Abstract
SARS-CoV is the causative agent of severe acute respiratory syndrome (SARS). The virally encoded 3C-like protease (3CLPro) has been presumed critical for the viral replication of SARS-CoV in infected host cells. In this study, we screened a natural product library consisting of 720 compounds for inhibitory activity against 3CLPro. Two compounds in the library were found to be inhibitive: tannic acid (IC50 = 3 µM) and 3-isotheaflavin-3-gallate (TF2B) (IC50 = 7 µM). These two compounds belong to a group of natural polyphenols found in tea. We further investigated the 3CLPro-inhibitory activity of extracts from several different types of teas, including green tea, oolong tea, Puer tea and black tea. Our results indicated that extracts from Puer and black tea were more potent than that from green or oolong teas in their inhibitory activities against 3CLPro. Several other known compositions in teas were also evaluated for their activities in inhibiting 3CLPro. We found that caffeine, (—)-epigallocatechin gallte (EGCg), epicatechin (EC), theophylline (TP), catechin (C), epicatechin gallate (ECg) and epigallocatechin (EGC) did not inhibit 3CLPro activity. Only theaflavin-3,3′-digallate (TF3) was found to be a 3CLPro inhibitor. This study has resulted in the identification of new compounds that are effective 3CLPro inhibitors.
Keywords: natural products, 3CLPro, black tea, TF2B, TF3
Introduction
Severe acute respiratory syndrome (SARS) is caused by a newly discovered coronavirus, SARS coronavirus (SARS-CoV) (1,2). From November 1, 2002 to June 18, 2003, a total of 8465 probable SARS cases were reported to the World Health Organization from 29 countries, and 801 people died from this disease. The case–fatality proportion was ∼10%. Although the spread of the virus has been averted, the re-emergence of SARS is still in question (3–6).
Like other related coronaviruses, SARS-CoV is an enveloped virus containing single-stranded RNA of positive polarity. Coronaviruses have the largest genomes known (∼30 kb) among RNA viruses [7]. The genome of SARS-CoV comprises one open reading frame (ORF) encoding two overlapping replicase polyproteins, polyprotein-1a (pp1a, ∼450 kDa) and poplyprotein-1ab (pp1ab, ∼750 kDa), responsible for virus replication (7). The rest of the genome comprises at least five ORFs encoding structural proteins and other genes with putative functions (8,9). Proteolytic processing, especially the processing of replicase polyproteins, is one of the crucial steps in the life cycle of many positive-stranded RNA viruses, including coronaviruses. All coronaviruses encode a papline-like protease (PLP) and a chymotrypsin-like (3CLPro) protease for proteolytic procession during virus maturation (10). Studies on other coronaviruses have shown that the PLP protein cleaves at no less than two sites on the pp1a polyprotein and that the 3CLPro protease cleaves at least 11 inter-domain sites on the pp1a and pp1ab polyproteins [7]. Thus, the 3CLPro protease of SARS-CoV has been considered as an important molecular target for anti-SARS-CoV drug discovery and developments. In this study, after screening of a natural product library and further confirmation, we found that SARS-3CLPro could be inhibited by compounds that are abundant in teas. We also examined crude extracts from various teas and a panel of representative natural products in teas for their inhibitory activities against SARS-3CLPro.
Methods
Preparation of SARS-CoV 3CLPro
Total RNA extracted from a throat swab of a SARS patient was provided by the Center for Disease Control (CDC), Taipei, Taiwan. All RNA samples were handled under bio-safety level 2 (BSL2) regulations. Two primers, 5′-GGCGGAATTCGCCTCTACCAACCACCACAGA-3′ and 5′-GGCGGAATTCA AAGCATCCAATGATGAGTGCC-3′, were used for reverse transcription–polymerase chain reaction (RT–PCR) to synthesize the cDNA of SARS-3CLPro using Ready-to-Go RT–PCR Beads (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's protocol. The PCR cycle used for the amplification of cDNA of SARS-3CLPro was as follows: 94°C for 5 min, 25 cycles of 94°C for 1 min, 58°C for 3 min, 72°C for 2 min and 72°C for 10 min. A full-length 3CLPro of SARS coronavirus was successfully expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein. Following cleavage by thrombin, 3CLPro was purified to homogeneity. A 15mer peptide with sequence H2N-SITSAVLQSGFRKMA-COOH corresponding to an autocleavage site of the SARS-3CLPro was synthesized and used for protease cleavage activity assay. The highly purified protein was shown by high-performance liquid chromatography (HPLC) analysis to exhibit proteolytic activity in trans.
