Abstract
Ayurvedic medicine is a time-tested system of medicine which has been in clinical use for centuries in India. Being a time-tested system, it has an edge over other existing systems of health management, especially for dealing with chronic disorders such as coronary artery disease, which is of a complex multi-etiological nature. Recently, we have shown that BHUx, a patented polyherbal formulation consisting of the aqueous fraction of five medicinal plants of the ayurvedic system, has significant anti-inflammatory properties through inhibition of cyclooxygenase-2 and lipoxygenase-15. Here we have investigated its effect on diet-induced atherosclerosis in albino rabbits. BHUx was given orally for 3 months to rabbits pre-treated with an atherogenic diet for 3 months. After 6 months, the dorsal aorta was processed for histological studies for calcium and collagen content. The results demonstrated a remarkable reduction in intimal thickening in the treated animals. In addition, there was less calcification at the intima–medial interface and increased intensity of collagen cap on the surface along with an increase in survival, compared with the sham control. We suggest that BHUx is a potent, multi-factorial formulation against atherosclerosis.
Keywords: atherosclerosis, polyherbal, ayurveda, diet-induced atherosclerosis
Introduction
Coronary artery disease (CAD) starts with the formation of atherosclerotic plaques in the coronary arteries. Abrupt occlusion of these atherosclerotic arteries due mainly to thrombosis leads to coronary heart diseases: unstable angina, acute myocardial infarction and sudden death (1). CAD is a disease of several risk factors, most notably among which are hyperlipidemia, hypertension, diabetes mellitus and tobacco smoking. Multiple pathogenic factors are known to be operating at the cellular level, such as enhanced oxidation of low-density lipoprotein (LDL) and proliferation of monocyte-derived macrophages and smooth muscle cells (SMCs). Atherogenesis is a complex process involving, among others, endothelial cell dysfunction such as increased endothelial permeability to lipoproteins. Such dysfunction is mediated by many factors such as nitric oxide (NO), platelet-derived growth factor (PDGF), prostacyclin, angiotensin II and endothelin. It also involves upregulation of endothelial adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and selectins. Inflammatory processes underlying atherogenesis have been the focus of intense studies, as migration of leukocytes and monocytes–macrophages is demonstrated in the subendothelial space (2–4). Atherosclerotic lesions first appear as deposits of lipid in the intima of large muscular and elastic arteries, which then progress through several stages before clinical manifestations of CAD emerge (5). Thus, abnormal lipid metabolism remains the crux of the pathogenesis of CAD, on which inflammatory and other mechanisms superimpose (6–8).
Several approaches have been proposed to counter progress of atherosclerosis: reduction of the risk factors, use of statins, β-blockers, angiotensin-converting enzyme (ACE) inhibitors, antithrombotic drugs and proper use of estrogen and antioxidants (9–11). However, there still seems no consensus as to the best pharmacological option to control atherosclerosis.
Thus, keeping in view these limitations and multi-etiological factors related to atherosclerosis, a combination drug has been developed as BHUx. It is a patented polyherbal formulation (12), simultaneously targeting oxidative stress and inflammation (13). It consists of a specific water-soluble extract of five medicinal plants, which are time tested and have been used in the ayurvedic system of medicine for many centuries (14). These plants are: Commiphora mukul (Hook. Ex Stock, Burseraceae) synonym: Balsamodendron mukul (15), Terminalia arjuna (W & A, Comberetaceae) (16), Boswellia serrata Roxb (Burseraceae) (17), Semecarpus anacardium Linn.f. (Anacardiaceae) (18) and Strychnos nux vomica Linn. (Loganiaceae) (19) in a particular ratio. CaCO3 (Shankha Bhasma) has been added to the finished product to reduce gastric irritation, if any. The finished product has been standardized by using high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC) fingerprinting (13).
These plants contain several phytochemicals, which may target several signaling pathways at the same time and may bring about benefits through a synergistic or additive action (20). In the present experiment, BHUx was orally given for 3 months to rabbits pre-treated with an atherogenic diet for 3 months. After 6 months, all the animals were sacrificed for histological study. The results of the groups each consisting of six animals were compared. It is demonstrated that BHUx significantly reduced intimal thickening and calcium deposition, and stabilized the collagen cap in the plaque in the dorsal aorta.
