Abstract
In their natural habitats, animals experience multiple ecological factors and regulate their social responses accordingly. To unravel the impact of two ecological factors on the immediate behavioural response of groups, we conducted experiments on wild zebrafish shoals in arenas with vegetation, predator cues, and both factors simultaneously or neither (control treatments). Analysis of 297 trials revealed that while shoals formed significantly larger subgroups in the presence of predator cues, their subgroup size was comparable to control treatments when they faced predator cues and vegetation. Shoals were highly polarized in open arenas, in the absence of either ecological factors and in the presence of predator cues (with/without vegetation). The presence of vegetation alone, however, significantly reduced shoal polarization. Furthermore, food intake was significantly reduced when predator cues and/or vegetation were present. Tracking individuals revealed that (i) individuals within shoals receiving predator cues had a significantly higher probability to continue being in a group compared with control treatments and (ii) individuals occupying the front positions deviated less from their median position within a shoal as compared with other individuals regardless of predator cues. The adaptability of animals depends on behavioural responses to changing environments, making this study significant in the context of environmental changes.
Keywords: behavioural plasticity, group behaviour, wild zebrafish, predation, vegetation
1. Introduction
Across taxa, ecological factors such as predation, vegetation, resource availability and habitat complexity shape behaviour [1–5]. Behavioural changes indirectly control a variety of large-scale ecological functions such as nutrient cycling, primary productivity, pathogen transfer, inter-species interactions and habitat provision [6]. Although variations in ecological factors over evolutionary timescales elicit long-term behavioural changes [7,8], sudden changes in these factors can trigger immediate behavioural responses. Immediate behavioural changes are reversible, can determine an individual’s success or adaptability in a new environment and, consequently, often guide evolutionary change [9]. As one of the quickest responses to environmental shifts, behavioural changes are crucial in determining an animal’s success in a changing environment, making their study essential.
In the wild, behavioural responses elicited by animals to environmental changes are dependent on a multitude of ecological variables. To comprehend an animal’s immediate responses to multiple factors, it is essential to grasp how ecological factors, either individually or in combination, exert distinct influences on behaviour. In the present study, using a shoaling cyprinid, the zebrafish (Danio rerio), we gain insight into immediate group-level behavioural response to changes in their habitat. We examine the immediate shoal responses of wild-caught zebrafish towards two key ecological factors: predation and vegetation cover.
The influence of predation and vegetation cover has been studied on several terrestrial taxa. For instance, in lions (Panthera leo), males rely on the ambush hunting strategy, and therefore to successfully hunt heavily rely on dense vegetation [10]. In the case of fox squirrels (Sciurus niger), the role of vegetation structure on anti-predator behaviour is dependent on the type of predators [11]. The water-drinking behaviour and frequency in red colobus monkeys (Procolobus kirkii) are significantly influenced by the time spent by them in mangroves [12]. Studies have long examined anti-predator responses, such as heightened group cohesion and increased group size (safety in numbers), heightened vigilance and improved predator confusion, as strategies to reduce individual vulnerability to attacks [13,14]. For instance, elks (Cervus elaphus) move into protective cover (timber) in response to wolf presence [15]. Meerkats (Suricata suricatta) produce several discrete call types to convey changes in predation risk [16].
In aquatic habitats too, behaviour is greatly influenced by both predation and vegetation cover. For instance, in fishes, studies have shown that predation strongly shapes social interactions and shoal properties within shoals [17–19]. The predator avoidance strategy in golden shiners (Notemigonus crysoleucas) is dependent on the attack strategy of their predator [20]. In other fish species, the presence of vegetation, on the other hand, decreases prey capture, swimming speed and shoaling tendencies [21,22].
Here, we aim to gain insight into the behavioural plasticity exhibited by shoals in response to ecological variables. We recorded the immediate group-level changes in the presence of vegetation and/or predation, we recorded the responses of wild zebrafish shoals in the presence of these ecological variables. In shoaling fishes, predator avoidance responses and their ability to forage are directly linked to their survival [23–26], and hence we examine these behaviours in response to vegetation/predation pressure. Previous literature suggests that fish shoals adhere to safety in numbers in the presence of a predator [14,27,28]. Shoal polarization or the alignment of shoal members in a common direction (exhibited for coordinated motion) is known to be disrupted in the presence of a predator. When shoals encounter a predator, shoal polarization either increases or decreases depending on the species [19,20,29–36].
Wild zebrafish occurring in freshwater streams along the Gangetic drainage in India experience variable predation risk, ranging from moderate to high dependent on habitat and vegetation characteristics (personal observation). These habitats frequently undergo dynamic changes, with temporal and/or spatial fluctuations in vegetation and predation pressure, influenced by factors such as seasonality or anthropogenic alterations. Hence, zebrafish shoals would be likely to exhibit notable plasticity in their shoaling properties as responses to variations in these ecological factors. We hypothesized that when individuals are exposed to sudden environmental changes in the form of predator cues they would (i) form large, polarized or polarized groups and (ii) move away less from the group. In the wild, zebrafish are known to shoal among vegetation (personal observation, [37–41]), and therefore it is likely that vegetation plays an important role in shaping anti-predator responses in zebrafish. We speculated that in the presence of vegetation, individuals would take refuge underneath vegetation—a common anti-predator response in fishes [39–42]. Furthermore, we also hypothesized that foraging (food intake) would reduce (i) in the presence of predator cues as individuals would engage in anti-predator behaviour (over foraging) and (ii) in the presence of vegetation as there would be a reduction in visual information on the presence of food.
2. Methods
Wild zebrafish shoals were collected from shallow water bodies on the Ganges drainage basin in West Bengal in December 2019 and January 2020 (habitat specifics detailed in electronic supplementary material, §S1). Shoals were brought to the laboratory and were maintained in bare, aerated 60 l tanks filled with aged filtered tap water. Shoals were maintained at a density of 100–120 individuals per tank. Four Channa spp. (snakeheads) individuals were also collected from the same habitat (mean length: 12 cm), brought to the laboratory and were kept in 18 l tanks (one individual per tank). A temperature range of 23–25°C and a constant lighting condition of 12 h dark : 12 h light in the laboratory were maintained. While the zebrafish were fed daily ad libitum with freeze-dried bloodworms or brine shrimp (Artemia spp.), the snakeheads were fed daily with pellet food or zebrafish that died of natural causes.
2.1. Experiments
Shoals were gently introduced into a 75 cm × 75 cm ×12 cm tank and were recorded for 20 min under the following four treatments. (i) Control treatments (CT) in which shoals were placed in an arena without predator cues or vegetation. (ii) Predator treatments (PT) in which, following previous studies, olfactory cues from their natural predator (6.5 l of tank water collected from a Channa tank) were gradually added to the arena centre [43]. Previous studies show that water from a tank housing a predator contains olfactory cues from the predator that evoke anti-predator responses in prey [44–47]. Thus, water from a Channa tank was added in treatments simulating the presence of a predator. Prior control experiments conducted in the laboratory have established that the gentle addition of water into the arena centre had no impact on shoaling properties (electronic supplementary material, figure S1). Prey species elicit anti-predator responses based on previous exposure to predators [48]. The test shoals were wild-caught and thus would recognize the danger of predator cues and elicit anti-predator responses. (iii) Vegetation treatments (VT) in which shoals were recorded in an arena with six identical aquarium plants, three on each diagonally opposed corner (two corners were devoid of vegetation). (iv) Predator and vegetation treatments (PVT) in which shoals were placed in an arena with vegetation, after which predator cues were gradually added.
