Abstract
We examined whether Brazilian green propolis, a widely used folk medicine, has a neuroprotective function in vitro and/or in vivo. In vitro, propolis significantly inhibited neurotoxicity induced in neuronally differentiated PC12 cell cultures by either 24 h hydrogen peroxide (H2O2) exposure or 48 h serum deprivation. Regarding the possible underlying mechanism, propolis protected against oxidative stress (lipid peroxidation) in mouse forebrain homogenates and scavenged free radicals [induced by diphenyl-p-picrylhydrazyl (DPPH). In mice in vivo, propolis [30 or 100 mg/kg; intraperitoneally administered four times (at 2 days, 1 day and 60 min before, and at 4 h after induction of focal cerebral ischemia by permanent middle cerebral artery occlusion)] reduced brain infarction at 24 h after the occlusion. Thus, a propolis-induced inhibition of oxidative stress may be partly responsible for its neuroprotective function against in vitro cell death and in vivo focal cerebral ischemia.
Keywords: focal cerebral ischemia, free radical, lipid peroxidation, middle cerebral artery occlusion, PC12 cell culture
Introduction
Ischemic stroke is a substantial public health problem. Indeed, it is the third leading cause of death, after heart disease and cancer, and the leading cause of long-term disability in major industrialized countries (1). A widely applicable treatment for cerebral ischemia would therefore have an enormous impact on public health: however, no such beneficial treatment has yet been found (2,3). Clinical and experimental data suggest that ischemic neuronal damage is at least partly induced by the free radicals and/or lipid peroxidation produced either during the ischemia itself or following reperfusion (4–6).
Propolis (honeybee glue), a resinous product consisting of sap, bark and bee excreta, accumulates in bee hives. It is currently used as a health food and for the treatment of various ailments. Indeed, it has been shown to have a wide range of biological activities, principally attributable to the presence of flavonoids (major component; rutin, quercetin, galangin, etc.) (7) and caffeic acid phenethyl ester (CAPE) (8). Hence, the putative therapeutic properties of propolis could be related to its antibacterial (9,10), anti-inflammatory (11), antioxidative (12,13) and/or tumoricidal (14,15) activities.
In total, at least 200 compounds have been identified in different samples of propolis, with >100 being present in any given sample. These include: fatty and phenolic acids and esters, substituted phenolic esters, flavonoids (flavones, flavanones, flavonols, dihydroflavonols, chalcones), terpenes, β-steroids, aromatic aldehydes and alcohols, and derivatives of sesquiterpenes, naphthalene and stilbenes (16–19). Propolis has a variety of botanical origins, and its chemical composition can also be variable. Baccharis dracunculifolia DC (Asteraceae), a native plant from Brazil, is the most important botanical source of Southeastern Brazilian propolis, known as green propolis because of its color (20–24). In recent years, green propolis has been widely studied because of its characteristic chemical composition and biological activities (25). In Japan in particular, Brazilian propolis is used extensively in foods and beverages with the aim of maintaining or improving human health (16,19). However, to our knowledge, no examination of the effects of green propolis has been carried out using PC12 cell cultures and/or a focal cerebral ischemia model.
To examine the function of Brazilian green propolis on neuronal damage in vivo and in vitro, we used, respectively: a middle cerebral artery (MCA) occlusion model in mice, and hydrogen peroxide (H2O2)- and serum deprivation-induced neurotoxicity in PC12 cells. In addition, we examined the effects of propolis (i) on lipid peroxidation in the mouse brain; and (ii) against diphenyl-p-picrylhydrazyl (DPPH)-induced free radicals.
Methods
Materials
Drugs and sources were as follows: Dulbecco's modified Eagle's medium (DMEM), DPPH, 2-thiobarbituric acid, 2,3,5-triphenyltetrazolium chloride (TTC), resazurin and Trolox (a derivative of α-tocopherol) were purchased from Sigma-Aldrich (St Louis, MO). Fetal bovine serum (FBS) and horse serum were from VALEANT (Costa Mesa, CA) and Sanko Junyaku (Tokyo, Japan), respectively. Collagen type IV was from Koken (Tokyo, Japan). Bovine serum albumin (BSA) was from Nacalai (Kyoto, Japan). H2O2 was from Wako (Osaka, Japan). Nerve growth factor (NGF), which was purified from male mouse submaxillary glands, was donated by Dr Shoei Furukawa (26), and isoflurane came from Nissan Kagaku (Tokyo, Japan). Brazilian green propolis (Brazil, Minas Gerais state) was extracted either with 95% ethanol at room temperature or with water at 50°C to yield the extract used. The plant of origin for Brazilian green propolis was B. dracunculifolia (21). The water extract was used in both in vitro and in vivo studies, while the ethanol extract was used only in the in vitro study. Hoechst 33342 and propidium iodide (PI) were from Molecular Probes (Eugene, OR).
