Abstract
Reef-building coral populations are at serious risk of collapse due to the combined effects of ocean warming and acidification. Nonetheless, many corals show potential to adapt to the changing ocean conditions. Here we examine the broad sense heritability (H2) of coral calcification rates across an ecologically and phylogenetically diverse sampling of eight of the primary reef-building corals across the Indo-Pacific. We show that all eight species exhibit relatively high heritability of calcification rates under combined warming and acidification (0.23–0.56). Furthermore, tolerance to each factor is positively correlated and the two factors do not interact in most of the species, contrary to the idea of trade-offs between temperature and pH sensitivity, and all eight species can co-evolve tolerance to elevated temperature and reduced pH. Using these values together with historical data, we estimate potential increases in thermal tolerance of 1.0–1.7°C over the next 50 years, depending on species. None of these species are probably capable of keeping up with a high global change scenario and climate change mitigation is essential if reefs are to persist. Such estimates are critical for our understanding of how corals may respond to global change, accurately parametrizing modelled responses, and predicting rapid evolution.
Keywords: coral reef, climate change, ocean acidification, evolution, heritability, adaptation
1. Background
Coral reef ecosystems and reef-building coral populations are at serious risk of collapse within the next few decades due to ocean warming and acidification [1–4]. Ocean warming can lead to coral bleaching, reduced growth, interruptions in reproduction, increased susceptibility to disease, and mortality [5], whereas acidification often results in lower calcification [6–9] and may reduce coral recruitment [10,11]. Projected changes in coral cover on reefs as well as the potential for coral persistence under future, global change rely on modelled responses [1,2,12–14]. Such predictions, however, are only as accurate as the parameter estimates and assumptions included in the models [15,16]. Most parameter estimates are based on a few species examined in the laboratory over relatively short timescales (days to weeks) and may not reflect the longer-term responses of these organisms under a changing climate [17–19]. While critical to our understanding, short-term studies may underestimate realistic levels of coral adaptation over decadal timescales and therefore may lead to more severe projected outcomes than should be expected. The scope for coral adaptation to warming and acidification (and especially the combination of these two factors simultaneously) remains largely unknown, as does the extent to which variation in warming and acidification is heritable.
The phenotype of an organism depends on both heritable and environmental influences, but only heritable variation can be passed to offspring and acted upon by selection. Hence, the heritability of a trait provides a critical predictor of the evolutionary potential of that species or population when experiencing selective pressure. Heat stress and ocean acidification both tend to reduce coral calcification rates, though responses to these stressors vary substantially among taxa [20] as well as among individuals within species [7,8,21,22]. Calcification appears to be intimately tied to coral fitness, and calcification responses under novel conditions may result in selection for phenotypes that better match the new environment [7,8,22]. Recent studies have shown that many corals exhibit surprisingly high heritability in a number of key traits under thermal stress [21,22], and our recent work demonstrates that the heritability of calcification rates under acidification is likewise high across a diverse group of coral species [7]. Still, it is unclear if corals will be able to mount adaptive responses to warming and acidification simultaneously, and if there might be trade-offs associated with these capacities. For example, reaction norms from three Hawaiian corals reveal that Pocillopora acuta and Montipora capitata shift their relative temperature tolerances under warmer conditions, whereas Porites compressa shifts its relative pH tolerance under more acidic conditions, but all three react differently to dual stressors under future ocean conditions [8].
Organisms often exhibit trade-offs among different aspects of their physiological performance. Shown frequently among plants [23,24], heavy investment into particular functions or strategies typically leaves fewer resources available to engage in others, which almost certainly happens in corals as well. For example, hosting certain types of thermally tolerant algal symbionts tends to result in higher bleaching thresholds for the host coral [25,26]. These thermally tolerant symbionts, however, tend to be less productive than their more sensitive counterparts, leading to reduced coral growth rates [25,27,28]. That is, selection for one trait (thermal tolerance) comes at the expense of another trait (growth). Likewise, a single trait may exhibit contrasting responses to different stressors. For example, for some corals, increased thermal tolerance and higher survivorship under heat stress are associated with lower resistance to pathogens and reduced survivorship under that stressor [29]. Hence, adaptive responses to one environmental challenge can result in a trade-off and reduced capacity to respond to a different challenge within the same trait. If trade-offs exist between warming and acidification on coral calcification rates, then corals may show reduced capacity or inability to adapt to both factors simultaneously.
On the other hand, a common life history strategy employed by some corals, and many other organisms, is one of general stress tolerance. Stress-tolerant species or individuals may invest heavily in processes such as cellular homeostasis, tissue repair and other aspects related to stress response, thereby allowing them to survive through environmental challenges where others may not. If corals exhibit calcification trade-offs between temperature and pH tolerance, then one would expect that their responses to experimental warming and acidification should be negatively correlated between the individual stressors and/or should show a synergistic negative effect under the combined stressors. That is, tolerance to one factor should be associated with a cost and increased sensitivity to the other, or increased sensitivity under the combination of factors. Conversely, if their calcification responses to each of warming and acidification are positively correlated and do not show a synergistic negative interaction, then it would suggest that individuals within these species show variable levels of general stress tolerance, and that some individuals are especially well equipped to deal with these future ocean stressors.
Heritability can be measured in the narrow or broad sense [30,31]. Narrow sense heritability (h2) includes only additive genetic variation and can differ significantly from broad sense (H2, based on the total genetic variance) if intra-locus dominance, maternal effects, epistatic or epigenetic interactions occur [30,31]. Narrow sense heritability (h2) is logistically difficult to measure, particularly in most natural populations, requiring precise pedigrees over multiple generations and/or substantial genomic information [32]. Even when this substantial effort is made, estimates of heritability are notoriously underpowered and require large sample sizes to estimate with confidence [33,34]. However, broad-sense heritability (H2) is commonly used to predict the response to selection in plant breeding trials or human quantitative genetics where identical twin studies can be used [30,31,34,35], because they focus on the proportion of a phenotypic response in a trait that is attributable to the underlying genotypic variance [36], and the large sample sizes needed can be more easily obtained using clonal replicates of each genotype. Furthermore, complex traits controlled by many loci tend to be dominated by additive genetic variation which typically exceeds over half, and often close to 100%, of the total genetic variance, lending further value to estimation of H2 as the upper limit for narrow sense heritability [37]. Regardless, whether H2 is a precise estimation or the upper limit of coral heritability, estimating thermal and acidification tolerances of scleractinian corals and their potential to respond to increasing global temperatures and declining pH remains a critical gap in our understanding of likely responses to future ocean conditions [21].
Here, we take advantage of the ability to sample genetically distinct coral colonies (genets) to produce genetically identical clonal fragments (ramets) and use an identical twin design to estimate the broad sense heritability (H2) and potential for adaptation of coral calcification rates under warming, acidification and the combination of both factors. Corals of eight species were exposed to one of four treatments: (i) control (present-day temperature and pH), (ii) ocean warming (+2°C and present-day pH), (iii) ocean acidification (present-day temperature and −0.2 pH units), or (iv) combined future ocean (+2°C and −0.2 pH units). This study was conducted over 1 year, providing the corals with time to acclimatize to the treatments and helping to ensure that the measured calcification responses were a result of heritable variation among them rather than differences in their short-term histories. Furthermore, these responses were assessed in biologically diverse reef mesocosms, to ensure that the environment was as realistic as possible. These eight species are ecologically and phylogenetically diverse, representing three of the most common reef-building coral families worldwide (Acroporidae, Pocilloporidae and Poritidae), both major evolutionary lineages of scleractinian corals (Complexa and Robusta), and all four of the major coral life history strategies including three competitive species (Montipora capitata, Porites compressa and Pocillopora meandrina), two generalist species (Montipora flabellata and M. patula), two stress-tolerant species (Porites evermanni and Porites lobata) and one weedy species (Pocillopora acuta). These species, or their close relatives [38,39], include some of the most common corals across the Indo-Pacific [40], yielding broad relevance for our study. In addition to estimates of H2, we test for possible trade-offs associated with temperature and pH tolerance within each of these species. Finally, we calculate selection coefficients for these corals, derived from our estimates of H2 along with historical data and estimate potential increases in thermal tolerance for these species over the next century.
