Abstract
The fundamental organization of the human brain is established before birth, with rapid growth continuing over the first postnatal years. Children exposed before or after birth to various biological (e.g., substance exposure) or psychosocial hazards (e.g., maltreatment) are at elevated likelihood of deviating from a typical developmental trajectory, which in turn can be associated with psychological, behavioral, and physical health sequelae. In the HEALthy Brain and Child Development (HBCD) Study, a multi-site prospective longitudinal cohort study, brain, physical, biological, cognitive, behavioral, social, and emotional development is being examined starting in pregnancy and planned through age 10 (data are sampled at varying degrees of granularity depending on age, with more dense sampling earlier in life). HBCD aims to determine the short- and long-term impacts of a variety of both harmful and protective factors, including prenatal substance use, on developmental trajectories through early childhood. Organized as a nationwide consortium across 27 sites, the HBCD Study will collect multimodal data that will be made publicly available on a yearly basis, through a data use application and approval process. Here we provide an overview of the HBCD Study design, sampling, protocol development, and data management. Data collected through HBCD will be fundamental to informing future prenatal and early childhood interventions and policies to promote wellbeing and resilience in all children.
Keywords: HBCD, Child development, Brain development, Prenatal substance use
1. Introduction
The human brain begins to develop three weeks after conception and does not reach adult maturity until approximately the third decade of life (Stiles and Jernigan, 2010). The brain’s basic organization is assembled over the first two trimesters of fetal life, with the last trimester and the first few postnatal years reserved for more global neural growth and pruning. The most prolonged set of developmental processes is the generation of the basic wiring of the brain (synaptogenesis), the fine-tuning of that wiring (pruning), and the modification of neural cells to improve synaptic efficiency (myelination). Indeed, marked changes in neural connectivity and function occur as development unfolds, from infancy through adolescence and young adulthood (Edde et al., 2021, Gao et al., 2017). Because so much of brain development begins before birth, for a study of typical brain development to succeed, it must begin prenatally, and in so doing, capture the myriad of both risk and protective factors that can impact the initial assembly and subsequent development of the brain.
Early experiences can radically impact the eventual structure and function of the brain, driving neurodevelopmental processes that occur in nearly all humans, as well as making individual brains unique and attuned to their particular environments. At a basic level, a relatively similar functional and structural brain organization arises for all humans undergoing typical development (i.e., those without serious developmental disorders or anomalies; (Stiles and Jernigan, 2010). This normative organization, shared across the human species, depends upon both the human genetic code and exposure to a key set of evolutionarily expected experiences--a category of environmental inputs experienced by nearly all members of the species during evolution (Fox et al., 2010). These are typically thought to include input of patterned light, sounds, and other sensory stimuli, as well as social exposures, including contact with human faces, voices, and touch. In the absence of these inputs, atypical neural organization can occur —examples include visual and auditory impairments, along with atypical social-emotional development (Ward, 2019). Unique features of an individual’s genetic background coupled with life experience and environmental exposures give rise to subtler heterogeneity in brain structure and function across the species, including variation in volume or microstructure of different brain regions or the richness of connectivity between such regions. Importantly, a child’s particular linguistic, cognitive, or social environment likely shapes individual variation in brain development outside of critical periods (McLaughlin et al., 2019, Tooley et al., 2021). These experiences may interact in the context of a child’s genetic background as development unfolds, contributing to the enormous range of individual differences in cognition and behavior observed across humans.
1.1. Neural plasticity and critical periods
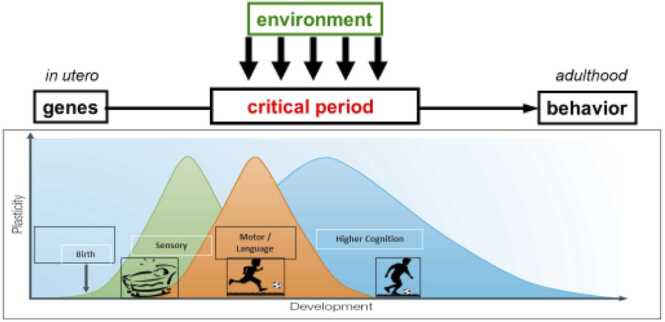
A core feature of human brain development is the fundamental ability to change and adapt in response to the environment. Protracted processes of neurodevelopmental maturation and fine-tuning, including neurogenesis, synaptic overproduction, and pruning, emerge from complex interactions between genes and experiences. Developing individuals thus have the potential to achieve exquisite specialization to the demands of their environments, and to benefit substantially from positive developmental environments. However, this protracted period of environmental susceptibility can also lead many children to be vulnerable to negative experiences, such as exposure to substances, maltreatment, and stress. Overall, while some degree of neural plasticity persists throughout life, enabling adult learning, changes and adaptations occurring early in life often emerge more readily and persist more stably after a given degree of experiential input (Reh et al., 2020). This is seen, for instance, in young children’s greater capacity for language learning (compared to adults) following comparable exposure (Werker and Hensch, 2015). Such periods of increased susceptibility of long-term neural structure and function to experiential input, deriving from their concurrence with processes such as massive early growth spurts and subsequent experience-responsive pruning, are often called developmental critical periods (see Fig. 1).
Fig. 1.
Depicts critical periods in brain development from in utero to adulthood. The Y axis illustrates the amount of plasticity (low to high), and the X axis illustrates age, from conception through the end of the life span. Two additional points are worth noting. First, different domains are governed by different critical periods, with critical periods regulating sensory functioning closing before those governing high cognitive functions. Second, the onset and offset (illustrated by the peaks) of critical periods differs by domain, such that the critical period for sensory systems occurs earlier and lasts more briefly than, say, language or higher cognitive functions. (Figure was adapted from a previous figure C. Nelson (author) created for Neurons to Neighborhoods).
Broadly, the complexity and protracted nature of human brain development and retention of some plasticity throughout life allows for ongoing adaptation. The timing of maximal plasticity across functional domains is thought to depend upon factors such as when peak synaptic overproduction occurs within relevant brain structures/circuits, and how long synaptic pruning and microstructural changes such as myelination persist—processes which, in turn, correspond roughly to the early versus late and brief versus protracted process of developing relatively more simple capacities (e.g., basic sensory function) or complex ones (e.g., higher cognition and planning). At a more granular level, the onset and offset of critical periods is regulated by a series of molecular cues and brakes that are becoming increasingly well understood (Hensch and Bilimoria, 2012).