Experiments were performed between 11.00 and 15.00. In their natural habitats, wild zebrafish typically form shoals with 10–20 individuals [49], and thus a shoal size of 10 individuals was maintained across all trials. Throughout the trials, the test arena maintained a consistent water depth of 5 cm. Shoals encountering predator cues were initially introduced into the arena with a water depth of approximately 4 cm. This initial depth was adjusted to reach 5 cm upon the addition of olfactory cues. To avoid the impact of sex of individuals on shoaling behaviour [50,51], we randomly chose individuals who constituted a shoal and thereby maintained the population sex ratio of a roughly equal number of males and females (as seen in natural populations). A 3.5 cm thermocol (polystyrene) sheet was placed under the arena to minimize ground vibrations. Two 20 W LED light bulbs on either side of the arena maintained a constant light source. Two minutes after the gradual addition of predator cues (to allow shoals to recover from disturbances—if any), or immediately after acclimatization in VT or CT, the shoal was video recorded for 20 min at 25 frames per second using an overhead camera (Canon Legria HF R306). Following this, 0.25 g of freeze-dried blood worms were introduced into the arena centre and the shoal was video recorded for another 5 min. The arena was emptied and rinsed with aged water between consecutive trials to remove cues from blood worms, from conspecifics or from a predator. Sixty unique shoals (30 across all treatments, 15 additional in PT and 15 additional in CT) were tested by performing 210 randomized trials (150 shoaling trials and 60 foraging trials). Each shoal was tested once per day and 6–8 trials were conducted daily. A shoal was tested across all four treatments in a randomized order over the course of four consecutive days. Between trials, each shoal was kept in a separate 25 l tank to maintain identity. A single observer (I.M.) blind to the treatment analysed the video recordings.
To extend the findings of the above experiment to field conditions, a follow-up experiment was performed in their natural habitat. In their natural habitats, predator cues may be less potent and occur with a variety of other cues. This study was conducted to compare the time taken for a shoal to emerge from underneath vegetation between treatments conducted in a controlled laboratory setup (in the absence of cues) and those carried out in the field, with the presence of various cues, including predator cues. The detailed experimental protocol for the follow-up study has been provided in electronic supplementary material, §S2.
2.2. Data preparation
From the video recording, shoaling behaviour was quantified by manually noting the size (number of individuals) of the largest subgroup and the polarization state of shoals every 30 s (750 frames) for 20 min. Individuals were regarded to be within a subgroup if any portion of their bodies was within two body lengths of another. The polarization score was calculated using the following as the proportion of the largest subgroup’s members aligned in a common direction [52,53], with an angle between −30° and +30° (manually calculated by inspecting each frame every 30 s on ImageJ [54]) and also as the mean heading vector of the whole group. We found the methods fetching comparable results (as detailed in electronic supplementary material, §S3). Owing to the fact that individuals in treatments with vegetation could not be tracked, we relied on the manual calculation to compare all four treatments. To check whether largest subgroup size and shoal polarization were consistent over time, the 20 min videos were divided into four 5 min sessions (sessions 1, 2, 3 and 4, in sequence of recording). Thereafter, the mean largest subgroup size and the mean polarization score for each session were calculated. The proportion of shoal underneath vegetation in VT and PVT treatments was determined every 30 s. To estimate foraging across the four treatments, the number of bites at bloodworms by each shoal in the first 2 min of the recording was counted.
To analyse temporal dynamics in shoals, individuals in CT and PT treatments were first tracked for the first 5 min (or 7500 frames) using idTracker. Thereafter, errors in their trajectories (if any) were manually corrected using an assisting software (idPlayer) to reach a tracking accuracy of almost 100% [55]. All individuals across 30 shoals (15 per treatment) were tracked to obtain 300 trajectories. Tracking in treatments with vegetation (VT and PVT) was not feasible as it was not possible to determine the precise position of fish underneath vegetation. Following Borner et al. [56] and Krause & Ruxton [57], we assigned solitary or group states to individuals every 10 s (250 frames). While individuals within four-body-length distance from other individuals were considered a group, individuals outside this zone were solitary. The rationale for setting the criteria of being within four body lengths to be in group state and setting the criteria of being within two body lengths to be a part of a subgroup is as follows: four body lengths is a looser cut-off and is optimum (as supported by other studies on schooling fish) to categorize whether an individual is within a group or is alone. On the other hand, two body lengths is a more stringent cut-off, and therefore was used to assign individuals into subgroups. The probability of not switching states, i.e. remaining solitary and remaining in group state, was calculated for each individual.
Next, following Doughty et al. [58], we manually noted down the movement order of individuals within the largest subgroup every 10 s (every 250 frames). The leader of the largest subgroup (of size n) received place 1, the individual spatially closest to the leader received position 2 and so forth. The last position was n. Following Fischhoff et al. [59], we normalized for variations in the largest subgroup size by calculating their order index using the following formula:
The order index was calculated every 10 s across 5 min i.e. a total of 30 times. Thereafter, from multiple order indices, the median order index of individuals or the mid-value of the all order indices was calculated. The standard deviation from their median order indexes was calculated using the following formula:
2.3. Statistical analysis
All analyses were performed using R Studio [60]. Generalized linear mixed models (GLMMs) were built (using ‘lme4’ [61] and ‘lmerTest’ [62]) to understand the effect of (i) treatment (CT, PT, VT or PVT) and session (sessions 1–4) on largest subgroup size, (ii) treatment, session and size of subgroup (subgroups comprising more than five individuals were considered big and fewer than five individuals were considered small) on shoal polarization, and (iii) treatment on percentage shoal under vegetation. In these GLMMs, shoal identity was incorporated as the random factor. Similarly, separate generalized linear models (GLMs) were built for parameters which did not involve repeated measures: GLMs were built to understand the effect of treatment on (i) foraging behaviour (number of bites at worms) and (ii) the probability of not switching states (remaining solitary or remaining in a group). We checked the distribution of our data using the ‘fitdistr’ function [63] and as our data were closest to the normal (Gaussian) distribution we ran models assuming the Gaussian distribution of the data. Model comparisons were performed using ANOVA in ‘car’ package [61] and post hoc paired tests (Tukey’s post hoc HSD test using the ‘multcomp’ package [64]) were performed for comparing the effects of factors that were significant. Spearman’s correlation tests were then performed to analyse the relationship between their median position index and standard deviation from the median position index. Mean ± s.e. values have been reported throughout the paper. Two-tailed p ≤ 0.05 were considered significantly different.
