The organophosphate metabolite malathion dicarboxylic acid (MDA) had positive associations with testosterone (βboys = 5.88% [1.21%, 10.78%], βgirls = 4.10% [−0.02%, 8.39%]), and cortisol (βboys = 6.06 [−0.23%, 12.75%]. Para-nitrophenol (organophosphate) had negatively-trending curvilinear associations, with testosterone (β2boys = −0.17 (−0.33, −0.003), p = 0.04) and DHEA (β2boys = −0.49 (−0.80, −0.19), p = 0.001) in boys. The neonicotinoid summary score (βboys = 5.60% [0.14%, 11.36%]) and the neonicotinoid acetamiprid-N-desmethyl (βboys = 3.90% [1.28%, 6.58%]) were positively associated with 17β-estradiol, measured in boys only. No associations between the pyrethroid 3-phenoxybenzoic acid and hormones were observed. In girls, bivariate response associations identified interactions of MDA, Para-nitrophenol, and 3,5,6-trichloro-2-pyridinol (organophosphates) with testosterone and DHEA concentrations. In boys, we observed an interaction of MDA and Para-nitrophenol with DHEA. No associations were identified for AChE.
Conclusions:
We observed evidence of endocrine disruption for specific organophosphate and neonicotinoid metabolite exposures in adolescents. Urinary organophosphate metabolites were associated with testosterone and DHEA concentrations, with stronger associations in boys than girls. Urinary neonicotinoids were positively associated with 17β-estradiol. Longitudinal repeat-measures analyses would be beneficial for causal inference.
Keywords: Pesticides, Endocrine disrupting chemicals, Environmental epidemiology, Organophosphates, Pyrethroids, Neonicotinoids, Metabolites, Acetylcholinesterase, Hormones
1. Introduction
There is growing evidence that organophosphate, pyrethroid, and neonicotinoid pesticides may be endocrine-disrupting chemicals (EDCs). Endocrine alterations by these three classes of insecticides have been observed in experimental studies of rats (Alaa-Eldin et al., 2017a; Hu et al., 2013; Bal et al., 2012; Tetsatsi et al., 2019), mice (Wang et al., 2019; Bhaskar et al., 2017), and fish (Tian et al., 2017; Crago and Schlenk, 2015; Ghasemzadeh et al., 2015). The limited epidemiological evidence of hormone disruption due to insecticide exposure has mostly been in adult populations and are focused on gonadal hormone outcomes, such as alterations in testosterone, estradiol, and dehydroepiandrosterone (DHEA) concentrations (Suárez et al., 2021; Mendy and Pinney, 2022; Suwannarin et al., 2021; Meeker et al., 2009a). Endocrine disruption can negatively affect human health, as it has been linked to health conditions including, but not limited to, depression and anxiety symptoms (Chronister et al., 2021), cancer, diabetes, and reproductive and developmental alterations (Kabir et al., 2015).
In epidemiologic studies, organophosphate exposure has also been linked to reduced concentrations of testosterone, total testosterone, free testosterone, and testosterone to estradiol ratios in adult males (Aguilar-Garduño et al., 2013; Dziewirska et al., 2018; Gravel et al., 2020; Omoike et al., 2015; Qin et al., 2020). In a cohort of men who were patients of the Massachusetts infertility clinic, there was a dose-dependent increase in the odds of being in the lowest estradiol quintile with increasing exposure to 3,5,6-Trichloro-2-pyridinol (TCPy) (Meeker et al., 2008). In a study of 116 Spanish male adolescents, non-occupational exposure to TCPy was also inversely associated with estradiol and follicle stimulating hormone (FSH) and positively associated with DHEA sulfate (DHEAS), while exposure to 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMPy) was directly associated with estradiol, DHEAS, and FSH (Suárez et al., 2021). Organophosphate pesticides are known to inhibit the enzymatic activity of acetylcholinesterase (AChE). The central cholinergic system has been shown to modulate the hypothalamic-pituitary-adrenal axis in rats, which controls adrenal hormone secretion (Rhodes et al., 2002). To our knowledge, no studies have directly assessed the effects of AChE disruption on testosterone, estradiol, DHEA, and cortisol hormones.
Research on the effects of neonicotinoid insecticides on endocrine alterations is limited. Using data from the 2015–2016 National Health and Nutrition Examination Study (NHANES) examination, in men, women, and children both n-desmethyl-acetamiprid and 5-hydroxy-imidacloprid (OHIM) were associated with decreased free androgen index which estimates physiologically active testosterone (Mendy and Pinney, 2022). OHIM was also associated with decreased serum total testosterone in women (Mendy and Pinney, 2022). In male farmworkers in Northern Thailand, imidacloprid exposure was associated with increased testosterone, dehydrocorticosterone, and DHEA (Suwannarin et al., 2021). N-desmethyl-acetamiprid exposure was associated with increased androstenedione, an endogenous weak androgen steroid hormone helping in the biosynthesis of estrone and testosterone from dehydroepiandrosterone (Suwannarin et al., 2021).
Exposures to pyrethroids have also been associated with endocrine alterations (Alaa-Eldin et al., 2017a; Hu et al., 2013; Wang et al., 2019; Crago and Schlenk, 2015). Pyrethroids have been found to induce significant estrogenicity in Var-I human endometrial cancer cell line (Suárez et al., 2021) and MCF-7 human breast carcinoma cell line (Go et al., 1999), and antagonize progesterone action in T47D human cell lines (Garey and Wolff, 1998). While in a study of adult men recruited from an infertility clinic observed that the urinary metabolites 3-phenoxybenzoic acid (3-PBA), cis-(cis-DCCA) and trans-3-(2, 2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (trans-DCCA) were positively associated with FSH and luteinizing hormones (LH) (Meeker et al., 2009b). In men, LH stimulates testosterone production from Leydig cells, while FSH stimulates spermatogenesis and Sertoli cell function (Dandona and Rosenberg, 2010). Trans-DCCA was also inversely associated with testosterone and free androgen index (Meeker et al., 2009b).
Adolescents living in agricultural communities have increased risk of exposure to pesticides, through pesticide drift from spray sites to nearby homes, direct contact with fumigated crops, parental take-home pathways, and behavioral activities (Simcox et al., 1995; Garry, 2004). Children and adolescents are at higher risk, as they are more susceptible to the effects of toxic exposures (Landrigan and Goldman, 2011) and endocrine disruption at this stage can have life-long effects (Kabir et al., 2015). Thus, it is imperative to identify whether organophosphate, neonicotinoids and pyrethroids contribute to endocrine disruption in adolescent populations. Considering the potential for hormone imbalances associated with pesticide exposures among adolescents, and the dearth of studies in this age-group, this study aimed evaluate whether biomarkers of exposures to organophosphate, neonicotinoids, and pyrethroid insecticides were associated with alterations in sex and adrenal hormones in Ecuadorian adolescents living in agricultural communities.
2. Methods
2.1. Participants
The Study of Secondary Exposures to Pesticides among Children and Adolescents (ESPINA) is a prospective cohort study, established in 2008, that examines the associations of subclinical pesticide exposures on child development. In 2008, 313 boys and girls aged 4–9 years living in Pedro Moncayo, Pichincha province, Ecuador were examined. Most participants (73%) were recruited using data from the 2004 Survey of Access and Demand of Health Services in Pedro Moncayo County, which was a representative survey of Pedro Moncayo County and procured data regarding 71% of the county’s population. This survey was carried out by Fundacion Cimas del Ecuador in collaboration with the Local ´ Rural Governments of Pedro Moncayo and community residents. The remaining participants (27%) were enrolled through community announcements given by governing councils, leaders, and by word-of-mouth. The need to develop this study was defined by the people of Pedro Moncayo County in participatory assemblies. Further details of participant ascertainment and recruitment in 2008 have been published previously (Suarez-Lopez et al., 2012). In 2016, we carried out a follow-up examination of ESPINA participants (n = 245) and recruited new adolescent participants for a total of 545 participants of ages 11–17 years. As in 2008, new participants were selected and recruited using the System of Local and Community Information (SILC) developed by Fundacion Cimas del Ecuador. SILC is a large geospatial database that ´ contains information of the 2016 Pedro Moncayo County Community Survey (formerly the Survey of Access and Demand of Health Services in Pedro Moncayo County).
We acquired informed consent of participation from parents and obtained parental permission and child assent of each of their selected children to participate. This study was approved by the institutional review boards at the University of California San Diego, University of Minnesota, Universidad San Francisco de Quito, and the Ministry of Public Health of Ecuador.
2.2. Setting
Pedro Moncayo County is located on the Ecuadorian Andes and has a mean altitude of 2952 m. The floriculture industry is vital to the economy of Pedro Moncayo County, employing 21% of all adults in the county (Suarez-Lopez et al., 2012) and using 4.47% of the geographic area (1495 ha) (Chronister et al., 2023). Flower plantations there have reported to use over 20 different insecticides, including organophosphates, pyrethroids, neonicotinoids, carbamates, and over 50 different fungicides (Grandjean et al., 2006; Suarez-Lopez et al., 2018).
2.3. Examination
In 2008, children were examined in 7 schools of Pedro Moncayo County in July and August when school was out of session. Parents and other adult residents were interviewed in their home to obtain socioeconomic status, demographic characteristics, and pesticide exposure history of household members. In 2016, children were examined in schools twice: first in April and again between July and October during the summer closure or during weekends. Examiners were uninformed of participants’ pesticide exposure status. Children’s height was measured following recommended procedures (World Health Organization. World Health Organization, 2008) to the nearest 1 mm, using a height board, and weight was measured using a digital scale (Tanita model 0108 MC; Corporation of America, Arlington Heights, IL, USA). Sexual maturation was estimated using self-reported Tanner Staging. Participants reported breast size and pubic hair growth for girls, and pubic hair growth for boys using standard modified tanner drawings from Rasmussen et al. as reference (Rasmussen et al., 2015; Emmanuel et al., 2020; Kormorniczak).
2.4. Sex and adrenal hormones
Levels of testosterone, 17β-estradiol (estradiol), DHEA, and cortisol were measured in passive-drool morning saliva using saliva enzyme-linked immunosorbent assays (ELISA) (Salimetrics, Carlsbad, CA) at the University of California San Diego Integrative Health and Mind-Body Biomarker Laboratory. Participants were given saliva collection kits prior to the examination day. Upon awakening the day of the examination, participants collected a saliva sample and were asked to refrigerate the sample. They then brought the sample to the examination, where they were stored at −80°C until assayed. Levels of testosterone, DHEA, and cortisol were measured in both girls and boys, while estradiol was only measured in boys. This is because estradiol concentrations vary according to menstrual cycle stage in girls.
2.5. Acetylcholinesterase activity
Erythrocyte AChE activity and hemoglobin concentration (mg/dL) were measured using the EQM Test-mate ChE Cholinesterase Test System 400 (EQM AChE Erythrocyte Cholinesterase Assay Kit 470; EQM Research, Inc, Cincinnati, OH). This system calculates these values utilizing a single finger-stick blood sample (EQM Research Inc, 2003). AChE measurement using this assay kit has a high correlation (r = 0.78) to AChE measured by venipuncture (EQM Research, 2003).
2.6. Metabolite assays
Urinary concentrations of creatinine and pesticide biomarkers were measured in the July–October 2016 examination. Participants were given urine collection kits to take home. Upon awakening on the day of the examination, participants collected a sample of their first morning void, and were asked to refrigerate it if possible. The samples brought to the examination site in the morning were aliquoted and frozen at −20 °C. At the end of each day, samples were transported to Quito for storage at −80 °C. Samples were then shipped frozen overnight to UCSD for long-term storage at −80 °C.