1.2. A framework for considering the effects of early adversity on brain development
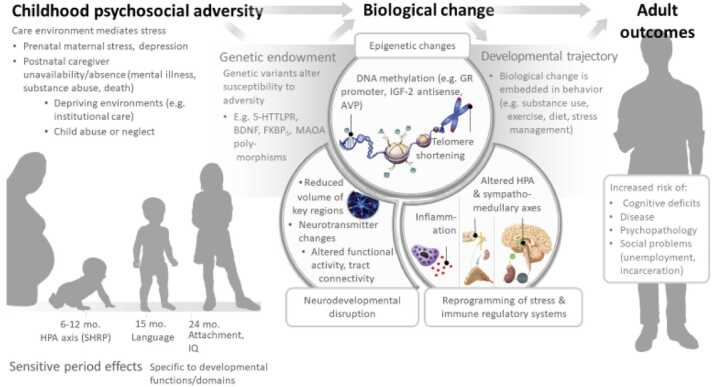
There is growing evidence that children exposed to various hazards or adverse events early in life, including before birth, are at increased risk for atypical variations in brain development that in turn are associated with a variety of psychological, behavioral, and physical health sequelae. Adversity generally involves exposure to biological hazards (e.g., malnutrition, environmental toxins, chronic infection, parental substance use), psychological hazards (e.g., maltreatment, neighborhood or domestic violence) and frequently, both. And, although one can be exposed to adversity at any point in the lifespan, the bulk of the evidence supports the view that exposure to adversity during critical periods of brain development are particularly hazardous to development (Berens et al., 2017; Nelson and Gabard-Durnam, 2020). Importantly, the effects of such exposures may become biologically embedded and extend across the entire life span, as illustrated in Fig. 2.
Fig. 2.
Biological effects of psychosocial adversity in critical periods of childhood.
Fig. 2 depicts biological effects of childhood adversity related to developmental trajectories and adult outcomes, set against one’s genetic background. The interaction of genes and environments can lead to epigenetic changes, changes in homeostatic systems and neurodevelopmental disruptions, which in turn can lead to a variety of maladaptive adult outcomes. (Figure was adapted from (Berens et al., 2017)).
Unfortunately, a great many children conceived and born around the world are exposed to a host of both pre- and postnatal risk factors that increase the likelihood of non-optimal developmental outcomes. For example, in the United States approximately 17 % of children live below the poverty line; nearly 700,000 children/year experience maltreatment (with the largest percentage being children <1 year); and at least 10–11 % of all live births are affected by prenatal exposure to risky levels of alcohol or illicit drugs (Substance Abuse and Mental Health Services Administation, 2020). This latter point is of increasing concern, as the use of alcohol, cannabis, opioids, and methamphetamines is rising among women of reproductive ages (Cook, 2022, Smid et al., 2019, Volkow et al., 2019), resulting in the potential for substantial exposure prior to, and in some cases after, recognition of the pregnancy. Although there is a substantial literature on the adverse birth and neurodevelopmental outcomes associated with some prenatal exposures, particularly alcohol and nicotine (Banderali et al., 2015, Mattson et al., 2019, May et al., 2018, Moore et al., 2020), other substances, including cannabis (which is increasingly being legalized across the US; (Corsi et al., 2020, Volkow et al., 2017), and opioids (Kiblawi et al., 2014; Nelson et al., 2020; Smith et al., 2008), are less well characterized. Additionally, prenatal polysubstance exposure is common (Jarlenski et al., 2020, Qato et al., 2020), and often is concomitant with other environmental adversities that may have effects on development. This has likely led to biased effect estimates for the individual substances. In order to rigorously evaluate these complex exposures occurring during a highly dynamic period, it is necessary to have a longitudinal study that uses multiple data sources to capture, with granularity, the timing, dose and duration of prenatal exposure to legal and illegal substances. Further, to address potential confounding, the sample must be enriched for other environmental adversities, and evaluate a broad and inclusive range of adversities in order to better isolate the effects of prenatal substance exposure (Brogly et al., 2014; Nelson et al., 2020). Also, as different substances and exposures at different developmental time points in gestation presumably would have differential effects, it is important to evaluate a broad range of structural, cognitive, emotional, and behavioral outcomes, longitudinally. This can best be accomplished by an integrated, coordinated consortium of investigators working together.
Although the hazards of some specific early exposures are increasingly well known, it is also important to emphasize that exposure to positive influences in the environment can confer protection from subsequent exposure to adversity. An example of a protective factor would be stable responsive caregiving (Miller and Commons, 2010). Thus, it would be naive to assume that exposure to adversity, particularly during critical periods, directly and necessarily mediates adverse outcomes; while true in many cases, there are a host of positive moderating variables that can mitigate a child’s response to adversity. Support for this assertion can be found in both the human and animal literatures; for example, that stable, responsive caregiving can mitigate the harms done by exposure to hazardous events, including prenatal substance exposure and poverty (Knop et al., 2017, Nunes Cauduro et al., 2021). Thus, in the HEALthy Brain and Child Development (HBCD) Study we will focus not only on the potential harms associated with exposure to adversity but also the protective factors that can buffer such children from compromised developmental sequelae.
In this introduction to the HBCD Study, we provide an overview of the study objectives, design, organizational structure, and briefly describe study measures. Additional detail about the many components of the study is provided in the 17 other papers in this special issue (see Table 1).
Table 1.
Articles Describing Components of the HBCD Study in this Special Issue in Developmental Cognitive Neuroscience.
