Abstract
Background
After an initial Takotsubo syndrome (TTS) event, there is growing recognition of adverse long-term outcomes, including recurrent TTS events. Recurrent events have been incompletely evaluated.
Objectives
The objective of this study was to characterize recurrent TTS events and evaluate variables associated with recurrence.
Methods
We studied 88 consecutive participants in the Cedars-Sinai Smidt Heart Institute Takotsubo Registry, an observational registry collecting retrospective and prospective data in TTS survivors. Detailed medical records are adjudicated. Standardized psychosocial questionnaires are administered remotely.
Results
Of 88 participants with adjudicated TTS, 15 (17%) experienced at least 1 recurrent TTS event (median 3.30 years to first recurrent event, range 0.13-18.56 years). In 9 of these 15 participants, there were different patterns of wall motion abnormalities observed between events. The recurrence-free survival significantly differed based on the pattern of wall motion abnormalities at the index TTS event. Clinical, electrocardiographic, echocardiographic, and invasive data obtained at the index TTS event were similar between participants who went on to have at least 1 recurrent event and those who did not.
Conclusions
Recurrent TTS episodes occurred in a significant proportion of cases, a median of 3.30 years after the index event. The recurrent episodes often had distinct triggers and different wall motion abnormalities compared to the index event. The wall motion pattern at the index event impacted the recurrence-free survival, though confirmatory studies are needed. TTS participants had a high rate of adverse psychosocial stress characteristics based on detailed questionnaires. (The Cedars-Sinai Smidt Heart Institute Takotsubo Registry & Proteomic Study; NCT03910569)
Key words: psychosocial stress, stress cardiomyopathy, Takotsubo syndrome
Central Illustration
Takotsubo syndrome (TTS) is a form of acute-onset heart failure associated with sympathetic activation that most often occurs following an intense emotional or physical stressor, and is predominantly seen in menopausal women.1,2 The presentation of TTS mimics an acute myocardial infarction—approximately 5% of women with suspected acute myocardial infarction who undergo coronary angiography actually have TTS.3 While the ventricular dysfunction in TTS is typically reversible, the initial presentation carries a risk of severe complications including cardiogenic shock, arrhythmias, and cardiac arrest, with an in-hospital mortality similar to acute myocardial infarction.3, 4, 5
After resolution of the acute event, more recent work has challenged the prior notion of a benign long-term prognosis in TTS. There is mounting evidence that the long-term mortality after TTS is higher than in the general population and similar to that of patients after an acute myocardial infarction.5, 6, 7, 8, 9 Reports suggest a longer-term heart failure phenotype with persistent cardiac symptoms and cardiac structural abnormalities on advanced imaging techniques more than 1 year after the TTS event,10 and increased heart failure hospitalizations compared with matched controls.11 A significant subset of patients, estimated at 4 to 12% in prior studies,7,8,12, 13, 14 experience a recurrent TTS event, even years after the initial event and are thereby re-exposed to the risk of severe complications during the acute period.
A characterization of recurrent TTS is therefore important to understand the risk of long-term morbidity and mortality associated with the disease. Furthermore, while population studies investigating incident disease are impractical in this relatively uncommon and under-recognized syndrome, the study of recurrent events can provide valuable insight into pathophysiology and prospective risk. In particular, we hypothesized that a detailed investigation of psychosocial factors would provide important insight, given the role for psychosocial stress in the pathophysiology of TTS,1,15,16 and the known importance of adverse psychosocial features in other cardiovascular conditions, including an association with recurrent myocardial infarction.17 There have not been consistent demographic, clinical, and echocardiographic predictors of recurrent TTS events in prior single-center studies and multicenter registries.12,13 Accordingly, in a population with prior TTS, we conducted a detailed examination of clinical, echocardiographic, and invasive parameters, as well as patient-reported measures of psychological stress, to evaluate recurrent TTS.