Compound Collection
The Pure Natural Products Library used to screen for 3CLPro inhibition was obtained from MicroSource Discovery Systems, Inc., Gaylordsville, CT. It consists of a unique collection of 720 pure natural products. The collection includes simple and complex oxygen heterocycles, alkaloids, sequiterpenes, diterpenes, pentacyclic triterpenes, sterols and many other diverse representatives. Initially, 10 different compounds were pooled, and the mixtures were tested for their inhibitory activities against SARS-3CLPro. Positive pools were then deconvoluted and single pure compounds that were able to inhibit >50% of peptide cleavage at 10 µM were scored as hits. Other compounds were obtained from Sigma (St Louis, MO). Theaflavin (TF1), TF2 [a mixture of TF-2A (theaflavin-3-gallate) and TF-2B (theaflavin-3′-gallate)] and theaflavin-3,3′-digallate (TF3) were kind gifts from Dr Yu-Chih Liang, School of Medical Technology, Taipei Medical University, Taipei, Taiwan, ROC.
Preparation of Crude Extracts from Oolong Tea, Green Tea, Black Tea and Puer Tea
Tea liquors were prepared from oolong tea, green tea, black tea and Puer tea (also called pu-er or pu-erh) samples as 2% (w/v) tea solutions. The water extracts of four different types of tea leaves were prepared by shaking for 10 min in boiling hot water in thermal flasks. The extracts were then filtered through a Millex-GS 0.22 µm filter (Millipore, Malsheim, France) to remove particulate matter. The water extracts were then evaporated under reduced pressure to obtain viscous masses. These materials were evaluated for their inhibitory activities against SARS-3CLPro.
Inhibition of Protease Activity of SARS-3CLPro by HPLC Assay
A general cysteine protease inhibitor, N-ethylmaleimide (NEM, protease inhibitor as positive control), was obtained from Sigma (St Louis, MO). This compound was tested for its inhibitory activities against SARS-3CLPro in the peptide cleavage assay. The activities of the proteases were measured in the absence or presence of different concentrations of these general inhibitors. The enzyme (2 µM) was pre-incubated with chemicals for 30 min. Peptide was then added at 100 µM to the reaction, and mixtures were incubated at 37°C for 1 h. The potency of the inhibitors was calculated. The HPLC analysis condition is: a C18 RP guard column (250 × 4.6 mm × 5 µm, Agilent Zorbax Extend). This method was chosen because it gave the best resolution among several methods that were tested. The column temperature was ambient, and the elution (0.8 ml/min) was performed using a solvent system comprising solvents A [10 MM NH4OAc, 0.1% trifluoroacetic acid (TFA)] and B (acetonitrile, 0.1% TFA) mixed using a gradient starting with 100% A, linearly decreasing to 10% A in 8 min, to 100% A in 16 min, and held at 100% A for 23 min. The injection volume was 40 ml. The UV detector was set at 214 nm.
Proteolytic Activity of SARS-3CLPro by Fluorogenic Substrate Peptide Assay
The development of a fluorescent protease activity assay has been reported previously (7). The enhanced fluorescence due to cleavage of the fluorogenic substrate peptide (Dabcyl-KTSAVLQSGFRKME-Edans) catalyzed by the protease was monitored at 538 nm with excitation at 355 nm using a fluorescence plate reader (Fluoroskan Ascent from ThermoLabsystems, Sweden). The fluorimetric assay was used to determine the IC50 of identified inhibitors on SARS 3CLPro activity. The protease was stored in the buffer containing 12 mM Tris–HCl (pH 7.5), 120 mM NaCl, 0.1 mM EDTA, 7.5 mM 2-mercaptoethanol and 1 mM dithiothreitol (DTT) at −70°C before use.