| HBCD Workgroup/ Committee | Authors | Title |
|---|---|---|
| National Institutes of Health (NIH) | Volkow et al. | The HEALthy Brain and Child Development Study (HBCD): NIH collaboration to understand the impacts of prenatal and early life experiences on brain development |
| Diversity, Equity, and Inclusion (DEI) | Murray et al. | Investment, integration, and innovation: Fostering diversity, equity, and inclusion across the HEALthy Brain and Child Development (Study) consortium |
| Ethics, Law, and Policy (ELP) | Kingsley et al. | Navigating ethical and legal requirements in the HEALthy Brain and Child Development (HBCD) Study: Lessons learned from the ethics, law, policy working group |
| Recruitment, Retention, and Community Engagement (RRC) | Harden et al. | The HEALthy Brain and Child Development Study (HBCD) experience: Recruiting and retaining diverse families in a longitudinal, multi-method early childhood study |
| Study Navigators (SN) | Hillard et al. | Establishing a model of peer support for pregnant persons with a substance use disorder as an innovative approach for engaging participants in longitudinal research |
| Communication, Engagement, and Dissemination(CED) | Cole et al. | Communications, engagement, and dissemination strategies for the HEALthy Brain and Child Development Study (HBCD) |
| Biostatistics (BST) and Design (DSN) | Si et al. | Advancing high quality longitudinal data collection: Implications for the HEALthy Brain and Child Development (HBCD) Study design and recruitment |
| Magnetic Resonance Imaging (MRI) | Dean et al. | Quantifying brain development in the HEALthy Brain and Child Development (HBCD) Study: The magnetic resonance imaging and spectroscopy protocol |
| Electroencephalography (EEG) | Fox et al. | The development and structure of the HEALthy Brain and Child Development (HBCD) Study EEG protocol |
| Pregnancy Exposures, including Substances (PRG) | Gurka et al. | Assessment of maternal health and behavior during pregnancy in the HEALthy Brain and Child Development (HBCD) Study: Rationale and approach |
| Biospecimens (BIO) | Sullivan et al. | Biospecimens in the HEALthy Brain and Child Development (HBCD) Study: Rationale and protocol |
| Novel Technology/ Wearable Sensors (NTW) | Pini et al. | Remote data collection of infant activity and sleep patterns via wearable sensors in the HEALthy Brain and Child Development (HBCD) Study |
| Physical Health (PHY) | Cioffredi et al. | Infant and early childhood physical health assessments in the HEALthy Brain and Child Development (HBCD) Study |
| Social and Environmental Determinants (SED) | Cioffredi et al. | Assessing prenatal and early childhood social and environmental determinants of health in the HEALthy Brain and Child Development (HBCD) Study |
| Child Behavior and Child-Caregiver Interactions (BEH) | Edwards et al. | Capturing the complexity of child behavior and caregiver-child interactions in HEALthy Brain and Child Development (HBCD) Study using a rigorous and equitable approach |
| Neurocognition and Language (NCL) | Kable et al. | WG-NCL: Measurement of emerging neurocognitive and language skills in the HEALthy Brain and Child Development Study |
| Spanish Language and Culture (SLC) | Anunziata et al. | ¿Donde están? Hispanic/Latine inclusion, diversity, and representation in the HEALthy Brain and Child Development (HBCD) Study |
2. HBCD study objectives
The HBCD Study will prospectively examine human brain, cognitive, behavioral, social, and emotional development beginning prenatally and planned through age 10. The overarching goal of the study is to understand the neurodevelopmental trajectories of children growing up in diverse and varied environments. We aim to determine the short- and long-term impacts of a variety of both harmful and protective environmental factors, including prenatal substance use, mental health, stress, sociodemographics, biological and genetic factors, parent/child interactions, and social conditions. This will enable us to identify the key developmental windows during which these exposures and protective factors may be more impactful on developmental outcomes.
3. HBCD study organizational structure
The HBCD Study entails building out a large, diverse national cohort of multimodal datasets of the developing child across the first 10 years after birth. Funding for the study is led by the National Institute of Drug Abuse (NIDA) with additional funding from 11 additional NIH Institutes and Centers including the Helping to End Addiction Long-term (HEAL) Initiative. The consortium, which includes an administrative core, a data coordinating center, and 27 recruitment sites nationwide (see https://hbcdstudy.org/; Fig. 3, Fig. 4), consists of investigators with extensive experience conducting longitudinal pregnancy cohort studies. The HBCD Administrative Core (HCAC) is responsible for primary leadership of the overall HBCD Study in collaboration with the NIH scientific staff. The HCAC leads the design and coordinated administration of the HBCD Study protocol, develops and administers HBCD-wide protocols and monitoring for site performance and fidelity, ensures recruitment and retention targets are met, manages the single Institutional Review Board (IRB) application for the study, manages the consortium’s Working Groups (WGs) and committees, and is responsible for communication with the sites, scientific community, and the public. Alongside the HCAC, the HBCD Data Coordinating Center (HDCC) leads the coordination of data collected across all sites and modalities. The HDCC is responsible for securing data receipt and monitoring data quality across modalities, including interview data, scored testing data, biospecimens, and wearable biosensors; ensuring the standardized implementation of MRI and EEG protocols; and reviews of structural MRIs and management of incidental findings. The HCAC and HDCC will ensure that at least annual HBCD data releases are provided to the public scientific community. Under the study’s open science data model, access to HBCD data for analysis and publication is only made available to HBCD investigators and the larger scientific community following data releases and after execution of the appropriate data use agreements. The HCAC and HDCC work in coordination with NIH to ensure appropriate and effective resource sharing and dissemination of study findings. The NIH scientific staff includes a Scientific Director for the study who guides the study progress and sits as a voting member on the HBCD Steering Committee. The HBCD Steering Committee is the governing body for the study. Standing members are the five HCAC and HDCC Directors, the NIH Scientific Director, and a rotating set of members drawn from the Consortium who serve staggered terms.
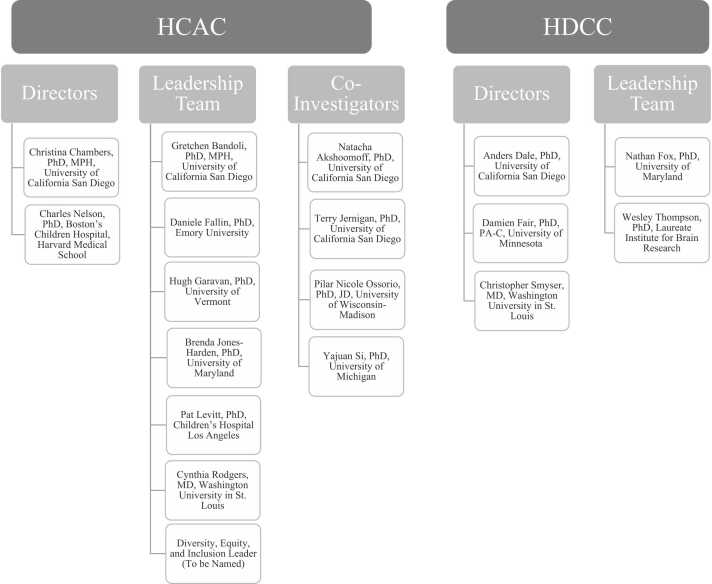
Fig. 3.
