Abstract
Addition of nitric oxide (NO) donors to mitogen-activated human immunodeficiency virus type 1 (HIV-1)-infected peripheral blood mononuclear cultures produced a significant increase in virus replication, and this effect was not associated with a change in cell proliferation. This effect was only observed with T-tropic X4 or X4R5 virus but not with R5 virus. Moreover, HIV-1 replication in mitogen-stimulated cultures was partially prevented by the specific inhibitors of the inducible nitric oxide synthase (iNOS). NO donors also enhanced HIV-1 infection of the human T-cell lines, Jurkat and MT-2. We have also observed that NO leads to an enhancement of HIV-1 replication in resting human T cells transfected with a plasmid carrying the entire HIV-1 genome and activated with phorbol ester plus ionomycin. Thus, in those cultures NO donors strongly potentiated HIV-1 replication in a dose-dependent manner, up to levels comparable to those with tumor necrosis factor alpha (TNF-α) stimulation. Furthermore, iNOS inhibitors decreased HIV-1 replication in HIV-1-transfected T cells to levels similar to those obtained with neutralizing anti-TNF-α antibodies. Moreover, HIV-1 replication induced iNOS and TNF-α transcription in T cells and T-cell lines. Interestingly, NO donors also stimulated long terminal repeat (LTR)-driven transcription whereas iNOS inhibitors partially blocked TNF-α-induced LTR transcription. Therefore, our results suggest that NO is involved in HIV-1 replication, especially that induced by TNF-α.
Nitric oxide (NO) is a free-radical gas produced by many cell types (22, 36). NO is synthesized from l-arginine by a family of three nitric oxide synthase (NOS) proteins: neuronal NOS (designated nNOS or NOS1), endothelial NOS (designated eNOS or NOS3), and inducible NOS (iNOS, also known as NOS2) (32). Cytokines and other proinflammatory stimuli induce this last enzyme (37). NO has a diverse repertoire of important functions (reviewed in references 8, 19, 41, and 42). Among those, NO acts as a neurotransmitter and as a regulator of blood pressure or vasodilation. Besides, NO has antiplatelet, tumoricidal, and microbicidal activities. It is also associated with several important pathological situations (29, 36, 46, 62).
More recently, NO has been shown to modulate immune functions (30, 41, 64). However, contrasting effects of NO, either activating or inhibiting immune cell activation, proliferation, cytokine synthesis, and cytokine signaling have been described (7). Thus, NO has been described as upregulating proliferation and increasing glucose uptake by T lymphocytes (35), whereas other reports have shown that NO inhibits T-cell activation (3, 5, 65).
NO also has controversial effects on cytokine synthesis. The synthesis of tumor necrosis factor α (TNF-α) is increased in human peripheral blood mononuclear cells (PBMC) (35) and lipopolysaccharide-stimulated neutrophil preparations (61) by exogenous NO. Endogenously produced NO was required for interleukin 12 (IL-12) production (56), whereas in other reports, exogenous NO decreased IL-12 production by macrophages (30). Interestingly, in iNOS knockout mice or in normal animals treated with iNOS inhibitors, it has been shown that IL-6 and granulocyte colony-stimulating factor mRNA production is decreased (28). Recently, NO has been shown to be involved in cytokine signaling, since in iNOS-deficient mice some cytokine signaling is lost. Thus, a functional iNOS is required for IL-12 regulation of T-cell proliferation and activation as well as for natural killer cell activation by alpha/beta interferon (15).
Although initially cyclic GMP was proposed as a second messenger of NO activity, recent findings have shown the existence of cyclic GMP-independent signal transduction pathways for NO (31). Thus, treatment of cell membranes with NO decreases cyclic AMP production by inhibiting calmodulin activation of adenylate cyclase type 1, presumably through thiol nitrosylation at the calmodulin-binding site (17). NO also increases TNF-α production in differentiated U937 cells by decreasing cyclic AMP (63). Several reports have shown a role for the activation of multiple mitogen-activated protein kinases (33–35), tyrosine kinases such as p56lck (35) or phosphatidylinositol 3-kinase (14), and p21ras (34) in the signaling pathways involved in the cellular response to NO. NO also induces nuclear translocation of the transcription factor NF-κB (35). Interestingly, iNOS knockout mice or normal animals treated with iNOS inhibitors have decreased NF-κB in vivo and STAT3 (for signal transducer and activator of transcription 3) activation upon inflammation, indicating that iNOS is important in controlling the levels of those transcription factors involved in T-cell activation (28). In addition, in natural killer cells iNOS is required for Tyk2 activation (15). However, other reports have shown that exogenous NO suppresses cytokine signaling by inhibiting various Jak/STAT pathways (5, 16). Thus, a direct interaction between NO and the Jak3/STAT5 signaling pathway in T cells has been described as responsible for the inhibition of T-cell proliferation. NO can reduce tyrosine phosphorylation of Jak3 and STAT5, thereby inactivating this signaling pathway (5). NO has been described as modulating apoptosis. Again, opposite effects have been described, with NO able to promote (27) or to prevent (57) apoptosis. The effect of NO in apoptosis may involve alteration of p53 levels (21, 40).
Human immunodeficiency virus type 1 (HIV-1)-infected patients often show elevated circulating levels of proinflammatory cytokines, particularly TNF-α and IL-6 (18, 26). Proinflammatory cytokines have been shown to be powerful activators of HIV-1 replication (20). In addition, those cytokines, produced as a result of cell activation, can be expected to stimulate iNOS in autocrine or paracrine fashion.
The importance of NO production in HIV-1 infection has already been established. HIV-1 infection has been associated with the accumulation of NO metabolites, nitrate and nitrite, in patients with central nervous system complications (23). On the other hand, other reports have shown an association between high levels of virus load and increased production of NO in the serum of HIV-1-infected patients (25, 60). So, production of large amounts of NO by macrophages has been proposed as a cause leading to the inactivation of lymphocytes and to the induction of a persistent immunosuppression (25, 59). In addition, it has been proposed that activation of NOS activity in the brain by gp120 envelope protein or by proinflammatory cytokines might explain the neurotoxicity of the virus (12, 44).
Recently, NO production and iNOS expression induced by HIV-1 infection in macrophages (9, 10) or neuroblastoma cell lines (49) have been described. However, neither high NO levels nor inhibition of NO synthesis seems to modify the outcome of virus replication in macrophages (27). In contrast, the role of NO in HIV-1-infected T cells is not clear. Here, we found that exogenous NO increases replication of HIV-1 T-tropic isolates in primary T cells or T-cell lines. More interestingly, HIV-1 infection induces iNOS expression in T cells, and iNOS inhibitors partially block HIV-1 replication, especially that induced by TNF-α. Moreover, the effect of NO seems to take place at the level of long terminal repeat (LTR) transcription.
MATERIALS AND METHODS
Reagents.
Recombinant human IL-2 was purchased from the Cetus Co.; recombinant human TNF-α (107 U/mg) was purchased from Promega (Madison, Wis.); l-NG-monomethyl-arginine (l-NMMA) and d-NG-monomethyl-arginine (d-NMMA) were purchased from Calbiochem-Behring Corp. (La Jolla, Calif.). l-N6-(1-iminoethyl)-lysine hydrochloride (l-NIL) was from Alexis Biochemicals, Laufelfinger, Switzerland. Sodium nitroprusside (SNP), S-nitroso-N-acetyl-penicillamine (SNAP), phorbol myristic acetate (PMA), phytohemagglutinin (PHA), and A23187 calcium ionophore (IONO) were purchased from Sigma Chemical Co. (St. Louis, Mo.). The neutralizing anti-TNF-α monoclonal antibody (MAb) B13.2 was developed in our laboratory. It was used in purified form obtained from ascitic fluid (52).