A total of 13 insecticide metabolites of organophosphates, pyrethroids, and neonicotinoids were measured. This included four organophosphates (IMPY, TCPy, para-nitrophenol [PNP], and malathion dicarboxylic acid [MDA]), three pyrethroid metabolites (3-PBA, 4-fluoro-3-phenoxybenzoic acid [4F-3PBA], and trans-DCCA), and six neonicotinoid metabolites (acetamiprid [ACET], AND, clothianidin [CLOT], imidacloprid [IMID], OHIM, and thiacloprid [THIA]). For this purpose, urine samples were shipped overnight at −20 °C from UCSD to the National Center for Environmental Health, Division of Laboratory Sciences of the CDC (Atlanta, GA) for quantification of all organophosphates, pyrethroids, and neonicotinoids, and to the Laboratory for Exposure Assessment and Development in Environmental Research at Emory University (Atlanta, GA) for quantification of creatinine. Quality control and quality assurance protocols were followed to ensure data accuracy and reliability of the analytical measurements. All the study samples were re-extracted if quality control failed the statistical evaluation (Baker et al., 2019; Caudill et al., 2008). Targeted organophosphate and pyrethroid metabolites were quantified using liquid chromatography coupled with tandem mass spectrometry and isotope dilution (Davis et al., 2013). The limit of detection (LOD) was 0.1 μg/L for TCPy, PNP, IMPy, 3-PBA, and 4F-3PBA, 0.5 μg/L for MDA, and 0.6 μg/L for trans-DCCA. To measure neonicotinoid metabolite concentrations, targeted metabolites were quantified from enzymatic hydrolysis of 0.5 mL of urine and online solid phase extraction to release, extract, and concentrate the target biomarkers, followed by reversed-phase high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) using electrospray ionization (ESI) (Baker et al., 2019). The LOD was 0.03 μg/L for THIA, 0.2 μg/L for AND and CLOT, 0.3 μg/L for ACET, and 0.4 μg/L for IMID and OHIM.
To assess the dilution of urine, creatinine measurement was conducted and controlled for in the analysis. Urinary creatinine was quantified using HPLC-MS/MS with ESI. A 10 μL aliquot of urine was diluted prior to analysis (Kwon et al., 2012). No further sample preparation was performed prior to analysis. The LOD was 5 mg/dL with a relative standard deviation (RSD) of 7%.
2.7. Imputation for values below the level of detection (LOD)
After excluding metabolites with a detection rate below 25%, six metabolites were included in our analysis. These included three organophosphates (PNP, TCPy, and MDA), one pyrethroid (3-PBA), and two neonicotinoids (AND and OHIM). We used two imputation methods: imputation using a constant and the model-based approach for single imputation (model-based single imputation), to estimate metabolite concentrations for individuals who had pesticide metabolite concentrations below the LOD. The model-based single imputation method was built as a log-logistic regression model that was fitted using backward selection where variables were retained if they had a significance level p < 0.10 (Tang et al., 2012). The initial model used for backwards stepwise regression included 14 variables: age, sex, race, BMI-for-age z-score (z-BMI-for-age), height-for-age z-score (z-height-for-age), monthly family income, tanner maturation score, creatinine concentration, acetylcholinesterase activity, urinary concentrations of 3,5,6-Trichloro-2-pyridinol (TCPy) and para-nitrophenol (PNP), flower crop area within 150 m of the participant’s home, distance from the house to the contour of the nearest flower crop, and cohabitation with a floricultural or agricultural worker. The imputation models were run 1000 times, and the median concentration values were outputted for each observation. Predicted concentrations were then rescaled between 0 and the LOD, and imputed for observations below the level of detection. Given that TCPy and PNP were detectable in all samples, model-based standard imputation was not conducted for these metabolites. Imputation for values below the LOD using a constant (LOD divided by the square root of two) was used to create organophosphateand neonicotinoid summary scores. Summary scores for each pesticide classification and overall pesticide exposure was created by natural log (ln) transforming the imputed metabolite concentrations plus 1, dividing it by the standard deviation, and then averaging the metabolite concentrations. The overall insecticide summary score contained TCPy, PNP, MDA, AND, OHIM, and 3-PBA. The organophosphate summary score contained TCPY, PNP and MDA, the neonicotinoid summary score contained AND and OHIM. As we only included one pyrethroid metabolite, 3-PBA, a summary score was not created for pyrethroids.
2.8. Statistical analysis
A total of 522 participants who had information on at least 1 urinary pesticide metabolite in addition to all covariates of interest were included in this analysis. Normality of outcome variables were determined by Anderson-Darling tests, histograms, and normal probability plots. All variables identified to be non-normally distributed were ln-transformed to meet the normality assumption of linear regression. As there was only 1 participant classified as being White, we combined this participant with the mestizo race category. We ln-transformed creatinine, testosterone, estradiol, DHEA, and cortisol, and all the pesticide metabolites and related summary scores. Descriptive statistics of the study population were calculated across tertiles of the overall pesticide summary score stratified by sex (Table 1). Metabolite concentrations, summary scores, and hormone concentrations were ln-transformed. We calculated means and standard deviations (SD), medians and interquartile ranges (IQR), or frequencies and column percentage for all characteristics as appropriate. Differences in covariate values across tertiles of the overall pesticide summary score were identified by unadjusted linear regression (p-trend). Geometric means and their 95% confidence intervals were calculated for each creatinine adjusted metabolite after imputation using a constant. To determine how exposure patterns differed between our cohort, we compared the biomarker concentrations to those that are presented in the National Health and Nutrition Examination Survey (NHANES) biomonitoring data (Ospina et al., 2019; Mage et al., 2004). The geometric means of the biomarker concentrations were compared to the NHANES 12–19-year-old participants, as it is the most similar age group to the participants in our study.
Table 1.
Participant Characteristics across tertiles of the overall pesticide summary score for boys.
| Overall Pesticide Summary Score |
|||||
|---|---|---|---|---|---|
| Variable | Overall | Tertile 1 | Tertile 2 | Tertile 3 | P-trend |
| (−2.00, −1.00)a | (−1.18, −0.57)a | (−0.78, 1.30)a | |||
|
| |||||
| N, % | 258 | 86 | 86 | 86 | |
| Age | 14.40 (1.74) | 14.36 (1.80) | 14.30 (1.67) | 14.53 (1.75) | 0.52 |
| Race | 0.009 | ||||
| Indigenous | 58 (22.48%) | 29 (33.7%) | 15 (17.4%) | 14 (16.3%) | |
| Mestizo or White | 200 (77.52%) | 57 (66.3%) | 71 (82.6%) | 72 (83.7%) | |
| Awakening time, hh:mm | 6:43 (0:53) | 6:47 (0:50) | 06:46 (00:58) | 06:37 (00:52) | 0.22 |
| Saliva collection time, hh:mm | 7:16 (1:13) | 07:46 (1:15) | 07:15 (1:19) | 07:09 (1:04) | 0.18 |
| Awakening– Saliva collection Time, hh:mm | 0:33 (0:59) | 00:37 (1:06) | 00:29 (00:53) | 00:32 (00:59) | 0.60 |
| BMI-for-age z-score, SD | 0.21 (0.89) | 0.24 (0.78) | 0.20 (0.98) | 0.20 (0.91) | 0.78 |
| Height-for-age z-score, SD | −1.50 (0.96) | −1.56 (0.90) | −1.51 (1.09) | 01.43 (0.90) | 0.36 |
| Tanner staging | 2.92 (1.12) | 2.84 (1.13) | 2.98 (1.17) | 2.93 (1.06) | 0.58 |
| April AChE, U/mL | 3.88 (0.50) | 3.82 (0.58) | 3.86 (0.46) | 3.96 (0.46) | 0.20 |
| July-October AChE, U/mL | 3.87 (0.58) | 3.83 (0.58) | 3.91 (0.50) | 3.86 (0.64) | 0.78 |
| Change of AChE/Hemoglobin concentration from April to July-October exam | −0.59 (2.85) | −0.40 (3.11) | −0.32 (2.08) | −1.14 (3.32) | 0.23 |
| Average Parental Education, years | 8.27 (3.53) | 7.65 (3.19) | 8.81 (4.15) | 8.35 (3.09) | 0.19 |
| Temperature during April AChE measurement, Celsius | 21.90 (2.54) | 21.17 (2.51) | 21.98 (2.49) | 22.64 (2.47) | 0.005 |
| Temperature during July-October AChE measurement, Celsius | 20.67 (3.41) | 20.08 (3.47) | 21.28 (3.37) | 20.65 (3.34) | 0.28 |
| Household income, USD/month | 500 (370 – 720) | 483 (365 – 700) | 500 (366 – 720) | 583 (375 – 750) | 0.1 |
| Creatinine, mg/dL | 88.15 (57.3 – 132.0) | 53.6 (39.0– 74.5) | 92.0 (70.5 – 118.0) | 136 (96.1 – 182.0) | <0.0001 |
| Testosterone, pg/mL | 58.38 (31.71 – 99.3) | 53.7 (26.3 – 91.1) | 55.1 (29.6 – 94.5) | 68.8 (42.2 – 109.2) | 0.05 |
| 17β-Estradiol, pg/mL | 0.43 (0.30, 0.59) | 0.42 (0.28 – 0.56) | 0.45 (0.30 – 0.64) | 0.44 (0.33 – 0.59) | 0.44 |
| DHEA, pg/mL | 42.4 (21.7 – 78.0) | 35.6 (16.1 – 64.7) | 51.3 (22.0 – 83.9) | 43.8 (27.8 – 83.0) | 0.05 |
| Cortisol, μg/dL | 0.21 (0.13, 0.30) | 0.17 (0.12 – 0.29) | 0.22 (0.15 – 0.30) | 0.21 (0.14 – 0.32) | 0.14 |
| Organophosphate Summary Score | 1.83 (1.44 – 2.22) | 1.33 (1.09 – 1.49) | 1.84 (1.71 – 2.07) | 2.42 (2.12 – 2.72) | <0.0001 |
| Neonicotinoid Summary Score | 0.53 (0.53 – 0.99) | 0.53 (0.53 – 0.59) | 0.57 (0.53 – 0.98) | 0.92 (0.53 – 1.82) | <0.0001 |
Value is a range.
Values are count (percent), mean (SD), or median (IQR).
hh:mm = hours and minutes, BMI = Body Mass Index, AChE=acetylcholinesterase, USD = United States Dollar, SD = Standard Deviation, IQR = Interquartile Range
General linear models (Proc GLM, SAS 9.4, SAS Institute Inc, SAS Institute Inc., Cary, NC, USA) were used to analyze the relationship of pesticide metabolites with concentrations of testosterone, 17β-estradiol (estradiol), DHEA, and cortisol. Potential confounders were identified a priori, and were included if they caused a 10% change in estimate of the main outcome β coefficient. The final model included age, race, saliva time minus awakening time, BMI-for-age z-score, and tanner score. All analyses were stratified by sex, due to biological differences in hormone levels (Oertelt-Prigione and Regitz-Zagrosek, 2013) and other physiological processes across sexes. Based on the literature, it is possible that pesticide exposure impacts hormone concentrations, and can potentially impact pubertal development. Thus, sensitivity analysis was conducted which removed tanner score from the covariates to assess its impact on the models. Given both the outcome and exposure were ln-transformed, model estimates were back-transformed to assess whether a 50% change in metabolite or summary score concentration was associated with a percent change in the hormone concentration. This was done by calculating 1.5 to the power of the coefficient, subtracting 1, and multiplying by 100. Curvilinearity was assessed by testing squared terms in the adjusted linear models and were reported if they reached a significance level of p < 0.05. The 50% difference in the independent variable depends on participants’ level of exposure. However, using the median value for each metabolite a 50% increase would equate to a difference of 0.215 μ/L for PNP, 1.35 μ/L for TCPy, 0.15 μ/L for MDA, 0.15 μ/L for AND, 0.05 μ/L for OHIM, and 0.19 μ/L for 3-PBA. For the pesticide metabolites that had concentrations below the LOD, we ran the same general linear models but using dichotomized variables for those who did or did not have detectable levels of the pesticide metabolite.
The linear associations of metabolite summary scores with each hormone were depicted by plotting the adjusted least squares means of hormone concentration for 225 ranks of pesticide summary score or metabolite concentrations. Due to low detectability for some of the neonicotinoids, less than 225 ranks were outputted. Then, we used locally weighted polynomial regression (LOESS) to graph the adjusted relationships between the ranks of natural log pesticide summary score concentrations and natural log transformed hormone concentrations by sex. To provide visual clarity of the relationships, the plotted points were removed, and only the LOESS curves are visible for each summary score and hormone relationship. If curvilinearity was identified and a threshold effect was suspected based on visualization of the LOESS figures, then the metabolite-hormone association was stratified according to the approximate metabolite concentration of the suspected threshold.