Leadership team of the HCAC and HDCC as of 2024. Figure depicts the leadership team of the HBCD Administrative Core (HCAC) and the Data Coordinating Center (HDCC).
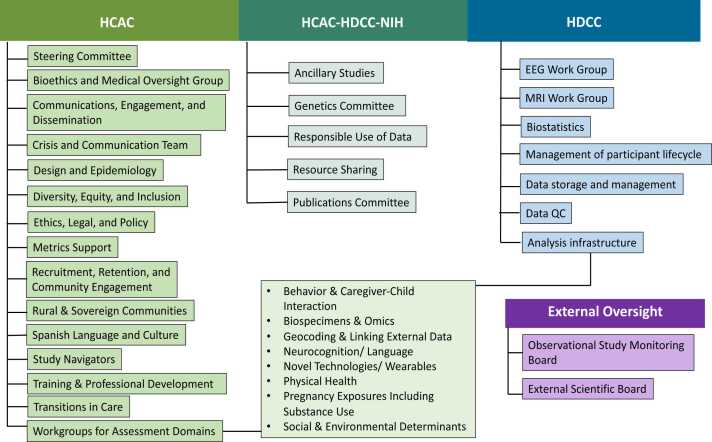
Fig. 4.
HBCD Organizational Structure. Figure depicts the organizational structure of the HBCD Consortium, including committees and workgroups under the Administrative Core (HCAC), the Data Coordinating Center (HDCC), and HCAC-HDCC and the National Institutes of Health (NIH), as well as the external oversight boards.
The overall goal of the Consortium is to collect multimodal data that will, at regular intervals, be made available to the public to address major knowledge gaps in developmental trajectories from infancy through the pre-adolescent period. Given the NIH-defined scale and interdisciplinary nature of this effort, to collect the highest quality data possible for broad use by the scientific community, our Consortium spent 12 months establishing working groups (WGs) to work out the details of the extensive battery of protocols that will be deployed (See Fig. 4). The WGs identified: 1) foundational principles for data harmonization across sites; 2) metrics for ensuring highest quality data that will facilitate the application of advanced analytical methods applied by the research community; 3) bioethical standards for all sites to create authentic partnerships with communities from which recruitment will occur, and with the families participating in the HBCD Study.
The HCAC manages the Bioethics and Medical Oversight (BMO) Committee which is responsible for identifying and addressing potential risks to participant safety and well-being such as processes for informing participants about incidental findings on MRI. The HBCD consortium also has in place external oversights in addition to the HCAC and HDCC. An HBCD Observational Study Monitoring Board (OSMB) reviews study protocols and addresses safety and ethical concerns as they arise. Finally, the External Scientific Board (ESB) reviews and provides scientific consultation for the HBCD consortium on study methods and progress.
Lastly, as of early 2024, the HBCD Consortium consists of 27 recruitment sites across the country (Table 2). Sites represent a diversity of catchment areas generally similar to the population of persons delivering newborn infants in the U.S. Each site determines their own team and organizational structure based on their need, but generally consists of MPIs, Co-Is, a project coordinator, research assistants, and study navigator(s).
Table 2.
HBCD Recruitment Sites as of 20241.
| Recruitment Site | Location | Principal Investigator(s) |
|---|---|---|
| Arkansas Children’s Research Institute | Little Rock, AR |
|
| University of Arkansas for Medical Sciences | Little Rock, AR |
|
| Boston Children’s Hospital, Harvard Medical School | Boston, MA |
|
| Cedars-Sinai Medical Center | Los Angeles, CA |
|
| Children’s Hospital Los Angeles | Los Angeles, CA |
|
| Children’s Hospital of Philadelphia | Philadelphia, PA |
|
| Cincinnati Children’s Hospital Medical Center | Cincinnati, OH |
|
| Emory University | Atlanta, GA |
|
| John Hopkins University/Kennedy Krieger Institute | Baltimore, MD |
|
| Northwestern University | Chicago, IL |
|
| New York University Langone Health | New York, NY |
|
| Oklahoma State University Center for Health Sciences | Tulsa, OK |
|
| Oregon Health and Science University | Portland, OR |
|
| Pennsylvania State University | University Park, PA |
|
| Pennsylvania State University College of Medicine | Hershey, PA |
|
| University of Alabama | Tuscaloosa, AL |
|
| University of Alabama at Birmingham | Birmingham, AL |
|
| University of California San Diego | San Diego, CA |
|
| University of Maryland, College Park | College Park, MD |
|
| University of Minnesota | Minneapolis, MN |
|
| University of New Mexico | Albuquerque, NM |
|
| University of North Carolina at Chapel Hill | Chapel Hill, NC |
|
| University of Vermont | Burlington, VT |
|
| University of Wisconsin-Madison | Madison, WI |
|
| Vanderbilt University | Nashville, TN |
|
| Virginia Tech | Roanoke, VA Blacksburg, VA |
|
| Washington University in St. Louis | St. Louis, MO |
|
1Table presents the current recruitment sites and principal investigators (PIs) at the time of manuscript submission (February 2024). PIs and institutions may change throughout the course of this 10-year study.
4. Methods
All study procedures and protocols have been reviewed and approved through a single application by the Institutional Review Board (sIRB) at the University of California San Diego. All adult participants provide written informed consent and parental permission for their child until the child reaches the age of 7 years.
4.1. Study design
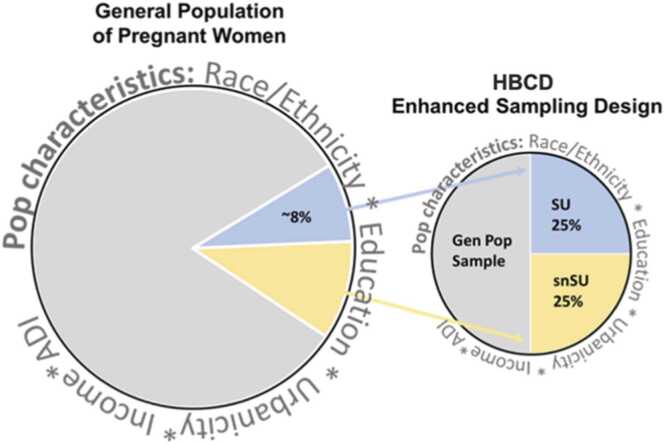
The HBCD Study uses a prospective, single cohort design aimed at collecting data from over 7000 mothers and infants starting during pregnancy and through the child’s first ten years of life. Across 27 recruitment sites nationwide, the cohort will examine neurodevelopmental trajectories of children across diverse populations, exposures, and environments. The sampling strategy is not a nationally representative design. Instead, the approach to sampling is intended to represent the characteristics of the population of persons who deliver infants in the catchment areas for each of the 27 sites, with oversampling of individuals with certain characteristics of focus in the study. However, sufficient data will be collected to support generalization to the U.S. population. For this reason, the HBCD cohort will involve: a) a general population cohort representing the diversity of 18–49 year old persons who deliver infants in the US, and b) a cohort with substance use (SU) exposure (alcohol, tobacco, cannabis, or opioids) during pregnancy. Within the general population cohort, participants who are demographically similar to those SU participants who do not use substances (“similar but no substance use” or snSU) are included as the appropriate comparison group to the SU participants (Fig. 5).