2. Methods
(a). Coral collection
Corals were collected using a hammer and chisel at 2 ± 1 m depth from a total of six locations around O‘ahu, Hawai‘i (figure 1). Each species was collected from three to five of the sites, depending on their local abundances and sizes, for a total of 22 colonies of Montipora flabellata and 30 colonies (genets) of the remaining seven species. The eight species examined in this study were Montipora capitata, Montipora flabellata, Montipora patula, Porites compressa, Porites evermanni, Porites lobata, Pocillopora acuta and Pocillopora meandrina.
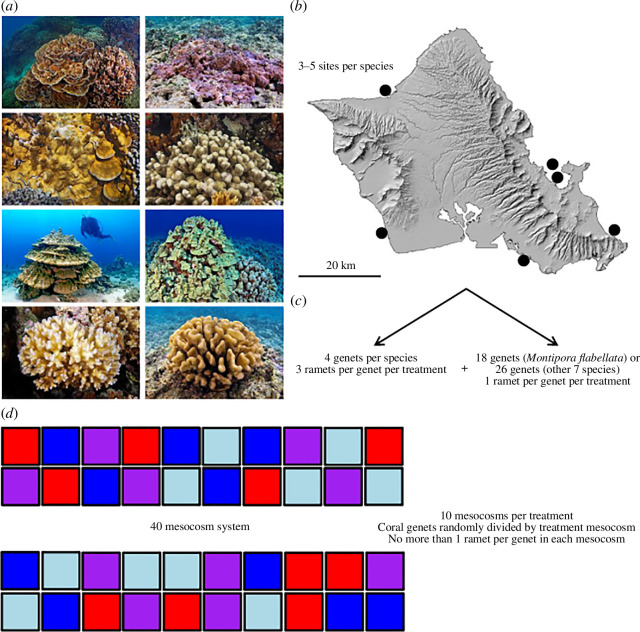
Figure 1.
Diagram illustrating the experimental design used in this study. Representative photos of each coral are shown in (a) From top-left to bottom-right these are: Montipora capitata, Montipora flabellata, Montipora patula, Porites compressa, Porites evermanni, Porites lobata, Pocillopora acuta and Pocillopora meandrina. Corals were collected from a total of six locations around O‘ahu, Hawai‘i as indicated by the black dots (b) with each species collected from three to five of the locations, depending on local abundance. The corals were then fragmented into a series of clonal nubbins (ramets) with four genotypes (genets) per species each contributing three ramets per treatment, whereas the remaining 18 genets (Montipora flabellata) or 26 genets (other seven species) each contributed one ramet per genet per treatment, yielding a total of 22 genets for Montipora flabellata and 30 genets for the remaining seven species (c) The ramets were randomly allocated among mesocosms, which were themselves randomly divided among treatments, and with no more than one ramet per coral genet in each mesocosm (d) Mesocosms are colour-coded according to treatment: control (blue), ocean acidification (light blue), ocean warming (red) and combined future ocean (purple). Photos courtesy of Keoki Stender.
After collection, corals were returned to the Hawai‘i Institute of Marine Biology (HIMB) on Moku o Lo‘e (Coconut Island), fragmented into 4–12 replicate nubbins (3–5 cm coral fragments, referred to as ‘ramets’) using a diamond-coated band saw, individually attached to a labelled ceramic tile using cyanoacrylate gel, and allowed to recover for 2.5 months in a common garden under present-day average temperature for O‘ahu and ambient pH conditions, with both temperature and pH following the seasonal cycle (electronic supplementary material, figure S1).
(b). Approach
There was a need to include both within-genet variation and among-genet variation in our estimates. Given logistical constraints about how many coral ramets could possibly be accommodated, we attempted to balance these conflicting needs by including (i) three replicate ramets from four genets for each coral species within each treatment, and (ii) one ramet per genet per treatment for the remaining genets (n = 22 total genets for Montipora flabellata; n = 30 for the other seven species, four of which were replicated within treatments per species). All coral ramets were randomly divided among mesocosms with no more than one ramet per genet in each mesocosm, resulting in three–four ramets per species within each mesocosm tank (figure 1).
This experiment was part of a larger mesocosm project, other components of which have been described elsewhere [41–47]. The experimental system received constant flow-through of unfiltered seawater from the adjacent reef and was initially set up with reef sand, rubble, algae, invertebrates and fish to provide a reef-like habitat (see electronic supplementary information for additional details regarding the mesocosm design). Temperature and pH of the incoming seawater were adjusted according to treatment in a series of header tanks, using aquarium heaters and CO2 gas injection, prior to flowing into the 70 l mesocosms at a rate of approximately 1.2 l min−1, for a residence time of approximately 1 h. Additional water circulation (4900 l h−1) was generated by seawater pumps within each mesocosm to provide water flow speeds (10–15 cm s−1) similar to those in situ. Both temperature and pH were allowed to vary according to natural daily and seasonal cycles while maintaining appropriate offsets according to treatment: control treatment (present-day temperature and pH), ocean warming treatment (+2°C and present-day pH), ocean acidification treatment (present-day temperature and −0.2 pH units), or combined future ocean treatment (+2°C and −0.2 pH units) with 10 replicate mesocosms per treatment in a 40 mesocosm system (see electronic supplementary material, figure S1). The corals were then randomly assigned to a mesocosm with either one or three replicate nubbins (ramets) per colony (genet) in each treatment, and no more than one ramet per genet in each mesocosm.
After 2.5 months of acclimatization in a common garden, temperature and pH were slowly adjusted starting on 1 February 2016 until target values were reached on 20 February 2016. The corals were then allowed 5.5 months to acclimatize to treatment conditions before calcification rates were evaluated, thereby excluding short-term history as a factor in their responses. Corals experienced heat stress during the final nine weeks of the study, during which the calcification assay was conducted (electronic supplementary material, figure S1). Calcification rates were assessed via the buoyant weighing technique [48], with initial weights taken 3–15 August 2016 (shortly after the onset of thermal stress in the heated treatments), final weights taken 26 September to 8 October 2016 (shortly after the seasonal peak in temperatures), and calcification rates were normalized to initial weight. In total, the study was conducted over nearly 1 year with approximately eight months of exposure under experimental treatment conditions, and the calcification assay was conducted over the last nine weeks of the experiment.
(c). Coral genotyping
Multi-locus genotyping of coral hosts was performed following published methods [49,50]. Briefly, total genomic DNA was isolated using the E.Z.N.A. Tissue DNA Kit (Omega Bio-tek, Inc., Norcross, GA, USA) following the manufacturer’s protocol. Amplicons were generated via polymerase chain reaction (PCR) using microsatellite primers [51], but with short unique barcodes [52] added to each primer to identify each position in a 96-well plate. Amplicons were pooled equimolarly, and a dual-index system of adaptors was used to identify individuals on each plate and libraries were sequenced on an Illumina MiSeq platform (v. 3 2 × 300 PE) at the Hawai‘i Institute of Marine Biology. We used a custom bioinformatic genotyping workflow pipeline [49] to call alleles, which were then converted to GenoDive v. 2.0b27 [53] file format for analyses. Individual genotypes were created using two different methods. First, we used sequence length (equivalent to peak calling in a microsatellite fragment analysis sensu [54]), such that all sequences of the same length, regardless of underlying sequence variation, would be scored as the same allele (sequence length). Second, we identified alleles by their sequence (ID) so that only two exactly identical alleles had the same ID, whereas alleles with the same length but differing in nucleotide composition would have different allelic IDs. Similar to previous findings [49], both approaches gave the same result. Using the ‘assign clones’ feature of GenoDive [55], we tested whether coral colonies sampled in the field had a unique multi-locus genotype. To be conservative, we allowed for up to two scoring errors among individuals and checked potential clones against the location of collection.