Methods
Takotsubo registry
The Cedars-Sinai Smidt Heart Institute Takotsubo Registry (NCT03910569) is an observational registry collecting retrospective and prospective data in TTS survivors.18 Participants with a prior episode of TTS are recruited through patient-centered modalities including social media outreach and physician referral.18 The registry has enrolled participants across 25 states in the United States and 3 additional countries (Canada, United Kingdom, and Australia).18 Participants enrolled between January 2019 and May 2021 were included in the present analysis. Ethical approval for the registry was obtained by the Cedars-Sinai Medical Center Institutional Review Board. Detailed TTS medical records, including laboratory data, echocardiographic data, angiographic data, and magnetic resonance imaging are adjudicated by 2 cardiology physician review according to InterTAK Diagnostic Criteria.4 After enrollment, these data are collected prospectively for all subsequent events. Participants complete e-surveys at enrollment to provide demographic information, medical and reproductive history, and data regarding their general and mental health. We obtain detailed data regarding physical and emotional stressors through chart review and the use of multiple standardized questionnaires. The questionnaires included at registry enrollment are the Spielberger State-Trait Anxiety Inventory (STAI) Form Y-2,19 Cardiac Anxiety Questionnaire,20 Post-Traumatic Stress Disorder Checklist-Civilian Form,21 Perceived Stress Scale (PSS),22 Patient Health Questionnaire 9,23 and Early Trauma Inventory Self Report-Short form (ETISR-SF).24 Participants complete annual follow-up surveys, including the aforementioned emotional stressor surveys, as well as surveys that provide information about their updated health data, including any recurrent TTS events.
Statistical analysis
Data are presented as mean SD for continuous variables with a normal distribution, median (IQR) for continuous variables with a non-normal distribution, and frequency (percentage) for categorical variables. Differences among demographic, clinical, and psychosocial characteristics were compared between subjects who developed at least 1 recurrent TTS event and those who did not have a recurrent TTS event. Differences were assessed using independent sample t-tests or Wilcoxon rank-sum tests for continuous variables, and Pearson’s chi-square test or Fisher’s exact test for categorical variables where appropriate.
Kaplan-Meier methods were used to estimate survival probabilities and survival curves were compared using the log-rank test. Time to first TTS recurrence was defined as the time from first adjudicated TTS event to first TTS recurrence or last follow-up. Univariate Cox proportional hazards regression was then performed to estimate the HRs of TTS recurrence in relation to selected predictors. Statistical analyses were performed using SAS software (SAS, version 9.4) and R statistical software (version 4.3.1, R Foundation) with 2-sided tests and a significance level of 0.05. The primary data underlying the analyses in this article will be provided on reasonable request.
Results
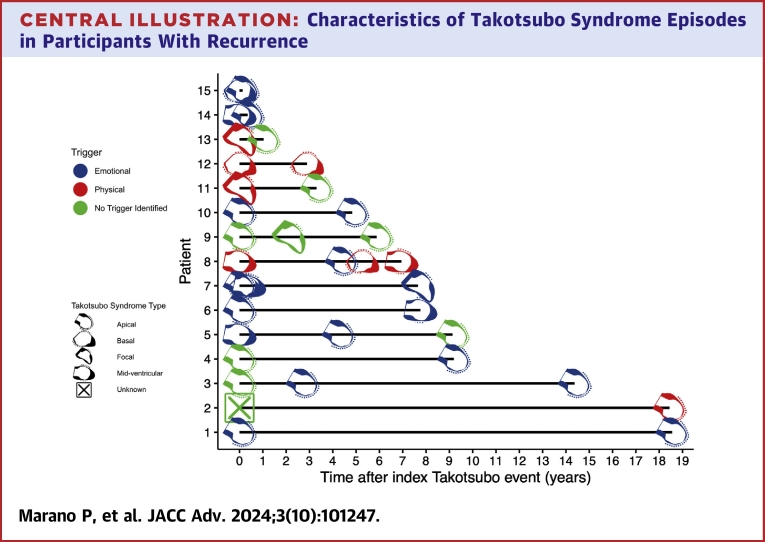
Of the 88 registry participants with adjudicated TTS, 15 (17%) experienced at least 1 recurrent TTS event (median 3.30 years to first recurrent event, range 0.13-18.56 years) and 73 had a single event with no recurrence to date over a median follow-up period of 2.32 years (range: 0.03-12.78 years). Of the patients with at least 1 recurrent event, 3/15 (20%) had 2 recurrent events and 2/15 (13%) had 3 recurrent events. The timing of recurrent events, the patterns of wall motion abnormalities of the index and recurrent events, and the trigger types of the index and recurrent events are summarized in the Central Illustration. In 9/15 (60%) participants with at least 1 recurrent event, there were different patterns of wall motion abnormalities observed between events (Central Illustration). In 7/15 (47%) participants, there were different trigger types (emotional, physical, or no trigger identified) among their multiple events (Central Illustration).