Fig. 5.
HBCD Enhanced Sampling Design. Figure depicts enhanced sampling design of HBCD from the target population, including the subcohorts: general population sample and a substance use (SU) cohort. Within the general population cohort, participants who are demographically similar to the SU cohort but without substance exposure (snSU) are included as the comparison.
Across recruitment sites, under the current core protocol, pregnant individuals may be enrolled and complete a total of eight study visits from prenatal life over the first four years. One visit occurs prenatally (visit 1). To allow for inclusion of those who have received minimal or no prenatal care, up to 10 % of the sample may be recruited shortly after delivery. During the child’s first 15 months of life, a period of accelerated brain development, three in-person study visits are planned. In subsequent years, at least annual visits are planned with a combination of in-person and remote data collection. To obtain a distribution of assessments across child ages in the 0–48-month range, participants are randomly assigned at delivery within site to specific time windows within each visit’s age range. This jittered timing approach to the assessment schedule is optimized to allow estimation of individual developmental trajectories in the presence of sparse longitudinal data collection, particularly over the highly dynamic period of growth that is being studied.
4.2. Participants
The HBCD cohort will consist of three subcohorts of pregnant persons and their infants, including 50 % of the sample who represent the diversity of the population of individuals giving birth, 25 % of the sample with SU exposure during pregnancy and 25 % of the sample who are demographically similar to the SU cohort but no SU in pregnancy (snSU). Inclusion criteria for
all subcohorts include being currently pregnant or being early postpartum with little to no prenatal care receipt, currently 18 years or older, and speak English or Spanish. Currently, postpartum individuals may be enrolled if they are within 5 days of delivery and they received 4 or fewer prenatal care visits. Individuals who do not plan to be a primary caregiver for the child (e.g., surrogates) will not be eligible for the study. Pregnant persons carrying twins or higher
order multiples and individuals who have more than one pregnancy over the study recruitment period are also eligible to enroll.
To be considered part of the SU cohort, individuals must meet inclusion criteria for alcohol, tobacco, cannabis, or opioid use in pregnancy. Preliminary threshold criteria for each substance are presented in Table 3. The target proportion of the sample meeting each of the substance thresholds is 12 % per specific substance. However, it is expected that co-exposure to multiple substances, or polysubstance use, will be common in the sample. Furthermore, the 50 % of the sample who do not meet the SU criteria are expected to represent the range of exposures to these substances seen in the general population of pregnant persons. Substance exposure will be based on both self-report and biospecimens (e.g., blood, urine and nails). Continuous active monitoring of the enrollment of the study participants in the cohort occurs through the Adaptive Enrollment Dashboard (see additional details in Recruitment section).
Table 3.
Recruitment Criteria to Identity Individuals with Substance Use Exposure in Pregnancy.
| Substance | Criteria |
|---|---|
| Alcohol |
|
| Tobacco/ Nicotine |
|
| Cannabis |
|
| Opioids |
|
4.3. Recruitment
Participants are being prospectively recruited from multiple venues at each of the 27 recruitment sites over an approximate four-year period. Recruitment strategies and venues are specific to each site. For example, at some sites potential participants are recruited through prescreening of electronic health records. Sites are also conducting screening at prenatal clinics, treatment centers, and other community venues. Healthcare providers in the catchment areas may also refer participants to the HBCD Study, including those serving pregnant persons with a history of substance use or those providing substance use treatment. In addition, HBCD sites are using social media messaging and the study website to share information about the HBCD Study with the community and encourage enrollment.
HBCD recruitment is monitored in real-time to ensure target recruitment goals are met with adequate representation of sampling cohorts, including those with SU. The study utilizes an Adaptive Enrollment Dashboard for this purpose. Data from the Dashboard are used to ensure that needed adjustments to recruitment can be implemented in a timely manner. Through this real-time monitoring, key maternal characteristics within site and overall will be compared for those enrolled relative to the population they are drawn from. Propensity score methods will also be used to evaluate balance on key characteristics for those who meet SU criteria and those who are similar but report no SU in pregnancy. This iterative process will provide timely information so that sites can modify recruitment strategies or venues to achieve the required balance.
4.4. Retention
The HBCD Consortium recognizes that participant retention will be one of the largest challenges in ensuring the success of the HBCD Study. Particular attention was given in designing the study protocol and procedures to participant burden, and a number of strategies were adopted to reduce this, including spreading visits over two days as needed and remote assessments prior to in-person visits. Comprehensive retention plans and staff training have been developed through the Consortium and WGs. All efforts are made to have participants maintain engagement in the study through a variety of strategies, including engagement and relationship development with staff, appointment reminders, provision of resources and referrals, thank you cards and certificates, and small gifts (e.g., diapers, coloring book, children’s books). Additionally, HBCD has developed a novel Study Navigator program. At each site, one or more staff members, some of whom may have substance use lived experience, have been trained and designated to serve in the role of Study Navigator. In this capacity, Navigators provide individualized support to study participants through interim contacts as frequently as needed or requested, referrals for services, and general support. The Study Navigators are integrated into the research team and work closely with research coordinators. The Navigator experience will be evaluated in terms of impact on participant retention as well as participant reflection on their study experience.
To address the circumstance where the custody of the participating child changes, including when a child is removed from parental custody and enters foster care or other placement, the Transitions in Care WG has established protocols for attempting to maintain children in the study by obtaining consent from the children’s new caregiver, whether that is another family member, foster parents, or the state. In the event that a participant moves out of the study catchment area, every attempt will be made to transition that participant to another study site closer to their new location and retain them in the study.