(d). Historical temperature tolerances
Over the last 50 years, the mean seawater temperature around O‘ahu has warmed at a rate of 1.9°C per century and acidified at a rate of 0.13 pH units per century [19,56] (but note that pH data first became available in 1992) (electronic supplementary material, figure S2). The rate of acidification will accelerate in the future as a consequence of reduced seawater buffer capacity [57]. In 1970, survivorship of corals exposed to temperatures near their upper thermal limits (31.0°C) was assessed for two of the species included in this study (Montipora capitata and Pocillopora acuta) [58]. In 2017, this study was repeated (31.4°C) [19] (data shown in the original publications). We examined changes in their temperature tolerances by fitting logistic regressions to survivorship using the R package ‘MASS’ in the following way (given differences in data overlap among datasets). For Montipora capitata, we assessed the LD20 (degree heating weeks, DHW, which results in 20% mortality at these upper thermal limits) and for Pocillopora acuta, we assessed the LD50 (DHW which result in 50% mortality at these upper thermal limits) in each of 1970 and 2017, again using ‘MASS’ to define these values. These fits resulted in the following estimates: LD20 for Montipora capitata of 1.30 ± 0.39 and 20.17 ± 2.46 DHW in 1970 and 2017, respectively; LD50 for Pocillopora acuta of 1.47 ± 0.56 and 18.68 ± 1.24 DHW in 1970 and 2017, respectively. We then estimated the change in these DHW tolerances for each species as proxies for their responses to selection (R) over the period 1970–2017, as described below.
(e). Statistics
To examine treatment effects on calcification rates, for each species individually, an ANOVA was fit with temperature, pH and collection site as fixed factors, and coral colony (genet), header tank and mesocosm as nested factors. Due to the smaller sample size for Montipora flabellata, there were an insufficient number of degrees of freedom to fit the full model. Instead, we first fit a model with all factors included except coral genet. Mesocosm effects were not significant, so this factor was dropped and a second model was then fit which included genet. Model fits were assessed via diagnostic plots of the residuals and in all cases the data adequately satisfied ANOVA assumptions. A Tukey honestly significant difference (HSD) was used as a post hoc to examine significant treatment effects.
Broad sense heritability (H2) was estimated using a Bayesian modelling approach, similar to that used in previous work [59,60]. The models were fit with the R package ‘MCMCglmm’ [61] with temperature and pH as fixed effects and coral genet, collection site, header tank and mesocosm as random effects. Models were run for 100 000 iterations, storing the Markov chain every 50 iterations, and with the first 15 000 iterations used as a burn-in period. Heritability was estimated as the proportion of the phenotypic variance which was explained by genotype [59,60].
To test for possible trade-offs between temperature and pH tolerance, and the hypothesis that these tolerances are related to a general stress-tolerance strategy, the association between temperature and pH response was examined for each species using Pearson’s correlation. Temperature tolerance was calculated as the change in mean calcification rate between the control and the ocean warming treatment, whereas pH tolerance was the difference between the control and the ocean acidification treatment. Furthermore, we considered if there were interactive effects between temperature and pH on calcification rates identified by the ANOVAs, as another indicator of trade-offs in these tolerances.
For Montipora capitata and Pocillopora acuta, we estimated their responses to selection (R) based on changes in their LD20 and LD50 values, respectively, from 1970 to 2017. The error associated with these estimates was determined from Monte Carlo simulations using the R package ‘propagate’ and derived from 100 000 simulations. Along with our heritability estimates, as described above, as well as the classical (univariate) breeder’s equation, R = H2S, we estimate the selection coefficients for these species under the selective pressure that they have experienced over the last 50 years, where H2 is the broad sense heritability (including additive, dominance and epistatic variance) which represents the proportion of the selection differential (S) that can be realized as the response (R) to selection [30,36,59,62], and with the error associated with S propagated in the same way. Again, we assume that broad sense heritability (H2) provides an upper bound for narrow sense heritability (h2), though the two values are probably similar if these traits are influenced by many genetic loci. Selection differentials for the remaining six species are unknown, so to be conservative we assumed that they experience selection similar to that for Montipora capitata (the lower of the two selection coefficients) and estimated their responses to selection (R) based on these assumed values and their measured heritabilities, with uncertainties propagated as above.
Analyses were performed in R v. 4.0.3 [63].
(f). Effects of unbalanced sampling design
We considered how an unbalanced sampling design might affect our estimates. In particular, our study was slightly unbalanced because most of the coral genets contributed one ramet per treatment whereas four genets per species contributed three ramets per treatment. In addition, a small percentage of the ramets died during the acclimatization phase (18 nubbins, or 1.5% of the total, and affecting 12 of the 232 genets) resulting in representation of 87–100% of the genets across all four treatments, depending on species.
For the ANOVAs, this slight unbalance has very little effect. ANOVA is highly robust to modest discrepancies in sample size and missing observations, such as occur in this study. With the heritability estimates, modelling work [36,62] suggests that our sampling design results in a less than 3% additional uncertainty in H2 for Pocillopora acuta and Pocillopora meandrina, and far less among the other species. This small uncertainty yields only a trivial effect on our estimates of selection coefficients (S) or responses to selection (R).
3. Results
(a). Coral collection and genotyping
Depending on local abundances, each coral species was sampled from three to five of the six collection locations around O‘ahu resulting in 22 parent colonies for Montipora flabellata and 30 parent colonies for each of the remaining seven species (figure 1, electronic supplementary material, table S1). Each of the 232 parent colonies samples exhibited a unique multi-locus genotype [42,64]. To be conservative, we allowed for up to two scoring errors in these microsatellite analyses which returned only a single potential clonal pair among these corals (Porites compressa colonies #1 and #3 from Waimānalo). These two corals, however, displayed highly distinctive coloration (yellow-grey vs. tan) and morphology (smoother vs. knobbier branches) which they maintained for a year while growing in a common garden with the other colony. This gave us confidence that none of the coral colonies sampled for this study were clonally derived and that each colony represents a distinct genet.
(b). Environmental conditions
We exposed the corals to the target treatment conditions, consisting of present-day mean temperature and pH as well as warming of +2°C and acidification of −0.2 pH units while preserving the natural daily and seasonal fluctuations of these parameters (electronic supplementary material, figure S1). In the heated treatments (ocean warming and combined future ocean) the corals accumulated approximately 14 DHW during the calcification assay.
(c). Coral survivorship
A small number of coral nubbins (18 ramets, or 1.5% of the 1184 ramets total) died during the acclimatization phase. Mortality was restricted primarily to one genet of Pocillopora meandrina, wherein 7 of the 12 ramets died. In addition, one ramet each from 11 of the 232 coral genets also died. These dead nubbins were excluded from the analyses. During the nine-week heat stress event, when the calcification assay was being conducted, an additional 14 nubbins (1.2% of the total) also died, but these ramets were included in the analyses to avoid biasing the data against the most thermally sensitive individuals.