Cell cultures.
PBMC from healthy HIV-1-seronegative donors were isolated from whole blood by Ficoll-Hypaque (Pharmacia Fine Chemicals, Uppsala, Sweden) centrifugation and resuspended in RPMI 1640 medium (Biochrom) supplemented with 10% fetal calf serum (FCS) basically as previously described (48). Human blood macrophages were separated by adherence to plastic dishes at 37°C for 2 h. T cells were further purified by passing the nonadherent population through a nylon fiber wool column as described (48). The purity of this population (detected by flow cytometry) was always greater than 95% CD3+ cells. Purified T cells (106/ml in RPMI medium containing 10% FCS) were cultured in six-well dishes and stimulated with 1 μg of PHA/ml or 10 ng of PMA/ml plus 1 μM IONO. For in vitro infections, the cells were infected or mock infected with the following isolates at a multiplicity of infection (MOI) of 0.5: primary 2308I (rapid-high, syncytium-inducing, lymphocytotropic phenotype; X4), 3002I (rapid-high, syncytium-inducing, dualtropic phenotype; X4R5), or 1641I (rapid-high, nonsyncytium-inducing, monocytotropic phenotype; R5) or established NL4.3 (X4) and Bal (R5), in the presence of different concentrations of SNP, SNAP, l-NIL, l-NMMA, d-NMMA, or anti-TNF-α MAb where indicated. The cultures were incubated at 37°C and maintained in a humidified atmosphere containing 5% CO2. At the third, sixth, and ninth days after infection, 50% of the culture supernatants were harvested, and the wells were replenished with an equivalent volume of fresh medium containing 20 U of recombinant human IL-2/ml to maintain a viable culture, together with the same concentration of the respective reagents. None of the reagents affected the viability of the cells at the concentrations used, as indicated by the trypan blue dye exclusion test. Proliferation was measured by [3H]thymidine incorporation during the last 14 h of culture (47).
The T-cell lines (Jurkat and MT-2) were routinely grown in RPMI 1640 supplemented with 10% FCS, 1% penicillin-streptomycin and 2 mM l-glutamine at 37°C in a humidified atmosphere of 5% CO2. They were infected with HIV-1 as mentioned above for normal T cells.
HIV-1 p24 Ag assay.
Culture supernatants harvested at different times postinfection (usually 3, 6, or 9 days) were assayed for viral p24 antigen (Ag) content using an Ag capture immunoassay (Innotest HIV Antigen Multiclonal Antibody assay; Innogenetics N.V., Ghent, Belgium).
Transfection assays.
Transcriptional activity was measured using reporter assays in transiently transfected resting human T cells and Jurkat and MT-2 cell lines. The plasmid TNF-α-luc contains a region 1,311 bp upstream from the transcriptional initiation site of the human TNF-α promoter (55) and was a generous gift of J. S. Economou. The reporter pLTRWT-luc expression plasmid was a generous gift of J. L. Virelizier and has been previously described (4). It carries the U3-R junction of the LTR of the LAI strain of HIV-1 from nucleotide −644 to +78.
For transfection assays, resting T cells were resuspended in RPMI supplemented with 10% FCS and electroporated at 320 V and 1,500 μF with a Bio-Rad Gene Pulser II with 1 μg of purified plasmid(s)/106 cells (2, 46, 47). After transfection the cells were cultured at 37°C for 14 h before being activated with PMA (10 ng/ml) plus IONO (1 μM). Cells were incubated for an additional 5-h period, harvested, and lysed. Luciferase activity was measured with a luminometer and expressed as relative luciferase units, calculated as light emission from experimental samples of untransfected cells divided by that from 106 cells. Resting T cells were infected with HIV-1 by transfecting them with a plasmid containing the entire coding sequence of the NL4.3 strain of HIV-1, basically as described above.
Jurkat and MT-2 cell lines (106 cells) were transfected in Optimen medium (Life Technologies) containing 5 μg of Lipofectin (Life Technologies) and 1 μg of plasmid DNA for 24 h. After removal of the Lipofectin-containing transfection mixture, cells were resuspended in completed medium and incubated at 37°C for 24 h. Then, transfected cells were exposed to different stimuli for 5 h, and luciferase activity was measured according to the instructions of a luciferase system kit (Promega Corp.). The light emission was measured in the luminometer (Monolight 2010; Analytical Luminescence Laboratory).
Determination of iNOS mRNA.
Determination of iNOS mRNA was carried out by reverse transcription (RT)-PCR. Cells (106/ml) were incubated in RPMI 1640 supplemented with 5% (vol/vol) FCS in the presence or absence of HIV-1NL4.3. At the indicated times, cells were washed in phosphate-buffered saline, and the pellet was frozen at −70°C until further analysis. The mRNA from 105 cells was isolated using oligo(dT)-coated magnetic beads and by subsequent RT analysis (PolyAtract series 9600 mRNA isolation and cDNA synthesis system; Promega), according to the manufacturer's instructions. PCR analysis was carried out with an automatic thermal cycler (GeneAmp PCR system 9600; Perkin-Elmer).
For amplification of the desired cDNA, the following gene-specific primers were used: iNOS sense (5′-CGGTGCTGTATTTCCTTACGAGGCGAAGAAGG-3′) and iNOS antisense (5′-GGTGCTGCTTGTTAGGAGGTCAAGTAAAGGGC-3′). The reaction mixture contained 5 μl of cDNA (1/6 of the isolated cDNA), 1 μM sense and antisense primers, 200 μM deoxynucleotide triphosphates, and 2.5 U of Taq DNA polymerase (Perkin-Elmer) in a final volume of 50 μl. The cycle program was set to denature at 94°C for 45 s, to anneal at 60°C for 45 s, and to extend at 72°C for 2 min for a total of 40 cycles. Electrophoresis of the PCR products was performed with 1.5% agarose gels containing 1 μg of ethidium bromide/ml. A 100-bp DNA ladder (GIBCO BRL) was used as a molecular weight marker. Glyceraldehyde-3-phosphate dehydrogenase mRNA was amplified as a control. The agarose gels were Southern blotted to a nitrocellulose filter and then incubated with a 32P-labeled cDNA human iNOS probe as previously described (50).
Statistical analysis.
Differences between HIV-1 p24 values obtained in the different experimental conditions were analyzed using the Student t test.
RESULTS
Effect of NO on HIV-1 replication in stimulated PBMC.
