Abstract
Chronic hepatitis B (CHB) is one of the important public health problems worldwide. Major advances have been made in the treatment of CHB during the past several years. This article systemically reviews advances in the application of HBV DNA quantitation and three approved drugs for HBV treatment, and presents an updated and practical clinical approach to managing CHB. Highly sensitive PCR-based quantitation of HBV DNA makes it possible to precisely determine pre-treatment HBV load and monitor HBV DNA response during treatment. HBV DNA level, HBeAg status, degree of hepatic histological activity and fibrosis, and serum transaminases are the most important parameters in determining indication, regimen, and duration of HBV treatment. Although interferon alfa-2b, lamivudine, and adefovir are all approved as initial HBV treatment, understanding the advantages and advantages of each agent is important in choosing the best treatment for each individual patient with CHB.
Keywords: Chronic Hepatitis B, Management, treatment
1. Introduction
It is estimated that 350 million of individuals are infected by hepatitis B virus (HBV) worldwide. Major advances have been made in the treatment of chronic hepatitis B (CHB) during the past several years. While increased available therapeutic agents improve efficacy of HBV treatment, it is also important to understand the general principles in optimizing these therapies. Several guidelines have been published on management of CHB that well summarized current standard practice 1-3. Through a systematic review of the literature, this article is aimed to provide a step-wise updated and practical clinical approach to management of CHB.
2. Clinical Presentation of Chronic HBV Infection
As discussed by Drs. Zhang and Pan in this special issue, the clinical presentation of chronic HBV infection can be quite different ranging from inactive carrier status to HBV-cirrhosis or hepatocellular carcinoma (HCC). Understanding the spectrum and clinical presentation of chronic HBV infection is prerequisite for optimizing HBV treatment. Chronic HBV infection is defined as positive HBsAg for > 6 months that has been traditionally classified as the following subgroups 4-6:
Inactive HBV Carrier State
This group of patients has positive HBsAg and a low level of HBV load (i.e. < 1 x 105 copies/ml). Clinically, these patients have normal transaminases (i.e. ALT and AST) and no symptoms related to HBV infection. Histologically, most patients do not have significant hepatitis.
Three different outcomes have been described in inactive HBV carriers. While 70-80% remain in inactive HBV carriers indefinitely, 10-20% may develop HBeAg-positive CHB presented with a high level of HBV DNA, elevated transaminases and hepatic inflammation. Anti-HBe-positive hepatitis may occur in inactive HBV carriers, but the frequency of this transition remains unknown. Approximately 0.5% of the inactive HBV carriers spontaneously lose HBsAg annually. It should be noted that all HBV carriers are at increased risk of developing HCC.
Chronic Hepatitis B (CHB)
CHB is characterized by positive HBsAg for greater than 6 months, serum HBV DNA ≥1 x 106 copies/ml, and persistent or intermittent elevation of serum ALT/AST. A liver biopsy in most of these patients shows chronic hepatitis. Depending on HBeAg status, these patients can be further classified as following two subgroups:
1) HBeAg-positive CHB
Patients with HBeAg-positive CHB present with positive HBsAg and HBeAg in serum that is associated with active HBV replication, infectivity, and hepatic inflammation. Depending on the mode of HBV transmission, spontaneous seroconversion from HBeAg to anti-HBe is variable. Most patients underwent seroconversion remain sustained remission of HBV infection that is associated with normal transaminases and a low or undetectable level of serum HBV DNA although serum HBsAg may remain positive.
2) HBeAg-negative CHB
Patients with HBeAg-negative CHB present with positive HBsAg, but negative HBeAg in serum that is associated with active HBV replication, elevated transaminases, and hepatic inflammation. Pathologically, it is secondary to mutant viral infection in HBV pre-core or pre-core promoter region. The prevalence of HBeAg-negative CHB varies worldwide from 14% to 33%. In patients with HBeAg-negative CHB, approximately half of the patients had serum HBV DNA < 105 copies/ml 19. HBeAg-negative CHB is usually progressive and less responsive to HBV treatment.
