Abstract
We have established an allergic dermatitis model in NC/Nga mice by repeated local exposure of mite antigen for analyzing atopic dermatitis. We examined how four Kampo medicines, Juzen-taiho-to, Hochu-ekki-to, Shofu-san and Oren-gedoku-to, on the dermatitis model to obtain basic information on their usefulness for treating atopic dermatitis. Mite antigen (Dermatophagoides farinae crude extract) solution at a concentration of 10 mg/ml was painted on the ear of NC/Nga mice after tape stripping. The procedure was repeated five times, at 7 day intervals. An apparent biphasic ear swelling was caused after the fourth and fifth antigen exposures with elevated serum IgE levels and accumulation of inflammatory cells. In the cervical lymph nodes and ear lobes, the five procedures of antigen exposure induced interleukin-4 mRNA expression but reduced interferon-γ mRNA expression. Oral administration of all four Kampo medicines inhibited the formation of ear swelling and inflammatory cell accumulation. Juzen-taiho-to and Hochu-ekki-to apparently prevented the elevation of serum IgE level. Furthermore, the four Kampo medicines showed a tendency to prevent not only the increase in interleukin-4 mRNA expression but also the decrease in interferon-γ mRNA expression. The present results indicate that Juzen-taiho-to, Hochu-ekki-to, Shofu-san and Oren-gedoku-to may correct the Th1/Th2 balance skewed to Th2, and this activity helps inhibit dermatitis in NC/Nga mice. The ability of the Kampo medicines to correct the Th1/Th2 balance seems to underlie their effectiveness in treating of atopic dermatitis.
Keywords: herbal medicine, IgE, Th1/Th2 balance
Introduction
Atopic dermatitis is a chronic eczematous skin disease accompanied by severe itch, episodes which are frequently repeated (1,2). In most cases, onset of the disease is observed in infants and is considered to be dependent on both genetic and environmental factors. Elevated serum IgE levels are also a characteristic feature in many patients (3,4). Itchiness is the most important problem for atopic patients and scratching worsens the dermatitis itself. Patients suffering from atopic dermatitis, especially adult patients with severe symptoms, have been increasing in Japan, and inappropriate usage of topical glucocorticoids has been emphasized as one of its main causes (1).
Topical glucocorticoids are important and effective remedies for treatment of atopic dermatitis. It is well known, however, that prolonged use of high dose glucocorticoids frequently causes a variety of adverse effects (5,6). Recently, tacrolimus, an immunosuppressant, has been introduced for treating of atopic dermatitis, but its efficacy is less than that of glucocorticoids (7,8). In contrast to these drugs, which are supposedly targeted at disease mechanisms, Kampo medicines essentially aim to correct the patient's constitution, hoping to bring about a cure of the disease secondarily (9,10). As Kampo medicines usually contain many different constituents, it is sometimes difficult to identify the active components and to elucidate the mechanism of their actions. However, the approach of Kampo medicines seems to be beneficial for treating of chronic diseases, including atopic dermatitis. Recently, several herbal medicines have been tried for treating atopic dermatitis, but the scientific evidence on their efficacy is rarely available (11,12).
We have established an allergic dermatitis model in NC/Nga mice by repeated local exposure of mite antigen (13). The dermatitis possesses some characteristic features observed in atopic dermatitis patients, such as eczematous skin lesion with inflammatory cell accumulation, a Th1/Th2 balance skewed to Th2 and elevated serum IgE levels. The Th1/Th2 imbalance has been considered to be an important feature of atopic disease (14,15).
In this study, we examined the effects of four Kampo medicines, Juzen-taiho-to, Hochu-ekki-to, Shofu-san and Oren- gedoku-to, on the mite antigen-induced dermatitis in NC/Nga mice to obtain basic information about their usefulness in the treatment of atopic dermatitis.
Materials and Methods
Mice
Female NC/Nga mice, 6 weeks of age, were obtained from Japan SLC (Hamamatsu, Japan) and maintained for 2 weeks before organizing the experiment. They were housed in an air-conditioned animal room at a temperature of 22 ± 1°C and humidity of 60 ± 5%, and fed a laboratory diet and water ad libitum. Experiments were undertaken following the guidelines for the care and use of experimental animals of the Japanese Association for Laboratory Animal Science (16).
Drugs
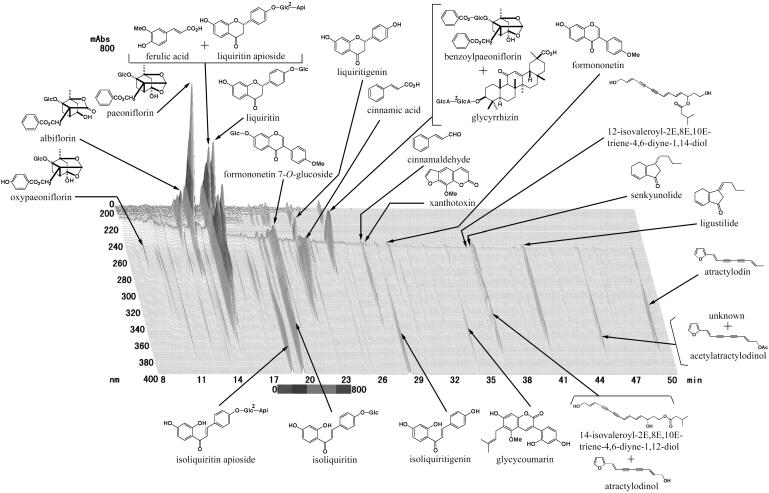
Dried extracts of Juzen-taiho-to (TJ-48), Hochu-ekki-to (TJ-41), Shofu-san (TJ-22) and Oren-gedoku-to (TJ-15) were provided by Tsumura & Co. (Tokyo, Japan). Constituent herbal medicines, their origins and their amounts per day are indicated in Table 1. The dried amount of extract obtained from each set of herbal medicines was roughly 5 g for Juzen-taiho-to, 5 g for Hochu-ekki-to, 4 g for Shofu-san and 1.5 g for Oren-gedoku-to. The quality of the extracts was checked by examining the quantity of key compounds using HPLC. The HPLC profile of Juzen-taiho-to is indicated in Fig. 1 as an example. The extracts were dissolved in distilled water and given to mice orally at doses of 100 and 300 mg/kg once a day throughout the experiment. Prednisolone (phosphate, Banyu, Tokyo, Japan) obtained commercially was used as a reference drug. It was dissolved in distilled water and given orally at a dose of 3 mg/kg once a day throughout the experiment. The doses of Kampo medicines and prednisolone were decided according to our preliminary experiments.