Cell Culture
PC12 cells were maintained in DMEM supplemented with 10% heat-inactivated horse serum and 5% heat-inactivated FBS. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere.
To examine the effect of propolis on 0.2 mM H2O2-induced cell death, cells were seeded at a density of 2 × 104 cells per well into collagen-coated 24-well plates, prepared by putting hydrochloric acid solution (pH 3.0) containing 30 mg/ml collagen into the wells, and left for 2 h. After incubating the cells for 1 day, they were differentiated into neuronal cells by adding 20 ng/ml NGF to the above medium for 3 days. To induce cell death, the differentiated cells were immersed in serum-free DMEM supplemented with 0.1% BSA. After pre-treatment with propolis or Trolox for 30 min, H2O2 was added to PC12 cell cultures for 24 h.
To examine how propolis acted on serum deprivation-induced cell death, cells were seeded into collagen-coated 24-well plates at a density of 1 × 104 cells per well. After incubating for 1 day, cells were differentiated into neuronal cells as described above. To induce cell death, the differentiated cells were immersed in serum-free DMEM supplemented with 0.1% BSA, and maintained in this condition for 2 days.
Cell Viability
To evaluate cell survival, we examined the change in fluorescence intensity following cellular reduction of resazurin to resorufin. All experiments were performed in DMEM at 37°C. Cell viability was assessed following immersion in 10% resazurin solution for 3 h at 37°C, and fluorescence was recorded at 560/590 nm.
Hoechst 33342 and PI Dual Staining
At the end of the cell culture, we added Hoechst 33342 (λex 350 nm, λem 461 nm) and PI (λex 535 nm, λem 617 nm) to the culture medium for 15 min at final concentrations of 8.1 and 1.5 μM, respectively. The viable cells were Hoechst 33342-positive and PI-negative, whereas dead cells were both Hoechst 33342-positive and PI-positive.
DPPH-induced Free Radicals
Free radical-scavenging activity was determined by the method of Mellors and Tappel (27), adding 0.25 ml of the drug dissolved in ethanol to 1.5 ml of ethanolic DPPH. The resulting decrease in DPPH absorption at 517 nm was measured after 30 min.
Lipid Peroxidation in Mouse Forebrain Homogenate
The supernatant fraction of mouse forebrain homogenate of male adult ddY mice, weighing 20–25 g (Japan SLC, Shizuoka, Japan), was prepared as described elsewhere (28). Brain tissues were homogenized in a glass–Teflon homogenizer in 4 vols of ice-cold phosphate saline buffer (50 mM, pH 7.4), and the homogenate was stored at −80°C. The stock brain homogenate was diluted 10-fold with the same buffer, then 2 ml portions of the diluted homogenate were added to 10 μl of the test compound and incubated at 37°C for 30 min. The reaction was stopped by adding 400 μl of 35% HClO4, followed by centrifugation at 2800 r.p.m. for 10 min. The supernatant (1 ml) was heated with 0.5 ml of thiobarbituric acid (TBA) solution (5 g/l in 50% acetic acid) for 15 min at 100°C. Absorbance was then measured at 532 nm.
Focal Cerebral Ischemia Model in Mice
Male adult ddY mice, weighing 20–27 g (Japan SLC), were kept under diurnal lighting conditions. Anesthesia was induced by 2.0% isoflurane and maintained with 1% isoflurane in 70% N2O and 30% O2 using an animal general anesthesia machine (Soft Lander; Sin-ei Industry Co. Ltd, Saitama, Japan), maintaining body temperature between 37.0 and 37.5°C with the aid of a heating pad and heating lump. A filament occlusion of the left MCA was performed as described previously (29). Briefly, the left MCA was occluded using an 8–0 nylon monofilament (Ethicon, Somerville, NJ) coated with a mixture of silicone resin (Xantopren; Bayer Dental, Osaka, Japan). Twenty-four hours after this occlusion, the forebrain was divided into five coronal (2 mm) sections using a mouse brain matrix (RBM-2000C; Activational Systems, Warren, MI), and the sections were stained with 2% TTC. All images of the infarcted areas were saved using a digital camera (Nikon Cool PIX4500) and quantitated using NIH Image software, calculations being performed as in our previous report (29). Brain swelling was calculated according to the following formula: (infarct volume + ipsilateral undamaged volume − contralateral volume) × 100/contralateral volume (%) (29).