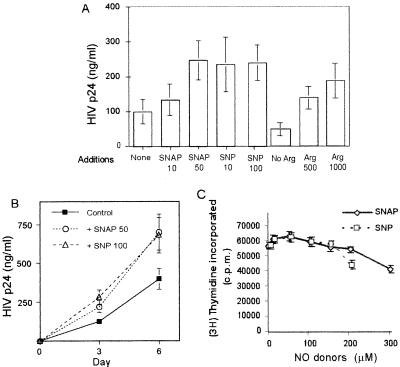
PHA-activated PBMC were infected in vitro with HIV-1NL4.3, and viral replication was monitored 3 days later by measuring HIV-1 p24 Ag formation. Although the results varied from donor to donor, the addition to the culture of different NO donors (SNP or SNAP) at low or moderate concentrations had a significant enhancing effect on HIV-1 replication (ranging from 130 to 600% of control). Figure 1A shows the average activity from four independent donors. Furthermore, this effect of the NO donors, SNP and SNAP, was observed in HIV-1-infected PBMC cultures at any time after infection (Fig. 1B). Interestingly, the replication of HIV-1 in PBMC was strongly dependent on the concentration of l-arginine (the substrate of NOS) in the culture medium (Fig. 1A). The increase in HIV-1 replication caused by NO in PHA-stimulated PBMC was associated neither with a substantial change in PBMC proliferation (Fig. 1C) nor with alterations in cell viability (data not shown). However, high concentrations of NO donors (greater than 300 μM) generally resulted in a decrease in cell viability.
FIG. 1.
Effect of NO donors and l-arginine on HIV-1 replication in PHA-activated PBMC. Human PBMC were stimulated with PHA (1 μg/ml) and infected with HIV-1. (A) Effect of NO donors and l-arginine (Arg) on viral replication. SNAP (10 or 50 μM) and SNP (10 or 100 μM) in normal medium were added to the cultures as indicated. None, control cultures in normal medium that contains 120 μM l-arginine; No Arg, cultures in medium lacking l-arginine, which was supplemented with 500 or 1,000 μM l-arginine as indicated. Three days later, HIV-1 p24 Ag in the culture supernatants was monitored. The differences are not statistically significant for 10 μM SNAP, but they are significant for 50 μM SNAP, 10 μM SNP, and 100 μM SNP (P < 0.01) with respect to the HIV-1-infected activated PBMC controls. (B) Kinetics of HIV-1 replication at 3 and 6 days after infection. Significant differences at day 3 and day 6 for 50 μM SNAP and 100 μM SNP with respect to the control (P < 0.01) were observed. HIV-1 p24 viral Ag content in the supernatants, measured by an Ag capture immunoassay, is shown. (C) Effect of NO donors on cell proliferation. Proliferation was measured by [3H]thymidine incorporation during the last 16 h of the 3-day cultures. Data shown are the means ± standard deviation (SD) of independent experiments from four blood donor samples.
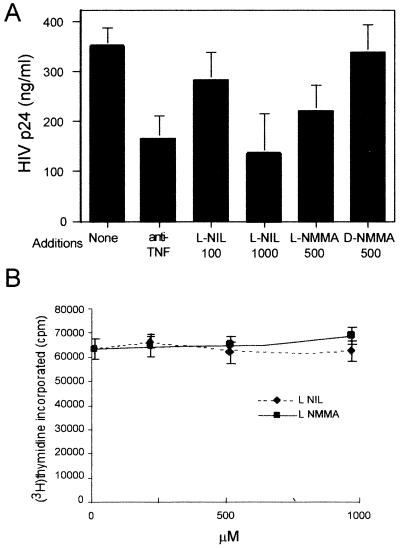
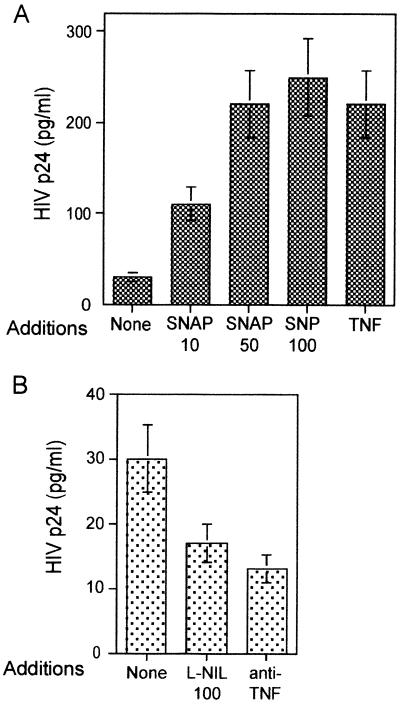
In order to study if endogenous NO played a role in HIV-1 replication, we assayed the effect of two specific and structurally unrelated NOS inhibitors on HIV-1 replication in PBMC. Results shown in Fig. 2 demonstrate that HIV-1 replication was partially prevented by the specific NOS inhibitor l-NMMA. As a control, the d-enantiomer d-NMMA, which is unable to inhibit NOS, had no effect (Fig. 2A). More interestingly, l-NIL, a highly specific inhibitor for the inducible NOS enzyme, was also an inhibitor of HIV-1 replication. Similar levels of inhibition were observed in the presence of a neutralizing anti-TNF-α MAb, in agreement with previous results (45). This effect of iNOS inhibitors was not due to a toxic effect, since they did not affect cell proliferation in the same cultures (Fig. 2B).
FIG. 2.
Effect of NOS inhibitors on HIV-1 replication in PHA-activated PBMC. Human PBMC were stimulated with PHA (1 μg/ml) and infected with HIV-1. l-NIL (100 or 1,000 μM), l-NMMA (500 μM), d-NMMA (500 μM), and anti-TNF-α (10 μg/ml) were added to the cultures as indicated. (A) Effect on HIV-1 replication. Three days after infection, cultures were assayed in triplicate for viral HIV-1 p24 Ag content in the supernatants by an Ag capture immunoassay. Differences are significant for anti-TNF-α (P < 0.01), 1,000 μM l-NIL (P < 0.01) and 500 μM l-NMMA (P < 0.05). (B) Effect on cell proliferation. Proliferation was measured by [3H]thymidine incorporation during the last 16 h of the 3-day cultures. Data shown are the means ± SD of four independent experiments from four blood donor samples.
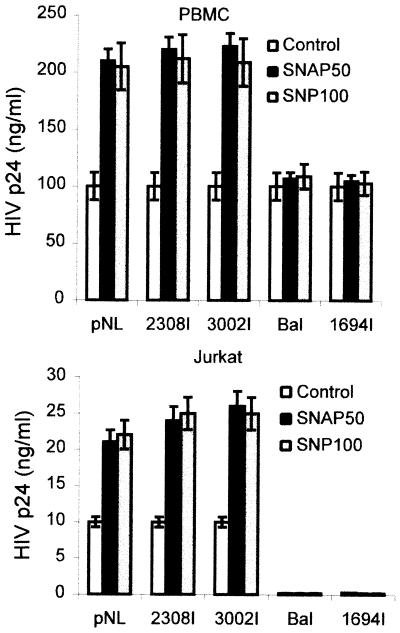
Interestingly, the enhancing effect of NO donors on HIV-1 replication was observed with X4 and X4R5 but not with R5 viral isolates (Fig. 3). This suggests that NO effect was primarily taking place in T cells, which express CXCR4, and not in macrophages.
FIG. 3.
Effect of NO donors on the replication of HIV-1 isolates with different tropism. The effect of NO donors on viral replication is shown. PBMC (A) or Jurkat cells (B) were infected with different HIV-1 strains. SNAP (50 μM) and SNP (100 μM) were added to the cultures as indicated. Three days later, HIV-1 p24 Ag was monitored in the culture supernatants. The differences of replication in the presence of NO donors are statistically significant (P < 0.01) for NL4.3, 2308I, and 3032I strains with respect to the untreated controls.
Effect of NO donors on HIV-1 replication in infected T-cell lines.