Central Illustration.
Characteristics of Takotsubo Syndrome Episodes in Participants With Recurrence
The trigger type is represented by the color of the icon, and the pattern of wall motion abnormalities is represented by the various icons. The time of the index event is referred to as time 0. Due to the short interval between their TTS events, there are overlapping icons for selected patients. The Takotsubo Syndrome types for the events in these patients are summarized below for clarity. For patient 15, the index event had a basal pattern followed by a recurrent event with an apical pattern. For patient 14, the index event had a mid-ventricular pattern, followed by a recurrent event with an apical pattern. For patient 7, the index event had an apical pattern, followed by a first recurrent event with a basal pattern, a second recurrent event with a mid-ventricular pattern, and a third recurrent event with a focal pattern.
The demographic, clinical, electrocardiographic, echocardiographic, and invasive data obtained at the index TTS event were similar between participants who went on to have at least 1 recurrent event and those who did not (Table 1). Comorbidities including chronic obstructive pulmonary disease, hypertension, and diabetes were present in a similar degree in both groups. There were no differences between the type of trigger (emotional or physical) of the index event, or the clinical severity of the index event, as assessed by the presence of cardiogenic shock or need for inotropes or vasopressors. There was no difference in the prescription of either angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and no difference in the prescription of beta blockers between the 2 groups at the time of index hospitalization discharge.
Table 1.
Clinical Characteristics
| No TTS Recurrence (n = 73) | TTS Recurrence (n = 15)a | |
|---|---|---|
| Demographics | ||
| Age at index TTS event, y | 59.7 ± 9.9 | 57.2 ± 8.8 |
| Female | 72 (98.6%) | 15 (100.0%) |
| Non-Hispanic White | 66 (90.4%) | 15 (100.0%) |
| Baseline clinical characteristics | ||
| Hypertension | 24 (36.4%) | 6 (40.0%) |
| Diabetes mellitus | 6 (9.2%) | 1 (6.7%) |
| Malignancy | 13 (18.1%) | 1 (6.7%) |
| Chronic obstructive pulmonary disease | 7 (9.7%) | 2 (13.3%) |
| Obstructive sleep apnea | 4 (6.1%) | 2 (13.3%) |
| Obesity | 8 (12.7%) | 1 (7.1%) |
| Index TTS event characteristics | ||
| Trigger for index TTS event | ||
| Emotional | 26 (36.1%) | 7 (46.7%) |
| Physical | 34 (47.2%) | 4 (26.7%) |
| Both | 7 (9.7%) | 0 (0.0%) |
| Cardiogenic shock at index TTS event | 6 (8.5%) | 1 (7.7%) |
| ACEI/ARB at discharge from index TTS event | 42 (60.9%) | 9 (64.3%) |
| BB at discharge from index TTS event | 58 (81.7%) | 12 (85.7%) |
| Echocardiogram at index TTS event | ||
| Left ventricular ejection fraction | 39.4 ± 14.2 | 38.3 ± 8.3 |
| Left ventricular end-diastolic diameter | 4.7 (4.1-5.1) | 5.0 (4.2-5.3) |
| Angiogram at index TTS event | ||
| Left ventricular end-diastolic pressure (mm Hg) | 20.5 ± 7.3 | 20.1 ± 9.9 |
| Nonobstructive coronary artery disease | 29 (42.6%) | 8 (53.3%) |
| Echocardiogram at recovery | ||
| Left ventricular ejection fraction | 60.0 (55.0-64.0) | 61.5 (52.5-65.0) |
Values are mean ± SD, n (%), or median (IQR).
ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blockers; BB = beta-blockers; TTS = Takotsubo syndrome.
All comparisons between the “no TTS recurrence” and “TTS recurrence” groups were nonsignificant with P > 0.05.
The echocardiographic data from the index TTS event were also similar between groups, with an ejection fraction of 38.3% ± 8.3% in the group that went on to experience TTS recurrence and 39.4% ± 14.2% in the group that did not, and no significant difference between left ventricular dimensions at end diastole and end systole (Table 1). In the group that went on to experience TTS recurrence, 7 (50%) had apical type TTS, 3 (21.4%) had a midventricular type TTS, and 2 (14.3%) had basal type TTS at the index presentation. In the group with no TTS recurrence, 52 (72.2%) had apical type TTS at their index presentation, 9 (12.5%) midventricular type TTS, and 2 (2.8%) basal type TTS.
The echocardiographic data at the time of recovery after the acute event were similar between groups, with a similar ejection fraction at recovery, and similar improvement in ejection fraction from the acute event (Table 1). Regarding angiographic data at the time of the index event, there was no difference in the left ventricular end-diastolic pressure or in the presence of nonobstructive coronary artery disease.
Scores on detailed psychosocial questionnaires assessing heart-focused anxiety, perceived stress, depression severity, and childhood trauma were not significantly different between participants in the 2 groups (Table 2). Using univariate Cox regression, no significant predictors of recurrent TTS were identified, although the ballooning pattern at index event approached statistical significance (Table 3).
Table 2.
Psychosocial Characteristics
| No TTS Recurrence (n = 73) | TTS Recurrence (n = 15)a | |
|---|---|---|
| History of depression per medical record | 25 (34.7%) | 4 (26.7%) |
| Antidepressant use | 22 (32.8%) | 5 (33.3%) |
| History of cigarette smoking | 24 (33.8%) | 9 (60.0%) |
| Cardiac Anxiety Questionnaire (score 0-4) | ||
| Total score | 1.8 (1.1-2.2) | 1.6 (1.4-2.1) |
| Spielberger State-Trait Anxiety Inventories (score 20-80) | 40.0 (29.0-48.0) | 35.0 (31.0-50.0) |
| Perceived Stress Scale (score 0-56) | 24.3 ± 10.9 | 20.8 ± 9.1 |
| PTSD Checklist-Civilian Form (score 17-85) | 32.0 (25.0-45.0) | 36.0 (20.0-43.0) |
| Patient Health Questionnaire– 9 (score 0-27) | 6.0 (2.0-11.0) | 4.5 (2.0-7.0) |
| Early Trauma Inventory Self-Report | ||
| Total ETI score (score 0-27) | 7.0 (3.0-12.0) | 6.5 (5.0-12.0) |
Values are n (%), median (IQR), or mean ± SD.
ETI = Early Trauma Inventory; PTSD = post-traumatic stress disorder; other abbreviation as in Table 1.
All comparisons between the “no recurrence” and “recurrence” groups were nonsignificant with P > 0.05. All variables assessed at the time of registry enrollment.
Table 3.