Mice were tested for neurological deficits at 24 h after the occlusion, scoring being as described in our previous report (29): 0, no observable neurological deficits (normal); 1, failure to extend right forepaw (mild); 2, circling to the contralateral side (moderate); 3, loss of walking or righting reflex (severe). The person doing the scoring was naïve to the treatment group.
Propolis, extracted with water, was administered intraperitoneally (i.p.) at doses of 30 or 100 mg/kg (0.1 ml/10 g) four times (at 2 days, 1 day and 60 min before, and at 4 h after the occlusion). Propolis was dissolved in purified water and made fresh daily.
Statistical Analysis
Data are presented as the mean ±SEM. Statistical comparisons were made by means of a one- or two-way analysis of variance (ANOVA) followed by a Student's t-test, Dunnett's test or Mann–Whitney U-test using STAT VIEW, version 5.0 (SAS Institute Inc., Cary, NC). A P < 0.05 was considered statistically significant.
Results
Propolis Inhibited H2O2- or Serum Deprivation-induced Cell Damage in PC12 Cell Culture
Propolis, extracted with ethanol, significantly inhibited both H2O2- and serum deprivation-induced cell death in PC12 cell culture at concentrations of 4 and 40 μg/ml (Tables 1 and 2). At 4 μg/ml, propolis extracted with water inhibited the serum deprivation-induced cell death as successfully as propolis extracted with ethanol.
Table 1.
Propolis and Trolox inhibited H2O2-induced neurotoxicity in PC12 cell cultures
| Treatments | Cell viability (% of no treatment) |
|---|---|
| No treatment | 100 ± 6.3# |
| Control | 77 ± 3.3 |
| Propolis 0.4 μg/ml | 83 ± 4.6 |
| Propolis 4 μg/ml | 108 ± 6.3# |
| Propolis 40 μg/ml | 110 ± 6.2# |
| Trolox 10 μM | 88 ± 4.7* |
Differentiated PC12 cells were immersed in serum-free DMEM supplemented with 0.1% BSA. After pre-treatment with propolis (extract with ethanol) or Trolox for 30 min, 0.2 mM H2O2 was added to the cell cultures for 24 h. Cell viability was assessed by adding 10% resazurin solution for 3 h at 37°C, and fluorescence was recorded at 560/590 nm. Values represent the mean ± SEM of six independent experiments.
*P < 0.05
#P < 0.01 versus control (H2O2 alone).
Table 2.
Propolis and NGF inhibited serum deprivation-induced neurotoxicity in PC12 cell cultures
| Treatments | Cell viability (% of no treatment) |
|---|---|
| No treatment | 100 ± 3.7# |
| Control | 44 ± 1.7 |
| Propolis 0.4 μg/mla | 46 ± 2.4 |
| Propolis 4 μg/ml | 52 ± 2.1* |
| Propolis 40 μg/ml | 50 ± 2.0* |
| Propolis 4 μg/mlb | 53 ± 2.1* |
| No treatment | 100 ± 9.3# |
| Control | 40 ± 2.3 |
| NGF 0.1 ng/ml | 52 ± 4.1 |
| NGF 1 ng/ml | 74 ± 3.8# |
| NGF 10 ng/ml | 83 ± 8.3# |
Differentiated PC12 cells were immersed in serum-free DMEM supplemented with 0.1% BSA, and then propolis or NGF was added to the cell cultures. Cells were maintained in this condition for 2 days. Cell viability was assessed by adding 10% resazurin solution for 3 h at 37°C, and fluorescence was recorded at 560/590 nm.
aExtract with ethanol.
bExtract with water.
Values represent the mean ± SEM of five independent experiments.
*P < 0.05
#P < 0.01 versus the respective control (serum deprivation alone).
Trolox at 10 μM (Table 1) and NGF at 1 and 10 ng/ml (Table 2) inhibited the cell death induced by H2O2 or serum deprivation, respectively.
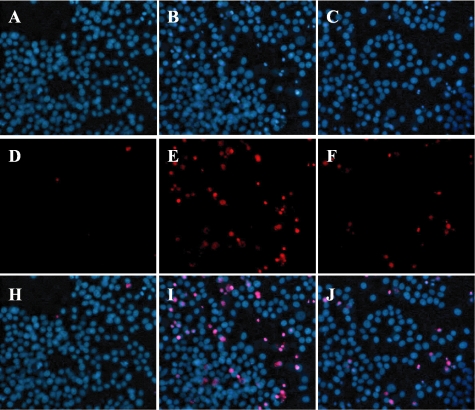
Figure 1 shows typical photographs of Hoechst 33342 and PI staining. Hoechst 33342 stained all cells (live and dead), while PI stained only dead cells. Propolis (4 μg/ml) decreased the number of cells showing PI staining following serum deprivation (versus vehicle treatment).