Methods
Materials
All the chemicals of AR grade were purchased from Merck and Central Drug House, Delhi. Male inbred albino rabbits of nearly the same age (60 days old) and weight (1.3 kg average) were from the central animal house of the Institute of Medical Sciences. The Institute's ethical committee for animal welfare approved the experimental protocol.
Preparation of BHUx
All the plants were purchased from the Surya Pharmaceuticals, D-17 Industrial area, Ramnagar, Varanasi (lot number 1992/4–8) and their authenticity was confirmed as per the standards given in the Indian Pharmacopoeia. They were also verified physically with the standard specimens kept in the museum of the Department of Dravyaguna, Faculty of Ayurveda. The samples of the plant materials used were preserved in the Department Herbarium as voucher # MC-Y-18 to 22 for future records. The aqueous extract of each medicinal plant was prepared separately as described in the Ayurvedic text (14) with special modifications (patented, 12). They were mixed in a particular ratio and dried at 46°C to produce a homogeneous powder. TLC and HPLC fingerprinting of each batch was carried out and maintained throughout the experiment, to avoid batch-to-batch variations (13). For biological experiments, BHUx was suspended in double-distilled water with 15% gum acacia and given orally.
Experiments with Albino Rabbits
After 1 week of acclimatization, rabbits were divided into two groups. One group was kept on the normal control diet and the other group was subjected to the atherogenic diet (40 g/day per animal) for 3 months along with added normal diet and water at libitum. Later on, the animals were randomly divided again into two groups. One group was the experimental sham control group, where animals were continued on the atherogenic diet schedule, along with drug vector (15% gum acacia), while the other group was the drug-treated experimental group where the animals were given BHUx (600 mg/kg body weight) along with the atherogenic diet for another 3 months. The number of animals in each group was 10. After 6 months, all the surviving animals were sacrificed and the heart and complete aortic tree were collected for histological study using standard operating procedures (21). In brief, tissues were dehydrated and wax blocks were prepared. Serial microsections of 5 µm thicknesses were cut out and divided into three groups. One group was processed for normal hematoxylin and eosin (H&E) staining and the remaining two groups were stained with Van-G for collagen content and with Alizarin red-S for calcium content visualization.
Statistical Analysis
Data were analyzed by using Student's t-test and analysis of variance.
Results
BHUx Inhibits Plaque Formation and Improves Survival
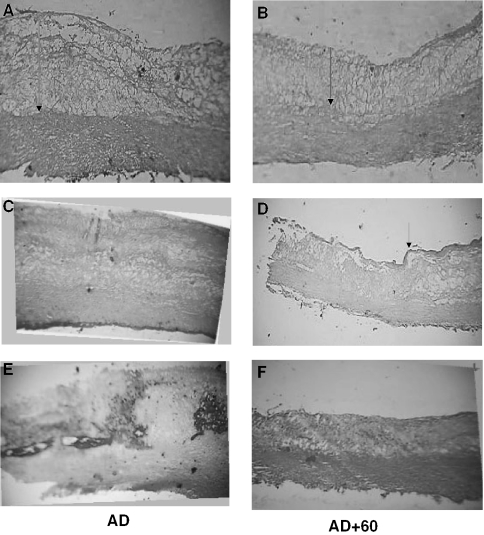
A significant reduction in the intimal thickening of the dorsal aorta was demonstrated in the drug-treated animals compared with the sham controls (H&E stain; Fig. 1A and B). The BHUx-treated animals were further shown to have more collagen content on the surface of the endothelium (Fig. 1C and D stained by Van-G) and less deposition of calcium in the arterial wall (Fig. 1E and F stained by Alizarin red-S). The overall survival rate of BHUx-treated animals was significantly higher (70–80%) than that of the control animals (35–50%) (Fig. 2).
Figure 1.