(d). Treatment effects on calcification rates
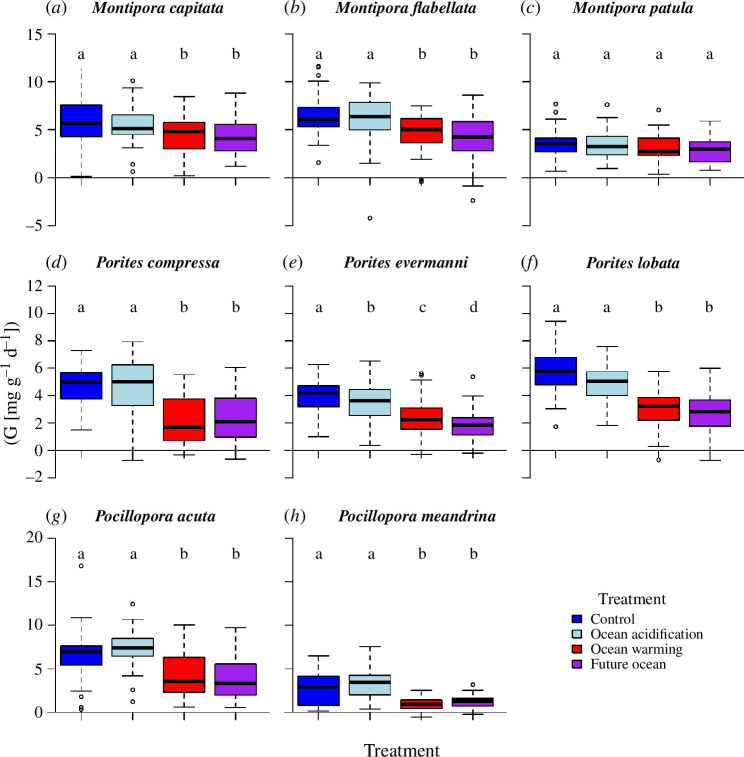
For all eight species, there were significant main effects of temperature on calcification rates, with all eight species experiencing reduced calcification at elevated temperature (figure 2, electronic supplementary material, table S2). In addition, five of the species also experienced a significant main effect of acidification, and calcification tended to decrease under reduced pH for Montipora capitata, Montipora flabellata, Porites evermanni and Porites lobata, yet increased for Pocillopora meandrina. Only one species, Pocillopora meandrina, exhibited a significant Temp × pH interaction, because the resistance of this species to acidification was diminished under heating. As is typical for most corals, linear extension rates ranged from approximately 1 to 10 cm yr− 1, depending on species and genet.
Figure 2.
Boxplot of treatment effects on coral calcification rate (G) for each species. All eight species experienced reduced calcification under warming. Three of the species (Montipora flabellata, Porites evermanni and Porites lobata) exhibited reduced calcification under acidification, whereas Pocillopora meandrina showed increased calcification, and the other four species did not respond significantly to reduced pH. For most of the species, temperature and pH effects were additive, whereas for Pocillopora meandrina there was a significant Temp × pH interaction such that the resistance of this species to acidification was diminished under elevated temperature. Horizontal black bar is the median, box edges are 75% confidence intervals, box whiskers are 95% confidence intervals, and points show outliers. Non-bolded letters above each box indicate Tukey HSD post hoc test results for pairwise contrasts among treatments; groups sharing a letter within each panel are not significantly different at α = 0.05. n = 22 genotypes (genets) for Montipora flabellata and n = 30 genets for the remaining seven species. Note differences in scale among rows.
(e). Heritability
Estimates of heritability (H2) ranged from a low of 0.23 in Pocillopora acuta to a high of 0.56 in Montipora capitata, and calcification rates were significantly heritable for all eight species (figure 3). See table 1 for a summary of major findings from this study according to species.
Figure 3.
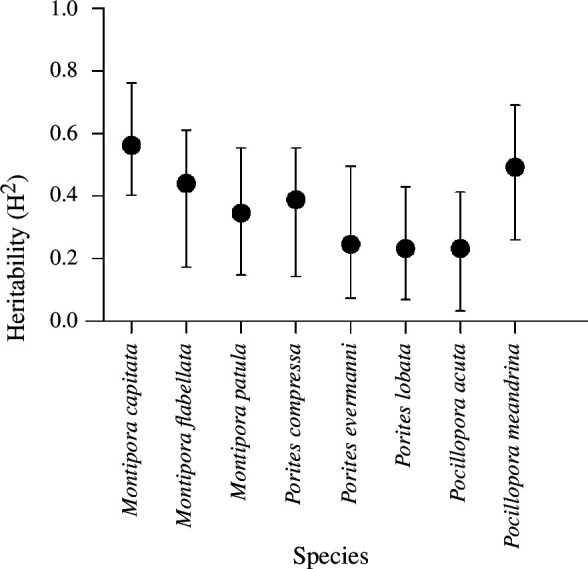
Broad sense heritability (H 2 ) for each coral species under combined warming and acidification. Points show best estimates and error bars are 95% confidence intervals. n = 22 genotypes (genets) for Montipora flabellata and n = 30 genets for the remaining seven species.
Table 1.
Summary of major findings from this study according to species. Columns show treatment effects on coral calcification due to heating (temp effects), acidification (pH effects), interactive effects between factors (temp × pH interaction) and estimates of broad sense heritability (H2).
| species | family | life history strategy | temp effects | pH effects | temp × pH interaction | H2 |
|---|---|---|---|---|---|---|
| Montipora capitata | Acroporidae | competitive | negative | negative | no | 0.56 |
| Montipora flabellata | Acroporidae | generalist | negative | negative | no | 0.44 |
| Montipora patula | Acroporidae | generalist | negative | neutral | no | 0.34 |
| Porites compressa | Poritidae | competitive | negative | neutral | no | 0.39 |
| Porites evermanni | Poritidae | stress tolerant | negative | negative | no | 0.24 |
| Porites lobata | Poritidae | stress tolerant | negative | negative | no | 0.23 |
| Pocillopora actua | Pocilloporidae | weedy | negative | neutral | no | 0.23 |
| Pocillopora meandrina | Pocilloporidae | competitive | negative | positive | yes | 0.49 |
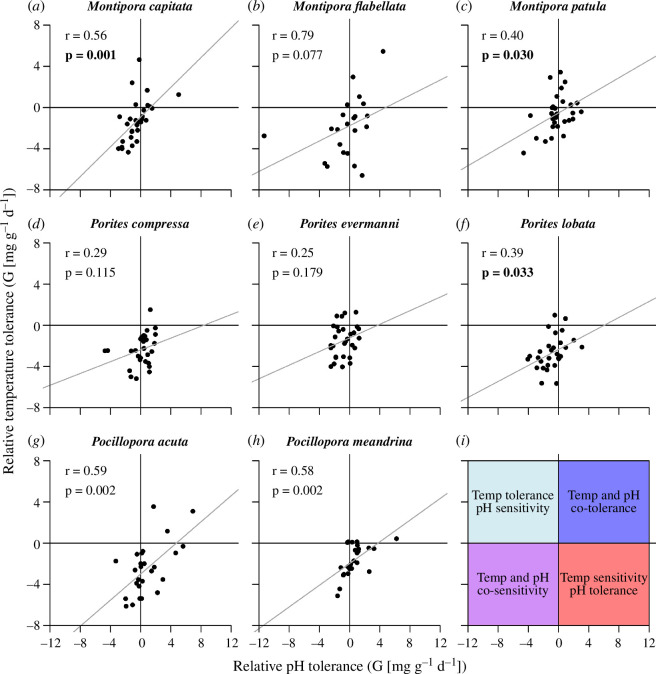
(f). Temperature and pH tolerance correlations
We define relative temperature tolerance as the change in calcification in the ocean warming treatment compared with the control, and relative acidification tolerance as the change in calcification in the ocean acidification treatment as compared with the control. Five of the species (Montipora capitata, Montipora patula, Porites lobata, Pocillopora acuta and Pocillopora meandrina) exhibited a significant positive correlation between temperature and pH tolerance, whereas the remaining three species (Montipora flabellata, Porites compressa and Porites evermanni) showed similar, non-significant trends (figure 4, electronic supplementary material, table S3). None of the corals exhibited negative correlations between temperature and acidification tolerance, as would be expected if there were trade-offs between these tolerances.