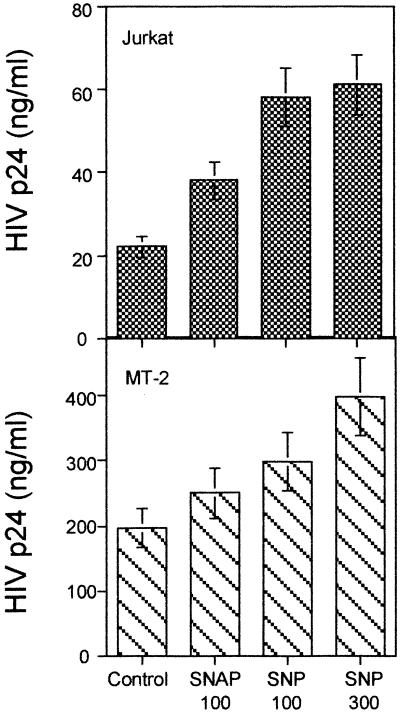
We assayed the effect of NO donors in several human T-cell lines. In those cell lines, HIV-1 replication takes place in the absence of exogenous stimuli not requiring mitogenic stimulation, thus avoiding the possible interference of NO-generating compounds with the T-cell activation process. The addition of SNAP or SNP to both Jurkat and MT-2 cell lines infected with HIV-1NL4.3 increased spontaneous HIV-1 replication, measured as HIV-1 p24 Ag 3 days after infection (Fig. 4). Similar results were found in HuT78 and CEM T-cell lines (data not shown). Besides, the enhancing effect of NO donors on HIV-1 replication on the Jurkat T-cell line was observed with T-tropic X4 virus (Fig. 3B). R5 virus did not significantly infect the Jurkat cell line, since it lacks CCR5.
FIG. 4.
Effect of NO donors on replication in infected T-cell lines. Jurkat and MT-2 cell lines infected with HIV-1 are shown. SNAP (100 μM) and SNP (100 or 300 μM) were added to the cultures as indicated. HIV-1 p24 Ag content in the supernatants was determined in triplicate cultures by an Ag capture immunoassay at day 3 after infection. Data shown are the means ± SD of triplicate cultures from three independent experiments. The differences are statistically significant (P < 0.01) for 100 μM SNAP, 100 μM SNP, and 300 μM SNP in Jurkat cells and for 100 μM SNP (P < 0.05) and 300 μM SNP (P < 0.01) in MT-2 cells with respect to the HIV-1 controls.
Effect of NO on HIV-1 replication in HIV-transfected resting T cells.
To assay the effect of NO in a system resembling in vivo resting T cells carrying the HIV-1 genome, we used resting human T cells transfected with a plasmid coding for the complete virus. The cells were then stimulated with PMA plus IONO. A direct effect of NO on HIV-1 replication in this system of transfected T cells was demonstrated by the addition of SNP or SNAP to the cultures (Fig. 5A). Thus, SNP and SNAP strongly potentiated HIV-1 replication in a dose-dependent manner. Similar higher levels of HIV-1 replication were observed when exogenous TNF-α was added to the cultures (Fig. 5A). Furthermore, addition of a highly specific inhibitor of iNOS (l-NIL) partially inhibited HIV-1 replication in pNL4.3 transiently transfected T cells. Similar levels of inhibition were observed in the presence of a neutralizing anti-TNF-α MAb, in agreement with previous published results (Fig. 5B).
FIG. 5.
Effect of NO donors and iNOS inhibitor on HIV-1 replication in T cells transfected with an HIV-1 plasmid. Cells were transfected with the pNL4.3 plasmid carrying the entire HIV-1 genome. After transfection, the cells were stimulated with PMA (10 ng/ml) plus IONO (1 μM) in the presence of SNAP (10 or 50 μM), SNP (10 μM), and TNF-α (100 U/ml) (A) or l-NIL (100 μM) or anti-TNF-α MAb (10 μg/ml) (B). At 3 days after infection, cultures were assayed in triplicate for HIV-1 p24 content in the supernatants by an Ag capture immunoassay. Data shown are the means ± SD of two independent experiments. All differences are statistically significant (P < 0.01).
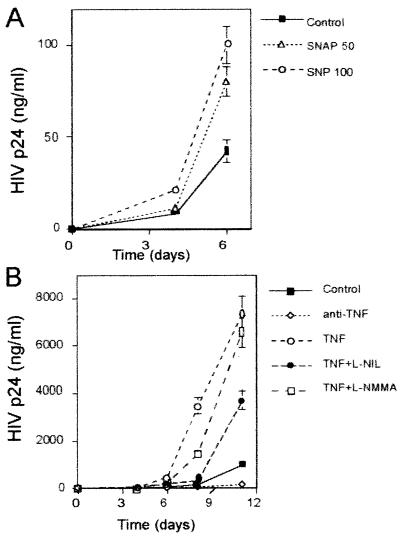
The enhancing effect of SNP and SNAP on HIV-1 replication induced by PMA plus IONO in resting T cells transfected with pNL4.3 plasmid was usually observed throughout the culture period (11 days) (Fig. 6A). We and others have described an autocrine effect of TNF-α in HIV-1 replication (20, 45). In agreement with this, induction of HIV-1 replication by PMA plus IONO in resting T cells transfected with the HIV pNL4.3 plasmid was dependent on autocrine TNF-α secretion, since anti-TNF-α strongly inhibited HIV-1 replication (up to 90%). Moreover, addition of exogenous TNF-α to the cultures significantly increased HIV-1 replication (up to 10-fold). Interestingly, l-NIL and l-NMMA partially reversed the enhancing effect of exogenous TNF-α on HIV-1 replication (Fig. 6B). The inactive enantiomer d-NMMA had no effect (data not shown).
FIG. 6.
Effect of NO donors and iNOS inhibitors on the kinetics of HIV-1 replication in T cells transfected with the pNL4.3 HIV-1 plasmid. Cells were transfected with the pNL4.3 plasmid carrying the entire HIV-1 genome. After transfection, the cells were stimulated with PMA (10 ng/ml) plus IONO (1 μM) in the presence of SNAP (50 μM) or SNP (100 μM) (A) or anti-TNF-α (10 μg/ml), TNF-α (200 U/ml), l-NIL (100 μM), and l-NMMA (500 μM) alone or in combination as indicated (B). Three, 6, 8, or 11 days after transfection, cultures were assayed in triplicate for HIV-1 p24 Ag. Data shown are the means ± SD of two independent experiments.
HIV-1 infection induces iNOS mRNA in T cells.
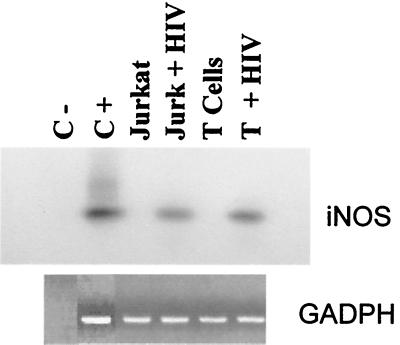
To determine whether iNOS expression in T cells and the Jurkat cell line correlates with HIV-1 infection, mRNA was extracted from HIV-1-infected and -uninfected T cells and Jurkat cells, and expression of the iNOS gene was analyzed by RT-PCR using iNOS-specific primers (Fig. 7). iNOS transcripts were undetectable in uninfected T cells and in the Jurkat cell line, even in Southern blotted gels. In contrast, HIV-infected T cells and Jurkat cells expressed iNOS mRNA. This effect seems to require productive infection, since a 100-fold-higher dose of heat-inactivated virus had no effect on iNOS mRNA expression by T cells (data not shown). MT-2 cells expressed iNOS mRNA even in the absence of HIV infection (data not shown), in agreement with a recent report (43).