Predictors of TTS Recurrence With Univariate Cox Regression
| Unadjusted HR (95% CI) | P Value | |
|---|---|---|
| Age | 0.989 (0.936-1.044) | 0.6789 |
| Hypertension | 1.025 (0.353-2.974) | 0.9645 |
| Diabetes | 0.829 (0.105-6.521) | 0.8585 |
| Chronic obstructive pulmonary disease | 0.419 (0.051-3.412) | 0.4160 |
| Trigger for index TTS event | 1.067 (0.323-3.520) | 0.9157 |
| Left ventricular ejection fraction (at index TTS event) | 1.014 (0.964-1.065) | 0.5962 |
| Ballooning pattern at index event | 0.0607 | |
| Apical | reference | - |
| Basal | 7.260 (1.421-37.086) | 0.0172 |
| Focal | 1.454 (0.291-7.258) | 0.6482 |
| Midventricular | 3.971 (0.963-16.373) | 0.0564 |
| History of depression | 1.197 (0.356-4.026) | 0.7720 |
| Antidepressant use | 1.566 (0.490-5.004) | 0.4497 |
| CAQ total Score | 1.092 (0.498-2.396) | 0.8261 |
| Spielberger State-Trait Anxiety Inventory score | 1.012 (0.971-1.055) | 0.5695 |
| Perceived stress scale | 0.984 (0.935-1.034) | 0.5204 |
| PTSD checklist | 1.020 (0.982-1.059) | 0.3096 |
| Patient Health Quetionnaire-9 score | 0.981 (0.890-1.081) | 0.6949 |
| Total ETI score | 1.080 (0.965-1.209) | 0.1822 |
Figure 1A demonstrates the recurrence-free survival after the index TTS event. In Figure 1B, the recurrence-free survival of participants with different initial patterns of wall motion abnormalities during the index TTS event is depicted. Using a log-rank test, the recurrence-free survival is different (P = 0.026) between groups of participants with an initial apical, midventricular, basal, or focal type TTS.
Figure 1.
Recurrence-Free Survival After Index Takotsubo Syndrome Event
(A) Kaplan-Meier survival curve demonstrating recurrence-free survival after the index Takotsubo syndrome event.
(B) Kaplan-Meier survival curves demonstrating recurrence-free survival for participants with varying patterns of wall motion abnormalities at the index takotsubo syndrome presentation. The log-rank test was used to evaluate for differences in the survival curves.
Discussion
Our work emphasizes the importance of longer-term follow-up in TTS patients, as recurrent TTS events often occurred multiple years after the index event. The adjudicated echocardiographic data in our registry provide insight into the importance of the different patterns of wall motion abnormalities. We observed a statistically significant difference in freedom from recurrence based on the pattern of wall motion abnormalities at the index TTS event, with a suggestion of increased risk of recurrence in participants with basal or midventricular wall motion abnormalities compared to the classical apical variant. In addition, we observed that a substantial proportion of participants with recurrent events had different patterns of wall motion abnormalities between their events.
Our data build on recent work from the International Takotsubo Registry, in which different ballooning patterns between recurrent events were also observed.25 Our study provides a further challenge to the prevailing pathophysiologic explanation for the classic apical ballooning pattern, which is that an apical to basal distribution of adrenoreceptors explains the predilection for apical hypokinesis in TTS.26,27 This theory does not explain the different patterns of wall motion abnormalities in multiple episodes in the same patient. Furthermore, we found that 47% experiencing recurrence had different triggers between their events. This again builds on data from the International Takotsubo Registry, which also reported different trigger types in a subset of patients with recurrent TTS.25 In addition, the Spanish Multicenter Registry of Takotsubo Syndrome (RETAKO) found that the absence of an identifiable trigger at the index TTS event was associated with an increased risk of recurrent TTS events.28 Taken together, our findings are supportive of a 2-hit hypothesis; there may be an underlying condition that renders a group of patients susceptible to TTS, which can be brought on by multiple different triggers. One possible pathophysiologic explanation that has been proposed is coronary microvascular dysfunction as an underlying condition, known to be prevalent among menopausal women, with acute triggers leading to varying regional microvascular spasm.29
Our data also show a difference in recurrence-free survival based on the pattern of wall motion abnormalities at the time of the index TTS event. This was not observed in the prior multicenter study of recurrent TTS,12 and the pathophysiology of this observation merits further investigation.