Results
Screening of a 720 Compound Library Against 3CLPro Activity by HPLC Assay
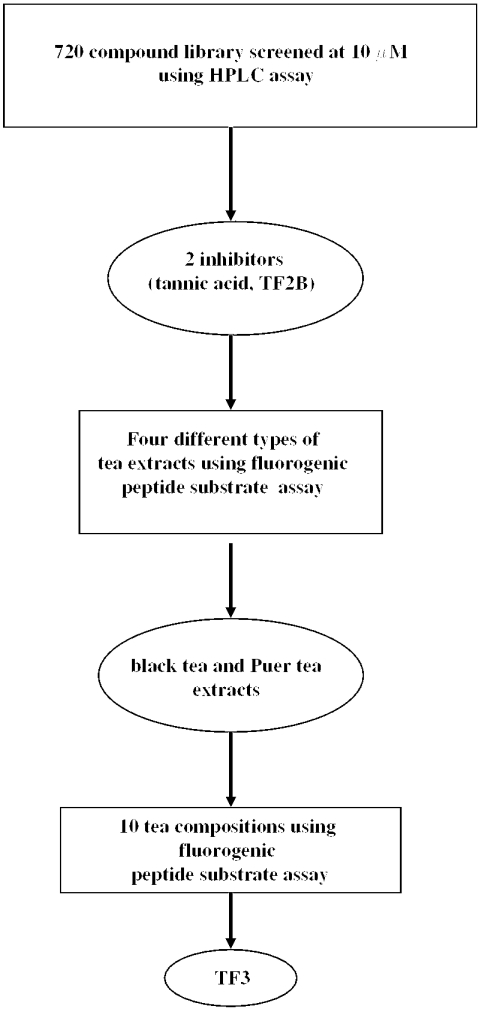
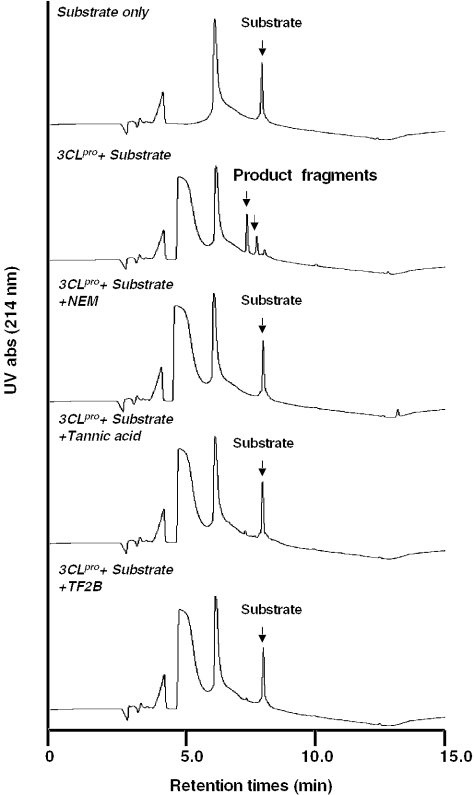
The spectrum collection, a library of 720 drugs and natural products, was screened for 3CLPro inhibitory activity. A flowchart of the screening procedure is shown in Fig. 1. Initially, 10 different compounds were pooled, and the 72 mixtures were then tested for their inhibitory activities against SARS-3CLPro. The HPLC assay was used in the primary screening because many compounds in the library are chromogenic. Single compounds in the positive pools were then evaluated for their activities. Two compounds, tannic acid and TF2B, were found to be active against 3CLPro (Fig. 2). Tannic acid is a water-soluble polyphenol present in plant food such as tea, red wine, beer and nuts. In addition, tea polyphenols and many tannin components are thought to be anticarcinogenic (14,15).