(A and B) Effect of BHUx on the intimal thickening in albino rabbits. (A) A rabbit on the atherogenic diet for 6 months (sham control). (B) A rabbit receiving BHUx 600 mg/kg body weight (last 3 months) along with the atherogenic diet (6 months). (C and D) Effect of BHUx on the collagen cap on the surface of the plaque in the dorsal aorta in albino rabbits. (C) A rabbit on the atherogenic diet for 6 months (sham control). (D) A rabbit receiving BHUx 600 mg/kg body weight (last 3 months) along with the atherogenic diet (6 months). (E and F) Effect of BHUx on the calcium deposition on the intima medial interface in the dorsal aorta in albino rabbits. (E) A rabbit on the atherogenic diet for 6 months (sham control). (F) A rabbit receiving BHUx 600 mg/kg body weight (last 3 months) along with the atherogenic diet (6 months).
Figure 2.
Effect of BHUx on the survival rate of rabbits kept on the atherogenic diet and BHUx treatment for 6 months. (1) Rabbits on a normal diet; (2) rabbits on the the atherogenic diet; (3) rabbits on the atherogenic diet and BHUx.
Discussion
Complex cellular mechanisms contribute to atherogeneis, including SMC migration [stimulated by PDGF and transforming growth factor (TGF)-β], T-cell activation [mediated by tumor necrosis factoe (TNF)-α and interleukin (IL)-2], formation of foam cells [from macrophages mediated by oxidized LDL, macrophage colony-stimulating factor (M-CSF), TNF-α and IL-1) and platelet adherence and aggregation (stimulated by thromboxane A2, tissue factors, etc.) (22).
As seen in the Results, BHUx remarkably prevented the formation of thickened intima loaded heavily with fatty vacuoles in the rabbits fed with the atherogenic diet. Several mechanisms can explain this effect. It may be due to the reduced formation of foam cells or induction of their apoptosis, or both. Another possibility could be its inhibitory effect on M-CSF, which is essential for viability and sustained growth of macrophages, embedded in the endothelium (4). Inhibition of proliferation of vascular SMCs and their migration to the intima is also possible (7). The inhibition of intimal thickening by BHUx may also be explained by its antioxidant and anti-inflammatory properties as shown in the case of probucol (11). BHUx has been shown to inhibit lipid peroxidation induced by cumene hydro peroxide and the activity of lipoxygenase-15 (responsible for LDL oxidation) in different experimental systems (13).
The histological sections from the control animals showed a very thin collagen cap on the inner surface of the plaque when stained with Van-G, while this cap was intact in the BHUx-treated animals. Matrix metalloproteinase (MMP) activation is one of the significant factors for dissolving this protective covering (23), leading to unstable atherosclerotic plaques. The ratio of lipid core to fibrous content (determined by the balance between SMC proliferation and extracellular matrix synthesis stabilizing the plaque, and macrophages which degrade collagen) is also important to determine the plaque vulnerability (24). Loss of the matrix-synthesizing SMCs is another possibility for a weak fibrous cap, because caps of ruptured coronary plaques contain a reduced number of SMCs. (25).
Current therapies to manage atherosclerosis are focused predominantly on the stabilization of plaques rather than their regression. It is assumed that the reduced frequency of acute vaso-occlusive events observed with interventions aimed to modify lipid and other risk factors is at least partially explained by the stabilization of plaques through alteration of the plaque composition or by inhibition of the release of MMPs and other proteases from mast cells (26). The potential mechanisms of this benefit include improvement in endothelial function and vasomotion, reduction of platelet aggregability and thrombus formation, fibrinolytic and antioxidant activity, as well as reduction of inflammation within plaques, reduced matrix degradation due to inhibition of macrophage MMP production and increasing collagen content. It is interesting to point out that ACE inhibitor therapy has been shown to reduce the risk of myocardial infarction and stroke by upregulating collagen synthesis (27). The prominent collagen cap seen in the BHUx-treated animals could be due to its anti-inflammatory effects, as the agent has been shown to exhibit significant inhibition of cyclooxygenase-2 activity and possess antioxidant activity (13), One of its components, S.anacardium, has also been shown to inhibit lipopolysaccharide (LPS)-induced NO production from activated peritoneal macrophages (28).
We have shown in the histological sections a significant reduction of calcium content in the dorsal aorta from the BHUx-treated animals compared with the control animals, in which a high degree of calcification both on the surface and within the plaque was observed when stained with Alizarin red-S. Vascular calcification is highly correlated with cardiovascular disease mortality, especially in patients with diabetes. Recent studies have shown that chemical composition and morphology, rather than anatomy (degree of stenosis), of atherosclerotic plaques determine their instability and predict disease progression (29). The increase in calcification is also associated with hyperlipidemia and elevated pH through alteration in the ratios of bicarbonate/CO2 (30).