Table 1.
Examination of Kampo medicines and their constituents
| Constituent | Origin | Family | Amount (g) |
|---|---|---|---|
| Juzen-taiho-to (TJ-48) | |||
| Astragali Radix | Astragalus membranaceus | Leguminosae | 3 |
| Glycyrrhizae Radix | Glycyrrhiza glabra | Leguminosae | 1.5 |
| Cinnamomi Cortex | Cinnamomum cassia | Lauraceae | 3 |
| Rehmanniae Radix | Rehmannia glutinosa | Scrophulariaceae | 3 |
| Paeoniae Radix | Paeonia lactiflora | Paeoniaceae | 3 |
| Cnidii Rhizoma | Cnidium officinale | Umbelliferae | 3 |
| Atractylodis Lanceae Rhizoma | Atractylodes lancea | Compositae | 3 |
| Angelicae Radix | Angelica acutiloba | Umbelliferae | 3 |
| Ginseng Radix | Panax ginseng | Araliaceae | 3 |
| Hoelen | Poria cocos | Polyporaceae | 3 |
| Hochu-ekki-to (TJ-41) | |||
| Astragali Radix | Astragalus membranaceus | Leguminosae | 4 |
| Glycyrrhizae Radix | Glycyrrhiza glabra | Leguminosae | 1.5 |
| Bupleuri Radix | Bupleurum falcatum | Umbelliferae | 2 |
| Zingiberis Rhizoma | Zingiber officinale | Zingiberaceae | 0.5 |
| Cimicifugae Rhizoma | Cimicifuga simplex | Ranunculaceae | 1 |
| Atractylodis Lanceae Rhizoma | Atractylodes lancea | Compositae | 4 |
| Zizyphi Fructus | Zizyphus jujuba | Rhamnaceae | 2 |
| Aurantii Nobilis Pericarpium | Citrus unshiu | Rutaceae | 2 |
| Angelicae Radix | Angelica acutiloba | Umbelliferae | 3 |
| Ginseng Radix | Panax ginseng | Araliaceae | 4 |
| Shofu-san (TJ-22) | |||
| Glycyrrhizae Radix | Glycyrrhiza glabra | Leguminosae | 1 |
| Sophorae Radix | Sophora flavescens | Leguminosae | 1 |
| Schizonepetae Spica | Schizonepeta tenuifolia | Labiatae | 1 |
| Arctii Fructus | Arctium lappa | Compositae | 2 |
| Sesami Semen | Sesamum indicum | Pedaliaceae | 1.5 |
| Rehmanniae Radix | Rehmannia glutinosa | Scrophulariaceae | 3 |
| Gypsum Fibrosum | 3 | ||
| Cicadae Periostracum | Cryptotympana pustulata | Cicadidae | 1 |
| Atractylodis Lanceae Rhizoma | Atractylodes lancea | Compositae | 2 |
| Anemarrhenae Rhizoma | Anemarrhena asphodeloides | Liliaceae | 1.5 |
| Angelicae Radix | Angelica acutiloba | Umbelliferae | 3 |
| Ledebouriellae Radix | Ledebouriella seseloides | Umbelliferae | 2 |
| Akebiae Caulis | Akebia quinata | Lardizabalaceae | 2 |
| Oren-gedoku-to (TJ-15) | |||
| Scutellariae Radix | Scutellaria baicalensis | Labiatae | 3 |
| Phellodendri Cortex | Phellodendron amurense | Rutaceae | 1.5 |
| Coptidis Rhizoma | Coptis japonica | Ranunculaceae | 2 |
| Gardeniae Fructus | Gardenia jasminoides | Rubiaceae | 2 |
The amount for each constituent indicates that for 1 day.
Figure 1.
HPLC profile of Juzen-taiho-to. Ingredients of Juzen-taiho-to (1.0 g of preparation) were extracted with 20 ml of methanol for 30 min under sonication and 30 μl of the extract was used for the HPLC analysis. Column, TSK-GEL 80TS (4.6 mm × 250mm, Tosoh, Japan); pump, LC-10ADVP (Shimadzu, Japan); detector, SPD-M10AVP (Shimadzu). Mobile phase: (A) 50 mM AcOH–AcONH4 buffer, (B) CH3CN, from 90% A + 10% B to 100% B in 60 min (linear gradient). Column temperature, 40°C; flow rate, 1.0 ml/min; wavelength range, 200–400 nm.
Mite Antigen
Dermatophagoides farinae crude extract (mite antigen, lyophilized, Torii, Tokyo, Japan) was used as an antigen (17). Mite antigen was dissolved in phosphate buffered saline (PBS) containing 0.5% Tween 20.