Figure 1.
Flowchart of the screening of the compound library and tea compositions.
Figure 2.
Inhibition of 3CL Pro activity by HPLC assay. Injection of 40 µl of incubation samples for C18 reverse phase column analysis. The substrate or product fragment peaks were analyzed by HPLC with UV absorbance detection at 214 nm. The HPLC system is described in Methods.
Evaluation of Tea Extracts and Pure Components from Teas for Their Inhibition of 3CLPro Activity
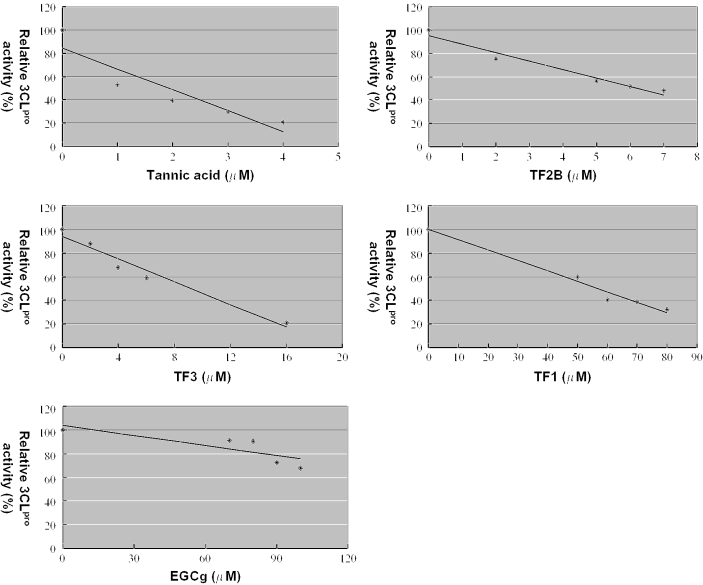
Since many other polyphenols related to tannic acid and TF2B are also present in various kinds of teas, we decided to examine the inhibition of activity by various tea extracts and several well known pure ingredients present in teas. Thus, water extracts from different types of teas were prepared and evaluated for their inhibitory activities against 3CLPro. As shown in Table 1, extracts from black and Puer teas inhibited 3CLPro activity while those from green or oolong teas did not. We further tested the possible involvement of several known ingredients present in teas, including caffeine, theophylline, catechin (C), epigallocatechin (EGC), (—)-epigallocatechin gallte (EGCg), epicatechin (EC), epicatechin gallate (ECg), TF1, TF2, TF2B, tannic acid and TF3. As shown in Table 1, the results showed that methylxanthine (caffeine and theophylline) did not influence 3CLPro activity; catechins, including C, EC, ECg, EGC and EGCg, present in green tea (unfermented) and oolong tea (partial fermented) also did not inhibit 3CLPro activity. Our results indicated that tannic acid, TF2B and TF3 are 3CLPro inhibitors as revealed by the fluorogenic substrate assay (Fig. 3). TF2A was not tested because of its unavailability.
Table 1.
Inhibition of SARS 3CLPro activity by natural products
| Compound library | IC50 (µM) |
| 3-Isotheaflavin-3-gallate (TF2B) | 7 |
| Tannic acid | 3 |
| Tea extract | IC50 (µg/ml) |
| Oolong tea | 125 |
| Green tea | 125 |
| Black tea | 70 |
| Puer tea | 25 |
| Other compositions in green tea | IC50 (µM) |
| Caffeine | 100 |
| Theophylline | 100 |
| Catechin (C) | 100 |
| Epigallocatechin (EGC) | 100 |
| (–)-Epigallocatechin gallte (EGCg) | 100 |
| Epicatechin (EC) | 100 |
| Epicatechin gallate (ECg) | 100 |
| Theaflavin composition in black tea | IC50 (µM) |
| Theaflavin (TF1) | 56 |
| Theaflavin-3-gallate,TF-2a and | 43 |
| Theaflavin-3′ -gallate,TF-2b mixture (TF2) | |
| Theaflavin-3,3′ -digallate (TF3) | 9.5 |
Figure 3.