Figure 1.
Typical photographs illustrating the effect of propolis on serum deprivation-induced cell damage in PC12 cell cultures [Hoechst 33342 and propidium iodide (PI) single or dual staining]. Differentiated PC12 cells were immersed in serum-free DMEM supplemented with 0.1% BSA, and then propolis was added to the cell cultures. Cells were maintained in this condition for 2 days. Viable cells are Hoechst 33342-positive and PI-negative, whereas dead cells are Hoechst 33342-positive and PI-positive. At 4 μg/ml, propolis (extract with ethanol) decreased the number of cells stained by PI (versus vehicle treatment). (A, D and H) Control (vehicle treatment). (B, E and I) Vehicle treatment + serum deprivation. (C, F and J) Propolis treatment + serum deprivation. (A–C) Hoechst 33342 staining. (D–F) PI staining. (G–I) Merged images (Hoechst 33342 + PI dual staining).
Propolis Reduced DPPH-induced Free Radicals and Lipid Peroxidation in Mouse Forebrain Homogenate
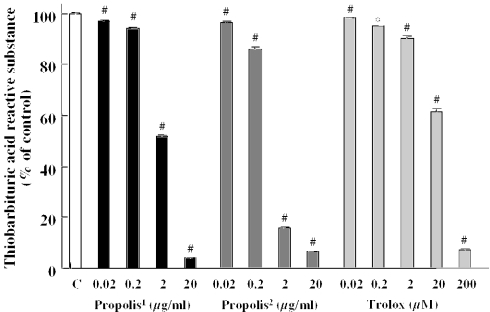
An inducer of stable free radicals, DPPH, was used to assess the radical-scavenging activities of propolis, which was extracted with ethanol, and Trolox (a derivative of α-tocopherol; vitamin E). Propolis and Trolox each reduced DPPH-induced free radical activity in a concentration-dependent manner, reaching significant levels at 2 μg/ml or more and 0.2 μM or more, respectively. The IC50 values [the concentrations causing 50% inhibition, with 95% confidence limits (in parentheses)] for propolis and Trolox were 4.2 (2.0–11.7) μg/ml and 1.4 (0.7–3.1) μM, respectively.
In the lipid peroxidation study, the malondialdehyde (MDA) level in the supernatant increased after 30 min incubation at 37°C, and propolis (extract with both ethanol and water) and Trolox inhibited the lipid peroxidation in a concentration-dependent manner (Fig. 2). The IC50 values (95% confidence limits) for propolis (extract with ethanol), propolis (extract with water) and Trolox were 1.4 (0.97–2.2) μg/ml, 0.76 (0.52–1.1) μg/ml and 16.8 (10.4–28.8) μM, respectively.
Figure 2.
Propolis and Trolox reduced (A) DPPH-induced free radicals and (B) lipid peroxidation in mouse forebrain homogenate. DPPH, diphenyl-p-picrylhydrazyl; TBARS, thiobarbituric acid-reactive substance. Values represent the mean ± SEM of 4–8 independent experiments. 1extract with ethanol; 2extract with water. *P < 0.05; #P < 0.01 versus control (vehicle-treated group).