3. Results
3.1. Shoaling and foraging behaviour
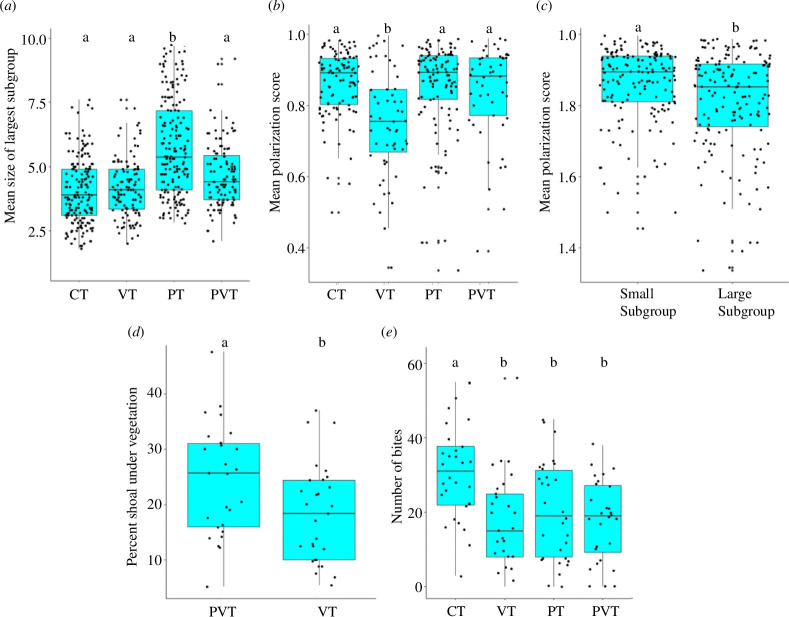
The GLMM revealed that the mean largest subgroup size was significantly impacted by treatment (Wald type IIχ2 = 163.65, d.f. = 3, p < 0.001) and that the mean largest subgroup size was comparable across sessions (Wald type IIχ2 = 1.79, d.f. = 4, p = 0.77; table 1a ). The mean largest subgroup size of PT shoals (mean = 5.72 ± 0.13) was significantly greater than the mean largest subgroup size of CT (mean = 4.06 ± 0.09), VT (mean = 4.18 ± 0.10) and PVT (mean = 4.64 ± 0.12) shoals (Tukey’s test results: CT versus PT: Z value = 11.24, p < 0.001; VT versus PT: Z value = −10.09, p < 0.001; PT versus PVT: Z value = −7.76, p < 0.001; figure 1a ).
Table 1a.
Results of the GLMM for predicting the effect of treatment and session on mean largest subgroup size. Model: mean size of largest subgroup ~ session + treatment + (1|shoalid).
| coefficients | ||||
|---|---|---|---|---|
| estimate | s.e. | t value | Pr (>|t|) | |
| (intercept) | <0.001 | <0.001 | 13.36 | <0.0001 |
| session 1 | <0.001 | <0.001 | −0.01 | 0.99 |
| session 2 | <0.001 | <0.001 | 0.64 | 0.52 |
| session 3 | <0.001 | <0.001 | 0.3 | 0.76 |
| session 4 | <0.001 | <0.001 | 0.19 | 0.85 |
| PT | <0.001 | <0.001 | 11.23 | <0.001 |
| PVT | <0.001 | <0.001 | 3.2 | <0.01 |
| VT | <0.001 | <0.001 | −0.01 | 0.99 |
Figure 1.
Box-and-whisker plots across treatments representing: (a) the mean size of largest subgroup, (b) the mean polarization score across treatments, (c) the mean polarization score across small and large subgroups, (d) the percent shoal under vegetation in the presence and absence of predator cues and (e) number of bites at worms. Each dot represents the average over one session of four sessions per group (a–c) or the average over all sessions (d) or the frequency of a unique group (e). The different letters (‘a’ and ‘b’) placed above the boxes represent significant differences between the treatments. The letters ‘ab’ indicate that the treatment is comparable to treatments ‘a’ and ‘b’. CT, control treatment; PT, predator treatment; PVT, predator vegetation treatment; VT, vegetation treatment. Comparisons were performed using Tukey’s HSD test (sample size: mean size of largest subgroup, mean polarization score: N CT = N PT = 45 shoals; N VT = N PVT = 30 shoals; p < 0.05).
The GLMM revealed that the mean polarization score was significantly impacted by treatment (Wald type IIχ2 = 57.77, d.f. = 3, p < 0.001), size of subgroup (Wald type IIχ2 = 15.38, d.f. = 1, p < 0.001) and session (Wald type IIχ2 = 25.46, d.f. = 4, p < 0.001; table 1b ). The mean polarization score of VT shoals (0.75 ± 0.02) was significantly lower than the mean polarization score of CT (mean = 0.86 ± 0.01), PT (mean = 0.85 ± 0.01) and PVT (mean = 0.83 ± 0.01) shoals (Tukey’s test results: CT versus VT: Z value = −6.79, p < 0.001; VT versus PT: Z value = −7.19, p < 0.001; VT versus PVT: Z value = −5.14, p < 0.001; figure 1b ). Small subgroups were more polarized (0.86 ± 0.01) than large subgroups (mean = 0.80 ± 0.01) (Tukey’s test results: small subgroups versus large subgroups: Z value = 3.92, p < 0.0001; figure 1c ) and the mean polarization score in session 4 (mean = 0.72 ± 0.02) was significantly lower than the mean polarization score in sessions 2 (mean = 0.82 ± 0.01) and 3 (mean = 0.86 ± 0.01) (Tukey’s test results: session 4 versus session 2: Z value = −4.47, p < 0.001; session 4 versus session 3: Z value = −4.10, p < 0.001).
Table 1b.
Results of the GLMM for predicting the effect of treatment, session and subgroup size on mean polarization score. Model: mean polarization score ~ session + treatment + size+ (1|shoalid).
| coefficients | |||||
|---|---|---|---|---|---|
| estimate | s.e. | d.f. | t value | Pr (>|t|) | |
| (intercept) | 0.89 | 0.03 | 303.4 | 22.72 | <0.0001 |
| session 1 | −0.07 | 0.03 | 273.49 | −1.99 | 0.04 |
| session 2 | −0.04 | 0.03 | 271.47 | −1.26 | 0.2 |
| session 3 | −0.05 | 0.03 | 273.25 | −1.46 | 0.14 |
| session 4 | −0.13 | 0.03 | 272.85 | −3.43 | <0.001 |
| PT | 0.009 | 0.01 | 309.99 | 0.54 | 0.58 |
| PVT | −0.02 | 0.02 | 298.32 | −1.03 | 0.30 |
| VT | −0.14 | 0.02 | 301.24 | −6.79 | <0.001 |
| small | 0.05 | 0.01 | 259.59 | 3.92 | <0.001 |
The GLMM revealed a significant effect of treatment on percentage shoal under vegetation (Wald type IIχ2 = 3.96, d.f. = 1, p = 0.04; table 1c )—a significantly greater percentage of individuals were under vegetation in PVT (mean = 18.23 ± 1.58%) as compared with VT (mean = 24.31 ± 1.89%) (Tukey’s test results: VT versus PVT: Z value = −1.99; p = 0.04; figure 1d ). Although statistically comparable, the time taken to emerge out of vegetation in the laboratory (in absence of cues) (mean = 181.56 ± 37.38 s) was less than that in the field in the presence of a variety of cues (mean = 301.43 ± 50.98 s) (Wilcox unpaired test results: W = 54, p = 0.07; electronic supplementary material, figure S2).