Figure 4.
Scatterplot of relative pH and temperature tolerances for each coral species. Relative pH tolerance is defined as the change in calcification rate in the ocean acidification treatment relative to the control treatment whereas relative temperature tolerance is defined as the change in calcification rate in the ocean warming treatment relative to the control treatment for each species (a–h) Grey line in each plot is a linear regression of the relationship between pH and temperature tolerance for each species. The plot in the lower right (i) is a visual guide to aid in interpretation of the panels. Correlation coefficients (r) and p-values for the regressions are shown in each panel; significant p-values at α = 0.05 are shown in bold. Five of the species (Montipora capitata, Montipora patula, Porites lobata, Pocillopora acuta and Pocillopora meandrina) show significant positive associations between pH and temperature tolerance, whereas the other three species (Montipora flabellata, Porites compressa and Porites evermanni) exhibit similar, non-significant trends. This analysis tests for possible calcification trade-offs between pH and temperature tolerance. Only Pocillopora meandrina exhibited a significant Temp × pH interaction in the combined future ocean treatment, which is likely to reduce the capacity of this species to adapt to the combined stressors. All eight species are expected to be capable of adapting to ocean warming, ocean acidification and the combination of both factors, though Pocillopora meandrina is likely to have diminished capacity to respond to the combination as compared with each factor individually. Due to mortality of a few coral ramets not all genets could be included in the analysis. n = 22 genets for Montipora flabellata, n = 26 for Pocillopora acuta and Pocillopora meandrina, and n = 30 for the remaining five species.
(g). Responses to selection
We project that all eight species are capable of evolving an increase in thermal tolerance over the next 50 years. These values ranged from a low of 8.5 DHW in Porites lobata to a high of 20.0 DHW in Montipora capitata (figure 5).
Figure 5.
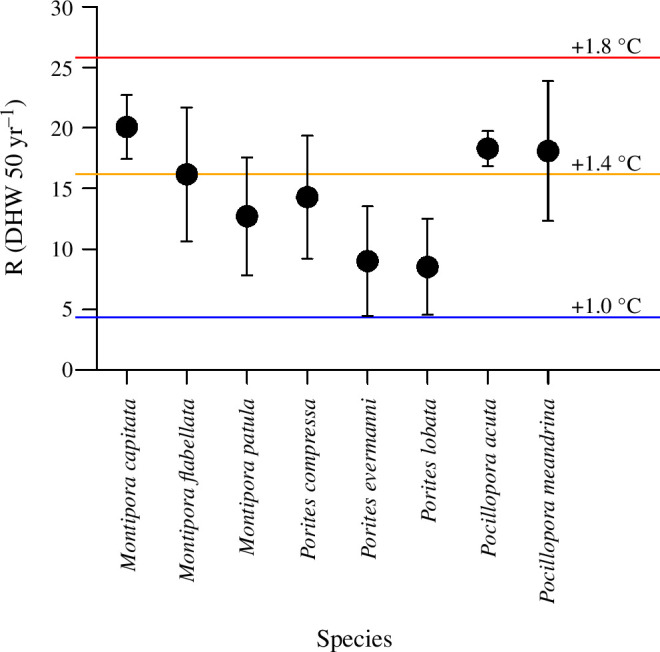
Estimated responses to selection for each species over the next 50 years. For Montipora capitata and Pocillopora acuta, values were empirically derived based on observed change in DHW tolerance between 1970 and 2017. For the remaining species, the values were estimated based on the heritability values derived here and assuming a selection coefficient similar to that for Montipora capitata. Horizontal lines show the mean annual DHW accumulation at warming levels of 1.0, 1.4, and 1.8°C, based on NOAA data for the main Hawaiian islands.
4. Discussion
The scope for corals to adapt to combined warming and acidification will play a key role in their responses to global change over coming decades [4,9,12,15,16,18,48]. While studies have sometimes assumed that corals exhibit low evolutionary potential and therefore the responses of future generations will mirror those of today [1,2,4,65,66], there is mounting evidence that many coral species harbour greater capacity to adapt to the changing climate than is often appreciated [8,19,21,22]. Because of the imminent threat that ocean warming poses to corals, most studies examining their capacity to adapt or acclimatize to novel conditions have focused on heat tolerance [21,22]. In contrast, and in spite of a rich literature characterizing coral responses to ocean acidification [6,9,18], far less is known about their capacity to adapt to reduced pH, and very few studies have examined their capacity to adapt to the combination of these two factors [59,60].
Corals often experience reduced calcification rates under acidification [9,18,48,65], but many of the species in this study showed comparatively small responses to reduced pH. Coral responses to acidification, however, are not necessarily linear [8,67–69]. Indeed, three of these species (Montipora capitata, Pocillopora acuta and Porites compressa) tend to achieve higher calcification rates under modest acidification (−0.15 pH units) [8] yet experience reduced calcification under more severe pH reductions (−0.3 to −0.4 pH units) [7], and all eight species tend to experience reduced calcification under severe acidification (−0.4 pH units) [7]. Coral responses to acidification, however, are complex and the mechanisms that govern them are not yet fully understood [9,18,70,71]. Under acidification, corals probably benefit from increased bicarbonate supply yet also suffer due to increased proton concentration [9,70]. The −0.2 pH unit change included in this study may roughly split the difference between the positive effects of carbon enrichment and the negative effects of higher proton concentration on calcification rates for many of these species.
None of the eight coral species examined here exhibited significant negative correlations between temperature and pH tolerance, and only one of the species (Pocillopora meandrina) exhibited a significant Temp × pH interaction, because its pH resistance was diminished under heating. Hence, none showed calcification responses consistent with there being clear trade-offs in their sensitivity to ocean warming and acidification and all eight species appear to be capable of adapting to each factor independently as well as the combination of both factors. For Pocillopora meandrina, however, the significant Temp × pH interaction suggests that this species has lower capacity to adapt to combined warming and acidification than it does to either factor by itself. If, however, there were no cost to maintaining comparatively high temperature or pH tolerance then these traits should have already gone to fixation within the populations, in which case we would have found very low heritability of this trait. Given the comparatively high heritability in all species, trade-offs may exist with unmeasured variables such as tolerance to other important environmental characteristics such as resistance to wave energy, reproductive output or disease tolerance. Rather, corals often show parabolic performance curves over a range of temperature and pH conditions [8,67–69], and there is no reason to think that all corals are best adapted to a particular set of conditions. Instead, these data suggest that coral individuals within populations exhibit a range of environmental tolerances and some corals are inherently better adapted to certain environments than are others.
In contrast to trade-offs, most of the species showed a significant positive correlation between temperature and pH tolerance. These responses are consistent with the hypothesis that sensitivity to both warming and acidification is related to general stress tolerance among colonies within these species. If that is the case, then these stress-tolerant individuals may also show higher performance under other sorts of environmental insults. Indeed, Wright et al. [59] found that tolerance to heat stress, acidification and pathogen exposure all tend to be positively correlated in an Australian coral. Furthermore, this relationship suggests that pH and temperature tolerance can co-evolve in all eight species.