4.5. Protocol
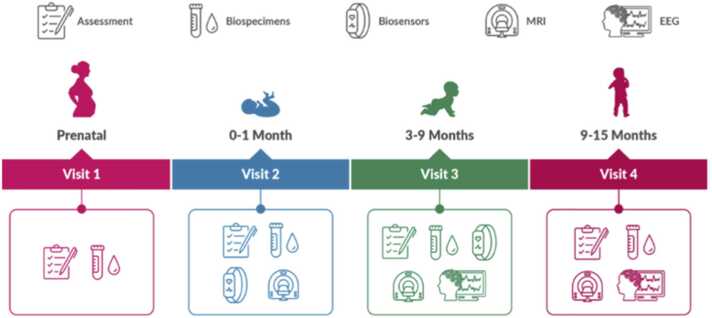
The study protocol was designed to capture more frequent assessments in the first months of life to best address the study objectives. Assessments will be collected, linked, and repeated over time focused on brain development, behavior, biospecimens, and environmental exposures. Assessments will include questionnaire-based surveys, neuroimaging, direct assessment of neurobehavioral performance, EEG, biospecimen collection, and wearable biosensor monitors (See Fig. 6). An overview of the assessments and timeline is presented in Table 4. Neuroimaging will consist of a total of four MRI-focused visits, starting at 0–1 month (Visit 2). EEG assessments will start at 3–9 months (Visit 3). Biospecimen collection will occur at multiple visits, including the prenatal Visit 1. Survey assessments will be conducted at all visits, and be the only data collected at remote visits. With parental consent, we also plan to obtain data from medical records. In addition, geocoded addresses will be linked to neighborhood-level data to capture environmental exposures and determinants. Details on study protocols can be found in the other articles included in this special issue as listed in Table 1.
Fig. 6.
HBCD Study Timeline and Assessments for Visits 1–4.Figure depicts major domains of assessments at visits 1 through 4, spanning from the prenatal period through 15 months of life.
Table 4.
HBCD measured constructs and assessments for visits 1–4.
| Construct | Measures | Visits1 |
|---|---|---|
| Parental and Family Factors, Environment, and Exposures | ||
| Demographics |
|
1, 4 1, 2, 3, 4 1 |
| Parental Physical Health, Current Pregnancy |
|
1 1 1 1 1 1, 2 |
| Parental Mental Health |
|
1, 2, 3, 4 1, 2, 3, 4 1, 2, 3 1 |
| Maternal Substance Use |
|
1, 2, 3, 4 1, 2 |
| Maternal Biomarkers2 -GWAS, EWAS, Transcriptome -Drug Panel -Toxins -Environmental Exposures -Metals, Nutrition, Proteins |
|
1, 2, 3, 4 1 1, 2 1 |
| Perceived Stress/Social Supports |
|
1, 2, 3, 4 |
| Intimate Partner Violence |
|
1 4 |
| Discrimination |
|
1 |
| Maternal Adverse Experiences |
|
4 |
| Neighborhoods |
|
1 4 |
| Protective Factors |
|
1 |
| Child Health and Development | ||
| Physical Health and Growth |
|
2, 4 2, 3, 4 4 4 4 |
| Nutrition |
|
2, 3, 4 2, 3, 4 4 |
| Sleep |
|
4 4 |
| Child Biomarkers2 -Metabolome, Microbiome -Environmental Exposures and Toxins |
|
2, 3, 4 2, 3, 4 2, 3, 4 |
| Brain Development- MRI |
|
2, 3, 4 2, 3, 4 2, 3, 4 2, 3, 4 2, 3, 4 2, 3, 4 |
| Brain Development - EEG |
|
3, 4 3, 4 3, 4 3, 4 3, 4 3, 4 |
| Motor Development |
|
2, 3 2, 3 4 4 |
| Neurocognition and Language Development |
|
3 3, 4 3, 4 3 3, 4 4 4 |
| Temperament -Self-regulation -Negative Affect -Positive Affect -Inhibition -Irritability |
|
2, 3 3 3 3 4 |
| Caregiver-Child Relationship -Parenting -Co-regulation |
|
3 3 4 4 |
| Social and Family Environmental Factors |
|
4 2, 3, 4 2, 4 4 4 4 4 |
1Some participants may be recruited postpartum if they had little to no prenatal care. In such cases, some assessments normally conducted in visit 1 (e.g., parental health assessments) are asked at theses participants’ first study visit, which corresponds to visit 2.
2At the present time (February 2024) some biospecimens, which can include saliva, stool, and blood, are being banked but not analyzed until plans and resources are obtained for appropriate analyses. Nail clippings and urine samples are currently being used for substance use assays. Proposed genetic analyses are optional in the informed consent; participants can opt in or out of genetic analyses for themselves or their child.
The HBCD Study protocol for Visits 1 through 4 has been developed, pilot tested and now implemented in the main study. However, protocol development is an iterative and standardized process across the Consortium. Final decisions regarding visits beyond 15 months are currently under review. WGs develop the assessments and measures relevant to their domain for each visit as appropriate. Each assessment is then submitted to the HBCD Diversity, Equity, and Inclusion (DEI) committee for review of issues around inclusion and cultural appropriateness, which is overseen by a DEI Associate Director (AD). The WG then presents recommended assessments to the Consortium investigators for their concurrence, and those accepted for adoption are presented to the HBCD Steering Committee. If approved, WGs then create standardized operating procedures around the assessment, develop the needed surveys, and address any overlapping constructs with other WGs. As needed, assessments are also sent for translation and approved by the Spanish Language and Culture committee. HDCC develops each measure in the data collection systems and tests for accuracy. Once ready, each measure is piloted with subjects for each study visit. Pilot data are then reviewed by each WG for data quality, completeness, participant burden, and feedback from both participants and research staff. Once approved, the assessment is added for inclusion in the final study protocol. If an assessment or procedure failed or a concern arose at any point in this process, the assessment is reevaluated, adapted, or removed. Additional measures added for each visit go through this process. This process ensures data is comprehensive, high quality, inclusive, and appropriate. The pilot procedures and evaluation also help ensure that participant burden, acceptability and feasibility are addressed before any protocol measure is adopted in the main study. Given that this is a 10-year longitudinal study, we anticipate that WGs will continue to be active throughout the lifespan of the study, as needed to develop new measures and/or revise existing measures.