Table 1c.
Results of the GLMM for predicting the effect of treatment on percent shoal under vegetation. Model: percent shoal under vegetation ~ treatment + (1|shoalid).
| estimate | s.e. | d.f. | t value | Pr (>|t| | |
|---|---|---|---|---|---|
| (intercept) | 23.69 | 1.92 | 52.87 | 12.34 | <0.0001 |
| VT | −4.94 | 2.48 | 29.64 | −1.99 | 0.05 |
The GLM revealed a significant effect of treatment (Wald type IIχ2 = 22.78, d.f. = 3, p < 0.001) on number of bites at worms (table 1d ): CT shoals bit significantly more worms (mean = 30.93 ± 2.31 bites) than shoals in VT (mean = 17.41 ± 2.32 bites), PT (mean = 20.33 ± 2.40 bites) or PVT (mean = 17.75 ± 2.01 bites) (Tukey’s test results: CT versus PT : Z value = −3.27, p < 0.01; CT versus PVT : Z value = −4.00, p < 0.001; CT versus VT: −4.14, p < 0.001; figure 1e ).
Table 1d.
Results of the GLM for predicting the effect of treatment on number of bites at prey (blood worms). Model: number of bites ~ treatment.
| coefficients | ||||
|---|---|---|---|---|
| estimate | s.e. | t value | Pr (>|t|) | |
| (intercept) | ||||
| PT | −10.6 | 3.23 | −3.27 | 0.001 |
| PVT | −13.18 | 3.29 | −4 | <0.001 |
| VT | −13.52 | 3.26 | −4.14 | <0.0001 |
3.2. Shoal dynamics within shoals receiving cues from a predator
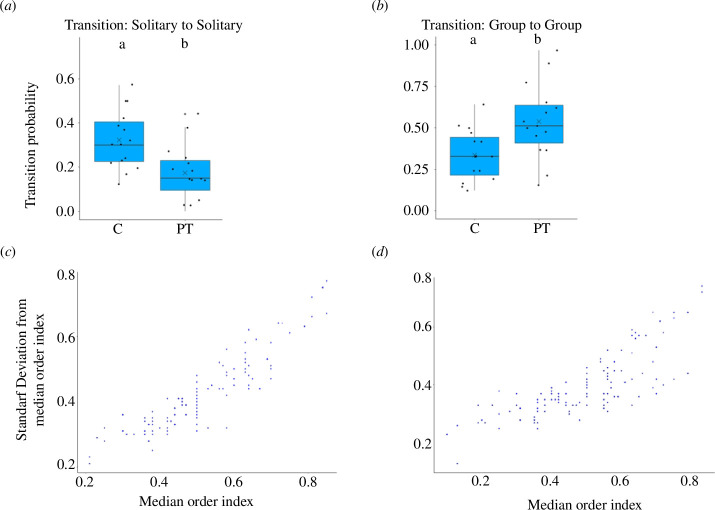
The probability of continuing to swim solitary or continuing to swim in a group was dependent on the treatment (GLM results for (i) continuing to swim solitary: Wald type IIχ2 = 10.03, d.f. = 1, p > 0.01, table 2a ; (ii) continuing to swim in group: Wald type IIχ2 = 8.13, d.f. = 1, p > 0.01; table 2b ). The probability of continuing to be in a solitary state was significantly smaller among individuals in PT shoals (mean = 0.17 ± 0.03) as compared with individuals in CT shoals (mean = 0.32 ± 0.01); figure 2a . Correspondingly, the probability of continuing to be in a group was significantly higher among individuals in PT shoals (mean = 0.53 ± 0.05) as compared with individuals in CT shoals (mean = 0.33 ± 0.03) (figure 2b ; Tukey’s test results in table 2c ).
Table 2a.
Results of the GLM for predicting the effect of treatment on mean transition probability for continuing to be in solitary state. Model: mean transition probability ~ treatment.
| estimate | s.e. | t value | Pr (>|t|) | |
|---|---|---|---|---|
| (intercept) | 0.32 | 0.03 | 9.7 | <0.0001 |
| PT | −0.14 | 0.04 | −3.16 | 0.01 |
Table 2b.
Results of the GLM for predicting the effect of treatment on mean transition probability for continuing to be in group state. Model: mean transition probability ~ treatment.
| estimate | s.e. | t value | Pr (>|t|) | |
|---|---|---|---|---|
| (intercept) | 0.33 | 0.04 | 6.72 | <0.0001 |
| PT | 0.2 | 0.07 | 2.85 | 0.008 |
Figure 2.
Shoal dynamics and deviation in individuals’ shoal position in control treatments and predator treatments. Box-and-whisker plots representing the probability of continuing to swim in: (a) solitary and (b) in group state for CT and PT. Each dot represents the mean probability of changing states by a unique group. The different letters (letters ‘a’ and ‘b’) placed above the boxes represent significant differences between the treatments. Comparisons were performed using Tukey’s HSD test (sample size: N C = N PT = 15 shoals; p < 0.05). Scatter plots representing the correlation between standard deviation from the median order index and the median order index in control treatments (c) and predator treatments (d). Each dot represents the median order index and the corresponding standard deviation from a median order index by an individual. The correlation was tested using Spearman’s correlation test (sample size: N C, N PT = 150 individuals; p < 0.05).
Table 2c.
Tukey’s test results for continuing to be in a given state.
| estimate | s.e. | Z value | Pr (>|Z|) | |
|---|---|---|---|---|
| continuing to be in solitary state | ||||
| PT - C | 0.14 | 0.04 | −3.17 | <0.01 |
| continuing to be in group state | ||||
| PT - C | 0.2 | 0.07 | 2.85 | <0.01 |
There was a strong correlation between the median order index and the standard deviation from the median order index for both treatments (CT: R 2 = 0.88; p < 0.0001; PT: R 2 = 0.83; p < 0.0001). Individuals in PT shoals and individuals in CT shoals showed a similar pattern with regards to their shoal position: individuals towards the front deviated less from their median position in the shoal as compared with other individuals (figure 2c,d ).