Propolis Reduced Infarction, Brain Swelling and Neurological Deficits Induced by MCA Occlusion in Mice
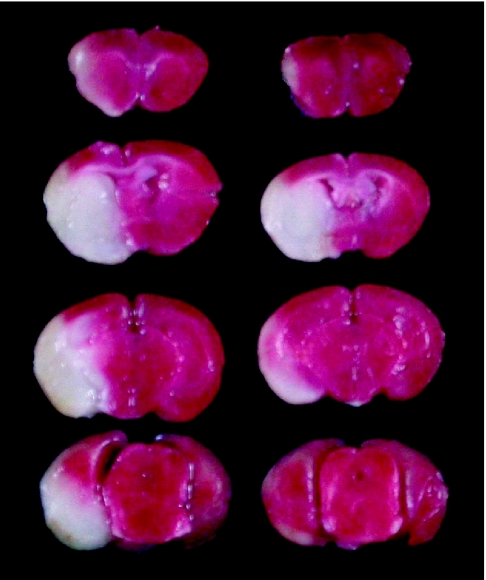
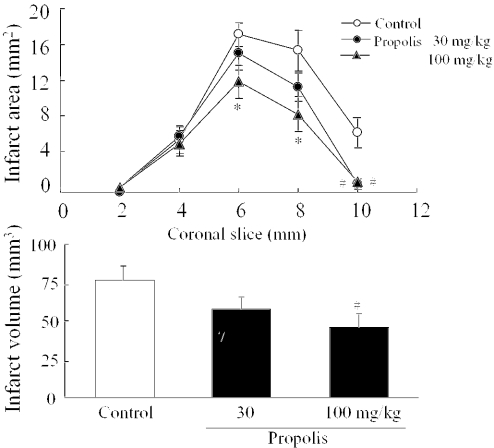
Animals treated with propolis at 30 or 100 mg/kg, i.p. showed no behavioral changes other than the neurological deficits induced by ischemia. Twenty-four hours after MCA occlusion, mice developed infarcts affecting both cortex and striatum (Fig. 3, left side). In this study, we used propolis extracted with water, because ethanol itself might influence behavior. Propolis (30 mg/kg) reduced the infarct area at the level of coronal slice 10 mm (‘0 mm’ being the rostral tip of the forebrain) (Fig. 4). At 100 mg/kg, propolis reduced the infarct areas at the levels of coronal slices 6, 8 and 10 mm, and also infarct volume and brain swelling (Fig. 3, right side, and Fig. 4). Brain swelling (see Materials and Methods) at 24 h after the occlusion was 41.9 ± 4.9% (mean ± SEM, n = 11), 32.3 ± 3.9% (n = 12) and 23.4 ± 4.5% (n = 12) following treatment with vehicle, propolis 30 mg/kg and propolis 100 mg/kg, respectively (the effect of propolis 100 mg/kg being significant versus vehicle).
Figure 3.
2,3,5-Triphenyltetrazolium chloride (TTC) staining of coronal brain sections (thickness, 2 mm) from representative mice at 24 h after permanent middle cerebral artery (MCA) occlusion. Damaged tissue shows as white areas. Sections are arranged from rostral (top) to caudal (bottom). Left and right sections are from vehicle-injected mouse (control) and propolis-treated mouse, respectively. Propolis (extract with water, 100 mg/kg) was administered i.p. four times (at 2 days, 1 day and 60 min before, and at 4 h after the occlusion).
Figure 4.
(A) Propolis reduced the brain infarct area at 24 h after permanent middle cerebral artery (MCA) occlusion in mice. The brains were removed and the forebrains sliced into five coronal 2 mm sections. Propolis, which was extracted with water, was administered i.p. at 30 or 100 mg/kg four times (at 2 days, 1 day and 60 min before, and at 4 h after the occlusion). Infarct areas were revealed by 2% 2,3,5-triphenyltetrazolium chloride (TTC) staining. Values represent the mean ± SEM of 11 or 12 independent experiments. *P < 0.05, #P < 0.01 versus control (vehicle treatment). (B) Propolis reduced the infarct volume at 24 h after permanent MCA occlusion in mice. Values represent the mean ± SEM of 11 or 12 independent experiments. #P < 0.01 versus control (vehicle treatment).
At 30 min after the occlusion, both vehicle- and propolis-treated mice displayed moderate neurological deficits (circling, decreases in resistance to lateral push and locomotor activity, flexion of contralateral torso, and forelimb upon lifting the animal by its tail and abnormal posture). Animals that did not show neurological deficits at this point were excluded from the study on the grounds that the MCA was not occluded successfully. The mean scores allocated for neurological deficits in the vehicle-, propolis 30 mg/kg- and propolis 100 mg/kg-treated groups were 1.45 ± 0.16 (n = 11), 1.33 ± 0.19 (n = 12) and 1.17 ± 0.11 (n = 12), respectively. Although the high dose of propolis tended to reduce the neurological deficits, statistical significance was not attained.
Discussion
Here, we compared the effects of propolis with those of Trolox or NGF (i) against H2O2- and serum deprivation-induced cell damage in neuronally differentiated PC12 cell cultures; and (ii) against lipid peroxidation in the mouse forebrain and DPPH-induced free radical production. Furthermore, we examined the function of propolis on the neuronal damage seen after permanent MCA occlusion in mice. Our results indicate that propolis inhibits neuronal damage both in vitro and in vivo.