We used Bayesian Kernel Machine Regression (BKMR), to evaluate nonlinear associations between the metabolites and hormone concentrations, and interactions between all pesticides assessed: TCPy, PNP, MDA, AND, OHIM, and 3-PBA (Bobb et al., 2018a; Bobb, 2017a). Imputation using a constant was conducted for MDA, AND, OHIM, and 3-PBA. We ran four BKMR models using testosterone, estradiol, DHEA, and cortisol as the outcome, stratified by sex. Given that missing values are not allowed for any of the exposures, our BKMR sample size was limited to participants who did not have interfering substances for any of the six metabolites, as these concentrations were unable to be imputed. For boys, our samples sizes were 213 for testosterone, 210 for estradiol, 198 for DHEA, and 216 for cortisol. For girls, our sample sizes were 224 for testosterone, DHEA, and cortisol models. BKMR models were run for 10,000 iterations and the Markov chain Monte Carlo algorithm was implemented. Model convergences were visually inspected using trace plots. Predictor response functions for each metabolite-hormone association were visualized by fixing all other exposures to the median. Posterior inclusion probabilities (PIPs) were estimated to identify the importance of each metabolite within the mixture with each hormone (Bobb et al., 2015a). A PIP is considered meaningful if it has a value of 0.5 or above (Barbieri and Berger, 2004). Interactions between individual pesticides were assessed using bivariate pesticide response associations with each hormone for a single pesticide metabolite (e.g. PNP) when a second metabolite is fixed to the 10th, 50th, and 90th percentile for each hormone (e.g. 3-PBA). More details regarding BKMR methods have been previously published (Bobb, 2017b; Bobb et al., 2015b, 2018b). These analyses were done using the BKMR R package (Bobb et al., 2018b), in the R statistical software (v4.1.2; R Core team 20211).
For our secondary analysis, we used general linear models to assess whether a 10% change in hemoglobin adjusted AChE activity (AChE/hemoglobin) from the April to July–October was associated with ln-transformed hormone concentrations for participants who had completed both assessments (n = 295), stratified by gender, and adjusted for confounders. Confounders were identified a priori, and included age, race, average parental education, tanner staging, BMI-for-age z-score, hemoglobin adjusted AChE concentration from the April exam, temperature during AChE measurement at the April and July–October exams, examination date, and saliva time minus awakening time. As our outcome was ln-transformed, we exponentiated the coefficient, subtracted one from this number, multiplied by 100, to determine the percentage change in hormone concentration for every 10% increase in hemoglobin-adjusted AChE activity from the April to the July–October assessment. Curvilinearity was assessed by introducing a squared term (AChE/hemoglobin* AChE/hemoglobin) and explored if the term was significant (p < 0.05). Effect modification by age was assessed by introducing an interaction term between AChE/hemoglobin and age. If it was found to be statistically significant (p < 0.05), we stratified the association by age median splits. The lower median of age are participants 14.38 years or younger, while the upper median of age are all participants aged 14.39 years and older.
3. Results
Participant characteristics for boys and girls are presented in Tables 1 and 2, respectively. The present study included observations from 522 participants (49% male) who did not have missing information for any of the covariates of interest and at least 1 urinary pesticide metabolite measured. The mean age at the time of assessment of all included participants was 14.2 years (SD = 1.77) and the cohort consisted of mostly mestizo or white individuals (78%). For boys, compared to the lowest tertile, those in the highest tertile of overall pesticide summary score were more likely to be Mestizo or White (Highest: n = 72 [83.7%] versus [vs] Lowest: n = 57 [66.3%]) and have higher temperatures during the April AChE measurement (Highest: 22.64 [2.47] vs Lowest: 21.17 [2.56]). Testosterone (Highest: 68.8 [42.2–109.2] vs Lowest: 53.7 [26.3–91.1]) and DHEA (Highest: 43.8 [27.8–83.0] vs Lowest: 35.6 [16.1–64.7]) concentration trended to being higher in the highest tertile of the overall pesticide summary score. For girls, compared to those in the lowest tertile of the overall pesticide summary score, those in the highest tertile were more likely to be Mestizo or White (Highest: 76 [86.4%] vs Lowest: 62 [70.5%]), have a higher tanner score (Highest: 3.08 [0.63] vs Lowest: 2.68 [0.79]), have higher monthly incomes (Highest: 600 [393–750] vs Lowest: 500 [366–700]), have higher creatinine concentrations (Highest: 125 [88.3–170.0] vs Lowest: 63.7 [43.3–92.3]), and have higher cortisol concentration (Highest: 0.21 [0.16–0.35] vs Lowest: 0.19 [0.12–0.26]).
Table 2.
Participant Characteristics across tertiles of the overall pesticide summary score for girls.
| Overall Pesticide Summary Score |
|||||
|---|---|---|---|---|---|
| Variable | Overall | Tertile 1 | Tertile 2 | Tertile 3 | P-trend |
| (−2.26, −0.98)a | (−1.12, −0.39)a | (−0.66, 1.13)a | |||
|
| |||||
| N, % | 264 | 88 | 88 | 88 | |
| Age | 14.52 (1.80) | 14.36 (1.80) | 14.30 (1.67) | 14.53 (1.75) | 0.52 |
| Race | 0.04 | ||||
| Indigenous | 57 (21.59%) | 26 (29.6%) | 19 (21.6%) | 12 (13.6%) | |
| Mestizo or White | 207 (78.41%) | 62 (70.5%) | 69 (78.4%) | 76 (86.4%) | |
| Awakening time, hh:mm | 6:41 (0:50) | 6:39 (0:51) | 6:40 (0:51) | 6:44 (50) | 0.47 |
| Saliva collection time, hh:mm | 7:11 (1:13) | 7:07 (1:14) | 7:16 (1:17) | 7:10 (1:07) | 0.80 |
| Awakening– Saliva collection Time, hh:mm | 0:29 (0:59) | 0:28 (58) | 0:35 (1:05) | 0:25 (0:54) | 0.76 |
| BMI-for-age z-score, SD | 0.53 (0.79) | 0.45 (0.72) | 0.58 (0.89) | 0.56 (0.75) | 0.32 |
| Height-for-age z-score, SD | −1.48 (−.90) | −1.46 (0.77) | −1.53 (1.02) | −1.46 (0.90) | 0.96 |
| Tanner staging | 2.85 (0.75) | 2.68 (0.79) | 2.78 (0.77) | 3.08 (0.63) | 0.0004 |
| April AChE, U/mL | 3.73 (0.53) | 3.76 (0.56) | 3.68 (0.51) | 2.75 (0.53) | 0.96 |
| July-October AChE, U/mL | 3.55 (0.47) | 0.17 (0.93) | 0.22 (0.64) | 0.35 (0.94) | 0.28 |
| Change of AChE/Hemoglobin concentration from April to July-October exam | −0.80 (2.37) | −0.61 (2.50) | −0.71 (2.07) | −1.08 (2.52) | 0.32 |
| Average Parental Education, years | 7.92 (3.41) | 7.79 (2.95) | 7.61 (3.80) | 8.34 (3.43) | 0.29 |
| Temperature during April AChE measurement, Celsius | 21.97 (2.58) | 21.86 (2.61) | 21.75 (2.41) | 22.26 (2.74) | 0.44 |
| Temperature during July-October AChE measurement, Celsius | 20.81 (2.98) | 20.67 (2.51) | 20.69 (2.71) | 21.05 (3.63) | 0.40 |
| Household income, USD/month | 500 (370 – 720) | 500 (366 – 700) | 466 (359 – 700) | 600 (393 – 750) | 0.009 |
| Creatinine, mg/dL | 88.4 (62.80 – 125.00) | 63.7 (43.3 – 92.3) | 87.3 (69.7 – 109.5) | 125 (88.3 – 170.0) | <0.0001 |
| Testosterone, pg/mL | 32.1 (23.1 – 42.9) | 26.8 (18.7 – 37.7) | 36.5 (24.5 – 46.91) | 32.1 (26.1 – 42.9) | 0.002 |
| DHEA, pg/mL | 81.4 (45.6 – 132.7) | 71.4 (39.8 – 111.4) | 82.9 (46.6 – 126.1) | 86.6 (53.4 – 163.9) | 0.06 |
| Cortisol, μg/dL | 0.20 (0.14 –0.30) | 0.19 (0.12 – 0.26) | 0.21 (0.15 – 0.30) | 0.21 (0.16 – 0.35) | 0.04 |
| Organophosphate Summary Score | 1.78 (1.48 – 2.21) | 1.38 (1.16 – 1.59) | 1.83 (1.68 – 2.02) | 2.35 (2.00 – 2.62) | <0.0001 |
| Neonicotinoid Summary Score | 0.74 (0.53 – 1.31) | 0.53 (0.53 – 0.67) | 0.74 (0.53 – 1.06) | 1.44 (0.84 – 2.01) | <0.0001 |
Value is a range.
Values are count (percent), mean (SD), or median (IQR).
hh:mm = hours and minutes, BMI = Body Mass Index, AChE=acetylcholinesterase, USD = United States Dollar, SD = Standard Deviation, IQR = Interquartile Range
The geometric means for all included metabolites can be found in Table 3. Mean concentrations for all metabolites were not different by sex (Table 3). Compared to NHANES, TCPy concentrations were three times higher in the ESPINA cohort (Table 3). Concentrations in ESPINA were higher for MDA, OHIM and AND, but were lower for PNP and 3-PBA. We did not observe associations between the overall pesticide summary score with any adrenal or gonadal hormones for boys nor girls (Table S3).
Table 3.
Geometric mean concentrations (μg per gram of creatinine) of organophosphate, neonicotinoid, and pyrethroid metabolites of the ESPINA and NHANES cohorts.
| Geometric Mean (95% CI) μg per g of creatinine |
NHANES |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Urinary Metabolite | Parent Chemical | Chemical Class | Overall | Boys | Girls | Detectablea | Year | Geometric Mean (95% CI) μg per g of creatinine |
|
| |||||||||
| PNP | para-Nitrophenol | Parathion, Methyl parathion | Organophosphate | 0.59 (0.57 – 0.62) | 0.60 (0.57, 0.64) | 0.58 (0.55, 0.62) | 100% | 2013–2014 | 0.66 (0.59, 0.74) |
| TCPy | 3,5,6-Trichloro-2-pyridinol | Chlorpyrifos, Chlorpyrifos-methyl | Organophosphate | 3.06 (2.88 – 3.25) | 3.02 (2.78, 3.29) | 3.09 (2.83, 3.37) | 100% | 2013–2014 | 1.08 (0.96, 1.21) |
| MDA | Malathion dicarboxylic acid | Malathion | Organophosphate | 0.58 (0.54 – 0.61) | 0.57 (0.52, 0.63) | 0.58 (0.53, 0.63) | 37.70% | 2009 – 2010 | 0.45 (0.41, 0.49) |
| IMPy | 2-isopropyl-4-methyl-6-hydroxypyrimidine | Diazinon | Organophosphate | - | - | - | 23.81% | ||
| OHIM | 5-Hydroxy imidacloprid | Imidacloprid | Neonicotinoid | 0.48 (0.44 – 0.52) | 0.46 (0.41, 0.51) | 0.50 (0.45, 0.55) | 28.80% | 2015–2016 | 0.36 (0.33, 0.39) |
| AND | Acetamiprid-N-desmethyl | Acetamiprid | Neonicotinoid | 0.31 (0.28 – 0.34) | 0.28 (0.24, 0.32) | 0.34 (0.29, 0.39) | 37.80% | 2015–2016 | 0.23 (0.21, 0.26) |
| ACET | Acetamiprid | Acetamiprid | Neonicotinoid | - | - | - | 1.14% | ||
| CLOT | Clothianidin | Clothianidin, Thiamethoxam | Neonicotinoid | - | - | - | 2.84% | ||
| IMID | Imidacloprid | Imidacloprid | Neonicotinoid | - | - | - | 7.72% | ||
| THIA | Thiacloprid | Thiacloprid | Neonicotinoid | - | - | - | 0.00% | ||
| 3-PBA | 3-Phenoxybenzoic acid | Cyhalothrin, Cypermethrin, Deltamethrin, Fenpropathrin, Permethrin, Tralomethrin | Pyrethroid | 0.45 (0.42 – 0.49) | 0.43 (0.39, 0.48) | 0.47 (0.42, 0.53) | 88.90% | 2013–2014 | 0.64 (0.53, 0.76) |
| TCC | Trans-3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid | Permethrin; Cypermethrin; Cyfluthrin | Pyrethroid | - | - | - | 16.28% | ||
| 4F-3PBA | 4-fluoro-3-phenoxybenzoic acid | Cyfluthrin, Flumethrin | Pyrethroid | - | - | - | 0.00% | ||
μg=microgram; g=gram
Detectable percentages reflect the overall cohort, and does not include observations that had interfering substances.
Geometric mean units are measured in μg per g of creatinine, and the values were calculated after imputation using a constant (LOD/√2) was conducted.
Mean concentrations of the metabolites were not significantly different by gender.