4. Discussion
Our study reveals immediate changes in group-level characteristics among wild zebrafish (Danio rerio) shoals in response to two ecological factors. Test shoals exhibited considerable behavioural plasticity in the form of changes in shoal size, polarization and foraging behaviour in the presence of predation and/or vegetation. Furthermore, temporal analysis revealed that the tendency of individuals to remain in a group or to remain solitary is also strongly dependent on the presence of predator cues. Our study thus demonstrates that fish perceive various ecological factors and adjust their shoaling characteristics accordingly. It is likely that such immediate behavioural responses may be necessary for their survival in freshwater habitats.
4.1. Shoaling and foraging behaviour
Wild zebrafish shoals respond to predation and vegetation with considerable plasticity in shoaling and foraging. Increased group size and/or group cohesion to escape predators has been shown to occur in several species, including fish. Our findings on increased shoal size among zebrafish in the presence of predator cues are in consensus with previous studies on three-spined stickleback (Gasterosteus aculeatus), bluntnose minnows (Pimephales notatus), Pacific salmon (Oncorhynchus spp.), mosquitofish (Gambusia affinis), guppies (Poecilia reticulata), wild piranha (Pygocentrus nattereri) and fathead minnows (Pimephales promelas) [29,40,65–69]. Wild zebrafish shoals were highly polarized in open tanks (CT) (similar to sticklebacks (Gasterosteus aculeatus) [70]) and in predator cue treatments (PT and PVT), suggesting that predation odour and absence of vegetation are considered risky. Similar to barred flagtails (Kuhlia mugil) [71], smaller subgroups were more polarized as compared with larger subgroups.
A previous study conducted by us revealed that zebrafish shoals modulate anti-predator strategies based on the magnitude and kind of predator cues present: while shoals adhered to safety in numbers in the presence of visual or olfactory cues of a predator, individuals within shoals underwent increased freezing in the simultaneous presence of both cues from a predator [72]. The percent individuals under vegetation was significantly more in the presence of predator cues indicating that in the presence of vegetation, zebrafish shoals elicit a different kind of anti-predator strategy, wherein individuals take refuge or hide under vegetation when detecting the presence of a predator. The fact that shoals take longer to emerge from underneath natural vegetation in their natural habitats (as compared with laboratory studies lacking predator cues) could be due to the presence of additional predator/alarm cues in their natural habitat. Differences between natural vegetation and plastic aquarium plants in the experimental tank could be another explanation for this difference. This follow-up study extends our laboratory-based findings to field conditions.
These findings highlight the adaptability exhibited by shoals, illustrating how they modify their behaviour in the presence of aquatic vegetation and cues from predators. The present study also reveals that school responses to a given ecological variable may also depend on other ecological factors present. We speculate that future studies, incorporating additional factors such as turbidity and water flow, may reveal further modifications in predator avoidance strategies.
While shoals forage most effectively in the absence of vegetation and predator odour, a reduction in foraging in the presence of a predator is likely to be an anti-predator response [67,73,74]. Test shoals might choose a refuge (in the form of vegetation) over foraging in the open arena. In the possibility of zebrafish being visual foragers [75,76], the presence of vegetation might also obstruct access to visual information about the presence/location of food, likely reducing foraging efficiency. In their natural habitats, zebrafish feed on algae and zooplankton in the water [77] and therefore in such habitats, vegetation and their food sources are often not spatially separated. While this study clearly shows that vegetation acts as a refuge and may be useful in the context of predator avoidance, the same factor may obstruct useful information such as the presence of food sources.
4.2. Shoal dynamics within shoals receiving cues from a predator
Ecological factors like habitat, prey availability and predation strongly control fission–fusion dynamics among schooling fishes [78,79]. As in guppies (Poecilia reticulata), the analyses of fission–fusion dynamics revealed that in the presence of predator cues, the tendency of individuals to leave the largest subgroup declined significantly [80]. Thus, shoals exposed to predator cues not only exhibit larger subgroup sizes but also exhibit minimal changes in membership within these subgroups. In fish species such as the Atlantic cod (Gadus morhua), golden shiner (Notemigonus crysoleucas), mosquitofish (Gambusia affinis) and guppies (Poecilia reticulata) specific individuals (termed as leaders) consistently occupy the front of a shoal [81–83]. Our results reveal that regardless of the presence of predator odour, individuals towards the front of a zebrafish shoal showed lesser deviation from their positions as compared with individuals who followed. Therefore, we establish that individual leadership within shoals remains intact even as they display anti-predator responses.
Anti-predator strategies (or responses to other kinds of environmental changes) in animals are tightly linked to other ecological variables of a given habitat. While anti-predator responses have been studied across species, these do not address the influence of other ecological variables (such as vegetation cover, presence of refuge, type and abundance of co-occurring species) on anti-predator strategies. Our experiments demonstrate that shoals not only modify behaviour in response to predators but their anti-predator strategies also depend on the presence of vegetation. Future studies aiming to achieve comprehensive understanding of anti-predator tactics in different fish species (or in other animals) should consider additional ecological variables encountered. As our results show that vegetation is likely to enable shoals to avoid predators these findings can also aid in the formulation of conservation strategies. Wild zebrafish habitats are shared by a variety of other small freshwater species and face additional threats from invasive fish species, that can further increase predation pressure on zebrafish/other similar sized freshwater fishes. Vegetation along the edges of their habitats may aid such small freshwater fishes to escape predator attacks.
Acknowledgements
The authors thank the Indian Institute of Science Education and Research Kolkata (IISER Kolkata), India, for providing infrastructural and financial support (through Academic Research funds to A.B. and research fellowship to I.M.). The authors also thank Prasenjit Pan for help in collection of the wild population and fish maintenance in the laboratory.
Contributor Information
Ishani Mukherjee, Email: im18rs098@iiserkol.ac.in.
Anuradha Bhat, Email: anuradhabhat@iiserkol.ac.in.
Ethics
Guidelines outlined by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Fisheries, Animal Husbandry and Dairying, Government of India were followed in all aspects of maintenance and experimentation. All experimental protocols followed here have been approved by the Institutional Animal Ethics Committee (IAEC) and guidelines of Indian Institute of Science Education and Research (IISER) Kolkata, Government of India (Approval number IISERK/IAEC/AP/2021/70).
Data accessibility
Data for this study are available online [84].
Supplementary material is available online [85].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
I.M.: conceptualization, formal analysis, investigation, methodology, writing—original draft, writing—review and editing; A.B.: conceptualization, funding acquisition, project administration, resources, software, supervision, writing—review and editing.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by Academic Research funding to A.B. from IISER Kolkata. I.M. received junior and senior graduate funding from IISER Kolkata.