Reactive oxygen species (ROS) such as H2O2, nitric oxide (NO), superoxide anion (O·2−) and hydroxyl radical (·OH) have been implicated in the regulation of many important cellular events, including transcription factor activation (30), gene expression (31) and cellular proliferation (32). However, excessive production of ROS gives rise to events that lead to death in several types of cells (33). In fact, ROS have induced death in vitro among cultured neurons (34) and cultured PC12 cells (35). In our experiments, low concentrations (≤0.1 mM) of H2O2 induced cellular proliferation, and consequently cell viability was increased. In contrast, high concentrations (≥0.2 mM) induced cell death in a concentration-dependent manner (data not shown). Therefore, we used 0.2 mM hydrogen peroxide in this study. It is known that cells possess antioxidant systems to control the redox state, which is important for their survival, and H2O2 is often used to investigate the mechanism underlying ROS-induced cell death (36,37).
We found that propolis at concentrations of 4 and 40 μg/ml inhibited H2O2- and serum deprivation-induced cell death in neuronal PC12 cell cultures. Thus, propolis was a more potent inhibitor of H2O2-induced cell death than serum deprivation-induced cell death. Furthermore, propolis acted against oxidative stress (lipid peroxidation) in mouse forebrain homogenates and exhibited a free radical-scavenging action (as assessed using DPPH). The potencies of propolis (extract with both ethanol and water) on lipid peroxidation were almost the same, with effective concentrations of propolis ranging from 2 to 40 μg/ml. Propolis has been reported to exhibit strong scavenging activity in vitro towards both the superoxide anion radical and the NO radical (38). Collectively, these findings indicate that the antioxidant function of propolis may contribute to its neuroprotective potential.
Such an antioxidant function may be an underlying mechanism by which propolis protects against neuronal damage (infarction and swelling) after focal ischemia. Excessive production of ROS is believed to play a critical role in the development of ischemic brain injury (5,39). In fact, ROS may contribute to brain injury directly (by attacking such macromolecules as proteins, lipids and DNA) and/or indirectly (by affecting cellular signaling pathways and gene regulation) (40–42). We evaluated the potential benefit of α-tocopherol in cerebral ischemia some years ago (43). Furthermore, edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), an antioxidative radical scavenger, was recently approved for the treatment of acute cerebral infarction in Japan (44). When propolis (30 or 100 mg/kg, i.p.) was administered four times in our cerebral ischemia experiment, it reduced infarction size at 24 h after the MCA occlusion. Recently, CAPE–which is an active component of propolis extracts and exhibits antioxidant properties–has been reported to reduce the neuronal damage induced by transient forebrain ischemia (45), myocardial ischemia (46) and spinal cord ischemia (47). These CAPE studies indicate that the protective function of propolis may stem from the antioxidant properties of CAPE.
Although CAPE is Poplar type propolis, Brazilian green propolis is Baccharis type—which contains various compounds (21). In a recent study, we showed that ethanol and water extracts of Brazilian green propolis contain artepillin C (14.0%), baccharin (6.8%), 3,4-di-O-caffeoylquinic acid (3.5%), 3,5-di-O-caffeoylquinic acid (2.7%) and p-coumaric acid (2.5%), and 3,4-di-O-caffeoylquinic acid (6.1%), 3,5-di-O-caffeoylquinic acid (4.9%), p-coumaric acid (3.7%) and chlorogenic acid (3.6%), respectively (48). In the present study, since both propolis extracts exhibited a neuroprotective function against in vitro cell death and in vivo ischemia-induced neuronal damage, the common constituents (such as 3,4-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid and p-coumaric acid) may be responsible.
Propolis reportedly has anti-inflammatory activity (11). There is substantial evidence that inflammation, such as neutrophil infiltration and release of interleukin-1 and tumor necrosis factor-α (TNFα), contributes to secondary brain injury after ischemia and reperfusion (49), and that pharmacological anti-inflammatory treatments are beneficial in focal ischemia models (50). Hence, we cannot exclude the possibility that the neuroprotection of propolis against focal ischemia-induced neuronal damage may depend to some extent on its anti-inflammatory activity.
In conclusion, in vitro propolis was neuroprotective in PC12 cell culture, and acted as an antioxidant against lipid peroxidation and free radical production. Furthermore, in vivo, propolis was neuroprotective against ischemic injury (cerebral infarction and swelling in mice). These findings are consistent with the observation that the anti-ischemic function of propolis derives, at least in part, from its antioxidant properties.
Acknowledgments
The authors wish to express their gratitude to Drs Shoei Furukawa and Hidefumi Fukumitsu for useful advice and support, and to Mr Yasuhisa Oida and Miss Noriko Otsu for skillful technical assistance.