HBV-cirrhosis
Approximately 20% of patients with CHB develop HBV-cirrhosis. Although HBV-cirrhosis represents a continued progression of CHB, we classify it as a different subgroup because of different treatment approach. HBV load varies in this group of patients although it is usually low. Like those with CHB, patients with HBV-cirrhosis can be either HBeAg or anti-HBe positive. Although thrombocytopenia, splenomegaly, and hypoalubuminemia are indicative of cirrhosis, a liver biopsy provides histological diagnosis. Patients with HBV-cirrhosis can be compensated or decompensated. The later presents with esophageal bleeding, ascites, hepatic encephalopathy, severe hyperbilirulinemia, and/or coagulopathy. Those with decompensated HBV-cirrhosis should be referred for liver transplant evaluation.
3. Available HBV Therapies
To date, the Food and Drug Administration (FDA) approved three treatments for chronic HBV infection in the United States. They are interferon (IFN) alfa-2b, lamivudine (LAM), and adefovir (ADV) 1-3, 7-10. Table 1 summarizes a comparison of these three therapeutic agents.
Table 1.
| Parameters | Interferon | Lamivudine | Adefovir |
|---|---|---|---|
| Dosing regimen | 5 MU/day, or 10 MU/TIW | 100 mg/day | 10 mg/day |
| Duration of treatment | |||
| HBeAg + CHB | 4-6 months | > 1 year | > 1 year |
| HBeAg - CHB | 1 year | > 1 year | > 1 year |
| HBeAg seroconversion (%) (In HBeAg + CHB) | 18 | 16-18 (After 1 year treatment) | 12 (After 1 year treatment) |
| Durability of response (%) (After HBeAg seroconversion) | 80-90 (At 4-8 years) | 77 (At 3 year) | 91 (At a mean of 55 weeks) |
| Route of administration | Subcutaneous | Oral | Oral |
| Tolerability | Poor | Good | Good |
| Cost | High | Low | Intermediate |
MU: Million international unit; TIW: Three times a week.
Interferon (IFN) alfa-2b
As a potent immune modulator, IFN has both direct and indirect anti-HBV effect although its precise mechanisms remain to be defined. IFN is the first FDA approved agent for HBV infection. A meta-analysis showed that 33% of treated patients loss HBeAg compared to only 12% of controls 11. In addition, 7.8% of treated subjects loss HBsAg versus only 1.8% of controls had spontaneous loss of HBsAg 11. The response rate to IFN treatment varies in different ethnic groups. Asian patients with normal ALT level respond poorly to this treatment. Studies indicated that a prolonged course of IFN treatment may improve response rate 12. One clinical trial has reported pegylated-IFN alfa-2a further improved response rate in patients with HBeAg-positive CHB 13. Although the results need to be reconfirmed by larger clinical trials, this might provide us with an additional option in managing CHB.
The course of IFN treatment is usually 16–24 weeks that offers a small, but certain opportunity of HBsAg clearance. Response to IFN treatment is usually durable. Reactivation of HBV infection occurs in approximately 10-15% of patients who responded to treatment, most commonly within a year after discontinuing IFN treatment. IFN is administrated by subcutaneous injection, associated with broad side effects, and represents an expensive treatment regimen.
Lamivudine (LAM)
As a nucleoside analogue, LAM inhibits HBV DNA replication by suppressing HBV DNA synthesis. As summarized in table 1, one-year of LAM treatment with 100 mg, once a day results in significant HBeAg seroconversion, suppression of HBV DNA, normalization of transaminases, and improved histology. The overall response rate to one year of LAM treatment as determined by HBeAg seroconvertion is approximately 16% (vs. 4% in the placebo group) that is comparable to a full course of IFN treatment 8, 11. A prolonged course of LAM treatment further improves the response rate 14. LAM represents a safe, cost-effective, and convenient HBV treatment regimen, but it does not result in HBsAg clearance and the response to LAM is less durable than that to IFN (Table 1). It is well known that LAM treatment is associated with YMDD mutation in the region of HBV DNA polymerase that causes LAM resistance. The incidence of YMDD mutation is treatment duration dependent, from 17% at 1-year treatment to 69% at 5-years treatment 1-3.