Induction of Dermatitis in the Mouse Ear
Both surfaces of mouse ear lobes were stripped three times using surgical tape (W129, Nichiban, Tokyo, Japan). One hour after the tape stripping, 25 μl of 10 mg/ml mite antigen solution was painted onto each surface of both ear lobes. Tape stripping and mite antigen painting was repeated five times at 7-day intervals and ear thickness was measured using a dial thickness gauge (R12-1A, Ozaki, Tokyo, Japan) immediately before each tape stripping, and 1, 4 and 24 h after each mite antigen application. In control mice, carrier solution only was painted instead of mite antigen solution. Results were expressed as increased ear thickness after subtracting the value obtained before the first tape stripping.
Blood samples were obtained 24 h after each antigen application and used for measuring serum IgE. Cervical lymph nodes and ears were removed for evaluating cytokine mRNA expression 4 h after the fifth antigen application. Ears for the histological observation were separated 24 h after the fifth antigen application.
Measurement of Serum IgE
Total serum IgE was measured using enzyme-linked immunosorbent assay. In brief, 100 μl of 5 μg/ml rat anti-mouse IgE heavy chain (Serotec, Oxford, UK) in PBS was placed in each well of an immunoplate (Nunc Immunoplate I, 96-well, Nalge Nunc International, Rochester, NY, USA) and the plate was kept overnight at 4°C. After washing the wells with PBS containing 0.1% Tween 20 (washing buffer) five times, 200 μl of PBS containing 1% bovine serum albumin (BSA–PBS) was placed in each well. After 1 h at room temperature, the wells were washed five times with washing buffer and 100 μl of serum samples diluted 30-fold with BSA–PBS were placed in the wells. After further incubation for 1 h at room temperature, wells were again washed five times with washing buffer, and then 100 μl of peroxide-labeled polyclonal anti-mouse IgE goat IgG antibody (Nordic Immunological Laboratory, Tilburg, Netherlands) diluted 5000-fold with washing buffer was added to the wells and the plate was kept for 1 h at room temperature. After washing five times with washing buffer, enzyme reaction was initiated by adding 100 μl of substrate solution (containing 0.1 M citric acid, 0.2 M Na2HPO4, o-phenylene diamine and H2O2) and the plate was kept for 30 min at room temperature in a dark place. The reaction was terminated by adding 50 μl of 2 M H2SO4 to each well, and absorbance at 492 nm was measured immediately using an immunoreader (Titertek Multiscan MCC/340, Dainippon, Osaka, Japan). Standard curve was prepared using monoclonal anti-DNP IgE (Sigma, St Louis, MO, USA) diluted with BSA–PBS.
Detection of Cytokine mRNA
Expression of interleukin-4 (IL-4) and interferon-γ (IFN-γ) mRNA in cervical lymph nodes and ears were examined. Excised lymph nodes and ears were homogenized in Isogen (Nippon Gene, Tokyo, Japan) using HG 30 Homogenizer (Hitachi, Tokyo, Japan). One milliliter of homogenate was mixed with 200 μl of chloroform (Nacalai Tesque, Kyoto, Japan) vigorously, and centrifuged at 13 000 r.p.m. for 15 min at 4°C using a microcentrifuge (Laboratory Centrifuge 1900, Kubota Co., Tokyo, Japan). The aqueous phase was separated and RNA in the phase was precipitated by mixing 0.5 ml of 2-propanol (Nacalai Tesque). The precipitate was washed with 75% ethanol (Nacalai Tesque) and dried, and then dissolved in diethyl pyrocarbonate (DEPC)-treated water (Nacalai Tesque). The total RNA content was calculated based on the absorbance at 260 nm and the quality was confirmed by electrophoresis.
Reverse transcriptase–polymerase chain reaction (RT–PCR) was employed for the detection of mRNA. A mixture of 11 μl containing 1 μg RNA, DEPC-treated water and random primer (GIBCO BRL, Grand Island, NY, USA) was heated at 70°C for 10 min and then mixed with 4 μl of 5× First Strand Buffer (GIBCO), 1 μl of 10 mM deoxynucleoside triphosphate (dNTP, GIBCO) and 2 μl of 0.1 M dithiothreitol (GIBCO). After 5 min at 25°C, 1 μl of reverse transcriptase (Superscript II, GIBCO) was added and RT, at 25°C for 10 min, 42°C for 50 min and then 70°C for 15 min, was performed on Trio-Thermoblock (Biometra, Goettingen, Germany). Then, cDNA at a volume of 1 μl was mixed with 100 mM Tris–HCl (pH 8.3), 500 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, 10 mM dNTP, 5 U/ml ampliTaq DNA polymerase (TaKaRa Taq, Takara, Kyoto, Japan) and 1 μM primers (Stratagene, La Jolla, CA, USA) (Table 2) and PCR (denaturation at 94°C for 1.5 min, annealing at 62°C for 1.5 min and extension at 72°C for 1.5 min, 35 cycles) was performed on Trio-Thermoblock. Products were electrophoresed on 2% agarose gel containing ethidium bromide. The bands were recorded by Polaroid camera (Polaroid 665 film, Nippon Polaroid, Tokyo, Japan) and densitometrically scanned for semiquantitative evaluation. Results were normalized by β-actin expression.
Table 2.
Primers employed for detecting cytokine mRNA
| Primer (product size) | Sequence |
|---|---|
| β-Actin (245 bp) | |
| Sense | 5′ GTG GGC CGC TAG GCA CCA 3′ |
| Antisense | 5′ CGG TTG GCC TTA GGG TTC AGG GGG G 3′ |
| IL-4 (279 bp) | |
| Sense | 5′ ACG GAG ATG GAT GTG CCA AAC GTC 3 |
| Antisense | 5′ CGA GTA ATC CAT TTG CAT GAT GC 3′ |
| IFN-γ (405 bp) | |
| Sense | 5′ TAC TGC CAC GGC ACA GTC ATT GAA 3′ |
| Antisense | 5′ GCA GCG ACT CCT TTT CCG CTT CCT 3′ |
Histopathological Observation
Excised ear lobes were fixed with 10% neutral formalin, embedded in paraffin, and thin, 5 μm, sections were prepared. The skin sections were stained with hematoxylin and eosin, and observed.