3.1. Organophosphates and hormones
Testosterone
Associations between organophosphate metabolite concentrations and hormone concentrations can be found in Fig. 1. The associations presented correspond to a percent change in hormone concentration per 50% increase in the exposure construct (β). A 50% increase in organophosphate summary score was associated with a 14.11% (2.12%, 27.52%) increase in testosterone concentration for boys (Fig. 1a); the association was weaker in girls (β = 7.88% [−2.61%, 19.50%]) (Fig. 1b). For the individual organophosphate metabolites, MDA had a positive association with testosterone, that was stronger in boys (β = 5.88% [1.21%, 10.78%], Fig. 1a), than girls (β = 4.10% [−0.02% 8.39%], Fig. 1b). Participants with detectable levels of MDA also had higher testosterone concentrations compared to participants whose concentrations were below the level of detection (β = 0.28 [−1.60, −0.04]) (Table S1). There was negatively-trending curvilinear association of PNP with testosterone among boys only (β2boys = −0.17 (−0.33, −0.003), pquadratic = 0.04, Fig. 1a). To assess the presence of a potential threshold, this PNP and testosterone association was stratified by a median split of PNP (PNP = 0.52 μg/g). The association was positive in the lower median of PNP concentration (β = 12.10% [−5.16%, 32.51%], p = 0.18), and negative in the upper median of PNP concentration (β = −7.03% [−17.0%, 4.13%], p = 0.21); however, although the curvature was significant, neither of the subgroup slopes was statistically significant.
Fig. 1.

Locally weighted polynomial regression (LOESS) of organophosphate metabolite concentrations with ln(hormone) concentration by sex.
Footnote for Fig. 1:
Values shown are percent increase in hormone concentration per 50% increase in pesticide metabolite concentration (β, 95% CI).
Adjusted for creatinine, age, race, saliva time minus awakening time, BMI-for-age z-score, and tanner score.
Each line represents the locally weighted fitted value (LOESS) of the adjusted least squares means of ln(hormone) concentration for 225 ranks of ln(pesticide biomarker) concentrations. Solid and thick lines represent either a linear or quadratic term with a p < 0.05, while dashed lines represent a p > 0.05.
**p < 0.05
Curvilinear terms:
a β = −0.17 (−0.40, 0.06), p = 0.15, β2 = −0.17 (−0.33, −0.003), p = 0.04
b β = −0.77 (−1.20, −0.34), p < 0.001, β2 = −0.49 (−0.80, −0.19), p = 0.001
c β = −0.69 (−1.51, 0.13), p = 0.09, β2 = 0.74 (0.08, 1.39), p = 0.03
DHEA = Dehydroepiandrosterone, OP = organophosphate summary score, TCPy = 3,5,6-Trichloro-2-pyridinol, PNP = para-Nitrophenol, MDA = malathion dicarboxylic acid.
DHEA
The organophosphate summary score, TCPy and MDA were not associated with DHEA. However, there was a negatively trending (upside-down J shape) significant curvilinear association between PNP and DHEA in boys which can be visualized in Fig. 1c. This association was then stratified by a median split of PNP (PNP = 0.52 μg/g). There was no association in the lower median of PNP (β = 3.96% [−23.9%, 42.1%], p = 0.81) with DHEA, but the association was strongly negative above the median of PNP (β = −27.0% [−41.0%, −9.75%], p = 0.004).
Cortisol
No significant linear associations with cortisol were seen for the organophosphate summary score, TCPy, or PNP. However, a significant curvature was identified for the association between organophosphate summary score and cortisol in girls (pquadratic = 0.03, Fig. 1f). MDA had borderline non-significant positive association with cortisol concentration in boys (β = 6.06% [−0.23%, 12.75%], p = 0.06, Fig. 1e).
Estradiol
There were no associations between any of the organophosphate biomarkers and estradiol concentration in boys (Fig. 1g).
3.2. Neonicotinoids and hormones
Testosterone
No neonicotinoid associations with testosterone were observed, except for a negatively-trending significant curvilinear association of the neonicotinoid summary score among girls (pquadratic = 0.006, Fig. 1b). In girls, however, participants with detectable levels of OHIM had higher testosterone concentrations (β = 0.14 [0.01, 0.28]) compared to those without detectable levels (Table S1).
DHEA and Cortisol
There were no associations observed between the neonicotinoid metabolites and DHEA and cortisol concentrations (Fig. 1c–f).
Estradiol
The neonicotinoid summary score was only associated with estradiol concentration among boys, as a 50% increase in the score led to a 5.60% (0.14%, 11.36%) increase in estradiol concentration (Fig. 2g). The biggest contributor to the association seen between the neonicotinoid summary score and estradiol was AND (β = 3.90% [1.28%, 6.58%], Fig. 2g). Compared to those who did not have detectable levels of AND, those with detectable levels of AND had higher estradiol concentration (β = 0.22 [0.05, 0.37],Table S1).
Fig. 2.
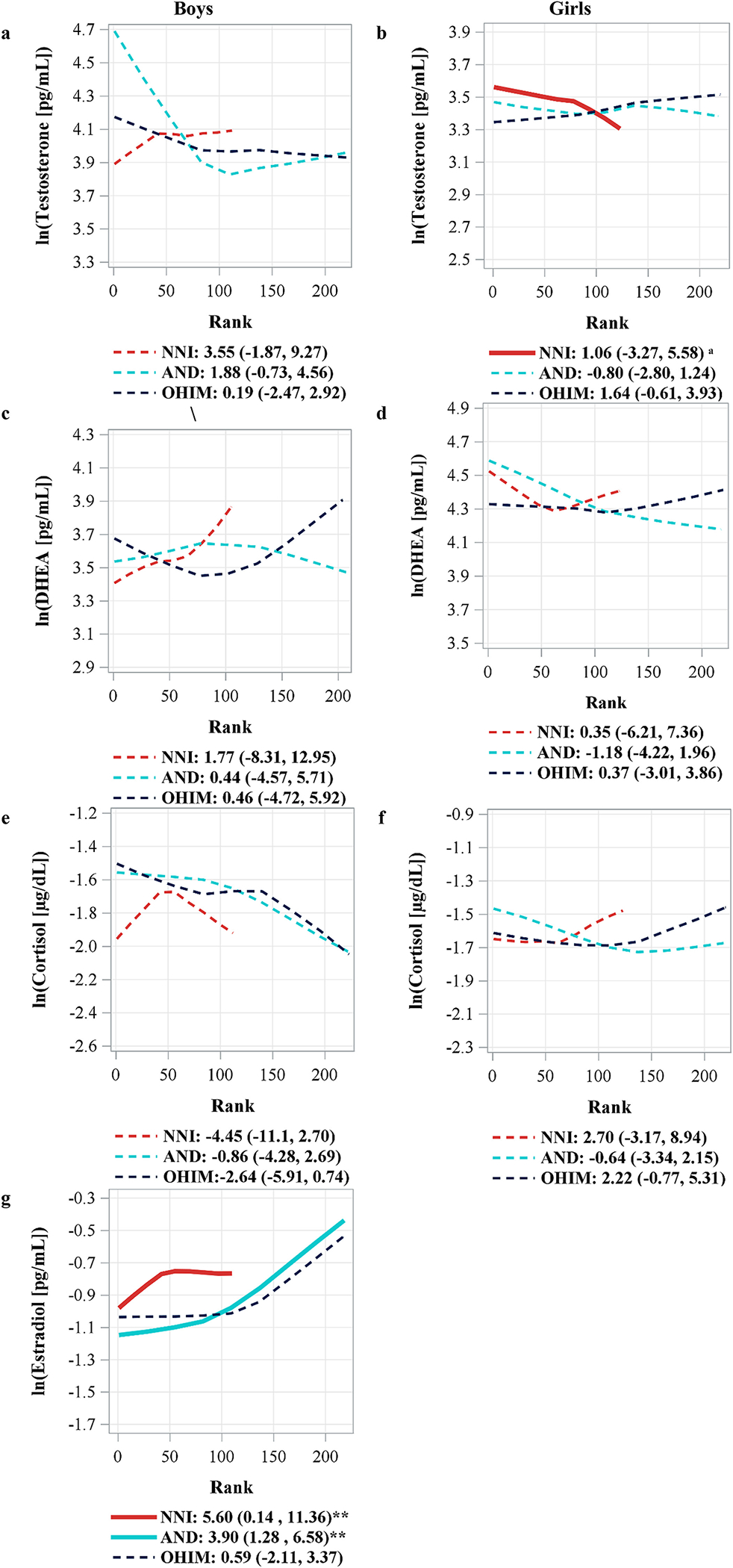
Locally weighted polynomial regression (LOESS) of neonicotinoid metabolite concentrations with ln(hormone) concentration by sex.
Footnote for Fig. 2
Values shown are percent increase in hormone concentration per 50% increase in pesticide metabolite concentration (β, 95% CI).
Adjusted for creatinine, age, race, saliva time minus awakening time, BMI-for-age z-score, and tanner score.
Each line represents the locally weighted fitted value (LOESS) of the adjusted least squares means of ln(hormone) concentration for 225 ranks of ln(pesticide biomarker) concentrations. Solid and thick lines represent either a linear or quadratic term with a p < 0.05, while dashed lines represent a p > 0.05.
**p < 0.05
Curvilinear terms:
a β = 0.06 (−0.05, 0.17), p = 0.30, β2 = −0.21 (−0.37, −0.06), p = 0.006
NNI = neonicotinoid summary score, AND = Acetamiprid-N-desmethyl, OHIM = 5-Hydroxy imidacloprid, DHEA = Dehydroepiandrosterone.
3.3. Pyrethroids and hormones
No linear or curvilinear associations were observed between 3-PBA and the four hormones assessed (Fig. 3).
Fig. 3.
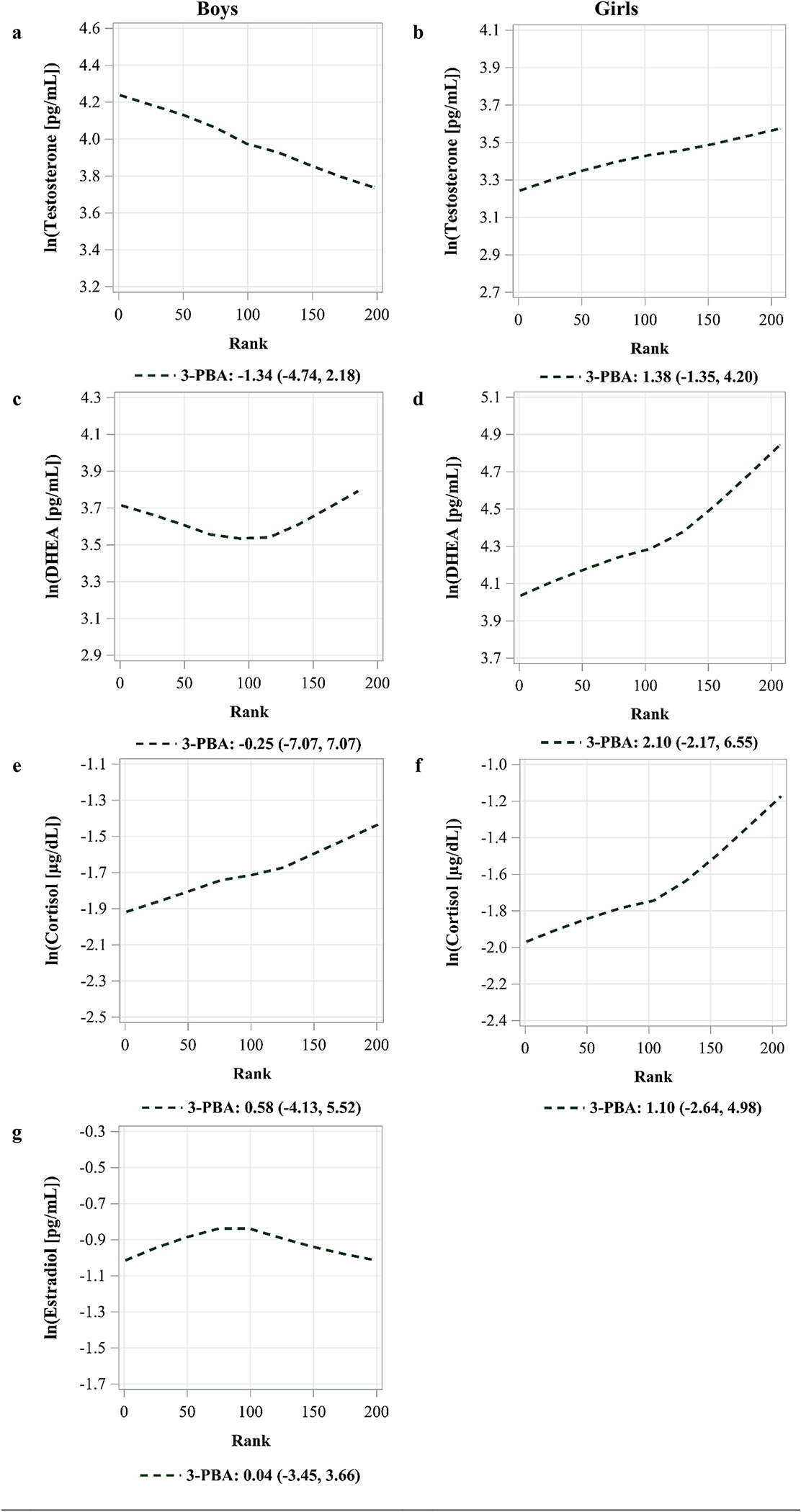
Locally weighted polynomial regression (LOESS) of pyrethroid 3-PBA metabolite concentration with ln(hormone) concentration by sex.