Adefovir (ADV) Dipivoxil
ADV dipivoxil is a prodrug of ADV and a nucleotide analogue of adenosine monophosphate that inhibits HBV DNA replication by suppressing HBV DNA polymerase. One year of ADV treatment with 10 mg, once a day results in significant HBeAg seroconversion, suppression of HBV DNA, normalization of transaminases, and improved histology. The overall response to one year of ADV treatment as determined by HBeAg seroconversion is approximately 12% (vs. 6% in placebo group) that could be increased to 23% by the end of 2-year treatment 9. In addition, ADV has been demonstrated as an effective treatment of HBeAg-negative CHB. In these patients, undetectable serum HBV DNA was achieved in 46% and 51% of these patients after 48 and 98 weeks of ADV treatment, respectively 10. Recent studies have demonstrated successful rescue therapy with ADV in patients who develop YMDD mutation 15, 16.
In contrast to LAM, the incidence of ADV-related HBV mutation and drug resistance is extremely low. For instance, the emergence of ADV resistant mutation, N236T, was only 1.8 and 3.9% in patients who received 2 and 3 years of this treatment, respectively 17, 18. Thus, ADV offers a unique advantage in patients who need a prolonged course of HBV treatment, especially those with HBeAg-negative CHB 10 and those with decompensated cirrhosis and are listed for or have received a liver transplantation 15. Patients who develop ADV resistant mutation remain sensitive to LAM treatment 16.
4. Indications of HBV Treatment
Historically, levels of HBV DNA and ALT, and histological activity of liver biopsy have been used as the three main factors to determine if a patient needs HBV treatment or not 1-3.
HBV DNA Levels
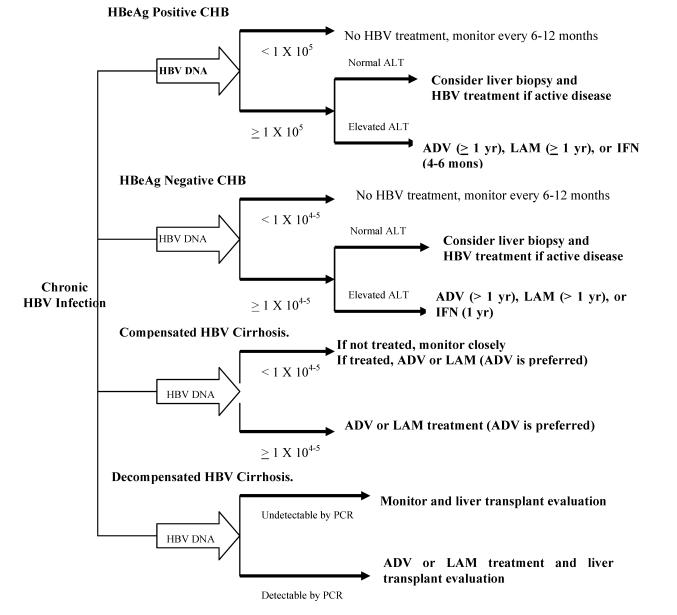
It is known that HBV is not directly pathogenic to hepatocytes and host immune response to HBV antigens expressed on the infected hepatocytes is the principle determinants of the liver injury. Thus, a high HBV load has been the primary indication for HBV infection. However, the threshold HBV level that is associated with progressive liver disease remains to be determined. In addition, patients with CHB may have fluctuating HBV DNA levels. A study revealed that only one third of patients with HBeAg-negative CHB and elevated ALT had HBV DNA levels persistently > 105 copies/ml 19. This suggested that an even lower HBV DNA threshold might be reasonable to indicate HBV treatment in patients HBeAg-negative CHB. Thus, it has recently been recommended that different HBV DNA levels should be used to determine HBV treatment depending on HBeAg status and clinical presentation of CHB 3. As summarized in Figure 1, HBV treatment should be considered in patients with HBeAg-positive CHB and HBV DNA ≥ 105 copies/ml in combination with elevated ALT level and histological activity. While in patients with HBeAg negative-CHB and patients with compensated HBV cirrhosis regardless HBeAg status, HBV treatment should be considered when HBV DNA ≥ 104 copies/ml 3. It should be noted that above cutoff HBV DNA levels are arbitrarily made based on limited research results, and not endorsed by all guidelines 1-3. Additional studies will be needed to verify these criteria.