Deposition of cholesterol and calcium on the elastic fiber, resulting in decreased elastin synthesis and cross-link formation, is directly related to calcification in SMCs. It is also reported that M-CSF is one of the important factors for arterial calcification, as it maintains the balance of osteoclasts with the osteoblasts, which are differentiated from the arterial SMCs (31).
Calcium antagonists have been one of the most relevant therapeutic tools for patients with hypertension, and their effects on calcium transport may influence the cellular changes leading to atherosclerosis (32). Calcification in arteriosclerosis is also inhibited by antioxidants, as aged garlic extract (AGE) and green tea have been shown to reduce arterial calcification through their antioxidant properties (33,34). Thus, we suggest that the BHUx-mediated reduction in the calcium content in the atherosclerotic plaque could also be attributed to its antioxidant property or to the calcium channel blocking property of T.arjuna (a component of BHUx) (35). It could also be explained by the modulation of the inflammatory cascade through the action of S.anacardium (28). Furthermore, the effect of BHUx may be exerted through downregulation of M-CSF, because it reduces calcification and intimal thickening without affecting the level of blood cholesterol (4).
We would like to emphasize that BHUx significantly enhanced the overall survival of animals (70–80%) as compared with the sham controls where survival was only 35–50% after 3 months of atherogenic diet administration.
We conclude that BHUx is capable of reducing the progress of atherosclerosis, possibly through its anti-inflammatory, calcium channel-modulatory and antioxidant properties. Further studies are warranted to test these claims in the clinical setting.
Acknowledgments
We thank the Department of Biotechnology, Government of India for financial assistance. We extend our sincere thanks to the late Professor Chakarvarti, FNA for his valuable suggestions in conducting different experiments, to the late Professor S. N. Tripathi, the pioneer worker on C.mukul, for suggesting this formulation as a medicine for inflammation and atherosclerosis, and to Surya Pharmaceuticals, Varanasi for preparing BHUx as per our specification.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1990;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Bhagat K, Vallance P. Inflammatory cytokines impair endothelium dependent dilation in human veins in vivo. Circulation. 1997;96:3042–7. doi: 10.1161/01.cir.96.9.3042. [DOI] [PubMed] [Google Scholar]
- 4.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang Xu-Ping, et al. Role of macrophage colony-stimulating factor in atherosclerosis. Am J Pathol. 1997;150:1687–99. [PMC free article] [PubMed] [Google Scholar]
- 5.Fuster V. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation. 1994;90:2126–46. doi: 10.1161/01.cir.90.4.2126. [DOI] [PubMed] [Google Scholar]
- 6.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin Invest. 1990;85:1234–41. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frishman WH, Lazer EJ. Reduction of mortality, sudden death and non-fatal reinfarction with beta-adrenergic blockers in survivors of acute myocardial infarction: a new hypothesis regarding the cardioprotective action of beta-adrenergic blockade. Am J Cardiol. 1990;66:66G–70G. doi: 10.1016/0002-9149(90)90401-l. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–8. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 9.Smith P. Antithrombotic therapy in the chronic phase myocardial infarction. In: Fuster V, Verstraete M, editors. Thrombosis in Cardiovascular Disorder. Philadelphia, PA: W. B. Saunders; 1992. pp. 343–62. [Google Scholar]
- 10.Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, et al. Association of hormone replacement therapy in various cardiovascular risk factors in postmenopausal women. N Engl J Med. 1993;328:1070–5. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
- 11.Carew TE, Schwenke DC, Steinberg D. Antiatherogenic efforts of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidant in vivo can selectively inhibit low-density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci USA. 1987;84:7725–9. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi YB, Singh BK, Pandey RS, Kumar M. Anti atherogenic role of BHUx: a patent polyherbal formulation. In Abstract Book of the 15th Annual Conference of the Indian Society for Atherosclerosis Research; Tirupati. 2002. [Google Scholar]
- 13.Tripathi YB, Reddy MM, Pandey RS, Subhashini J, Tiwari OP, Singh BK, et al. Anti-inflammatory properties of BHUx, a polyherbal formulation to prevent atherosclerosis. Inflammopharmacology. 2004;12:131–52. doi: 10.1163/1568560041352301. [DOI] [PubMed] [Google Scholar]
- 14.Pandey GS, Chunekar KC. Bhavaprakash Bhava prakash nighantu (Chaukhambha Vidya Bhawan, Varanasi) 1967. pp. 139–41.