Footnote for Fig. 3
Values shown are percent increase in hormone concentration per 50% increase in pesticide metabolite concentration (β, 95% CI).
Adjusted for creatinine, age, race, saliva time minus awakening time, BMI-for-age z-score, and tanner score.
Each line represents the locally weighted fitted value (LOESS) of the adjusted least squares of ln(hormone) concentration for 225 ranks of ln(biomarker) concentrations. Solid and thick lines represent either a linear or quadratic term with a p < 0.05, while dashed lines represent a p > 0.05.
**p < 0.05
3-PBA = 3-phenoxybenzoic acid, DHEA = dehydroepiandrosterone.
3.4. Sensitivity analysis
Linear regression models were run which removed tanner score as a covariate (Table S2). For associations that were previously found to be statistically significant, there was less than a 5% change in estimate when we removed the tanner score from the model. An example this is for the association between MDA and testosterone in boys where we only saw a 1% change in estimate comparing models with tanner score (5.88 [1.21, 10.78], Fig. 1a) versus the model without tanner score (5.92 [1.14, 10.93],Table S2). In girls, we saw that there was a positive association between MDA and testosterone that was statistically significant (4.30 [0.19, 8.58],Table S2), however the model with tanner score was also positive (4.10 [−0.02, 8.39], Fig. 1b).
3.5. Pesticide metabolite interactions
The adjusted joint associations of pesticide mixtures with hormone concentrations are depicted in Fig. S1 for boys and girls. There was no statistical evidence of joint effects of the organophosphate, pyrethroid, and neonicotinoid mixtures on any of the outcomes for boys nor girls. Yet, there were some trends which suggest that associations might strengthen in higher quantiles of pesticide exposure, although the confidence intervals are wide. For boys, concentrations above the 70th percentile had more positive estimates for testosterone (Fig. S1a) and estradiol (Fig. S1g). For DHEA concentration, the association became more negative with increasing quantiles of pesticide metabolite exposure (Fig. S1e), while the association with cortisol became more positive as the quantile of exposure increased (Fig. S1e). For girls, pesticide metabolite exposure above the 65th percentile had more positive associations with testosterone (Fig. S1b) and cortisol (Fig. S1f).
The posterior inclusion probabilities (PIPs) of individual metabolites from the BKMR models can be found inTable S4. Overall, the estimated PIPs were small (<0.50). For girls, PNP had the largest PIP value for the DHEA outcome (0.56). The second largest PIP was observed in girls, for OHIM for the cortisol outcome (0.52); all other metabolites had a PIP<0.16 for this model. Looking at univariate exposure-response plots (Fig. S2[boys] and S3 [girls]), we did not observe evidence of a nonlinear relationship between any of the metabolites and hormone concentrations.
Assessing the bivariate relationship between biomarkers (Figs. 4 and 5), we observed some interaction effects. For boys, higher concentrations of MDA strengthened the negative association of PNP with DHEA (Fig. 4), while with higher concentrations of PNP we observed more negative associations between MDA and DHEA (Fig. 4). Bivariate pesticide response associations for girls can be found in Fig. 5. For testosterone, MDA concentrations at the 90th quantile led to more positive associations for TCPy and PNP (Fig. 5a). Similar effects were seen in the 90th quantiles of TCPy and PNP on the association between MDA and testosterone (Fig. 5a). Stronger negative associations between TCPy and testosterone were observed at higher concentrations of PNP (Fig. 5a). For DHEA (Fig. 5b), at higher concentrations of MDA we observed more positive associations for PNP, TCPy, AND, and 3-PBA. At higher concentrations of PNP there were more negative associations for TCPy, and more positive associations for MDA. Lastly, greater concentrations of TCPy led to more negative associations for PNP, and more positive associations for MDA. No other bivariate interactions were observed for boys (Fig. S4) and girls (Fig. S5).
Fig. 4.

Bivariate pesticide response associations with DHEA for a single pesticide metabolite, when a second metabolite is fixed to the 10th, 50th, and 90th percentile for boys. Models are adjusted for age, race, saliva time minus awakening time, BMI-for-age z-score, and tanner score.
Footnote for Fig. 4: DHEA = dehydroepiandrosterone, Est = estimate, TCPY = 3,5,6-trichloro-2-pyridinol, PNP = para-nitrophenol, MDA = malathion dicarboxylic acid, AND = acetamiprid-N-desmethyl, OHIM = 5-hydroxy imidacloprid, 3-PBA = 3-phenoxybenzoic acid.
Fig. 5.

Bivariate pesticide response associations with testosterone and DHEA for a single pesticide metabolite, when a second metabolite is fixed to the 10th, 50th, and 90th percentile for girls. Models are adjusted for age, race, saliva time minus awakening time, BMI-for-age z-score, and tanner score.
Footnote for Fig. 5: DHEA = dehydroepiandrosterone, Est = estimate, TCPY = 3,5,6-trichloro-2-pyridinol, PNP = para-nitrophenol, MDA = malathion dicarboxylic acid, AND = acetamiprid-N-desmethyl, OHIM = 5-hydroxy imidacloprid, 3-PBA = 3-phenoxybenzoic acid.
3.6. Acetylcholinesterase and hormones
In boys, there were no associations between a 10% increase in hemoglobin adjusted AChE concentration with testosterone (β = 6.53% [−5.72%, 20.39%], p = 0.31), estradiol (β = 2.23% [−9.44%, 15.42%], p = 0.72), DHEA (β = −0.49% [−23.20%, 28.94%], p = 0.97), nor cortisol concentration (β = 0.09% [−14.07%, 16.59%], p = 0.99). This was also true in girls, as we did not observe associations between a 10% change in hemoglobin adjusted AChE concentration with testosterone (β = 11.23% [−2.64%, 27.08%], p = 0.12), DHEA (β = 13.05% [−8.28%, 39.34%], p = 0.25), nor cortisol (β = 16.76% [−2.64%, 40.02%], p = 0.09). We did not observe any curvilinearity for any of the models. In boys for the cortisol outcome, we identified a significant interaction between age and hemoglobin adjusted AChE concentration (β = −0.10 95%CI [−0.18, −0.01], p = 0.03). There was qualitative effect modification by age, as a 10% increase in hemoglobin adjusted AChE concentration was associated with a 21.07% ([0.87%, 45.31%], p = 0.04) higher cortisol concentration in the lower median of age, but a 20.48% [−38.96%, 3.59%], p = 0.09 lower cortisol concentration in the upper median of age.
4. Discussion
This study identified that select urinary metabolites of organophosphate and neonicotinoid insecticides were associated with hormone concentrations in adolescent boys but not girls. The organophosphate summary score and MDA concentration were positively associated with testosterone concentration in boys, whereas PNP had a negative association. For DHEA, there was a curvilinear relationship with PNP resembling a threshold, in which there was a negative association among boys above the 50th percentile of PNP concentration. Estradiol had positive associations with both the neonicotinoid summary score and the neonicotinoid metabolite AND in boys, whereas cortisol concentrations were not associated with any pesticide metabolite, other than a flat U-shaped association with OPs among girls. Our findings suggest that background exposures to pesticides in agricultural settings could alter endocrine levels in adolescents, which is concerning given the pervasive use of pesticides in agriculture worldwide. To our knowledge, this is the first study to characterize the relationship of neonicotinoid urinary metabolites with estradiol concentrations and AChE activity with hormone concentration in humans, and amongst the first to characterize the relationship of organophosphate, neonicotinoid and pyrethroid exposure with endocrine concentrations. As developmental plasticity occurs in childhood and adolescence, endocrine disruption may lead to lifelong imbalances and subsequent health consequences (Gore et al., 2006; Barker, 2003; Crews and McLachlan, 2006). More specifically, the hormones measured in our study, including testosterone, estradiol, DHEA, and cortisol are essential for the development of sexual characteristics, behaviors, inflammation modulation, and other physiological processes (Khonsary, 2017), and alterations in their concentrations can affect many organs and systems within an individual.
It is known that there are gonadal and adrenal steroid differences that help to account for differences in male and female characteristics (Vigil et al., 2016). Thus, it was expected that our associations would need to be stratified by sex. The results consistently showed that associations between pesticide metabolite concentrations and hormone concentrations were stronger in boys than girls. This indicates that the endocrine systems of boys potentially are more sensitive to the effects of pesticide exposure compared to girls, considering that the pesticide metabolite concentrations among boys and girls were similar. Animal and epidemiologic studies looking at the effects of pesticide exposure on hormonal changes have commonly only included one sex; therefore, we were unable to identify studies that assessed sex effect modification of pesticide-endocrine associations. Outside of endocrine effects, there has been evidence of differential effects of pesticide exposure by sex for cognitive abilities (Benavides-Piracón et al., 2022), depression symptoms (Suarez-Lopez et al., 2019), and child development (Boucher et al., 2013). Our results highlight the importance of identifying the role of sex on pesticide-endocrine associations.
4.1. Organophosphates
We observed a positive association between organophosphate summary score and MDA concentration with testosterone concentrations in boys but not girls. Our findings are concordant with two studies where rural farmers who had occupational exposure to organophosphate pesticides and organophosphate manufacturing plant workers had increased testosterone concentrations compared to men who did not have organophosphate pesticide exposure (Ghafouri-Khosrowshahi et al., 2019a; Waheed et al., 2017). Likewise, in a study of 88 Pakistani cotton farmers, FSH and testosterone levels were higher in cotton pickers during high pesticide spray seasons (Khan et al., 2013), but did not measure malathion specifically. However, research on juvenile and adult rat models generally have observed decreased plasma or testosterone concentrations after malathion exposure, a parent pesticide of MDA (Salahshoor et al., 2020; Geng et al., 2015; Erthal et al., 2020; Slimen et al., 2014; Sarabia et al., 2009; Krause, 1977; Krause et al., 1976; Choudhary et al., 2008). Effect modification by sex has been observed in fish models, as malathion exposure at 2 and 20 μg/L in Gobiocypris rarus minnows decreased testosterone levels in males, but inhibited 17B-estradiol concentrations and increased testosterone levels in females (Zhang et al., 2016a). In an epidemiologic analysis of Thai farmers, malathion exposure measured by urinary metabolites without imputation, was not linked to changes in total nor free testosterone, but had a small sample size which limited the power of their analysis (n = 133) (Panuwet et al., 2018).
There was an indication of the presence of synergistic interactions between MDA, TCPy, and PNP on their associations with testosterone and DHEA in girls. Higher concentrations of MDA led to more positive associations of TCPy and PNP with testosterone, while higher concentrations of PNP led to more negative associations of TCPy with testosterone. To our knowledge, there are no studies that have assessed the joint effects of PNP, MDA, and TCPy on testosterone concentration. Synergistic interactions could be plausible considering that organophosphates are potent inhibitors of cytochrome P450 (CYP) (Usmani et al., 2003a; Abass et al., 2009a), which includes CYP3A4 (Ghafouri-Khosrowshahi et al., 2019b; Abass et al., 2009b; Usmani et al., 2003b). Additionally, phosphorothioate organophosphates, such as parathion and diazinon, are metabolized by CYP enzymes through dearylation resulting in an inactive metabolite or through desulfuration resulting in an oxon metabolite with potent cholinesterase inhibitor properties (Ellison et al., 2012). Mixtures of organophosphate pesticides could plausibly potentiate this metabolite-CYP-testosterone loop.