Figure 1.
Histological Activity and Stage
Liver biopsy provides us with valuable information of histological activity and stage of HBV disease in patients with HBV infection. It is known that patients with chronic HBV infection and active hepatic inflammation carry a significantly higher risk of disease progression. Histological staging of HBV disease is clinically very valuable in assessing degree of fibrosis and predicting disease progression. Good response to HBV treatment has been well associated with improved histology. Therefore, a pre-HBV treatment liver biopsy is usually preferred by most hepatologists. However, for those with clear-cut indications for HBV treatment, a liver biopsy may not be necessary. As it will be discussed below, histological assessment is very valuable in determining HBV treatment in patients with persistently normal transaminases.
ALT Levels
For many years, ALT has been used as a standard surrogate for the activity of CHB. Thus, ALT level in combination with HBV DNA level and histological activity has been used as a determinant for HBV treatment 1-3. It is well accepted that elevated ALT should be used as one of the determinants for HBV treatment in patients with CHB. However, it remains controversial on at what level of ALT elevation a patient with CHB should be considered for HBV treatment 3.
On the other hand, patients with detectable HBV load (i.e. by HBV hybridization or HBV ≥ 105 copies/ml), but persistently normal transaminases have historically not been considered as candidates for HBV treatment based on the assumption that these patients usually have a slow progression and evidence that these patients usually have a low response rate to HBV treatments. However, the extent of liver injury and fibrosis is not always correlated with ALT levels 20. Furthermore, a sustained suppression of HBV replication is now more achievable than before. In addition, an active HBV replication has been associated with increased risk for HCC 21. Thus, it has become more accepted that a normal ALT level alone should not be used to determine HBV treatment in these patients. Instead, in patients with HBV DNA ≥ 105 copies/ml and persistently normal ALT levels, a liver biopsy might be considered (Figure 1). If active liver inflammation or advanced fibrosis is histologically confirmed, the patient should be considered for HBV treatment. Additional studies will be needed to further assess the efficacy and benefit of HBV treatment in this group of patients.
5. Goals of HBV Treatment
The rationale of HBV treatment is to significantly suppress HBV replication and prevent the progression of HBV-mediated liver injury that may cause cirrhosis, liver failure, or HCC. Therefore, the primary goal of HBV treatment should focus on maintaining sustained HBV DNA suppression. This will lead to the other benefits, i.e. the secondary aims of therapy, including normalization of transaminases, histological improvement, reduction of cirrhosis and the related complications, and the need of liver transplantation 1-3.
For patients with HBeAg-positive CHB, HBeAg seroconversion to anti-HBe has been associated with a durable and sustained HBV suppression. Hence, this has been used as an end point of the treatment in this group of patients. It has been reported that cirrhosis may occur in those who discontinued HBV treatment after achieved HBeAg seroconversion. However, it remains unclear whether maintenance HBV treatment will further slow down disease progression in these patients. For patients with HBeAg-negative CHB, seroconversion of HBeAg is absent and discontinuation of this treatment is associated with high incidence of HBV relapse. Thus, a long-term treatment to maintain sustained HBV suppression is usually necessary in these patients. Although loss of HBsAg is desirable, it is rarely achieved with currently available HBV treatments. Thus, loss of HBsAg is not one of the common goals for HBV treatments.
6. Predictors of HBV Treatment Response
Knowing clinical, biochemical, and virological predictive factors to HBV treatment response is important for planning and monitoring HBV treatment. A low level of pre-treatment serum HBV DNA, high levels of ALT and histological activity, a history of adulthood HBV infection, and non-Asian ethnic origin have been associated with higher sustained response rates to IFN treatment 22. Duration of IFN treatment also affects response. For instance, 32- weeks treatment is superior to 16 weeks treatment 12. The predictors of response to LAM and ADV therapies are similar to those for IFN except that baseline HBV DNA may not be very important.