Inhibition of 3CLPro activity by fluorogenic substrate peptide assay. Inhibition of 3CLPro activity by tannic acid, TF1, TF2B, TF3 and EGCg. The enhanced fluorescence due to cleavage of the fluorogenic substrate peptide catalyzed by the protease was monitored at 538 nm with exicitation at 355 nm using a fluorescence plate reader. The determining assay is described in Methods.
TF2B and TF3 as Potent Agents Against 3CLPro Inhibitory Activity
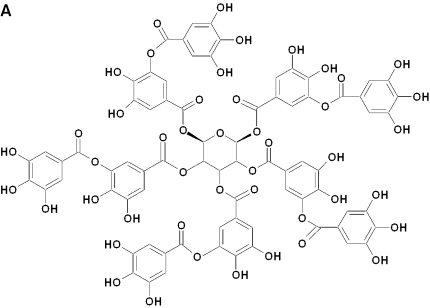
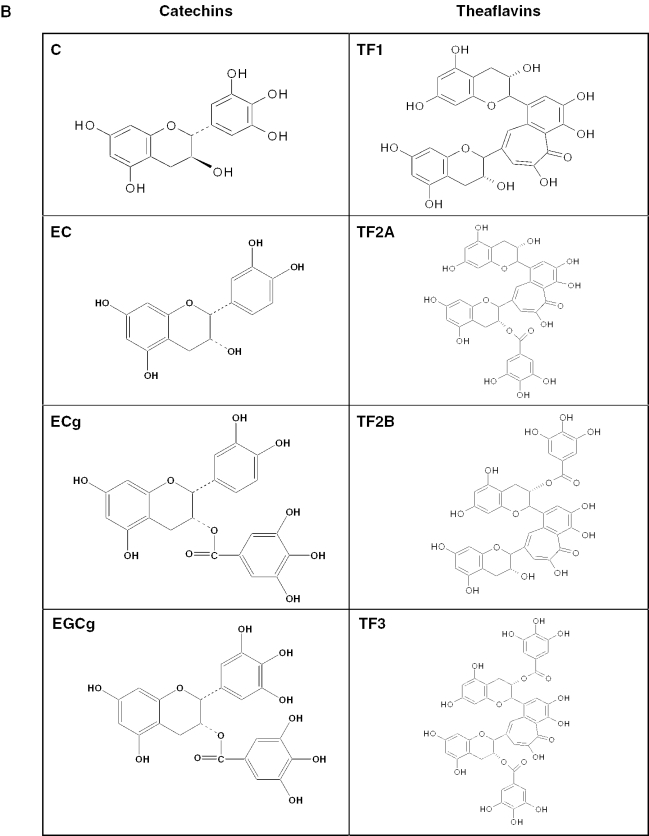
The natural product screened library consisted of simple and complex oxygen heterocycles, alkaloids, sequiterpenes, diterpenes, pentacyclic triterpenes, sterols and many other diverse representatives. Numerous black tea polyphenols (especially those of TF1, TF2 and TF3) were found to inhibit 3CLPro. In black tea, TF3 was the most abundant (1.05%) followed by TF2A (0.34%), TF2B (0.11%) and TF1 (0.08%) (16). It is interesting to note that TF3 contained two gallate groups attached to the 3,3′ positions as shown in Fig. 4b. TF2A and TF2B, on the other hand, consisted of only one gallate group attached either to the 3 or the 3′ positions, respectively (Fig. 4b). In contrast, no gallate group was attached to TF1. Results in Table 1, indicating that the gallate group attached to the 3′ position to TF2B and TF3, might be important for interaction with the 3CLPro active site. EC, EGCg, ECG and EGC were much less active (IC50 ≥ 100 µM) than theaflavins.