Statistics
Results of ear swelling and serum IgE levels were expressed as the means ± SEM. Statistical evaluation of data was performed using InStat Program (GraphPad Software, San Diego, CA, USA). Comparison of data among Kampo medicine-treated and control groups was performed using Dunnett's or Dunn's multiple comparison test after confirming the variance of data by Bartlet's test. Comparison of data between prednisolone-treated and control groups was performed by Student's or Welch's t-test based on the variance of data examined by F-test. When the P value was <0.05 the difference was considered to be significant.
Results
Ear Swelling Inhibited by Varied Doses of Kampo and Prednisolone
Results of ear swelling are shown in Fig. 2. After the fourth and fifth mite-antigen exposure, an apparent biphasic ear swelling was induced. Daily administration of Juzen-taiho-to at a dose of 100 mg/kg inhibited the swelling, and a tendency of inhibition was observed at a dose of 300 mg/kg. Hochu-ekki-to at a dose of 300 mg/kg strongly inhibited the swelling and at a dose of 100 mg/kg showed a tendency of inhibition. In the case of Shofu-san, although an apparent inhibition of the ear swelling was induced at a dose of 300 mg/kg, no effect was observed at a dose of 100 mg/kg. Oren-gedoku-to strongly inhibited the ear swelling at both doses of 100 and 300 mg/kg. Prednisolone at a dose of 3 mg/kg strongly inhibited the swelling in all four experiments.
Figure 2.
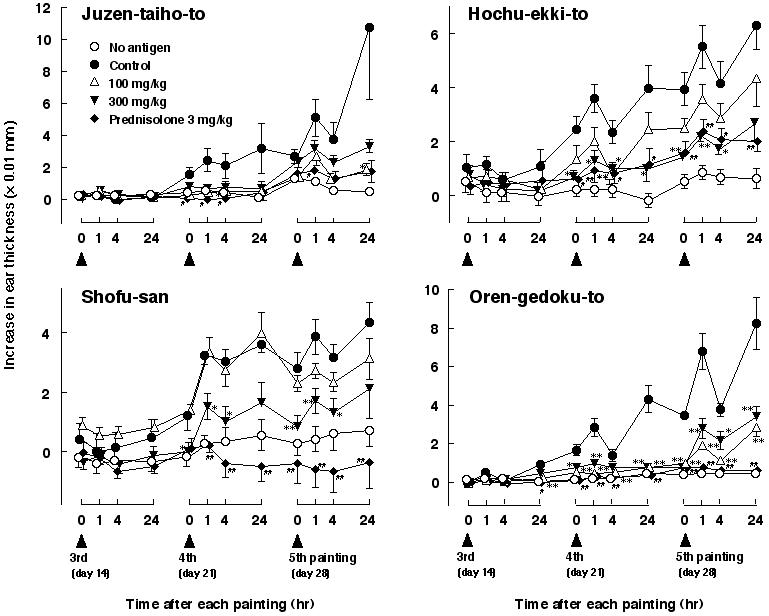
Varied doses of Juzen-taiho-to, Hochu-ekki-to, Shofu-san, Oren-gedoku-to and prednisolone influence ear thickness from repeated painting with mite antigen solution in NC/Nga mice. Ear thickness was measured immediately before, and 1, 4 and 24 h after each antigen application. Results after the first and the second antigen applications were omitted because increased ear thickness and drug effects were negligible. Each value represents the mean ± SEM of 5–8 mice. *P < 0.05, **P < 0.01 for Kampo medicine-administered groups by multiple comparison test, #P < 0.05, ##P < 0.01 for prednisolone-administered group by t-test.
Changes in the Serum IgE Levels
Resulting changes in serum IgE levels are shown in Fig. 3. Apparent elevation of serum IgE was observed after the third or fourth mite antigen exposure. Juzen-taiho-to and Hochu-ekki-to significantly depressed the elevation, whereas Shofu-san and Oren-gedoku-to failed to affect the serum IgE levels. Prednisolone inhibited the elevation of serum IgE levels in only one of four experiments. In three of four experiments, IgE levels increased in the no antigen group.
Figure 3.
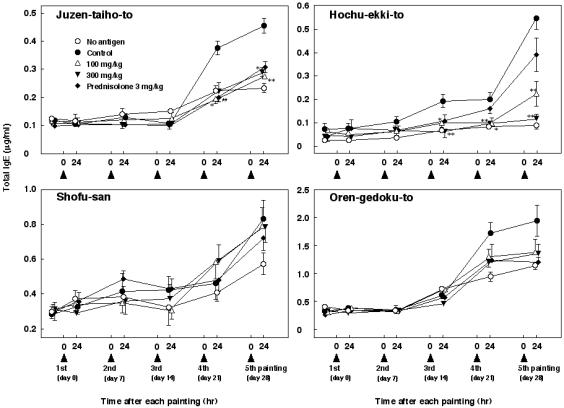
Mixed results of Juzen-taiho-to, Hochu-ekki-to, Shofu-san, Oren-gedoku-to and prednisolone administration on serum total IgE levels in NC/Nga mice repeatedly treated with mite antigen. Serum samples were obtained 24 h after each antigen application and serum IgE was quantified by enzyme-linked immunosorbent assay. Each value indicates the mean ± SEM of 5–8 mice. *P < 0.05, **P < 0.01 for Kampo medicine-administered groups by multiple comparison test; ##P < 0.01 for prednisolone-administered group by t-test.