7. Individualization of HBV Treatment
As summarized in Table 1 and Figure 1, although IFN, LAM, and ADV are all FDA approved agents for HBV treatment, the differences in drug administration, duration and efficacy of the treatment, frequency of drug resistance, and cost do exist in these three drugs. Thus, an HBV treatment should be tailored based on the type of chronic HBV infection, host, virus, and drug-related factors. Although it remains controversial, most published guidelines recommend the following principles in choosing HBV treatment 1-3.
In patients with HBeAg-positive CHB, LAM, ADV, and IFN are all the first line of HBV treatment. Overall, LAM and ADV are probably more favorable due to convenient administration, better tolerance, and less cost. When planning a LAM treatment, medical adherence and regular follow-up should be assured because of a relatively high frequency of drug-resistant mutation and flare of HBV disease. IFN treatment might be an option for those who prefer a shorter and definitive course of HBV treatment.
To date, there is no study indicating a significant difference of treatment response in these three drugs in patients with HBeAg-negative CHB. However, these patients carry a high incidence of relapse after the treatment is discontinued. Thus, these patients usually need a prolonged course of HBV treatment. As a convenient regimen with extremely low frequency of HBV mutation and drug resistance, ADV is a preferred treatment regimen in these patients.
Likewise, patients with HBV cirrhosis, especially hepatic decompensation and listed for liver transplantation, and patients who underwent liver transplantation usually need a prolonged course of HBV treatment. In addition, any episode of HBV disease flare induced by drug resistance could negatively impact outcomes in these patients. Clearly, ADV is a preferred treatment regimen in these patients. A recent study has shown that ADV treatment is effective and well tolerated in this group of patients 15. A high frequency of YMDD mutation and flare of HBV disease has been demonstrated when LAM was used in this group of patients. IFN is usually poorly tolerated and could induce hepatic decompensation, and therefore relatively contraindicated in this group of patients.
The value of combination HBV treatment remains to be determined. Results on efficacy of IFN and LAM combination have been inconsistent. Combination of LAM with ADV appears not synergistic. However, a brief course of combination of LAM with ADV is thought reasonable to reduce flare when dealing with drug resistance and planning switch these two drugs.
8. On-treatment Monitoring and Duration of Therapy
Patients who receive HBV treatment should be monitored regularly. The interval of follow-up visits may vary depending on regimen and duration of the HBV treatment. For instance, those on IFN therapy may need to be monitored every 4-6 weeks, but those on LAM or ADV should be monitored every 3-6 months. On-treatment monitoring should focus on medical adherence and tolerance, biochemical and virological responses to these treatments. Comparing to HCV treatment, the frequency needed for follow-up HBV DNA measurement is less defined during HBV treatment. However, it is recommended whenever a breakthrough of ALT and other liver tests or clinical deterioration occurs during the treatment. A rising or relapse of HBV DNA level indicates drug resistance. Special assays of drug resistance related HBV DNA mutation provide virological evidence of this diagnosis.
Currently recommended duration of IFN treatment is 16-24 weeks. It remains to be determined whether a prolonged course of this treatment may further improve the response rate, but this might not be practical due to a high frequency of IFN- induced adverse effects. For patients with HBeAg-positive CHB, HBeAg seroconversion to anti-HBe has been used as an end point of both ALM and ADV treatments. The finding that the virologic response to LAM treatment is less durable than that to IFN treatment raised a question whether LAM treatment should be continued after HBeAg seroconversion to anti-HBe. It was reported that additional 3-6 months of LAM treatment after HBeAg seroconversion could further sustain virologic response in these patients 3. Thus, it is recommended that LAM treatment should be continued for additional 3-6 months after HBeAg seroconversion is confirmed 2, 3. Preliminary data suggested that HBeAg seroconversion appears associated with a durable virologic response to ADV treatment 23. Long-term ADV or LAM treatment is usually needed in patients with HBeAg-negative CHB and those with decompensated HBV liver disease no matter their candidacy for liver transplantation. Additional studies are needed on how to individualize HBV treatment in these patients.