We observed inverse associations between PNP with both testosterone and DHEA concentrations. A limited number of animal studies have evaluated these associations, with conflicting findings. Administration of parathion, the parent compound of PNP, led to a decrease in serum testosterone concentration in roosters (Ren et al., 2021; Ahmed et al., 2015), or led to higher testosterone concentrations in rats (Li et al., 2009a, 2009b; Zhang et al., 2016b). Our findings align with another analysis which assessed the associations of pesticide exposure with hormones in the NHANES III study, in which PNP exposure was associated with decreased total testosterone concentrations in girls (n = 96, 6–11 years) and female adolescents (n = 129, 12–19 years) (Liang et al., 2022). Given that DHEA can be converted to testosterone or estradiol in certain tissues, it is possible that reduced testosterone levels associated with PNP are downstream effects of reduced DHEA levels (Peiris and Dhanushka, 2017; Alaa-Eldin et al., 2017b). Our results did not indicate that there was a relationship between TCPy, a metabolite of chlorpyrifos and chlorpyrifos-methyl, with the hormones measured. Exposure to chlorpyrifos has been associated with decreased LH and FSH levels, reduced weight of testis (epididymis), and decreased sperm count, motility, and viability (Alaa-Eldin et al., 2017a; Peiris and Dhanushka, 2017). TCPy concentration has been negatively associated with decreased estradiol concentration in male (Suárez et al., 2021) and female (Liang et al., 2022) adolescents, negatively associated with total testosterone in females (Liang et al., 2022), and positively associated with DHEAS in male adolescents (Liang et al., 2022).
Higher AChE concentration, suggestive of lower organophosphate or carbamate exposure, was not associated with any of the hormones. However, we observed qualitative effect modification by age for boys with cortisol, where a 10% increase in hemoglobin adjusted AChE had positive associations in the lower median of age, but negative associations in the upper median of age. To our knowledge, this is the first study that has characterized the relationship between AChE activity and cortisol concentration. Although the direct association between AChE activity and cortisol has not been previously studied, a study mimicked AChE activity depression in mice to look at its effects on cortisol concentration. Acute exposure of mice to the AChE-inhibitor called Physostigmine depressed AChE activity in the hippocampus and prefrontal cortex, which subsequently led to an increase in cortisol levels (Goad et al., 2004). Exposure to organophosphates and carbamates results in the diminished breakdown of acetylcholine (ACH), and its subsequent accumulation in neurologic pathways and neuromuscular junctions (Kwong, 2002). AChE inhibition can potentially influence adrenal hormone secretion by reducing the breakdown of the ACH neurotransmitters. The presence of ACH can stimulate the adrenal medulla to release catecholamines, like epinephrine and norepinephrine, which interact with the hypothalamic-pituitary-adrenal axis (HPA) and subsequently can affect the secretion of adrenal hormones like cortisol (Fink, 2000).
4.2. Neonicotinoids
This is among the first human studies to characterize a relationship between neonicotinoid metabolites and hormones in adolescents. The positive association we found between the neonicotinoid summary score and estradiol concentration has been observed in animal and in vitro studies. A co-culture of H29Hr human adrenocortical carcinoma cells with fetal characteristics and BeWo human choriocarcinoma cells with villous cytotrophoblast characteristics had increased estrogen production following 24-h exposure to three neonicotinoids: thiacloprid, thiamethoxam, and imidacloprid (parent chemical of OHIM and IMID) (Caron-Beaudoin et al., 2017). In rat models, repeated prenatal exposure with a no-observed-adverse-effect-level (NOAEL) dose of neonicotinoid acetamiprid (parent pesticide of AND and ACET) increased estrone and estradiol production (Kagawa and Nagao, 2018); this finding coincides with the positive association we identified between AND concentration with estradiol concentration in boys. The positive association we observed between AND and estradiol had been also observed in zebrafish, in whom nominal acetamiprid exposures ranging from 0.15 to 1500 μg/L induced increases in 17β-estradiol concentration, leading to the feminization of the males even at the lowest observed doses (Ma et al., 2022). The half maximal effective concentration (EC50) of acetamiprid in zebrafish embryo malformation at 120 hpf was reported to be 323 mg/L, thus these effects were seen above and below this threshold (Ma et al., 2019, 2022). Although we measured biomarkers of thiacloprid (metabolite: THIA), thiamethoxam (metabolite: CLOT), ACET, and IMID exposure, they had low detectability that limited our ability to estimate the associations of these neonicotinoids with estradiol concentration. However, given that they belong to the same classification of pesticides, we would anticipate similar mechanisms of action.
AND is a common biomarker for acetamiprid exposure. The null association we observed between AND concentration and testosterone was not concordant with the existing animal literature, in which most studies have identified an inverse relationship. In male adult Sprague Dawley rats, low (NOAEL dose) and high doses (13.7% of the median lethal dose [LD50]) acetamiprid exposure caused oxidative stress and mitochondrial damage to Leydig cells; high acetamiprid doses inhibited testosterone (Kong et al., 2017a). Testosterone levels also decreased in a dose dependent manner with increasing acetamiprid exposure, while levels of FSH, LH, GnRH and GSH either were unchanged or increased (Arican et al., 2020; Kong et al., 2017b).
Unlike the null associations we observed, imidacloprid exposure, the parent pesticide of OHIM and IMID, has been associated with altered gonadal hormone concentrations. In a study of farmworkers, imidacloprid exposure was positively associated with testosterone and DHEA levels (Suwannarin et al., 2021). Imidacloprid, however, was negatively associated with testosterone in the NHANES study (Mendy and Pinney, 2022). IMID had a low percent detectable, therefore was not included in this analysis. The geometric mean of OHIM concentration (0.48 μg/g [0.44 μg/g – 0.52 μg/g]) was lower in the ESPINA cohort compared to these farmworkers (imidacloprid geometric mean: 17.4 μg/g), which could explain the null association we observed of imidacloprid metabolite concentration with testosterone and DHEA concentrations. The negative relationship between OHIM and cortisol observed in males has been observed in animal models. Daily oral exposure to 1/10th of the LD50 of imidacloprid in male rats over 28 days increased levels of adrenocorticotropic hormone (ACTH), a signaling hormone which stimulates adrenal cortisol production (Annabi et al., 2015). In fish, sublethal exposure (1/10th of the median lethal concentration [LC50]) to imidacloprid also led to decreased cortisol (Pandya et al., 2018). Yet, the positive association between OHIM and cortisol concentration observed in females has not been characterized in animal nor epidemiological studies.
4.3. Pyrethroids
We observed no associations between 3-PBA and any of the measured hormones. These findings are concordant with two studies. In a study of male university students in Japan who had comparable (minimally higher) 3-PBA concentrations than our study, they had 3-PBA concentrations that were not associated with any serum hormone levels (Yoshinaga et al., 2014). Additionally, in a study of 161 adult men, whose median concentration was lower than those reported in our study, no associations of 3-PBA urinary concentrations with estradiol nor testosterone concentration were observed in adjusted linear regression models (Meeker et al., 2009a). Both studies contrasted with the other existing human study which reported inverse associations of 3-PBA urinary concentrations with estradiol among Chinese (n = 212) adult men (Han et al., 2008). The latter study reported higher 3-PBA concentrations as those in our cohort (Han et al.: 0.93 μg/g of creatinine versus ESPINAboys: 0.43 μg/g of creatinine), which could explain why they observed an association, and we did not.
4.4. Strengths and Limitations
Strengths of this study include our ability to characterize pesticide exposures using specific urinary pesticide metabolite measurements, and a study sample size which is currently among the largest to evaluate the health effects of pesticide exposures amongst children and adolescents. Limitations of the present study include that the cross-sectional nature of the analysis restricts our ability to determine causation. There was low detectability for some of the metabolites included in this study. We were unable to confirm adolescent stage with LH or FSH concentration, but self-reported tanner staging has been found to be an appropriate research method to obtain puberty stage approximation (Rapkin et al., 2006; Schlossberger et al., 1992). Given that endocrine changes occur dynamically, our methods would be improved if additional signaling hormones were measured, such as LH, FSH, and GnRH. Enzymatic hormone measurement has been found to have lower accuracy compared to serum measurements, but the test kits used for this study have high correlations with serum measures of testosterone (r = 0.96, p <0.001), 17β-estradiol (r = 0.80, p = <0.001), cortisol (r = 0.91, p < 0.0001), and DHEA (0.86, p < 0.0001) (Salimetrics, 2019a; Salimetrics, 2016; Salimetrics, 2019b; Salimetrics. Salimetrics L.L.C., 2006). Measuring salivary hormone concentrations are valuable. Most steroid hormones in the bloodstream are bound to specific proteins, thus hormone concentrations measured in serum can include both total and free circulating hormones. It is the free fraction of sex hormones, which accounts for less than 5% of the total, that accounts for their actual activity at the tissue level. These free sex hormone molecules, being smaller relative to their bound protein–steroid complex, can penetrate small membranes, such as those found in the salivary gland. Therefore, salivary measures can reflect not only the free (bioactive) sex hormone levels (Lu et al.; Worthman et al., 1990), but the amount of such hormones that have entered the organs. Reliance on self-reported waketime and saliva sample times is subject to recall bias and measurement error.
5. Conclusion
Our study identified that urinary concentrations of organophosphate metabolites are associated with testosterone and DHEA concentrations in boys but not girls, and neonicotinoid insecticide metabolites were associated with estradiol concentrations in boys (estradiol was not measured in girls). This study is amongst the first to characterize the relationship of organophosphate, neonicotinoid and pyrethroid urinary metabolites with hormones in humans. Furthering research on the long-term effects of pesticide-related endocrine and developmental alterations in pediatric populations as well as replication of our findings on diverse human populations is advised.
Supplementary Material
Funding
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Numbers (R01ES025792, R01ES030378, R21ES026084, U2CES026560). Briana Chronister was funded by the Institute of Mental Health (5T32MH122376). We thank ESPINA study staff, Fundación Cimas del Ecuador, the Parish Governments of Pedro Moncayo County, community members of Pedro Moncayo, and the Education District of Pichincha-Cayambe-Pedro Moncayo counties for their contributions and support on this project. We would like to thank Andrea Cardenas for her help preparing the manuscript for submission.
Footnotes
CRediT authorship contribution statement
Briana N.C. Chronister: Writing – review & editing, Writing – original draft, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Denise Justo: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Robert Wood: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Dolores Lopez-Paredes: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Conceptualization. Eduardo Gonzalez: Writing – review & editing, Formal analysis, Conceptualization. Jose Suarez-Torres: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Sheila Gahagan: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. Danilo Martinez: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Data curation. David R. Jacobs: Writing – review & editing, Methodology, Investigation, Conceptualization. Harvey Checkoway: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. Marta M. Jankowska: Writing – review & editing, Supervision, Methodology, Conceptualization. Jose R. Suarez-Lopez: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2024.114386.