- 15.Tripathi YB, Tripathi P, Malhotra OP, Tripathi SN. Thyroid stimulatory action of guggulsterone: mechanism of action. Planta Med. 1988;4:271–76. doi: 10.1055/s-2006-962431. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi YB, Tripathi VP, Tripathi P. Effect of T.arjuna-extract on KCl induced contraction on rat was deferens. Phytother Res. 1989;13:162–64. [Google Scholar]
- 17.Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthitis of knee—a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3–7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- 18.Tripathi YB, Singh AV. Effect of Semecarpus anacardium nuts on lipid peroxidation. Indian J Exp Biol. 2001;39:798–801. [PubMed] [Google Scholar]
- 19.Tripathi YB, Chaurasia S. Effect of S.nuxvomica alcoholic extract on lipid peroxidation in rat liver. Int J Pharmacog. 1996;34:295–99. [Google Scholar]
- 20.Rastogi RP, Mehrotra BN. New Delhi, India: PID, CSIR; Compendium of Indian Medicinal Pants. [Google Scholar]
- 21.Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. 1996.
- 22.Clinton SK, Underwood LH, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–16. [PMC free article] [PubMed] [Google Scholar]
- 23.Leskinen MJ, Kovanen PT, Lindstedt KA. Regulation of smooth muscle cell growth, function, death in vitro by activated mast cells—a potential mechanism for the weakening and rupture of atherosclerotic plaques. Biochem Pharmacol. 2003;15;66:1493–8. doi: 10.1016/s0006-2952(03)00503-3. [DOI] [PubMed] [Google Scholar]
- 24.Galis ZS, Sukhova GK, Lark MW. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotoudeh M, Li YS, Yajima N, et al. Induction of apoptosis in vascular smooth muscle cells by mechanical stretch. Am J Physiol. 2002;282:H1709–16. doi: 10.1152/ajpheart.00744.2001. [DOI] [PubMed] [Google Scholar]
- 26.Davies MJ, Richardson PD, Woolf N, et al. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–81. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfon J, Pueyo Palazon C, Royo T, Badimon L. Effects of statins in thrombosis and aortic lesion development in a dyslipemic rabbit model. Thromb Haemostasis. 1999;81:822–7. [PubMed] [Google Scholar]
- 28.Tripathi YB, Pandey RS. Semecarpus anacardium L, nuts inhibit lipopolysaccharide induced NO production in rat macrophages along with its hypolipidemic property. Indian J Exp Biol. 2004;42:432–6. [PubMed] [Google Scholar]
- 29.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 30.Hsu HH, Abbo BG. Role of bicarbonate/CO2 buffer in the initiation of vesicle-mediated calcification: mechanisms of aortic calcification related to atherosclerosis. Biochim Biophys Acta. 2004;1690:118–23. doi: 10.1016/j.bbadis.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Bostrom K, Watson, Stanford W, Demer L. Atherosclerotic calcification: relation to developmental osteogenesis. Am J Cardiol. 1995;75:88–91. doi: 10.1016/0002-9149(95)80020-s. [DOI] [PubMed] [Google Scholar]
- 32.Hirata K, Yokoyama M. Calcium channel blockers and atherosclerosis. Clin Calcium. 2004;14:128–32. [PubMed] [Google Scholar]
- 33.Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol. 2005;173:271–5. doi: 10.1097/01.ju.0000141311.51003.87. [DOI] [PubMed] [Google Scholar]
- 34.Budoff MJ, Takasu J, Flores FR, Niihara Y, Lu B, Lau BH, et al. In patients receiving statin therapy: a preliminary study. Prev Med. 2004;39:985–91. doi: 10.1016/j.ypmed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Tripathi YB. T.arjuna extract modulates the concentration of rat aorta induced by KCl and norepinephrine. Phytother Res. 1993;7:320–2. [Google Scholar]