The opportunity for adaptive change of populations in response to natural or artificial selection relies on the genetic variance underlying phenotypic traits, and predictive models of adaptation to global change require estimates of the proportion of that trait variance which is explained by heritable genetic factors [30,31]. This explanative proportion is most accurately estimated by narrow sense heritability (h2), which depends strictly on additive genetic variance and provides the fodder for natural selection [33]. Such studies, however, require enormous investment of resources to produce precise pedigrees or substantial genomic information [34]. In contrast, broad sense heritability (H2), as we measure here, is easier and faster to estimate, but also includes other heritable factors, such as dominance, epistatic, maternal and epigenetic effects. Thus, H2 provides an upper bound for narrow sense (h2) heritability [30,31,72]. However, both theory and data demonstrate that non-additive interactions at the level of individual genes are unlikely to greatly impact variance for complex traits controlled by many genes, such as thermal and acidification tolerances, and often close to 100% of the total observed variation is additive [37]. Despite the importance of heritability estimates, they remain elusive for scleractinian corals [21]. Thus, broad sense heritability (H2) provides a reasonable first pass at estimating the underlying genetic variance for coral thermal and pH tolerance, and at worst, provides an upper bound for the value. Here we show that the broad sense heritability of coral calcification rates under combined ocean warming and acidification is fairly high, ranging from 0.23 to 0.56, and consistent with other organisms in which life history traits are linked directly to fitness [73–75]. These values are also consistent with previous reports examining the heritability of scleractinian calcification under warming alone (H2 approx. 0.25–0.5) [21] or acidification alone (H2 approx. 0.32–0.61) [7]. A useful parameter is derived from the classical breeder’s equation, R = h2S, where R is the response to selection, h2 is the narrow sense heritability and S is the selection coefficient. Given caveats such as the fact that most coral populations are very large [76], the breeder’s equation provides a useful metric for our understanding of how coral populations may evolve in the future. While it is commonly assumed that corals are under strong selective pressure for higher temperature tolerances, given their recent and predicted future declines due to heating [1–4], and that they might be under selective pressure due to acidification [7], the paucity of quantitative data for selection coefficients contributes uncertainty to model predictions regarding coral responses to global change and the potential for adaptation.
Here, we empirically estimate not only the broad sense heritability (H2) of coral calcification rates under combined warming and acidification but also the selection coefficients (S) and responses to selection (R) for two of the species (Montipora capitata and Pocillopora acuta), derived from historical data, and assuming that h2 and H2 are similar. It is important to note that these selection coefficients represent the average strength of selection over the period 1970–2017. Without a doubt, both selection and responses to selection will vary over space and time depending on the environmental characteristics that coral populations experience. Given these caveats, and assuming that the remaining six coral species exhibit similar selection coefficients, we estimate that these coral species can probably increase their thermal tolerances by a mean of 8.5 DHW (Porites lobata) to 20.0 DHW (Montipora capitata) over the next 50 years. Considering the uncertainties in these estimates, these values correspond to an increase of approximately 1.0–1.7°C, depending on species. Under a high CO2 emissions scenario [77], none of these species are probably capable of keeping up with the greater than 3°C of warming expected by the end of the century. In contrast, if climate change is limited to no more than 2°C above the pre-industrial (approx. 0.8°C above present-day), in line with Paris Climate Agreement targets [78], then all of these species might be able to persist, albeit very likely with changes in coral reef community structure [47,64]. Hence, while these data show that diverse coral taxa possess heritable variation with clear capacity to respond to selection for both ocean warming and acidification, substantial climate change mitigation efforts remain essential if coral reefs are to persist over the twenty-first century and beyond.
Acknowledgements
Thanks to PL Jokiel, KS Rodgers, EW Barba, ‘A Dudoit, EC Johnston, RM Wright, the Coral Reef Ecology Lab and the ToBo lab.
Contributor Information
Christopher P. Jury, Email: jurycp@hawaii.edu.
Robert J. Toonen, Email: toonen@hawaii.edu.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Data and code are freely accessible through Dryad [79].
Supplementary material is available online [80].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
C.P.J.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, validation, visualization; R.J.T.: conceptualization, data curation, funding acquisition, methodology, project administration, resources, supervision.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Funding was provided by the National Science Foundation grant OA 1416889 (C.P.J., R.J.T.), Hawaiʻi Sea Grant Omnibus 2014–2016, Project ID 2180 (C.P.J., R.J.T.), and the National Oceanic and Atmospheric Administration’s Ocean Acidification Program (C.P.J., R.J.T.). This paper is funded in part by a grant/cooperative agreement from the National Oceanic and Atmospheric Administration, Project R/IR-23 which is sponsored by the University of Hawai‘i Sea Grant College Program, SOEST, under Institutional grant no. NA09OAR4170060 from NOAA Office of Sea Grant, Department of Commerce. The views expressed herein are those of the author(s) and do not necessarily reflect the views of NOAA or any of its subagencies. UNIHI-SEAGRANT-4938.
References
- 1. Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W. 2016. Climate change disables coral bleaching protection on the great barrier reef. Science 352 , 338–342. ( 10.1126/science.aac7125) [DOI] [PubMed] [Google Scholar]
- 2. Hoeke RK, Jokiel PL, Buddemeier RW, Brainard RE. 2011. Projected changes to growth and mortality of Hawaiian corals over the next 100 years. PLoS One 6 , e18038. ( 10.1371/journal.pone.0018038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennedy EV, et al. 2013. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 23 , 912–918. ( 10.1016/j.cub.2013.04.020) [DOI] [PubMed] [Google Scholar]
- 4. Cornwall CE, et al. 2021. Global declines in coral reef calcium carbonate production under ocean acidification and warming. Proc Natl Acad. Sci. USA 118 , e2015265118. ( 10.1073/pnas.2015265118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jokiel PL, Coles SL. 1990. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 8 , 155–162. ( 10.1007/BF00265006) [DOI] [Google Scholar]
- 6. Jokiel PL, Jury CP, Kuffner IB. 2016. Coral calcification and ocean acidification. In Coral reefs at the crossroads (eds Hubbard DK, Rogers CS, Lipps J, Stanley GD), pp. 7–45. Dordrecht, The Netherlands: Springer. ( 10.1007/978-94-017-7567-0_2) [DOI] [Google Scholar]