References
- Abass K, Turpeinen M, Pelkonen O, 2009a. An evaluation of the cytochrome P450 inhibition potential of selected pesticides in human hepatic microsomes. J Environ Sci Health B 44 (6), 553–563. 10.1080/03601230902997766. [DOI] [PubMed] [Google Scholar]
- Abass K, Turpeinen M, Pelkonen O, 2009b. An evaluation of the cytochrome P450 inhibition potential of selected pesticides in human hepatic microsomes. J Environ Sci Health B 44 (6), 553–563. 10.1080/03601230902997766. [DOI] [PubMed] [Google Scholar]
- Aguilar-Garduño C, Lacasaña M, Blanco-Muñoz J, et al. , 2013. Changes in male hormone profile after occupational organophosphate exposure. A longitudinal study. Toxicology 307, 55–65. 10.1016/j.tox.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Ahmed E, Nagaoka K, Fayez M, Abdel-Daim MM, Samir H, Watanabe G, 2015. Suppressive effects of long-term exposure to P-nitrophenol on gonadal development, hormonal profile with disruption of tissue integrity, and activation of caspase-3 in male Japanese quail (Coturnix japonica). Environ. Sci. Pollut. Control Ser. 22 (14), 10930–10942. 10.1007/s11356-015-4245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaa-Eldin EA, El-Shafei DA, Abouhashem NS, 2017a. Individual and combined effect of chlorpyrifos and cypermethrin on reproductive system of adult male albino rats. Environ. Sci. Pollut. Control Ser. 24 (2), 1532–1543. 10.1007/s11356-016-7912-6. [DOI] [PubMed] [Google Scholar]
- Alaa-Eldin EA, El-Shafei DA, Abouhashem NS, 2017b. Individual and combined effect of chlorpyrifos and cypermethrin on reproductive system of adult male albino rats. Environ. Sci. Pollut. Control Ser. 24 (2), 1532–1543. 10.1007/s11356-016-7912-6. [DOI] [PubMed] [Google Scholar]
- Annabi A, Dhouib IEB, Dkhili H, et al. , 2015. Mechanisms of imidacloprid-induced alteration of hypothalamic-pituitary-adrenal (HPA) Axis after subchronic exposure in male rats. Recent Adv. Biol. Med. 1, 51. 10.18639/rabm.2015.01.195931. [DOI] [Google Scholar]
- Arican EY, Gökçeoğlu Kayali D, Ulus Karaca B, et al. , 2020. Reproductive Effects of Subchronic Exposure to Acetamiprid in Male Rats, 10. Nature Research. 10.1038/s41598-020-65887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SE, Serafim AB, Morales-Agudelo P, Vidal M, Calafat AM, Ospina M, 2019. Quantification of DEET and neonicotinoid pesticide biomarkers in human urine by online solid-phase extraction high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 411 (3), 669–678. 10.1007/s00216-018-1481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal R, Naziroǧlu M, Türk G, et al. , 2012. Insecticide imidacloprid induces morphological and DNA damage through oxidative toxicity on the reproductive organs of developing male rats. Cell Biochem. Funct. 30 (6), 492–499. 10.1002/cbf.2826. [DOI] [PubMed] [Google Scholar]
- Barbieri MM, Berger JO, 2004. Optimal predictive model selection. Ann. Stat. 32 (3), 870–897. 10.1214/009053604000000238. [DOI] [Google Scholar]
- Barker DJP, 2003. The developmental origins of adult disease. Eur. J. Epidemiol. 18 (8), 733–736. 10.1023/A:1025388901248. [DOI] [PubMed] [Google Scholar]
- Benavides-Piracón JA, Hernández-Bonilla D, Menezes-Filho JA, et al. , 2022. Prenatal and postnatal exposure to pesticides and school-age children’s cognitive ability in rural Bogotá, Colombia. Neurotoxicology 90 (March), 112–120. 10.1016/j.neuro.2022.03.008. [DOI] [PubMed] [Google Scholar]
- Bhaskar R, Mishra AK, Mohanty B, 2017. Neonatal exposure to endocrine disrupting chemicals impairs learning behaviour by disrupting hippocampal organization in male Swiss albino mice. Basic Clin. Pharmacol. Toxicol. 121 (1), 44–52. 10.1111/bcpt.12767. [DOI] [PubMed] [Google Scholar]
- Bobb JF, 2017a. Package “Bkmr” Title Bayesian Kernel Machine Regression. Maintainer. https://cran.r-project.org/web/packages/bkmr/bkmr.pdf. [Google Scholar]
- Bobb JF, 2017b. Package “Bkmr” Title Bayesian Kernel Machine Regression. Maintainer. [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B., et al. , 2015a. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16 (3), 493–508. 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B., et al. , 2015b. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16 (3), 493–508. 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA, 2018a. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 17 (1), 1–10. 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA, 2018b. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 17 (1), 1–10. 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Simard MN, Muckle G, et al. , 2013. Exposure to an organochlorine pesticide (chlordecone) and development of 18-month-old infants. Neurotoxicology 35 (1), 162–168. 10.1016/j.neuro.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Caron-Beaudoin E, Viau R, Hudon-Thibeault AA, Vaillancourt C, Sanderson JT, 2017. The use of a unique co-culture model of fetoplacental steroidogenesis as a screening tool for endocrine disruptors: the effects of neonicotinoids on aromatase activity and hormone production. Toxicol. Appl. Pharmacol. 332, 15–24. 10.1016/j.taap.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Caudill SP, Schleicher RL, Pirkle JL, 2008. Multi-rule quality control for the age-related eye disease study. Stat. Med. 27 (20), 4094–4106. 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- Choudhary N, Goyal R, Joshi SC, 2008. Effect of malathion on reproductive system of male rats. J. Environ. Biol. 29 (2), 259–262. [PubMed] [Google Scholar]
- Chronister BNC, Gonzalez E, Lopez-Paredes D, et al. , 2021. Testosterone, estradiol, DHEA and cortisol in relation to anxiety and depression scores in adolescents. J. Affect. Disord. 294 (November 2020), 838–846. 10.1016/j.jad.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronister BNC, Yang K, Yang AR, Lin T, Tu XM, Lopez-Paredes D, Checkoway H, Suarez-Torres J, Gahagan S, Martinez D, Barr D, Moore RC, Suarez-Lopez JR, 2023. Urinary Glyphosate, 2,4-D and DEET Biomarkers in Relation to Neurobehavioral Performance in Ecuadorian Adolescents in the ESPINA Cohort. Environ. Health Perspect. 131 (10). 10.1289/EHP11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago J, Schlenk D, 2015. The effect of bifenthrin on the dopaminergic pathway in juvenile rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 162, 66–72. 10.1016/j.aquatox.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Crews D, McLachlan JA, 2006. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology 147 (6), 4–10. 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- Dandona P, Rosenberg MT, 2010. A practical guide to male hypogonadism in the primary care setting. Int. J. Clin. Pract. 64 (6), 682–696. 10.1111/j.1742-1241.2010.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Wade EL, Restrepo PR, et al. , 2013. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 929, 18–26. 10.1016/j.jchromb.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Dziewirska E, Hanke W, Jurewicz J, 2018. Environmental non-persistent endocrine-disrupting chemicals exposure and reproductive hormones levels in adult men. Int. J. Occup. Med. Environ. Health 31 (5), 551–573. 10.13075/ijomeh.1896.01183. [DOI] [PubMed] [Google Scholar]
- Ellison CA, Tian Y, Knaak JB, Kostyniak PJ, Olson JR, 2012. Human hepatic cytochrome P450-specific metabolism of the organophosphorus pesticides methyl parathion and diazinon. Drug Metabol. Dispos. 40 (1), 1–5. 10.1124/dmd.111.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel M, Bokor BR, Bornstein MH, 2020. Tanner Stages. StatPearls Publishing, Treasure Island (FL). 10.4135/9781506307633.n814. [DOI] [PubMed] [Google Scholar]
- EQM Research, 2003. Test-Mate ChE Cholinesterase Test System (Model 400). EQM Research. www.eqmresearch.com. (Accessed 21 October 2020). [Google Scholar]
- EQM Research Inc, 2003. Test-mate ChE Cholinesterase Test System (Model 400). Published online.
- Erthal RP, Staurengo-Ferrari L, Fattori V, et al. , 2020. Exposure to low doses of malathion during juvenile and peripubertal periods impairs testicular and sperm parameters in rats: role of oxidative stress and testosterone. Reprod. Toxicol. 96, 17–26. 10.1016/j.reprotox.2020.05.013. [DOI] [PubMed] [Google Scholar]
- Fink G (Ed.), 2000. Encyclopedia of Stress, 1. Academic Press. [Google Scholar]
- Garey J, Wolff MS, 1998. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochem. Biophys. Res. Commun. 251 (3), 855–859. 10.1006/bbrc.1998.9569. [DOI] [PubMed] [Google Scholar]
- Garry VF, 2004. Pesticides and children. Toxicol. Appl. Pharmacol. 198 (2), 152–163. 10.1016/j.taap.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Geng X, Shao H, Zhang Z, Ng JC, Peng C, 2015. Malathion-induced testicular toxicity is associated with spermatogenic apoptosis and alterations in testicular enzymes and hormone levels in male Wistar rats. Environ. Toxicol. Pharmacol. 39 (2), 659–667. 10.1016/j.etap.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Ghafouri-Khosrowshahi A, Ranjbar A, Mousavi L, et al. , 2019a. Chronic exposure to organophosphate pesticides as an important challenge in promoting reproductive health: a comparative study. J. Educ. Health Promot. 8 (1), 1–6. 10.4103/jehp.jehp_148_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri-Khosrowshahi A, Ranjbar A, Mousavi L, et al. , 2019b. Chronic exposure to organophosphate pesticides as an important challenge in promoting reproductive health: a comparative study. J. Educ. Health Promot. 8 (1), 1–6. 10.4103/jehp.jehp_148_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh J, Sinaei M, Bolouki M, 2015. Biochemical and histological changes in fish, spotted scat (Scatophagus argus) exposed to diazinon. Bull. Environ. Contam. Toxicol. 94 (2), 164–170. 10.1007/s00128-014-1454-8. [DOI] [PubMed] [Google Scholar]
- Go V, Garey J, Wolff MS, Pogo BGT, 1999. Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line. Environ. Health Perspect. 107 (3), 173–177. 10.1289/ehp.99107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad RT, Goad JT, Atieh BH, Gupta RC, 2004. Carbofuran-induced endocrine disruption in adult male rats. Toxicol. Mech. Methods 14 (4), 233–239. 10.1080/15376520490434476. [DOI] [PubMed] [Google Scholar]
- Gore AC, Heindel JJ, Zoeller RT, 2006. Endocrine disruption for endocrinologists (and others). Endocrinology 147 (6), 2005–2007. 10.1210/en.2005-1367. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Harari R, Barr DB, Debes F, 2006. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics 117 (3), e546–e556. 10.1542/peds.2005-1781. [DOI] [PubMed] [Google Scholar]
- Gravel S, Lavoué J, Bakhiyi B, et al. , 2020. Multi-exposures to suspected endocrine disruptors in electronic waste recycling workers: associations with thyroid and reproductive hormones. Int. J. Hyg Environ. Health 225. 10.1016/j.ijheh.2019.113445. [DOI] [PubMed] [Google Scholar]
- Han Y, Xia Y, Han J, et al. , 2008. The relationship of 3-PBA pyrethroids metabolite and male reproductive hormones among non-occupational exposure males. Chemosphere 72 (5), 785–790. 10.1016/j.chemosphere.2008.03.058. [DOI] [PubMed] [Google Scholar]
- Hu JX, Li YF, Li J, et al. , 2013. Toxic effects of cypermethrin on the male reproductive system: with emphasis on the androgen receptor. J. Appl. Toxicol. 33 (7), 576–585. 10.1002/jat.1769. [DOI] [PubMed] [Google Scholar]
- Kabir ER, Rahman MS, Rahman I, 2015. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 40 (1), 241–258. 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Kagawa N, Nagao T, 2018. Neurodevelopmental toxicity in the mouse neocortex following prenatal exposure to acetamiprid. J. Appl. Toxicol. 38 (12), 1521–1528. 10.1002/jat.3692. [DOI] [PubMed] [Google Scholar]
- Khan DA, Ahad K, Ansari WM, Khan H, 2013. Pesticide exposure and endocrine dysfunction in the cotton crop agricultural workers of Southern Punjab, Pakistan. Asia Pac. J. Publ. Health 25 (2), 181–191. 10.1177/1010539511417422. [DOI] [PubMed] [Google Scholar]
- Khonsary S, 2017. Guyton and Hall: textbook of medical physiology. Surg. Neurol. Int. 10.4103/sni.sni_327_17. Published online. [DOI] [Google Scholar]
- Kong D, Zhang J, Hou X, et al. , 2017a. Acetamiprid inhibits testosterone synthesis by affecting the mitochondrial function and cytoplasmic adenosine triphosphate production in rat Leydig cells. Biol. Reprod. 96 (1), 254–265. 10.1095/biolreprod.116.139550. [DOI] [PubMed] [Google Scholar]
- Kong D, Zhang J, Hou X, et al. , 2017b. Acetamiprid inhibits testosterone synthesis by affecting the mitochondrial function and cytoplasmic adenosine triphosphate production in rat Leydig cells. Biol. Reprod. 96 (1), 254–265. 10.1095/biolreprod.116.139550. [DOI] [PubMed] [Google Scholar]
- Kormorniczak M Tanner Scale. Wikipedia. doi: 10.32388/8rd4dl. [DOI] [Google Scholar]