4.6. Participant compensation
The HBCD consortium aims to ensure that adult and child participants are adequately compensated for their time and effort in the study without being coercive. Participant compensation is determined at the site level based on local context, as well as the complexity and time commitment involved for each visit. Financial compensation and age-appropriate small gifts that are integral to the study visit are provided. Participants are also reimbursed for travel and transportation costs as needed, provided access to child-care services at the study visit as needed, and provided or are reimbursed for food or refreshments for the time period they are participating in study activities.
4.7. Data management
Following recommendations for clinical data warehouse design and management by Healthcare Information and Management Systems Society and HIPPA regulations, the HDCC manages all data through systems that incorporate validated secure data transfer, storage, management, and analysis to ensure data security. Data management includes continuous quality control checks for study measures that are conducted by the measure experts, and ongoing validation that study data collection, transfer and storage procedures are functioning correctly. The HDCC ensures that a curated HBCD cumulative dataset is prepared for release at least annually for public data access. Access to the dataset will be managed through a data use application process and is expected to begin by 4th quarter 2024 or 1st quarter 2025. Specific guidance to the field on approaches to how data after release may be analyzed is provided in the paper by Si et al. in this special issue (Table 4).
4.8. Diversity, equity and inclusion
Particular attention throughout study development was paid to issues around diversity, equity and inclusion and all HBCD consortium members are required to agree to the HBCD DEI values statement. Under the leadership of the DEI AD, the HBCD DEI committee is involved in all study procedure development, recruitment, and protocol design plans. They are responsible for reviewing and making suggestions for each assessment submitted by WGs for inclusion in the study protocols. All procedures are reviewed for inclusive language, cultural appropriateness, and understanding. Along with the DEI committee, the HBCD Spanish Language and Culture committee reviews each assessment for translation accuracy as well as continued cultural competency and appropriateness for each measure. Moreover, the DEI committee hosts regular staff trainings around DEI issues and aims to ensure the HBCD consortium is equitable in all research plans, participant and community engagement, and training and leadership in the scientific community.
4.9. Community advisory boards
Each recruitment site has invited and hosts one or more external community advisory boards (CABs) for the purposes of engaging the community in every step of the study planning and implementation. CAB members may consist of relevant community agency representatives such as child welfare services, individuals with substance use histories, early childhood education groups, clinicians, and ethicists. CAB members meet regularly with the site study teams, and are consulted ad hoc by the sites as well as the consortium as a whole for advice on study progress and challenges.
5. Limitations
In any study with as many moving parts as HBCD, and that involves such an enormous commitment on the part of families, the HBCD Study has a number of limitations which include the following:
First, given the dramatic changes that occur in both body and brain from conception through birth, it is unfortunate that only one prenatal visit has been planned for. Like the entirety of HBCD, we needed to attempt to reduce the burden on both pregnant persons and later on children and families, and the decision was made to limit what we obtained prenatally. In a perfect world, of course, we would ideally have sampled more finely across the entire prenatal period. Doing so, however, would come at some cost, including imposing on individuals at a uniquely demanding period of life.
Second, a great deal of discussion was directed at how often we should collect data after birth, and how extensive data collection should be at individual time points. WGs paid close attention to the time involved in navigating each visit, consulted with CABs, reviewed pilot participant feedback and staff burden, and settled on a protocol that is still very time consuming but considerate of families. Evaluation of participant satisfaction, retention, measure completion, and site burden is ongoing and will inform future modifications as necessary.
Third, we recognize that the most challenging participants to recruit and retain will be those facing various stressors including lack of resources or social support, overwhelming responsibilities, and in some cases complicated by substance use. Here our Study Navigators and CABs will play a key role in supporting participants, and in helping guide recruitment and retention.
Finally, we recognize that families are making an enormous commitment to HBCD by agreeing to participate until their children are 10 years old. A study of this scope and ambition has never been done before and thus, we are making a concerted effort to encourage our families to remain involved in HBCD. Only time will tell if we are being successful and if we detect that retaining our sample is being compromised, we will take corrective action.
6. Conclusions
In this special issue of the Journal, specific components of the HBCD Study are described in more detail by the various teams that have developed and are implementing these aspects of study design, measures, and oversight (Table 4). The HBCD Study is the first of its kind with longitudinal data collection planned for over a 10-year period across multiple domains using state of the art techniques in a national sample of parent/child pairs. The sample size and study design will enable appropriately powered statistical analyses that can address the core research questions posed by the study. In addition, for the scientific community at large, this study will support hypothesis-testing for a wide array of other research questions, some of which may only be conceived in future years. Importantly, data collected through the HBCD Study will help inform interventions and policies leading to better health and development for all children.
Funding
This work was supported by the National Institutes of Health (NIH), National Institute of Drug Abuse (NIDA) grants U24DA055325 and T32DA023356.
CRediT authorship contribution statement
Christina D Chambers: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition, Conceptualization. Jessica Frankeberger: Writing – review & editing, Writing – original draft, Visualization. Charles A Nelson: Writing – review & editing, Writing – original draft, Visualization, Methodology, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Charles Nelson reports financial support was provided by National Institute on Drug Abuse. Jessica Frankeberger reports financial support was provided by National Institute on Drug Abuse. Christina Chambers reports financial support was provided by National Institute on Drug Abuse. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
Concepts used in the preparation of this article were obtained from the HEALthy Brain and Child Development (HBCD) Study (https://hbcdstudy.org/). This is a multisite, longitudinal study designed to recruit over 7000 families and follow them from pregnancy to early childhood. The HBCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA055352, U01DA055353, U01DA055366, U01DA055365, U01DA055362, U01DA055342, U01DA055360, U01DA055350, U01DA055338, U01DA055355, U01DA055363, U01DA055349, U01DA055361, U01DA055316, U01DA055344, U01DA055322, U01DA055369, U01DA055358, U01DA055371, U01DA055359, U01DA055354, U01DA055370, U01DA055347, U01DA055357, U01DA055367, U24DA055325, U24DA055330. A full list of supporters is available at https://hbcdstudy.org/about/federal-partners/. A listing of participating sites and a complete listing of the study investigators can be found at https://hbcdstudy.org/study-sites/. HBCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or HBCD consortium investigators.
Disclosures
The authors declare no competing interests.