9. HBV Treatment in Special Patient Groups
Chronic HBV Infection with Persistently Normal Transaminases
Chronic HBV infection may present with high level of serum HBV DNA, but persistently normal transaminases. These patients usually have milder hepatic inflammation and tend to have a poor serological response to antiviral therapy. Thus, this group of patients is traditionally not considered for HBV treatment 1-3. However, it is well known that there is increased risk of developing HCC and flare of HBV disease in these patients 4, 21. Histologically, some of these patients have significant hepatic inflammation and fibrosis 20. Therefore, it is now recommended that a liver biopsy should be considered in these patients 3. For those who have histological evidence of active and/or advanced HBV disease, HBV treatment should be considered (Figure 1) 3. Although it is known that these patients respond a standard course of HBV treatment poorly, additional study is necessary to assess whether a prolonged course of HBV treatment improves virological response in these patients.
HBV and HIV Coinfection
It is known that HIV infection negatively impact the natural course of CHB, resulting in a more progressive HBV disease, increased incidence of HBV cirrhosis, and liver-related mortality. Although HBV treatment is usually highly indicated, clinical trials of HBV treatment remains limited in this special group of patients. The available preliminary studies indicated that the response rate of these patients to HBV treatment is low and less durable, especially those with a low CD4 account. However, it should be noted that most of these studies involved small cohort of patients, lacking a proper control, or reported a short-term observation. Thus, effort should be made to conduct multicenter, randomized clinical trials to assess the efficacy of available HBV treatments and define optimal treatment regimens in this group of patients. Several articles have systematically reviewed current literatures and provided recommendations on managing HBV disease in these patients 24, 25.
HBV Infection and Immunosuppressive Therapy or Cancer Chemotherapy
Approximately 20-50% of HBV carriers undergoing immunosuppressive therapy or cancer chemotherapy develop reactivation of HBV replication presented with hepatitis flare and rarely hepatic decompensation 26, 27. This may occur even in those with occult HBV infection 28. Administration of LAM prior to these treatments is associated with reduced frequency and severity of hepatitis B flare and improved survival in these patients 26, 29.
HBV Infection and Pregnancy
Both LAM and ADV are classified as category C, meaning certain risk for fetus during pregnancy. Although these is no consensus on managing HBV disease in female patients who plan to become pregnancy, several options have been proposed: (1) postpone HBV treatment if HBV DNA is low and disease activity is mild; (2) A full course of IFN treatment may be attempted for a possible virologic response; (3) start HBV treatment during third trimester to prevent HBV transmission. Two small studies, including a randomized study, suggested that LAM treatment started in third trimester is safe and effective in reducing HBV perinatal transmission 30, 31. However, LAM treatment should be combined with hepatitis B immnoglobulin and HBV vaccination of the newborns. In addition, another study indicated that it appears safe to continue LAM treatment in patients who became pregnant while receiving LAM treatment 32. The safety of ADV in pregnant patients remains unknown. It is recommended that any patients treated with LAM or ADV should be reported to the respective pregnancy registry 3.
HBV Infection and Liver Transplantation
Patients with HBV end-stage liver disease carry an increased risk of recurrent HBV infection after liver transplantation. In addition, utilization of anti-HBc positive organs could develop HBV infection in the recipients. Management of these two clinical entities will be discussed by Dr. Vierling in a separated article in this special HBV issue.
10. Research Directions
Although significant advances have been made in managing HBV infection, challenge remains in optimizing HBV therapy. First, further understanding HBV dynamics and correlation of the level of HBV replication with liver injury will be invaluable for us to define an evidence-based indication of HBV treatment in HBeAg-positive and HBeAg-negative chronic HBV infection. Second, several nucleoti(si)de analogues are being tested as new anti-HBV agents, such as entecavir, telbivudine, and clevudine 33, 34. This may offer us possibility to better individualize HBV treatment based on patient's condition and viral profile (i.e. drug resistance). Third, although early studies did not demonstrate improved treatment response 35, ongoing studies using modified regiments of combination HBV therapy may prove to be more effective and less drug resistant. All these will further improve the management of CHB.