We did not observe significant differences in the scores on detailed psychosocial questionnaires completed at the time of registry enrollment by participants who developed at least 1 recurrent TTS event compared to those who did not have a recurrent event. These similar scores on detailed instruments may suggest that there are underlying conditions separate from any psychosocial factors that impact susceptibility to TTS; prior work has suggested there may be a genetic predisposition30 and that microvascular dysfunction may be an important predisposing condition.31 In addition to this detailed psychosocial data, the clinical, echocardiographic, and angiographic data obtained at the time of the acute event were similar between these groups.
While our detailed psychosocial data were not able to distinguish participants who developed recurrence from those who did not, there was notably a high burden of adverse mental health symptoms in all participants with a prior episode of TTS. The symptoms of heart-focused anxiety based on the Cardiac Anxiety Questionnaire were higher in our study compared with observations in prior trials of post-myocardial infarction patients.32 The median score of our participants on the STAI was above the cutoff consistent with anxiety,33 the median score on the post-traumatic stress disorder (PTSD) civilian checklist in both groups was above the cutoff suggesting the presence of PTSD,34 and the scores we observed on the Early Trauma Inventory Self-Report were consistent with scores observed in psychiatric populations.24
Compared to prior studies,12,16,25,35 our registry entails a deeper phenotyping of the psychosocial profile of participants with TTS. Our surveys assessed multiple domains of the participants’ psychosocial status, including their heart-focused anxiety, anxiety more broadly, depression, PTSD symptoms, and early trauma. It also allowed for a quantitative measure of the degree of these psychosocial conditions, rather than a binary presence or absence of a diagnosis. While this deep phenotyping did not predict recurrence, it is important to note that the psychosocial profile we observed across all participants may portend an increased risk for adverse outcomes. There have been multiple prior studies associating increased scores on psychosocial questionnaires with adverse outcomes in patients with cardiovascular disease. Increased anxiety trait based on the STAI was independently associated with a composite outcome of all-cause mortality, readmission or death in a population of patients with heart failure with preserved ejection fraction.36 A meta-analysis including studies of patients after myocardial infarction demonstrated worse short- and long-term outcomes in patients with anxiety.37 Using the PSS, higher stress levels were associated with an increased risk of all-cause mortality in a cohort of 765 patients with peripheral arterial disease.38 In addition, early life trauma assessed using the ETISR-SF has been associated with increased microvolt T-wave alternans during mental stress, an electrocardiographic sign of increased arrhythmic risk.39 Furthermore, depression has been associated with increased mortality in patients with chronic heart failure.40 Given the association of psychosocial stress as defined by these scales with adverse outcomes in other cardiovascular diseases, our observation of significant psychosocial stress in TTS participants should be explored in future studies.
Limitations of our study include the relatively small sample size and therefore limited power to detect differences between the groups. We note, however, that even the largest published series of recurrent TTS only included 66 such patients.25 Due to our recruitment strategy in which participants enroll remotely after an episode of TTS, we are not able to infer an incidence of recurrent Takotsubo, and may select for a subset of TTS patients who are interested in participating in a trial. Furthermore, our recruitment strategy resulted in a longer follow-up period in the group with recurrence than the group without recurrence, which may introduce additional bias between groups. Since our psychological assessment is conducted at registry enrollment and after the index event, we cannot distinguish whether the adverse psychosocial characteristics we observed are cause or effect of the TTS episode. The lack of difference in these characteristics between participants with and without recurrence may reflect a common psychological state after a TTS event. A strength of our study relative to prior work is the deep phenotyping of the psychosocial profile of participants. Our study is also the first to our knowledge to measure detailed psychosocial stress markers including the STAI, PSS, and ETISR-SF in TTS participants.
Conclusions
In conclusion, we observed at least one recurrent TTS episode in 17% of registry participants, including multiple years after the index event. Importantly, in many cases recurrent TTS episodes were found to be brought on by different triggers and had different wall motion abnormalities between events. The wall motion abnormality pattern at the index event impacted the recurrence-free survival, though larger studies are needed to confirm this finding. TTS participants had a high rate of adverse psychosocial stress characteristics, which may play an important role in poor long-term outcomes. Further investigation is needed to establish predictors of recurrent events and identify preventive strategies to lower the risk of recurrence in these patients.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with a history of TTS and new cardiac symptoms after recovering from the index event, clinicians should consider the possibility of a recurrent Takotsubo event. Recurrent Takotsubo events may present differently from the index event, with different triggers and a different pattern of wall motion abnormalities.