References
- 1.Stapf C, Mohe JP. Ischemic stroke therapy. Annu Rev Med. 2002;53:453–75. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- 2.Sandercock P, Willems H. Medical treatment of acute ischaemic stroke. Lancet. 1992;339:537–9. doi: 10.1016/0140-6736(92)90348-7. [DOI] [PubMed] [Google Scholar]
- 3.Sila CA. Prophylaxis and treatment of stroke. Drugs. 1993;45:329–37. doi: 10.2165/00003495-199345030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Flamm ES, Demopoulos HB, Seligman ML, Poser RG, Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978;9:445–7. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- 5.Hara H, Sukamoto T, Kogure K. Mechanism and pathogenesis of ischemia-induced neuronal damage. Prog Neurobiol. 1993;40:645–70. doi: 10.1016/0301-0082(93)90009-h. [DOI] [PubMed] [Google Scholar]
- 6.Siesjo BK. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1:155–84. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- 7.Isla MI, Nieva Moreno MI, Sampietro AR, Vattuone MA. Antioxidant activity of Argentine propolis extracts. J Ethnopharmacol. 2001;76:165–70. doi: 10.1016/s0378-8741(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan K, Singh S, Burker TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankova V, Marcucci MC, Simova S, Nikolova N, Kujumgiev A. Anti-bacterial diterpenic acids from Brazilian propolis. Z Naturforsch [C] 1996;51:277–80. doi: 10.1515/znc-1996-5-602. [DOI] [PubMed] [Google Scholar]
- 10.Drago B, Mombelli B, Vecchi EDE, Fassina MC, Tocalli L, Gismondo MR. In vitro antimicrobial activity of propolis dry extract. J Chemother. 2000;12:390–5. doi: 10.1179/joc.2000.12.5.390. [DOI] [PubMed] [Google Scholar]
- 11.Mizoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996;55:441–9. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Krol W, Czuba Z, Scheller S, Gabrys J, Grabiec S, Shani J. Anti-oxidant property of ethanolic extract of propolis (EEP) as evaluated by inhibiting the chemiluminescence oxidation of luminal. Biochem Int. 1990;21:593–7. [PubMed] [Google Scholar]
- 13.Scheller S, Wilczok T, Imielski S, Krol W, Gabrys J, Shani J. Free radical scavenging by ethanol extract of propolis. Int J Radiat Biol. 1990;57:461–5. doi: 10.1080/09553009014552601. [DOI] [PubMed] [Google Scholar]
- 14.Chen CN, Weng MS, Wu CL, Lin JK. Comparison of radical scavenging activity, cytotoxic effects and apoptosis induction in human melanoma cells by Taiwanese propolis from different sources. eCAM. 2004;1:175–85. doi: 10.1093/ecam/neh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuno T. Isolation and characterization of the tumoricidal substance from Brazilian propolis. Honeybee Sci. 1992;13:49–54. [Google Scholar]
- 16.Aga H, Shibuya T, Sugimoto T, Kurimoto M, Nakajima SH. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci Biotechnol Biochem. 1994;58:945–6. [Google Scholar]
- 17.Bankova V, Christov R, Kujumgiev A, Marcucci MC, Popov S. Chemical composition and antibacterial activity of Brazilian propolis. Z Naturforsch [C] 1995;50:167–72. doi: 10.1515/znc-1995-3-402. [DOI] [PubMed] [Google Scholar]
- 18.Greenaway W, May J, Scaysbrook T, Whatley FR. Identification by gas chromatography-mass spectrometry of 150 compounds in propolis. Z Naturforsch [C] 1991;46:111–21. [Google Scholar]
- 19.Marcucci MC, De Camargo FA, Lopes CMA. Identification of amino acids in Brazilian propolis. Z Naturforsch [C] 1996;51:11–4. [Google Scholar]
- 20.Kumazawa S, Yoneda M, Shibata I, Kanaeda J, Hamasaka T, Nakayama T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem Pharm Bull. 2003;51:740–2. doi: 10.1248/cpb.51.740. [DOI] [PubMed] [Google Scholar]
- 21.Kumazawa S, Yoneda M, Nakayama T. Constituents in Brazilian propolis and its plant of origin. FFI J. 2004;209:132–9. [Google Scholar]
- 22.Midorikawa K, Banskota AH, Tezuka Y, Nagaoka T, Matsushige K, Message D, Huertas AA, Kadota S. Liquid chromatography–mass spectrometry analysis of propolis. Phytochem Anal. 2001;12:366–73. doi: 10.1002/pca.605. [DOI] [PubMed] [Google Scholar]