References
- Banderali G., Martelli A., Landi M., Moretti F., Betti F., Radaelli G., Verduci E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J. Transl. Med. 2015;13:327. doi: 10.1186/s12967-015-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens A.E., Jensen S.K., Nelson C.A. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15(1):1–12. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogly S.B., Saia K.A., Walley A.Y., Du H.M., Sebastiani P. Prenatal buprenorphine versus methadone exposure and neonatal outcomes: systematic review and meta-analysis. Am. J. Epidemiol. 2014;180(7):673–686. doi: 10.1093/aje/kwu190. [DOI] [PubMed] [Google Scholar]
- Cook J.L. Epidemiology of opioid use in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2022;85:12–17. doi: 10.1016/j.bpobgyn.2022.07.008. doi:https://doi.org/10.1016/j.bpobgyn.2022.07.008. [DOI] [PubMed] [Google Scholar]
- Corsi D.J., Donelle J., Sucha E., Hawken S., Hsu H., El-Chaâr D., Walker M. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 2020;26(10):1536–1540. doi: 10.1038/s41591-020-1002-5. [DOI] [PubMed] [Google Scholar]
- Edde M., Leroux G., Altena E., Chanraud S. Functional brain connectivity changes across the human life span: from fetal development to old age. J. Neurosci. Res. 2021;99(1):236–262. doi: 10.1002/jnr.24669. [DOI] [PubMed] [Google Scholar]
- Fox S.E., Levitt P., Nelson C.A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Lin W., Grewen K., Gilmore J.H. Functional connectivity of the infant human brain:plastic and modifiable. Neuroscientist. 2017;23(2):169–184. doi: 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2012. Hensch, T.K., & Bilimoria, P.M. (2012). Re-opening windows: manipulating critical periods for brain development. Paper presented at the Cerebrum: the Dana forum on brain science.. [PMC free article] [PubMed]
- JarleNski, M.p., Paul, N.c., Krans, E.e., 2020. Polysubstance Use among pregnant women with opioid use disorder in the United States, 2007-2016. Obstet. Gynecol. 136 (3), 556–564. 10.1097/aog.0000000000003907.. [DOI] [PMC free article] [PubMed]
- Kiblawi Z.N., Smith L.M., Diaz S.D., LaGasse L.L., Derauf C., Newman E., Lester B. Prenatal methamphetamine exposure and neonatal and infant neurobehavioral outcome: results from the IDEAL study. Subst. Abus. 2014;35(1):68–73. doi: 10.1080/08897077.2013.814614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop J., Joëls M., van der Veen R. The added value of rodent models in studying parental influence on offspring development: opportunities, limitations and future perspectives. Curr. Opin. Psychol. 2017;15:174–181. doi: 10.1016/j.copsyc.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Mattson S.N., Bernes G.A., Doyle L.R. Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol Clin. Exp. Res. 2019;43(6):1046–1062. doi: 10.1111/acer.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P.A., Chambers C.D., Kalberg W.O., Zellner J., Feldman H., Buckley D., Hoyme H.E. Prevalence of fetal alcohol spectrum disorders in 4 US communities. Jama. 2018;319(5):474–482. doi: 10.1001/jama.2017.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 2019;1(1):277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P.M., Commons M.L. The benefits of attachment parenting for infants and children: a behavioral developmental view. Behav. Dev. Bull. 2010;16(1):1–14. doi: 10.1037/h0100514. [DOI] [Google Scholar]
- Moore B.F., Shapiro A.L., Wilkening G., Magzamen S., Starling A.P., Allshouse W.B., Dabelea D. Prenatal exposure to tobacco and offspring neurocognitive development in the healthy start study. J. Pedia. 2020;218:28–34. doi: 10.1016/j.jpeds.2019.10.056. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Gabard-Durnam L.J. Early adversity and critical periods: neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 2020;43(3):133–143. doi: 10.1016/j.tins.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L.F., Yocum V.K., Patel K.D., Qeadan F., Hsi A., Weitzen S. Cognitive outcomes of young children after prenatal exposure to medications for opioid use disorder: a systematic review and meta-analysis. JAMA Netw. Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes Cauduro G., de Mendonça Filho E.J., Pandolfo Silveira N., Ruschel Bandeira D. Direct and indirect effects of socio-economic status on child development: is developmental parenting a relevant mediator? Early Child Dev. Care. 2021;191(11):1715–1728. doi: 10.1080/03004430.2019.1673384. [DOI] [Google Scholar]
- Qato D.M., Zhang C., Gandhi A.B., Simoni-Wastila L., Coleman-Cowger V.H. Co-use of alcohol, tobacco, and licit and illicit controlled substances among pregnant and non-pregnant women in the United States: findings from 2006 to 2014 National Survey on drug use and health (NSDUH) data. Drug Alcohol Depend. 2020;206 doi: 10.1016/j.drugalcdep.2019.107729. [DOI] [PubMed] [Google Scholar]
- Reh R.K., Dias B.G., Nelson III C.A., Kaufer D., Werker J.F., Kolb B., Hensch T.K. Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. 2020;117(38):23242–23251. doi: 10.1073/pnas.1820836117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid M.C., Metz T.D., Gordan A.J. Stimulant use in pregnancy: an under-recognized epidemic among pregnant women. Clin. Obstet. Gynecol. 2019;62(1):168–184. doi: 10.1097/grf.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.M., Lagasse L.L., Derauf C., Grant P., Shah R., Arria A., Lester B.M. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30(1):20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol. Rev. 2010;20(4):327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administation. (2020). 2020 National Survey on Drug Use and Health: Women. Retrieved from https://www.samhsa.gov/data/report/2020-nsduh-women.
- Tooley U.A., Bassett D.S., Mackey A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021;22(6):372–384. doi: 10.1038/s41583-021-00457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Compton W.M., Wargo E.M. The risks of marijuana use during pregnancy. Jama. 2017;317(2):129–130. doi: 10.1001/jama.2016.18612. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Han B., Compton W.M., McCance-Katz E.F. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. Jama. 2019;322(2):167–169. doi: 10.1001/jama.2019.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. Individual differences in sensory sensitivity: a synthesizing framework and evidence from normal variation and developmental conditions. Cogn. Neurosci. 2019;10(3):139–157. doi: 10.1080/17588928.2018.1557131. [DOI] [PubMed] [Google Scholar]
- Werker J.F., Hensch T.K. Critical periods in speech perception: new directions. Annu. Rev. Psychol. 2015;66(1):173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]