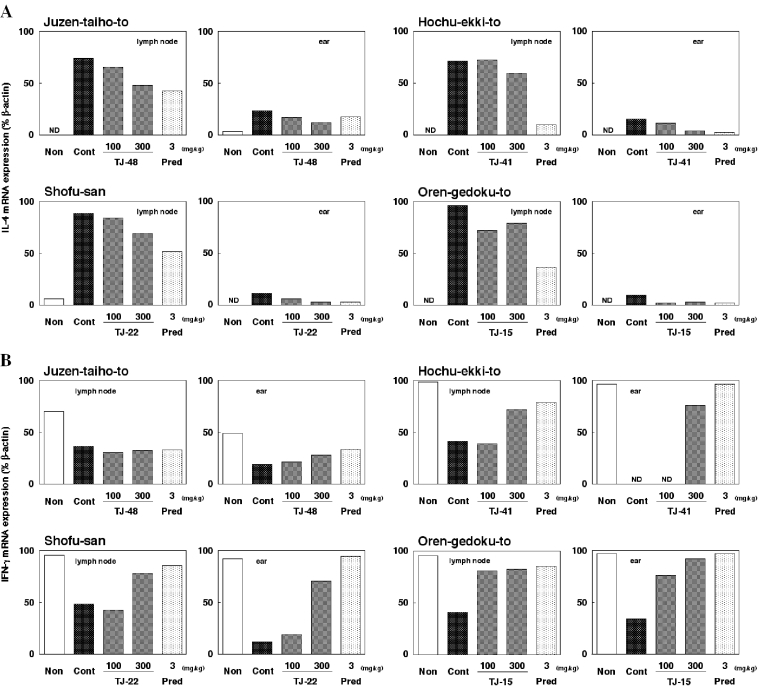
Cytokine mRNA Expression
IL-4 and IFN-γ mRNA expression in cervical lymph nodes and ears at 4 h after the fifth antigen exposure was examined. Results are shown in Fig. 4. Under the present experimental conditions, IL-4 mRNA expression was minimal or undetectable in both cervical lymph nodes and ears of non-stimulated mice. After repeated exposure of mite antigen, however, an apparent mRNA expression was induced in both tissues, although the expression in ears was relatively weak (Fig. 4A). In contrast, IFN-γ mRNA expression almost comparable to that of β-actin was detectable in mice without antigen exposure. However, repeated exposure of mite antigen resulted in an apparent depression of IFN-γ mRNA expression in both lymph nodes and ears (Fig. 4B).
Figure 4.
Application of Juzen-taiho-to, Hochu-ekki-to, Shofu-san, Oren-gedoku-to and prednisolone influence the expression of IL-4 and IFN-γ mRNA in the cervical lymph nodes and ears of NC/Nga mice treated repeatedly with mite antigen. Cervical lymph nodes and ears were obtained 4 h after the fifth antigen application. Expression of mRNA was examined using RT–PCR. Resultant electrophoretic bands were semi-quantitatively evaluated using NIH Image software. Experiments were repeated at least twice and a representative experiment is indicated. Results are expressed as a ratio of (A) IL-4 and (B) IFN-γ to β-actin mRNA expression. Non, no antigen; Cont, control; TJ-48, Juzen-taiho-to; TJ-41, Hochu-ekki-to; TJ-22, Shofu-san; TJ-15, Oren-gedoku-to; Pred, prednisolone; ND, not detected.
Administration of all four Kampo medicines tended to inhibit the increased expression of IL-4 mRNA in both cervical lymph nodes and ears (Fig. 4A). In contrast, Hochu-ekki-to, Shofu-san and Oren-gedoku-to clearly recovered the depressed expression of IFN-γ mRNA in both tissues. In the experiment with Juzen-taiho-to, partial recovery of IFN-γ mRNA expression was observed in the ear but not in cervical lymph nodes (Fig. 4B). Similarly, prednisolone inhibited the increase in IL-4 mRNA expression and the decrease in IFN-γ mRNA expression.
Histopathologic Data Clearly Show that Kampo Medicines Inhibited Inflammatory Reactions
Histopathological specimens were prepared using mouse ear lobes separated 24 h after the fifth antigen exposure. Histological pictures are shown in Fig. 5. Repeated mite-antigen exposure caused potent inflammatory changes, such as thickening of the epidermis, fibrosis in the dermis and accumulation of inflammatory cells such as lymphocytes, eosinophils and neutrophils in the ear tissues (Fig. 5B). Administration of all four Kampo medicines clearly inhibited the signs of inflammation (Fig. 5C–J). Prednisolone also clearly inhibited the inflammation (Fig. 5K).
Figure 5.
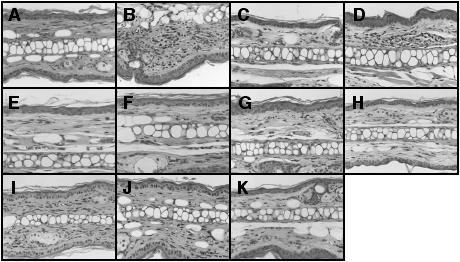
Application of Juzen-taiho-to, Hochu-ekki-to, Shofu-san, Oren-gedoku-to and prednisolone initiates histological changes in ear tissues of NC/Nga mice treated repeatedly with mite antigen. Mouse ears were separated 24 h after the fifth antigen application. Tissue sections were stained with hematoxylin–eosin. (A) no antigen; (B) control; (C) Juzen-taiho-to 100 mg/kg; (D) Juzen-taiho-to 300 mg/kg; (E) Hochu-ekki-to 100 mg/kg; (F) Hochu-ekki-to 300 mg/kg; (G) Shofu-san 100 mg/kg; (H) Shofu-san 300 mg/kg; (I) Oren-gedoku-to 100 mg/kg; (J) Oren-gedoku-to 300 mg/kg; (K) prednisolone 3 mg/kg.