TRANSLATIONAL OUTLOOK: Further investigation utilizing biomarkers and imaging modalities are needed to understand the pathophysiology of adverse long-term outcomes, including recurrence, after an initial TTS event. Additional studies will be needed to assess whether medical and psychosocial treatments can lower the risk of recurrent Takotsubo events.
Funding support and author disclosures
This work was supported by R01HL124649, U54 AG065141, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, the Adelson Family Foundation, and the Chester and Susan Zerlin Family Charitable Fund, Cedars-Sinai Medical Center, Los Angeles, California. Dr Bairey Merz has a relationship with SNL Telemedicine and iRhythm. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Ghadri J.-R., Wittstein I.S., Prasad A., et al. International expert consensus document on takotsubo syndrome (Part I): clinical characteristics, diagnostic Criteria, and pathophysiology. Eur Heart J. 2018;39:2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharkey S.W., Lesser J.R., Zenovich A.G., et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 3.Redfors B., Vedad R., Angerås O., et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction — a report from the SWEDEHEART11Swedish web system for enhancement of evidence-based care in heart disease evaluated according to recommended therapies. registry. Int J Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 4.Ghadri J.-R., Wittstein I.S., Prasad A., et al. International expert consensus document on takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharkey S.W., Pink V.R., Lesser J.R., Garberich R.F., Maron M.S., Maron B.J. Clinical profile of patients with high-risk tako-tsubo cardiomyopathy. Am J Cardiol. 2015;116:765–772. doi: 10.1016/j.amjcard.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 6.Stiermaier T., Moeller C., Oehler K., et al. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail. 2016;18:650–656. doi: 10.1002/ejhf.494. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey S.W., Windenburg D.C., Lesser J.R., et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–341. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 8.Templin C., Ghadri J.R., Diekmann J., et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 9.Ghadri J.R., Kato K., Cammann V.L., et al. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–882. doi: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Scally C., Rudd A., Mezincescu A., et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (takotsubo) cardiomyopathy. Circulation. 2018;137:1039–1048. doi: 10.1161/CIRCULATIONAHA.117.031841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt J.H., Bang L.E., RØRth R., et al. Long-term risk of death and hospitalization in patients with heart failure and takotsubo syndrome: insights from a nationwide cohort. J Card Fail. 2022;28:1534–1544. doi: 10.1016/j.cardfail.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 12.El-Battrawy I., Santoro F., Stiermaier T., et al. Incidence and clinical impact of recurrent takotsubo syndrome: results from the GEIST registry. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau C., Chiu S., Nayak R., Lin B., Lee M.-S. Survival and risk of recurrence of takotsubo syndrome. Heart. 2021;107:1160–1166. doi: 10.1136/heartjnl-2020-318028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh K., Carson K., Usmani Z., Sawhney G., Shah R., Horowitz J. Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. Int J Cardiol. 2014;174:696–701. doi: 10.1016/j.ijcard.2014.04.221. [DOI] [PubMed] [Google Scholar]
- 15.Lyon A.R., Bossone E., Schneider B., et al. Current state of knowledge on takotsubo syndrome: a position statement from the taskforce on takotsubo syndrome of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 16.Nayeri A., Rafla-Yuan E., Farber-Eger E., et al. Pre-existing psychiatric illness is associated with increased risk of recurrent takotsubo cardiomyopathy. Psychosomatics. 2017;58:527–532. doi: 10.1016/j.psym.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtman J.H., Froelicher E.S., Blumenthal J.A., et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations. Circulation. 2014;129:1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 18.Obrutu O., Maughan J., Tjoe B., et al. Smidt heart Institute takotsubo registry – study design and baseline characteristics. Am Heart J: Cardiol Res Pract. 2022;13 doi: 10.1016/j.ahjo.2022.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spielberger C., Gorsuch R., Lushene R., Vagg P.R., Jacobs G. In: Manual for the State-Trait Anxiety Inventory (Form Y1 – Y2) Charles D.S., Richard L.G., Robert E.L., Vagg P.R., Jacobs G.A., editors. CA:Consulting Psychologists Press; 1983. [Google Scholar]