FIG. 7.
Induction of iNOS mRNA by HIV-1 in T cells and Jurkat cells. (Top) T cells and Jurkat cells were infected or mock infected with HIV-1 where indicated. One day later, iNOS mRNA was detected by RT-PCR with specific primers and Southern blotted with a human iNOS-specific probe. Shown are the results of a representative experiment. A positive control sample from a human cell line expressing iNOS mRNA (C+) and a negative control with no cells (C−) are also shown. (Bottom) A control of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA is shown.
HIV-1 replication induces TNF-α transcription in T-cell lines.
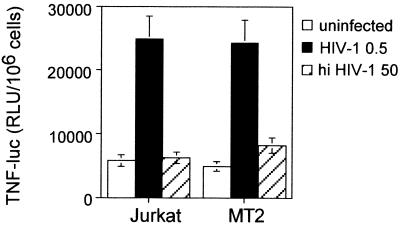
To test the effect of HIV-1 infection on TNF-α production in Jurkat and MT-2 cell lines, we transiently transfected the TNF-α-luc plasmid into both T-cell lines. Again, we observed an enhancing effect after adding infectious virus but not after a 100-fold-higher dose of heat-inactivated HIV-1, indicating that this effect requires infectious HIV-1 (Fig. 8). Thus, HIV-1 infection induces both iNOS and TNF-α transcription.
FIG. 8.
Activation of the TNF-α promoter by HIV-1 infection in Jurkat and MT-2 cells. Cell lines were transfected with the reporter plasmid TNF-α-luc. After transfection, cells were infected with HIV-1 (MOI, 0.5) (HIV-1 0.5) or treated with heat inactivated HIV-1 (MOI, 50) (hi HIV-1 50). Luciferase activity in relative light units (RLU) was determined 16 h later. Data shown are the means ± SD of two independent experiments.
NO donors activate the transcription of HIV-1 LTR.
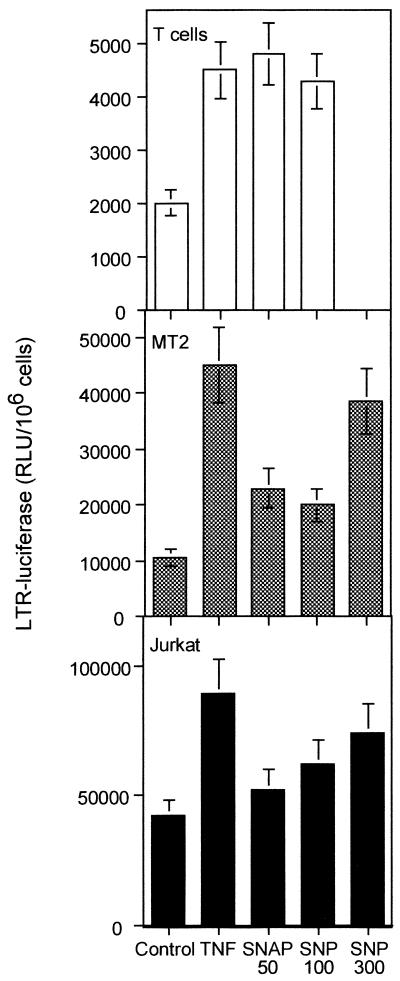
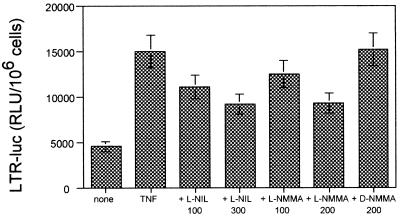
To test if the effect of NO was taking place at the transcriptional level, we transiently transfected the LTR-luc plasmid into resting T cells or MT-2 or Jurkat T-cell lines. Then, the cells were stimulated with TNF-α or different NO donors (SNAP and SNP). NO donors were able to increase transcription of HIV LTR in the three types of cells, albeit to different degrees (Fig. 9). More interestingly, both iNOS inhibitors, l-NIL and l-NMMA, partially inhibited (up to 60% after subtraction of the unstimulated response) LTR-dependent transcription induced by TNF-α in a dose-response manner (Fig. 10). As a control, the inactive enantiomer d-NMMA had no effect.
FIG. 9.
Effect of NO donors on LTR transcription. Resting human T cells and Jurkat and MT-2 cell lines were transfected with LTR-luc. After 14 h, the cells were stimulated in the presence of TNF-α (200 U/ml), SNAP (50 μM), and SNP (100 or 300 μM) as indicated. Luciferase activity in relative light units (RLU) was determined 5 h later. Data shown are the means ± SD of two independent experiments.
FIG. 10.
Effect of iNOS inhibitors on TNF-α-induced LTR-driven transcription. Jurkat cells were transfected with LTR-luc. After transfection, cells were stimulated with TNF-α (200 U/ml) in the presence or absence of l-NIL (100 or 300 μM), l-NMMA (100 or 200 μM) or d-NMMA (200 μM), and 5 h later luciferase activity in relative light units (RLU) was determined. Results are the means ± SD of two independent experiments. There are statistically significant differences with respect to the TNF-α alone: l-NIL 100 (P < 0.05), l-NIL 300 (P < 0.01), l-NMMA-100 (P < 0.05), and l-NMMA-200 (P < 0.01).
DISCUSSION
NO is a pleiotropic mediator that has been shown to have multiple physiological activities. Here, we have shown that NO acts as an autocrine factor that mediates HIV-1 replication. Thus, several NO-generating compounds at low to moderate concentrations were able to activate HIV-1 replication in normal T cells as well as in human T-cell lines. Although we found variations from donor to donor of PBMC, we always observed stimulation of HIV-1 replication. Since NO is a highly reactive gas, these differences may be explained by the redox status of the cells from the different human blood donors. Besides, the concentrations of the NO donors used are also important, since higher concentrations were inhibitory, probably because they cause apoptosis (27). More interestingly, two structurally unrelated inhibitors of iNOS, l-NIL and l-NMMA, partially abrogated HIV-1 replication induced by PHA, PMA plus IONO, or TNF-α.
At the molecular level, NO seems to act through activation of LTR-mediated transcription. The reduction observed with iNOS inhibitors l-NIL and l-NMMA on TNF-α-dependent LTR transcription and HIV-1 replication indicates that endogenous NO production plays a role in controlling LTR and HIV-1 replication induced by this cytokine. This effect of NO on the LTR may be due to its reported ability to activate NF-κB in T cells (35) and neural cells (49). Since NF-κB activity is absolutely required for LTR transcription in T cells (2), an enhancing effect of NO on NF-κB activity may explain the increase in HIV-1 replication observed in infected T cells. In support of this, there is increasing evidence that NO derived from iNOS may be involved in controlling some aspects of immune activation. Thus, iNOS knockout mice are defective in NF-κB activity induced by inflammation (28). Our results suggest that iNOS is also involved in some aspects of TNF-α-mediated signaling.