Discussion
In the present study, we investigated the effects of four Kampo medicines, Juzen-taiho-to, Hochu-ekki-to, Shofu-san and Oren-gedoku-to on the atopic dermatitis model in mice, and found that all were able to inhibit mite antigen-induced allergic dermatitis in NC/Nga mice and correct the Th1/Th2 balance skewed to Th2. It is this correction of the Th1/Th2 imbalance that seems to play a role in inhibiting dermatitis. Similar results were also obtained for prednisolone.
Kampo medicines are used primarily to correct a patient's constitution, with the aim of curing a disease (9,10). This approach is in clear contrast to that of modern Western medicines, which are targeted to a symptom or an organ directly. Although the usage of Kampo medicines is not disease-oriented, the approach seems to be beneficial for the treatment of chronic diseases including atopic dermatitis. Recently, some Kampo medicines, such as Juzen-taiho-to, Hochu-ekki-to, Shofu-san and Oren-gedoku-to have shown promise in treating atopic dermatitis, although no literature in English on their clinical efficacy is available at present. Juzen-taiho-to and Hochu-ekki-to are typical prescriptions used to aid recovery from an exhausted or weakened condition. Shofu-san is a unique prescription used for eczematous skin diseases accompanying pruritus. Oren-gedoku-to is prescribed to alleviate fever and is used for both acute and chronic phases of various diseases. Some of the herbs involved in these prescriptions, such as Glycyrrhizae Radix, Angelicae Radix, Bupleuri Radix, Scutellariae Radix and Coptidis Rhizoma (Table 1), or their constituents, are actually reported to exhibit modulating activities on inflammation and immunity (18–24).
In the present study, we examined these four named Kampo medicines using a recently established allergic dermatitis model (13). Repeated mite antigen application coupled with previous tape stripping produces a dermatitis possessing a Th2-dominant background in NC/Nga mice. Elevation of serum IgE level, and induction or potentiation of IL-4 mRNA expression and depression of IFN-γ mRNA expression in cervical lymph nodes and ear lobes as well as potent ear swelling were observed. The type of dermatitis induced reflects some of the characteristic features of atopic dermatitis and thus this model seems to be appropriate for the basic study of atopic dermatitis. Daily oral administration of the four Kampo medicines apparently inhibited the formation of the ear swelling and inflammatory changes in the ear lesion. It is interesting to note that these Kampo medicines inhibited the increase in IL-4 mRNA expression and decrease in IFN-γ mRNA expression caused by mite-antigen exposure. Although the inhibition of IL-4 mRNA expression was partial, recovery of IFN-γ mRNA expression was apparent, except for the experiment with Juzen-taiho-to. In the results of Juzen-taiho-to, IFN-γ mRNA expression in mice without antigen-treatment (non) was relatively low, and recovery of depressed expression in prednisolone-administered mice was not apparent. Although the reason has not yet been determined, the positive effect of Juzen-taiho-to on the depressed expression of IFN-γ mRNA may be underestimated, similar to that of prednisolone. These results strongly suggest that the Kampo medicines examined in the present study are able to correct the Th1/Th2 balance skewed to Th2, and that this activity contributes to the inhibition of the dermatitis in NC/Nga mice.
In 1999, Iijima et al. (25) reported that Juzen-taiho-to and its component, Hoelen, shift the Th1/Th2 balance toward the Th1 dominant state in ovalbumin-sensitized old BALB/c mice. Furthermore, Matsumoto and Yamada (26) examined the influence of oral administration of Juzen-taiho-to on the concanavalin A-stimulated cytokine production in mouse lymphocytes and reported that the ratio of produced IFN-γ and IL-4 is shifted to Th1 dominant in mesenteric lymph node cells and Peyer's patch cells. These reports suggest that Juzen-taiho-to could modulate the Th1/Th2 balance and support our present results on Juzen-taiho-to. On the other hand, Hochu-ekki-to is also reported to regulate the Th1/Th2 balance in sensitized mice (27). Hochu-ekki-to inhibits IgE production as well as IL-4 production (28,29), but enhances IFN-γ production (30,31). Furthermore, not only IgE production but also spontaneous dermatitis in NC/Nga mice is inhibited by Hochu-ekki-to (32). Therefore, the present results on Hochu-ekki-to coincide well with these earlier results.
In contrast to Juzen-taiho-to and Hochu-ekki-to, modulating effects of Shofu-san and Oren-gedoku-to on Th1 and Th2 responses, and their balance, have rarely been investigated, although some anti-allergic and ant-inflammatory effects have been reported (33–36). In this study, we have indicated that these two Kampo medicines exhibit an activity which corrects the Th1/Th2 balance skewed to Th2 in mite antigen-treated NC/Nga mice, similar to Juzen-taiho-to and Hochu-ekki-to.