- 23.Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50:2502–6. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira EW, Negri G, Meira PMSA, Message D, Slatino A. Plant origin of green propolis: bee behavior, plant anatomy and chemistry. eCAM. 2005;2:85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcucci MC, Ferreres F, Garcia-Viguera C, Bankova VS, De Castro SL, Dantas AP, Valente PH, Paulino N. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol. 2001;74:105–12. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa S, Kamo I, Furukawa Y, Akazawa S, Satoyoshi E, Itoh K, Hayashi K. A highly sensitive enzyme immunoassay for mouse beta nerve growth factor. J Neurochem. 1983;40:734–44. doi: 10.1111/j.1471-4159.1983.tb08040.x. [DOI] [PubMed] [Google Scholar]
- 27.Mellors A, Tappel AL. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J Biol Chem. 1966;241:4353–6. [PubMed] [Google Scholar]
- 28.Hara H, Kogure K. Prevention of hippocampus neuronal damage in ischemic gerbils by a novel lipid peroxidation inhibitor (quinazoline derivative) J Pharmacol Exp Ther. 1990;255:906–13. [PubMed] [Google Scholar]
- 29.Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metabol. 1996;16:605–11. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–30. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- 32.Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–65. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe JT, Ross D, Cohen GM. A role for metals and free radicals in the induction of apoptosis in thymocytes. FEBS Lett. 1994;352:58–62. doi: 10.1016/0014-5793(94)00920-1. [DOI] [PubMed] [Google Scholar]
- 34.Ratan RR, Murphy TH, Baraban JM. Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem. 1994;62:376–9. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- 35.Vimard F, Nouvelot A, Duval D. Cytotoxic effects of an oxidative stress on neuronal-like pheochromocytoma cells (PC12) Biochem Pharmacol. 1996;51:1389–95. doi: 10.1016/0006-2952(96)00065-2. [DOI] [PubMed] [Google Scholar]
- 36.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H2O2. Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:46379–85. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura Y, Ota T, Matsuoka Y, Tooyama I, Kimura H, Shimohama S, Nomura Y, Gebicke-Haerter PJ, Taniguchi T. Hydrogen peroxide-induced apoptosis mediated by p53 protein in glial cells. Glia. 1999;25:154–64. [PubMed] [Google Scholar]
- 38.Ichikawa H, Satoh K, Tobe T, Yasuda I, Ushio F, Matsumoto K, Endo K, Ookubo C. Free radical scavenging activity of propolis. Redox Rep. 2002;7:347–50. doi: 10.1179/135100002125000965. [DOI] [PubMed] [Google Scholar]
- 39.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–9. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–45. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 41.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci USA. 1990;87:5144–7. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–95. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 43.Hara H, Kato H, Kogure K. Protective effect of α-tocopherol on ischemic neuronal damage in the gerbil hippocampus. Brain Res. 1990;510:335–8. doi: 10.1016/0006-8993(90)91386-u. [DOI] [PubMed] [Google Scholar]
- 44.Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–9. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 45.Irmak MK, Fadillioglu E, Sogut S, Erdogan H, Gulec M, Ozer M, Yagmurca M, Gozukara ME. Effects of caffeic acid phenethyl ester and alpha-tocopherol on reperfusion injury in rat brain. Cell Biochem Funct. 2003;21:283–9. doi: 10.1002/cbf.1024. [DOI] [PubMed] [Google Scholar]
- 46.Ozer MK, Parlakpinar H, Acet A. Reduction of ischemia–reperfusion induced myocardial infarct size in rats by caffeic acid phenethyl ester (CAPE) Clin Biochem. 2004;37:702–5. doi: 10.1016/j.clinbiochem.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Ilhan A, Koltuksuz U, Ozen S, Uz E, Ciralik H, Akyol O. The effects of caffeic acid phenethyl ester (CAPE) on spinal cord ischemia/reperfusion injury in rabbits. Eur J Cardiothorac Surg. 1999;16:458–63. doi: 10.1016/s1010-7940(99)00246-8. [DOI] [PubMed] [Google Scholar]
- 48.Mishima S, Narita Y, Chikamatsu S, Inoh Y, Ohta S, Yoshida C, Araki Y, Akao Y, Suzuki K, Nozawa Y. Effects of propolis on cell growth and gene expression in HL-60 cells. J Erthnopharmacol. 2005 doi: 10.1016/j.jep.2005.02.005. in press. [DOI] [PubMed] [Google Scholar]
- 49.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia:the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–96. doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
- 50.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–36. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]