However, our results are contradictory with a previous report that has shown the opposite effect of NO on the LTR (58), although it is noteworthy that the same authors have shown that NO reactivates virus in latently infected cells. In addition, it has been reported that the NO donor, S-nitrosoglutathione (GSNO), decreases viral replication in PBMC (39). The reasons for the discrepancies with our results are unknown, but they may depend on the different NO donors used that varied in the speed and amount of NO released. In this regard, several lines of evidence have shown that NO may have apparently contradictory effects. Thus, NO has been shown to either promote or prevent apoptosis (66). An explanation for all those conflicting results might be related to the different fluxes of NO used in these experiments. In general, low concentrations of NO donors or NO gas are sufficient to activate NF-κB (35). On the other hand, high NO output rates, 2 to 4 μM NO2−/h, were found to be inhibitory (58). In agreement with that hypothesis, the two NO donors used in our studies, SNAP and SNP, have been previously shown to activate NF-κB in T cells (35). Besides, our results with NO donors have been demonstrated over a long period of culture and have been obtained with low to moderate concentrations of NO. At those concentrations, no effect on cell proliferation or cell survival was observed. In contrast, others have shown that high concentrations of NO (as those supplied by GSNO) inhibit NF-κB activation (13, 51, 58) and HIV-1 replication (39). At those concentrations, NO has been shown to reduce cell proliferation and to cause apoptosis (3, 65). Therefore, it is likely that high rates of NO flux are inhibiting where low to moderate rates are able to activate NF-κB and, subsequently, LTR-dependent transcription. More importantly, the specific reduction observed with iNOS inhibitors l-NIL and l-NMMA of HIV-1 replication and TNF-α-dependent LTR transcription indicates that endogenous NO production plays a role in controlling LTR activation and HIV-1 replication. Those results are important, since they avoid the controversy among the different studies mentioned above regarding the different NO donors and concentrations used.
On the other hand, our results indicate that HIV-1 infection was able to induce iNOS in T cells and T-cell lines. There are three different NOS enzymes, one of them (iNOS) being inducible by cytokines such as TNF-α and other proinflammatory stimuli in macrophages and several other cell types (37). However, reports of the expression of NOS enzymes in T cells are scarce. Thus, expression of neuronal NOS (65) and endothelial NOS (54) in T cells has been reported. In this regard, it is worth mentioning that T-cell lines express iNOS after infection with another retrovirus, human T-cell leukemia virus type 1 (HTLV-1) (24, 43). Moreover, this effect was shown to depend on HTLV-1 Tax. In agreement with that, the MT-2 cell line used in our studies constitutively expresses HTLV-1 Tax as well as iNOS (data not shown). Recently, HIV-1 Tat has also been shown to induce iNOS in macrophages (11). Therefore, it is tempting to speculate that Tat may be involved in the observed effect on iNOS induction by HIV-1 infection.
Previous reports have demonstrated that HIV-1 infection induces iNOS in macrophages (10) and in neural cells (1, 49), thus supporting the ability of HIV-1 to induce iNOS expression. In contrast, Hermann et al. have found no increases in nitrite accumulation (a measure of NO production) upon HIV-1 infection of PBMC or macrophages (27) However, nitrite accumulation requires a large amount of NO production, and it is well known that human cells are poor producers of NO (37). Therefore, measurement by RT-PCR is a much more sensitive way than nitrite accumulation to detect iNOS induction.
Increased TNF-α production has been described upon productive HIV-1 infection of several cell types, and it has been ascribed to HIV-1 Tat protein (6). In agreement with this, we have found that HIV-1 productive infection induces the transcription of the TNF-α promoter in HIV-1-infected T cells. On the other hand, previous reports have shown that NO increases TNF-α production in monocytes and neutrophils (35, 61, 63). Since TNF-α augmented HIV-1 replication in our cultures, it was theoretically possible that induction of TNF-α by NO donors contributes to their enhancing effect on HIV-1 replication. Although we have not specifically addressed that effect in our system and therefore we cannot discard it, we think that this is unlikely, since NOS inhibitors prevented TNF-α-mediated induction of HIV-1 replication and LTR-dependent transcription. This suggests that NO plays a role downstream of the signal transduction pathways induced by TNF-α.
We and others have previously shown that in T cells HIV-1 replication is dependent on autocrine TNF-α production (20, 45). Since iNOS inhibitors partially inhibited TNF-α activation in HIV-1-transfected T cells, this can be taken as an indication that TNF-α mediates its activity on HIV-1 replication, at least partially through NO production. Thus, it is likely that HIV-1 infection and/or T-cell activation induces TNF-α, which in turn induces iNOS in T cells.
In PBMC cultures, HIV-1 can infect both T cells and macrophages, although the enhancing effect of NO was observed with X4 but not with R5 virus. This, together with the NO-enhancing effect in T-cell lines, leads us to believe that the effect of NO observed in PBMC is at the T-cell level. In support of this, neither NO donors nor NOS inhibitors have an effect on HIV-1 replication in macrophages (27). An explanation might be that CXCR4, the receptor of T-tropic virus, supplies different signals than CCR5 that in turn activate iNOS. However, we think this is unlikely, since heat-inactivated virus able to bind CCR4 did not induce iNOS (data not shown) or TNF-α activation. Rather, we believe that it is an intrinsic property of T cells in which HIV-1 proteins (i.e., Tat) induce NF-κB and subsequently iNOS, as has been described for HTLV Tax protein (43). Besides, macrophages usually produce higher levels of NO than T cells (32, 37), and this may result in inhibition, rather than activation, of HIV-1 replication in those cells. On the contrary, in T cells the smaller amounts of NO produced may be beneficial for the virus. Additional interpretations cannot be ruled out. For example, NO may induce NF-κB and therefore HIV-1 LTR only in T cells and not in macrophages. Whatever the mechanism, this fact may have important consequences, since X4 strains are associated with a poorer prognosis and with a more advanced stage of AIDS. Thus, the above data suggest that iNOS inhibitors may result in some benefit in HIV-1 infection but only in patients with X4 strains.
It is somewhat surprising that NO enhanced HIV-1 replication, since it is generally accepted that NO and related species have antiviral activity (38, 53). However, those antiviral effects have been shown to take place at high NO concentrations. In contrast, our results suggest that NO neutralization may be a target against HIV-1 infection. In this regard, the detrimental role of NO in HIV-1 infection in vivo has been extensively documented. Thus, increased nitrite levels that correlate with virus load in the sera of HIV-1-infected individuals have been shown (25, 60), especially in those suffering neurological complications (23). Moreover, NO directly induced by HIV-1 infection or by HIV-1 products such as gp120 or indirectly through HIV-1-induced cytokines such as TNF-α has been regarded as the main cause of AIDS dementia (44). In addition to those effects, we have shown here that autocrine NO may also be detrimental in HIV-1 infection by increasing HIV-1 replication in T cells. Taken together, those results indicate that NO plays a deleterious role in HIV-1 infection and suggest that NOS inhibitors may have some therapeutic benefit in AIDS treatment, most likely in combination with antiretroviral drugs.
ACKNOWLEDGMENTS
This work was supported by grants from the Programa Nacional de Salud (SAF 99-0022), the Comunidad Autonóma de Madrid, the Fondos de Investigación Sanitaria (FIS 00/0207), and the Fundación para la Investigación y Prevención del SIDA in Spain (FIPSE 3008/99) to M.A.M.-F. and by grants from the Ministerio de Educación y Cultura, the Fondo de Investigaciones Sanitarias, the Comunidad Autónoma de Madrid, FIPSE, and the Fundación Ramón Areces to M.F.