Juzen-taiho-to and Hochu-ekki-to inhibited the elevation of serum IgE levels as well as the dermatitis in NC/Nga mice. Actually, as mentioned above, many investigators have recognized the inhibitory effect of Hochu-ekki-to on IgE production (28,29,32). In contrast, Shofu-san and Oren-gedoku-to did not affect IgE levels, suggesting that the correction of Th2-skewed Th1/Th2 balance may not be a direct cause for the inhibition of IgE production under the experimental conditions described herein. Similarly, inhibition of IgE production by prednisolone was inconsistent, in spite of its apparent inhibition of dermatitis and correction of the Th1/Th2 imbalance. NC/Nga mice produce an atopic dermatitis-like skin lesion spontaneously accompanied by elevated serum IgE (32,37). It has been suggested that NC/Nga mice possess some genetic factors that facilitate the induction of IgE production. The factors seem to be independent of genetic factors that facilitate the formation of dermatitis (38). Therefore, regulation of IgE production in NC/Nga mice may be complicated. In contrast to the IgE production, the four Kampo medicines examined here inhibited the dermatitis and corrected the Th1/Th2 imbalance in NC/Nga mice, suggesting that the inhibition of dermatitis may have some correlation to the correction of Th1/Th2 imbalance.
In conclusion, the four Kampo medicines, Juzen-taiho-to, Hochu-ekki-to, Shofu-san and Oren-gedoku-to, that have been successfully applied in treating atopic dermatitis, inhibit the dermatitis and inflammatory changes in the lesions and correct the Th1/Th2 balance skewed to Th2 in mite antigen-treated NC/Nga mice. The correcting activity of Kampo medicines for the Th1/Th2 imbalance seems to contribute to the inhibition of dermatitis in NC/Nga mice. This activity may also play a role in their effectiveness for treating atopic dermatitis. Furthermore, the mite antigen-induced allergic dermatitis model in NC/Nga mice seems to be useful for the basic study of atopic dermatitis.
Acknowledgments
The authors would like to thank Tsumura & Co. (Tokyo, Japan) for providing the extracts of Kampo medicines and their HPLC profiles.
References
- 1.Furue M, Furukawa F, Hide M, Takehara K. Guidelines for therapy for atopic dermatitis 2004. Jpn J Dermatol. 2004;114:135–42. [Google Scholar]
- 2.Wahlgren CF. Pathophysiology of itching in urticaria and atopic dermatitis. Allergy. 1992;47:65–75. doi: 10.1111/j.1398-9995.1992.tb05091.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman DR, Yamamoto FY, Geller B, Haddad Z. Specific IgE antibodies in atopic eczema. J Allergy Clin Immunol. 1975;55:256–67. [Google Scholar]
- 4.Sampson HA, Albergo R. Comparison of results of prick skin tests, RAST, and double-blind placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 1984;74:26–33. doi: 10.1016/0091-6749(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 5.Barnetson RS, White AD. The use of corticosteroids in dermatological practice. Med J Aust. 1992;156:428–31. doi: 10.5694/j.1326-5377.1992.tb139850.x. [DOI] [PubMed] [Google Scholar]
- 6.Leung AK, Barber KA. Managing childhood atopic dermatitis. Adv Ther. 2003;20:129–37. doi: 10.1007/BF02850199. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa H, Etoh T, Ishibashi Y, et al. Tacrolimus ointment for atopic dermatitis. Lancet. 1994;344:883. doi: 10.1016/s0140-6736(94)92855-x. [DOI] [PubMed] [Google Scholar]
- 8.Bieber T. Topical tacrolimus (FK 506): a new milestone in the management of atopic dermatitis. J Allergy Clin Immunol. 1998;102:555–7. doi: 10.1016/s0091-6749(98)70270-2. [DOI] [PubMed] [Google Scholar]
- 9.Shih FJ. Concepts related to Chinese patients' perceptions of health, illness and person: issues of conceptual clarity. Accid Emerg Nurs. 1996;4:208–15. doi: 10.1016/s0965-2302(96)90086-7. [DOI] [PubMed] [Google Scholar]
- 10.Meng A. Principles of traditional Chinese medicine. Wien Med Wochenschr. 2000;150:310–6. [PubMed] [Google Scholar]
- 11.Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther. 2000;86:191–8. doi: 10.1016/s0163-7258(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 12.Koo J, Desai R. Traditional Chinese medicine in dermatology. Dermatol Ther. 2003;16:98–105. doi: 10.1046/j.1529-8019.2003.01617.x. [DOI] [PubMed] [Google Scholar]
- 13.Gao XK, Nakamura N, Fuseda K, Tanaka H, Inagaki N, Nagai H. Establishment of an allergic dermatitis in NC/Nga mice as a model for severe atopic dermatitis. Biol Pharm Bull. 2004;27:1376–81. doi: 10.1248/bpb.27.1376. [DOI] [PubMed] [Google Scholar]
- 14.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 15.McGeady SJ. Immunocompetence and allergy. Pediatrics. 2004;113:1107–13. [PubMed] [Google Scholar]
- 16.Japanese Association for Laboratory Animal Science. Guideline for animal experimentation. Exp Anim. 1987;36:285–8. [Google Scholar]
- 17.Koda A, Inagaki N, Tsuruoka N, et al. Specific suppression of antigen-antibody reactions by a dialysate from Dermatophagoides farinae. J Pharmacobio Dyn. 1987;10:104–11. doi: 10.1248/bpb1978.10.104. [DOI] [PubMed] [Google Scholar]