- Krause W, 1977. Influence of DDT, DDVP and malathion on FSH, LH and testosterone serum levels and testosterone concentration in testis. Bull. Environ. Contam. Toxicol. 18 (2), 231–242. 10.1007/BF01686072. [DOI] [PubMed] [Google Scholar]
- Krause W, Hamm K, Weissmüller J, 1976. Damage to spermatogenesis in juvenile rat treated with DDVP and malathion. Bull. Environ. Contam. Toxicol. 15 (4), 458–462. 10.1007/BF01685072. [DOI] [PubMed] [Google Scholar]
- Kwon W, Kim JY, Suh S, In MK, 2012. Simultaneous determination of creatinine and uric acid in urine by liquid chromatography – tandem mass spectrometry with polarity switching electrospray ionization. Forensic Sci. Int. 221 (1–3), 57–64. 10.1016/j.forsciint.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Kwong TC, 2002. Organophosphate pesticides: biochemistry and clinical toxicology. Ther. Drug Monit. 24 (1), 144–149. 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Goldman LR, 2011. Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff. 30 (5), 842–850. 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- Li X, Li C, Suzuki AK, Taneda S, Watanabe G, Taya K, 2009a. 4-Nitrophenol isolated from diesel exhaust particles disrupts regulation of reproductive hormones in immature male rats. Endocrine 36 (1), 98–102. 10.1007/s12020-009-9192-0. [DOI] [PubMed] [Google Scholar]
- Li X, Li C, Suzuki AK, Watanabe G, Taneda S, Taya K, 2009b. Endocrine disruptive effect of 3-Methyl-4-nitrophenol Isolated from diesel exhaust particles in hershberger assay using castrated immature rats. Biosci. Biotechnol. Biochem. 73 (9), 2018–2021. 10.1271/bbb.90204. [DOI] [PubMed] [Google Scholar]
- Liang H, Wu X, Yao H, et al. , 2022. Association of urinary metabolites of non-persistent pesticides with serum sex hormones among the US females: NHANES 2013–2014. Chemosphere 300. 10.1016/j.chemosphere.2022.134577. [DOI] [PubMed] [Google Scholar]
- Lu YC, Bentley GR, Gann PH, Hodges KR, Chatterton RT. Salivary Estradiol and Progesterone Levels in Conception and Nonconception Cycles in Women: Evaluation of a New Assay for Salivary Estradiol. [DOI] [PubMed]
- Ma X, Li H, Xiong J, Mehler WT, You J, 2019. Developmental toxicity of a neonicotinoid insecticide, acetamiprid to zebrafish embryos. J. Agric. Food Chem. 67 (9), 2429–2436. 10.1021/acs.jafc.8b05373. [DOI] [PubMed] [Google Scholar]
- Ma X, Xiong J, Li H, Brooks BW, You J, 2022. Long-term exposure to neonicotinoid insecticide acetamiprid at environmentally relevant concentrations impairs endocrine functions in zebrafish: bioaccumulation, feminization, and transgenerational effects. Environ. Sci. Technol. 56 (17), 12494–12505. 10.1021/acs.est.2c04014. [DOI] [PubMed] [Google Scholar]
- Mage DT, Allen RH, Gondy G, Smith W, Barr DB, Needham LL, 2004. Estimating pesticide dose from urinary pesticide concentration data by creatinine correction in the Third National Health and Nutrition Examination Survey (NHANES-III). J. Expo. Anal. Environ. Epidemiol. 14 (6), 457–465. 10.1038/sj.jea.7500343. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Ravi SR, Barr DB, Hauser R, 2008. Circulating estradiol in men is inversely related to urinary metabolites of nonpersistent insecticides. Reprod. Toxicol. 25 (2), 184–191. 10.1016/j.reprotox.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Hauser R, 2009a. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod. Toxicol. 27 (2), 155–160. 10.1016/j.reprotox.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Hauser R, 2009b. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod. Toxicol. 10.1016/j.reprotox.2008.12.012. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A, Pinney SM, 2022. Exposure to neonicotinoids and serum testosterone in men, women, and children. Environ. Toxicol. 37 (6), 1521–1528. 10.1002/TOX.23503. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S, Regitz-Zagrosek V, 2013. Sex and Gender Aspects in Clinical Medicine. 10.1007/978-0-85729-832-4. [DOI]
- Omoike OE, Lewis RC, Meeker JD, 2015. Association between urinary biomarkers of exposure to organophosphate insecticides and serum reproductive hormones in men from NHANES 1999–2002. Reprod. Toxicol. 53, 99–104. 10.1016/j.reprotox.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina M, Wong LY, Baker SE, Serafim AB, Morales-Agudelo P, Calafat AM, 2019. Exposure to neonicotinoid insecticides in the U.S. general population: data from the 2015–2016 national health and nutrition examination survey. Environ. Res. 176 (February), 108555 10.1016/j.envres.2019.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya P, Upadhyay A, Thakkar B, Parikh P, 2018. Evaluating the toxicological effects of agrochemicals on glucocorticoid receptor and serum cortisol level in Mozambique tilapia. Cogent Biol 4 (1), 1480338. 10.1080/23312025.2018.1480338. [DOI] [Google Scholar]
- Panuwet P, Ladva C, Barr DB, et al. , 2018. Investigation of associations between exposures to pesticides and testosterone levels in Thai farmers. Arch. Environ. Occup. Health 73 (4), 205–218. 10.1080/19338244.2017.1378606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris DC, Dhanushka T, 2017. Low doses of chlorpyrifos interfere with spermatogenesis of rats through reduction of sex hormones. Environ. Sci. Pollut. Control Ser. 24 (26), 20859–20867. 10.1007/s11356-017-9617-x. [DOI] [PubMed] [Google Scholar]
- Qin K, Zhang Y, Wang Y, et al. , 2020. Prenatal organophosphate pesticide exposure and reproductive hormones in cord blood in Shandong, China. Int. J. Hyg Environ. Health 225 (October 2019), 113479. 10.1016/j.ijheh.2020.113479. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Tsao JCI, Turk N, Anderson M, Zeltzer LK, 2006. Relationships among self-rated tanner staging, hormones, and psychosocial factors in healthy female adolescents. J. Pediatr. Adolesc. Gynecol. 19 (3), 181–187. 10.1016/j.jpag.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Rasmussen AR, Wohlfahrt-Veje C, De Renzy-Martin KT, et al. , 2015. Validity of self-assessment of pubertal maturation. Pediatrics 135 (1), 86–93. 10.1542/peds.2014-0793. [DOI] [PubMed] [Google Scholar]
- Ren S, Li Y, Li C, 2021. Effects of P-Nitrophenol Exposure on the Testicular Development and Semen Quality of Roosters, 301. Academic Press Inc. 10.1016/j.ygcen.2020.113656 [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Balestreire EM, Czambel RK, Rubin RT, 2002. Estrous Cycle Influences on Sexual Diergism of HPA Axis Responses to Cholinergic Stimulation in Rats, 59. [DOI] [PubMed] [Google Scholar]
- Salahshoor MR, Abdolmaleki A, Faramarzi A, Jalili C, Shiva R, 2020. Does Tribulus terrestris improve toxic effect of Malathion on male reproductive parameters? J. Pharm. BioAllied Sci. 12 (2), 183–191. 10.4103/jpbs.JPBS_224_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimetrics, 2016. Salivary Testosterone Protocol.
- Salimetrics, 2019a. Salivary DHEA Enzyme Immunoassay Kit.
- Salimetrics, 2019b. High Sensitivity Salivary 17β-Estradiol Enzyme Immunoassay Kit. [Google Scholar]
- Salimetrics. Salimetrics L.L.C., 2006. Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit [Brochure]. https://www.salimetrics.com/assets/documents/1-3002.pdf. [Google Scholar]
- Sarabia L, Maurer I, Bustos-Obregón E, 2009. Melatonin prevents damage elicited by the organophosphorous pesticide diazinon on the mouse testis. Ecotoxicol. Environ. Saf. 72 (3), 938–942. 10.1016/j.ecoenv.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Schlossberger NM, Turner RA, Irwin CE, 1992. Validity of self-report of pubertal maturation in early adolescents. J. Adolesc. Health 13 (2), 109–113. 10.1016/1054-139X(92)90075-M. [DOI] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Wolz S a, Lee IC, Kalman DA, 1995. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ. Health Perspect. 103 (12), 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimen S, Saloua EF, Najoua G, 2014. Oxidative stress and cytotoxic potential of anticholinesterase insecticide, malathion in reproductive toxicology of male adolescent mice after acute exposure. Iran J Basic Med Sci 17 (7), 522–530. 10.22038/ijbms.2014.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez B, Vela-Soria F, Castiello F, et al. , 2021. Organophosphate pesticide exposure, hormone levels, and interaction with PON1 polymorphisms in male adolescents. Sci. Total Environ. 769, 144563 10.1016/j.scitotenv.2020.144563. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR, Himes JH, Alexander BH, Lazovich D, Gunnar M, 2012. Lower acetylcholinesterase activity among children living with flower plantation workers. Environ. Res. 114 (1), 53–59. 10.1016/j.envres.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Butcher CR, Gahagan S, Checkoway H, Alexander BH, Al-Delaimy WK, 2018. Acetylcholinesterase activity and time after a peak pesticide-use period among Ecuadorian children. Int. Arch. Occup. Environ. Health 91 (2), 175–184. 10.1007/s00420-017-1265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Hood N, Suárez-Torres J, Gahagan S, Gunnar MR, López-Paredes D, 2019. Associations of acetylcholinesterase activity with depression and anxiety symptoms among adolescents growing up near pesticide spray sites. Int. J. Hyg Environ. Health 222 (7), 981–990. 10.1016/j.ijheh.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwannarin N, Prapamontol T, Isobe T, et al. , 2021. Exposure to organophosphate and neonicotinoid insecticides and its association with steroid hormones among male reproductive-age farmworkers in Northern Thailand. Int. J. Environ. Res. Publ. Health 18 (11), 1–16. 10.3390/ijerph18115599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, He H, Tu X, 2012. Applied categorical and count data analysis. 1st Editio. Chapman and Hall/CRC. 10.1201/b12123. [DOI] [Google Scholar]
- Tetsatsi ACM, Nkeng-Effouet PA, Alumeti DM, Bonsou GRF, Kamanyi A, Watcho P, 2019. Colibri® insecticide induces male reproductive toxicity: alleviating effects of Lannea acida (Anacardiaceae) in rats. Basic Clin Androl 29 (1). 10.1186/s12610-019-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Sun Y, Wang H, Bing X, Wang W, Ru S, 2017. Monocrotophos pesticide affects synthesis and conversion of sex steroids through multiple targets in male goldfish (Carassius auratus). Sci. Rep. 7 (1), 2306. 10.1038/s41598-017-01935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani KA, Rose RL, Hodgson E, 2003a. Inhibition and activation of the human liver microsomal and human cytochrome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metabol. Dispos. 31 (4), 384–391. 10.1124/dmd.31.4.384. [DOI] [PubMed] [Google Scholar]
- Usmani KA, Rose RL, Hodgson E, 2003b. Inhibition and activation of the human liver microsomal and human cytochrome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metabol. Dispos. 31 (4), 384–391. 10.1124/dmd.31.4.384. [DOI] [PubMed] [Google Scholar]
- Vigil P, del Río JP, Carrera B, Aránguiz FC, Rioseco H, Cortés ME, 2016. Influence of sex steroid hormones on the adolescent brain and behavior: an update. Linacre Q. 83 (3), 308–329. 10.1080/00243639.2016.1211863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed S, Halsall C, Sweetman AJ, Jones KC, Malik RN, 2017. Pesticides contaminated dust exposure, risk diagnosis and exposure markers in occupational and residential settings of Lahore, Pakistan. Environ. Toxicol. Pharmacol. 56, 375–382. 10.1016/j.etap.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang HX, Shen JY, et al. , 2019. The anti-androgenic effects of cypermethrin mediated by non-classical testosterone pathway activation of mitogen-activated protein kinase cascade in mouse Sertoli cells. Ecotoxicol. Environ. Saf. 177, 58–65. 10.1016/j.ecoenv.2019.03.109. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Organization, 2008. Training course on child growth assessment. Geneva. World Health Organization Training Course on Child Growth Assessment Geneva WS; 103 (0), 1–116. [Google Scholar]
- Worthman CM, Stallings JF, Hofman LF, 1990. Sensitive salivary estradiol assay for monitoring ovarian function. Clin. Chem. 36 (10), 1769–1773. 10.1093/clinchem/36.10.1769. [DOI] [PubMed] [Google Scholar]
- Yoshinaga J, Imai K, Shiraishi H, et al. , 2014. Pyrethroid insecticide exposure and reproductive hormone levels in healthy Japanese male subjects. Andrology 2 (3), 416–420. 10.1111/j.2047-2927.2014.00202.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu L, Zhang G, Guan Y, Wang Z, 2016a. Effect of low-dose malathion on the gonadal development of adult rare minnow Gobiocypris rarus. Ecotoxicol. Environ. Saf. 125, 135–140. 10.1016/j.ecoenv.2015.11.041. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao Y, Wang F, et al. , 2016b. 4-Nitrophenol induces activation of Nrf2 antioxidant pathway and apoptosis of the germ cells in rat testes. Environ. Sci. Pollut. Control Ser. 23 (13), 13035–13046. 10.1007/s11356-016-6470-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