- 7. Jury CP, Delano MN, Toonen RJ. 2019. High heritability of coral calcification rates and evolutionary potential under ocean acidification. Sci. Rep. 9 , 20419. ( 10.1038/s41598-019-56313-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jury CP, Toonen RJ. 2019. Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proc. R. Soc. B 286 , 20190614. ( 10.1098/rspb.2019.0614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jury CP, Whitehead RF, Szmant AM. 2010. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (= Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob. Chang. Biol. 16 , 1632–1644. ( 10.1111/j.1365-2486.2009.02057.x) [DOI] [Google Scholar]
- 10. Albright R. 2011. Reviewing the effects of ocean acidification on sexual reproduction and early life history stages of reef-building corals. J. Mar. Biol. 2011 , 1–14. ( 10.1155/2011/473615) [DOI] [Google Scholar]
- 11. Fabricius KE, Noonan SHC, Abrego D, Harrington L, De’ath G. 2017. Low recruitment due to altered settlement substrata as primary constraint for coral communities under ocean acidification. Proc. R. Soc. B 284 , 20171536. ( 10.1098/rspb.2017.1536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donner SD. 2009. Coping with commitment: projected thermal stress on coral reefs under different future scenarios. PLoS One 4 , e5712. ( 10.1371/journal.pone.0005712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frieler K, Meinshausen M, Golly A, Mengel M, Lebek K, Donner SD, Hoegh-Guldberg O. 2013. Limiting global warming to 2°C is unlikely to save most coral reefs. Nat. Clim. Chang. 3 , 165–170. ( 10.1038/nclimate1674) [DOI] [Google Scholar]
- 14. McManus LC, Vasconcelos VV, Levin SA, Thompson DM, Kleypas JA, Castruccio FS, Curchitser EN, Watson JR. 2020. Extreme temperature events will drive coral decline in the Coral Triangle. Glob. Chang. Biol. 26 , 2120–2133. ( 10.1111/gcb.14972) [DOI] [PubMed] [Google Scholar]
- 15. McManus LC, et al. 2021. Evolution and connectivity influence the persistence and recovery of coral reefs under climate change in the Caribbean, Southwest Pacific, and Coral Triangle. Glob. Chang. Biol. 27 , 4307–4321. ( 10.1111/gcb.15725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McManus LC, et al. 2021. Evolution reverses the effect of network structure on metapopulation persistence. Ecology 102 , e03381. ( 10.1002/ecy.3381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Logan CA, Dunne JP, Eakin CM, Donner SD. 2014. Incorporating adaptive responses into future projections of coral bleaching. Glob. Chang. Biol. 20 , 125–139. ( 10.1111/gcb.12390) [DOI] [PubMed] [Google Scholar]
- 18. Jury CP, Jokiel PL. 2016. Climate change, ocean chemistry, and the evolution of reefs through time. In Coral reefs at the crossroads(eds Hubbard DKRogers C, Lipps J, Stanley G), pp. 197–223. Dordrecht, The Netherlands: Springer. ( 10.1007/978-94-017-7567-0_9) [DOI] [Google Scholar]
- 19. Coles SL, Bahr KD, Rodgers KS, May SL, McGowan AE, Tsang A, Bumgarner J, Han JH. 2018. Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ 6 , e5347. ( 10.7717/peerj.5347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes TP, et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301 , 929–933. ( 10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- 21. Bairos‐Novak KR, Hoogenboom MO, van Oppen MJH, Connolly SR. 2021. Coral adaptation to climate change: meta‐analysis reveals high heritability across multiple traits. Glob. Chang. Biol. 27 , 5694–5710. ( 10.1111/gcb.15829) [DOI] [PubMed] [Google Scholar]
- 22. Humanes A, et al. 2022. Within-population variability in coral heat tolerance indicates climate adaptation potential. Proc. R. Soc. B 289 , 20220872. ( 10.1098/rspb.2022.0872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Angilletta MJ Jr, Wilson RS, Navas CA, James RS. 2003. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol. Evol. 18 , 234–240. ( 10.1016/S0169-5347(03)00087-9) [DOI] [Google Scholar]
- 24. Puglielli G, Pavanetto N, Laanisto L. 2022. Towards a ‘periodic table’ of abiotic stress tolerance strategies of woody plants. Flora 292 , 152089. ( 10.1016/j.flora.2022.152089) [DOI] [Google Scholar]
- 25. Stat M, Gates RD. 2011. Clade D symbiodinium in scleractinian corals: a ‘nugget of hope’, a selfish opportunist, an ominous sign, or all of the above? J. Mar. Biol. 2011 , 1–9. ( 10.1155/2011/730715) [DOI] [Google Scholar]
- 26. Berkelmans R, van Oppen MJH. 2006. The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proc. R. Soc. B 273 , 2305–2312. ( 10.1098/rspb.2006.3567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cunning R, Gillette P, Capo T, Galvez K, Baker AC. 2015. Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs 34 , 155–160. ( 10.1007/s00338-014-1216-4) [DOI] [Google Scholar]
- 28. Cunning R, Silverstein RN, Baker AC. 2015. Investigating the causes and consequences of symbiont shuffling in a multi-partner reef coral symbiosis under environmental change. Proc. R. Soc. B 282 , 20141725. ( 10.1098/rspb.2014.1725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein AM, Sturm AB, Eckert RJ, Walker BK, Neely KL, Voss JD. 2024. Algal symbiont genera but not coral host genotypes correlate to stony coral tissue loss disease susceptibility among Orbicella faveolata colonies in South Florida. Front. Mar. Sci. 11 . ( 10.3389/fmars.2024.1287457) [DOI] [Google Scholar]
- 30. Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics, 4th edn. Harlow, UK: Longmans Green. [Google Scholar]
- 31. Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits, 1st edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 32. Allendorf FW, Hohenlohe PA, Luikart G. 2010. Genomics and the future of conservation genetics. Nat. Rev. Genet. 11 , 697–709. ( 10.1038/nrg2844) [DOI] [PubMed] [Google Scholar]
- 33. Visscher PM, Hill WG, Wray NR. 2008. Heritability in the genomics era — concepts and misconceptions. Nat. Rev. Genet. 9 , 255–266. ( 10.1038/nrg2322) [DOI] [PubMed] [Google Scholar]
- 34. Klein TW. 1974. Heritability and genetic correlation: statistical power, population comparisons, and sample size. Behav. Genet. 4 , 171–189. ( 10.1007/BF01065758) [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Vik JO, Omholt SW, Gjuvsland AB. 2013. Effect of regulatory architecture on broad versus narrow sense heritability. PLoS Comput. Biol. 9 , e1003053. ( 10.1371/journal.pcbi.1003053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt P, Hartung J, Rath J, Piepho HP. 2019. Estimating broad‐sense heritability with unbalanced data from agricultural cultivar trials. Crop Sci. 59 , 525–536. ( 10.2135/cropsci2018.06.0376) [DOI] [Google Scholar]
- 37. Hill WG, Goddard ME, Visscher PM. 2008. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 4 , e1000008. ( 10.1371/journal.pgen.1000008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ. 2009. Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol. Biol. 9 , 45. ( 10.1186/1471-2148-9-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forsman ZH, Concepcion GT, Haverkort RD, Shaw RW, Maragos JE, Toonen RJ. 2010. Ecomorph or endangered coral? DNA and microstructure reveal Hawaiian species complexes: Montipora dilatata/flabellata/turgescens & M. patula/verrilli. PLoS One 5 , e15021. ( 10.1371/journal.pone.0015021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Veron JEN, Stafford-Smith MG, Turak E, DeVantier LM. 2016. Corals of the world. See http://coralsoftheworld.org.