- 20.Eifert G.H., Thompson R.N., Zvolensky M.J., et al. The cardiac anxiety questionnaire: development and preliminary validity. Behav Res Ther. 2000;38:1039–1053. doi: 10.1016/s0005-7967(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 21.Weathers F.W., Litz B., Herman D., Juska J., Keane T. PTSD Checklist—Civilian Version (PCL-C) [Database record]. Washington, DC: APA PsycTests. 1993 [Google Scholar]
- 22.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 23.Spitzer R.L., Kroenke K., Williams J.B. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 24.Bremner J.D., Bolus R., Mayer E.A. Psychometric properties of the early trauma inventory-self report. J Nerv Ment Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato K., Vece D.D., Cammann V.L., et al. Takotsubo recurrence. J Am Coll Cardiol. 2019;73:982–984. doi: 10.1016/j.jacc.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Pelliccia F., Kaski J.C., Crea F., Camici P.G. Pathophysiology of takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 27.Paur H., Wright P.T., Sikkel M.B., et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi–dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126:697–706. doi: 10.1161/CIRCULATIONAHA.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-Cordón C., Núñez-Gil I.J., Martín de Miguel I., et al. Takotsubo syndrome, stressful triggers, and risk of recurrence. Am J Cardiol. 2023;205:58–62. doi: 10.1016/j.amjcard.2023.07.155. [DOI] [PubMed] [Google Scholar]
- 29.Galiuto L., De Caterina A.R., Porfidia A., et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31:1319–1327. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 30.Limongelli G., Masarone D., Maddaloni V., et al. Genetics of takotsubo syndrome. Heart Fail Clin. 2016;12:499–506. doi: 10.1016/j.hfc.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Dong F., Yin L., Sisakian H., et al. Takotsubo syndrome is a coronary microvascular disease: experimental evidence. Eur Heart J. 2023;44:2244–2253. doi: 10.1093/eurheartj/ehad274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leissner P., Held C., Rondung E., Olsson E.M.G. The factor structure of the cardiac anxiety questionnaire, and validation in a post-MI population. BMC Med Res Methodol. 2022;22:338. doi: 10.1186/s12874-022-01820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ercan I., Hafizoglu S., Ozkaya G., Kirli S., Yalcintas E., Akaya C. Examining cut-off values for the state-trait anxiety inventory. Rev Argent Clin. Psicol. 2015;24:143–148. [Google Scholar]
- 34.Bovin M.J., Marx B.P., Weathers F.W., et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28:1379–1391. doi: 10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- 35.Shaw K.E., Lund P.G., Witt D., et al. Super recurrence of takotsubo syndrome: clinical characteristics and late cardiac outcomes. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.029144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin T.K., Hsu B.C., Li Y.D., et al. Prognostic value of anxiety between heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen Y., Yang Y., Shen J., Luo S. Anxiety and prognosis of patients with myocardial infarction: a meta-analysis. Clin Cardiol. 2021;44:761–770. doi: 10.1002/clc.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik A.O., Peri-Okonny P., Gosch K., et al. Association of perceived stress levels with long-term mortality in patients with peripheral artery disease. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah A.J., Weeks V., Lampert R., et al. Early life trauma is associated with increased microvolt T-wave alternans during mental stress challenge: a substudy of mental stress ischemia: prognosis and genetic influences. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W., Kuchibhatla M., Cuffe M.S., et al. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation. 2004;110:3452–3456. doi: 10.1161/01.CIR.0000148138.25157.F9. [DOI] [PubMed] [Google Scholar]