REFERENCES
- 1.Adamson D C, Wildemann B, Sasaki M, Glass J D, McArthur J C, Christov V I, Dawson T M, Dawson V L. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 2.Alcami J, Lain de Lera T, Folgueira L, Pedraza M A, Jacque J M, Bachelerie F, Noriega A R, Hay R T, Harrich D, Gaynor R B, et al. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allione A, Bernabei P, Bosticardo M, Ariotti S, Forni G, Novelli F. Nitric oxide suppresses human T lymphocyte proliferation through IFN-gamma-dependent and IFN-gamma-independent induction of apoptosis. J Immunol. 1999;163:4182–4191. [PubMed] [Google Scholar]
- 4.Bachelerie F, Alcami J, Arenzana-Seisdedos F, Virelizier J L. HIV enhancer activity perpetuated by NF-kappa B induction on infection of monocytes. Nature. 1991;350:709–712. doi: 10.1038/350709a0. [DOI] [PubMed] [Google Scholar]
- 5.Bingisser R M, Tilbrook P A, Holt P G, Kees U R. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- 6.Biswas D K, Salas T R, Wang F, Ahlers C M, Dezube B J, Pardee A B. A Tat-induced auto-up-regulatory loop for superactivation of the human immunodeficiency virus type 1 promoter. J Virol. 1995;69:7437–7444. doi: 10.1128/jvi.69.12.7437-7444.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdan C. The multiplex function of nitric oxide in (auto)immunity. J Exp Med. 1998;187:1361–1365. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bredt D S, Snyder S H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 9.Bukrinsky M, Schmidtmayerova H, Zybarth G, Dubrovsky L, Sherry B, Enikolopov G. A critical role of nitric oxide in human immunodeficiency virus type 1-induced hyperresponsiveness of cultured monocytes. Mol Med. 1996;2:460–468. [PMC free article] [PubMed] [Google Scholar]
- 10.Bukrinsky I M, Nottet H S, Schmidtmayerova H, Dubrovsky L, Flanagan C R, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F, Lu Y, Castranova V, Rojanasakul Y, Miyahara K, Shizuta Y, Vallyathan V, Shi X, Demers L M. Nitric oxide inhibits HIV tat-induced NF-kappaB activation. Am J Pathol. 1999;155:275–284. doi: 10.1016/s0002-9440(10)65121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson V L, Dawson T M, Uhl G R, Snyder S H. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci USA. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Caterina R, Libby P, Peng H B, Thannickal V J, Rajavashisth T B, Gimbrone M A, Jr, Shin W S, Liao J K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Investig. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deora A A, Win T, Vanhaesebroeck B, Lander H M. A redox-triggered ras-effector interaction. Recruitment of phosphatidylinositol 3′-kinase to Ras by redox stress. J Biol Chem. 1998;273:29923–29928. doi: 10.1074/jbc.273.45.29923. [DOI] [PubMed] [Google Scholar]
- 15.Diefenbach A, Schindler H, Rollinghoff M, Yokoyama W M, Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science. 1999;284:951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 16.Duhe R J, Evans G A, Erwin R A, Kirken R A, Cox G W, Farrar W L. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc Natl Acad Sci USA. 1998;95:126–131. doi: 10.1073/pnas.95.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duhe R J, Nielsen M D, Dittman A H, Villacres E C, Choi E J, Storm D R. Oxidation of critical cysteine residues of type I adenylyl cyclase by o-iodosobenzoate or nitric oxide reversibly inhibits stimulation by calcium and calmodulin. J Biol Chem. 1994;269:7290–7296. [PubMed] [Google Scholar]
- 18.Emilie D, Fior R, Jarrousse B, Marfaing-Koka A, Merrien D, Devergne O, Crevon M C, Maillot M C, Galanaud P. Cytokines in HIV infection. Int J Immunopharmacol. 1994;16:391–396. doi: 10.1016/0192-0561(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 19.Evans C H. Nitric oxide: what role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995;47:107–116. doi: 10.1007/978-3-0348-7343-7_9. [DOI] [PubMed] [Google Scholar]
- 20.Fauci A S. Host factors in the pathogenesis of HIV disease. Antibiot Chemother. 1996;48:4–12. doi: 10.1159/000425151. [DOI] [PubMed] [Google Scholar]
- 21.Forrester K, Ambs S, Lupold S E, Kapust R B, Spillare E A, Weinberg W C, Felley-Bosco E, Wang X W, Geller D A, Tzeng E, Billiar T R, Harris C C. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci USA. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forstermann U, Kleinert H, Gath I, Schwarz P, Closs E I, Dun N J. Expression and expressional control of nitric oxide synthases in various cell types. Adv Pharmacol. 1995;34:171–186. doi: 10.1016/s1054-3589(08)61085-6. [DOI] [PubMed] [Google Scholar]
- 23.Giovannoni G, Miller R F, Heales S J, Land J M, Harrison M J, Thompson E J. Elevated cerebrospinal fluid and serum nitrate and nitrite levels in patients with central nervous system complications of HIV-1 infection: a correlation with blood-brain-barrier dysfunction. J Neurol Sci. 1998;156:53–58. doi: 10.1016/s0022-510x(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 24.Goto H, Nakamura T, Shirabe S, Ueki Y, Nishiura Y, Furuya T, Tsujino A, Nakane S, Eguchi K, Nagataki S. Up-regulation of iNOS mRNA expression and increased production of NO in human monoblast cell line, U937 transfected by HTLV-I tax gene. Immunobiology. 1997;197:513–521. doi: 10.1016/s0171-2985(97)80083-6. [DOI] [PubMed] [Google Scholar]
- 25.Groeneveld P H, Kroon F P, Nibbering P H, Bruisten S M, van Swieten P, van Furth R. Increased production of nitric oxide correlates with viral load and activation of mononuclear phagocytes in HIV-infected patients. Scand J Infect Dis. 1996;28:341–345. doi: 10.3109/00365549609037916. [DOI] [PubMed] [Google Scholar]
- 26.Gurram M, Chirmule N, Wang X P, Ponugoti N, Pahwa S. Increased spontaneous secretion of interleukin 6 and tumor necrosis factor alpha by peripheral blood lymphocytes of human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1994;13:496–501. doi: 10.1097/00006454-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Hermann E, Idziorek T, Kusnierz J P, Mouton Y, Capron A, Bahr G M. Role of nitric oxide in the regulation of lymphocyte apoptosis and HIV-1 replication. Int J Immunopharmacol. 1997;19:387–397. doi: 10.1016/s0192-0561(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 28.Hierholzer C, Harbrecht B, Menezes J M, Kane J, MacMicking J, Nathan C F, Peitzman A B, Billiar T R, Tweardy D J. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzmann A. Nitric oxide and sepsis. Respir Care Clin N Am. 1997;3:537–550. [PubMed] [Google Scholar]
- 30.Huang F P, Niedbala W, Wei X Q, Xu D, Feng G J, Robinson J H, Lam C, Liew F Y. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur J Immunol. 1998;28:4062–4070. doi: 10.1002/(SICI)1521-4141(199812)28:12<4062::AID-IMMU4062>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Ignarro L J, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Knowles R G, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lander H M, Jacovina A T, Davis R J, Tauras J M. Differential activation of mitogen-activated protein kinases by nitric oxide-related species. J Biol Chem. 1996;271:19705–19709. doi: 10.1074/jbc.271.33.19705. [DOI] [PubMed] [Google Scholar]