Figure 4.
Chemical structures of tannic acid, catechins and TFs.
Discussion
Human coronaviruses (HCoVs) are major causes of upper respiratory tract illness in animals including humans. It has been demonstrated that a novel coronavirus causes SARS. The main protease, frequently called 3C-like protease (3CLPro), has been identified as an attractive drug target (17). Of the 720 natural products screened in this study, tannic acid (Fig. 4a) and TF2B were identified using the HPLC proteolytic assay to inhibit 50% of the proteolytic acitivity of 3CLPro at concentrations ≤10 µM. Both tannic acid and TF2B belong to the group of natural polyphenols found in teas. Low concentrations of tannic acid were found to inhibit proteases including tissue-type plasminogen activator, urokinase-type plasminogen activator and plasmin activity [13]. Over 300 different kinds of tea are now produced, but they are classified into three main forms: green tea is manufactured by drying fresh tea leaves, with emphasis on the prevention of oxidation of the tea polyphenols during the manufacturing process. In contrast, the manufacture of black tea is characterized by a high degree of fermentation, which produces a series of chemical condensations among the ingredients present in tea. Oolong tea is partially fermented, and the tea compositions are more similar to those found in green tea (18). In evaluating different types of tea, we found that extracts from several different types of tea, including Puer and black tea, could inhibit 3CLPro activity while green or oolong tea could not. However, the chemical composition in Puer tea is very complex and it is difficult to isolate pure ingredients for structural identification. Purification and identification of Puer tea ingredients should be carried out in a separate study.
We next examined whether other well-known ingredients present in tea could also inhibit 3CLPro activity. We found that methylxanthine (caffeine and theophylline) and catechins (EGCg, EC, C, ECg and EGC) were not able to inhibit 3CLPro activity at concentrations up to 100 µM (Table 1). However, TF1, TF2 and TF3 were more potent 3CLPro inhibitors than catechins in green tea. During the fermentation step in the production of black tea, most of the catechins are oxidized and condensed into theaflavins through dimerization and into thearubigins through polymerization. Green tea contains ∼30% of catechins (dry mass base) while black tea contains ∼9% of catechins and 4% of theaflavins (19). In another study, Leung et al. (16) reported that TF3 was the most abundant (1.05%) theaflavin in black tea followed by TF2A (0.34%), TF2B (0.11%) and TF1 (0.08%). Tannic acid is a class of polyphenolic in plants but the quantification of its levels in green or black tea is difficult because tannic acid constitutes a wide range of polymers with mol. wts of 500–3000 Da.
It is interesting to note that TF2B and TF3 are more potent inhibitors of 3CLPro than TF1 (Table 1). Unlike TF2B and TF3, TF1 does not contain a gallate group (Fig. 4b). Thus, the addition of a gallate group attached at the 39 position to TF2B and TF3 might be important for their inhibitory activity against 3CLPro. These results suggest that TF2B and TF3 might be good starting points for the design of more active inhibitors for the 3CLPro encoded by SARS-CoV.
Finally, this study has identified three compounds (TF2B, TF3 and tannic acid) that are effective 3CLPro inhibitors (IC50 ≤10 µM). These compounds are abundant in the extract of black tea (16,19). Black tea is a popular beverage in the world. Results from this study warrant further investigation to examine the effect of these natural products in inhibition of SARS-CoV replication in cell culture. Clark et al. reported that theaflavins extracted from black tea were able to neutralize bovine coronavirus and rotavirus infections (20). Thus, it will be very interesting to evaluate, in a separate study, whether drinking black tea can prevent or alleviate the infection of an enteric form of coronavirus since SARS-CoV is known to actively replicate in the intestinal tract (21).
Acknowledgments
We thank Dr Tzong-Der Way for providing excellent technical support. This work was supported in part by funds and facilities of NHRI of Taiwan and by Grants NSC92-2751-B-400-006-Y to X.C and J.T.A.H. and NSC92-2751-B-001-012-Y to P.H.L. from the National Science Council, Taiwan.