Biography
Ke-Qin Hu, MD, is the Director of Hepatology Services and Associate Professor of Clinical Medicine, Divisions of Gastroenterology and Transplantation, University of California, Irvine, California, USA. His current researches include natural history and management of hepatitis B and C and chemoprevention of hepatocellular carcinoma.
References
- 1.The EASL Jury. EASL international consensus conference on hepatitis B: Consensus statement. J Hepatol. 2003;39:S3–S25. [PubMed] [Google Scholar]
- 2.Lok ASF, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology. 2004;39:857–861. doi: 10.1002/hep.20110. [DOI] [PubMed] [Google Scholar]
- 3.Keeffe EB, Dieterich DT, Han S-H, Jacobson IM, Martin P, Schiff ER. et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol. 2004;2:87–106. doi: 10.1016/s1542-3565(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G. Natural history and prognosis of hepatitis B. Semin Liv Dis. 2003;23:47–58. doi: 10.1055/s-2003-37590. [DOI] [PubMed] [Google Scholar]
- 5.McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liv Dis. 2004;24(Suppl 1):17–21. doi: 10.1055/s-2004-828674. [DOI] [PubMed] [Google Scholar]
- 6.Tram TT, Martin P. Hepatitis B: epidemiology and natural history. Clin Liver Dis. 2004;8:255–266. doi: 10.1016/j.cld.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Perrillo RP, Schiff ER, Davis GL, Bodenheimer Jr HC, Lindsay K, Payne J. et al. A randomized, controlled trial of interferon alfa-2b alone after prednisone withdrawal for the treatment of chronic hepatitis B. The hepatitis International Therapy Group. N Engl J Med. 1990;19:295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- 8.Lai C-L, Chie R-N, Leung NWY, Chang T-T, Guan R, Tai D-I. et al. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 9.Marcellin P, Chang TT, Tong MJ, Sievert W, Shiffman ML, Jeffers L. et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 10.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang T-T, Kitis G, Rizzetto M. et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 11.Wong DKH, Cheung AM, O'Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. Ann Inter Med. 1993;119:312–323. doi: 10.7326/0003-4819-119-4-199308150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Janssen HL, Gerken G, Carreno V, Marcellin P, Naoumov NV, Craxi A. et al. Interferon alpha for chronic hepatitis B infection: increased efficacy of prolonged treatment. Hepatology. 1999;30:238–243. doi: 10.1002/hep.510300113. [DOI] [PubMed] [Google Scholar]
- 13.Cooksley WGE, Piratvisuth T, Lee S-D, Mahachai V, Chao V-C, Tanwandee T. et al. Pegiterferon α-a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepatitis. 2003;10:298–305. doi: 10.1046/j.1365-2893.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 14.Leung NWY, Lai CL, Chang TT, Guan R, Tai DI, Ng KY. et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e ahtigen seroconversion rates: results after 3 years of therapy. Hepatology. 2002;22:1527–1532. doi: 10.1053/jhep.2001.25084. [DOI] [PubMed] [Google Scholar]
- 15.Schiff ER, Lai C-L, Hadziyannis S, Neuhaus P, Terrault N, Colombo M. et al. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419–1427. doi: 10.1016/j.hep.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Peters MG, Hann Hw H, Martin P, Heathcote EJ, Buggisch P, Rubin R. et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:343–347. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 17.Westland CE, Villeneuve J-P, Terrault NA, Zoulim F, Brosgart CL, Wulfsohn M. et al. Resistance surveillance of liver transplantation patients with lamivudine resitant hepatitis B virus (HBV) after 96 weeks of adefovir dipivoxil treatment. Hepatology. 2003;38(suppl. 1):160A. [Google Scholar]
- 18.Qi X, Snow A, Thibault V, Zhu Y, Curtis M, Hadziyannis S. et al. Week 144 resistance surveillance of adefovir dipivoxil-treated chronic hepatitis B patients. Gastroenterol. 2004;123(supp 2):A–660. [Google Scholar]