- 18.Hsiang CY, Lai IL, Chao DV, Ho TY. Differential regulation of activator protein 1 activity by glycyrrhizin. Life Sci. 2002;70:1643–56. doi: 10.1016/s0024-3205(01)01556-9. [DOI] [PubMed] [Google Scholar]
- 19.Wei F, Zou S, Young A, Dubner R, Ren K. Effects of four herbal extracts on adjuvant-induced inflammation and hyperalgesia in rats. J Altern Complement Med. 1999;5:429–36. doi: 10.1089/acm.1999.5.429. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh CC, Lin WC, Lee MR, et al. Dang-Gui-Bu-Xai-Tang modulate the immunity of tumor bearing mice. Immunopharmacol Immunotoxicol. 2003;25:259–71. doi: 10.1081/iph-120020474. [DOI] [PubMed] [Google Scholar]
- 21.Oka H, Ohno N, Iwanaga S, et al. Characterization of mitogenic substances in the hot water extracts of bupleuri radix. Biol Pharm Bull. 1995;18:757–65. doi: 10.1248/bpb.18.757. [DOI] [PubMed] [Google Scholar]
- 22.Koda A, Nagai H, Wada H. Pharmacological actions of baicalin and baicalein. I. On active anaphylaxis. Nippon Yakurigaku Zasshi. 1970;66:194–213. doi: 10.1254/fpj.66.194. [DOI] [PubMed] [Google Scholar]
- 23.Chi YS, Lim H, Park H, Kim HP. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression. Biochem Pharmacol. 2003;66:1271–8. doi: 10.1016/s0006-2952(03)00463-5. [DOI] [PubMed] [Google Scholar]
- 24.Ckless K, Schlottfeldt JL, Pasqual M, Moyna P, Henriques JA, Wajner M. Inhibition of in-vitro lymphocyte transformation by the isoquinoline alkaloid berberine. J Pharm Pharmacol. 1995;47:1029–31. doi: 10.1111/j.2042-7158.1995.tb03291.x. [DOI] [PubMed] [Google Scholar]
- 25.Iijima K, Sun S, Cyong JC, Jyonouchi H. Juzen-taiho-to, a Japanese herbal medicine, modulates type 1 and type 2 T cell responses in old BALB/c mice. Am J Clin Med. 1999;27:191–203. doi: 10.1142/S0192415X99000239. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Yamada H. Orally administered Kampo (Japanese herbal) medicine, “Juzen-taiho-to” modulates cytokine secretion in gut associated lymphoreticular tissues in mice. Phytomedicine. 2000;6:425–30. doi: 10.1016/S0944-7113(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 27.Ishimitsu R, Nishimura H, Kawauchi H, Kawakita T, Yoshikai Y. Dichotomous effect of a traditional Japanese medicine, bu-zhong-yi-qi-tang on allergic asthma in mice. Int Immunopharmacol. 2001;1:857–65. doi: 10.1016/s1567-5769(01)00022-4. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko M, Kishihara K, Kawakita T, Nakamura T, Takimoto H, Nomoto K. Suppression of IgE production in mice treated with a traditional Chinese medicine, bu-zhong-yi-qi-tang (Japanese name: hochu-ekki-to) Immunopharmacology. 1997;36:79–85. doi: 10.1016/s0162-3109(96)00162-2. [DOI] [PubMed] [Google Scholar]
- 29.Nakada T, Watanabe K, Matsumoto T, Santa K, Triizuka K, Hanawa T. Effect of orally administered Hochu-ekki-to, a Japanese herbal medicine, on contact hypersensitivity caused by repeated application of antigen. Int Immunopharmacol. 2002;2:901–11. doi: 10.1016/s1567-5769(02)00027-9. [DOI] [PubMed] [Google Scholar]
- 30.Yamaoka Y, Kawakita T, Kishihara K, Nomoto K. Effect of a traditional Chinese medicine, Bu-zhong-yi-qi-tang on the protection against an oral infection with Listeria monocytogenes. Immunopharmacology. 1998;39:215–23. doi: 10.1016/s0162-3109(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Tamada K, Abe K, et al. The restoration of the antitumor T cell response from stress-induced suppression using a traditional Chinese herbal medicine Hochu-ekki-to (TJ-41: Bu-Zhong-Yi-Qi-Tang) Immunopharmacology. 1999;43:11–21. doi: 10.1016/s0162-3109(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi H, Mizuno N, Kutsuna H, et al. Hochu-ekki-to suppresses development of dermatitis and elevation of serum IgE level in NC/Nga mice. Drugs Exp Clin Res. 2003;29:81–4. [PubMed] [Google Scholar]
- 33.Akamatsu H, Asada Y, Horio T. Inhibitory effect of shofu-san, a Japanese kampo medicine, on neutrophil functions in vitro. Am J Clin Med. 1998;26:57–64. doi: 10.1142/S0192415X98000087. [DOI] [PubMed] [Google Scholar]
- 34.Nose M, Sakushima J, Harada D, Ogihara Y. Comparison of immunopharmacological actions of 8 kinds of kampo-hozai clinically used in atopic dermatitis on delayed-type hypersensitivity in mice. Biol Pharm Bull. 1999;22:48–54. doi: 10.1248/bpb.22.48. [DOI] [PubMed] [Google Scholar]
- 35.Wang LM, Yamamoto T, Wang XX, et al. Effects of oren-gedoku-to and unsei-in, Chinese traditional medicines, on interleukin-8 and superoxide dismutase in rats. J Pharm Pharmacol. 1997;49:102–4. doi: 10.1111/j.2042-7158.1997.tb06760.x. [DOI] [PubMed] [Google Scholar]
- 36.Fukutake M, Miura N, Yamamoto M, et al. Suppressive effect of the herbal medicine Oren-gedoku-to on cyclooxygenase-2 activity and azoxymethane-induced aberrant crypt foci development in rats. Cancer Lett. 2000;157:9–14. doi: 10.1016/s0304-3835(00)00432-8. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda H, Watanabe N, Geba GP, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–6. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 38.Tsidzuki M, Watanabe N, Wada A, Nakane Y, Hiroi J, Matsuda H. Genetic analyses for dermatitis and IgE hyperproduction in the NC/Nga mouse. Immunogenetics. 1997;47:88–90. doi: 10.1007/s002510050330. [DOI] [PubMed] [Google Scholar]