- 41. Bahr KD, Tran T, Jury CP, Toonen RJ. 2020. Abundance, size, and survival of recruits of the reef coral Pocillopora acuta under ocean warming and acidification. PLoS One 15 , e0228168. ( 10.1371/journal.pone.0228168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McLachlan RH, Price JT, Muñoz-Garcia A, Weisleder NL, Levas SJ, Jury CP, Toonen RJ, Grottoli AG. 2022. Physiological acclimatization in Hawaiian corals following a 22-month shift in baseline seawater temperature and pH. Sci. Rep. 12 , 3712. ( 10.1038/s41598-022-06896-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henley EM, Bouwmeester J, Jury CP, Toonen RJ, Quinn M, Lager CVA, Hagedorn M. 2022. Growth and survival among Hawaiian corals outplanted from tanks to an ocean nursery are driven by individual genotype and species differences rather than preconditioning to thermal stress. PeerJ 10 , e13112. ( 10.7717/peerj.13112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Timmers MA, Vicente J, Webb M, Jury CP, Toonen RJ. 2022. Sponging up diversity: evaluating metabarcoding performance for a taxonomically challenging phylum within a complex cryptobenthic community. Environmental DNA 4 , 239–253. ( 10.1002/edn3.163) [DOI] [Google Scholar]
- 45. Vicente J, Timmers MA, Webb MK, Bahr KD, Jury CP, Toonen RJ. 2022. Ecological succession of the sponge cryptofauna in Hawaiian reefs add new insights to detritus production by pioneering species. Sci. Rep. 12 , 15093. ( 10.1038/s41598-022-18856-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vicente J, Webb MK, Paulay G, Rakchai W, Timmers MA, Jury CP, Bahr K, Toonen RJ. 2022. Unveiling hidden sponge biodiversity within the Hawaiian reef cryptofauna. Coral Reefs 41 , 727–742. ( 10.1007/s00338-021-02109-7) [DOI] [Google Scholar]
- 47. Timmers MA, Jury CP, Vicente J, Bahr KD, Webb MK, Toonen RJ. 2021. Biodiversity of coral reef cryptobiota shuffles but does not decline under the combined stressors of ocean warming and acidification. Proc. Natl Acad. Sci. USA 118 , e2103275118. ( 10.1073/pnas.2103275118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT. 2008. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27 , 473–483. ( 10.1007/s00338-008-0380-9) [DOI] [Google Scholar]
- 49. Cros A, Toonen RJ, Davies SW, Karl SA. 2016. Population genetic structure between Yap and Palau for the coral Acropora hyacinthus. PeerJ 4 , e2330. ( 10.7717/peerj.2330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cros A, Toonen RJ, Donahue MJ, Karl SA. 2017. Connecting Palau’s marine protected areas: a population genetic approach to conservation. Coral Reefs 36 , 735–748. ( 10.1007/s00338-017-1565-x) [DOI] [Google Scholar]
- 51. Concepcion GT, Polato NR, Baums IB, Toonen RJ. 2010. Development of microsatellite markers from four Hawaiian corals: Acropora cytherea, Fungia scutaria, Montipora capitata and Porites lobata. Conserv. Genet. Resour. 2 , 11–15. ( 10.1007/s12686-009-9118-4) [DOI] [Google Scholar]
- 52. Faircloth BC, Glenn TC. 2012. Not all sequence tags are created equal: designing and validating sequence identification tags robust to indels. PLoS One 7 , e42543. ( 10.1371/journal.pone.0042543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meirmans P. In press. Genodive version 2.b14. [Google Scholar]
- 54. Selkoe KA, Toonen RJ. 2006. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol. Lett. 9 , 615–629. ( 10.1111/j.1461-0248.2006.00889.x) [DOI] [PubMed] [Google Scholar]
- 55. Meirmans PG, Tienderen PHV. 2004. Genotype and genodive: two programs for the analysis of genetic diversity of asexual organisms. Mol. Ecol. Notes 4 , 792–794. ( 10.1111/j.1471-8286.2004.00770.x) [DOI] [Google Scholar]
- 56. Dore JE, Lukas R, Sadler DW, Church MJ, Karl DM. 2009. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proc. Natl Acad. Sci. USA 106 , 12 235–12 240. ( 10.1073/pnas.0906044106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jury CP, Thomas FIM, Atkinson MJ, Toonen RJ. 2013. Buffer capacity, ecosystem feedbacks, and seawater chemistry under global change. Water 5 , 1303–1325. ( 10.3390/w5031303) [DOI] [Google Scholar]
- 58. Jokiel PL, Coles SL. 1977. Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar. Biol. 43 , 201–208. ( 10.1007/BF00402312) [DOI] [Google Scholar]
- 59. Wright RM, Mera H, Kenkel CD, Nayfa M, Bay LK, Matz MV. 2019. Positive genetic associations among fitness traits support evolvability of a reef-building coral under multiple stressors. Glob. Chang. Biol. 25 , 3294–3304. ( 10.1111/gcb.14764) [DOI] [PubMed] [Google Scholar]
- 60. Muller EM, Dungan AM, Million WC, Eaton KR, Petrik C, Bartels E, Hall ER, Kenkel CD. 2021. Heritable variation and lack of tradeoffs suggest adaptive capacity in Acropora cervicornis despite negative synergism under climate change scenarios. Proc. R. Soc. B 288 , 20210923. ( 10.1098/rspb.2021.0923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33 , 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 62. Piepho HP, Möhring J. 2007. Computing heritability and selection response from unbalanced plant breeding trials. Genetics 177 , 1881–1888. ( 10.1534/genetics.107.074229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. R Core Team . 2020. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org. [Google Scholar]
- 64. Jury C, et al. Submitted. Experimental reef communities persist under future ocean acidification and warming. ( 10.21203/rs.3.rs-640089/v1) [DOI]
- 65. Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. 2011. Projecting coral reef futures under global warming and ocean acidification. Science 333 , 418–422. ( 10.1126/science.1204794) [DOI] [PubMed] [Google Scholar]
- 66. Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ. 2013. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16 , 1488–1500. ( 10.1111/ele.12185) [DOI] [PubMed] [Google Scholar]
- 67. Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37 , 1131–1134. ( 10.1130/G30210A.1) [DOI] [Google Scholar]
- 68. Castillo KD, Ries JB, Bruno JF, Westfield IT. 2014. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. R. Soc. B 281 , 20141856. ( 10.1098/rspb.2014.1856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bove CB, Ries JB, Davies SW, Westfield IT, Umbanhowar J, Castillo KD. 2019. Common Caribbean corals exhibit highly variable responses to future acidification and warming. Proc. R. Soc. B 286 , 20182840. ( 10.1098/rspb.2018.2840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jokiel PL. 2011. Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. bms 87 , 639–657. ( 10.5343/bms.2010.1107) [DOI] [Google Scholar]
- 71. Jokiel PL. 2016. Predicting the impact of ocean acidification on coral reefs: evaluating the assumptions involved. ICES J. Mar. Sci. 73 , 550–557. ( 10.1093/icesjms/fsv091) [DOI] [Google Scholar]
- 72. Singh M, Ceccarelli S, Hamblin J. 1993. Estimation of heritability from varietal trials data. Theoret. Appl. Genetics 86 , 437–441. ( 10.1007/BF00838558) [DOI] [PubMed] [Google Scholar]
- 73. Mousseau TA, Roff DA. 1987. Natural selection and the heritability of fitness components. Heredity 59 , 181–197. ( 10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- 74. Price T, Schulter D. 1991. On the low heritability of life-history traits. Evolution 45 , 853. ( 10.2307/2409693) [DOI] [PubMed] [Google Scholar]
- 75. Stirling DG, Réale D, Roff DA. 2002. Selection, structure and the heritability of behaviour. J. Evol. Biol. 15 , 277–289. ( 10.1046/j.1420-9101.2002.00389.x) [DOI] [Google Scholar]
- 76. Tsounis G, Edmunds PJ. 2016. The potential for self-seeding by the coral Pocillopora spp. in Moorea, French Polynesia. PeerJ 4 , e2544. ( 10.7717/peerj.2544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. IPCC . 2023. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (eds.)], pp. 35–115. Geneva, Switzerland: IPCC. ( 10.59327/IPCC/AR6-9789291691647) [DOI] [Google Scholar]
- 78. Rogelj J, et al. 2016. Paris agreement climate proposals need a boost to keep warming well below 2 °C. Nature 534 , 631–639. ( 10.1038/nature18307) [DOI] [PubMed] [Google Scholar]
- 79. Jury C, Toonen R. 2024. Data for: Environmental and calcification data for widespread scope for coral adaptation under combined ocean warming and acidification. Dryad Digital Repository. ( 10.5061/dryad.kwh70rzdc) [DOI] [PubMed]
- 80. Jury CP, Toonen R. 2024. Data from: Widespread scope for coral adaptation under combined ocean warming and acidification. Figshare. ( 10.6084/m9.figshare.c.7449541) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code are freely accessible through Dryad [79].
Supplementary material is available online [80].