References
- 1.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 2.Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Enserink M. Infectious diseases: calling all coronavirologists. Science. 2003;300:413–4. doi: 10.1126/science.300.5618.413. [DOI] [PubMed] [Google Scholar]
- 4.Enserink M, Vogel G. Infectious diseases: hungry for details, scientists zoom in on SARS genomes. Science. 2003;300:715–7. doi: 10.1126/science.300.5620.715. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel RP, Edmond MB. Managing SARS amidst uncertainty. N Engl J Med. 2003;348:1947–8. doi: 10.1056/NEJMp030072. [DOI] [PubMed] [Google Scholar]
- 6.Holmes KV, Enjuanes L. Virology: the SARS coronavirus: a postgenomic era. Science. 2003;300:1377–8. doi: 10.1126/science.1086418. [DOI] [PubMed] [Google Scholar]
- 7.Hegyi A, Friebe A, Gorbalenya AE, Ziebuhr J. Mutational analysis of the active centre of coronavirus 3C-like proteases. J Gen Virol. 2002;83:581–93. doi: 10.1099/0022-1317-83-3-581. [DOI] [PubMed] [Google Scholar]
- 8.Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, et al. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 9.Rota PA, Oberste MS, Monroe SS, Nix WA. Campagnoli R, Icenogle JP et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 10.Herold J, Gorbalenya AE, Thiel V, Schelle B, Siddell SG. Proteolytic processing at the amino terminus of human coronavirus 229E gene 1-encoded polyproteins: identification of a papain-like proteinase and its substrate. J Virol. 1998;72:910–8. doi: 10.1128/jvi.72.2.910-918.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziebuhr J, Thiel V, Gorbalenya AE. The autocatalytic release of a putative RNA virus transcription factor from its polyprotein precursor involves two paralogous papain-like proteases that cleave the same peptide bond. J Biol Chem. 2001;276:33220–2. doi: 10.1074/jbc.M104097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziebuhr J, Siddell SG. Processing of the human coronavirus 229E replicase polyproteins by the virus-encoded 3C-like proteinase: identification of proteolytic products and cleavage sites common to pp1a and pp1ab. J Virol. 1999;73:177–85. doi: 10.1128/jvi.73.1.177-185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taitzoglou IA, Tsantarliotou M, Zervos I, Kouretas D, Kokolis NA. Inhibition of human and ovine acrosomal enzymes by tannic acid in vitro. Reproduction. 2001;121:131–7. [PubMed] [Google Scholar]
- 14.Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–21. [PubMed] [Google Scholar]
- 15.Kazi A, Urbizu DA, Kuhn DJ, Acebo AL, Jackson ER, Greenfelder GP, et al. A natural musaceas plant extract inhibits proteasome activity and induces apoptosis selectively in human tumor and transformed, but not normal and non-transformed, cells. Int J Mol Med. 2003;12:879–87. [PubMed] [Google Scholar]
- 16.Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131:2248–51. doi: 10.1093/jn/131.9.2248. [DOI] [PubMed] [Google Scholar]
- 17.Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–7. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 18.Chen CN, Liang CM, Lai JR, Tsai YJ, Tsay JS, Lin JK. Capillary electrophoretic determination of theanine, caffeine, and catechins in fresh tea leaves and oolong tea and their effects on rat neurosphere adhesion and migration. J Agric Food Chem. 2003;51:7495–503. doi: 10.1021/jf034634b. [DOI] [PubMed] [Google Scholar]
- 19.Harbowy ME, Balentine DA. Tea chemistry. Crit Rev Plant Sci. 1997;16:415–80. [Google Scholar]
- 20.Clark KJ, Grant PG, Sarr AB, Belakere JR, Swaggerty CL, Phillips TD, et al. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Vet Microbiol. 1998;63:147–57. doi: 10.1016/S0378-1135(98)00242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–7. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]