References
- 1. He P, Maldonado-Chaparro AA, Farine DR. 2019. The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behav. Ecol. Sociobiol. 73 , 1–4. ( 10.1007/s00265-018-2602-7) [DOI] [Google Scholar]
- 2. Réale D, Festa-Bianchet M. 2003. Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 65 , 463–470. ( 10.1006/anbe.2003.2100) [DOI] [PubMed] [Google Scholar]
- 3. Mettke‐Hofmann C, Winkler H, Leisler B. 2002. The significance of ecological factors for exploration and neophobia in parrots. Ethology 108 , 249–272. ( 10.1046/j.1439-0310.2002.00773.x) [DOI] [Google Scholar]
- 4. Brumm H, Todt D. 2002. Noise-dependent song amplitude regulation in a territorial songbird. Anim. Behav. 63 , 891–897. ( 10.1006/anbe.2001.1968) [DOI] [Google Scholar]
- 5. Brooke PN, Alford RA, Schwarzkopf L. 2000. Environmental and social factors influence chorusing behaviour in a tropical frog: examining various temporal and spatial scales. Behav. Ecol. Sociobiol. 49 , 79–87. ( 10.1007/s002650000256) [DOI] [Google Scholar]
- 6. Wilson MW, Ridlon AD, Gaynor KM, Gaines SD, Stier AC, Halpern BS. 2020. Ecological impacts of human-induced animal behaviour change. Ecol. Lett. 23 , 1522–1536. ( 10.1111/ele.13571) [DOI] [PubMed] [Google Scholar]
- 7. Huizinga M, Ghalambor CK, Reznick DN. 2009. The genetic and environmental basis of adaptive differences in shoaling behaviour among populations of Trinidadian guppies, Poecilia reticulata. J. Evol. Biol. 22 , 1860–1866. ( 10.1111/j.1420-9101.2009.01799.x) [DOI] [PubMed] [Google Scholar]
- 8. Miller N, Gerlai R. 2007. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 184 , 157–166. ( 10.1016/j.bbr.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 9. Caspi T, Johnson JR, Lambert MR, Schell CJ, Sih A. 2022. Behavioral plasticity can facilitate evolution in urban environments. Trends Ecol. Evol. 37 , 1092–1103. ( 10.1016/j.tree.2022.08.002) [DOI] [PubMed] [Google Scholar]
- 10. Loarie SR, Tambling CJ, Asner GP. 2013. Lion hunting behaviour and vegetation structure in an African savanna. Anim. Behav. 85 , 899–906. ( 10.1016/j.anbehav.2013.01.018) [DOI] [Google Scholar]
- 11. Potash AD, Conner LM, McCleery RA. 2019. Vertical and horizontal vegetation cover synergistically shape prey behaviour. Anim. Behav. 152 , 39–44. ( 10.1016/j.anbehav.2019.04.007) [DOI] [Google Scholar]
- 12. Nowak K. 2008. Frequent water drinking by Zanzibar red colobus (Procolobus kirkii) in a mangrove forest refuge. Am. J. Primatol. 70 , 1081–1092. ( 10.1002/ajp.20605) [DOI] [PubMed] [Google Scholar]
- 13. Landeau L, Terborgh J. 1986. Oddity and the ‘confusion effect’ in predation. Anim. Behav. 34 , 1372–1380. ( 10.1016/S0003-3472(86)80208-1) [DOI] [Google Scholar]
- 14. Lehtonen J, Jaatinen K. 2016. Safety in numbers: the dilution effect and other drivers of group life in the face of danger. Behav. Ecol. Sociobiol. 70 , 449–458. ( 10.1007/s00265-016-2075-5) [DOI] [Google Scholar]
- 15. Winnie J, Creel S. 2007. Sex-specific behavioural responses of elk to spatial and temporal variation in the threat of wolf predation. Anim. Behav. 73 , 215–225. ( 10.1016/j.anbehav.2006.07.007) [DOI] [Google Scholar]
- 16. Rauber R, Manser MB. 2017. Discrete call types referring to predation risk enhance the efficiency of the meerkat sentinel system. Sci. Rep. 7 , 44436. ( 10.1038/srep44436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dugatkin LA, Godin JG. 1992. Prey approaching predators: a cost-benefit perspective. Ann. Zool. Fenn. 29 , 233–252. [Google Scholar]
- 18. Seppälä O, Karvonen A, Valtonen ET. 2008. Shoaling behaviour of fish under parasitism and predation risk. Anim. Behav. 75 , 145–150. ( 10.1016/j.anbehav.2007.04.022) [DOI] [Google Scholar]
- 19. Herbert-Read JE, et al. 2017. How predation shapes the social interaction rules of shoaling fish. Proc. R. Soc. B 284 , 20171126. ( 10.1098/rspb.2017.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jolles JW, Sosna MMG, Mazué GPF, Twomey CR, Bak-Coleman J, Rubenstein DI, Couzin ID. 2022. Both prey and predator features predict the individual predation risk and survival of schooling prey. eLife 11 , e76344. ( 10.7554/eLife.76344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Priyadarshana T, Asaeda T, Manatunge J. 2001. Foraging behaviour of planktivorous fish in artificial vegetation: the effects on swimming and feeding. Hydrobiologia 442 , 231–239. ( 10.1023/A:1017578524578) [DOI] [Google Scholar]
- 22. Ghoshal A, Bhat A. 2021. Group size and aquatic vegetation modulates male preferences for female shoals in wild zebrafish, Danio rerio. Sci. Rep. 11 , 1236. ( 10.1038/s41598-020-80913-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Figueiredo BRS, Mormul RP, Chapman BB, Lolis LA, Fiori LF, Benedito E. 2016. Turbidity amplifies the non‐lethal effects of predation and affects the foraging success of characid fish shoals. Freshw. Biol. 61 , 293–300. ( 10.1111/fwb.12703) [DOI] [Google Scholar]
- 24. Paijmans KC, Booth DJ, Wong MYL. 2020. Predation avoidance and foraging efficiency contribute to mixed-species shoaling by tropical and temperate fishes. J. Fish Biol. 96 , 806–814. ( 10.1111/jfb.14277) [DOI] [PubMed] [Google Scholar]
- 25. Rooker JR, Holt GJ, Holt SA. 1998. Vulnerability of newly settled red drum (Sciaenops ocellatus) to predatory fish: is early-life survival enhanced by seagrass meadows? Mar. Biol. 131 , 145–151. ( 10.1007/s002270050305) [DOI] [Google Scholar]
- 26. Li A, Richardson JML, Helen Rodd F. 2022. Shoaling in the Trinidadian guppy: costs, benefits, and plasticity in response to an ambush predator. Behav. Ecol. 33 , 758–766. ( 10.1093/beheco/arac038) [DOI] [Google Scholar]
- 27. Roy T, Bhat A. 2018. Population, sex and body size: determinants of behavioural variations and behavioural correlations among wild zebrafish Danio rerio. R. Soc. Open Sci. 5 , 170978. ( 10.1098/rsos.170978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoare DJ, Couzin ID, Godin JGJ, Krause J. 2004. Context-dependent group size choice in fish. Anim. Behav. 67 , 155–164. ( 10.1016/j.anbehav.2003.04.004) [DOI] [Google Scholar]
- 29. Wilson ADM, Schaerf TM, Ward AJW. 2022. Individual and collective behaviour of fish subject to differing risk-level treatments with a sympatric predator. Behav. Ecol. Sociobiol. 76 . ( 10.1007/s00265-022-03269-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katz Y, Tunstrøm K, Ioannou CC, Huepe C, Couzin ID. 2011. Inferring the structure and dynamics of interactions in schooling fish. Proc. Natl Acad. Sci. USA 108 , 18720–18725. ( 10.1073/pnas.1107583108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJT, Ward AJW. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. USA 108 , 18726–18731. ( 10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sbragaglia V, Roy T, Thörnqvist PO, López-Olmeda JF, Winberg S, Arlinghaus R. 2022. Evolutionary implications of size-selective mortality on the ontogenetic development of shoal cohesion: a neurochemical approach using a zebrafish, Danio rerio, harvest selection experiment. Behav. Ecol. Sociobiol. 76 , 154. ( 10.1007/s00265-022-03258-7) [DOI] [Google Scholar]