- 34.Lander H M, Ogiste J S, Teng K K, Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 35.Lander H M, Sehajpal P, Levine D M, Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993;150:1509–1516. [PubMed] [Google Scholar]
- 36.Liew F Y. Regulation of lymphocyte functions by nitric oxide. Curr Opin Immunol. 1995;7:396–399. doi: 10.1016/0952-7915(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 37.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 38.Mannick J B. The antiviral role of nitric oxide. Res Immunol. 1995;146:693–697. doi: 10.1016/0923-2494(96)84920-0. [DOI] [PubMed] [Google Scholar]
- 39.Mannick J B, Stamler J S, Teng E, Simpson N, Lawrence J, Jordan J, Finberg R W. Nitric oxide modulates HIV-1 replication. J Acquir Immune Defic Syndr. 1999;22:1–9. doi: 10.1097/00042560-199909010-00001. [DOI] [PubMed] [Google Scholar]
- 40.Messmer U K, Brune B. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem J. 1996;319:299–305. doi: 10.1042/bj3190299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moilanen E, Vapaatalo H. Nitric oxide in inflammation and immune response. Ann Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- 42.Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 43.Mori N, Nunokawa Y, Yamada Y, Ikeda S, Tomonaga M, Yamamoto N. Expression of human inducible nitric oxide synthase gene in T-cell lines infected with human T-cell leukemia virus type-I and primary adult T-cell leukemia cells. Blood. 1999;94:2862–2870. [PubMed] [Google Scholar]
- 44.Muñoz-Fernández M A, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 45.Muñoz-Fernández M A, Navarro J, García A, Punzón C, Fernández-Cruz E, Fresno M. Replication of human immunodeficiency virus-1 in primary human T cells is dependent on the autocrine secretion of tumor necrosis factor through the control of nuclear factor-kappa B activation. J Allergy Clin Immunol. 1997;100:838–845. doi: 10.1016/s0091-6749(97)70282-3. [DOI] [PubMed] [Google Scholar]
- 46.Nathan C, Xie Q W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 47.Navarro J, Punzón C, Jiménez J L, Fernández-Cruz E, Pizarro A, Fresno M, Muñoz-Fernández M A. Inhibition of phosphodiesterase type IV suppresses human immunodeficiency virus type 1 replication and cytokine production in primary T cells: involvement of NF-kappaB and NFAT. J Virol. 1998;72:4712–4720. doi: 10.1128/jvi.72.6.4712-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navarro J, Punzon M C, Pizarro A, Fernandez-Cruz E, Fresno M, Munoz-Fernandez M A. Pentoxifylline inhibits acute HIV-1 replication in human T cells by a mechanism not involving inhibition of tumour necrosis factor synthesis or nuclear factor-kappa B activation. AIDS. 1996;10:469–475. doi: 10.1097/00002030-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Obregón E, Punzón C, Fernández-Cruz E, Fresno M, Muñoz-Fernández M A. HIV-1 infection induces differentiation of immature neural cells through autocrine tumor necrosis factor and nitric oxide production. Virology. 1999;261:193–204. doi: 10.1006/viro.1999.9848. [DOI] [PubMed] [Google Scholar]
- 50.Obregon E, Punzon M C, Gonzalez-Nicolas J, Fernandez-Cruz E, Fresno M, Munoz-Fernandez M A. Induction of adhesion/differentiation of human neuroblastoma cells by tumour necrosis factor-alpha requires the expression of an inducible nitric oxide synthase. Eur J Neurosci. 1997;9:1184–1193. doi: 10.1111/j.1460-9568.1997.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 51.Peng H B, Libby P, Liao J K. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 52.Pimentel-Muiños F X, Muñoz-Fernández M A, Fresno M. Control of T lymphocyte activation and IL-2 receptor expression by endogenously secreted lymphokines. J Immunol. 1994;152:5714–5722. [PubMed] [Google Scholar]
- 53.Powell K L, Baylis S A. The antiviral effects of nitric oxide. Trends Microbiol. 1995;3:81–82. doi: 10.1016/s0966-842x(00)88884-8. [DOI] [PubMed] [Google Scholar]
- 54.Reiling N, Kroncke R, Ulmer A J, Gerdes J, Flad H D, Hauschildt S. Nitric oxide synthase: expression of the endothelial, Ca2+/calmodulin-dependent isoform in human B and T lymphocytes. Eur J Immunol. 1996;26:511–516. doi: 10.1002/eji.1830260302. [DOI] [PubMed] [Google Scholar]
- 55.Rhoades K L, Golub S H, Economou J S. The regulation of the human tumor necrosis factor alpha promoter region in macrophage, T cell, and B cell lines. J Biol Chem. 1992;267:22102–22107. [PubMed] [Google Scholar]
- 56.Rothe H, Hartmann B, Geerlings P, Kolb H. Interleukin-12 gene-expression of macrophages is regulated by nitric oxide. Biochem Biophys Res Commun. 1996;224:159–163. doi: 10.1006/bbrc.1996.1000. [DOI] [PubMed] [Google Scholar]
- 57.Sciorati C, Rovere P, Ferrarini M, Heltai S, Manfredi A A, Clementi E. Autocrine nitric oxide modulates CD95-induced apoptosis in gammadelta T lymphocytes. J Biol Chem. 1997;272:23211–23215. doi: 10.1074/jbc.272.37.23211. [DOI] [PubMed] [Google Scholar]
- 58.Sekkai D, Aillet F, Israel N, Lepoivre M. Inhibition of NF-kappaB and HIV-1 long terminal repeat transcriptional activation by inducible nitric oxide synthase 2 activity. J Biol Chem. 1998;273:3895–3900. doi: 10.1074/jbc.273.7.3895. [DOI] [PubMed] [Google Scholar]
- 59.Torre D, Ferrario G. Immunological aspects of nitric oxide in HIV-1 infection. Med Hypotheses. 1996;47:405–407. doi: 10.1016/s0306-9877(96)90221-2. [DOI] [PubMed] [Google Scholar]
- 60.Torre D, Ferrario G, Bonetta G, Speranza F, Zeroli C. Production of nitric oxide from peripheral blood mononuclear cells and polymorphonuclear leukocytes of patients with HIV-1. AIDS. 1995;9:979–980. doi: 10.1097/00002030-199508000-00027. [DOI] [PubMed] [Google Scholar]
- 61.Van Dervort A L, Yan L, Madara P J, Cobb J P, Wesley R A, Corriveau C C, Tropea M M, Danner R L. Nitric oxide regulates endotoxin-induced TNF-alpha production by human neutrophils. J Immunol. 1994;152:4102–4109. [PubMed] [Google Scholar]
- 62.Vladutiu A O. Role of nitric oxide in autoimmunity. Clin Immunol Immunopathol. 1995;76:1–11. doi: 10.1006/clin.1995.1081. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Yan L, Wesley R A, Danner R L. Nitric oxide increases tumor necrosis factor production in differentiated U937 cells by decreasing cyclic AMP. J Biol Chem. 1997;272:5959–5965. doi: 10.1074/jbc.272.9.5959. [DOI] [PubMed] [Google Scholar]
- 64.Wei X Q, Charles I G, Smith A, Ure J, Feng G J, Huang F P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 65.Williams S M, Noguchi S, Henkart P A, Osawa Y. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J Immunol. 1998;161:6526–6531. [PubMed] [Google Scholar]
- 66.Xu W, Liu L, Smith G C, Charles I G. Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. Nat Cell Biol. 2000;2:339–345. doi: 10.1038/35014028. [DOI] [PubMed] [Google Scholar]