- 19.Chu CJ, Hussain M, Lok ASF. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology. 2002;36:1408–1415. doi: 10.1053/jhep.2002.36949. [DOI] [PubMed] [Google Scholar]
- 20.Yang LM, Xu KC, Zhao YL, Wu ZR, Chen TF, Qin ZY. et al. Clinical significance of liver biopsy in chronic hepatitis B patients with persistently normal transaminase. Chinese J Dig Dis. 2002;3:150–153. [Google Scholar]
- 21.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY. et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 22.Cooksley WG. Treatment of hepatitis B with interferon and combination therapy. Clin Liv Dis. 2004;8:353–370. doi: 10.1016/j.cld.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Chang TT, Shiffman M, Tong M, Marcellin P, Liaw YF, Luengrojanakul P. et al. Durability of HBeAg seroconversion after adefovir dipivoxil (ADV) treatment for chronic hepatitis B (CHB) J Hepatol. 2004;40(Suppl. 1):424. [Google Scholar]
- 24.Nunez M, Puoti M, Camino N, Soriano V. Treatment of hepatitis B in the human immunodeficiency virus-infected patients: present and future. Clin Infect Dis. 2003;37:1678–1685. doi: 10.1086/379774. [DOI] [PubMed] [Google Scholar]
- 25.Benhamou Y, Poynard T. Treatment of chronic hepatitis B virus infection in patients co-infected with human immunodeficiency virus. J Hepatol. 2003;39:S194–S199. doi: 10.1016/s0168-8278(03)00321-0. [DOI] [PubMed] [Google Scholar]
- 26.Lau GKK, He ML, Fong DYT, Bartholomeusz A, Au WY, Lie AKW. et al. Preemptive use of lamivudine reduced hepatitis B exacerbation after allogeneic hematopoetic cell transplantation. Hepatology. 2002;36:702–709. doi: 10.1053/jhep.2002.35068. [DOI] [PubMed] [Google Scholar]
- 27.Rossi G, Pelizzari A, Motta M, Puoti M. Primary prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HBsAg carriers with lymphoid malignancies treated with chemotherapy. Br J Haematol. 2001;115:58–62. doi: 10.1046/j.1365-2141.2001.03099.x. [DOI] [PubMed] [Google Scholar]
- 28.Hu K-Q. Occult HBV infection and its clinical implications. J Viral Hepatitis. 2002;9:245–254. doi: 10.1046/j.1365-2893.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- 29.Chan TM, Fang GX, Tang CSO, Cheng IKP, Lai KN, Ho SKN. Preemptive lamivudine therapy based on HBV DNA level in HBsAg-positive kidney allograft recipients. Hepatology. 2002;36:1246–1252. doi: 10.1053/jhep.2002.36156. [DOI] [PubMed] [Google Scholar]
- 30.Van Zonneveld M, Nunen AB, Niesters HGM, de Man RA, Schalm SW, Janssen HLA. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepatitis. 2003;10:294–297. doi: 10.1046/j.1365-2893.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 31.Li X-M, Yang Y-B, Hou H-Y, Shi Z-J, Shen H-M, Teng B-Q. et al. Intrerruption of HBV intrauterine transmission: A clinical study. World J Gastroenterol. 2003;9:1501–1503. doi: 10.3748/wjg.v9.i7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su G-G, Pan K-H, Zhao N-F, Fang S-H, Yang D-H, Zhou Y. Efficacy and safety of lamivudine treatment for chronic hepatitis B in pregnancy. World J Gastroenterol. 2003;10:910–912. doi: 10.3748/wjg.v10.i6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan DJ, Peters MG. Antiviral therapy: nucleotide and nucleoside analogs. Clin Liver Dis. 2004;8:371–385. doi: 10.1016/j.cld.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Schiff ER, Karayalcin S, Grimm I, Perillo R, Dienstag J, Husa P. et al. A placebo controlled study of lamivudine and interferon alpha-2b in patients with chronic hepatitis B who previously failed interferon therapy. Hepatology. 1998;28:388A. [Google Scholar]
- 35.Schalm SW, Heathcote H, Cianciara J, Farrell G, Sherman M, Willems B. et al. Lamivudine and interferon alpha combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:565–568. doi: 10.1136/gut.46.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]