- 33. Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. 2017. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 27 , 2862–2868. ( 10.1016/j.cub.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez-Pinto II, Rieucau G, Handegard NO, Boswell KM. 2020. Environmental context elicits behavioural modification of collective state in schooling fish. Anim. Behav. 165 , 107–116. ( 10.1016/j.anbehav.2020.05.002) [DOI] [Google Scholar]
- 35. Calovi DS, Litchinko A, Lecheval V, Lopez U, Pérez Escudero A, Chaté H, Sire C, Theraulaz G. 2018. Disentangling and modeling interactions in fish with burst-and-coast swimming reveal distinct alignment and attraction behaviors. PLoS Comput. Biol. 14 , e1005933. ( 10.1371/journal.pcbi.1005933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zienkiewicz AK, Ladu F, Barton DAW, Porfiri M, Bernardo MD. 2018. Data-driven modelling of social forces and collective behaviour in zebrafish. J. Theor. Biol. 443 , 39–51. ( 10.1016/j.jtbi.2018.01.011) [DOI] [PubMed] [Google Scholar]
- 37. Suriyampola PS, Sykes DJ, Khemka A, Shelton DS, Bhat A, Martins EP. 2017. Water flow impacts group behavior in zebrafish (Danio rerio). Behav. Ecol. 28 , 94–100. ( 10.1093/beheco/arw138) [DOI] [Google Scholar]
- 38. Wright D, Rimmer LB, Pritchard VL, Butlin RK, Krause J. 2003. inter and intra‐population variation in shoaling and boldness in the zebrafish (Danio rerio). J. Fish Biol. 63 , 258–259. ( 10.1111/j.1095-8649.2003.216bw.x) [DOI] [PubMed] [Google Scholar]
- 39. Ajemian MJ, Sohel S, Mattila J. 2015. Effects of turbidity and habitat complexity on antipredator behavior of three-spined sticklebacks (Gasterosteus aculeatus). Environ. Biol. Fishes 98 , 45–55. ( 10.1007/s10641-014-0235-x) [DOI] [Google Scholar]
- 40. Chivers DP, Smith RJF. 1995. Free-living fathead minnows rapidly learn to recognize pike as predators. J. Fish Biol. 46 , 949–954. ( 10.1111/j.1095-8649.1995.tb01399.x) [DOI] [Google Scholar]
- 41. Snickars M, Sandström A, Mattila J. 2004. Antipredator behaviour of 0+ year Perca fluviatilis: effect of vegetation density and turbidity. J. Fish Biol. 65 , 1604–1613. ( 10.1111/j.0022-1112.2004.00570.x) [DOI] [Google Scholar]
- 42. Rangeley RW, Kramer DL. 1998. Density-dependent antipredator tactics and habitat selection in juvenile pollock. Ecology 79 , 943–952. ( 10.1890/0012-9658(1998)079[0943:DDATAH]2.0.CO;2) [DOI] [Google Scholar]
- 43. Miyai CA, Sanches FHC, Pinho-Neto CF, Barreto RE. 2016. Effects of predator odour on antipredator responses of Nile tilapia. Physiol. Behav. 165 , 22–27. ( 10.1016/j.physbeh.2016.06.033) [DOI] [PubMed] [Google Scholar]
- 44. Ferrari MC, Wisenden BD, Chivers DP. 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 88 , 698–724. ( 10.1139/Z10-029) [DOI] [Google Scholar]
- 45. Kelley JL, Magurran AE. 2003. Learned predator recognition and antipredator responses in fishes. Fish Fish. 4 , 216–226. ( 10.1046/j.1467-2979.2003.00126.x) [DOI] [Google Scholar]
- 46. Brown GE. 2003. Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 4 , 227–234. ( 10.1046/j.1467-2979.2003.00132.x) [DOI] [Google Scholar]
- 47. Fischer S, Oberhummer E, Cunha-Saraiva F, Gerber N, Taborsky B. 2017. Smell or vision? The use of different sensory modalities in predator discrimination. Behav. Ecol. Sociobiol. 71 , 143. ( 10.1007/s00265-017-2371-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stratmann A, Taborsky B. 2014. Antipredator defences of young are independently determined by genetic inheritance, maternal effects and own early experience in mouthbrooding cichlids. Funct. Ecol. 28 , 944–953. ( 10.1111/1365-2435.12224) [DOI] [Google Scholar]
- 49. Spence R, Fatema MK, Reichard M, Huq KA, Wahab MA, Ahmed ZF, Smith C. 2006. The distribution and habitat preferences of the zebrafish in Bangladesh. J. Fish Biol. 69 , 1435–1448. ( 10.1111/j.1095-8649.2006.01206.x) [DOI] [Google Scholar]
- 50. Snekser JL, Ruhl N, Bauer K, McRobert SP. 2010. The influence of sex and phenotype on shoaling decisions in zebrafish. Int. J. Comp. Psychol. 23 , 70–81. ( 10.46867/IJCP.2010.23.01.04) [DOI] [Google Scholar]
- 51. Schons RF, Vitt S, Thünken T. 2021. Environmental habituation and sexual composition affect juveniles’ shoaling activity in a cichlid fish (Pelvicachromis taeniatus). J. Fish Biol. 99 , 1307–1317. ( 10.1111/jfb.14836) [DOI] [PubMed] [Google Scholar]
- 52. Allan JR, Pitcher TJ. 1986. Species segregation during predator evasion in cyprinid fish shoals. Freshw. Biol. 16 , 653–659. ( 10.1111/j.1365-2427.1986.tb01007.x) [DOI] [Google Scholar]
- 53. Kastelein RA, van der Heul S, van der Veen J, Verboom WC, Jennings N, de Haan D, Reijnders PJH. 2007. Effects of acoustic alarms, designed to reduce small cetacean bycatch in gillnet fisheries, on the behaviour of North Sea fish species in a large tank. Mar. Environ. Res. 64 , 160–180. ( 10.1016/j.marenvres.2006.12.012) [DOI] [PubMed] [Google Scholar]
- 54. Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 18 , 529. ( 10.1186/s12859-017-1934-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pérez-Escudero A, Vicente-Page J, Hinz RC, Arganda S, de Polavieja GG. 2014. IdTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 11 , 743–748. ( 10.1038/nmeth.2994) [DOI] [PubMed] [Google Scholar]
- 56. Borner KK, Krause S, Mehner T, Uusi-Heikkilä S, Ramnarine IW, Krause J. 2015. Turbidity affects social dynamics in Trinidadian guppies. Behav. Ecol. Sociobiol. 69 , 645–651. ( 10.1007/s00265-015-1875-3) [DOI] [Google Scholar]
- 57. Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 58. Doughty AK, Horton BJ, Huyen NTD, Ballagh CR, Corkrey R, Hinch GN. 2018. The influence of lameness and individuality on movement patterns in sheep. Behav. Process. 151 , 34–38. ( 10.1016/j.beproc.2018.03.008) [DOI] [PubMed] [Google Scholar]
- 59. Fischhoff IR, Sundaresan SR, Cordingley J, Larkin HM, Sellier MJ, Rubenstein DI. 2007. Social relationships and reproductive state influence leadership roles in movements of plains zebra, Equus burchellii. Anim. Behav. 73 , 825–831. ( 10.1016/j.anbehav.2006.10.012) [DOI] [Google Scholar]
- 60. RStudio Team . 2020. RStudio: integrated development for R. Boston, MA: RStudio, PBC. See https://www.rstudio.com/. [Google Scholar]
- 61. Fox J, Weisberg S. 2015. Using car functions in other functions. See http://ftp.zut.edu.pl/dsk0/CRAN/web/packages/car.
- 62. Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82 , 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 63. Delignette-Muller ML, Dutang C. 2015. fitdistrplus: an R package for fitting distributions. J. Stat. Softw. 64 , 1–34. ( 10.18637/jss.v064.i04) [DOI] [Google Scholar]
- 64. Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50 , 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 65. Jolles JW, Laskowski KL, Boogert NJ, Manica A. 2018. Repeatable group differences in the collective behaviour of stickleback shoals across ecological contexts. Proc. R. Soc. B 285 , 20172629. ( 10.1098/rspb.2017.2629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morgan MJ. 1988. The effect of hunger, shoal size and the presence of a predator on shoal cohesiveness in bluntnose minnows, Pimephales notatus Rafinesque. J. Fish Biol. 32 , 963–971. ( 10.1016/j.anbehav.2008.01.016) [DOI] [Google Scholar]
- 67. Polyakov AY, Quinn TP, Myers KW, Berdahl AM. 2022. Group size affects predation risk and foraging success in Pacific salmon at sea. Sci. Adv. 8 , eabm7548. ( 10.1126/sciadv.abm7548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seghers BH. 1974. Schooling behavior in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution 28 , 486. ( 10.2307/2407174) [DOI] [PubMed] [Google Scholar]
- 69. Queiroz H, Magurran AE. 2005. Safety in numbers? Shoaling behaviour of the Amazonian red-bellied piranha. Biol. Lett. 1 , 155–157. ( 10.1098/rsbl.2004.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. MacGregor HEA, Ioannou CC. 2021. Collective motion diminishes, but variation between groups emerges, through time in fish shoals. R. Soc. Open Sci. 8 , 210655. ( 10.1098/rsos.210655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gautrais J, Ginelli F, Fournier R, Blanco S, Soria M, Chaté H, Theraulaz G. 2012. Deciphering interactions in moving animal groups. PLoS Comput. Biol. 8 , e1002678. ( 10.1371/journal.pcbi.1002678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mukherjee I, Malakar A, Das D, Bhat A. 2024. Use of multimodal sensory cues in predator avoidance by wild-caught zebrafish shoals. Biol. J. Linnean Soc. 141 , 364–378. ( 10.1093/biolinnean/blad103) [DOI] [Google Scholar]
- 73. Morgan MJ, Colgan PW. 1987. The effects of predator presence and shoal size on foraging in bluntnose minnows, Pimephales notatus. Environ. Biol. Fish. 20 , 105–111. ( 10.1007/BF00005290) [DOI] [Google Scholar]
- 74. Siepielski AM, Fallon E, Boersma K. 2016. Predator olfactory cues generate a foraging–predation trade-off through prey apprehension. R. Soc. Open Sci. 3 , 150537. ( 10.1098/rsos.150537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sekhar MA, Singh R, Bhat A, Jain M. 2019. Feeding in murky waters: acclimatization and landmarks improve foraging efficiency of zebrafish (Danio rerio) in turbid waters. Biol. Lett. 15 , 20190289. ( 10.1098/rsbl.2019.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Howe HB, McIntyre PB, Wolman MA. 2018. Adult zebrafish primarily use vision to guide piscivorous foraging behavior. Behav. Process. 157 , 230–237. ( 10.1016/j.beproc.2018.10.005) [DOI] [PubMed] [Google Scholar]
- 77. Spence R, Gerlach G, Lawrence C, Smith C. 2008. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 83 , 13–34. ( 10.1111/j.1469-185X.2007.00030.x) [DOI] [PubMed] [Google Scholar]
- 78. Bierbach D, Krause S, Romanczuk P, Lukas J, Arias-Rodriguez L, Krause J. 2020. An interaction mechanism for the maintenance of fission-fusion dynamics under different individual densities. PeerJ 8 , e8974. ( 10.7717/peerj.8974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zheng YH, Fu SJ. 2021. Effects of fasting on collective movement and fission-fusion dynamics in both homogeneous and heterogeneous shoals of a group-living cyprinid fish species. J. Fish Biol. 99 , 1640–1649. ( 10.1111/jfb.14872) [DOI] [PubMed] [Google Scholar]
- 80. Kelley JL, Morrell LJ, Inskip C, Krause J, Croft DP. 2011. Predation risk shapes social networks in fission-fusion populations. PLoS ONE 6 , e24280. ( 10.1371/journal.pone.0024280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Burns AL, Herbert-Read JE, Morrell LJ, Ward AJW. 2012. Consistency of leadership in shoals of mosquitofish (Gambusia holbrooki) in novel and in familiar environments. PLoS ONE 7 , e36567. ( 10.1371/journal.pone.0036567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Krause J, Hoare D, Krause S, Hemelrijk CK, Rubenstein DI. 2000. Leadership in fish shoals. Fish Fish. 1 , 82–89. ( 10.1111/j.1467-2979.2000.tb00001.x) [DOI] [Google Scholar]
- 83. Reebs SG. 2000. Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 59 , 403–409. ( 10.1006/anbe.1999.1314) [DOI] [PubMed] [Google Scholar]
- 84. Mukherjee I, Bhat A. 2024. Data from: The impact of predators and vegetation on shoaling in wild zebrafish [Dataset]. Dryad. ( 10.5061/dryad.dncjsxm46) [DOI]
- 85. Mukherjee I, Bhat A. 2024. Data from: The impact of predators and vegetation on shoaling in wild zebrafish. Figshare. ( 10.6084/m9.figshare.c.7452030) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study are available online [84].
Supplementary material is available online [85].